Food Safety Assessment: Overview of Metrological Issues and Regulatory Aspects in the European Union
Abstract
:1. Introduction
2. COVID-19 and Food Safety
3. Food Safety—The European Framework
3.1. Food Safety: Definitions, Policy, Mission, and Approach of the European Commission
3.2. Contaminants
3.2.1. Mycotoxins
3.2.2. Contaminants of Emerging Concern
3.2.3. Nanomaterials
3.2.4. Process Contaminants
3.3. Official Controls
4. Metrology for Food Safety
4.1. Method Validation
4.2. Proficiency Testing
- external and independent evaluation and monitoring of performance on a continuous basis, which results in the quality of routine analyses being verified;
- identification of any problems in performing analyses and possibilities for corrective action. Therefore the return information can stimulate the continuous improvement of the laboratory [103];
- evaluation of the efficacy and comparability of the test or measurement methods used by the laboratory;
- guarantee of reliability for customers;
- training or retraining of staff on the basis of the results of such comparisons.
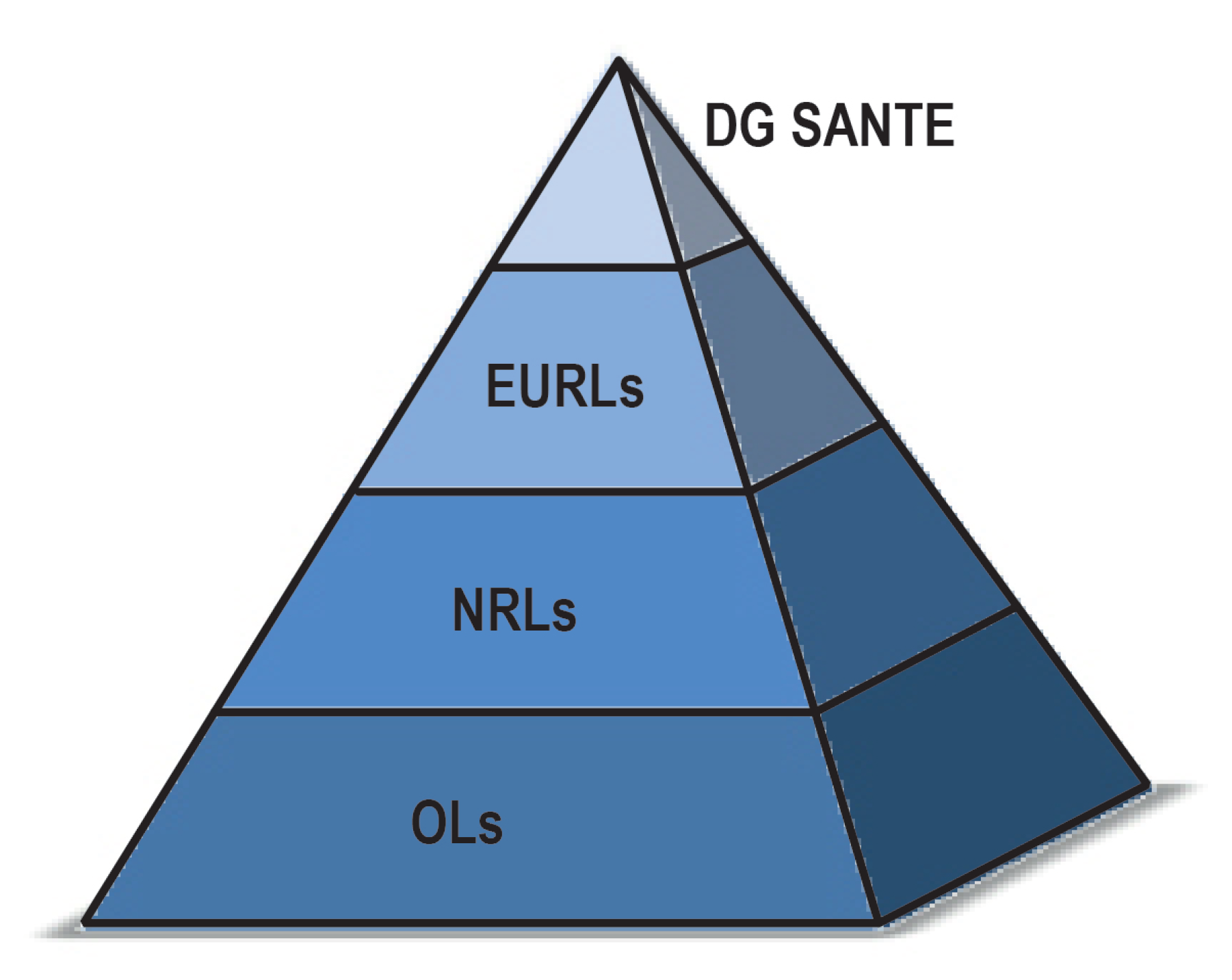
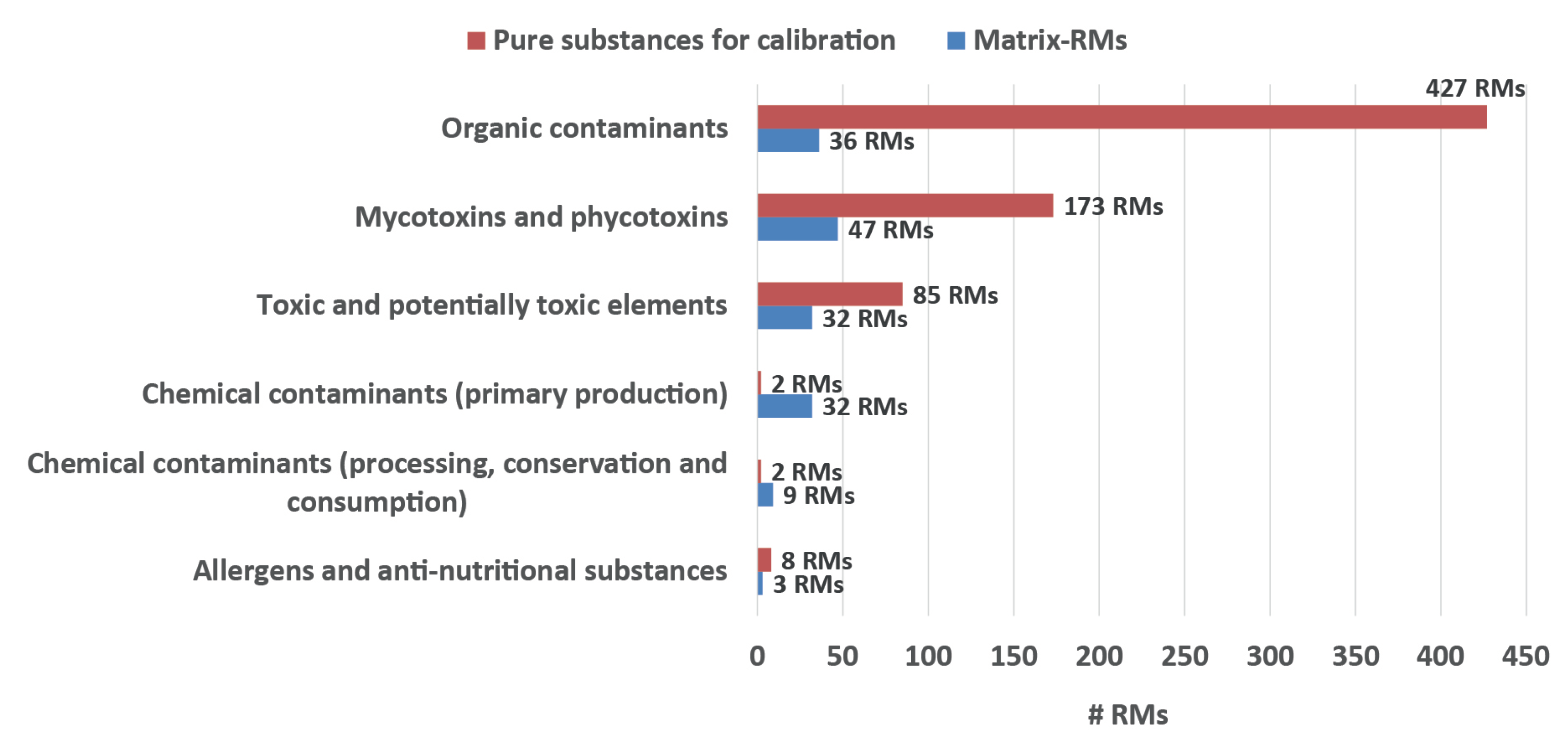
4.3. Reference Materials
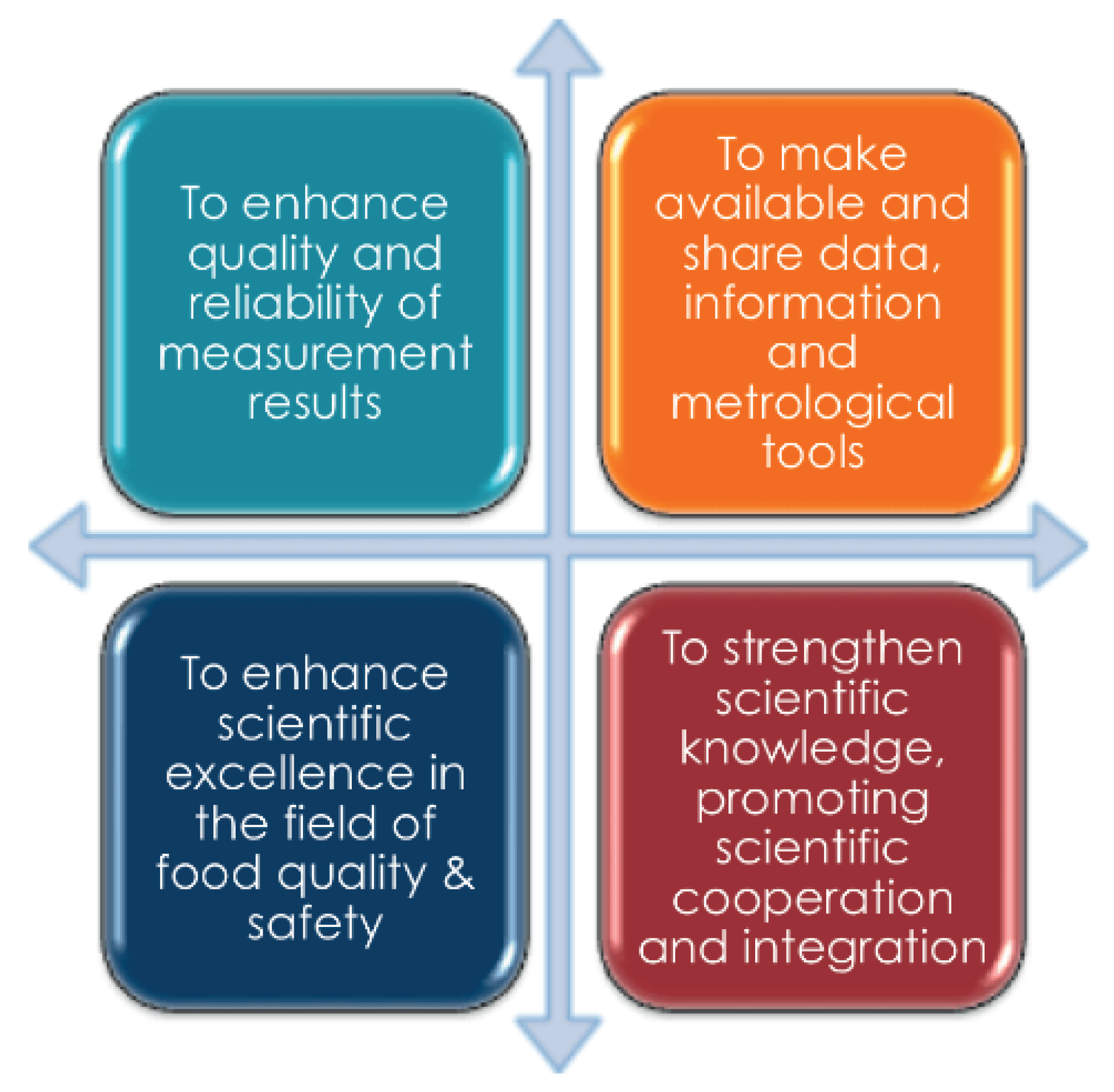
4.4. METROFOOD-RI as an Opportunity to Support Metrology in Food Safety
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gizaw, Z. Public health risks related to food safety issues in the food market: A systematic literature review. Environ. Health Prev. Med. 2019, 24, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Conference on Nutrition; World Health Organization. Nutrition Unit and Food and Agriculture Organization of the United Nations. In World Declaration and Plan of Action for Nutrition, Rome, December 1992; World Health Organization: Rome, Italy, 1992; Issued in collaboration with the Food and Agriculture Organization of the United Nations; Available online: https://apps.who.int/iris/handle/10665/61051 (accessed on 26 January 2022).
