Abstract
Serratula L. and Klasea Cass. are two systematically related genera of the family Asteraceae, which are distributed in most of the Eurasia area and are used as food and colorants and in traditional medicines as a drug. Since 1967, 261 metabolites have been isolated and identified from five Serratula species and 21 Klasea species. This review provides information on the chemodiversity of the terpenes, penolics, lipids, and other compounds found in both genera and their occurrence in individual species. Among the studied species, the most studied are S. coronata subsp. coronata, K. centauroides, and K. centauroides subsp. centauroides. This review also provides information on the methods of extraction, isolation, and analysis of ecdysteroids and flavonoids as the most valuable metabolites. For the first time, we provide general information about the biological activity of these extracts and individual compounds. The data presented in this review demonstrate the prospects of Serratula and Klasea species as sources of bioactive metabolites.
Keywords:
Serratula; Klasea; ecdysteroids; flavonoids; chemodiversity; isolation; chromatography; bioactivity 1. Introduction
The genus Serratula L. of the Asteraceae family, which is distributed in a wide range of temperature zones from Western Europe to the Far East, Japan, and Korea, currently includes four species and some subspecies [1]. The distribution area of the systematically closely related genus Klasea Cass. differs in a more southern location with centers of species diversity in the mountains of Central Asia, Western Iran, and the Iberian Peninsula, and it includes more than 50 species [2]. The systematic position of Serratula and Klasea has changed several times over the past two centuries. In 1825, A.H.G. Cassini proposed a theory about the presence of phylogenetic differences between the genera Serratula and Klasea, which was disproved by a number of authors [3]. To date, the existence of Serratula and Klasea as two separate genera has been confirmed based on morphological [3] and molecular data [4]. However, despite their differences, plants included in Serratula and Klasea have similar appearances and ethnopharmacological uses; thus, both genera are regarded in this paper together. Botanically, serratulas and klaseas are perennial herbs with erect stems and are usually branched in the upper half (Figure 1) [5]. The leaves are generally pinnate and rarely undivided and have a margin dentate or serrate. Capitulas are hetero- or homogamous, one or many in the panicle, paniculate, or corymbose, solitary at the end of stems and branches, and have an ovoid, hemispheric, or bowl-shaped involucre. Phyllaries are usually darker toward the apex, imbricate, and apex acute; the inner phyllaries are the longest, and the corolla is purple to pink or rarely almost white or yellow.

Figure 1.
Serratula L. (S.) and Klasea Cass. (K.) species in their natural habitat: (a) S. coronata; (b) S. coronata subsp. coronata; (c) S. tinctoria; (d) K. cardunculus; (e) K. erucifolia; (f) K. centauroides; (g) K. latifolia; (h) K. lycopifolia; (i) K. lyratifola; (j) K. procumbens; (k) K. quinquefolia; (l) K. radiata; (m) K. gmelinii; (n) K. sogdiana.
The wide distribution of Serratula and Klasea in Europe and Asia meadows, steppes, and forests determines its medical, food, and economic importance. Probably the best-known use of the herb of plumeless sawwort (S. coronata) has been reported to be medicinal uses among the indigenous peoples of the Far East for curing epilepsy, neurosis, and fatigue, as well as having anti-tumor and wound healing effects [6].
In Tibetan medicine, the herbs K. centauroides and K. cardunculus have been used as hemostatic and wound-healing remedies [7], and the fermented leaves of K. centauroides in Siberia are applied as a tea surrogate [8]. In Mongolia, a decoction of K. cardunculus flowers can be added to bath water to treat scrofulous and dropsy [9]. The herb powder of K. sogdiana has been known as an insecticide against spider mites and cotton aphids in Middle Asia [10]. In Europe, the roots and herb of S. tinctoria or sawwort were a source of wood, cotton, and flax textile dyes until the 19th century [11].
While studying the biological activity of Serratula and Klasea, it was shown that their preparations have adaptogenic, anti-stress, and anabolic activity due to the presence of ecdysteroids and flavonoids [12]. These substances are characterized by high biological activity due to their tonic and stimulating effect on the human body [13]. Taking into account the unquenchable scientific interest in the study of plant adaptogens, as well as the prospects and practical significance of Serratula and Klasea species as sources of valuable compounds, we have analytically studied the scientific literature data related to chemodiversity, methods for isolating and analyzing metabolites, as well as the bioactivity of both genera.
2. Review Strategy
The resources of international databases (e.g., Scopus, Web of Science, PubMed, and Google Scholar) were used, and only original papers written in English and published in journals prior to November 2022 were considered. The search keywords used included plant names (e.g., “Serratula”, “Klasea”, etc.) and metabolite names. Metabolites with a tentative structure (e.g., “luteolin-O-hexoside”, etc.) were excluded from the study. The structures of well-known metabolites (e.g., monoterpenes, sesquiterpenes, fatty acids, amino acids, etc.) are not discussed in this paper. The list of compounds includes secondary metabolites mostly correlated to ethnopharmacological uses and bioactivities of plants, and for a more complete picture, information about primary metabolites is also mentioned in this manuscript.
3. Chemodiversity of Serratula L. and Klasea Cass. Genera
The study of the chemical composition of the still unified genus Serratula began in the late 1960s after the discovery of polyacetylenes in S. coronata [14], which was continued later for other species of this genus. In the early stages of the scientific history of the genus arbutin [15], some flavonoids [16] were described in plants, and only after the discovery of 20-hydroxyecdysone in K. sogdiana (syn. S. sogdiana) in the early 70s [17] did plants of this genus began to become interesting for scientists as promising sources of ecdysteroids. As a result, more than 50 years of study of both genera resulted in the discovery of 261 metabolites in 5 Serratula and 21 Klasea species (Table 1). To date, the largest number of compounds has been found in K. centauroides (S. centauroides) (123 compounds) as well as in S. coronata subsp. coronata (S. manshurica Kitag., S. wolffii Andrae) (57 compounds) and in K. centauroides subsp. centauroides (S. komarovii) (32 compounds).

Table 1.
Synopsis of Serratula (S.) and Klasea (K.) species included in the review and total count of compounds found.
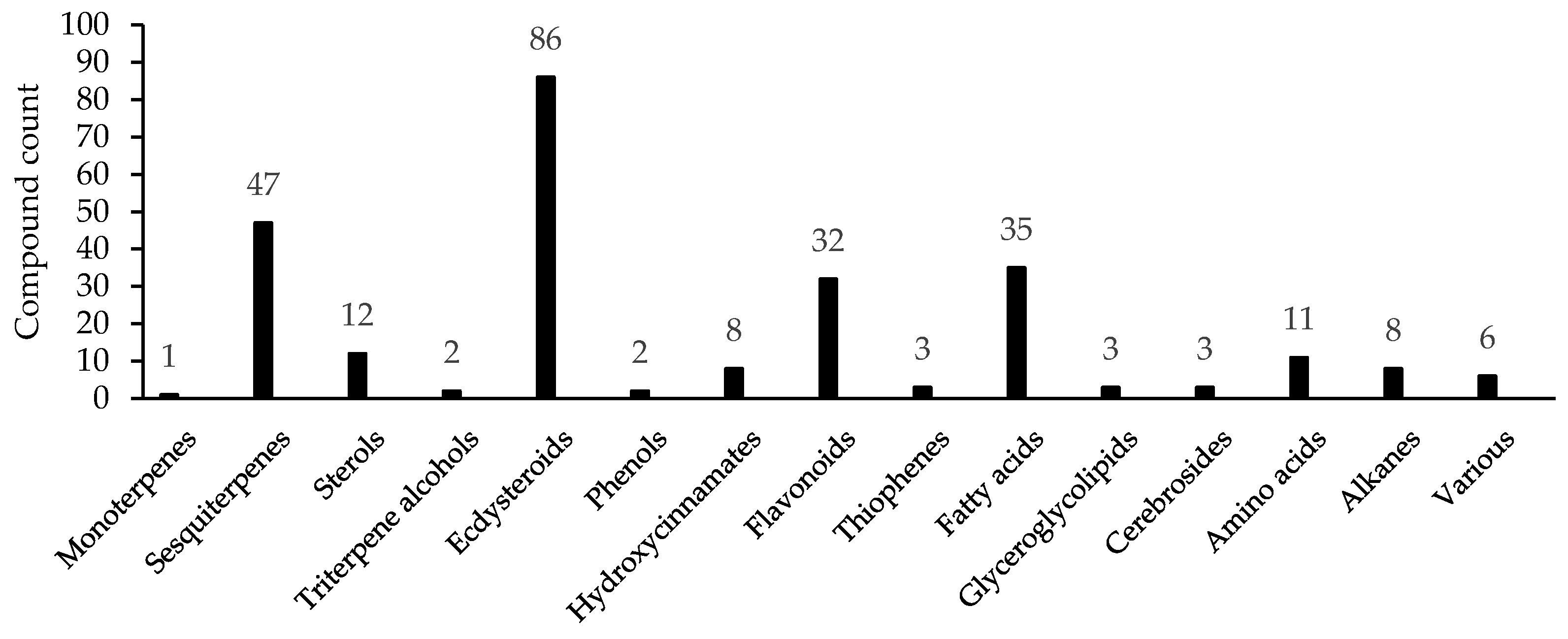
From 1967 to 2022, compounds 1–261 were identified for two genera, including monoterpene 1, sesquiterpenes (2–48), sterols (49–60), triterpene alcohols (61, 62), ecdysteroids (63–148), phenols (149, 150), hydroxycinnamates (151–158), flavonoids (159–190) and other phenolics (191, 192), thiophenes (193–195), fatty acids (196–230), glyceroglycolipids (231–233), cerebrosides (234–236), amino acids (237–247), alkanes (248–255), and other groups (256–261) [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77] (Table 2). The largest groups of metabolites are ecdysteroids, which include 86 compounds (ca. 33% of total compound), followed by the sesquiterpenes (47 comp.; ca. 18%), fatty acids (35 comp.; ca. 13%), and flavonoids (32 comp.; ca. 12%) (Figure 2).

Table 2.
Compounds 1–261 found in Serratula (S.) and Klasea (K.) plants.
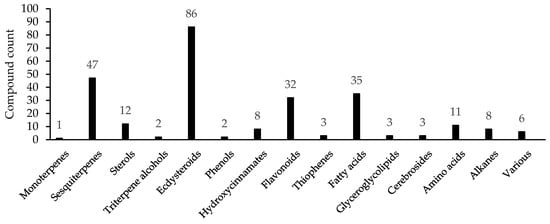
Figure 2.
Total count of compounds found in Serratula and Klasea.
