PVA-Based MMMs for Ethanol Dehydration via Pervaporation: A Comparison Study between Graphene and Graphene Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PAN Porous Membranes
2.3. Preparation of PVA-Based MMMs
2.4. Characterization
2.5. Pervaporation Performance Measurement
3. Results
3.1. Characterization of PVA-Based MMMs
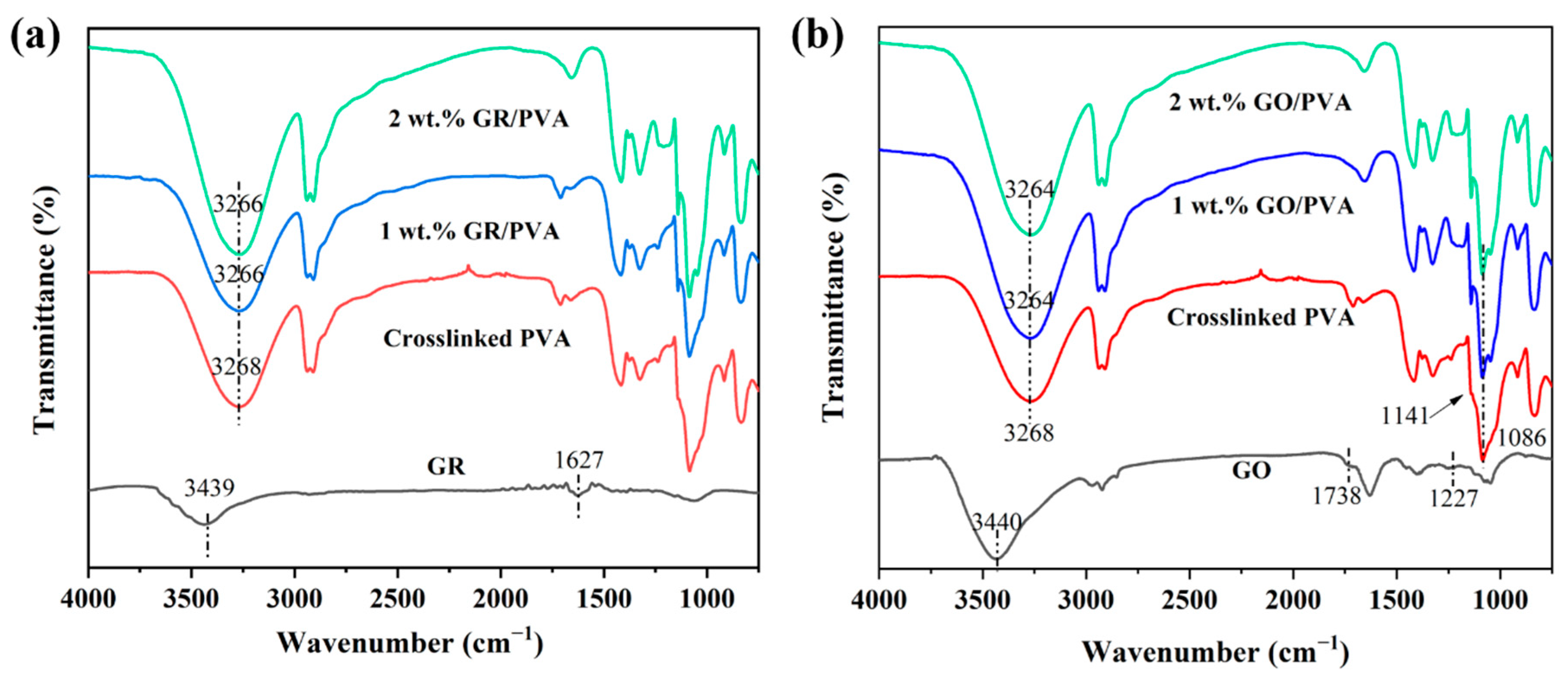
3.1.1. Chemical Structures of PVA-Based MMMs
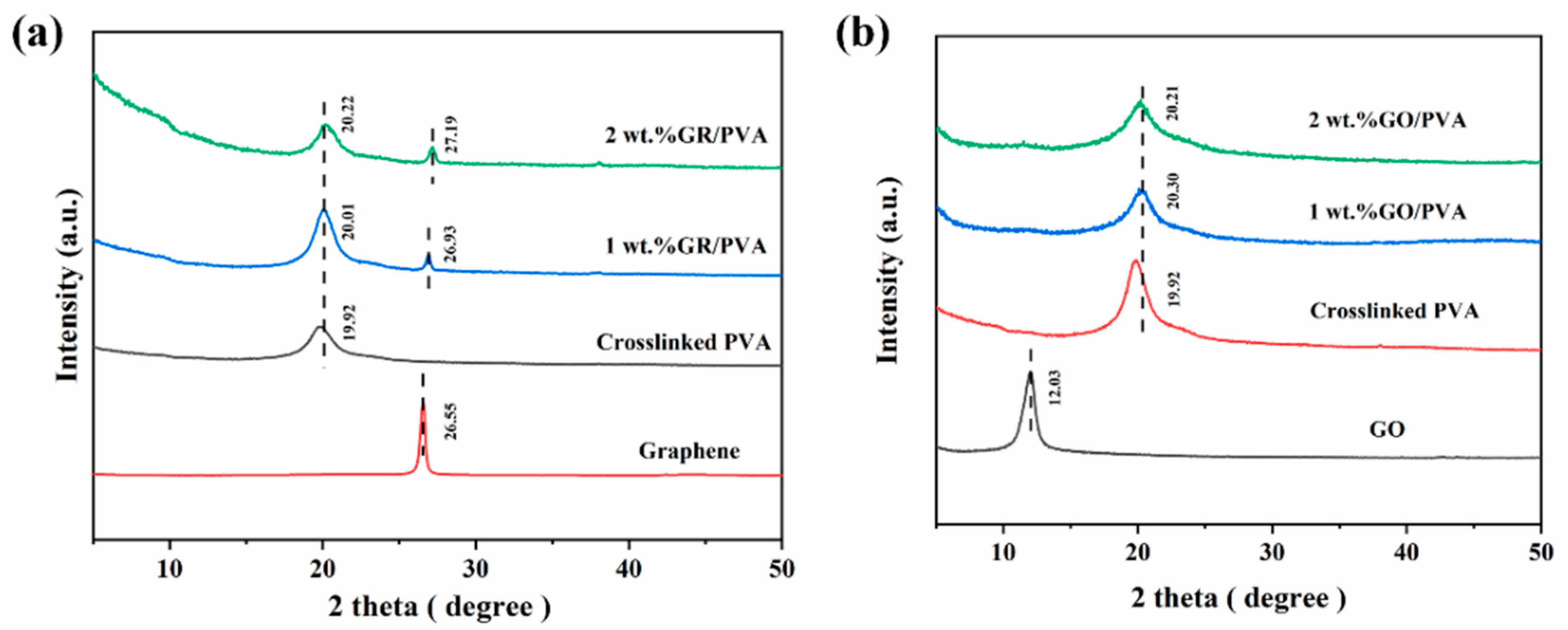
3.1.2. Crystal Structures of PVA-Based MMMs
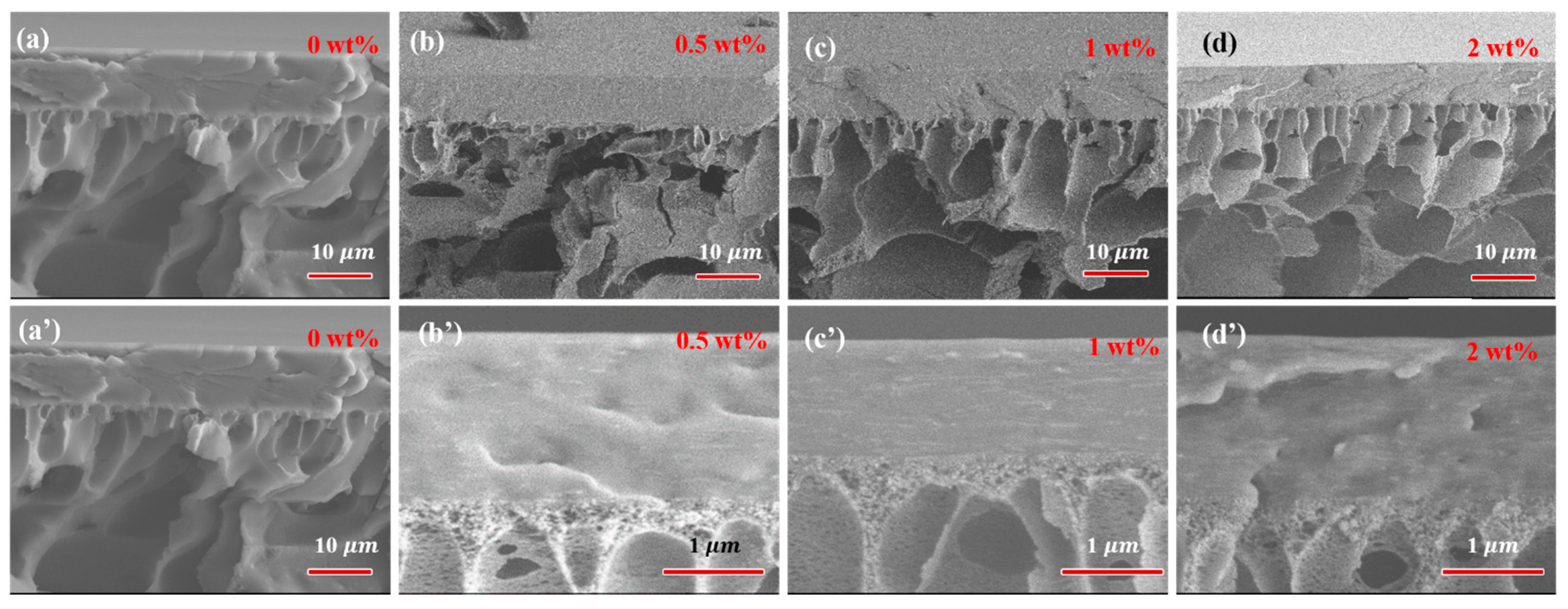
3.1.3. Morphology of PVA-Based MMMs
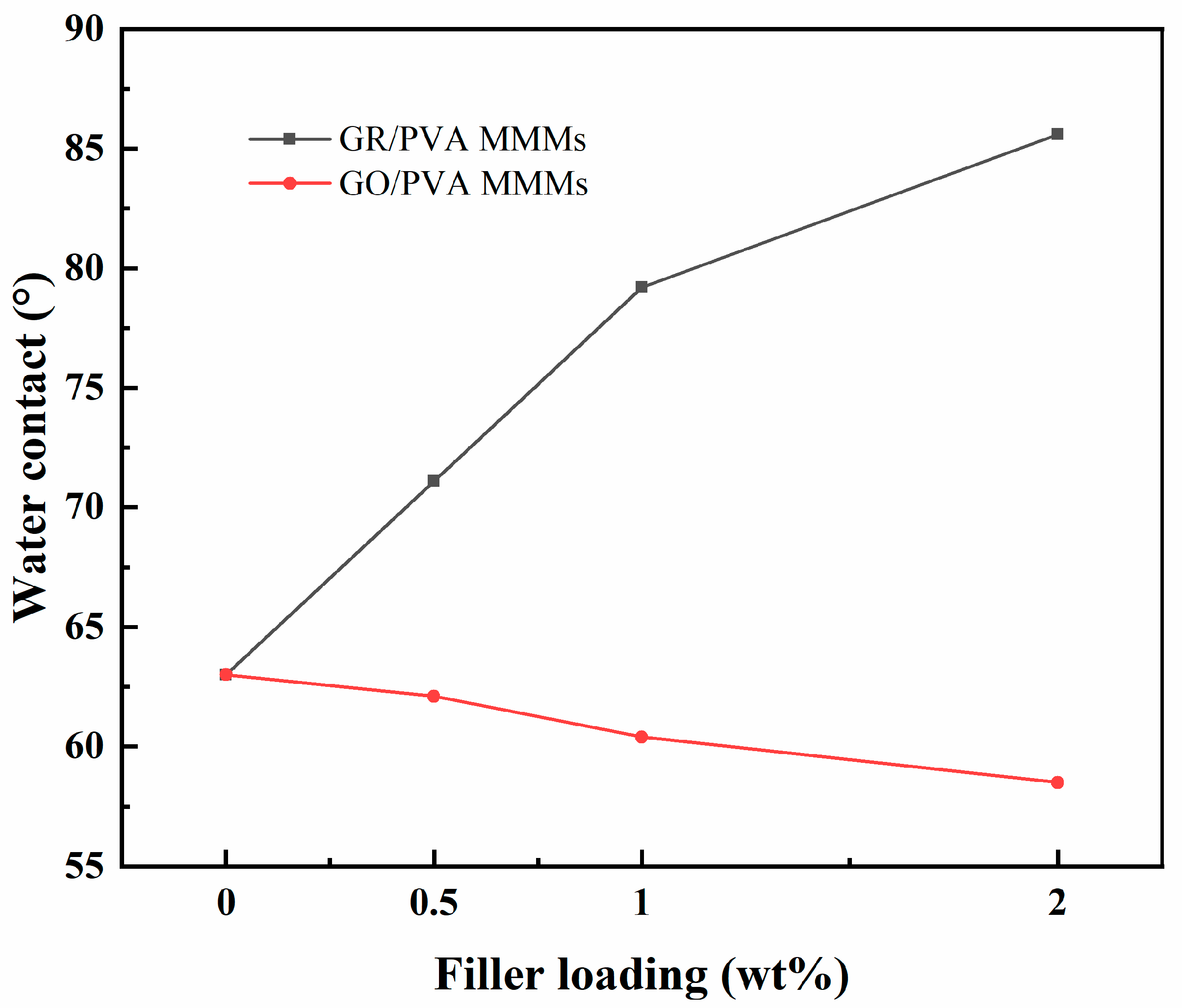