- The Burden of Foodborne Diseases in the WHO European Region; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.euro.who.int/en/health-topics/disease-prevention/food-safety/publications/2017/the-burden-of-foodborne-diseases-in-the-who-european-region-2017 (accessed on 26 January 2022).
- Safe and Healthy Food in Traditional Food Markets in the WHO European Region; WHO Regional Office for Europe: Copenhagen, Denmark, 2021.
- Fung, F.; Wang, H.S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bendeković, J.; Naletina, D.; Iva, N. Food safety and quality in the supply chain. In Trade perspectives 2015: Innovations in Food Retailing; Knego, N., Renko, S., Kneževic, B., Eds.; Faculty of Economics and Business Zagreb & Croatian Chamber of Economy: Zagreb, Croatia, 2015; Available online: https://www.bib.irb.hr/786708/download/786708.TP_Bendekovic_Naletina_Nola.pdf (accessed on 26 January 2022).
- Dulio, V.; van Bavel, B.; Brorström-Lundén, E.; Harmsen, J.; Hollender, J.; Schlabach, M.; Slobodnik, J.; Thomas, K.; Koschorreck, J. Emerging pollutants in the EU: 10 years of NORMAN in support of environmental policies and regulations. Environ. Sci. Eur. 2018, 30, 5. [Google Scholar] [CrossRef] [PubMed]
- Farré, M.; Barceló, D. Analysis of emerging contaminants in food. TrAC Trend. Anal. Chem. 2013, 43, 240–253. [Google Scholar] [CrossRef]
- Kantiani, L.; Llorca, M.; Sanchís, J.; Farré, M.; Barceló, D. Emerging food contaminants: A review. Anal. Bioanal. Chem. 2010, 398, 2413–2427. [Google Scholar] [CrossRef] [PubMed]
- Campo, J.; Picó, Y. Emerging contaminants and toxins. In Chemical Analysis of Food, 2nd ed.; Pico, Y., Ed.; Academic Press: Cambridge, MA, USA, 2020; Chapter 17; pp. 729–758. [Google Scholar] [CrossRef]
- White Paper on Food Safety; EU Commission: Brussels, Belgium, 2000; Available online: https://op.europa.eu/en/publication-detail/-/publication/6d4b523b-dad8-4449-b2b4-9fa9b0d6e2be (accessed on 26 January 2022).
- King, T.; Cole, M.; Farber, J.M.; Eisenbrand, G.; Zabaras, D.; Fox, E.M.; Hill, J.P. Food safety for food security: Relationship between global megatrends and developments in food safety. Trends Food Sci. Technol. 2017, 68, 160–175. [Google Scholar] [CrossRef]
- Vågsholm, I.; Arzoomand, N.S.; Boqvist, S. Food Security, Safety, and Sustainability-Getting the Trade-Offs Right. Front. Sustain. Food Syst. 2020, 4, 16. [Google Scholar] [CrossRef]
- FAO. The Future of Food Safety; FAO: Rome, Italy, 2019; Available online: https://www.fao.org/3/ca4289en/CA4289EN.pdf (accessed on 26 January 2022).
- Food Security and Nutrition and Sustainable Agriculture. Available online: https://sdgs.un.org/topics/food-security-and-nutrition-and-sustainable-agriculture (accessed on 26 January 2022).
- Durazzo, A.; Souto, E.B.; Lombardi-Boccia, G.; Santini, A.; Lucarini, M. Metrology, Agriculture and Food: Literature Quantitative Analysis. Agriculture 2021, 11, 889. [Google Scholar] [CrossRef]
- What Is Metrology? 2017. Available online: https://www.bipm.org/en/home (accessed on 26 January 2022).
- Rychlik, M.; Zappa, G.; Añorga, L.; Belc, N.; Castanheira, I.; Donard, O.F.X.; Kouřimská, L.; Ogrinc, N.; Ocké, M.C.; Presser, K.; et al. Ensuring Food Integrity by Metrology and FAIR Data Principles. Front. Chem. 2018, 6, 49. [Google Scholar] [CrossRef] [Green Version]
- COVID-19 and Food Safety: Guidance for Competent Authorities Responsible for National Food Safety Control Systems; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Food_Safety_authorities-2020.1 (accessed on 26 January 2022).
- COVID-19 and Food Safety: Guidance for Food Businesses. 2020. Available online: https://www.who.int/publications/i/item/covid-19-and-food-safety-guidance-for-food-businesses (accessed on 26 January 2022).
