Metabolites of Serratula L. and Klasea Cass. (Asteraceae): Diversity, Separation Methods, and Bioactivity
Abstract
1. Introduction
2. Review Strategy
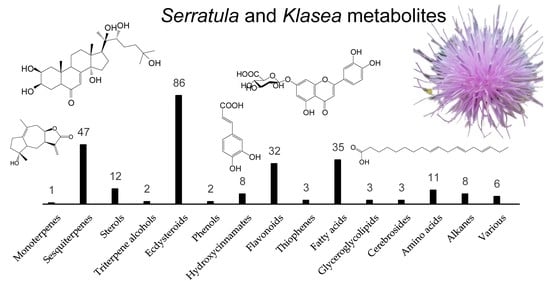
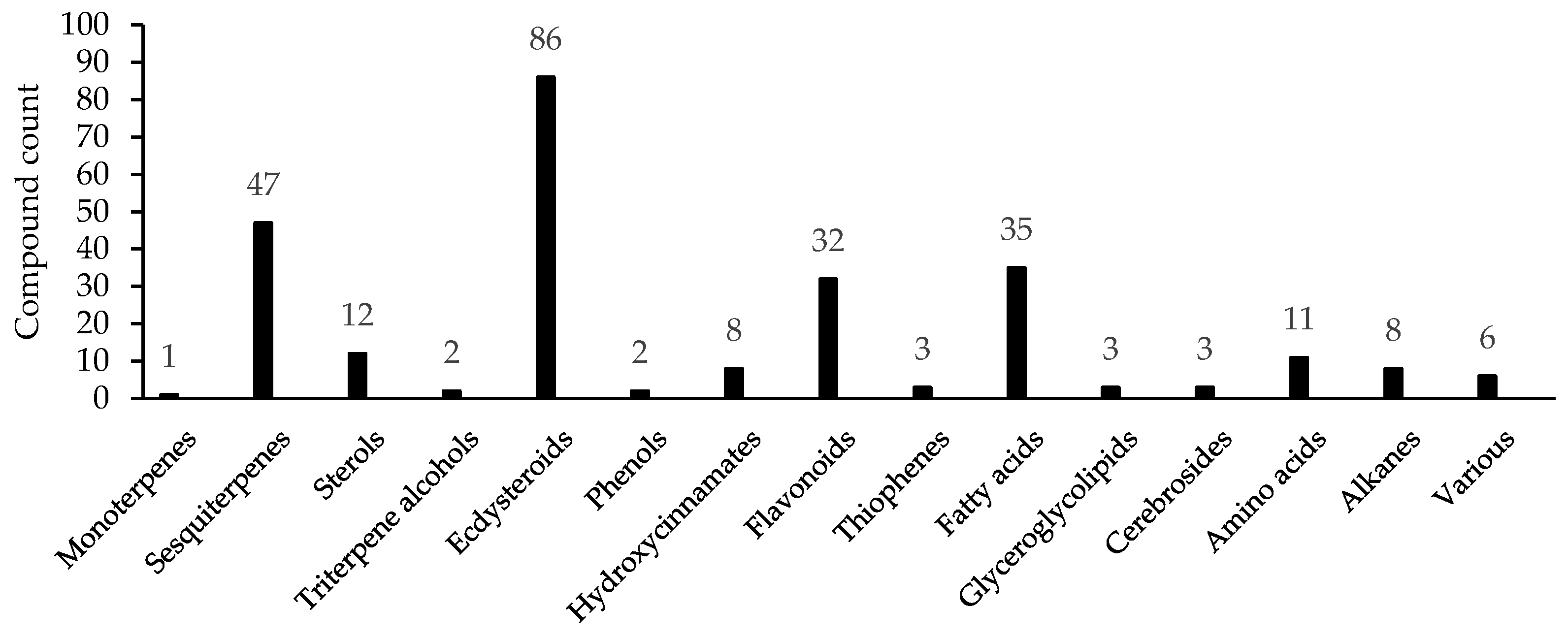
3. Chemodiversity of Serratula L. and Klasea Cass. Genera
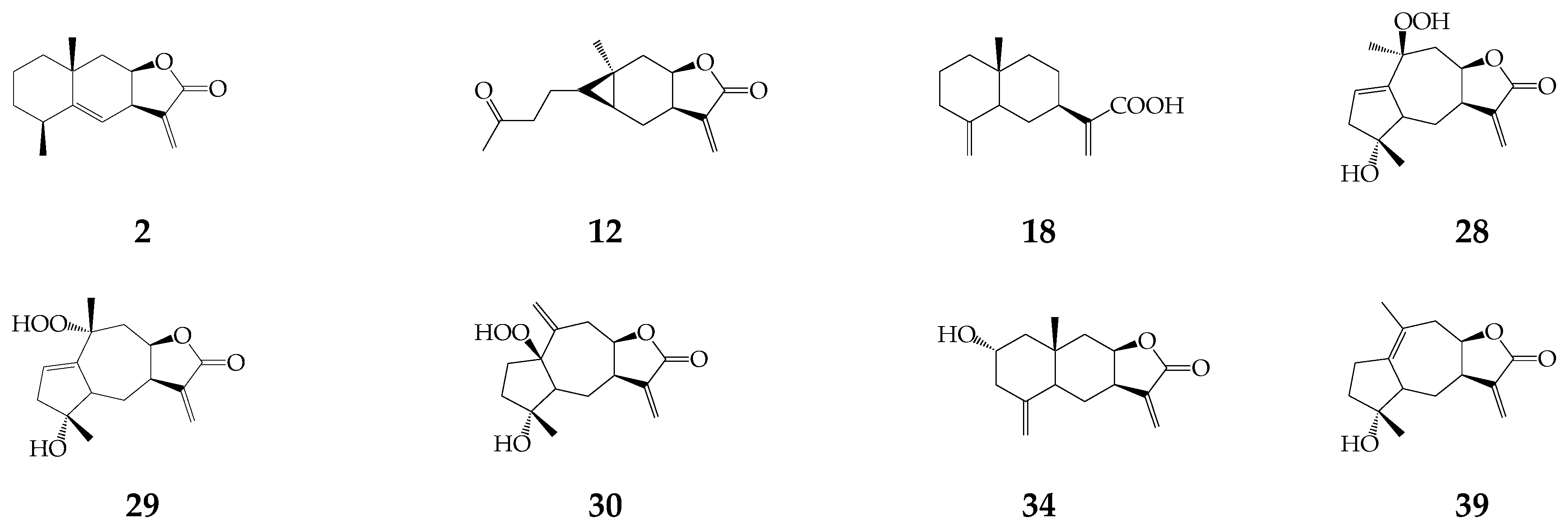
3.1. Mono-, Sesquiterpenes, Sterols and Triterpene Alcohols
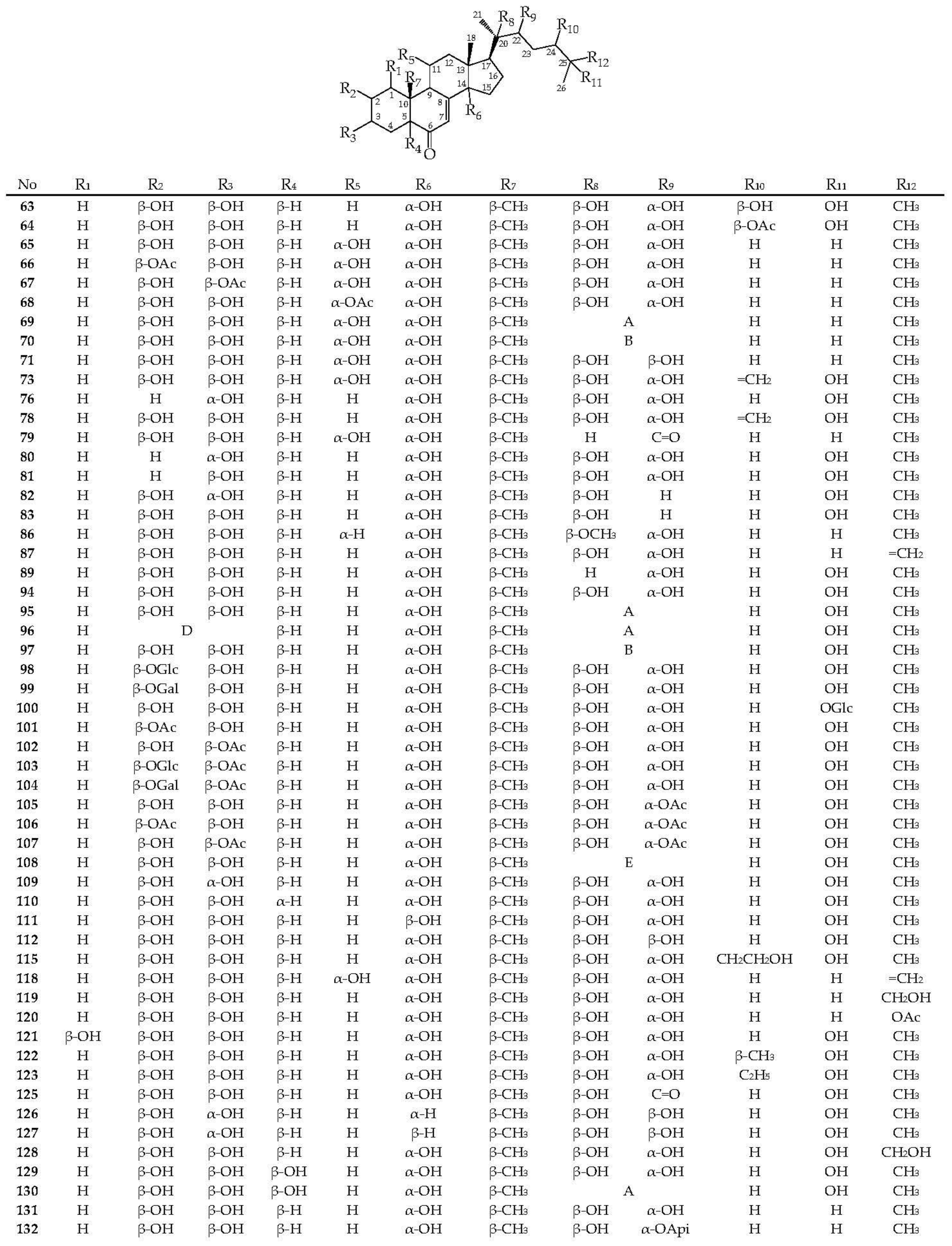
3.2. Ecdysteroids
3.3. Distribution of Ecdysteroids in the Genera Serratula and Klasea
3.4. Phenols and Hydroxycinnamates
3.5. Flavonoids
3.6. Other Groups
4. Extraction and Separation of Ecdysteroids and Flavonoids of Serratula и Klasea
4.1. Ecdysteroids
4.2. Flavonoids
5. Bioactivity of Serratula and Klasea
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plant of the World Online. Serratula L. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:11002-1 (accessed on 30 October 2022).
- Plant of the World Online. Klasea Cass. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:9583-1 (accessed on 30 October 2022).