3.1.4. Hydrophilicity of PVA-Based MMMs
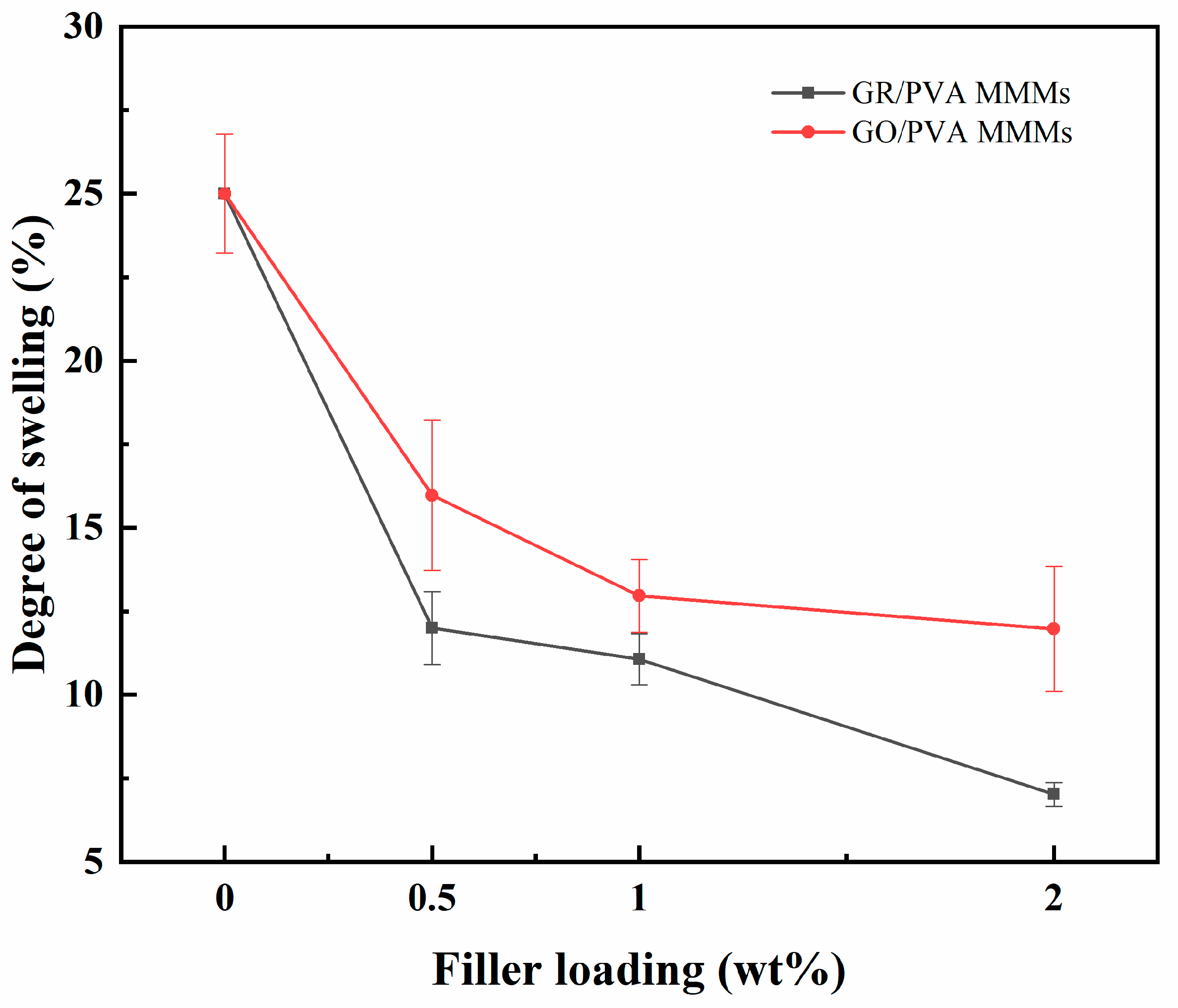
3.1.5. Swelling Properties of PVA-Based MMMs
3.1.6. Thermal Properties of PVA-Based MMMs
3.2. Pervaporation Performance of PVA-Based MMMs
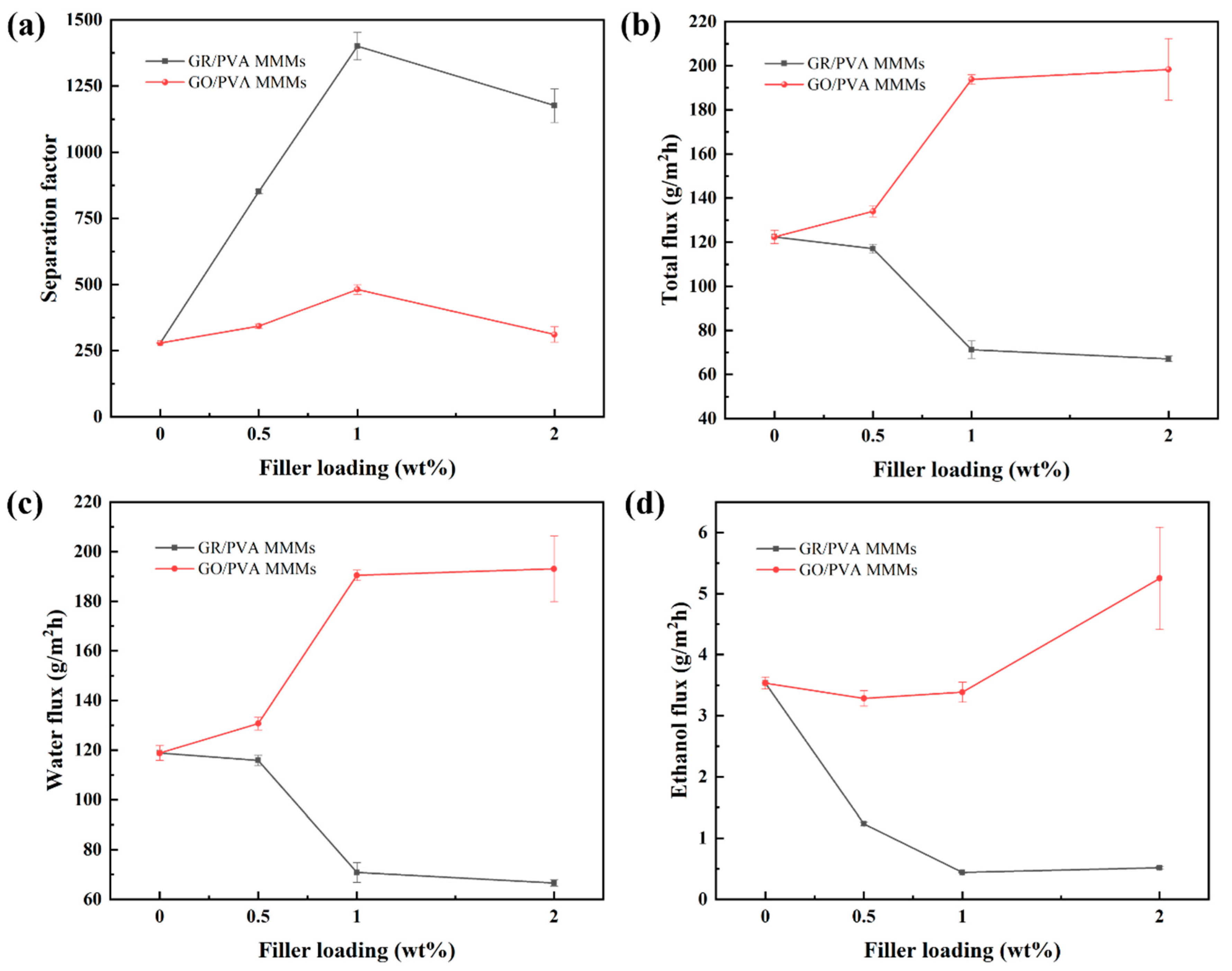
3.2.1. Effect of GR/GO Filler Loading on PV Performance
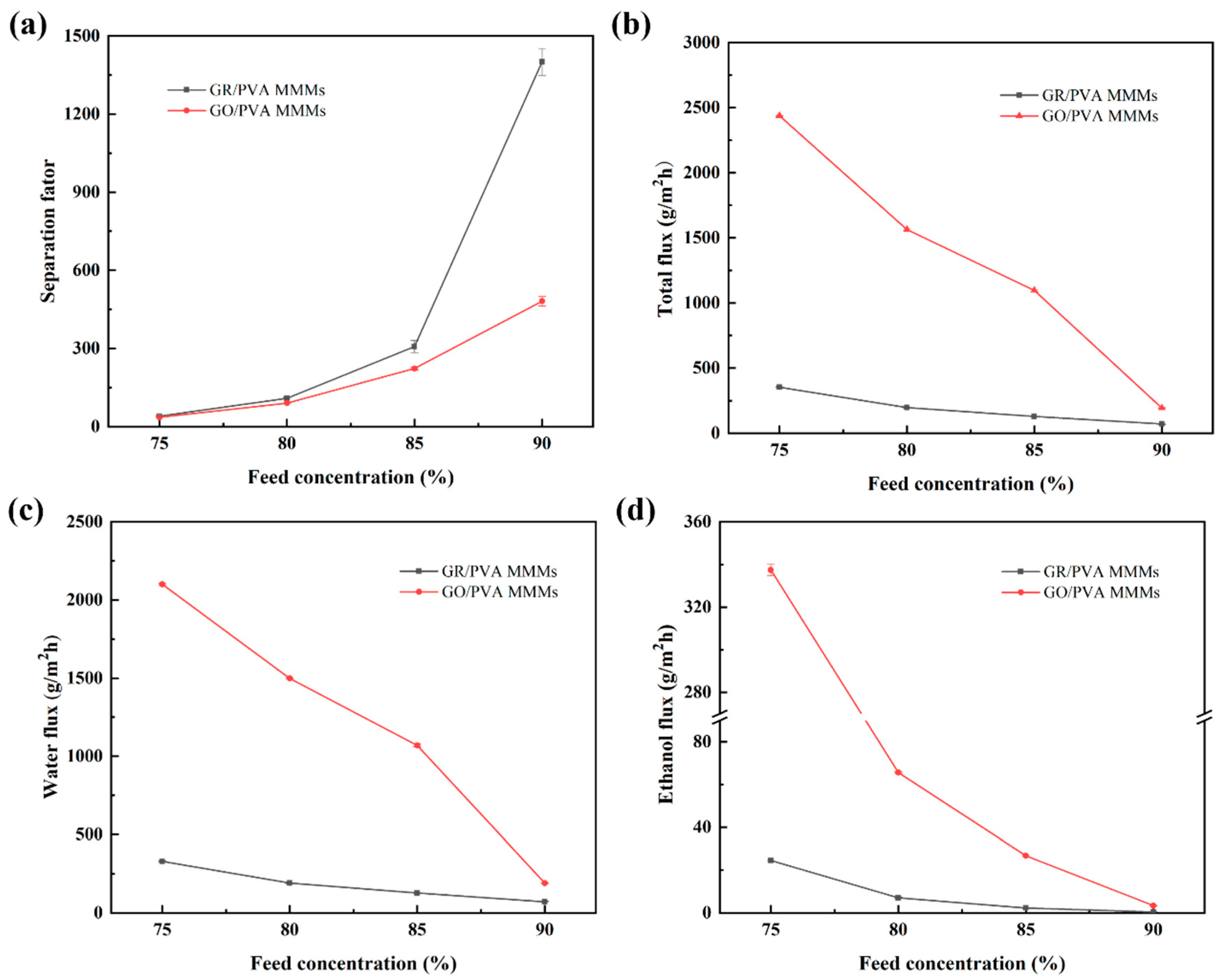
3.2.2. Effect of Feed Concentration on PV Performance
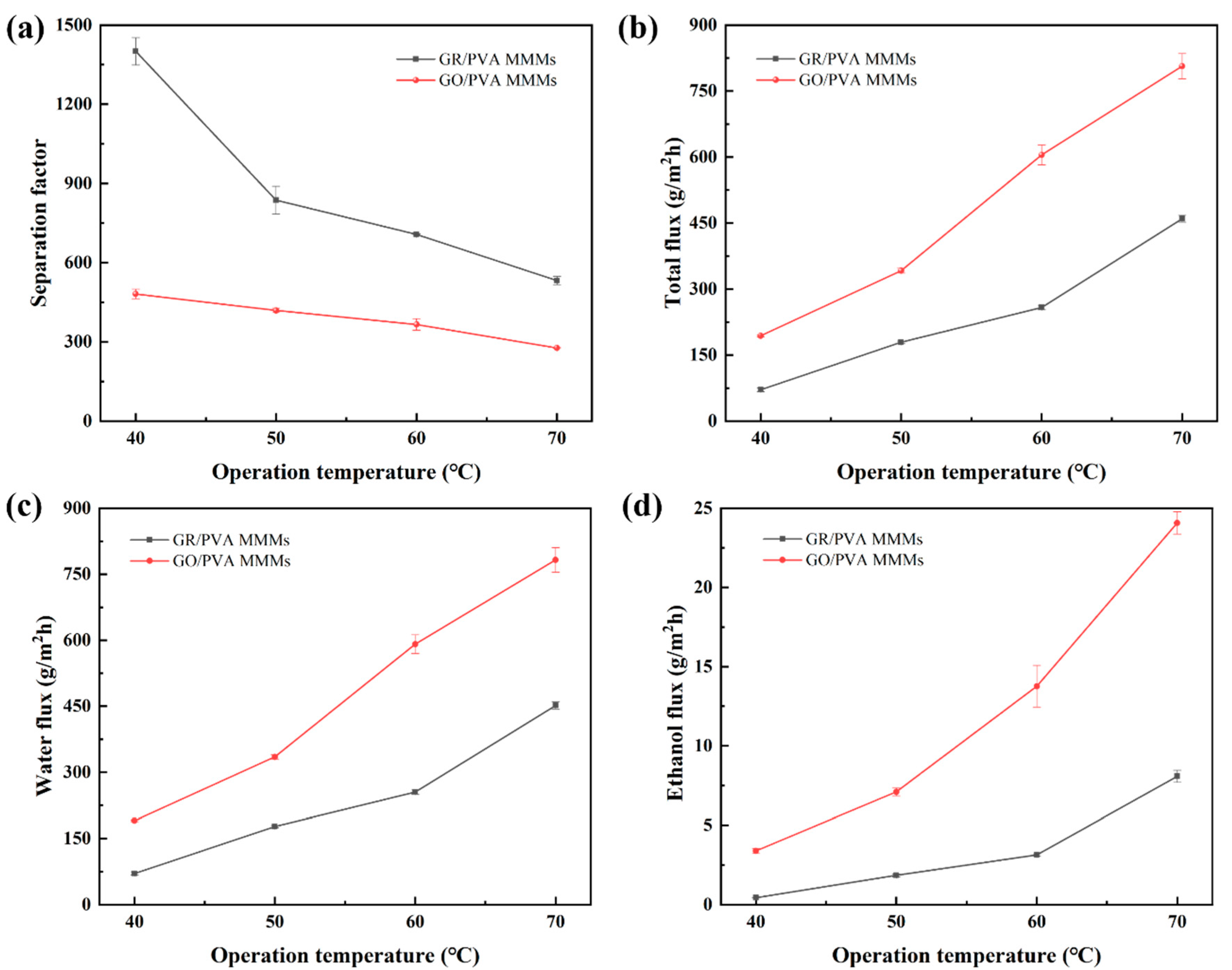
3.2.3. Effect of Operation Temperature on PV Performance
3.2.4. Comparison of PV Performance of PVA-Based MMMs
4. Conclusions
- (1)