- Djekic, I.; Nikolić, A.; Uzunović, M.; Marijke, A.; Liu, A.; Han, J.; Brnčić, M.; Knežević, N.; Papademas, P.; Lemoniati, K.; et al. Covid-19 pandemic effects on food safety - Multi-country survey study. Food Control 2021, 122, 107800. [Google Scholar] [CrossRef]
- Trmčić, A.; Demmings, E.; Kniel, K.; Wiedmann, M.; Alcaine, S. Food Safety and Employee Health Implications of COVID-19: A Review. J. Food Prot. 2021, 84, 1973–1989. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Shahbaz, H.M.; Fatima, N.; Munir, S.; Holley, R.A. Food Safety During and After the Era of COVID-19 Pandemic. Front. Microbiol. 2020, 11, 1854. [Google Scholar] [CrossRef] [PubMed]
- Ouabdesselam, L.; Sayad, A. Impact of COVID-19 on Food Safety. Eur. J. Basic Med. Sci. 2020, 10, 27–32. [Google Scholar]
- Ray, L.C.; Collins, J.P.; Griffin, P.M.; Shah, H.J.; Boyle, M.M.; Cieslak, P.R.; Dunn, J.; Lathrop, S.; McGuire, S.; Rissman, T.; et al. Decreased Incidence of Infections Caused by Pathogens Transmitted Commonly Through Food During the COVID-19 Pandemic - Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2017–2020. MMWR Morb. Mortal. Wkly Rep. 2021, 70, 1332–1336. [Google Scholar] [CrossRef]
- Ståhl, K. (Ed.) Surveillance of Infectious Diseases in Animals and Humans in Sweden 2020; National Veterinary Institute (SVA): Uppsala, Sweden, 2020. [Google Scholar]
- A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. 2020. Available online: https://ec.europa.eu/food/horizontal-topics/farm-fork-strategy_en (accessed on 26 January 2022).
- Vittuari, M.; Bazzocchi, G.; Blasioli, S.; Cirone, F.; Maggio, A.; Orsini, F.; Penca, J.; Petruzzelli, M.; Specht, K.; Amghar, S.; et al. Envisioning the Future of European Food Systems: Approaches and Research Priorities After COVID-19. Front. Sustain. Food Syst. 2021, 5, 58. [Google Scholar] [CrossRef]
- Zhang, W.; He, H.; Zhu, L.; Liu, G.; Wu, L. Food Safety in Post-COVID-19 Pandemic: Challenges and Countermeasures. Biosensors 2021, 11, 71. [Google Scholar] [CrossRef]
- RASFF-Food and Feed Safety Alerts. Available online: https://ec.europa.eu/food/safety/rasff-food-and-feed-safety-alerts_en (accessed on 26 January 2022).
- Food Safety; European Commission: Brussels, Belgium, 2014. [CrossRef]
- European Parliament, Council of the European Union. Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying down Procedures in Matters of Food Safety. 2002. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32002R0178 (accessed on 26 January 2022).
- European Parliament, Council of the European Union. Regulation (EU) 2019/1381 of the European Parliament and of the Council of 20 June 2019 on the Transparency and Sustainability of the EU Risk Assessment in the Food Chain and Amending Regulations (EC) No 178/2002, (EC) No 1829/2003, (EC) No 1831/2003, (EC) No 2065/2003, (EC) No 1935/2004, (EC) No 1331/2008, (EC) No 1107/2009, (EU) 2015/2283 and Directive 2001/18/EC. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019R1381 (accessed on 26 January 2022).
- Codex Alimentarius Commission. General Principles of Food Hygiene CXC 1-1969. 2021. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/fr/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXC%2B1-1969%252FCXC_001e.pdf (accessed on 26 January 2022).
- EUR-Lex-Food Safety. Available online: https://eur-lex.europa.eu/summary/chapter/30.html?expand=3010,3011,3012,301006,301007#arrow_301007 (accessed on 26 January 2022).
- FAO. One Health: Food and Agricolture of the United Nations Strategic Action Plan; FAO: Rome, Italy, 2011; Available online: https://www.fao.org/documents/card/en/c/e193889c-28d5-55a0-9c03-beeac89e5960/ (accessed on 26 January 2022).
- One Health. Available online: https://www.fao.org/one-health/en/ (accessed on 26 January 2022).
- King, L.J. One health and food safety. In Institute of Medicine (US). Improving Food Safety Through a One Health Approach: Workshop Summary; National Academies Press (US): Washington, DC, USA, 2012. [Google Scholar]
- Boqvist, S.; Söderqvist, K.; Vågsholm, I. Food safety challenges and One Health within Europe. Acta Vet. Scand. 2018, 60, 1. [Google Scholar] [CrossRef]
- Contaminants. Available online: https://ec.europa.eu/food/safety/chemical-safety/contaminants_en (accessed on 26 January 2022).
- Sadiku, M.N.O.; Ashaolu, T.J.; Musa, S.M. Food Contamination: A Primer. Int. J. Adv. Sci. Res. Eng. (IJASRE) 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Di Stefano, V.; Avellone, G. Food Contaminants. J. Food Stud. 2014, 3, 88–103. [Google Scholar] [CrossRef] [Green Version]
- Nerín, C.; Aznar, M.; Carrizo, D. Food contamination during food process. Trends Food Sci. Technol. 2016, 48, 63–68. [Google Scholar] [CrossRef]
- Food Contaminants. 2008. Available online: https://ec.europa.eu/food/system/files/2016-10/cs_contaminants_factsheet_en.pdf (accessed on 26 January 2022).
- Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 Supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as Regards the Specific Compositional and Information Requirements for Infant Formula and Follow-On Formula and as Regards Requirements on Information Relating to Infant and Young Child Feeding. 2016. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2016.025.01.0001.01.ENG (accessed on 26 January 2022).
- Council of the European Union. Council Directive 2001/110/EC of 20 December 2001 Relating to Honey. 2001. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32001L0110 (accessed on 26 January 2022).
- Wenzl, T.; Haedrich, J.; Schaechtele, A.; Robouch, P.; Stroka, J. (Eds.) Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food. 2016. Available online: https://ec.europa.eu/food/system/files/2017-05/cs_contaminants_sampling_guid-doc-lod-loq.pdf (accessed on 26 January 2022).
- Eppe, G.; Schaechtele, A.; Haedrich, J.; Fernandes, A. (Eds.) Guidance Document on Measurement Uncertainty for Laboratories Performing PCDD/F and PCB Analysis Using Isotope Dilution Mass Spectrometry. 2017. Available online: https://ec.europa.eu/food/system/files/2017-05/cs_contaminants_sampling_guid-doc-pcdd-f-pcb.pdf (accessed on 26 January 2022).
- Guidance Document on Identification of Mycotoxins in Food and Feed. 2017. Available online: https://ec.europa.eu/food/system/files/2017-05/cs_contaminants_sampling_guid-doc-ident-mycotoxins.pdf (accessed on 26 January 2022).