- Hellwig, F.H. Centaureinae (Asteraceae) in the Mediterranean-history of ecogeographical radiation. Plant Syst. Evol. 2004, 246, 137–162. [Google Scholar] [CrossRef]
- Martins, L.; Hellwig, F.H. Systematic position of the genera Serratula and Klasea within Centaureinae (Cardueae, Asteraceae) inferred from ETS and ITS sequence data and new combinations in Klasea. Taxon 2005, 54, 632–638. [Google Scholar] [CrossRef]
- Shi, Z.; Chen, Y.; Chen, Y.; Lin, Y.; Liu, S.; Ge, X.; Gao, T.; Zhu, S.; Liu, Y.; Yang, Q.; et al. Asteraceae (Compositae). In Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2011; Volume 20, pp. 177–180. [Google Scholar]
- Shreter, A.I. Medical Flora of Soviet Far East; Nauka: Moscow, Russia, 1975; pp. 315–317. [Google Scholar]
- Batorova, S.M.; Yakovlev, G.P.; Aseeva, T.A. Reference Book of Traditional Tibetan Medicine Herbs; Nauka: Novosibirsk, Russia, 2013; pp. 135–136. [Google Scholar]
- Aseeva, T.A. Tibetan Medicine of Buryats; SO RAN: Novosibirsk, Russia, 2008; pp. 308–309. [Google Scholar]
- Khaidav, T.S. Medical Plants of Mongolian Medicine; Gosizdatelstvo: Ulan-Bator, Mongolia, 1985; pp. 282–285. [Google Scholar]
- Belenovskaya, L.M.; Korkhov, V.V.; Mats, M.N.; Medvedeva, L.I. Plant Resources of USSR; Nauka: Moscow, Russia, 1993; Volume 7, pp. 181–183. [Google Scholar]
- Guinot, P.; Gargadennec, A.; La Fisca, P.; Fruchier, A.; Andary, C.; Mondolot, L. Serratula tinctoria, a source of natural dye: Flavonoid pattern and histolocalization. Ind. Crop. Prod. 2009, 29, 320–325. [Google Scholar] [CrossRef]
- Punegov, V.V.; Sychov, R.L.; Zainullin, V.G.; Fedorov, V.N.; Punegova, N.V. Extraction of ecdysteron-80 from Serratula coronata L. and assessment of its pharmacological action. Part I. Adaptogenic, gastroprotective, thermoprotective, and antihypoxic activity. Pharm. Chem. J. 2008, 42, 446–451. [Google Scholar] [CrossRef]
- Báthori, M.; Tóth, N.; Hunyadi, A.; Márki, A.; Zádor, E. Phytoecdysteroids and anabolic-androgenic steroids – Structure and effects on humans. Curr. Med. Chem. 2008, 15, 75–91. [Google Scholar] [CrossRef]
- Bohlmann, F.; Rode, K.-M.; Waldau, E. Polyacetylenverbindungen, CXXIX. Über die ersten pflanzlichen Polyinglykoside. Chem. Ber. 1967, 100, 1915–1926. [Google Scholar] [CrossRef]
- Yatsyuk, Y.K.; Lyashenko, S.S.; Batyuk, V.S. The content of arbutin in some species of the genus Serratula. Chem. Nat. Compd. 1968, 4, 46–47. [Google Scholar] [CrossRef]
- Yatsyuk, Y.K.; Lyashenko, S.S. Flavonoids of Serratula inermis. Chem. Nat. Compd. 1969, 5, 46–47. [Google Scholar] [CrossRef][Green Version]
- Yatsyuk, Y.K.; Segel, G.M. The isolation of ecdysterone. Chem. Nat. Compd. 1970, 6, 284. [Google Scholar] [CrossRef]
- Tsybiktarova, L.P.; Taraskin, V.V.; Nikolaeva, I.G.; Radnaeva, L.D.; Gereltu, B. Constituent composition of essential oil from Serratula centauroides. Chem. Nat. Compd. 2016, 52, 1123–1124. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Faramarzi, S. Sesquiterpene lactones from Serratula latifolia. Phytochemistry 1988, 27, 479–481. [Google Scholar] [CrossRef]
- Bohlmann, F.; Czerson, H. Polyacetylenverbindungen, 240. Über die Inhaltsstoffe von Serratula wolfii Andrae. Chem. Berichte. 1976, 109, 2291–2295. [Google Scholar] [CrossRef]
- Dai, J.-Q.; Hou, Z.-F.; Zhu, Q.-X.; Yang, L.; Li, Y. Sesquiterpenes and Flavonoids from Serratula strangulata. J. Chin. Chem. Soc. 2001, 48, 249–252. [Google Scholar] [CrossRef]
- Dai, J.Q.; Zhu, Q.X.; Zhao, C.Y.; Yang, L.; Li, Y. Glyceroglycolipids from Serratula strangulata. Phytochemistry 2001, 58, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Corio-Costet, M.; Chapuis, L.; Mouillet, J.; Delbecque, J. Sterol and ecdysteroid profiles of Serratula tinctoria (L.): Plant and cell cultures producing steroids. Insect Biochem. Mol. Biol. 1993, 23, 175–180. [Google Scholar] [CrossRef]
- Tsybiktarova, L.P.; Taraskin, V.; Nikolaeva, I.G.; Radnaeva, L.D.; Garmaeva, L.L. Lipids from Serratula centauroides. Chem. Nat. Compd. 2016, 52, 294–295. [Google Scholar] [CrossRef]
- Tang, H.-J.; Fan, C.-L.; Wang, G.-Y.; Wei, W.; Wang, Y.; Ye, W.-C. Chemical constituents from roots tubers of Serratula chinensis. Chin. Trad. Herb. Drugs 2014, 45, 906–912. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Yang, W.-Q.; Fan, C.-L.; Zhao, H.-N.; Huang, X.-J.; Wang, Y.; Ye, W.-C. New ecdysteroid and ecdysteroid glycosides from the roots of Serratula chinensis. J. Asian Nat. Prod. Res. 2016, 19, 208–214. [Google Scholar] [CrossRef]
- Dinan, L.; Balducci, C.; Guibout, L.; Lafont, R. Small-scale analysis of phytoecdysteroids in seeds by HPLC-DAD-MS for the identification and quantification of specific analogues, dereplication and chemotaxonomy. Phytochem. Anal. 2020, 31, 643–661. [Google Scholar] [CrossRef]
- Volodin, V.V.; I Alexeeva, L.; A Kolegova, N.; Sarker, S.D.; Šik, V.; Lafont, R.; Dinan, L. Further ecdysteroids from Serratula coronata L. (Asteraceae). Biochem. Syst. Ecol. 1998, 26, 459–461. [Google Scholar] [CrossRef]
- Miladera, K.; Saatov, Z.; Kholodova, Y.D.; Gorovits, M.B.; Shashkov, A.S.; Abubakirov, N.K. Phytoecdysteroids of plants of the genus Serratula. Ajugasterone C 20,22-monoacetonide from Serratura wolffii. Chem. Nat. Compd. 1992, 28, 59–63. [Google Scholar] [CrossRef]
- Hunyadi, A.; Gergely, A.; Simon, A.; Tóth, G.; Veress, G.; Báthori, M. Preparative-scale chromatography of ecdysteroids of Serratula wolffii Andrae. J. Chromatogr. Sci. 2007, 45, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Aliouche, L.; Larguet, H.; Amrani, A.; Leon, F.; Brouard, I.; Benayache, S.; Zama, D.; Meraihi, Z.; Benayache, F. Isolation, antioxidant and antimicrobial activities of ecdysteroids from Serratula cichoracea. Curr. Bioact. Compd. 2018, 14, 60–66. [Google Scholar] [CrossRef]
- Galyautdinov, I.V.; Sadretdinova, Z.R.; Muslimov, Z.S.; Gareev, V.F.; Khalilov, L.M.; Odinokov, V.N. New minor phytoecdysteroids from the juice of Serratula coronata L. (Asteraceae). J. Med. Plants Stud. 2016, 4, 30–34. [Google Scholar]
- Odinokov, V.; Kumpun, S.; Galyautdinov, I.; Evrard-Todeschi, N.; Veskina, N.A.; Khalilov, L.M.; Girault, J.-P.; Dinan, L.; Maria, A.; Lafont, R. Low-polarity phytoecdysteroids from the juice of Serratula coronata L. (Asteraceae). Collect. Czechoslov. Chem. Commun. 2005, 70, 2038–2052. [Google Scholar] [CrossRef]
- Ling, T.; Zhang, Z.; Xia, T.; Ling, W.; Wan, X. Phytoecdysteroids and other constituents from the roots of Klaseopsis chinensis. Biochem. Syst. Ecol. 2009, 37, 49–51. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. Phytoecdysteroids of Serratula centauroides herb from Cisbaikalia. Russ. J. Bioorganic Chem. 2019, 45, 913–919. [Google Scholar] [CrossRef]
- Takács, M.; Simon, A.; Liktor-Busa, E.; Báthori, M.; Zsila, F.; Bikádi, Z.; Horváth, P.; Veress, G.; Gergely, A.; Tóth, G. Structure and stereochemistry of novel ecdysteroids from the roots of Serratula wolffii. Magn. Res. Chem. 2010, 48, 386–391. [Google Scholar] [CrossRef]
- Odinokov, V.; Galyautdinov, I.; Fatykhov, A.; Khalilov, L.M. A new phytoecdysteroid. Russ. Chem. Bull. 2000, 49, 1923–1924. [Google Scholar] [CrossRef]
- Vorob’Eva, A.N.; Rybin, V.G.; Zarembo, E.V.; Boltenkov, E.V. Phytoecdysteroids from Serratula centauroides. Chem. Nat. Compd. 2005, 41, 105–106. [Google Scholar] [CrossRef]
- Vorob’Eva, A.N.; Rybin, V.G.; Zarembo, E.V.; Boltenkov, E.V.; Verbitskii, G.A. Phytoecdysteroids from Serratula komarovii. Chem. Nat. Compd. 2004, 40, 492–495. [Google Scholar] [CrossRef]
- Ványolós, A.; Béni, Z.; Dékány, M.; Simon, A.; Báthori, M. Novel Ecdysteroids from Serratula wolffii. Sci. World J. 2012, 2012, 651275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, S.; Dai, J.; Chen, X.; Hu, Z. Identification and determination of ecdysones and flavonoids in Serratula strangulata by micellar electrokinetic capillary chromatography. Planta Med. 2002, 68, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-Q.; Cai, Y.-J.; Shi, Y.-P.; Zhang, Y.-H.; Liu, Z.-L.; Yang, L.; Li, Y. Antioxidant activity of ecdysteroids from Serratula strangulata. Chin. J. Chem. 2010, 20, 497–501. [Google Scholar] [CrossRef]