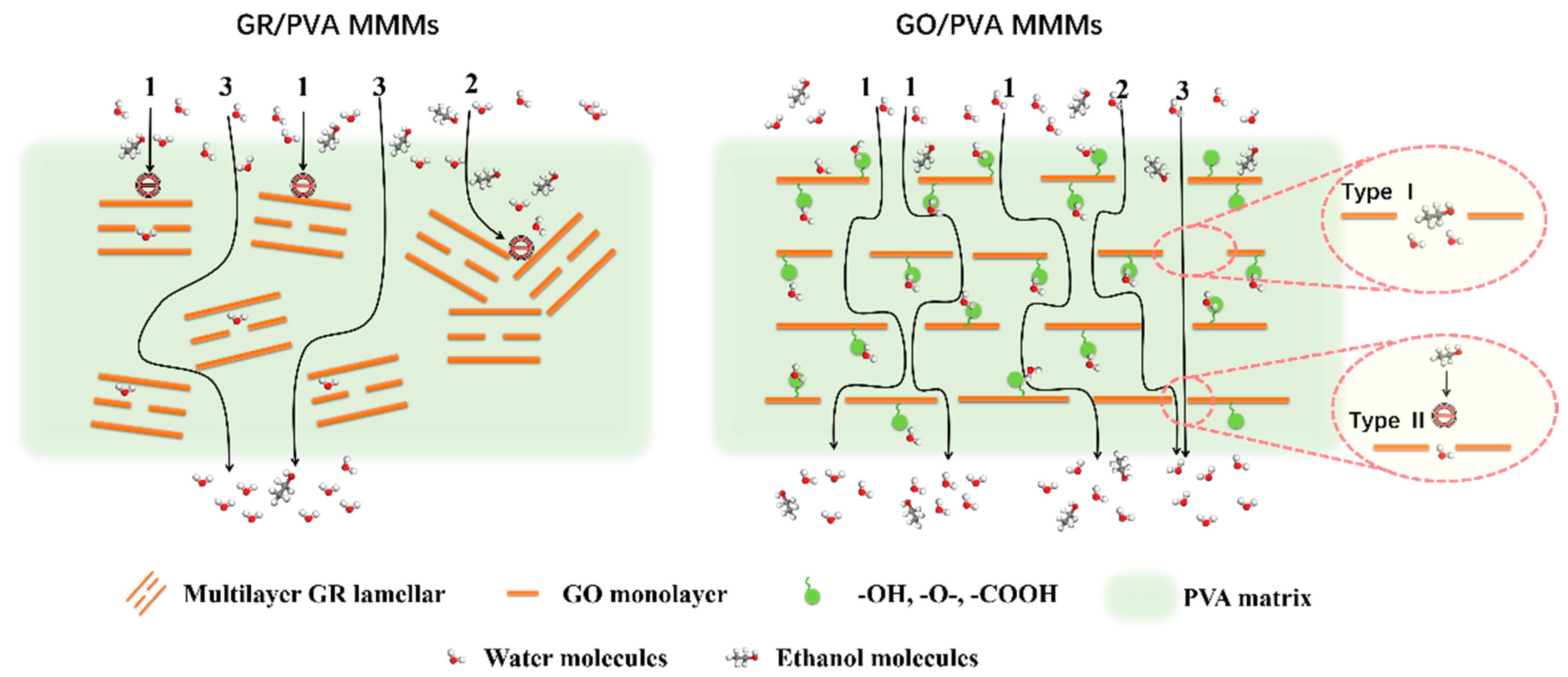
- GR lamellae were dispersed in the PVA matrix in the form of multilayer aggregates due to the strong π–π interaction between GR layers and weak interaction between GR and PVA. GO was well exfoliated by PVA and homogeneously dispersed in the PVA matrix, benefiting from the good solubility of GO, and a strong interaction existed between the GO nanosheet and PVA polymer due to oxygen-containing groups. The incorporation of GO nanosheets into PVA reduced PVA’s crystallinity and enhanced the membrane hydrophilicity, while the incorporation of GR into PVA led to the opposite results. The incorporation of GR/GO into PVA depressed the PVA membrane swelling degree, and the incorporation of GR showed a more obvious depression effect.
- (2)
- GR/PVA MMMs showed a much higher separation factor than GO/PVA MMMs, while they exhibited much lower permeation flux than GO/PVA MMMs and pristine PVA membranes. The huge difference in microstructure and performance between GO/PVA and GR/PVA MMMs was strongly associated with the oxygen-containing groups on graphene lamellae. The higher permeation flux of GO/PVA MMMs was ascribed to the facilitated transport of water molecules induced by oxygen-containing groups and exclusive channels provided by GO lamellae, while the much lower permeation flux and higher separation factor GR/PVA MMMs resulted from the smaller GR interplanar spacing (0.33 nm) and hydrophobicity as well as barrier effect of GR lamellae on the sorption and diffusion of water molecules. With the augment of filler loading, the separation factor of both GR/PVA MMMs and GO/PVA MMMs increased first and subsequently decreased, which reached maximum values of 1400 and 481 respectively at 1 wt% filler loading, increasing by 402% and 72% compared with that of the pristine PVA membrane.