- Method for the Determination of Ergot (Claviceps purpurea Tul.). Available online: https://ec.europa.eu/food/system/files/2017-05/cs_contaminants_sampling_guid-doc-determination-ergot.pdf (accessed on 26 January 2022).
- Guidance Document for Competent Authorities for the Control of Compliance with eu Legislation on Aflatoxins. 2010. Available online: https://ec.europa.eu/food/system/files/2016-10/cs_contaminants_sampling_analysis-guidance-2010_en.pdf (accessed on 26 January 2022).
- Guidance Document for the Implementation of Commission Regulation (EU) No 519/2014 of 16 May 2014 Amending Regulation (EC) No 401/2006 Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Food. 2014. Available online: https://ec.europa.eu/food/system/files/2016-10/cs_contaminants_sampling_guidance-sampling-final_en.pdf (accessed on 26 January 2022).
- Wright, C. Analytical methods for monitoring contaminants in food—An industrial perspective. J. Chromatogr. A 2009, 1216, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S. (Ed.) Food Analysis; Springer: Boston, MA, USA, 2010. [Google Scholar]
- Ismail, B.; Reuhs, B.L.; Nielsen, S.S. Analysis of Food Contaminants, Residues, and Chemical Constituents of Concern. In Food Analysis; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; pp. 317–349. [Google Scholar] [CrossRef]
- Chiou, J.; Leung, A.H.H.; Lee, H.W.; Wong, W.T. Rapid testing methods for food contaminants and toxicants. J. Integr. Agric. 2015, 14, 2243–2264. [Google Scholar] [CrossRef] [Green Version]
- Adebo, O.A.; Molelekoa, T.; Makhuvele, R.; Adebiyi, J.A.; Oyedeji, A.B.; Gbashi, S.; Adefisoye, M.A.; Ogundele, O.M.; Njobeh, P.B. A review on novel non-thermal food processing techniques for mycotoxin reduction. Int. J. Food Sci. Technol. 2021, 56, 13–27. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, S.; Du, H.; Kebede, H.; Liu, Y.; Xing, F. Contamination status of major mycotoxins in agricultural product and food stuff in Europe. Food Control 2021, 127, 108120. [Google Scholar] [CrossRef]
- Iqbal, S.Z. Mycotoxins in food, recent development in food analysis and future challenges; a review. Curr. Opin. Food Sci. 2021, 42, 237–247. [Google Scholar] [CrossRef]
- EFSA-Topics-Mycotoxins. Available online: https://www.efsa.europa.eu/en/topics/topic/mycotoxins (accessed on 26 January 2022).
- Tolosa, J.; Rodríguez-Carrasco, Y.; Ruiz, M.J.; Vila-Donat, P. Multi-mycotoxin occurrence in feed, metabolism and carry-over to animal-derived food products: A review. Food Chem. Toxicol. 2021, 158, 112661. [Google Scholar] [CrossRef]
- Nobili, C.; De Acutis, A.; Reverberi, M.; Bello, C.; Leone, G.P.; Palumbo, D.; Natella, F.; Procacci, S.; Zjalic, S.; Brunori, A. Buckwheat Hull Extracts Inhibit Aspergillus flavus Growth and AFB1 Biosynthesis. Front. Microbiol. 2019, 10, 1997. [Google Scholar] [CrossRef] [Green Version]
- Nobili, C.; D’Angeli, S.; Altamura, M.M.; Scala, V.; Fabbri, A.A.; Reverberi, M.; Fanelli, C. ROS and 9-oxylipins are correlated with deoxynivalenol accumulation in the germinating caryopses of Triticum aestivum after Fusarium graminearum infection. Eur. J. Plant Pathol. 2014, 139, 429–444. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef]
- Braun, D.; Eiser, M.; Puntscher, H.; Marko, D.; Warth, B. Natural contaminants in infant food: The case of regulated and emerging mycotoxins. Food Control 2021, 123, 107676. [Google Scholar] [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Malule, H.; Quiñones-Murillo, D.H.; Manotas-Duque, D. Emerging contaminants as global environmental hazards. A bibliometric analysis. Emerg. Contam. 2020, 6, 179–193. [Google Scholar] [CrossRef]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Tushara Chaminda, G.G.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- ANSES- French Agency for Food, Environmental and Occupational Health & Safety, France; Anastasi, E.; Riviere, G.; Teste, B. Nanomaterials in Food—Prioritisation & Assessment. EFSA J. 2019, 17, e170909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. Commission Recommendation of 18 October 2011 on the Definition of Nanomaterial. 2011. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32011H0696 (accessed on 26 January 2022).
- ISO/TS 80004-1:2015; Nanotechnologies—Vocabulary—Part 1: Core Terms. ISO: Geneva, Switzerland, 2015.
- Ameta, S.K.; Rai, A.K.; Hiran, D.; Ameta, R.; Ameta, S.C. Use of Nanomaterials in Food Science. In Biogenic Nano-Particles and Their Use in Agro-Ecosystems; Ghorbanpour, M., Bhargava, P., Varma, A., Choudhary, D., Eds.; Springer: Singapore, 2020; pp. 457–488. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; Cubadda, F.; Dini, L.; Terranova, M.L.; Aureli, F.; Sorbo, A.; Passeri, D. Scientific basis of nanotechnology, implications for the food sector and future trends. Trends Food Sci. Technol. 2014, 40, 127–148. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; Passeri, D.; Sinibaldi, A.; Angjellari, M.; Tamburri, E.; Sorbo, A.; Carata, E.; Dini, L. Nanotechnology for Food Packaging and Food Quality Assessment. Adv. Food Nutr. Res. 2017, 82, 149–204. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liang, J.; Tang, L.; Li, H.; Zhu, Y.; Jiang, D.; Song, B.; Chen, M.; Zeng, G. Nano-pesticides: A great challenge for biodiversity? Nano Today 2019, 28, 100757. [Google Scholar] [CrossRef]
- Wilson, N. Nanoparticles: Environmental Problems or Problem Solvers? BioScience 2018, 68, 241–246. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Scientific Opinion on Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain. EFSA J. 2011, 9, 2140. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; et al. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA J. 2018, 16, e05327. [Google Scholar] [CrossRef] [Green Version]
- EUON—European Union Observatory for Nanomaterials—Regulation. Available online: https://euon.echa.europa.eu/regulation (accessed on 26 January 2022).