- Liktor-Busa, E.; Simon, A.; Tóth, G.; Fekete, G.; Kele, Z.; Báthori, M. Ecdysteroids from Serratula wolffii roots. J. Nat. Prod. 2007, 70, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Abubakirov, N.K. Ecdysteroids of flowering plants (Angiospermae). Chem. Nat. Compd. 1981, 17, 489–503. [Google Scholar] [CrossRef]
- Bathori, M.; Mathe, I.; Girault, J.-P.; Kalasz, A.H.; Lafont, R. Isolation and structural elucidation of two plant ecdysteroids, gerardiasterone and 22-epi-20-hydroxyecdysone. J. Nat. Prod. 1998, 61, 415–417. [Google Scholar] [CrossRef]
- Hunyadi, A.; Tóth, G.; Simon, A.; Mák, M.; Kele, Z.; Máthé, A.I.; Báthori, M. Two new ecdysteroids from Serratula wolffii. J. Nat. Prod. 2004, 67, 1070–1072. [Google Scholar] [CrossRef]
- Kasterova, E.; Zibareva, L.; Revushkin, A. Secondary metabolites of some Siberian species of plants tribe Cynareae (Asteraceae). S. Afr. J. Bot. 2019, 125, 24–29. [Google Scholar] [CrossRef]
- Tuleuov, B.I. 20-Hydroxyecdysone content of several representatives of the families Asteraceae and Caryophyllaceae. Chem. Nat. Compd. 2009, 45, 762. [Google Scholar] [CrossRef]
- Rudel, D.; Bathori, M.; Gharbi, J.; Girault, J.-P.; Racz, I.; Melis, K.; Szendrei, K.; Lafont, R. New ecdysteroids from Serratula tinctoria. Planta Med. 1992, 58, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, I.G.; Tsybiktarova, L.P.; Garmaeva, L.L.; Nikolaeva, G.G.; Olennikov, D.N.; Matkhanov, I.E. Determination of ecdysteroids in Fornicium unflorum (L.) and Serratula centauroides (L.) raw materials by chromatography–UV spectrophotometry. J. Anal. Chem. 2017, 72, 854–861. [Google Scholar] [CrossRef]
- Kholodova, Y.D.; Baltaev, U.; Volovenko, V.O.; Gorovits, M.B.; Abubakirov, N.K. Phytoecdysones of Serratula xeranthemoides. Chem. Nat. Compd. 1979, 15, 144–146. [Google Scholar] [CrossRef]
- Odinokov, V.; Galyautdinov, I.; Mel’Nikova, D.A.; Muslimov, Z.S.; Khalilov, L.M.; Denisenko, O.N.; Mogilenko, T.G.; Zaripova, E.R.; Zakirova, L.M. Isolation and identification of phytoecdysteroids from juice of Serratula quinquefolia. Chem. Nat. Compd. 2013, 49, 392–394. [Google Scholar] [CrossRef]
- Zatsny, I.L.; Gorovits, M.B.; Abubakirov, N.K. Ecdysterone from Serratula sogdiana. Chem. Nat. Compd. 1971, 7, 822. [Google Scholar] [CrossRef][Green Version]
- Odinokov, V.; Galyautdinov, I.; Nedopekin, D.; Khalilov, L.; Shashkov, A.; Kachala, V.; Dinan, L.; Lafont, R. Phytoecdysteroids from the juice of Serratula coronata L. (Asteraceae). Insect Biochem. Mol. Biol. 2002, 32, 161–165. [Google Scholar] [CrossRef]
- Simon, A.; Liktor-Busa, E.; Tóth, G.; Kele, Z.; Groska, J.; Báthori, M. Additional minor phytoecdysteroids of Serratula wolffii. Helv. Chim. Acta 2008, 91, 1640–1645. [Google Scholar] [CrossRef]
- Simon, A.; Tóth, G.; Liktor-Busa, E.; Kele, Z.; Takács, M.; Gergely, A.; Báthori, M. Three new steroids from the roots of Serratula wolffii. Steroids 2007, 72, 751–755. [Google Scholar] [CrossRef]
- Liktor-Busa, E.; Simon, A.; Tóth, G.; Báthori, M. The first two ecdysteroids containing a furan ring from Serratula wolffii. Tetrahedr. Lett. 2008, 49, 1738–1740. [Google Scholar] [CrossRef]
- Novosel’Skaya, I.L.; Gorovits, M.B.; Abubakirov, N.K. Phytoecdysones of Serratula IV. Sogdysterone. Chem. Nat. Compd. 1975, 11, 445–446. [Google Scholar] [CrossRef]
- Shantanova, L.N.; Olennikov, D.N.; Matkhanov, I.E.; Gulyaev, S.M.; Toropova, A.A.; Nikolaeva, I.G.; Nikolaev, S.M. Rhaponticum uniflorum and Serratula centauroides extracts attenuate emotional injury in acute and chronic emotional stress. Pharmaceuticals 2021, 14, 1186. [Google Scholar] [CrossRef] [PubMed]
- Zatsny, I.L.; Gorovits, M.B.; Abubakirov, N.K. Phytoecdysones of serratula II. Viticosterone E from Serratula sogdiana and its partial synthesis. Chem. Nat. Compd. 1973, 9, 170–173. [Google Scholar] [CrossRef]
- Myagchilov, A.V.; Mineev, S.A.; Sokolova, L.I.; Gerdasova, E.D.; Gorovoi, P.G. Arbutin content in the Far-Eastern species Serratula komarovii Iljin. Pharm. Chem. J. 2020, 54, 377–379. [Google Scholar] [CrossRef]
- Nowak, G.; Nawrot, J.; Latowski, K. Arbutin in Serratula quinquefolia M.B. (Asteraceae). Acta Soc. Bot. Pol. 2009, 78, 137–140. [Google Scholar] [CrossRef]
- Kusano, K.; Iwashina, T.; Kitajima, J.; Mishio, T. Flavonoid diversity of Saussurea and Serratula species in Tien Shan mountains. Nat. Prod. Commun. 2007, 2, 1121–1128. [Google Scholar] [CrossRef]
- Zatsny, I.L.; Gorovits, M.B.; Abubakirov, N.K. Arbutin from Serratula sogdiana. Chem. Nat. Compd. 1973, 9, 415–416. [Google Scholar] [CrossRef]
- Lech, K.; Witkoś, K.; Jarosz, M. HPLC-UV-ESI MS/MS identification of the color constituents of sawwort (Serratula tinctoria L.). Anal. Bioanal. Chem. 2014, 406, 3703–3708. [Google Scholar] [CrossRef]
- Báthori, M.; Zupkó, I.; Hunyadi, A.; Gácsné-Baitz, E.; Dinya, Z.; Forgó, P. Monitoring the antioxidant activity of extracts originated from various Serratula species and isolation of flavonoids from Serratula coronata. Fitoterapia 2004, 75, 162–167. [Google Scholar] [CrossRef]
- Myagchilov, A.V.; Sokolova, L.I.; Gorovoy, P.G. Flavonoids of the East Asian species Serratula manshurica Kitag. Khim. Rastit. Syr’ya 2021, 167–173. [Google Scholar] [CrossRef]
- Aliouche, L.; Zater, H.; Zama, D.; Bentamene, A.; Seghiri, R.; Mekkiou, R.; Benayache, S.; Benayache, F. Flavonoids of Serratula cichoracea and their antioxidant activity. Chem. Nat. Compd. 2007, 43, 618–619. [Google Scholar] [CrossRef]
- Myagchilov, A.V.; Gorovoi, P.G.; Sokolova, L.I. Flavonoids from Serratula komarovii Iljin (the Asteraceae Family). Russ. J. Bioorg. Chem. 2021, 47, 1418–1423. [Google Scholar] [CrossRef]
- Nikolaev, S.M.; Nikolaeva, I.G.; Razuvaeva, Y.G.; Matkhanov, I.E.; Tsybiktarova, L.P.; Shantanova, L.N.; Nikolaeva, G.G. Phenolic compounds of Serratula centauroides and anxiolytic effect. Farmacia 2019, 67, 504–510. [Google Scholar] [CrossRef]
- Myagchilov, A.V.; Sokolova, L.I.; Gorovoy, P.G.; Kechaikin, A.A. Features of the composition of flavonoids in the crowned sawwort (Serratula coronata L.) from Siberia and the Far East of Russia. Khim. Rastit. Syr’ya 2020, 171–179. [Google Scholar] [CrossRef]
- Glyzin, V.I.; Ban’Kovskii, A.I.; Mel’Nikova, T.M. 3-O-Methylquercetin from Serratula inermis. Chem. Nat. Compd. 1972, 8, 383. [Google Scholar] [CrossRef]
- Dai, J.Q.; Shi, Y.P.; Yang, L.; Li, Y. Two new components from Serratula strangulata Iljin. Chin. Chem. Lett. 2002, 13, 143–146. [Google Scholar]
- Bohlmann, F.; Waldau, E. Polyacetylenverbindungen, CXXV. Über die Inhaltsstoffe von Serratula radiata Bieb. Eur. J. Inorg. Chem. 1967, 100, 1206–1208. [Google Scholar] [CrossRef]
- Tel, G.; Öztürk, M.; Duru, M.E.; Doǧan, B.; Harmandar, M. Fatty acid composition, antioxidant, anticholinesterase and tyrosinase inhibitory activities of four Serratula species from Anatolia. Rec. Nat. Prod. 2013, 7, 86–95. [Google Scholar]
- Ling, T.; Xia, T.; Wan, X.; Li, D.; Wei, X. Cerebrosides from the Roots of Serratula chinensis. Molecules 2006, 11, 677–683. [Google Scholar] [CrossRef]
- Tsybiktarova, L.P.; Nikolaeva, I.G. Amino acids from Serratula centauroides. Chem. Nat. Compd. 2017, 53, 203–204. [Google Scholar] [CrossRef]
- Napierała, M.; Nawrot, J.; Gornowicz-Porowska, J.; Florek, E.; Moroch, A.; Adamski, Z.; Kroma, A.; Miechowicz, I.; Nowak, G. Separation and HPLC characterization of active natural steroids in a standardized extract from the Serratula coronata Herb with antiseborrheic dermatitis activity. Int. J. Environ. Res. Public Health 2020, 17, 6453. [Google Scholar] [CrossRef]