3.1. Mono-, Sesquiterpenes, Sterols and Triterpene Alcohols
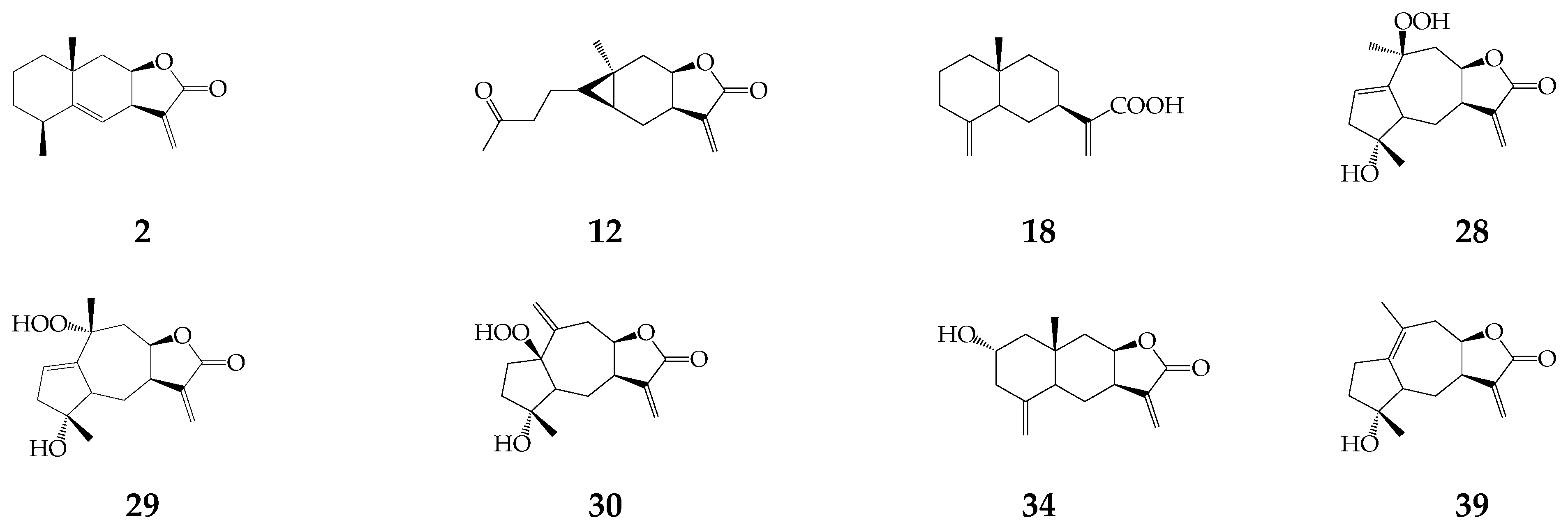
One monoterpene geranyl acetate (1) and forty-seven sesquiterpenes (2–48) were detected in essential oils of herbs and roots of K. centauroides [18], aerial part of K. latifolia [19], roots of S. coronata subsp. coronata [20] and K. centauroides subsp. strangulata [21,22]. Non-volatile sesquiterpenes were alantolactone (2), carabrone (12), costic acid (18), three guaianolides 28–30, ivalin (34), and pseudoivalin (39) from K. latifolia [19], and cetaurepensin (15) and its ester 16 from K. centauroides subsp. strangulata [21,22] (Figure 3).
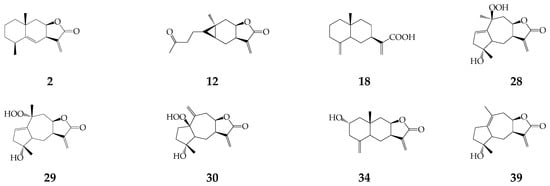
Figure 3.
Non-volatile sesquiterpenes (2), (12), (18), (28–30), (34), and (39).
Sterols of S. tinctoria [23] and K. centauroides [24] are derivatives of cholestane (50–52), ergostane (49, 53), and stigmastane (54–60), and two triterpene alcohols include α- (61) and β-amyrins (62) [24].
3.2. Ecdysteroids
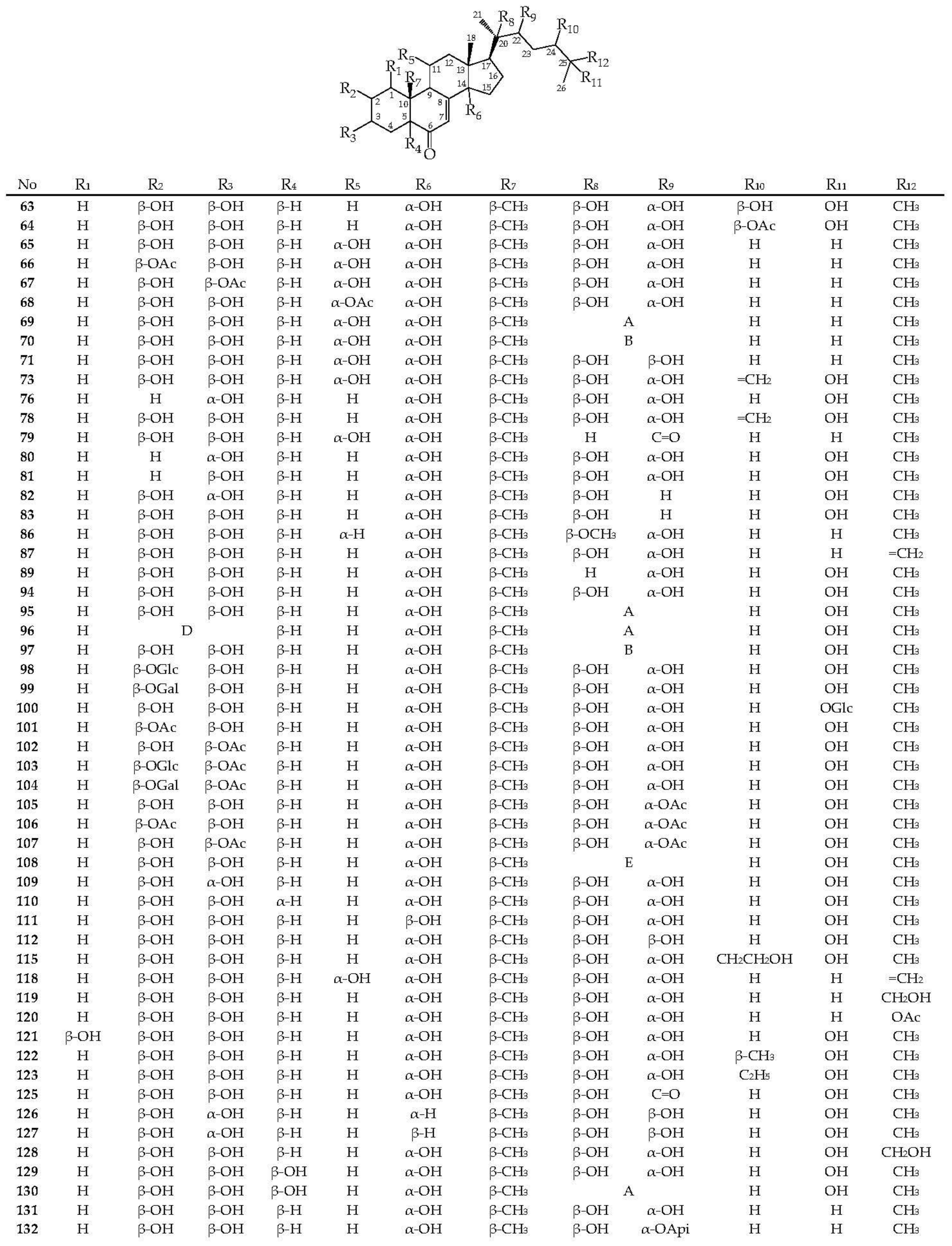
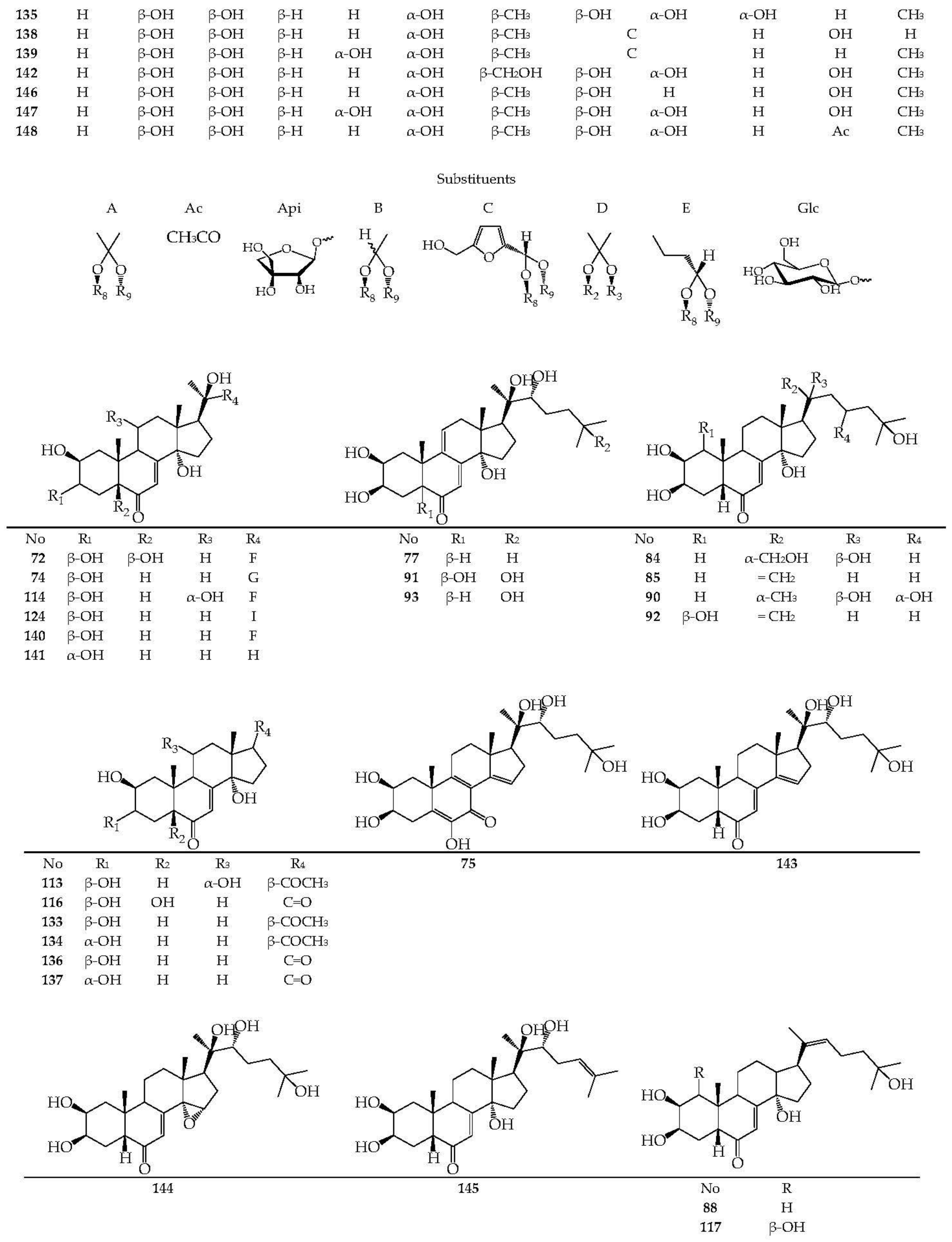
To date, 86 ecdysteroids have been isolated from Serratula and Klasea species (63–148) (Figure 4). Most compounds have structures with complete side chains, and six compounds are characterized by C-20–C-22 bond breaks (113, 116, 133, 134, 136, and 137). The presence of a hydroxyl group, free or substituted, at the C-3 position of the steroid nucleus, was noted for all compounds.