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Process Contaminants. Available online: https://www.efsa.europa.eu/en/topics/topic/process-contaminants (accessed on 26 January 2022).
- Castle, L.; Eriksson, S. Analytical methods used to measure acrylamide concentrations in foods. J. AOAC Int. 2005, 88, 274–284. [Google Scholar] [CrossRef]
- European Food Safety Authority. Analysis of occurrence of 3-monochloropropane-1, 2-diol (3-MCPD) in food in Europe in the years 2009-2011 and preliminary exposure assessment. EFSA J. 2013, 11, 3381. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic Aromatic Hydrocarbons in Foods: Biological Effects, Legislation, Occurrence, Analytical Methods, and Strategies to Reduce Their Formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef] [PubMed]
- Anklam, E.; Battaglia, R. Food analysis and consumer protection. Trends Food Sci. Technol. 2001, 12, 197–202. [Google Scholar] [CrossRef]
- de la Calle Guntiñas, M.B.; Wysocka, I.; Quétel, C.; Vassileva, E.; Robouch, P.; Emteborg, H.; Taylor, P. Proficiency test for heavy metals in feed and food in Europe. TrAC Trend. Anal. Chem. 2009, 28, 454–465. [Google Scholar] [CrossRef]
- Broothaerts, W.; Cordeiro, F.; Robouch, P.; Emons, H. Ten years of proficiency testing reveals an improvement in the analytical performance of EU National Reference Laboratories for genetically modified food and feed. Food Control 2020, 114, 107237. [Google Scholar] [CrossRef]
- Sorbo, A.; Ciprotti, M.; Colabucci, A.; D’Amato, M.; Di Gregorio, M.; Fornari Luswergh, G.; Turco, A.C.; Ciaralli, L. Proficiency testing as a tool to assess quality of data: The experience of the EU Reference Laboratory for chemical elements in food of animal origin. Pure Appl. Chem. 2020, 92, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, C.; Lozano, A.; Uclés, S.; Valverde, A.; Fernández-Alba, A.R. European Union proficiency tests for pesticide residues in fruit and vegetables from 2009 to 2016: Overview of the results and main achievements. Food Control 2017, 82, 101–113. [Google Scholar] [CrossRef]
- Sorbo, A.; Colabucci, A.; Ciaralli, L. Control charts to evaluate long-term performance in proficiency tests. Accred. Qual. Assur. 2013, 18, 291–298. [Google Scholar] [CrossRef]
- CCQM Strategy Document 2021–2030 Version 1.0 21.06.2021. 2021. Available online: https://www.bipm.org/documents/20126/41532413/CCQM+Strategy/31283069-94f4-f2c7-bbfc-7d652c9b3de8 (accessed on 26 January 2022).
- Iyengar, V. Metrological concepts for enhancing the reliability of food and nutritional measurements. J. Food Compos. Anal. 2007, 20, 449–450. [Google Scholar] [CrossRef]
- Brown, R.J.C. Measuring measurement—What is metrology and why does it matter? Measurement 2021, 168, 108408. [Google Scholar] [CrossRef]
- Ulberth, F. Metrological concepts required for food safety and quality testing. Accred. Qual. Assur. 2011, 16, 607–613. [Google Scholar] [CrossRef]
- Magnusson, B.; Örnemark, U. (Eds.) Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics. 2014. Available online: https://www.eurachem.org/images/stories/Guides/pdf/MV_guide_2nd_ed_EN.pdf (accessed on 26 January 2022).
- Barwick, V. (Ed.) Planning and Reporting Method Validation Studies—Supplement to Eurachem Guide on the Fitness for Purpose of Analytical Methods. 2019. Available online: https://www.eurachem.org/index.php/publications/guides/planning-validation-studies (accessed on 26 January 2022).
- Barwick, V. (Ed.) Eurachem/CITAC Guide: Guide to Quality in Analytical Chemistry: An Aid to Accreditation. 2016. Available online: https://www.eurachem.org/images/stories/Guides/pdf/Eurachem_CITAC_QAC_2016_EN.pdf (accessed on 26 January 2022).
- ISO/IEC 17043:2010; Conformity Assessment—General Requirements for Proficiency Testing. ISO: Geneva, Switzerland, 2010.
- ISO 13528:2015; Statistical Methods for Use in Proficiency Testing by Inter-Laboratory Comparison. ISO: Geneva, Switzerland, 2015.