- Báthori, M.; Máthé, I.; Guttman, A. Determination of 20-hydroxyecdysone content by thin-layer chromatography and micellar electrokinetic chromatography. Chromatographia 1998, 48, 145–148. [Google Scholar] [CrossRef]
- Báthori, M.; Gergely, A.; Kalász, H.; Nagy, G.; Dobos, Á.; Máthé, I. Liquid chromatographic monitoring of phytoecdysteroid production of Serratula wolffii. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 281–294. [Google Scholar] [CrossRef]
- Nawrot, J.; Budzianowski, J.; Nowak, G.; Micek, I.; Budzianowska, A.; Gornowicz-Porowska, J. Biologically active compounds in Stizolophus balsamita inflorescences: Isolation, phytochemical characterization and effects on the skin biophysical parameters. Int. J. Mol. Sci. 2021, 22, 4428. [Google Scholar] [CrossRef] [PubMed]
- Chabannyĭ, V.N.; Levitskiĭ, E.L.; I Gubskiĭ, I.; Kholodova, I.D.; E. Vistunova, I.; I Budmaska, M.I. Genoprotective effect of drugs based on ecdysteroids in poisoning of rats with tetrachloromethane and chlorophos. Biochem. J. 1994, 66, 67–77. [Google Scholar]
- Trenin, D.S.; Volodin, V.V.; Beĭkin, I.B.; Shlykova, A.B. The ecdysteroid fraction of the above-ground portion of Serratula coronata L. in the spontaneous E-rosette formation reaction and the agar migration test in vitro. Exp. Clin. Pharm. 1996, 59, 55–57. [Google Scholar]
- Amosova, E.N.; Zueva, E.P.; Razina, T.G.; Krylova, S.G.; Goldberg, E.D. The search for new antiulcerative drugs from plants of Siberia and the Far East. Exp. Clin. Pharmacol. 1998, 61, 35–36. [Google Scholar]
- Selyaskin, K.E.; Sidorova, Y.S.; Zorin, S.N.; Vasilevskaya, L.S.; Volodina, S.O.; Volodin, V.V.; Mazo, V.K. Effect of Serratula coronata extract on apoptosis activity in rats. Pharm. Chem. J. 2016, 50, 315–319. [Google Scholar] [CrossRef]
- Quilantang, N.G.; Ryu, S.H.; Park, S.H.; Byun, J.S.; Chun, J.S.; Lee, J.S.; Rodriguez, J.P.; Yun, Y.-S.; Jacinto, S.D.; Lee, S. Inhibitory activity of methanol extracts from different colored flowers on aldose reductase and HPLC-UV analysis of quercetin. Hortic. Environ. Biotechnol. 2018, 59, 899–907. [Google Scholar] [CrossRef]
- Fedorov, V.N.; Punegova, N.V.; Zainullin, V.G.; Punegov, V.V.; Sychev, R.L. Extraction of Ecdysterone-80 from Serratula coronata L. and evaluation of its pharmacological actions. II. Cardioprotective properties. Effects on hormone- transmitter balance in chronic cardiac failure. Pharm. Chem. J. 2009, 43, 36–40. [Google Scholar] [CrossRef]
- Shaposhnikov, M.V.; Shilova, L.A.; Plyusnina, E.N.; Volodina, S.O.; Volodin, V.V.; Moskalev, A. Influence of preparations containing phytoecdysteroids and plant steroid glycosides on the life span and stress resistance of Drosophila melanogaster. Russ. J. Genet. Appl. Res. 2016, 6, 215–224. [Google Scholar] [CrossRef]
- Markova, K.V.; Toropova, A.A.; Razuvaeva, Y.G.; Olennikov, D.N. Studying of the anti-ischemic action of Rhaponticum uniflorum and Serratula centauroides dry extracts on a model of bilateral occlusion of the carotid arteries. Acta Biomed. Sci. 2022, 7, 28–36. [Google Scholar] [CrossRef]
- Markova, K.V.; Razuvaeva, Y.G.; Toropova, A.A.; Olennikov, D.N. Morphological assessment of neuroprotective effects of Rhaponticum uniflorum and Serratula centauroides dry extracts in hypoxia/reoxygenation. J. Biomed. 2022, 18, 56–62. [Google Scholar] [CrossRef]
- Razuvaeva, Y.G.; Markova, K.V.; Toropova, A.A.; Olennikov, D.N. Influence of Serratula centauroides dry extract on the white rats in positive supported tests. Rev. Clin. Pharmacol. Med. Ther. 2021, 19, 237–242. [Google Scholar] [CrossRef]
- Savchenko, R.G.; Kostyleva, S.A.; Odinokov, V.N.; Akhmetkireeva, T.T.; Benkovskaya, G.V. Stress- and geroprotective properties of 20-hydroxyecdysone and its derivatives. Adv. Gerontol. 2015, 5, 247–251. [Google Scholar] [CrossRef]
- Shirshova, T.I.; Politova, N.K.; Burtseva, S.A.; Beshlei, I.V.; Volodin, V.V. Antimicrobial activity of natural ecdysteroids from Serratula coronata L. and their acyl derivatives. Pharm. Chem. J. 2006, 40, 268–271. [Google Scholar] [CrossRef]

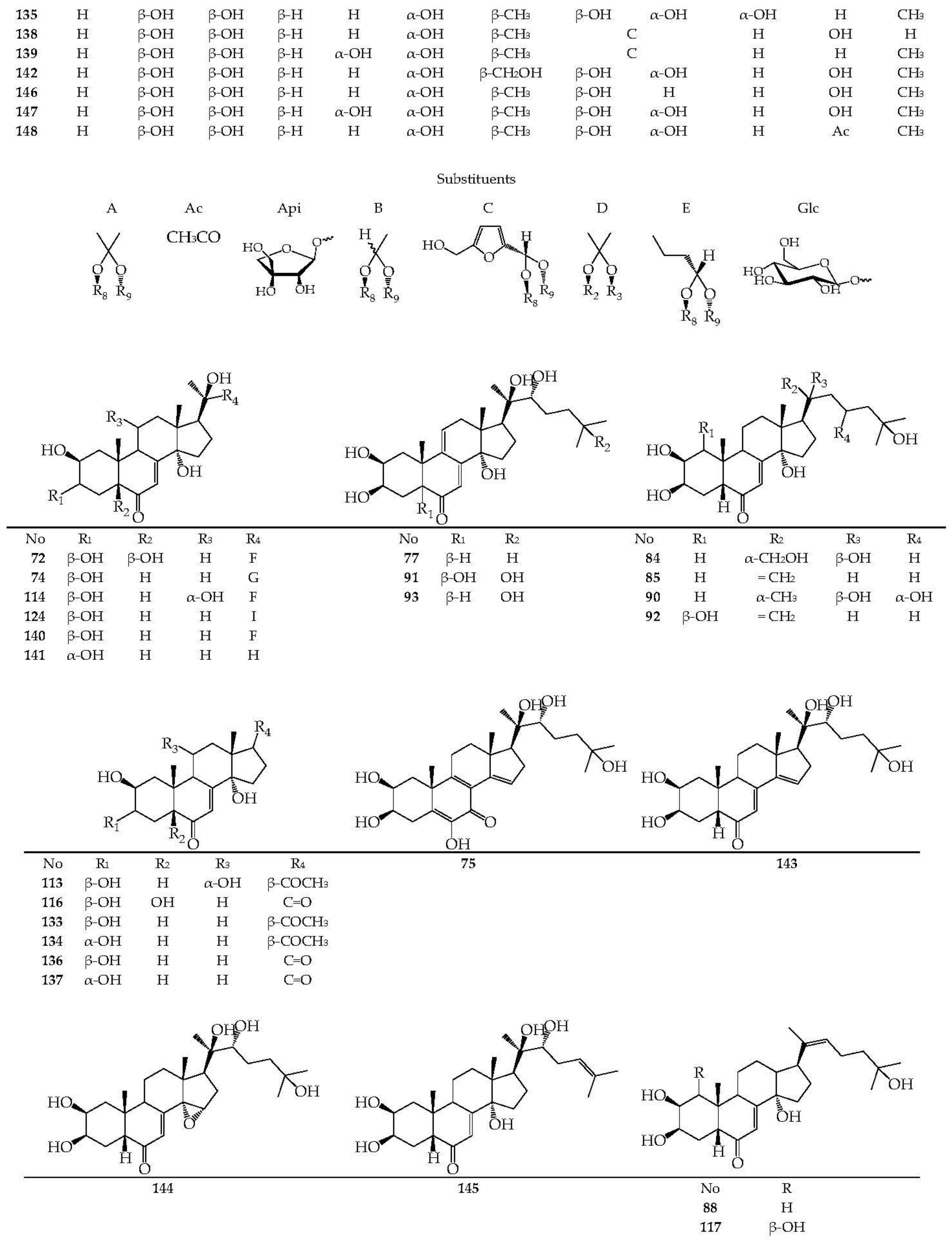
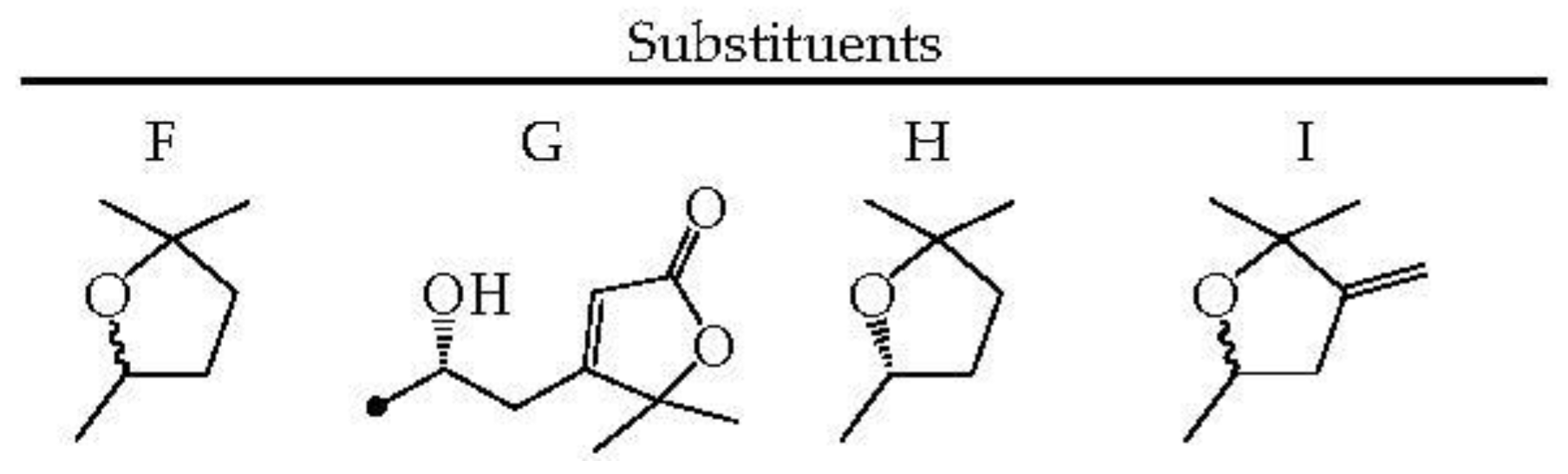
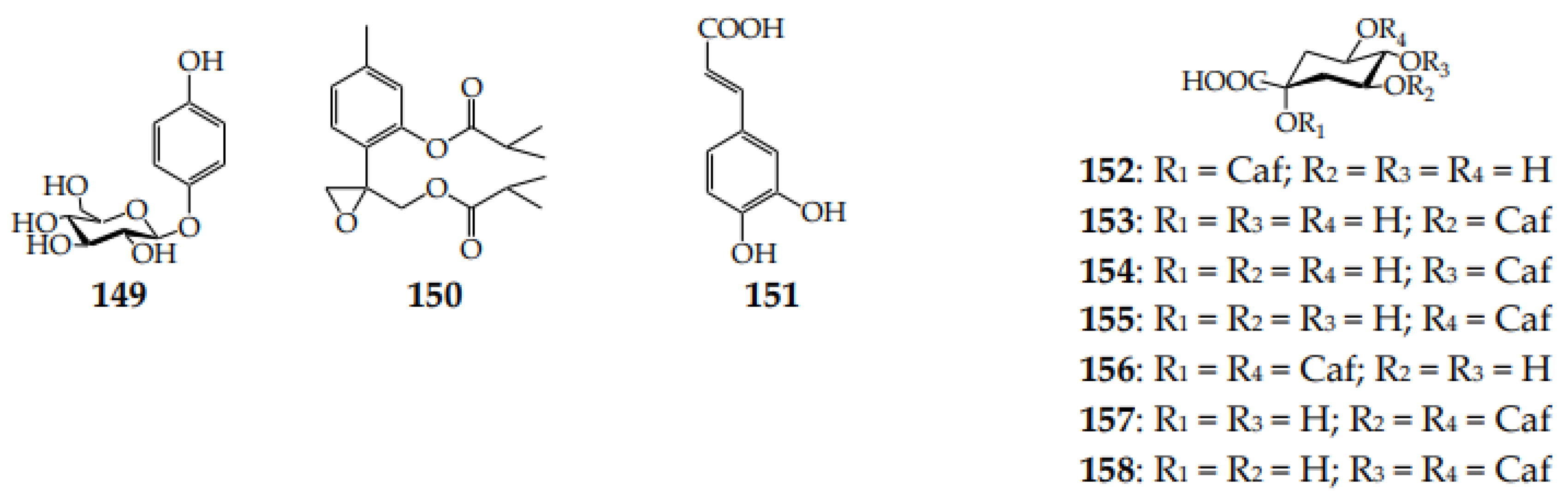
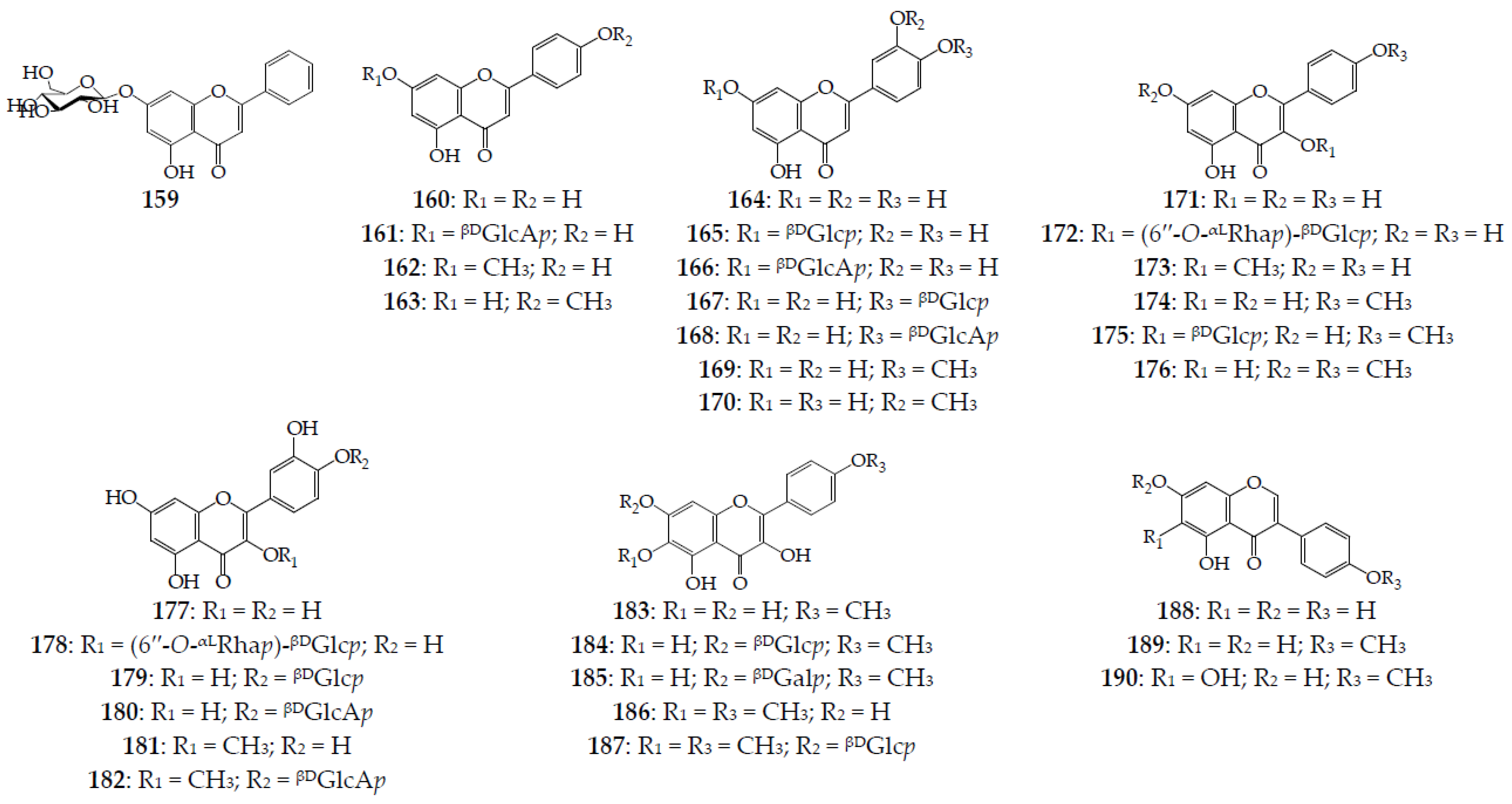
| Species (Synonyms) | Compound Count |
|---|---|
| Serratula species | |
| S. coronata L. | 30 |
| S. coronata subsp. coronata = syn. S. manshurica Kitag., S. martini Vaniot, S. wolffii Andrae | 57 |
| S. kirghisorum Iljin | 2 |
| S. tinctoria L. | 31 |
| S. tinctoria subsp. tinctoria = syn. S. inermis Gilib., S. pinnata Kit., S. pumila Thore ex DC | 4 |
| Klasea species | |
| K. algida (Iljin) Hidalgo = syn. S. algida Iljin, S. dshungarica Iljin | 3 |
| K. cardunculus (Pall.) Holub = syn. S. cardunculus (Pall.) Schischk., S. nitida Fisch. ex Spreng | 1 |
| K. centauroides (L.) Cass. ex Kitag. = syn. S. centauroides L. | 123 |
| K. centauroides subsp. centauroides = syn. S. komarovii Iljin, S. pectinata Turcz. ex Herder | 32 |
| K. centauroides subsp. strangulata (Iljin) L.Martins = syn. S. strangulata Iljin | 22 |
| K. chinensis (S.Moore) Kitag. = syn. S. chinensis S.Moore | 26 |
| K. erucifolia (L.) Greuter & Wagenitz = syn. S. erucifolia (L.) Boriss., S. xeranthemoides M.Bieb | 16 |
| K. flavescens subsp. cichoracea (L.) Greuter & Wagenitz = syn. S. cichoracea (L.) DC | 8 |
| K. hakkiarica (P.H.Davis) Greuter & Wagenitz = syn. S. hakkiarica P.H.Davis | 15 |
| K. lasiocephala (Bornm.) Greuter & Wagenitz = syn. S. lasiocephala Bornm | 15 |
| K. latifolia (Boiss.) L.Martins = syn. S. latifolia Boiss | 9 |
| K. lycopifolia (Vill.) Á.Löve & D.Löve = syn. S. lycopifolia (Vill.) Wettst., S. nitida Besser | 3 |
| K. lyratifolia (Schrenk) L.Martins = syn. S. lyratifolia Schrenk ex Fisch. & C.A.Mey., S. rugulosa Iljin | 5 |
| K. pinnatifida (Cav.) Talavera = syn. S. pinnatifida Poir | 2 |
| K. procumbens (Regel) Holub = syn. S. procumbens Regel | 2 |
| K. quinquefolia (Willd.) Greuter & Wagenitz = S. quinquefolia Willd | 4 |
| K. radiata (Waldst. & Kit.) Á.Löve & D.Löve = syn. S. radiata (Waldst. & Kit.) DC | 8 |
| K. radiata subsp. biebersteiniana (Grossh.) Greuter = syn. S. radiata subsp. biebersteiniana Grossh | 14 |
| K. radiata subsp. gmelinii (Tausch) L.Martins = S. gmelinii Tausch | 5 |
| K. radiata subsp. radiata = S. bracteifolia (Iljin) Stankov, S. heterophylla Vill, S. isophylla Claus | 3 |
| K. sogdiana (Bunge) L.Martins = syn. S. sogdiana Bunge | 4 |
| No | Compound a | Species (Organ) b | Ref. |
|---|---|---|---|
| Monoterpenes | |||
| 1 | Geranyl acetate | K. centauroides (h) | [18] |
| Sesquiterpenes | |||
| 2 | Alantolactone | K. latifolia (ae) | [19] |
| 3 | cis-α-Bergamotene | K. centauroides (h) | [18] |
| 4 | trans-α-Bergamotene | K. centauroides (h) | [18] |
| 5 | Bicyclogermacrene | K. centauroides (h) | [18] |
| 6 | (Z)-α-Bisabolene | K. centauroides (h) | [18] |
| 7 | (6S,7R)-Bisabolone | K. centauroides (h) | [18] |
| 8 | trans-Cadina-1,4-diene | K. centauroides (h) | [18] |
| 9 | α-Cadinene | K. centauroides (h) | [18] |
| 10 | δ-Cadinene | K. centauroides (h) | [18] |
| 11 | α-Calacorene | K. centauroides (h) | [18] |
| 12 | Carabrone | K. latifolia (ae) | [19] |
| 13 | Caryophyllene | S. coronata subsp. coronata (r) | [20] |
| K. centauroides (h, r) | [18] | ||
| 14 | Caryophyllene oxide | S. coronata subsp. coronata (r) | [20] |
| K. centauroides (h, r) | [18] | ||
| 15 | Centaurepensin | K. centauroides subsp. strangulata (rz,w) | [22] |
| 16 | Centaurepensin 17-O-(p-hydroxyphenylethanol) | K. centauroides subsp. strangulata (w) | [21] |
| 17 | α-Copaene | K. centauroides (h) | [18] |
| 18 | Costic acid | K. latifolia (ae) | [19] |
| 19 | β-Elemene | K. centauroides (h) | [18] |
| 20 | γ-Elemene | K. centauroides (h) | [18] |
| 21 | Eudesma-4(15),7-dien-1β-ol | K. centauroides (h, r) | [18] |
| 22 | γ-Eudesmol | K. centauroides (h) | [18] |