- (3)
- The pervaporation performance of GR/PVA MMMs showed similar variation trends with that of GO/PVA as the water feed concentration or operation temperature increased. As the operation temperature or water feed concentration increased, the separation factor decreased, while the total flux and ethanol/water partial flux increased.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, T.; Xia, Q.; Zuo, J.; Liu, S.; Yu, X.; Wang, Y. Recent advances of thin film composite membranes for pervaporation applications: A comprehensive review. Adv. Membr. 2021, 1, 100008. [Google Scholar] [CrossRef]
- Lin, G.-S.; Chen, Y.-R.; Chang, T.-H.; Huang, T.-C.; Zhuang, G.-L.; Huang, W.-Z.; Liu, Y.-C.; Matsuyama, H.; Wu, K.C.W.; Tung, K.-L. A high ZIF-8 loading PVA mixed matrix membrane on alumina hollow fiber with enhanced ethanol dehydration. J. Membr. Sci. 2021, 621, 118935. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W. Pervaporation membrane materials: Recent trends and perspectives. J. Membr. Sci. 2021, 636, 119557. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Zhou, S.; Xue, A.; Wu, F.; Zhao, Y. Polyacrylonitrile-supported self-aggregation crosslinked poly (vinyl alcohol) pervaporation membranes for ethanol dehydration. Eur. Polym. J. 2020, 122, 109359. [Google Scholar] [CrossRef]
- Thorat, G.B.; Gupta, S.; Murthy, Z.V.P. Synthesis, characterization and application of PVA/ionic liquid mixed matrix membranes for pervaporation dehydration of isopropanol. Chin. J. Chem. Eng. 2017, 25, 1402–1411. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Missyul, A.B.; Kuzminova, A.I.; Markelov, D.A.; Ermakov, S.S.; Roizard, D. Development and investigation of mixed-matrix PVA-fullerenol membranes for acetic acid dehydration by pervaporation. Sep. Purif. Technol. 2017, 187, 343–354. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zou, Y.; Wei, T.Y.; Mu, C.X.; Liu, X.J.; Tong, Z.F. Pervaporation dehydration of binary and ternary mixtures of n-butyl acetate, n-butanol and water using PVA-CS blended membranes. Sep. Purif. Technol. 2017, 173, 314–322. [Google Scholar] [CrossRef]
- Li, S.; Dai, J.; Geng, X.; Li, J.; Li, P.; Lei, J.; Wang, L.; He, J. Highly selective sodium alginate mixed-matrix membrane incorporating multi-layered MXene for ethanol dehydration. Sep. Purif. Technol. 2020, 235, 116206. [Google Scholar] [CrossRef]
- Nigiz, F.U.; Dogan, H.; Hilmioglu, N.D. Pervaporation of ethanol/water mixtures using clinoptilolite and 4A filled sodium alginate membranes. Desalination 2012, 300, 24–31. [Google Scholar] [CrossRef]
- Lecaros, R.L.G.; Ho, S.-Y.; Tsai, H.-A.; Hung, W.-S.; Hu, C.-C.; Huang, S.-H.; Lee, K.-R.; Lai, J.-Y. Ionically cross-linked sodium alginate and polyamidoamine dendrimers for ethanol/water separation through pervaporation. Sep. Purif. Technol. 2021, 275, 119125. [Google Scholar] [CrossRef]
- Kononova, S.V.; Kruchinina, E.V.; Petrova, V.A.; Baklagina, Y.G.; Klechkovskaya, V.V.; Orekhov, A.S.; Vlasova, E.N.; Popova, E.N.; Gubanova, G.N.; Skorik, Y.A. Pervaporation membranes of a simplex type with polyelectrolyte layers of chitosan and sodium hyaluronate. Carbohydr. Polym. 2019, 209, 10–19. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Baldasso, C.; Tessaro, I.C. Potential of chitosan-based membranes for the separation of essential oil components by target-organophilic pervaporation. Carbohydr. Polym. 2020, 247, 116676. [Google Scholar] [CrossRef]
- Xu, Y.M.; Japip, S.; Chung, T.-S. Mixed matrix membranes with nano-sized functional UiO-66-type MOFs embedded in 6FDA-HAB/DABA polyimide for dehydration of C1-C3 alcohols via pervaporation. J. Membr. Sci. 2018, 549, 217–226. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y. Novel thermally cross-linked polyimide membranes for ethanol dehydration via pervaporation. J. Membr. Sci. 2015, 496, 142–155. [Google Scholar] [CrossRef]
- Le, N.L.; Wang, Y.; Chung, T.S. Synthesis, cross-linking modifications of 6FDA-NDA/DABA polyimide membranes for ethanol dehydration via pervaporation. J. Membr. Sci. 2012, 415–416, 109–121. [Google Scholar] [CrossRef]
- Shi, G.M.; Yang, T.; Chung, T.S. Polybenzimidazole (PBI)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of alcohols. J. Membr. Sci. 2012, 415-416, 577–586. [Google Scholar] [CrossRef]
- Shi, G.M.; Chen, H.M.; Jean, Y.C.; Chung, T.S. Sorption, swelling, and free volume of polybenzimidazole (PBI) and PBI/zeolitic imidazolate framework (ZIF-8) nano-composite membranes for pervaporation. Polymer 2013, 54, 774–783. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Buera-González, J.; Iglesia, Ó.D.L.; Galiano, F.; Fíla, V.; Malankowska, M.; Rubio, C.; Figoli, A.; Téllez, C.; Coronas, J. Towards the dehydration of ethanol using pervaporation cross-linked poly (vinyl alcohol)/graphene oxide membranes. J. Membr. Sci. 2019, 582, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Tsebriienko, T.; Popov, A.I. Effect of Poly (Titanium Oxide) on the Viscoelastic and Thermophysical Properties of Interpenetrating Polymer Networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Peters, T.; Benes, N.; Buijs, H.; Vercauteren, F.; Keurentjes, J. Thin high flux ceramic-supported PVA membranes. Desalination 2006, 200, 37–39. [Google Scholar] [CrossRef]