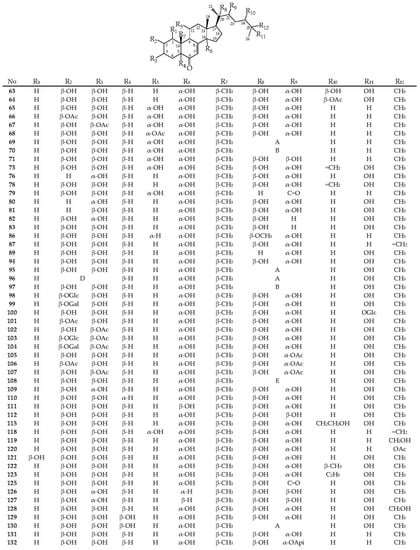
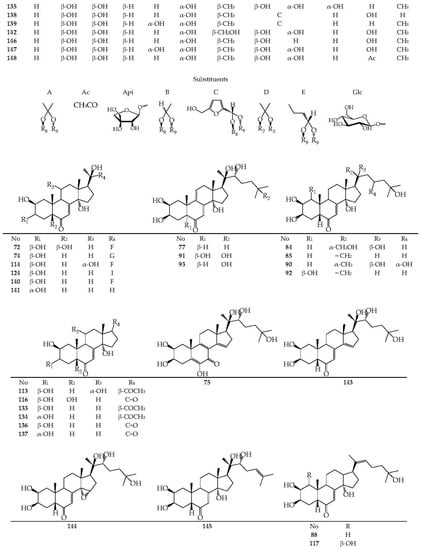
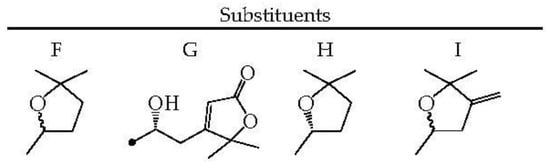
Figure 4.
Ecdysteroids 66–148.
The frequently encountered positions of the hydroxyl group also include C-2 (82 comp.), C-14 (76 comp.), C-20 (64 comp.), C-22, and C-25 (54 comp. each). Components with a hydroxy function at positions C-1 (2 comp.), C-24 (3 comp.), C-5 (5 comp.), and C-11 (14 comp.) are rare. The keto group at C-6 is an obligatory structural feature of all phytoecdysteroids, except calonisterone (75) from S. coronata [32] has a hydroxyl in this position. Component 75 also differs from other compounds in the presence of an unsaturated Δ5–6 bond, which is uncharacteristic of other compounds with an unsaturated Δ7–8 bond. The presence of the Δ9–11 dehydro function was found in herkesterone (91), dacryhainansterone (77), and its 25-hydroxy derivative (93), while stachysterone B (143) has an unsaturated Δ bond14–15. The presence of a large number of hydroxyl groups in the structures of phytoecdysteroids indicates the possibility for the synthesis of conjugates, esters, and other derivatives in plants. The formation of monoesters with acetic acid was revealed for ajugasterone C (65) at positions C-2 (66), C-3 (67), and C-11 (68), for inokosterone (119) at position C-26 (120), as well as for 20-hydroxyecdysone (94) at positions C-2 (101), C-3 (102), C-22 (105), and C-25 (148). Two 20-hydroxyecdysone diacetates were isolated from S. tinctoria containing acyl groups at C-2 and C-22 (106) and C-3 and C-22 (107) [49]. The existence of 1,2-diol fragments in the structures of ajugasterone C (65), polypodin B (129), and 20-hydroxyecdysone (94) at C2–C3 and C20–C22 allows the existence of isopropylidene ketals in the form of monoacetonides 69, 95, and 130 and diacetonide 96 found in S. chinensis [25], S. coronata [29,30,33], and S. strangulata [42]. Ethylidene-substituted ajugasterone C (70) and 20-hydroxyecdysone (97) were identified as components of S. tinctoria [33], while 20,22-O-butylidene-acetal of 20-hydroxyecdysone (108) was found in S. chinensis [25,34].
The side chain of some compounds may contain a furan ring attached via an ethylidene group to the C-20–C-22 diol fragment, as in serfusterone A (138) and serfusterone B (139) from S. coronata [57]. In ajugasterone D (72), shidasterone (140), and its derivatives 114, 124, and 141 from S. chinensis [25,34] and S. coronata [40,55,56], the furan fragment is present at C-22, as in carthamosterone (74) from S. chinensis at C-24 [25,34]. The only 14,15-epoxide of stachysterone B (144) was found in S. coronata [56]. Glycosides of phytoecdysteroids remain a rare group of derivatives for the genera Serratula and Klasea. The isolations of ponasterone A 22-O-apioside (132) from S. coronata [40] and 2-O- (98), 25-O-glucosides 20-hydroxyecdysone (100) [25], 2-O-galactoside 20-hydroxyecdysone (99), 2-O-glucoside (103), and 2-O-galactoside (104) of 20-hydroxyecdysone 3-O-acetate from S. chinensis [26] have been found. In general, the phytoecdysteroids Serratula and Klasea are characterized by the presence of a complete side chain and a large number of hydroxyl groups (5–7) both in the steroid core and in the side chain.
The content of ecdysteroids varied in plant organs of Serratula and Klasea species (Table 3). A high level of 20-hydroxyecdysone (190.1 mg/g) and polypodine B (35.8 mg/g) was detected in S. coronata extract [78], and 20-hydroxyecdysone was quantified in S. coronata subsp. coronata leaves (1.0–8.5 mg/g) and stems (0.2–3.4 mg/g) [79,80] and in S. tinctoria leaves (1.6–13.4 mg/g) [79]. Three Klasea species showed a high content of 20-hydroxyecdysone in leaves (8.8–16.8 mg/g) [35,38], stems (4.5–14.2 mg/g) [35,38], and seeds (4.7–5.5 mg/g) [39,41]. The minor Klasea ecdysteroids are integristerone A (0.02–2.20 mg/g) [35,38,39], 2-desoxy-20-hydroxyecdysone (0.1–2.9 mg/g) [35,38,39], and inokosterone (0.9–2.5 mg/g) [35,59].

Table 3.
Ecdysteroid content in Serratula (S.) and Klasea (K.) species a.
3.3. Distribution of Ecdysteroids in the Genera Serratula and Klasea
Data on the presence of phytoecdysteroids in the genus Serratula are known for % species, as well as for 14 species of the genus Klasea. Literature reports indicate that S. coronata subsp. coronata (S. wolffii, S. manshurica) is the most studied species in which 42 compounds were found (Table 4).

Table 4.
Ecdysteroid distribution on Serratula and Klasea species.
The probable reason for this interest is the availability of raw materials, which are widely distributed in Europe, European Russia, Siberia, and the Far East. From S. chinensis, S. coronata, and S. tinctoria, 23, 21, and 18 compounds were isolated, respectively. For the majority of species studied, information on the presence of ecdysteroids is limited to the presence of 20-hydroxyecdysone (94) and two to three other compounds. Such information cannot be considered sufficient for understanding the biological functions of these compounds for a plant or revealing the chemotaxonomic features of their accumulation within the genus; therefore, additional research is needed in this area. Considering the distribution of individual compounds among species, the most common is 20-hydroxyecdysone (94) found in all studied species. Inokosterone (119), polypodin B (129), and viticosterone E (148) were found in five species; ecdysone (89), integristerone A (121), and makisterone C (123) were found in four species; ajugasterone C (65), 20-hydroxyecdysone 20,22-monoacetonide (95), and 20-hydroxyecdysone 2-O-, 3-O- and 22-O-acetates (101, 102, 105) were found in three species.
3.4. Phenols and Hydroxycinnamates
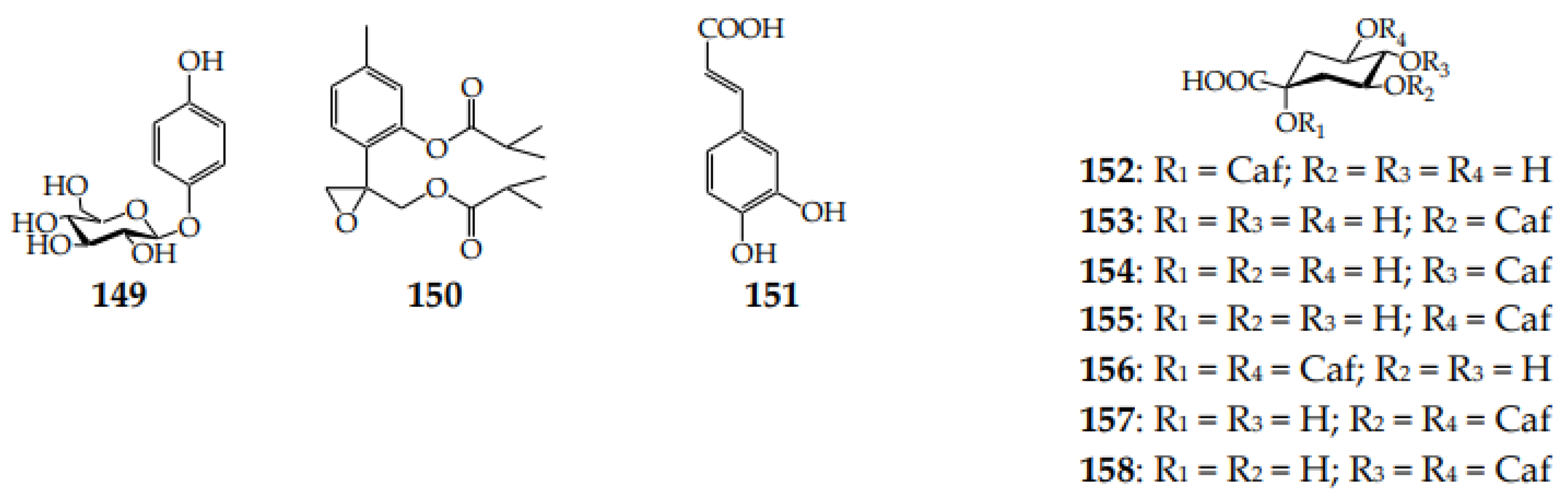
Arbutin (hydroquinone O-glucoside; 149), a rare Asteraceae metabolite, which was detected in only nine Klasea species [15,62,63,64] and never in Serratula, was also found in other Centaureinae members, such as Centaurea [41] and Stizolophus [81] (Figure 5). The known data demonstrated the high level of arbutin in K. centauroides subsp. centauroides leaves (3.3%) and stems (0.5%) [61] and in K. centauroides herb extract (2.5%) [59]. One thymol derivative, 150, was isolated from the K. latifolia herb [19]. The known hydroxycinnamates of Serratula and Klasea include caffeic acid (151) and caffeoylquinic acids (152–158) found in S. tinctoria [65] and three klaseas [47,59].