| 23 | (E,E)-α-Farnesene | K. centauroides (h) | [18] |
| 24 | (E)-β-Farnesene | K. centauroides (h, r) | [18] |
| 25 | Germacrene B | K. centauroides (h) | [18] |
| 26 | Germacrene D | S. coronata subsp. coronata (r) | [20] |
| 27 | Hexahydroxyfarnesyl | K. centauroides (h) | [18] |
| 28 | 4α-Hydroxy-1β-hydroperoxyguaia-10(14),11(13)-dien-12,8β-olide | K. latifolia (ae) | [19] |
| 29 | 4α-Hydroxy-10α-hydroperoxyguaia-1,11(13)-dien-12,8β-olide | K. latifolia (ae) | [19] |
| 30 | 4α-Hydroxy-10β-hydroperoxyguaia-1,11(13)-dien-12,8β-olide | K. latifolia (ae) | [19] |
| 31 | Humulene | K. centauroides (h, r) | [18] |
| 32 | Humulen-6,7-epoxide | K. centauroides (h, r) | [18] |
| 33 | Isospathulenol | K. centauroides (h) | [18] |
| 34 | Ivalin | K. latifolia (ae) | [19] |
| 35 | Junenol | K. centauroides (h, r) | [18] |
| 36 | Mint oxide | K. centauroides (h, r) | [18] |
| 37 | Mint sulfide | K. centauroides (r) | [18] |
| 38 | E-Nerolidol | K. centauroides (h) | [18] |
| 39 | Pseudoivalin | K. latifolia (ae) | [19] |
| 40 | Salviadienol | K. centauroides (h, r) | [18] |
| 41 | Salvial-4(14)-en-1-one | K. centauroides (h, r) | [18] |
| 42 | Selina-4,11-diene | K. centauroides (h) | [18] |
| 43 | 7-epi-α-Selinene | K. centauroides (h) | [18] |
| 44 | β-Selinene | K. centauroides (h, r) | [18] |
| 45 | E-Sesquilavandulol | K. centauroides (h) | [18] |
| 46 | Spathulenol | K. centauroides (h, r) | [18] |
| 47 | Valencene | K. centauroides (h) | [18] |
| 48 | epi-Zonarene | K. centauroides (h) | [18] |
| Sterols | |||
| 49 | Campesterol | S. tinctoria (r) | [23] |
| K. centauroides (h) | [24] | ||
| 50 | Cholesterol | S. tinctoria (r) | [23] |
| 51 | Desmosterol | S. tinctoria (r) | [23] |
| 52 | Lathosterol | S. tinctoria (r) | [23] |
| 53 | 24-Methylene-cholesterol | S. tinctoria (r) | [23] |
| 54 | β-Sitosterol | S. tinctoria (r) | [23] |
| 55 | β-Sitosterol acetate | K. centauroides (h, r) | [24] |
| 56 | γ-Sitosterol | K. centauroides (h, r) | [24] |
| 57 | Sitostanol | S. tinctoria (r) | [23] |
| 58 | Stigmasterol | S. tinctoria (r) | [23] |
| K. centauroides (h, r) | [24] | ||
| 59 | Stigmastanol | S. tinctoria (r) | [23] |
| 60 | Stigmast-4-en-3-one | K. centauroides (h) | [24] |
| Triterpene alcohols | |||
| 61 | α-Amyrin | K. centauroides (h) | [24] |
| 62 | β-Amyrin | K. centauroides (h) | [24] |
| Ecdysteroids | |||
| 63 | epi-Abutasterone | K. chinensis (r) | [25] |
| 64 | epi-Abutasterone 24-O-acetate | K. chinensis (r) | [26] |
| 65 | Ajugasterone C | S. coronata (l, s) | [27,28] |
| S. coronata subsp. coronata (h) | [29,30] | ||
| K. flavescens subsp. cichoracea (f) | [31] | ||
| 66 | Ajugasterone C 2-O-acetate | S. coronata (j) | [32] |
| 67 | Ajugasterone C 3-O-acetate | S. coronata (j) | [32] |
| 68 | Ajugasterone C 11-O-acetate | S. coronata (j) | [32] |
| 69 | Ajugasterone C 20,22-monoacetonide | S. coronata (j) | [33] |
| S. coronata subsp. coronata (h) | [29] | ||
| 70 | Ajugasterone C 20,22-ethylidene | S. coronata (j) | [33] |
| 71 | 22-epi-Ajugasterone C | K. flavescens subsp. cichoracea (f) | [31] |
| 72 | Ajugasterone D | S. coronata subsp. coronata (h) | [30] |
| K. chinensis (r) | [25] | ||
| 73 | Atrotosterone C | K. chinensis (r) | [34] |
| 74 | Carthamosterone | K. chinensis (r) | [25,34] |
| 75 | Calonisterone | S. coronata (j) | [32] |
| 76 | Coronatasterone | K. chinensis (r) | [25] |
| 77 | Dacryhainansterone | S. coronata (l) | [28] |
| S. coronata subsp. coronata (h) | [30] | ||
| 78 | 24(28)-Dehydromakisterone | K. centauroides (h) | [35] |
| 79 | 22-Dehydro-20-desoxymakisterone C | S. coronata subsp. coronata (r) | [36] |
| 80 | 2-Deoxy-3-epi-4β,20-dihydroxycedysone | S. coronata (h) | [37] |
| 81 | 2-Deoxy-20-hydroxyecdysone | K. centauroides (f, h, l, st) | [35,38] |
| K. centauroides subsp. centauroides (l,r,s,st) | [39] | ||
| 82 | 3-epi-2-Deoxy-20-hydroxyecdysone | S. coronata subsp. coronata (r) | [40] |
| 83 | 22-Deoxy-20-hydroxyecdysone | S. coronata subsp. coronata (h) | [30] |
| 84 | 22-Deoxy-20,21-dihydroxyecdysone | S. coronata subsp. coronata (h) | [30] |
| 85 | 22-Deoxy-20,21-didehydroecdysone | S. coronata subsp. coronata (r) | [36] |
| 86 | 25-Deoxy-11,20-dihydroxyecdysone | K. centauroides subsp. strangulata (l, r, rz, s) | [22,41,42] |
| 87 | 25,26-Didehydroponasterone A | K. chinensis (r) | [34] |
| 88 | 20,22-Didehydrotaxisterone | S. coronata subsp. coronata (r) | [43] |
| 89 | Ecdysone | S. coronata subsp. coronata (h) | [29,30] |
| K. lycopifolia (h) | [44] | ||
| K. radiata (h) | [44] | ||
| K. radiata subsp. gmelinii (h) | [44] | ||
| 90 | Gerardiasterone | S. tinctoria (h) | [45] |
| 91 | Herkesterone | S. coronata subsp. coronata (h) | [30,46] |
| 92 | 1-Hydroxy-22-desoxy-20,21-didehydroecdysone | S. coronata subsp. coronata (r) | [36] |
| 93 | 25-Hydroxydacryhainansterone | S. coronata subsp. coronata (h) | [30] |
| 94 | 20-Hydroxyecdysone | S. coronata (s) | [27] |
| S. coronata subsp. coronata (h) | [29,30] | ||
| S. kirghisorum (f, h) | [47,48] | ||
| S. tinctoria (r) | [49] | ||
| S. tinctoria subsp. tinctoria (f) | [17] | ||
| K. algida (h) | [44,47] | ||
| K. centauroides (f, h, l, r, st) | [35,38,50] | ||
| K. centauroides subsp. centauroides (l, r, s, st) | [39] | ||
| K. centauroides subsp. strangulata (l, r, rz, s) | [22,41,42] | ||
| K. chinensis (r) | [25,34] | ||
| K. cardunculus (f, h) | [48] | ||
| K. ericifolia (f, h) | [48,51] | ||
| K. flavescens subsp. cichoracea (f) | [31] | ||
| K. procumbens (h) | [44] | ||
| K. quinquefolia (f, j, l, st) | [48,52] | ||
| K. lycopifolia (h) | [44] | ||
| K. radiata (f, l, st) | [48] | ||
| K. radiata subsp. gmelinii (f, l, st) | [48] | ||
| K. sogdiana (f) | [53] | ||
| 95 | 20-Hydroxyecdysone 20,22-monoacetonide | S. coronata (j) | [33] |
| S. coronata subsp. coronata (h) | [29,30] | ||
| K. centauroides (h) | [35] | ||
| K. centauroides subsp. strangulata (rz, w) | [22,42] | ||
| K. chinensis (r) | [25] | ||
| 96 | 20-Hydroxyecdysone 2,3;20,22-diacetonide | S. coronata subsp. coronata (h) | [29] |
| 97 | 20-Hydroxyecdysone 20,22-ethylidene | S. coronata (j) | [33] |
| 98 | 20-Hydroxyecdysone 2-O-βDGlcp | K. chinensis (r) | [25] |
| 99 | 20-Hydroxyecdysone 2-O-βDGalp | K. chinensis (r) | [26] |
| 100 | 20-Hydroxyecdysone 25-O-βDGlcp | K. chinensis (r) | [25] |
| 101 | 20-Hydroxyecdysone 2-O-acetate | S. coronata (j) | [33] |
| S. tinctoria (r) | [49] | ||
| K. chinensis (r) | [25] | ||
| 102 | 20-Hydroxyecdysone 3-O-acetate | S. coronata (j) | [33] |
| S. tinctoria (r) | [49] | ||
| K. chinensis (r) | [25,34] | ||
| 103 | 20-Hydroxyecdysone 3-O-acetate 2-O-βDGlcp | K. chinensis (r) | [26] |
| 104 | 20-Hydroxyecdysone 3-O-acetate 2-O-βDGalp | K. chinensis (r) | [26] |
| 105 | 20-Hydroxyecdysone 22-O-acetate | S. coronata (j) | [33,54] |
| S. tinctoria (r) | [49] | ||
| K. centauroides (h) | [35] | ||
| 106 | 20-Hydroxyecdysone 2,22-di-O-acetate | S. tinctoria (r) | [49] |
| 107 | 20-Hydroxyecdysone 3,22-di-O-acetate | S. tinctoria (r) | [49] |
| 108 | 20-Hydroxyecdysone 20,22-butylidene acetal | K. chinensis (r) | [25,34] |
| 109 | 3-epi-20-Hydroxyecdysone | S. coronata subsp. coronata (h) | [30] |
| 110 | 5α-20-Hydroxyecdysone | S. coronata subsp. coronata (h) | [30] |
| 111 | 14-epi-20-Hydroxyecdysone | S. coronata subsp. coronata (h) | [30] |
| 112 | 22-epi-20-Hydroxyecdysone | S. coronata subsp. coronata (h) | [30] |
| S. tinctoria (h) | [45] | ||
| 113 | 11α-Hydroxypoststerone | S. coronata subsp. coronata (h) | [30,46] |
| 114 | 11α-11-Hydroxyshidasterone | S. coronata subsp. coronata (r) | [55] |
| 115 | 24-(2-Hydroxyethyl)-20-hydroxyecdysone | K. centauroides subsp. strangulata (w) | [42] |
| 116 | 5β-Hydroxyrubrosterone | S. tinctoria (r) | [49] |
| 117 | 1-Hydroxy-20,22-didehydrotaxisterone | S. coronata subsp. coronata (r) | [43] |
| 118 | Isovitexirone | S. coronata subsp. coronata (h) | [30] |