- Miller, W.G.; Jones, G.R.D.; Horowitz, G.L.; Weykamp, C. Proficiency Testing/External Quality Assessment: Current Challenges and Future Directions. Clin. Chem. 2011, 57, 1670–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juniper, I. Quality issues in proficiency testing. Accred. Qual. Assur. 1999, 4, 336–341. [Google Scholar] [CrossRef]
- Sykes, M. Proficiency testing for the improvement of analytical practice. Accred. Qual. Assur. 2012, 17, 467–471. [Google Scholar] [CrossRef]
- Stoyke, M.; Polzer, J.; Radeck, W.; Hamann, F.; Gowik, P. Proficiency testing in food control: An evaluation of more than 13 years. J. Verbr. Lebensm. 2015, 10, 273–279. [Google Scholar] [CrossRef]
- Rose, M.; Poms, R.; Macarthur, R.; Pöpping, B.; Ulberth, F. What is the best way to ensure that valid analytical methods are used for food control? Qual. Assur. Saf. Crop. 2011, 3, 123–134. [Google Scholar] [CrossRef]
- Sorbo, A.; Ciprotti, M.; Colabucci, A.; Zoani, C.; Di Gregorio, M.; Turco, A.C.; Ciaralli, L. Preparation of an infant formula proficiency testing material and assessment of its homogeneity and stability. Accred. Qual. Assur. 2015, 20, 373–380. [Google Scholar] [CrossRef]
- Ciaralli, L.; Turco, A.C.; Ciprotti, M.; Colabucci, A.; Di Gregorio, M.; Sorbo, A. Honey as a material for proficiency testing. Accred. Qual. Assur. 2015, 20, 359–365. [Google Scholar] [CrossRef]
- Thompson, M.; Ellison, S.L.R.; Wood, R. The International Harmonized Protocol for the proficiency testing of analytical chemistry laboratories. Pure Appl. Chem. 2006, 78, 145–196. [Google Scholar] [CrossRef]
- Brunetti, L.; Oberto, L.; Sellone, M.; Terzi, P. Establishing reference value in high frequency power comparisons. Measurement 2009, 42, 1318–1323. [Google Scholar] [CrossRef]
- Priel, M.; Amarouche, S.; Fisicaro, P. Metrological traceability is not always a straight line. Accred. Qual. Assur. 2015, 14, 593. [Google Scholar] [CrossRef]
- Ferrero, C.; Casaril, M. Proficiency testing programs to improve traceability in chemical analysis. Measurement 2009, 42, 1502–1509. [Google Scholar] [CrossRef]
- Kaarls, R.; Mackay, L.; Samuel, A.; Sin, D.W.M.; Mok, C.S.; Wong, Y.L.; Yip, Y.C. Laboratory capacity building through the use of metrologically traceable reference values in proficiency testing programmes. Accred. Qual. Assur. 2017, 22, 659–663. [Google Scholar] [CrossRef]
- Kuselman, I. Nonparametric assessment of comparability of analytical results obtained in proficiency testing based on a metrological approach. Accred. Qual. Assur. 2006, 10, 321–334. [Google Scholar] [CrossRef]
- Côté, I.; Robouch, P.; Robouch, B.; Bisson, D.; Gamache, P.; LeBlanc, A.; Dumas, P.; Pedneault, M. Determination of the standard deviation for proficiency assessment from past participant’s performances. Accred. Qual. Assur. 2012, 17, 389–393. [Google Scholar] [CrossRef] [Green Version]
- Thompson, M.; Mathieson, K.; Damant, A.P.; Wood, R. A general model for interlaboratory precision accounts for statistics from proficiency testing in food analysis. Accred. Qual. Assur. 2008, 17, 223–230. [Google Scholar] [CrossRef]
- Fisicaro, P.; Amarouche, S.; Lalere, B.; Labarraque, G.; Priel, M. Approaches to uncertainty evaluation based on proficiency testing schemes in chemical measurements. Accred. Qual. Assur. 2008, 13, 361–366. [Google Scholar] [CrossRef]
- Désenfant, M.; Priel, M. Road map for measurement uncertainty evaluation. Measurement 2006, 39, 841–848. [Google Scholar] [CrossRef]
- Magnusson, B.; Näykki, T.; Hovind, H.; Krysell, M.; Sahlin, E. Handbook for Calculation of Measurement Uncertainty in Environmental Laboratories. NT TR 537-Edition 4. 2017. Available online: http://www.nordtest.info/wp/2017/11/29/handbook-for-calculation-of-measurement-uncertainty-in-environmental-laboratories-nt-tr-537-edition-4/ (accessed on 26 January 2022).
- EUROLAB. Measurement Uncertainty Revisited: Alternative Approaches to Uncertainty Evaluation. Technical Report No. 1/2007. 2007. Available online: https://eurolab-d.de/files/measurement_uncertainty_revisited_-_alternative_approaches_to_uncertainty_evaluation.pdf (accessed on 26 January 2022).
- Astrua, M.; Pisani, M. Validation of a novel technique for the measurement of the refractive index of a prism by means of interlaboratory comparison. Measurement 2009, 42, 1546–1549. [Google Scholar] [CrossRef]
- Detaille, R.; Maetz, P. Practical uses of proficiency testing as valuable tools for validation and performance assessment in environmental analysis. Accred. Qual. Assur. 2006, 11, 408–413. [Google Scholar] [CrossRef]
- Analytical Method Committee, The Royal Society of Chemistry. The role of proficiency testing in method validation. Accred. Qual. Assur. 2010, 15, 73–79. [Google Scholar] [CrossRef]
- Wilrich, P.T. The determination of precision of qualitative measurement methods by interlaboratory experiments. Accred. Qual. Assur. 2010, 15, 439–444. [Google Scholar] [CrossRef]
- Ciprotti, M.; Sorbo, A.; Orlandini, S.; Ciaralli, L. Preparation of liquid milk for proficiency test and internal quality control for chemical elements in food. Accred. Qual. Assur. 2013, 18, 333–339. [Google Scholar] [CrossRef]
- ISO GUIDE 30:2015; Reference Materials—Selected Terms and Definitions. ISO: Geneva, Switzerland, 2015.
- International Vocabulary of Metrology—Basic and General Concepts and Associated Terms (VIM). BIPM. 2012. Available online: https://www.bipm.org/documents/20126/2071204/JCGM_200_2012.pdf/f0e1ad45-d337-bbeb-53a6-15fe649d0ff1?version=1.15&t=1641292389029&download=true (accessed on 26 January 2022).
- ISO/IEC 17025; General Requirements for the Competence of Testing and Calibration Laboratories. ISO: Geneva, Switzerland, 2017.
- Grombe, R.; Allmaier, G.; Charoud-Got, J.; Dudkiewicz, A.; Emteborg, H.; Hofmann, T.; Huusfeldt Larsen, E.; Lehner, A.; Llinàs, M.; Loeschner, K.; et al. Feasibility of the development of reference materials for the detection of Ag nanoparticles in food: Neat dispersions and spiked chicken meat. Accred. Qual. Assur. 2015, 20, 3–16. [Google Scholar] [CrossRef]
- METROFOOD—Infrastructure for Promoting Metrology in Food and Nutrition. Available online: https://www.metrofood.eu/ (accessed on 26 January 2022).
- European Strategy Forum on Research Infrastructures (ESFRI). Available online: www.esfri.eu (accessed on 26 January 2022).