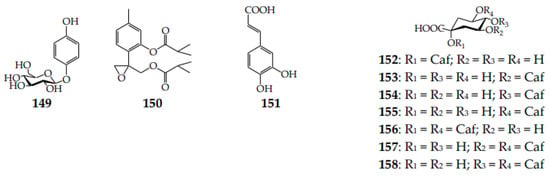
Figure 5.
Phenols 149 and 150 and hydroxycinnamates 151–158. Caf–caffeoyl.
3.5. Flavonoids
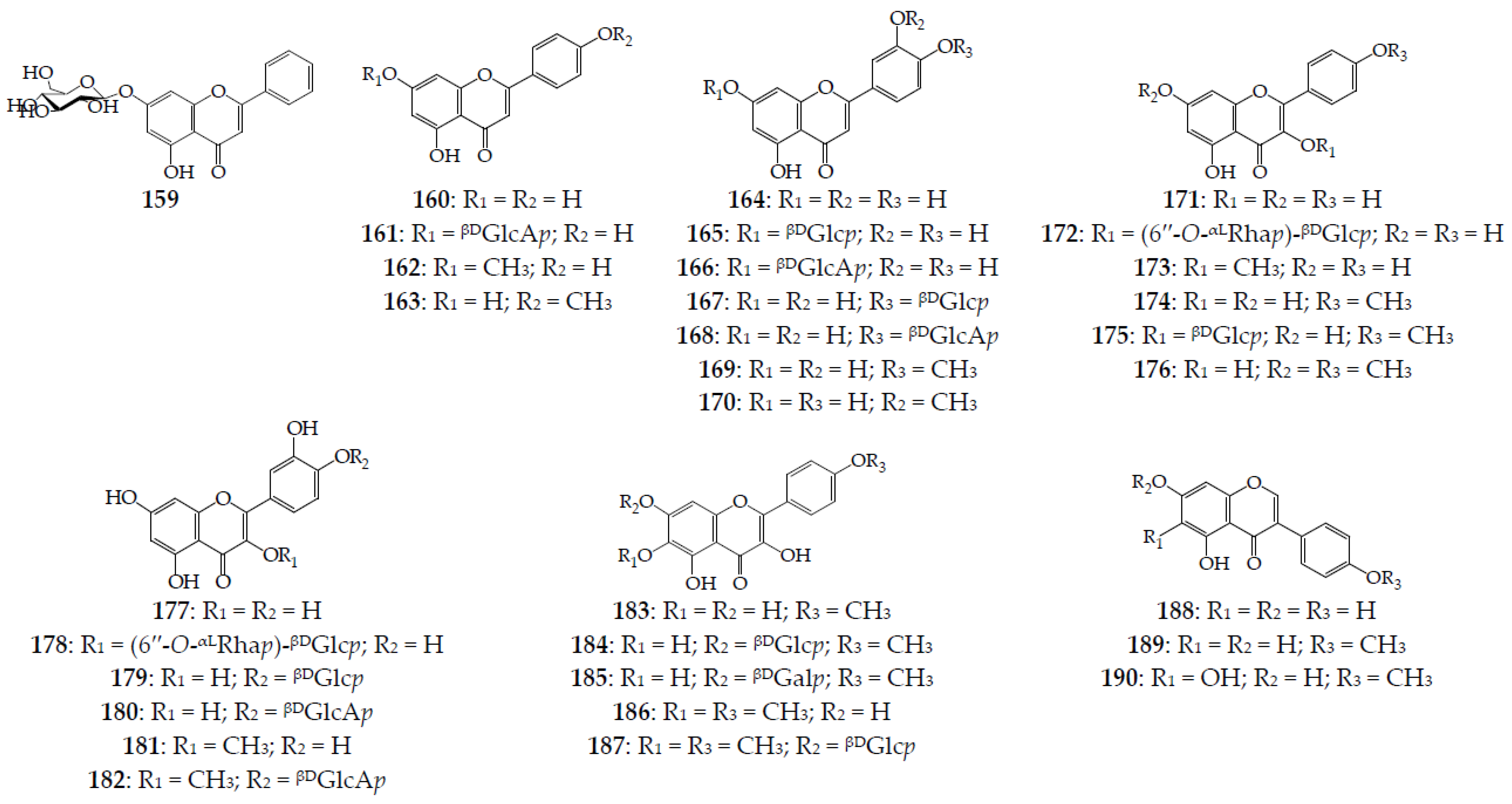
Three groups of flavonoids, including flavones (159–170), flavonols (171–187), and isoflavones (188–190), are distributed in different organs of four serratulas and seven klaseas (Figure 6). Flavones are derivatives of chrysin (159) [47], apigenin (160, 161) [16,67,68], acacetin (162) [68], genkwanin (163) [68], luteolin (164–168) [67,70], diosmetin (169) [65], and chrysoeriol (170) [69] in the form of aglycones (6 comp.) and O-glycosides (7 comp.). All glycosides have one carbohydrate (monoglycosides) that is β-D-glucose or β-D-glucouronic acid attached at the C-7 or C-4′ position of aglycone. The most common flavone is luteolin (164), found in three Serratula, and three Klasea, followed by the apigenin (160) and apigenin 7-O-glucuronide detected in four and three species, respectively. The highest number of flavones have been found in K. centauroides (5 comp.), with four found in S. coronata subsp. coronata, K. centauroides subsp. centauroides, and K. flavescens subsp. cichoriacea, three found in S. tinctoria, and two found in S. coronata and S. tinctoria subsp. tinctoria. The chemodiversity of seventeen flavonols include derivatives of kaempferol (171, 172) [63], its methyl esters at the C-3 (kaempferide, 173) [14], C-4′ (174, 175) [21] and C-7/C-4′ positions (176) [21], quercetin (177–180) [70] and 3-O-methyl ester (181, 182) [11], 6-hydroxykaempferol 4′-O-methyl ester (183–185) [21], and 6,4′-di-O-methyl ester (186, 187) [21].
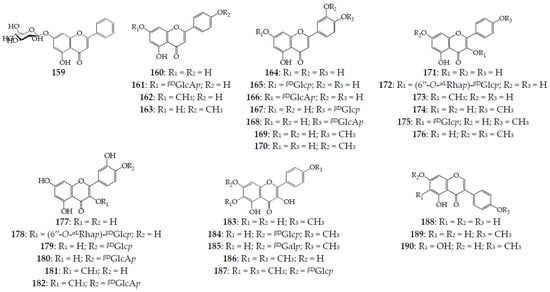
Figure 6.
Flavonoids 159–190. βDGalp–β-D-galactopyranose; βDGlcp–β-D-glucopyranose; βDGlcAp–β-D-glucuronopyranose; αLRhap–α-L-rhamnopyranose.
Seven glycosides are monoglycosides with β-D-glucose (175, 179, 184, and 187), β-D-galactose (185), and β-D-glucouronic acid (180 and 182) at the C-7 and C-4′ positions, and two biosides 172 and 178 containing rutinose moiety were found. From K. centauroides subsp. strangulata, eight compounds were isolated, and five flavonols were detected in S. coronata subsp. coronata; however, in other species, no more than two compounds were found. Non-glycosidic quercetin 3-O-methyl ester and quercetin have been described in five and four species, respectively. Additionally, three isoflavones have been mentioned for the whole plant of K. centauroides subsp. strangulata [21,41]. The total contents of flavonoids in wild S. coronata leaves and stems are 6.7–8.3% and 0.5–0.9%, respectively [71]; in S. tinctoria leaves, they are 1.2–1.6% [11], and in K. centauroides subsp. centauroides leaves, flowers, and stems, they are 9.0%, 4.6%, and 2.0%, respectively [69]. The dominant flavonoids in K. centauroides subsp. centauroides after HPLC quantification are apigenin-7-O-glucuronide (161) in flowers (1.1%), and luteolin-7-O-glucuronide (166) in leaves (4.9%) and stems (1.2%) [69].