- Chaudhari, S.; Baek, M.; Kwon, Y.; Shon, M.; Nam, S.; Park, Y. Surface-modified halloysite nanotube-embedded polyvinyl alcohol/polyvinyl amine blended membranes for pervaporation dehydration of water/isopropanol mixtures. Appl. Surf. Sci. 2019, 493, 193–201. [Google Scholar] [CrossRef]
- Selim, A.; Toth, A.J.; Fozer, D.; Haaz, E.; Valentínyi, N.; Nagy, T.; Keri, O.; Bakos, L.P.; Szilágyi, I.M.; Mizsey, P. Effect of silver-nanoparticles generated in poly (vinyl alcohol) membranes on ethanol dehydration via pervaporation. Chin. J. Chem. Eng. 2019, 27, 1595–1607. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.H.; To, L.A.; Ngo, N.P. Fabrication and characterization of graphene/graphene oxide based poly (vinyl alcohol) nanocomposite membranes for pervaporation dehydration of ethanol. Can Tho. Univ. J. Sci. 2016, 36–45, Special issue: Renewable Energy. [Google Scholar] [CrossRef]
- Suhas, D.P.; Aminabhavi, T.M.; Raghu, A.V. Mixed matrix membranes of H-ZSM5-loaded poly (vinyl alcohol) used in pervaporation dehydration of alcohols: Influence of silica/alumina ratio. Polym. Eng. Sci. 2014, 54, 1774–1782. [Google Scholar] [CrossRef]
- Asvadi, F.; Raisi, A.; Aroujalian, A. Preparation of multi-layer pervaporation membrane by electrospraying of nano zeolite X. Microporous Mesoporous Mater. 2017, 251, 135–145. [Google Scholar] [CrossRef]
- Huang, Z.; Ru, X.-f.; Zhu, Y.-T.; Guo, Y.-h.; Teng, L.-j. Poly (vinyl alcohol)/ZSM-5 zeolite mixed matrix membranes for pervaporation dehydration of isopropanol/water solution through response surface methodology. Chem. Eng. Res. Des. 2019, 144, 19–34. [Google Scholar] [CrossRef]
- Samei, M.; Iravaninia, M.; Mohammadi, T.; Asadi, A.A. Solution diffusion modeling of a composite PVA/fumed silica ceramic supported membrane. Chem. Eng. Process. 2016, 109, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Jegal, J.; Kim, W.N. Modification of performances of various membranes using MWNTs as a modifier. Macromol. Symp. 2007, 249–250, 610–617. [Google Scholar] [CrossRef]
- Panahian, S.; Raisi, A.; Aroujalian, A. Multilayer mixed matrix membranes containingmodified-MWCNTs for dehydration of alcohol by pervaporation process. Desalination 2015, 355, 45–55. [Google Scholar] [CrossRef]
- Choi, J.H.; Jegal, J.; Kim, W.-N.; Choi, H.-S. Incorporation of multiwalled carbon nanotubes into poly (vinyl alcohol) membranes for use in the pervaporation of water/ethanol mixtures. J. Appl. Polym. Sci. 2009, 111, 2186–2193. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y. Poly (vinyl alcohol)/ZIF-8-NH2mixed matrix membranes for ethanol dehydration via pervaporation. AlChE J. 2016, 62, 1728–1739. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Q.; Wu, C.; Wang, H.; Wang, H. Viscosity-driven in situ self-assembly strategy to fabricate cross-linked ZIF-90/PVA hybrid membranes for ethanol dehydration via pervaporation. Sep. Purif. Technol. 2018, 201, 256–267. [Google Scholar] [CrossRef]
- Jiang, H.J.; Shi, W.X.; Liu, Q.; Wang, H.Y.; Li, J.L.; Wu, C.L.; Li, Y.C.; Wei, Z. Intensification of water/ethanol separation by PVA hybrid membrane with different functional ligand UiO-66-X nanochannels in pervaporation process. Sep. Purif. Technol. 2021, 256, 117802. [Google Scholar] [CrossRef]
- Kudasheva, A.; Sorribas, S.; Zornoza, B.; Téllez, C.; Coronas, J. Pervaporation of water/ethanol mixtures through polyimide based mixed matrix membranes containing ZIF-8, ordered mesoporous silica and ZIF-8-silica core-shell spheres. J. Chem. Technol. Biotechnol. 2015, 90, 669–677. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, F.; Wang, M.; Cao, C.; Zhang, Z.; Wang, H.; Liu, X.; Li, Y.; Jiang, Z. Vertically oriented Fe3O4 nanoflakes within hybrid membranes for efficient water/ethanol separation. J. Membr. Sci. 2021, 620, 118916. [Google Scholar] [CrossRef]
- Selim, A.; Toth, A.J.; Fozer, D.; Szanyi, A.; Mizsey, P. Pervaporative dehydration of methanol using PVA/nanoclay mixed matrix membranes: Experiments and modeling. Membranes 2020, 10, 435. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef] [PubMed]
- Guan, K.; Liu, G.; Matsuyama, H.; Jin, W. Graphene-based membranes for pervaporation processes. Chin. J. Chem. Eng. 2020, 28, 1755–1766. [Google Scholar] [CrossRef]
- Cheng, X.; Cai, W.; Chen, X.; Shi, Z.; Li, J. Preparation of graphene oxide/poly (vinyl alcohol) composite membrane and pervaporation performance for ethanol dehydration. RSC Adv. 2019, 9, 15457–15465. [Google Scholar] [CrossRef] [Green Version]
- Bian, Q.B.; Tian, H.F.; Wang, Y.R.; Liu, Q.; Ge, X.; Rajulu, A.V.; Xiang, A.M. Effect of graphene oxide on the structure and properties of poly (vinyl alcohol) composite films. Polym. Sci. Ser. A Polym. Phys. 2015, 57, 836–844. [Google Scholar] [CrossRef]