| K. centauroides (f, l, st) | [35] | ||
| 119 | Inokosterone | K. centauroides (f, l, st) | [35] |
| K. lycopifolia (h) | [44] | ||
| K. quinquefolia (j) | [52] | ||
| K. radiata (h) | [44] | ||
| K. radiata subsp. gmelinii (h) | [44] | ||
| 120 | Inokosterone 26-O-acetate | S. coronata (j) | [33] |
| 121 | Integristerone A | S. coronata (s) | [27] |
| S. coronata subsp. coronata (h) | [29] | ||
| K. centauroides (f, h, l, st) | [35,38] | ||
| K. centauroides subsp. centauroides (l,r,s,st) | [39] | ||
| K. ericifolia (f) | [51] | ||
| 122 | Makisterone A | S. coronata subsp. coronata (h) | [30] |
| 123 | Makisterone C | S. coronata (j, l) | [28,33] |
| S. coronata subsp. coronata (h) | [30] | ||
| S. tinctoria (r) | [49] | ||
| K. centauroides (h) | [35] | ||
| 124 | 24-Methyleneshidasterone | S. coronata subsp. coronata (r) | [56] |
| K. chinensis (r) | [25,34] | ||
| 125 | 22-Oxo-20-hydroxyecdysone | S. tinctoria (r) | [49] |
| 126 | (2β,3α,5β,22R)-2,3,20,22,25-Pentahydroxycholest-7-en-6-one | S. coronata subsp. coronata (r) | [55] |
| 127 | (2β,3α,5β,14β,22R)-2,3,20,22,25-Pentahydroxycholest-7-en-6-one | S. coronata subsp. coronata (r) | [55] |
| 128 | Podecdysone C | S. coronata subsp. coronata (h) | [30] |
| K. chinensis (r) | [34] | ||
| 129 | Polypodine B | S. coronata (s) | [27] |
| S. coronata subsp. coronata (h) | [29,30] | ||
| S. tinctoria (r) | [49] | ||
| K. chinensis (r) | [25] | ||
| K. quinquefolia (j) | [52] | ||
| 130 | Polypodine B 20,22-monoacetonide | K. chinensis (r) | [25] |
| 131 | Ponasterone A | S. coronata subsp. coronata (r) | [55] |
| 132 | Ponasterone A 22-O-βDApif | S. coronata subsp. coronata (r) | [40] |
| 133 | Poststerone | S. tinctoria (r) | [49] |
| 134 | 3-epi-Poststerone | S. tinctoria (r) | [49] |
| 135 | Pterosterone | S. coronata subsp. coronata (h) | [29,30] |
| S. tinctoria (r) | [49] | ||
| 136 | Rubrosterone | S. tinctoria (r) | [49] |
| 137 | 3-epi-Rubrosterone | S. tinctoria (r) | [49] |
| 138 | Serfurosterone A | S. coronata subsp. coronata (r) | [57] |
| 139 | Serfurosterone B | S. coronata subsp. coronata (r) | [57] |
| 140 | Shidasterone | K. chinensis (r) | [25,34] |
| 141 | 3-epi-Shidasterone | S. coronata subsp. coronata (r) | [40] |
| 142 | Sogdisterone | K. sogdiana (f) | [58] |
| 143 | Stachysterone B | S. coronata subsp. coronata (r) | [56] |
| 144 | Stachysterone B 14,15-α-epoxide | S. coronata subsp. coronata (r) | [56] |
| 145 | Stachysterone C | K. chinensis (r) | [34] |
| 146 | Taxisterone | S. coronata (j) | [54] |
| 147 | Turkesterone | S. coronata subsp. coronata (h) | [30] |
| 148 | Viticosterone E | S. coronata (j) | [33] |
| S. tinctoria (r) | [49] | ||
| K. centauroides (h) | [59] | ||
| K. procumbens (h) | [44] | ||
| K. sogdiana (l) | [60] | ||
| Phenols | |||
| 149 | Arbutin | K. centauroides (h) | [59] |
| K. centauroides subsp. centauroides (l, st) | [61] | ||
| K. erucifolia (l) | [15] | ||
| K. quinquefolia (h, j) | [52,62] | ||
| K. lyratifolia (l) | [63] | ||
| K. radiata (l) | [62] | ||
| K. radiata subsp. radiata (l) | [15] | ||
| K. radiata subsp. gmelinii (l) | [62] | ||
| K. sogdiana (l) | [64] | ||
| 150 | 7-Isobutyryloxy-8,9-epoxy-thymol-isobutyrate | K. latifolia (ae) | [19] |
| Hydroxycinnamates | |||
| 151 | Caffeic acid | K. centauroides (h) | [47] |
| K. algida (h) | [47] | ||
| 152 | 1-O-Caffeoylquinic acid | K. centauroides (h) | [59] |
| 153 | 3-O-Caffeoylquinic acid | S. tinctoria (l, st) | [65] |
| 154 | 4-O-Caffeoylquinic acid | K. centauroides (h) | [59] |
| 155 | 5-O-Caffeoylquinic acid | S. kirghisorum (h) | [47] |
| S. tinctoria (l, st) | [65] | ||
| K. centauroides (h) | [47,59] | ||
| K. algida (h) | [47] | ||
| 156 | 1,5-Di-O-caffeoylquinic acid | K. centauroides (h) | [59] |
| 157 | 3,5-Di-O-caffeoylquinic acid | K. centauroides (h) | [59] |
| 158 | 4,5-Di-O-caffeoylquinic acid | K. centauroides (h) | [59] |
| Flavones | |||
| 159 | Chrysin 7-O-βDGlcp | K. centauroides (h) | [47] |
| 160 | Apigenin | S. coronata (h) | [66] |
| S. coronata subsp. coronata (h) | [67] | ||
| S. tinctoria (f) | [16] | ||
| S. tinctoria subsp. tinctoria (f) | [16] | ||
| K. flavescens subsp. cichoracea (f) | [68] | ||
| 161 | Apigenin 7-O-βDGlcAp | S. coronata subsp. coronata (h) | [67] |
| K. centauroides (h) | [59] | ||
| K. centauroides subsp. centauroides (h) | [69] | ||
| 162 | Acacetin | K. flavescens subsp. cichoracea (f) | [68] |
| 163 | Genkwanin | K. flavescens subsp. cichoracea (f) | [68] |
| 164 | Luteolin | S. coronata (h) | [66] |
| S. coronata subsp. coronata (h) | [67] | ||
| S. tinctoria (f, st) | [16,65] | ||
| S. tinctoria subsp. tinctoria (f) | [16] | ||
| K. centauroides (h, r) | [70] | ||
| K. centauroides subsp. centauroides (h) | [69] | ||
| K. flavescens subsp. cichoracea (f) | [68] | ||
| 165 | Luteolin 7-O-βDGlcp | K. centauroides (h) | [47] |
| 166 | Luteolin 7-O-βDGlcAp | S. coronata subsp. coronata (h) | [67] |
| K. centauroides subsp. centauroides (h) | [69] | ||
| 167 | Luteolin 4′-O-βDGlcp | S. coronata (h) | [66,71] |
| S. tinctoria (l, st) | [11] | ||
| 168 | Luteolin 4′-O-βDGlcAp | S. coronata (h) | [71] |
| 169 | Diosmetin | S. tinctoria (l, st) | [65] |
| 170 | Chrysoeriol 7-O-βDGlcAp | K. centauroides (h) | [59] |
| K. centauroides subsp. centauroides (h) | [69] | ||
| Flavonols | |||
| 171 | Kaempferol | K. lyratifolia (l) | [63] |
| 172 | Kaempferol 3-O-(6′′-αLRhap)-βDGlcp (nicotoflorin) | K. lyratifolia (l) | [63] |
| 173 | Kaempferol 3-O-methyl ester (kaempferide) | S. heterophilla (r) | [14] |
| S. coronata (r) | [14] | ||
| S. coronata subsp. coronata (h, r) | [20,67] | ||
| K. pinnatifida (r) | [14] | ||
| K. radiata subsp. gmelinii (r) | [14] | ||
| K. radiata subsp. radiata (r) | [14] | ||
| 174 | Kaempferol 4′-O-methyl ester | K. centauroides subsp. strangulata (w) | [21] |
| 175 | Kaempferol 4′-O-methyl ester 7-O-βDGlcp (mumemin) | K. centauroides subsp. strangulata (w) | [21] |
| 176 | Kaempferol 7,4′-di-O-methyl ester | K. centauroides subsp. strangulata (l, r, rz, s) | [21,41] |
| 177 | Quercetin | S. coronata subsp. coronata (h) | [67] |
| K. centauroides (h, r) | [70] | ||
| K. centauroides subsp. centauroides (h) | [69] | ||
| K. lyratifolia (l) | [63] | ||
| 178 | Quercetin 3-O-(6′′-αLRhap)-βDGlcp (rutin) | K. centauroides (h, r) | [70] |
| K. lyratifolia (l) | [63] | ||
| 179 | Quercetin 4′-O-βDGlcp | S. coronata (h) | [66,71] |
| 180 | Quercetin 4′-O-βDGlcAp | S. coronata (h) | [71] |
| S. coronata subsp. coronata (h) | [67] | ||
| 181 | Quercetin 3-O-methyl ester | S. coronata (h) | [66] |
| S. coronata subsp. coronata (h) | [67] | ||
| S. tinctoria (l, st) | [11] | ||
| S. tinctoria subsp. tinctoria (f) | [72] | ||
| K. centauroides subsp. centauroides (h) | [69] | ||
| K. flavescens subsp. cichoracea (f) | [68] | ||
| 182 | Quercetin 3-O-methyl ester 4′-O-βDGlcAp | S. coronata subsp. coronata (h) | [67] |
| 183 | 6-Hydroxykaempferol 4′-O-methyl ester | K. centauroides subsp. strangulata (w) | [21] |
| 184 | 6-Hydroxykaempferol 4′-O-methyl ester 7-O-βDGlcp | K. centauroides subsp. strangulata (w) | [21] |
| 185 | 6-Hydroxykaempferol 4′-O-methyl ester 7-O-βDGalp | K. centauroides subsp. strangulata (w) | [21] |
| 186 | 6-Hydroxykaempferol 6,4′-di-O-methyl ester | K. centauroides subsp. strangulata (w) | [21] |
| 187 | 6-Hydroxykaempferol 6,4′-di-O-methyl ester 7-O-βDGlcp | K. centauroides subsp. strangulata (w) | [21] |
| Isoflavones | |||
| 188 | Genistein | K. centauroides subsp. strangulata (w) | [21] |
| 189 | 5,7-Dihydroxy-4′-methoxyisoflavone (biochanin A) | K. centauroides subsp. strangulata (l, r, s) | [41] |
| 190 | 5,6,7-Trihydroxy-4′-methoxy-isoflavone | K. centauroides subsp. strangulata (w) | [21] |
| Various phenolics | |||
| 191 | Strangusin A | K. centauroides subsp. strangulata (w) | [73] |
| 192 | Strangusin B | K. centauroides subsp. strangulata (w) | [73] |