3.6. Other Groups
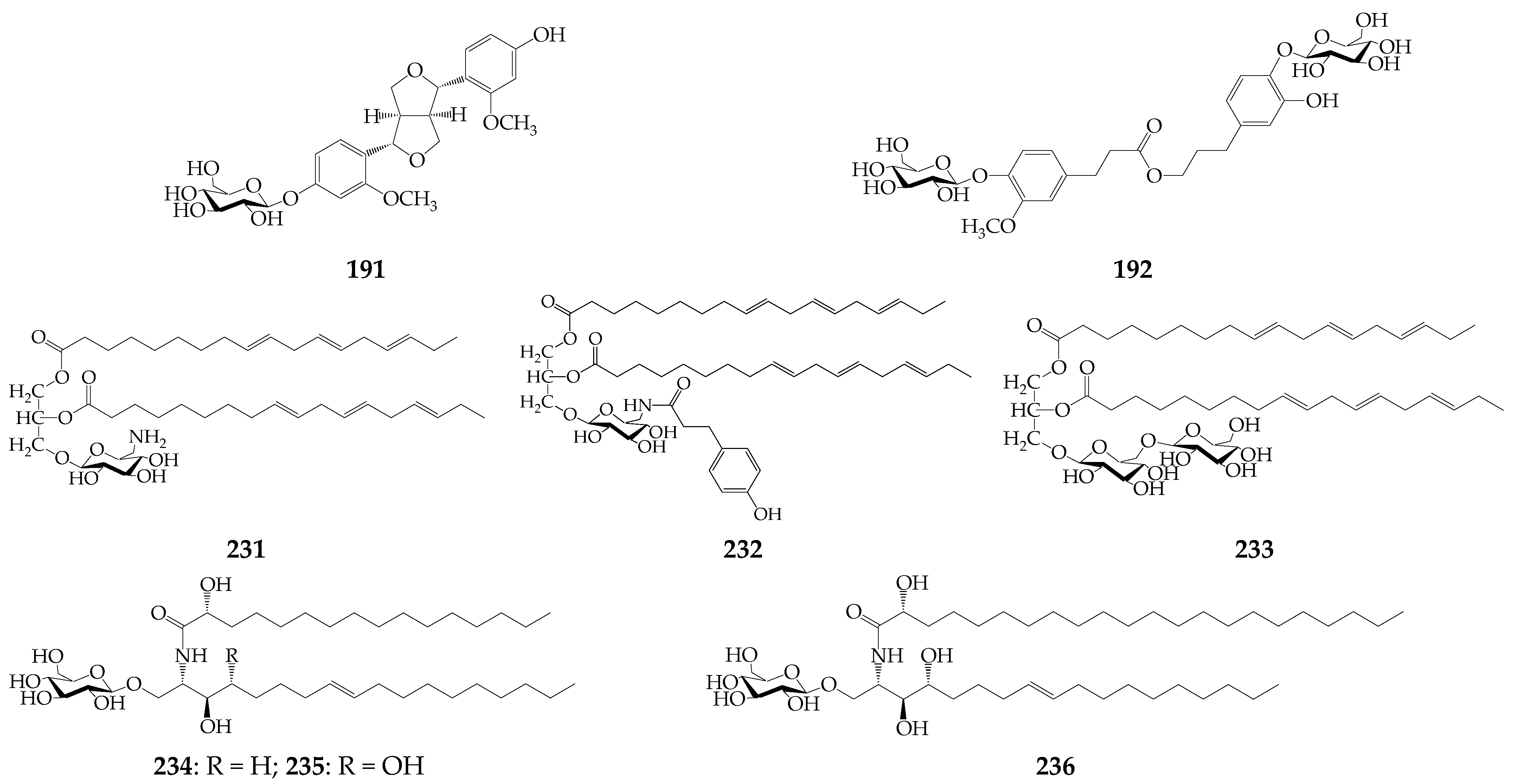
Lignan strangusin A (191) and phenolic glycoside strangusin B (192) have been isolated from the whole herb of K. centauroides subsp. strangulata [73]. The lipophilic nature metabolites include thiophenes 193–195 from K. radiata roots [74], fatty acids (196–230) from S. coronata subsp. coronata [20] and five klaseas [18,75], three rare glycerolipids strangulatosides A (231), B (232), and C (233) from K. centauroides subsp. strangulata rhizomes [22], rare cerebrosides 234–236 from K. chinensis roots [76], alkanes 248–255 found in essential oils of K. centauroides [18,24] and other groups (256–261) (Figure 7). Additionally, 11 amino acids were quantified in the herb and roots of K. centauroides [77].
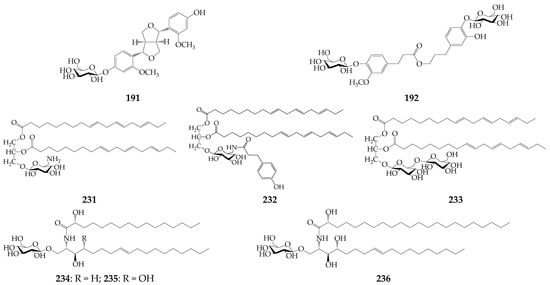
Figure 7.
Strangusin A (191), strangusin B (192), glycerolipids (231–233), and cerebrosides (234–236).
4. Extraction and Separation of Ecdysteroids and Flavonoids of Serratula и Klasea
Despite the diversity of metabolites found in Serratula and Klasea, the real highlights are ecdysteroids and flavonoids, featuring the most frequently isolated and separated compounds with proven data about their bioactivity, making them valuable metabolites (Figure 2). Sesquiterpenes and fatty acids have been characterized after a routine chromatographic procedure by gas chromatography-mass spectrometry (GC-MS) and have been described elsewhere. For other compounds, it is too early to draw conclusions about the features of isolation and separation.
4.1. Ecdysteroids
The general isolation procedure for Serratula and Klasea ecdysteroids includes the steps of extracting plant materials, followed by liquid–liquid/solid-phase extraction and/or purification of the resulting extract to obtain target-enriched fractions for which chromatographic separation is performed (Table 5). Herb and roots used for the extraction and the main solvents are low-molecular-weight alcohols (ethanol and methanol) as well as water. In rare cases, extraction is performed from freshly squeezed plant juice to prevent the thermal degradation of compounds [32,33,52,54]. Depending on the tasks set, the extraction temperature can vary from 0 °C [38] to the boiling point of the extractant [53,55,56,60]. After extraction and additional concentration, the aqueous residue is subjected to liquid–liquid extraction by lipophilic organic solvents (e.g., petroleum ether and hexane) to remove interfering lipophilic components.

Table 5.
Extraction conditions of ecdysteroid from Serratula and Klasea plant material.
Liquid–liquid extraction of target compounds is performed, as a rule, with ethyl acetate or n-butanol. In rare cases, multistage purification schemes of plant extracts are used, including multiple precipitations of ballast components from a methanol solution using acetone [30]. As an additional stage of purification, the procedure of liquid–liquid extraction of ecdysteroids by benzene followed by re-extraction of the organic phase with 50% methanol can be applied [49]. As the final stage of the purification, the solid-phase extraction [38] or ion exchange [34] leads to better results.
To isolate separate compounds from the mixture, it is not enough to use extraction procedures. An obligatory step is a chromatographic separation, various variants of which are widely used for the study of Serratula and Klasea ecdysteroids (Table 6).

Table 6.
Chromatographic conditions used for the separation of Serratula и Klasea ecdysteroids.
Atmospheric pressure chromatography (APC), as the main large-scale separation method, is realized on various sorbents, including alumina, normal- and reverse-phase silica gel, polyamide, and Sephadex LH-20. For separation on alumina, two-component solvent systems are often used, which are mixtures of chloroform and methanol/ethanol, and are less often three-component systems, including ethyl acetate and low-molecular-weight alcohols. Silica gel, as the most commonly used sorbent for APC of ecdysteroids, allows elution with mixtures of chloroform or dichloroethane with alcohols, as well as water–methanol solvent systems. To perform the separation on the epoxy-modified dextran Sephadex LH-20, one-component (methanol), two-component (methanol–ethyl acetate), and three-component systems (ethyl acetate–methanol–water) are used. The possibility of gradient elution with water–alcohol mixtures is a distinctive feature of APC on polyamide and reversed-phase silica gel.
Thin layer chromatography (TLC), as both a detection (analysis) and a separation method, is widely used to isolate the ecdysteroids of Serratula and Klasea. The use of TLC variants on normal-phase (NP) silica gel and its modifications, such as reversed-phase (RP) and cyano-derivatized layers, are well known. The compositions of the solvent systems used on NP-silica gel are close to those used in APC, including mixtures of chloroform with alcohols, as well as complex alkaline systems containing an ammonia solution, allowing more selective separation of individual components. For TLC on RP-silica gel, two-component systems are used, which are mixtures of water with methanol, acetonitrile, and tetrahydrofuran, while for TLC on cyano-derivatized-silica gel, mixtures of hexane and acetone can be used.
The proximity of the physicochemical and chromatographic properties of Serratula ecdysteroids, which make it difficult to isolate individual compounds, has led to using the various HPLC options, including its preparative mode. The normal-phase sorbent Zorbax-SIL has performed well in the process of ecdysteroid separation for S. tinctoria [45,49] and S. coronata [30,33,36,40,57,58]. The use of the isocratic mode for solvent systems containing dichloroethane/cyclohexane mixed with aqueous solutions of isopropanol made it possible to satisfactorily separate more than 30 compounds.
Traditional variants of preparative HPLC have been realized on RP-silica gel columns, and the Zorbax SB-C18 column has been used most often as a tool for the isolation of S. coronata ecdysteroids [30,33,36,40,57,58]. The positive results obtained with the use of Diasorb 130 C16 T [28], Reprosil-Pur C18-AQ [32], and Separon C18 [52,54] columns are also worth noting. For RP-HPLC, the use of mixtures of methanol and acetonitrile with water as eluents is common. An analytical version of HPLC was implemented on columns with normal (Zorbax-SIL) [30,33,56] and reversed-phase Separon C18 (Spherisorb 5ODS2, Zorbax ODS) [30,38,54,56] using mixtures of cyclohexane/dichloroethane/isooctane with isopropanol as eluents for NP-silica gels and mixtures of methanol/acetonitrile with water for RP-silica gels.
The presented information demonstrates the breadth of options for the chromatographic separation of Serratula and Klasea ecdysteroids, which explains the large number of compounds isolated from the genera.
4.2. Flavonoids
The extraction procedure of flavonoids of the herb and roots of Serratula and Klasea plants may include treatment by lipophilic solvents, such as ethyl ether–petroleum ether mixture [14] or methanol/ethanol infusion [21,68], boiling [67,71], or sonication [11] (Table 7). The resulting extracts have been subjected to liquid–liquid extraction by low- and medium-polarity solvents for purification and isolation of the target compounds in most cases [67,68,69] or re-crystallized from the alcohols to isolate the dominant component [67]. Finally, the enriched fractions can be separated by chromatographic techniques using atmospheric pressure, thin-layer, and high-performance chromatography (Table 8).

Table 7.
Extraction conditions of flavonoids from Serratula and Klasea plant material.

Table 8.
Chromatographic conditions used for the separation of Serratula and Klasea flavonoids.
Separation of Serratula and Klasea flavonoids by APC has been realized on cellulose [11], silica [21,68,71], Sephadex LH-20 [63,66], and polyamide sorbents [66], as well as preparative thin-layer chromatography on the cellulose if necessary [66]. Thus, although there was far less interest in flavonoids than in ecdysteroids, more than thirty compounds were successfully isolated and characterized. Additionally, some high-performance assays were applied to the profiling and quantitative analysis of Serratula and Klasea flavonoids. All known HPLC methods used reversed-phase sorbents (packed in 150-mm [59,63,65] and 250-mm columns [47,69,70,71]) and various combinations of high-polarity solvents (such as water [63] and organic acid solutions [47,59,65]) and medium-polarity solvents (such as acetonitrile [47,59] and methanol [67,71]). The elution mode is isocratic [63,70] or gradient [47,59,67,69,71]. Traditionally, the methods of flavonoid separation used for Serratula and Klasea plants are similar to the known methodological approach applied in flavonoid chemistry.
5. Bioactivity of Serratula and Klasea
The earliest mentions of the bioactivity of Serratula that we were able to find were inaccessible papers from the 1990s that demonstrated the genoprotective [82], immunostimulant [83], and anti-ulcer potential of S. coronata extracts [84]. Later, these studies were slightly expanded for total extracts and individual compounds [12,31,42,59,66,68,70,78,85,86,87,88,89,90,91,92,93] (Table 9). Pharmacological in vitro and in vivo animal studies of methanol, ethanol, and water extracts showed that S. coronata and K. centauroides have various activities, such as stress-protective [59], antioxidative [66], anxyolitic [70], adaptogenic [85], aldose reductase inhibitory [86], anti-ishemic [89], antihypoxic, and neuroprotective effects [90]. The only human experiment showed the antiseborrheic dermatitis effect of methanol extract of S. coronata herb [78]. The ecdysteroid fraction of the S. coronata herb containing 20-hydroxyecdysone as a dominant component proved to be an antihypoxic, adaptogenic, termoprotective [12], and cardioprotective agent [87]. Pure ecdysteroids and flavonoids isolated from S. coronata, K. centauroides subsp. strangulata, and K. flavescens subsp. cichoracea have shown antioxidant [31,42,68], stress-protective [92], and antibacterial effects [31,93].