- Tsimidou, M.Z.; Ordoudi, S.A.; Mantzouridou, F.T.; Nenadis, N.; Stelzl, T.; Rychlik, M.; Belc, N.; Zoani, C. Scientific plan of the European Research Infrastructure METROFOOD-RI for promoting metrology in food and nutrition. Foods, 2022; accepted. [Google Scholar]
- METROFOOD—e-Services. Available online: https://www.metrofood.eu/access/e-services.html (accessed on 26 January 2022).
| Reference | Topic |
|---|---|
| EEC 315/1993 (consolidated version 2009) | Definition of contaminant |
| EC 178/2002 | General Food Law Regulation |
| EC 882/2004 | Establishment of official controls system |
| EC 852/2004 | Hygiene of foodstuff |
| EC 853/2004 | Specific hygiene rules for food of animal origin |
| EU 2017/625 | Official control regulation (repealing EC 854/2004) |
| EU 2019/624 | Official controls of products of animal origin |
| EU 2019/625 | Import conditions |
| EU 2019/627 | Practical arrangement of official controls of products of animal origin |
| EU 2020/2235 | Import certificates |
| EU 2021/405 | Lists of third countries authorized to import products of animal origin |
| EC 2073/2005 | Microbiological criteria for foodstuffs |
| EU 2015/1375 | Specific rules on official controls for Trichinella in meat |
| EU 2021/382 | Food allergen management (amending Annexes to EC 852/2004), redistribution of food, concept of food safety culture |
| EC 1881/2006 (consolidated version 2021) | Maximum permitted levels in food for some specific contaminants |
| EU 2017/2158 | Regulation specific to acrylamide |
| EU 2019/1793 | Regulation specific to acrylamide |
| EC 401/2006 | Mycotoxin control |
| EU 2017/644 | Sampling and analysis methods for the control of levels of dioxins, dioxin-like and non-dioxin-like PCBs in certain foodstuffs |
| EC 333/2007 | Control of levels of lead, cadmium, mercury, inorgainc tin, 3-MCPD and benzo(a)pyrene in foodstuffs |
| EC 1882/2006 | Control of levels of nitrates |
| 2011/696/EU | Recommendation on definition of nanomaterials |
| EU 2015/2283 | Novel foods |
| EC 1333/2008 | Novel foods, food additives |
| EC 1223/2009 | Biocides and cosmetics |
| EC 1169/2011 | Food information to consumers |
| EC 10/2011 | Plastic food contact materials |
| EC 450/2009 | Active and intelligent materials |
| EC 1223/2009 | Biocides and cosmetics |
| Parameter | Definition and Discussion |
|---|---|
| Selectivity | …refers to the ‘extent to which the method can be used to determine particular analytes in mixtures or matrices without interference from other components with similar characteristics’. The recovery of the analyte(s) of interest shall be determined, and any suspicious interference and any restrictions on the applicability of the method shall be indicated in the validation report [97,98]. |
| Working range & linearity | …determined by examining samples containing the analyte at different concentrations and calculating the regression statistics from the results, usually by the method of least squares in order to establish the range within which acceptable uncertainty can be reached. Before that, a calibration function for the instrument needs to be defined, therefore the working range of the method should be examined separately from that of the instrument. For this reason, it may be appropriate to consider separately the working range of the method and that of the instrument. |
| Limit of detection (LOD) | …is the lowest amount of the analyte that can be detected by the method at a specified level of confidence. Its value is different depending on the type of sample. |
| Limit of quantification (LOQ) | …is the lowest concentration of analyte that can be determined with an acceptable level of uncertainty and can, therefore, be set arbitrarily as the required lower end of the method working range [98]. Estimates of LOD and LOQ may be different among different matrices covered by the same analytical method; for this reason, they need to be determined for each matrix [47]. |
| Precision | …is a measure of the concordance between mutually independent measurement results obtained under specified conditions. It is usually expressed by a standard deviation. Repeatability is a type of precision representing the smallest variation in results [98]. |
| Trueness | …is an expression of how close the mean of an infinite number of results (produced by the method) is to a reference value. Since it is not possible to take an infinite number of measurements, trueness cannot be measured but it is generally estimated as bias, that is, the systematic error [97,98]. Three approaches are commonly used during validation for bias determination: the analysis of RMs, recovery experiments using spiked samples, and the comparison with results obtained using another method [98]. |
| Ruggedness (or robustness) | …provides an indication of reliability of a method that has the ability to remain unaltered by small variations in the parameters of the method [98]. |
| Uncertainty | …characterizes the range of values attributable to the measurand with a specified level of confidence. Every measurement result has an uncertainty associated with it, deriving from errors arising in the various stages of sampling and analysis and from imperfect knowledge of factors affecting the result. A statement of the uncertainty associated conveys the ‘quality’ of the result [97,98]. |
| PT Material | Assigned Value | Main Limitations |
|---|---|---|
| Certified reference material | Certified property value |
|
| Formulation (mixing materials in specific proportions if the levels of a properties are known or adding a certain amount of a substance to the blank material) | Calculation on the basis of the proportions used and the known analyte content |
|
| Other materials | Results from a single laboratory using a reference method |
|
| Other materials | Consensus value from expert laboratories |
|
| Other materials | Consensus value from participant results (location estimate such as robust mean, median or arithmetic mean) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorbo, A.; Pucci, E.; Nobili, C.; Taglieri, I.; Passeri, D.; Zoani, C. Food Safety Assessment: Overview of Metrological Issues and Regulatory Aspects in the European Union. Separations 2022, 9, 53. https://doi.org/10.3390/separations9020053
Sorbo A, Pucci E, Nobili C, Taglieri I, Passeri D, Zoani C. Food Safety Assessment: Overview of Metrological Issues and Regulatory Aspects in the European Union. Separations. 2022; 9(2):53. https://doi.org/10.3390/separations9020053
Chicago/Turabian StyleSorbo, Angela, Emilia Pucci, Chiara Nobili, Isabella Taglieri, Daniele Passeri, and Claudia Zoani. 2022. "Food Safety Assessment: Overview of Metrological Issues and Regulatory Aspects in the European Union" Separations 9, no. 2: 53. https://doi.org/10.3390/separations9020053
APA StyleSorbo, A., Pucci, E., Nobili, C., Taglieri, I., Passeri, D., & Zoani, C. (2022). Food Safety Assessment: Overview of Metrological Issues and Regulatory Aspects in the European Union. Separations, 9(2), 53. https://doi.org/10.3390/separations9020053