- Zhan, X.; Lu, J.; Xu, H.; Liu, J.; Liu, X.; Cao, X.; Li, J. Enhanced pervaporation performance of PDMS membranes based on nano-sized octa[(trimethoxysilyl)ethyl]-POSS as macro-crosslinker. Appl. Surf. Sci. 2019, 473, 785–798. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, M.; Gao, T.; Lu, J.; He, Y.; Li, J. A highly selective sorption process in POSS-g-PDMS mixed matrix membranes for ethanol recovery via pervaporation. Sep. Purif. Technol. 2020, 236, 116238. [Google Scholar] [CrossRef]
- Toth, A.J.; Szilagyi, B.; Fozer, D.; Haaz, E.; Selim, A.K.M.; Szori, M.; Viskolcz, B.; Mizsey, P. Membrane Flash Index: Powerful and Perspicuous Help for Efficient Separation System Design. ACS Omega 2020, 5, 15136–15145. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.J.; Haaz, E.; Valentinyi, N.; Nagy, T.; Tarjani, A.J.; Fozer, D.; Andre, A.; Khaled Mohamed, S.A.; Solti, S.; Mizsey, P. Selection between Separation Alternatives: Membrane Flash Index (MFLI). Ind. Eng. Chem. Res. 2018, 57, 11366–11373. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Liu, Y.; Lu, F.; Qu, J.; Chen, H.; Dai, L. Functionalization of graphene oxide with polyhedral oligomeric silsesquioxane (POSS) for multifunctional applications. J. Phys. Chem. Lett. 2012, 3, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Abo-Hamad, A.; AlSaadi, M.A.; Hashim, M.A. Functionalization of graphene using deep eutectic solvents. Nanoscale Res. Lett. 2015, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.; Pratihar, S.K.; Behera, S.K. Strong and ductile graphene oxide reinforced PVA nanocomposites. J. Alloys Compd. 2016, 684, 254–260. [Google Scholar] [CrossRef]
- Shameli, A.; Ameri, E. Synthesis of cross-linked PVA membranes embedded with multi-wall carbon nanotubes and their application to esterification of acetic acid with methanol. Chem. Eng. J. 2017, 309, 381–396. [Google Scholar] [CrossRef]
- Xia, L.L.; Li, C.L.; Wang, Y. In-situ crosslinked PVA/organosilica hybrid membranes for pervaporation separations. J. Membr. Sci. 2016, 498, 263–275. [Google Scholar] [CrossRef]
- Le, N.L.; Wang, Y.; Chung, T.S. Pebax/POSS mixed matrix membranes for ethanol recovery from aqueous solutions via pervaporation. J. Membr. Sci. 2011, 379, 174–183. [Google Scholar] [CrossRef]
- Sun, D.; Li, B.B.; Xu, Z.L. Pervaporation of ethanol/water mixture by organophilic nano-silica filled PDMS composite membranes. Desalination 2013, 322, 159–166. [Google Scholar] [CrossRef]
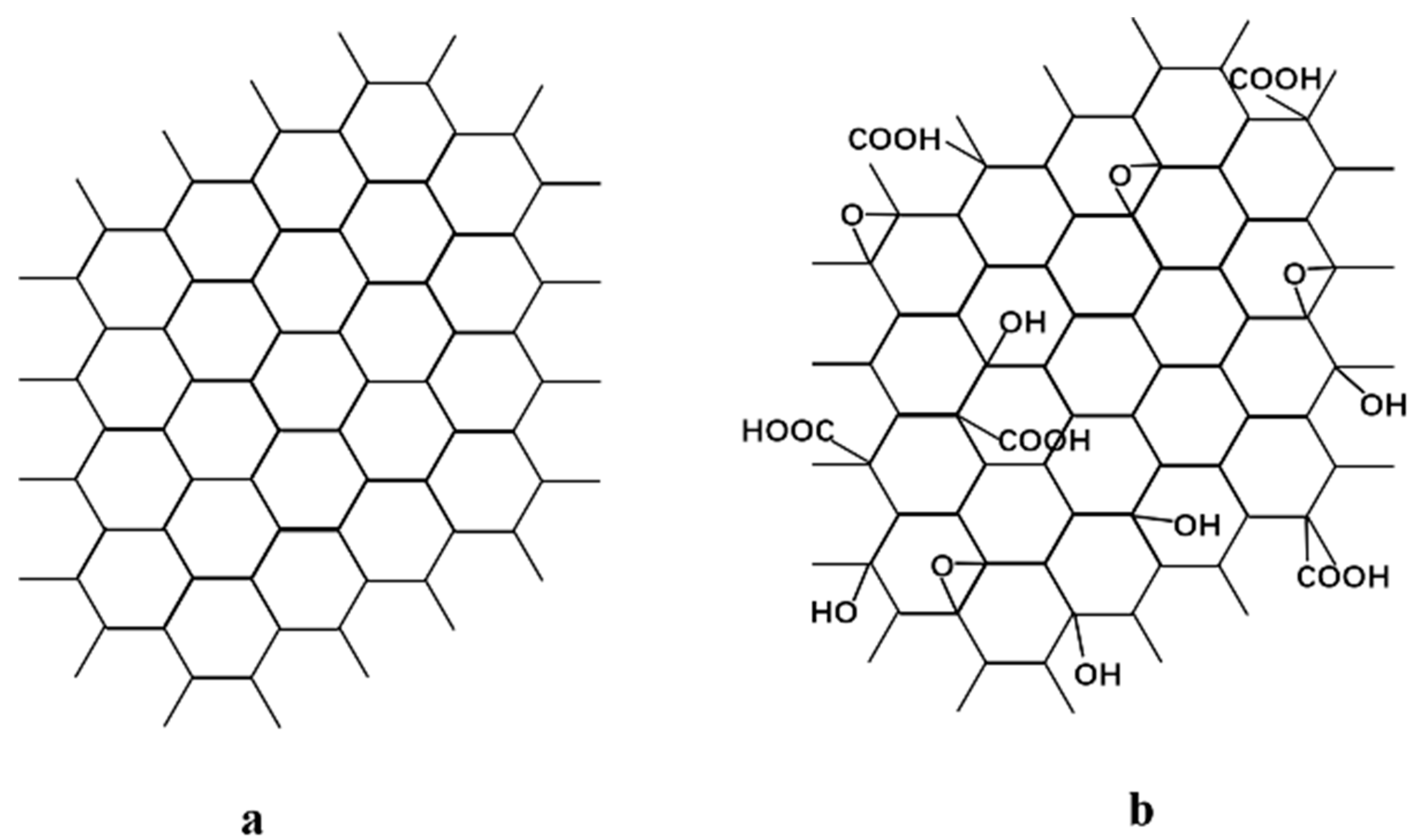


| Sample | C (wt%) | O (wt%) | H (wt%) | Oxidation Degree (%) |
|---|---|---|---|---|
| GR | 98.1 | 1.2 | 0.6 | 0.9 |
| GO | 46.3 | 46.9 | 1.9 | 75.9 |
| Membrane | Filler Loading (wt%) | Feed Temperature (°C) | Ethanol Concentration (wt%) | Total Flux (g/m2h) | Separation Factor | PSI (kg/m2h) | MFLI | Ref. |
|---|---|---|---|---|---|---|---|---|
| Zirconia/PVA | 42 | 50 | 90 | 105 | 142 | 14.8 | 10.45 | [20] |
| silica/PVA | 2 | 30 | 90 | 40 | 180 | 7.2 | 10.58 | [27] |
| H-ZSM-5/PVA | 7 | 30 | 96 | 125–118 | 349–236 | 43.5–27.7 | 22.69–23.39 | [25] |
| Zeolite X/PVA | 5 | 30 | 90 | 374 | 178 | 66.2 | 10.58 | [26] |
| c-alumina/PVA | - | 50 | 90 | 330 | 56 | 18.2 | 9.57 | [21] |
| CNT/PVA | 1 | 40 | 90 | 82 | 460 | 37.6 | 10.90 | [28] |
| CNT/PVA | 5 | 30 | 90 | 50 | 780 | 39.0 | 10.98 | [30] |
| CNT/PVA | 5 | 40 | 90 | 80 | 500 | 39.9 | 10.91 | [30] |
| ZIF-90/PVA | - | 30 | 90 | 268 | 1379 | 369.3 | 11.04 | [33] |
| ZIF-8-NH2/PVA | 7.5 | 40 | 85 | 120 | 200 | 23.9 | 10.80 | [31] |
| AgNP /PVA | 0.5 | 50 | 90 | 89 | 101.7 | 9.0 | 10.21 | [23] |
| UiO-66/PVA | 8 | 30 | 90 | 979 | 2084 | 2039.3 | 11.06 | [34] |
| rGO/PVA | 0.3 | 50 | 80 | 56 | 51.2 | 2.8 | 10.31 | [36] |
| GO/PVA | 2 | 70 | 90 | 185 | 65.9 | 12.0 | 9.78 | [19] |
| GO/PVA | 1 | 40 | 90 | 137 | 263 | 35.9 | 10.74 | [19] |
| GO/PVA | 1 | 40 | 90 | 194 | 481 | 93.1 | 10.91 | This work |
| GR/PVA | 1 | 40 | 90 | 71 | 1400 | 99.3 | 11.04 | This work |
| GR/PVA | 1 | 50 | 90 | 179 | 837 | 149.6 | 10.99 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, X.; Ge, R.; Gao, Z.; Gao, T.; Wang, L.; Li, J. PVA-Based MMMs for Ethanol Dehydration via Pervaporation: A Comparison Study between Graphene and Graphene Oxide. Separations 2022, 9, 26. https://doi.org/10.3390/separations9020026
Zhan X, Ge R, Gao Z, Gao T, Wang L, Li J. PVA-Based MMMs for Ethanol Dehydration via Pervaporation: A Comparison Study between Graphene and Graphene Oxide. Separations. 2022; 9(2):26. https://doi.org/10.3390/separations9020026
Chicago/Turabian StyleZhan, Xia, Rui Ge, Zhongyong Gao, Teng Gao, Luying Wang, and Jiding Li. 2022. "PVA-Based MMMs for Ethanol Dehydration via Pervaporation: A Comparison Study between Graphene and Graphene Oxide" Separations 9, no. 2: 26. https://doi.org/10.3390/separations9020026
APA StyleZhan, X., Ge, R., Gao, Z., Gao, T., Wang, L., & Li, J. (2022). PVA-Based MMMs for Ethanol Dehydration via Pervaporation: A Comparison Study between Graphene and Graphene Oxide. Separations, 9(2), 26. https://doi.org/10.3390/separations9020026