Table 9.
Bioactivity data of Serratula and Klasea extracts and pure compounds.
6. Conclusions
Plant species of the Serratula and Klasea genera are a source of various groups of biologically active and economically valuable natural compounds with a chemodiversity of approximately 300 compounds. Despite this, to date, it is not possible to make conclusions about the chemical differentiation of Serratula and Klasea using the features of the chemical composition due to insufficient scientific information. Acceptance of 20-hydroxyecdysone, found in most of the studied species (in 19 of 26 studied), as a chemical marker of the genera cannot be considered correct because this compound is present in all ecdysteroid-containing plants. The distribution of other compounds does not allow the creation of an adequate chemical picture for these genera. It is necessary to note the complete absence of chemical information about the carbohydrate components (including polysaccharides) of both genera and the poor knowledge of flavonoids, which are rich in species of the Asteraceae family, as well as non-volatile sesquiterpenes, which are characteristic of plants belonging to the Cardueae tribe, including Serratula and Klasea. Because some species (K. centauroides and others) are used as food and functional products, in-depth studies of nutrients and their safety in humans are needed. In this regard, the relevance of the further in-depth study of the metabolites of Serratula and Klasea species is beyond doubt. Among the Serratula and Klasea plants, there are still few objects used for the industrial production of medicinal substances; only S. coronata is used to manufacture drugs containing 20-hydroxyecdysone and other ecdysteroids. Given the good natural resources of some species of Klasea, as well as the possibility of their cultivation in artificial conditions, it is necessary to expand the range of industrial plants. For the industrial species S. coronata, there is no information on the processing of waste products from the production of ecdysteroid-containing substances and the use of the plant’s industrial waste, which should remain after the end of industrial cycles. Regarding the methods of isolating and separating the metabolites of Serratula and Klasea, they are diverse and allow the required level of selectivity to be achieved. The use of massive and multistage schemes for the chromatographic separation of total fractions is justified in most cases because this leads to the isolation of new compounds and the refinement of the chemical composition of individual species. However, there is still little information about the quantitative content of individual groups of compounds, especially those that are important as target bioactive agents. Extracts and individual compounds of Serratula and Klasea require an extended and in-depth study of biological activity, as well as the study of the mechanisms of biological effects. To date, spot studies of bioactivity have been realized due to adaptogenic and anti-stress effects. There are no data on the effect of Serratula and Klasea preparations on the immune, cardiovascular, excretory, and other systems of humans or on the safety and possibility of mutagenic effects. When summarizing data on the metabolites of the genera Serratula and Klasea, it should be clarified that despite the gaps in scientific knowledge and the need for further research, plants of both genera are promising sources of drugs and possible food products. Although the history of the study of these plants began in the 20th century, it can be confidently stated that great achievements in the field of studying metabolites, their methods of separation, and their biological activity are still ahead.
Funding
This research was funded by the Ministry of Education and Science of Russia, grant number 121030100227-7.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The author is very thankful to colleagues for kindly providing the photos of plants: Oleg Syomin—Serratula coronata (authenticated by Elena V. Pismarkina, Botanical Garden, Ural Branch, Russian Academy of Sciences; https://www.plantarium.ru/page/image/id/503355.html, accessed on 30 October 2022); Vadim E. Prokhorov (Kazan Federal University, Kazan, Russia)—Serratula tinctoria (https://www.plantarium.ru/page/image/id/162446.html, accessed on 30 October 2022); Galina F. Darman (Amur branch of the Botanical Garden-Institute, Blagoveshchensk, Russia)—Serratula coronata subsp. coronata (https://www.plantarium.ru/page/image/id/219023.html, accessed on 30 October 2022); Andrey N. Efremov (Omsk State Pedagogical University, Omsk, Russia)—Klasea cardunculus (https://www.plantarium.ru/page/image/id/512873.html, accessed on 30 October 2022); Anton V. Popovich (Krasnodar Regional Branch, Russian Geographical Society, Krasnodar, Russia)—Klasea erucifolia (https://www.plantarium.ru/page/image/id/705743.html, accessed on 30 October 2022); Alexander V. Pavlenko (Serdar Branch, Center for Prevention of Especially Dangerous Infectious Diseases, State Sanitary and Epidemiological Service, Ministry of Health and Medical Industry of Turkmenistan, Serdar, Turkmenistan)—Klasea latifolia (https://www.plantarium.ru/page/image/id/682724.html, accessed on 30 October 2022); Maxim L. Zaitsev—Klasea lycopifolia (https://www.plantarium.ru/page/image/id/12730.html, accessed on 30 October 2022); Aleksandr L. Ebel (Tomsk State University, Tomsk, Russia)—Klasea lyratifola (https://www.plantarium.ru/page/image/id/631211.html, accessed on 30 October 2022); Evgeny Komarov—Klasea procumbens (https://www.plantarium.ru/page/image/id/626215.html, accessed on 30 October 2022); Gennadiy Okatov—Klasea quinquefolia (https://www.plantarium.ru/page/image/id/584433.html; accessed on 30 October 2022); Alyona Ruchka—Klasea radiata (authenticated by Natalia S. Gamova, Lomonosov Moscow State University, Moscow, Russia; https://www.plantarium.ru/page/image/id/661648.html, accessed on 30 October 2022); Elvir Izmaylov—Klasea gmelinii (authenticated by Vadim E. Prokhorov, Kazan Federal University, Kazan, Russia; https://www.plantarium.ru/page/image/id/59870.html, accessed on 30 October 2022); Alexander N. Naumenko—Klasea sogdiana (authenticated by Georgii A. Lazkov, National Academy of Sciences, Bishkek, Kyrgyzstan; https://www.plantarium.ru/page/image/id/225743.html, accessed on 30 October 2022).
Conflicts of Interest
The author declares no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Plant of the World Online. Serratula L. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:11002-1 (accessed on 30 October 2022).
- Plant of the World Online. Klasea Cass. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:9583-1 (accessed on 30 October 2022).
- Hellwig, F.H. Centaureinae (Asteraceae) in the Mediterranean-history of ecogeographical radiation. Plant Syst. Evol. 2004, 246, 137–162. [Google Scholar] [CrossRef]
- Martins, L.; Hellwig, F.H. Systematic position of the genera Serratula and Klasea within Centaureinae (Cardueae, Asteraceae) inferred from ETS and ITS sequence data and new combinations in Klasea. Taxon 2005, 54, 632–638. [Google Scholar] [CrossRef]
- Shi, Z.; Chen, Y.; Chen, Y.; Lin, Y.; Liu, S.; Ge, X.; Gao, T.; Zhu, S.; Liu, Y.; Yang, Q.; et al. Asteraceae (Compositae). In Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2011; Volume 20, pp. 177–180. [Google Scholar]
- Shreter, A.I. Medical Flora of Soviet Far East; Nauka: Moscow, Russia, 1975; pp. 315–317. [Google Scholar]
- Batorova, S.M.; Yakovlev, G.P.; Aseeva, T.A. Reference Book of Traditional Tibetan Medicine Herbs; Nauka: Novosibirsk, Russia, 2013; pp. 135–136. [Google Scholar]
- Aseeva, T.A. Tibetan Medicine of Buryats; SO RAN: Novosibirsk, Russia, 2008; pp. 308–309. [Google Scholar]
- Khaidav, T.S. Medical Plants of Mongolian Medicine; Gosizdatelstvo: Ulan-Bator, Mongolia, 1985; pp. 282–285. [Google Scholar]
- Belenovskaya, L.M.; Korkhov, V.V.; Mats, M.N.; Medvedeva, L.I. Plant Resources of USSR; Nauka: Moscow, Russia, 1993; Volume 7, pp. 181–183. [Google Scholar]
- Guinot, P.; Gargadennec, A.; La Fisca, P.; Fruchier, A.; Andary, C.; Mondolot, L. Serratula tinctoria, a source of natural dye: Flavonoid pattern and histolocalization. Ind. Crop. Prod. 2009, 29, 320–325. [Google Scholar] [CrossRef]
- Punegov, V.V.; Sychov, R.L.; Zainullin, V.G.; Fedorov, V.N.; Punegova, N.V. Extraction of ecdysteron-80 from Serratula coronata L. and assessment of its pharmacological action. Part I. Adaptogenic, gastroprotective, thermoprotective, and antihypoxic activity. Pharm. Chem. J. 2008, 42, 446–451. [Google Scholar] [CrossRef]
- Báthori, M.; Tóth, N.; Hunyadi, A.; Márki, A.; Zádor, E. Phytoecdysteroids and anabolic-androgenic steroids – Structure and effects on humans. Curr. Med. Chem. 2008, 15, 75–91. [Google Scholar] [CrossRef]