| Thiophenes | |||
| 193 | 5-(1,2-Dihydroxyethyl)-2-[hepten-(5)-diin-(1,3)-yl]-thiophene | K. radiata (r) | [74] |
| 194 | 5-(1,2-Diacetoxyethyl)-2-[hepten-(5)-diin-(1,3)-yl]-thiophene | K. radiata (r) | [74] |
| 195 | 5-(1-Hydroxy-2-acetoxyethyl)-2-[hepten-(5)-diin-(1,3)-yl]-thiophene | K. radiata (r) | [74] |
| Fatty acids | |||
| 196 | 9:0 (pelargonic acid) | K. erucifolia (h) | [75] |
| 197 | 10:0 (capric acid) | K. erucifolia (h) | [75] |
| K. lasiocephala (h) | [75] | ||
| 198 | 12:0 (lauric acid) | K. centauroides (h, r) | [18] |
| K. erucifolia (h) | [75] | ||
| K. hakkiarica (h) | [75] | ||
| K. lasiocephala (h) | [75] | ||
| K. radiata subsp. biebersteiniana (h) | [75] | ||
| 199 | 14:0 (myristic acid) | K. centauroides (h, r) | [18] |
| K. erucifolia (h) | [75] | ||
| K. hakkiarica (h) | [75] | ||
| K. lasiocephala (h) | [75] | ||
| K. radiata subsp. biebersteiniana (h) | [75] | ||
| 200 | 15:0 (pentadecanoic acid) | K. centauroides (r) | [18] |
| K. erucifolia (h) | [75] | ||
| K. hakkiarica (h) | [75] | ||
| K. lasiocephala (h) | [75] | ||
| K. radiata subsp. biebersteiniana (h) | [75] | ||
| 201 | 16:0 (palmitic acid) | S. coronata subsp. coronata (r) | [20] |
| K. centauroides (h, r) | [18] | ||
| K. erucifolia (h) | [75] | ||
| K. hakkiarica (h) | [75] | ||
| K. lasiocephala (h) | [75] | ||
| K. radiata subsp. biebersteiniana (h) | [75] | ||
| 202 | 17:0 (margaric acid) | K. centauroides (h, r) | [18] |
| K. erucifolia (h) | [75] | ||
| K. lasiocephala (h) | [75] | ||
| K. radiata subsp. biebersteiniana (h) | [75] | ||
| 203 | 18:0 (stearic acid) | K. centauroides (h, r) | [18] |
| K. erucifolia (h) | [75] | ||
| K. hakkiarica (h) | [75] | ||
| K. lasiocephala (h) | [75] | ||
| K. radiata subsp. biebersteiniana (h) | [75] | ||
| 204 | 19:0 (nonadecanoic acid) | K. centauroides (h, r) | [18] |
| 205 | 20:0 (arachidic acid) | K. centauroides (h, r) | [18] |
| K. erucifolia (h) | [75] | ||
| K. hakkiarica (h) | [75] | ||
| K. lasiocephala (h) | [75] | ||
| K. radiata subsp. biebersteiniana (h) | [75] | ||
| 206 | 21:0 (heneicosanoic acid) | K. centauroides (h) | [18] |
| K. hakkiarica (h) | [75] | ||
| K. lasiocephala (h) | [75] | ||
| 207 | 22:0 (behenic acid) | K. centauroides (h, r) | [18] |
| K. hakkiarica (h) | [75] | ||
| K. lasiocephala (h) | [75] | ||
| K. radiata subsp. biebersteiniana (h) | [75] | ||
| 208 | 23:0 (tricosanoic acid) | K. centauroides (h, r) | [18] |
| K. lasiocephala (h) | [75] | ||
| 209 | 24:0 (lignoceric acid) | K. centauroides (h, r) | [18] |
| K. hakkiarica (h) | [75] | ||
| K. lasiocephala (h) | [75] | ||
| K. radiata subsp. biebersteiniana (h) | [75] | ||
| 210 | 2-OH-24:0 (2-hydroxytetracosanoic acid) | K. centauroides (h, r) | [18] |
| 211 | 25:0 (pentacosanoic acid) | K. centauroides (h) | [18] |
| 212 | 26:0 (cerotic acid) | K. centauroides (h, r) | [18] |
| 213 | 27:0 (carboceric acid) | K. centauroides (h) | [18] |
| 214 | 28:0 (montanic acid) | K. centauroides (h) | [18] |
| 215 | 29:0 (nonacosanoic acid) | K. centauroides (h) | [18] |
| 216 | 30:0 (melissic acid) | K. centauroides (h) | [18] |
| 217 | 14:1n5 (myristoleic acid) | K. centauroides (h) | [18] |
| 218 | 16:1n7 (palmitoleic acid) | K. erucifolia (h) | [75] |
| K. radiata subsp. biebersteiniana (h) | [75] | ||
| 219 | 16:1n9 (hypogeic acid) | K. centauroides (h, r) | [18] |
| 220 | 17:1 (heptadecenoic acid) | K. centauroides (h) | [18] |
| 221 | 18:1n9 (oleic acid) | K. centauroides (h) | [18] |
| K. erucifolia (h) | [75] | ||
| K. hakkiarica (h) | [75] | ||
| K. lasiocephala (h) | [75] | ||
| K. radiata subsp. biebersteiniana (h) | [75] | ||
| 222 | 20:1n9 (gondoic acid) | K. centauroides (h) | [18] |
| K. hakkiarica (h) | [75] | ||
| 223 | 18:2n6 (linoleic acid) | S. coronata subsp. coronata (r) | [20] |
| K. centauroides (h, r) | [18] | ||
| K. erucifolia (h) | [75] | ||
| K. hakkiarica (h) | [75] | ||
| K. lasiocephala (h) | [75] | ||
| K. radiata subsp. biebersteiniana (h) | [75] | ||
| 224 | 18:3n3 (α-linolenic acid) | S. coronata subsp. coronata (r) | [20] |
| K. centauroides (h, r) | [18] | ||
| K. hakkiarica (h) | [75] | ||
| K. radiata subsp. biebersteiniana (h) | [75] | ||
| 225 | 18:3n6 (γ-linolenic acid) | K. centauroides (h, r) | [18] |
| 226 | 18:4n3 (stearidonic acid) | K. centauroides (h) | [18] |
| 227 | 20:2n6 (eicosadienoic acid) | K. centauroides (h, r) | [18] |
| 228 | 20:4n6 (arachidonic acid) | K. hakkiarica (h) | [75] |
| 229 | 22:2n6 (docosahexaenoic acid) | K. centauroides (h, r) | [18] |
| 230 | 24:1n9 (nervonic acid) | K. centauroides (r) | [18] |
| K. erucifolia (h) | [75] | ||
| K. hakkiarica (h) | [75] | ||
| K. lasiocephala (h) | [75] | ||
| K. radiata subsp. biebersteiniana (h) | [75] | ||
| Glyceroglycolipids | |||
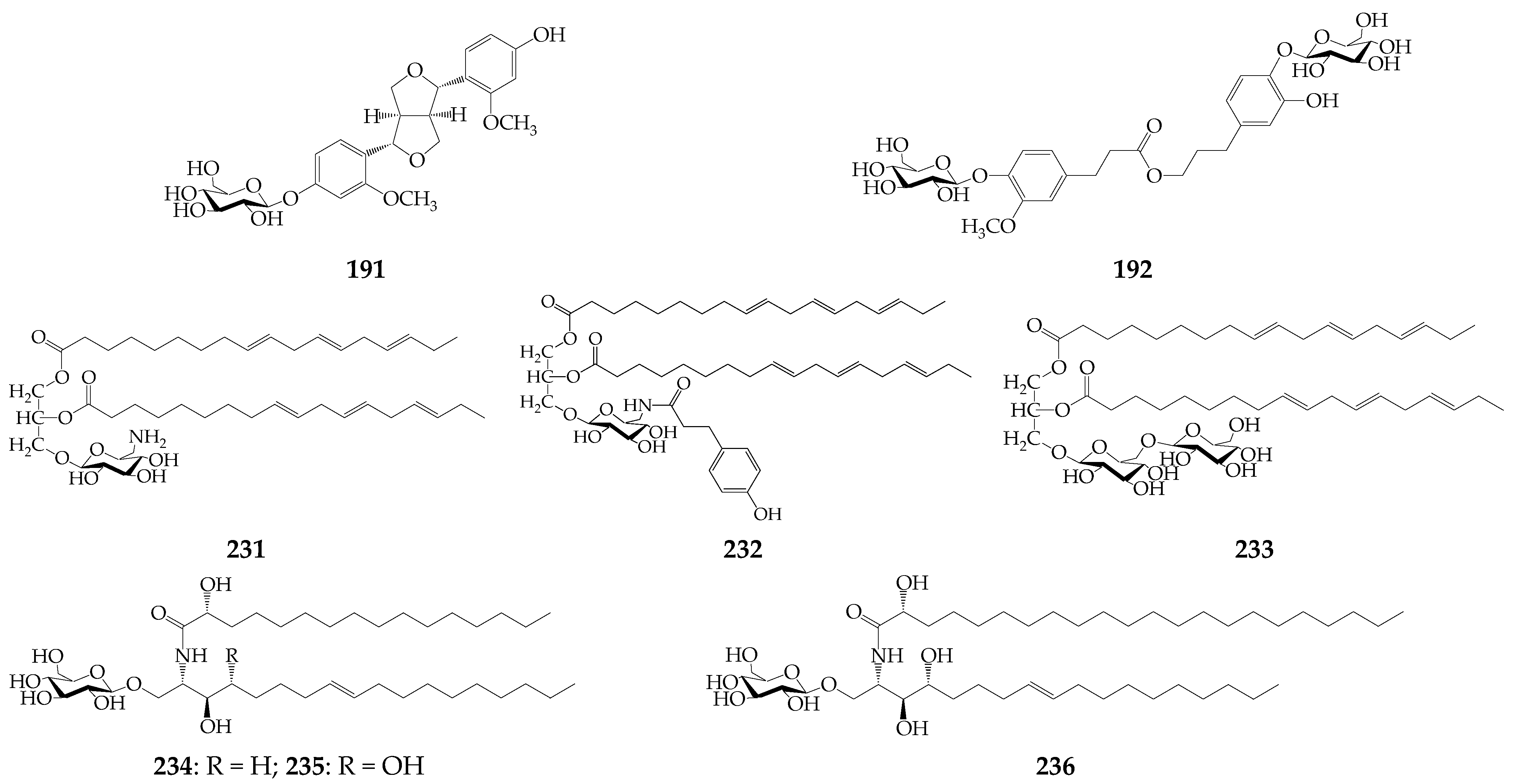
| 231 | 1,2-Di-O-(9Z,12Z,15Z-octadecatrienoyl)-3-O-(6-amine-6-deoxy-α-D-glucosyl)-glycerol (strangulatoside A) | K. centauroides subsp. strangulata (rz) | [22] |
| 232 | 1,2-Di-O-(9Z,12Z,15Z-octadecatrienoyl)-3-O-(6-p-hydroxy-phenyl-propionamido-6-deoxy-α-D-glucosyl)-glycerol (strangulatoside B) | K. centauroides subsp. strangulata (rz) | [22] |
| 233 | 1,2-Di-O-(9Z,12Z,15Z-octadecatrienoyl)-3-O-[α-D-glucose(1→6)-β-D-allose]-glycerol (strangulatoside C) | K. centauroides subsp. strangulata (rz) | [22] |
| Cerebrosides | |||
| 234 | 1-O-β-D-Glucopyranosyl-(2S,3R,8E)-2-[(2′R)-2-hydroxylpalmitoylamino]-8-octadecene-1,3-diol | K. chinensis (r) | [76] |
| 235 | 1-O-β-D-Glucopyranosyl-(2S,3S,4R,8E)-2-[(2′R)-2-hydroxybehenoyl-amino]-8-octadecene-1,3,4-triol | K. chinensis (r) | [76] |
| 236 | 1-O-β-D-Glucopyranosyl-(2S,3S,4R,8E)-2-[(2′R)-2-hydroxypalmitoyl-amino]-8-octadecene-1,3,4-triol (aralia cerebroside) | K. chinensis (r) | [76] |
| Amino acids | |||
| 237 | Alanine | K. centauroides (h) | [77] |
| 238 | Arginine | K. centauroides (h, r) | [77] |
| 239 | Histidine | K. centauroides (r) | [77] |
| 240 | Glycine | K. centauroides (h, r) | [77] |
| 241 | Lysine | K. centauroides (h, r) | [77] |
| 242 | Methionine | K. centauroides (h) | [77] |
| 243 | Phenylalanine | K. centauroides (h, r) | [77] |
| 244 | Proline | K. centauroides (h, r) | [77] |
| 245 | Serine | K. centauroides (h, r) | [77] |
| 246 | Tyrosine | K. centauroides (h, r) | [77] |
| 247 | Valine | K. centauroides (h, r) | [77] |
| Alkanes | |||
| 248 | Tricosane | K. centauroides (h) | [18] |
| 249 | Pentacosane | K. centauroides (h, r) | [24] |
| 250 | Heptacosane | K. centauroides (h, r) | [18,24] |
| 251 | Octacosane | K. centauroides (h, r) | [24] |
| 252 | Nonacosane | K. centauroides (h) | [18] |
| 253 | Docosane | K. centauroides (h, r) | [24] |
| 254 | Triacontane | K. centauroides (h, r) | [24] |
| 255 | Hentriacontane | K. centauroides (h) | [18] |
| Other groups | |||
| 256 | Tridec-1-ene | K. centauroides (r) | [18] |
| 257 | Hexadecanol | K. centauroides (h, r) | [18] |
| 258 | 2E,4E-Heptadienal | K. centauroides (r) | [18] |
| 259 | Cuparene | K. centauroides (h) | [18] |
| 260 | Tetradeca-2,12-diene-4,6,8,10-tetrayne | K. radiata (r) | [74] |
| 261 | Trideca-12-ene-2,4,6,8,10-pentayne | S. coronata (r) | [14] |
| K. pinnatifida (r) | [14] | ||
| K. radiata subsp. radiata (r) | [14] |
| Species | 20-Hydroxyecdysone | Integristerone A | 2-Desoxy-20-hydroxyecdysone | Polypodine B | Inokosterone |
|---|---|---|---|---|---|
| S. coronata | 18.4 mg/g (s) [27] 190.1 mg/g (e) [78] | 35.8 mg/g (e) [78] | |||
| S. coronata subsp. coronata | 1.0–8.5 mg/g (l) [79,80] 0.2–3.4 mg/g (st) [80] | ||||
| S. tinctoria | 1.6–13.4 mg/g (l) [79] | ||||
| K. centauroides | 1.5–6.5 mg/g (f) [35,38] 10.1 mg/g (h) [50] 8.8–16.8 mg/g (l) [35,38] 0.5 mg/g (r) [50] 4.5–14.2 mg/g (st) [35,38] 27.4 mg/g (e) [59] | 0.1–1.3 mg/g (f) [35,38] 2.2 mg/g (h) [50] 0.1–1.6 mg/g (l) [35,38] 0.1 mg/g (r) [50] 0.1–1.3 mg/g (st) [35,38] | 0.9–1.1 mg/g (f) [35,38] 1.8 mg/g (h) [50] 1.0–2.9 mg/g (l) [35,38] 0.1 mg/g (r) [50] 1.0–3.2 mg/g (st) [35,38] | 0.9 mg/g (f) [35] 2.5 mg/g (l) [35] 1.1 mg/g (st) [35] 5.7 mg/g (e) [59] | |