- Bohlmann, F.; Rode, K.-M.; Waldau, E. Polyacetylenverbindungen, CXXIX. Über die ersten pflanzlichen Polyinglykoside. Chem. Ber. 1967, 100, 1915–1926. [Google Scholar] [CrossRef]
- Yatsyuk, Y.K.; Lyashenko, S.S.; Batyuk, V.S. The content of arbutin in some species of the genus Serratula. Chem. Nat. Compd. 1968, 4, 46–47. [Google Scholar] [CrossRef]
- Yatsyuk, Y.K.; Lyashenko, S.S. Flavonoids of Serratula inermis. Chem. Nat. Compd. 1969, 5, 46–47. [Google Scholar] [CrossRef]
- Yatsyuk, Y.K.; Segel, G.M. The isolation of ecdysterone. Chem. Nat. Compd. 1970, 6, 284. [Google Scholar] [CrossRef]
- Tsybiktarova, L.P.; Taraskin, V.V.; Nikolaeva, I.G.; Radnaeva, L.D.; Gereltu, B. Constituent composition of essential oil from Serratula centauroides. Chem. Nat. Compd. 2016, 52, 1123–1124. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Faramarzi, S. Sesquiterpene lactones from Serratula latifolia. Phytochemistry 1988, 27, 479–481. [Google Scholar] [CrossRef]
- Bohlmann, F.; Czerson, H. Polyacetylenverbindungen, 240. Über die Inhaltsstoffe von Serratula wolfii Andrae. Chem. Berichte. 1976, 109, 2291–2295. [Google Scholar] [CrossRef]
- Dai, J.-Q.; Hou, Z.-F.; Zhu, Q.-X.; Yang, L.; Li, Y. Sesquiterpenes and Flavonoids from Serratula strangulata. J. Chin. Chem. Soc. 2001, 48, 249–252. [Google Scholar] [CrossRef]
- Dai, J.Q.; Zhu, Q.X.; Zhao, C.Y.; Yang, L.; Li, Y. Glyceroglycolipids from Serratula strangulata. Phytochemistry 2001, 58, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Corio-Costet, M.; Chapuis, L.; Mouillet, J.; Delbecque, J. Sterol and ecdysteroid profiles of Serratula tinctoria (L.): Plant and cell cultures producing steroids. Insect Biochem. Mol. Biol. 1993, 23, 175–180. [Google Scholar] [CrossRef]
- Tsybiktarova, L.P.; Taraskin, V.; Nikolaeva, I.G.; Radnaeva, L.D.; Garmaeva, L.L. Lipids from Serratula centauroides. Chem. Nat. Compd. 2016, 52, 294–295. [Google Scholar] [CrossRef]
- Tang, H.-J.; Fan, C.-L.; Wang, G.-Y.; Wei, W.; Wang, Y.; Ye, W.-C. Chemical constituents from roots tubers of Serratula chinensis. Chin. Trad. Herb. Drugs 2014, 45, 906–912. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Yang, W.-Q.; Fan, C.-L.; Zhao, H.-N.; Huang, X.-J.; Wang, Y.; Ye, W.-C. New ecdysteroid and ecdysteroid glycosides from the roots of Serratula chinensis. J. Asian Nat. Prod. Res. 2016, 19, 208–214. [Google Scholar] [CrossRef]
- Dinan, L.; Balducci, C.; Guibout, L.; Lafont, R. Small-scale analysis of phytoecdysteroids in seeds by HPLC-DAD-MS for the identification and quantification of specific analogues, dereplication and chemotaxonomy. Phytochem. Anal. 2020, 31, 643–661. [Google Scholar] [CrossRef]
- Volodin, V.V.; I Alexeeva, L.; A Kolegova, N.; Sarker, S.D.; Šik, V.; Lafont, R.; Dinan, L. Further ecdysteroids from Serratula coronata L. (Asteraceae). Biochem. Syst. Ecol. 1998, 26, 459–461. [Google Scholar] [CrossRef]
- Miladera, K.; Saatov, Z.; Kholodova, Y.D.; Gorovits, M.B.; Shashkov, A.S.; Abubakirov, N.K. Phytoecdysteroids of plants of the genus Serratula. Ajugasterone C 20,22-monoacetonide from Serratura wolffii. Chem. Nat. Compd. 1992, 28, 59–63. [Google Scholar] [CrossRef]
- Hunyadi, A.; Gergely, A.; Simon, A.; Tóth, G.; Veress, G.; Báthori, M. Preparative-scale chromatography of ecdysteroids of Serratula wolffii Andrae. J. Chromatogr. Sci. 2007, 45, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Aliouche, L.; Larguet, H.; Amrani, A.; Leon, F.; Brouard, I.; Benayache, S.; Zama, D.; Meraihi, Z.; Benayache, F. Isolation, antioxidant and antimicrobial activities of ecdysteroids from Serratula cichoracea. Curr. Bioact. Compd. 2018, 14, 60–66. [Google Scholar] [CrossRef]
- Galyautdinov, I.V.; Sadretdinova, Z.R.; Muslimov, Z.S.; Gareev, V.F.; Khalilov, L.M.; Odinokov, V.N. New minor phytoecdysteroids from the juice of Serratula coronata L. (Asteraceae). J. Med. Plants Stud. 2016, 4, 30–34. [Google Scholar]
- Odinokov, V.; Kumpun, S.; Galyautdinov, I.; Evrard-Todeschi, N.; Veskina, N.A.; Khalilov, L.M.; Girault, J.-P.; Dinan, L.; Maria, A.; Lafont, R. Low-polarity phytoecdysteroids from the juice of Serratula coronata L. (Asteraceae). Collect. Czechoslov. Chem. Commun. 2005, 70, 2038–2052. [Google Scholar] [CrossRef]
- Ling, T.; Zhang, Z.; Xia, T.; Ling, W.; Wan, X. Phytoecdysteroids and other constituents from the roots of Klaseopsis chinensis. Biochem. Syst. Ecol. 2009, 37, 49–51. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. Phytoecdysteroids of Serratula centauroides herb from Cisbaikalia. Russ. J. Bioorganic Chem. 2019, 45, 913–919. [Google Scholar] [CrossRef]
- Takács, M.; Simon, A.; Liktor-Busa, E.; Báthori, M.; Zsila, F.; Bikádi, Z.; Horváth, P.; Veress, G.; Gergely, A.; Tóth, G. Structure and stereochemistry of novel ecdysteroids from the roots of Serratula wolffii. Magn. Res. Chem. 2010, 48, 386–391. [Google Scholar] [CrossRef]
- Odinokov, V.; Galyautdinov, I.; Fatykhov, A.; Khalilov, L.M. A new phytoecdysteroid. Russ. Chem. Bull. 2000, 49, 1923–1924. [Google Scholar] [CrossRef]
- Vorob’Eva, A.N.; Rybin, V.G.; Zarembo, E.V.; Boltenkov, E.V. Phytoecdysteroids from Serratula centauroides. Chem. Nat. Compd. 2005, 41, 105–106. [Google Scholar] [CrossRef]
- Vorob’Eva, A.N.; Rybin, V.G.; Zarembo, E.V.; Boltenkov, E.V.; Verbitskii, G.A. Phytoecdysteroids from Serratula komarovii. Chem. Nat. Compd. 2004, 40, 492–495. [Google Scholar] [CrossRef]
- Ványolós, A.; Béni, Z.; Dékány, M.; Simon, A.; Báthori, M. Novel Ecdysteroids from Serratula wolffii. Sci. World J. 2012, 2012, 651275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, S.; Dai, J.; Chen, X.; Hu, Z. Identification and determination of ecdysones and flavonoids in Serratula strangulata by micellar electrokinetic capillary chromatography. Planta Med. 2002, 68, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-Q.; Cai, Y.-J.; Shi, Y.-P.; Zhang, Y.-H.; Liu, Z.-L.; Yang, L.; Li, Y. Antioxidant activity of ecdysteroids from Serratula strangulata. Chin. J. Chem. 2010, 20, 497–501. [Google Scholar] [CrossRef]
- Liktor-Busa, E.; Simon, A.; Tóth, G.; Fekete, G.; Kele, Z.; Báthori, M. Ecdysteroids from Serratula wolffii roots. J. Nat. Prod. 2007, 70, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Abubakirov, N.K. Ecdysteroids of flowering plants (Angiospermae). Chem. Nat. Compd. 1981, 17, 489–503. [Google Scholar] [CrossRef]
- Bathori, M.; Mathe, I.; Girault, J.-P.; Kalasz, A.H.; Lafont, R. Isolation and structural elucidation of two plant ecdysteroids, gerardiasterone and 22-epi-20-hydroxyecdysone. J. Nat. Prod. 1998, 61, 415–417. [Google Scholar] [CrossRef]
- Hunyadi, A.; Tóth, G.; Simon, A.; Mák, M.; Kele, Z.; Máthé, A.I.; Báthori, M. Two new ecdysteroids from Serratula wolffii. J. Nat. Prod. 2004, 67, 1070–1072. [Google Scholar] [CrossRef]
- Kasterova, E.; Zibareva, L.; Revushkin, A. Secondary metabolites of some Siberian species of plants tribe Cynareae (Asteraceae). S. Afr. J. Bot. 2019, 125, 24–29. [Google Scholar] [CrossRef]
- Tuleuov, B.I. 20-Hydroxyecdysone content of several representatives of the families Asteraceae and Caryophyllaceae. Chem. Nat. Compd. 2009, 45, 762. [Google Scholar] [CrossRef]
- Rudel, D.; Bathori, M.; Gharbi, J.; Girault, J.-P.; Racz, I.; Melis, K.; Szendrei, K.; Lafont, R. New ecdysteroids from Serratula tinctoria. Planta Med. 1992, 58, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, I.G.; Tsybiktarova, L.P.; Garmaeva, L.L.; Nikolaeva, G.G.; Olennikov, D.N.; Matkhanov, I.E. Determination of ecdysteroids in Fornicium unflorum (L.) and Serratula centauroides (L.) raw materials by chromatography–UV spectrophotometry. J. Anal. Chem. 2017, 72, 854–861. [Google Scholar] [CrossRef]
- Kholodova, Y.D.; Baltaev, U.; Volovenko, V.O.; Gorovits, M.B.; Abubakirov, N.K. Phytoecdysones of Serratula xeranthemoides. Chem. Nat. Compd. 1979, 15, 144–146. [Google Scholar] [CrossRef]
- Odinokov, V.; Galyautdinov, I.; Mel’Nikova, D.A.; Muslimov, Z.S.; Khalilov, L.M.; Denisenko, O.N.; Mogilenko, T.G.; Zaripova, E.R.; Zakirova, L.M. Isolation and identification of phytoecdysteroids from juice of Serratula quinquefolia. Chem. Nat. Compd. 2013, 49, 392–394. [Google Scholar] [CrossRef]
- Zatsny, I.L.; Gorovits, M.B.; Abubakirov, N.K. Ecdysterone from Serratula sogdiana. Chem. Nat. Compd. 1971, 7, 822. [Google Scholar] [CrossRef]
- Odinokov, V.; Galyautdinov, I.; Nedopekin, D.; Khalilov, L.; Shashkov, A.; Kachala, V.; Dinan, L.; Lafont, R. Phytoecdysteroids from the juice of Serratula coronata L. (Asteraceae). Insect Biochem. Mol. Biol. 2002, 32, 161–165. [Google Scholar] [CrossRef]
- Simon, A.; Liktor-Busa, E.; Tóth, G.; Kele, Z.; Groska, J.; Báthori, M. Additional minor phytoecdysteroids of Serratula wolffii. Helv. Chim. Acta 2008, 91, 1640–1645. [Google Scholar] [CrossRef]
- Simon, A.; Tóth, G.; Liktor-Busa, E.; Kele, Z.; Takács, M.; Gergely, A.; Báthori, M. Three new steroids from the roots of Serratula wolffii. Steroids 2007, 72, 751–755. [Google Scholar] [CrossRef]
- Liktor-Busa, E.; Simon, A.; Tóth, G.; Báthori, M. The first two ecdysteroids containing a furan ring from Serratula wolffii. Tetrahedr. Lett. 2008, 49, 1738–1740. [Google Scholar] [CrossRef]
- Novosel’Skaya, I.L.; Gorovits, M.B.; Abubakirov, N.K. Phytoecdysones of Serratula IV. Sogdysterone. Chem. Nat. Compd. 1975, 11, 445–446. [Google Scholar] [CrossRef]
- Shantanova, L.N.; Olennikov, D.N.; Matkhanov, I.E.; Gulyaev, S.M.; Toropova, A.A.; Nikolaeva, I.G.; Nikolaev, S.M. Rhaponticum uniflorum and Serratula centauroides extracts attenuate emotional injury in acute and chronic emotional stress. Pharmaceuticals 2021, 14, 1186. [Google Scholar] [CrossRef] [PubMed]
- Zatsny, I.L.; Gorovits, M.B.; Abubakirov, N.K. Phytoecdysones of serratula II. Viticosterone E from Serratula sogdiana and its partial synthesis. Chem. Nat. Compd. 1973, 9, 170–173. [Google Scholar] [CrossRef]
- Myagchilov, A.V.; Mineev, S.A.; Sokolova, L.I.; Gerdasova, E.D.; Gorovoi, P.G. Arbutin content in the Far-Eastern species Serratula komarovii Iljin. Pharm. Chem. J. 2020, 54, 377–379. [Google Scholar] [CrossRef]
- Nowak, G.; Nawrot, J.; Latowski, K. Arbutin in Serratula quinquefolia M.B. (Asteraceae). Acta Soc. Bot. Pol. 2009, 78, 137–140. [Google Scholar] [CrossRef]
- Kusano, K.; Iwashina, T.; Kitajima, J.; Mishio, T. Flavonoid diversity of Saussurea and Serratula species in Tien Shan mountains. Nat. Prod. Commun. 2007, 2, 1121–1128. [Google Scholar] [CrossRef]
- Zatsny, I.L.; Gorovits, M.B.; Abubakirov, N.K. Arbutin from Serratula sogdiana. Chem. Nat. Compd. 1973, 9, 415–416. [Google Scholar] [CrossRef]
- Lech, K.; Witkoś, K.; Jarosz, M. HPLC-UV-ESI MS/MS identification of the color constituents of sawwort (Serratula tinctoria L.). Anal. Bioanal. Chem. 2014, 406, 3703–3708. [Google Scholar] [CrossRef]
- Báthori, M.; Zupkó, I.; Hunyadi, A.; Gácsné-Baitz, E.; Dinya, Z.; Forgó, P. Monitoring the antioxidant activity of extracts originated from various Serratula species and isolation of flavonoids from Serratula coronata. Fitoterapia 2004, 75, 162–167. [Google Scholar] [CrossRef]
- Myagchilov, A.V.; Sokolova, L.I.; Gorovoy, P.G. Flavonoids of the East Asian species Serratula manshurica Kitag. Khim. Rastit. Syr’ya 2021, 167–173. [Google Scholar] [CrossRef]
- Aliouche, L.; Zater, H.; Zama, D.; Bentamene, A.; Seghiri, R.; Mekkiou, R.; Benayache, S.; Benayache, F. Flavonoids of Serratula cichoracea and their antioxidant activity. Chem. Nat. Compd. 2007, 43, 618–619. [Google Scholar] [CrossRef]
- Myagchilov, A.V.; Gorovoi, P.G.; Sokolova, L.I. Flavonoids from Serratula komarovii Iljin (the Asteraceae Family). Russ. J. Bioorg. Chem. 2021, 47, 1418–1423. [Google Scholar] [CrossRef]
- Nikolaev, S.M.; Nikolaeva, I.G.; Razuvaeva, Y.G.; Matkhanov, I.E.; Tsybiktarova, L.P.; Shantanova, L.N.; Nikolaeva, G.G. Phenolic compounds of Serratula centauroides and anxiolytic effect. Farmacia 2019, 67, 504–510. [Google Scholar] [CrossRef]
- Myagchilov, A.V.; Sokolova, L.I.; Gorovoy, P.G.; Kechaikin, A.A. Features of the composition of flavonoids in the crowned sawwort (Serratula coronata L.) from Siberia and the Far East of Russia. Khim. Rastit. Syr’ya 2020, 171–179. [Google Scholar] [CrossRef]
- Glyzin, V.I.; Ban’Kovskii, A.I.; Mel’Nikova, T.M. 3-O-Methylquercetin from Serratula inermis. Chem. Nat. Compd. 1972, 8, 383. [Google Scholar] [CrossRef]
- Dai, J.Q.; Shi, Y.P.; Yang, L.; Li, Y. Two new components from Serratula strangulata Iljin. Chin. Chem. Lett. 2002, 13, 143–146. [Google Scholar]
- Bohlmann, F.; Waldau, E. Polyacetylenverbindungen, CXXV. Über die Inhaltsstoffe von Serratula radiata Bieb. Eur. J. Inorg. Chem. 1967, 100, 1206–1208. [Google Scholar] [CrossRef]
- Tel, G.; Öztürk, M.; Duru, M.E.; Doǧan, B.; Harmandar, M. Fatty acid composition, antioxidant, anticholinesterase and tyrosinase inhibitory activities of four Serratula species from Anatolia. Rec. Nat. Prod. 2013, 7, 86–95. [Google Scholar]
- Ling, T.; Xia, T.; Wan, X.; Li, D.; Wei, X. Cerebrosides from the Roots of Serratula chinensis. Molecules 2006, 11, 677–683. [Google Scholar] [CrossRef]
- Tsybiktarova, L.P.; Nikolaeva, I.G. Amino acids from Serratula centauroides. Chem. Nat. Compd. 2017, 53, 203–204. [Google Scholar] [CrossRef]
- Napierała, M.; Nawrot, J.; Gornowicz-Porowska, J.; Florek, E.; Moroch, A.; Adamski, Z.; Kroma, A.; Miechowicz, I.; Nowak, G. Separation and HPLC characterization of active natural steroids in a standardized extract from the Serratula coronata Herb with antiseborrheic dermatitis activity. Int. J. Environ. Res. Public Health 2020, 17, 6453. [Google Scholar] [CrossRef]
- Báthori, M.; Máthé, I.; Guttman, A. Determination of 20-hydroxyecdysone content by thin-layer chromatography and micellar electrokinetic chromatography. Chromatographia 1998, 48, 145–148. [Google Scholar] [CrossRef]
- Báthori, M.; Gergely, A.; Kalász, H.; Nagy, G.; Dobos, Á.; Máthé, I. Liquid chromatographic monitoring of phytoecdysteroid production of Serratula wolffii. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 281–294. [Google Scholar] [CrossRef]
- Nawrot, J.; Budzianowski, J.; Nowak, G.; Micek, I.; Budzianowska, A.; Gornowicz-Porowska, J. Biologically active compounds in Stizolophus balsamita inflorescences: Isolation, phytochemical characterization and effects on the skin biophysical parameters. Int. J. Mol. Sci. 2021, 22, 4428. [Google Scholar] [CrossRef] [PubMed]
- Chabannyĭ, V.N.; Levitskiĭ, E.L.; I Gubskiĭ, I.; Kholodova, I.D.; E. Vistunova, I.; I Budmaska, M.I. Genoprotective effect of drugs based on ecdysteroids in poisoning of rats with tetrachloromethane and chlorophos. Biochem. J. 1994, 66, 67–77. [Google Scholar]
- Trenin, D.S.; Volodin, V.V.; Beĭkin, I.B.; Shlykova, A.B. The ecdysteroid fraction of the above-ground portion of Serratula coronata L. in the spontaneous E-rosette formation reaction and the agar migration test in vitro. Exp. Clin. Pharm. 1996, 59, 55–57. [Google Scholar]
- Amosova, E.N.; Zueva, E.P.; Razina, T.G.; Krylova, S.G.; Goldberg, E.D. The search for new antiulcerative drugs from plants of Siberia and the Far East. Exp. Clin. Pharmacol. 1998, 61, 35–36. [Google Scholar]
- Selyaskin, K.E.; Sidorova, Y.S.; Zorin, S.N.; Vasilevskaya, L.S.; Volodina, S.O.; Volodin, V.V.; Mazo, V.K. Effect of Serratula coronata extract on apoptosis activity in rats. Pharm. Chem. J. 2016, 50, 315–319. [Google Scholar] [CrossRef]
- Quilantang, N.G.; Ryu, S.H.; Park, S.H.; Byun, J.S.; Chun, J.S.; Lee, J.S.; Rodriguez, J.P.; Yun, Y.-S.; Jacinto, S.D.; Lee, S. Inhibitory activity of methanol extracts from different colored flowers on aldose reductase and HPLC-UV analysis of quercetin. Hortic. Environ. Biotechnol. 2018, 59, 899–907. [Google Scholar] [CrossRef]
- Fedorov, V.N.; Punegova, N.V.; Zainullin, V.G.; Punegov, V.V.; Sychev, R.L. Extraction of Ecdysterone-80 from Serratula coronata L. and evaluation of its pharmacological actions. II. Cardioprotective properties. Effects on hormone- transmitter balance in chronic cardiac failure. Pharm. Chem. J. 2009, 43, 36–40. [Google Scholar] [CrossRef]
- Shaposhnikov, M.V.; Shilova, L.A.; Plyusnina, E.N.; Volodina, S.O.; Volodin, V.V.; Moskalev, A. Influence of preparations containing phytoecdysteroids and plant steroid glycosides on the life span and stress resistance of Drosophila melanogaster. Russ. J. Genet. Appl. Res. 2016, 6, 215–224. [Google Scholar] [CrossRef]
- Markova, K.V.; Toropova, A.A.; Razuvaeva, Y.G.; Olennikov, D.N. Studying of the anti-ischemic action of Rhaponticum uniflorum and Serratula centauroides dry extracts on a model of bilateral occlusion of the carotid arteries. Acta Biomed. Sci. 2022, 7, 28–36. [Google Scholar] [CrossRef]
- Markova, K.V.; Razuvaeva, Y.G.; Toropova, A.A.; Olennikov, D.N. Morphological assessment of neuroprotective effects of Rhaponticum uniflorum and Serratula centauroides dry extracts in hypoxia/reoxygenation. J. Biomed. 2022, 18, 56–62. [Google Scholar] [CrossRef]
- Razuvaeva, Y.G.; Markova, K.V.; Toropova, A.A.; Olennikov, D.N. Influence of Serratula centauroides dry extract on the white rats in positive supported tests. Rev. Clin. Pharmacol. Med. Ther. 2021, 19, 237–242. [Google Scholar] [CrossRef]
- Savchenko, R.G.; Kostyleva, S.A.; Odinokov, V.N.; Akhmetkireeva, T.T.; Benkovskaya, G.V. Stress- and geroprotective properties of 20-hydroxyecdysone and its derivatives. Adv. Gerontol. 2015, 5, 247–251. [Google Scholar] [CrossRef]
- Shirshova, T.I.; Politova, N.K.; Burtseva, S.A.; Beshlei, I.V.; Volodin, V.V. Antimicrobial activity of natural ecdysteroids from Serratula coronata L. and their acyl derivatives. Pharm. Chem. J. 2006, 40, 268–271. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).