| K. centauroides subsp. centauroides | 0.7 mg/g (f) [39] 0.1–3.1 mg/g (l) [39] 0.6–1.5 mg/g (r) [39] 4.7 mg/g (s) [39] 0.1–2.9 mg/g (st) [39] | 23–150 μg/g (l) [39] 30–94 μg/g (r) [39] 20–24 μg/g (st) [39] | 0.1–0.4 mg/g (l) [39] 0.3–0.5 mg/g (r) [39] 0.8 mg/g (s) [39] 0.1–0.2 mg/g (st) [39] | ||
| K. centauroides subsp. strangulata | 0.7 mg/g (l) [41] 0.6 mg/g (r) [41] 5.5 mg/g (s) [41] |
| Species | Total Count | Compound Numbers |
|---|---|---|
| Serratula | ||
| S. coronata | 21 | 65–70, 75, 77, 80, 94, 95, 97, 101, 102, 105, 120, 121, 123, 129, 149, 148 |
| S. coronata subsp. coronata | 42 | 65, 69, 72, 77, 79, 82–85, 88, 89, 91–96, 109–114, 117, 118, 121–124, 126–129, 131, 132, 135, 138, 139, 141, 143, 144, 147 |
| S. kirghisorum | 1 | 94 |
| S. tinctoria | 18 | 90, 94, 101, 102, 105–107, 112, 116, 123, 125, 129, 133–137, 148 |
| S. tinctoria subsp. tinctoria | 1 | 94 |
| Klasea | ||
| K. algida | 1 | 94 |
| K. cardunculus | 1 | 94 |
| K. centauroides | 10 | 78, 81, 94, 95, 105, 118, 119, 121, 123, 148 |
| K. centauroides subsp. centauroides | 3 | 81, 94, 121 |
| K. centauroides subsp. strangulata | 4 | 86, 94, 95, 115 |
| K. chinensis | 23 | 63, 64, 72–74, 76, 87, 94, 95, 98–104, 108, 124, 128–130, 140, 145 |
| K. ericifolia | 2 | 94, 121 |
| K. flavescens subsp. cichoracea | 3 | 94, 65, 71 |
| K. lycopifolia | 3 | 89, 94, 119 |
| K. procumbens | 2 | 94, 148 |
| K. quinquefolia | 3 | 94, 119, 129 |
| K. radiata | 3 | 89, 94, 119 |
| K. radiata subsp. gmelinii | 3 | 89, 94, 119 |
| K. sogdiana | 3 | 94, 142, 148 |
| Species | Source, Solvent, Extraction Conditions * | Ref. |
|---|---|---|
| S. coronata | Leaves → H2O-extr. (1:10; 50 °C) → LLE EtOAc–MeOH (4:1)/H2O → EtOAc–MeOH-extr. RP-APC (Diasorb 130 C16 T), prep. RP-HPLC (Diasorb 130 C16 T) | [28] |
| Herb → EtOH-extr. (1:6) → LLE CHCl3/H2O, BuOH/H2O → BuOH-extr. NP-APC (Al2O3, SiO2) | [29] | |
| Herb juice → LLE EtOAc/H2O → EtOAc-extr. NP-APC (SiO2), prep. TLC (SiO2) | [32,33,54] | |
| Herb → MeOH-extr. (1:10; 20 °C) → dissol. MeOH → mixing with Me2CO → LLE Hex/H2O → water phase NP-APC (SiO2, polyamide), prep. NP-HPLC (Zorbax SIL), RP-HPLC (Zorbax-ODS), APC Sephadex LH-20 | [30] | |
| Roots → MeOH-extr. (1:10; 20 °C) → RP-APC (octadecyl silica), prep. NP-HPLC (Zorbax SIL), RP-HPLC (Zorbax SB C18) | [36,40,49,57,58] | |
| S. tinctoria | Herb → MeOH-extr. (boiling) → LLE Benz/H2O → LLE Benz-extr./50% MeOH → MeOH-extr. mixing with Me2CO → NP-APC (Al2O3, SiO2), prep. RP-TLC (RP-18 F254S) | [45] |
| Roots → MeOH-extr. (1:17; boiling) → LLE Benz/H2O → LLE Benz.-extr./50% MeOH → MeOH-extr. NP-APC (Al2O3, SiO2, Sephadex LH-20, polyamide), prep. NP-HPLC (Zorbax SIL), RP-HPLC (Spherisorb®-50DS2) | [49] | |
| K. centauroides | Roots, herb → 70% EtOH-extr. (1:25–1:50; 0 °C) → SPE → NP-APC (SiO2), RP-HPLC (LiChrospher PR-18), RP-HPLC (LiChrospher PR-18) | [35,38] |
| K. chinensis | Roots → 95% EtOH-extr. (20 °C) → LLE PE/H2O, CHCl3/H2O, BuOH/H2O → BuOH-extr. NP-APC (SiO2, Sephadex LH-20), RP-HPLC (Diaion HP-20) | [34] |
| K. erucifolia | Flowers → MeOH-extr. (20 °C) → LLE Hex/H2O, BuOH/H2O → BuOH-extr. NP-APC (Al2O3, SiO2) | [51] |
| K. quinquefolia | Herb juice → LLE EtOAc/H2O → EtOAc-extr. NP-APC (Al2O3, SiO2) | [52] |
| K. sogdiana | Flowers → EtOH-extr. (boiling) → NP-APC (Al2O3, SiO2) | [53] |
| Leaves → MeOH-extr. (1:6; boiling) → LLE PE/H2O, BuOH/H2O → BuOH-extr. NP-APC (polyamide), LP-RP-CC (octadecyl silica), prep. NP-HPLC (SiO2) | [55,60] |
| Sorbent, Column | Eluent | Ref. |
|---|---|---|
| Atmospheric Pressure Chromatography | ||
| Al2O3 | CHCl3-MeOH (30:1) | [55,60] |
| CHCl3-MeOH (10:1) | [51] | |
| CHCl3-MeOH (2:1, 15:1) | [29] | |
| CHCl3-MeOH | [28] | |
| CHCl3-MeOH (95:5 → 90:10); CH2Cl2-EtOH (90:10 → 70:30); CHCl3-EtOH (90:10 → 60:40) | [30] | |
| EtOAc-EtOH-H2O (80:10:2) | [56] | |
| EtOAc-MeOH-H2O (85:10:5) | [49] | |
| SiO2 | CHCl3-EtOH (90:10) | [53] |
| CHCl3-MeOH (90:10) | [34] | |
| CHCl3-MeOH (20:1) | [32] | |
| CHCl3-MeOH (10:1) | [52] | |
| CHCl3-MeOH (5:1) | [54] | |
| CHCl3-MeOH (25:1), CHCl3-MeOH (10:1 → 5:1) | [33] | |
| CHCl3-MeOH (25:1, 9:1, 4:1); CHCl3-MeOH-H2O (4:1:0.1) | [29] | |
| CHCl3-MeOH-H2O (60:32:6) | [51] | |
| CH2Cl2; CH2Cl2-EtOH (98:2 → 80:20) | [56] | |
| CH2Cl2-EtOH (90:10 → 50:50) | [49] | |
| EtOAc-MeOH-H2O (85:10:5); CH2Cl2; CH2Cl2-EtOH (98:2 → 90:10); MeOH | [30] | |
| MeOH-H2O (55:45) | [40] | |
| MeOH-H2O (50:40) | [57,58] | |
| MeOH-H2O (45:55) | [45] | |
| Sephadex LH-20 | MeOH-EtOAc (50:50), MeOH | [56] |
| EtOAc-MeOH-H2O (16:2:1); EtOAc-MeOH (2:1) | [30] | |
| Polyamide | H2O | [36,40,49,56,57,58] |
| H2O-MeOH (100:0 → 0:100) | [30] | |
| Kovasil C18 | MeOH-H2O (30:70 → 60:40) | [30] |
| Superclean C18 | EtOH-H2O (60:40) | [38] |
| Thin Layer Chromatography | ||
| NP-SiO2 | CHCl3-EtOH (4:1) | [53,55,60] |
| CHCl3-MeOH (4:1) | [51] | |
| CHCl3-MeOH (5:1) | [32,52,54] | |
| CHCl3-MeOH (8:1) | [33] | |
| CHCl3-MeOH (25:1) | [29] | |
| CH2Cl2-EtOH (85:15), EtOAc-MeOH-NH3 (85:10:5), EtOAc-EtOH-H2O (80:10:2) | [49,56] | |
| CH2Cl2-EtOH (8:2), Toluene-Me2CO-EtOH-NH3 (100:140:32:9), CH2Cl2-MeOH-C6H6 (25:5:3), EtOAc-EtOH-H2O (16:2:1) | [30] | |
| RP-SiO2 | MeOH-H2O (65:35) | [49] |
| MeOH-H2O (4:6), MeCN-H2O (35:65), 0.1% TFA in MeCN-H2O (35:65), Tetrahydrofuran-H2O (45:55) | [30] | |
| Cy-SiO2 | Hexane-Me2CO (6:4), MeCN-H2O (2:8) | [30] |
| Preparative NP-HPLC | ||
| Zorbax-SIL (250 mm × 9.4 mm × 5 μm) | CH2Cl2-iPrOH-H2O (125:25:2, 125:15:1) | [33,56] |
| CH2Cl2-iPrOH-H2O (125:40:3); CH2Cl2-iPrOH-H2O (125:25:2); Cyclohexane-iPrOH-H2O (80:40:3). | [49] | |
| Cyclohexane-iPrOH-H2O (100:40:3) | [40] | |
| Zorbax SIL (250 mm × 4.6 mm × 5 μm) | CH2Cl2-iPrOH-H2O (125:50:5; 125:40:3; 125:30:2; 125:25:2), Cyclohexane-iPrOH-H2O (100:40:3) | [30,36,49,57] |
| Preparative RP-HPLC | ||
| Diasorb 130 C16 T (250 mm × 15 mm × 7.5 μm) | H2O-MeOH-BuOH (45:30:1); MeOH-H2O (45:55) | [28] |
| Reprosil-Pur C18-AQ (250 mm × 10 mm × 10 μm) | MeCN-H2O | [32] |
| Separon C18 (125 mm × 25 mm × 10 μm) | MeOH-H2O (60:40) | [54] |
| Separon C18 (250 mm × 10 mm × 5 μm) | MeOH-H2O (60:40) | [52] |
| Zorbax SB-C18 (250 mm × 4.6 mm × 5 μm) | MeCN-H2O (23:77) | [30] |
| MeCN-H2O (35:65) | [40,49,58] | |
| MeOH-H2O (80:20) | [36] | |
| Analytic NP-HPLC | ||
| Zorbax-SIL (250 mm × 4.6 mm × 5 μm) | Isooctane-iPrOH-H2O (100:30:2) | [56] |
| Cyclohexane-iPrOH-H2O (100:30:2) | [33] | |
| CH2Cl2-iPrOH-H2O (125:50:5; 125:40:3; 125:30:2; 125:25:2), Cyclohexane-iPrOH-H2O (100:40:3) | [30] | |
| Analytic RP-HPLC | ||
| Separon C18 (125 mm × 4 mm × 5 μm) | MeOH-H2O (60:40) | [54] |
| Spherisorb 5ODS2 (250 mm × 4.6 mm × 5 μm) | MeCN-0.1% TFA in H2O (20:80 → 70:30; 23:77) | [56] |
| MeOH-H2O (60:40, 50:50) | [33] | |
| Zorbax ODS (250 mm × 4.6 mm × 5 μm) | MeCN-H2O (20:80) | [38] |
| MeCN-H2O (23:77) | [30] | |
| Species | Source, Solvent, Extraction Conditions * | Ref. |
|---|---|---|
| S. coronata | Roots → Et2O-PE-extr. (1:2) → pecip. | [14] |
| Herb → 50% MeOH-extr. (US) → MeOH-extr. NP-APC (polyamide, Sephadex LH-20), prep. TLC (polyamide) | [66] | |
| Leaves, stems → 70% EtOH-extr. (boiling) → LLE CCl4/H2O, EtOAc/H2O, BuOH/H2O → BuOH-extr. NP-APC (SiO2) | [71] | |
| S. coronata subsp. coronata | Leaves, flowers → 70% EtOH-extr. (boiling) → LLE CCl4/H2O, EtOAc/H2O, BuOH/H2O → BuOH-extr. recry. | [67] |
| S. tinctoria | Leaves → 30% EtOH-extr. (1:10, US) → LLE Benz/H2O → Water phase MPLC (cellulose), NP-APC (Sephadex LH-20) | [11] |
| K. centauroides subsp. centauroides | Leaves, flowers → 70% EtOH-extr. (boiling) → LLE CCl4/H2O, EtOAc/H2O, BuOH/H2O → BuOH-extr. NP-APC (SiO2), prep. RP-HPLC (Discovery C18) | [69] |
| K. centauroides subsp. strangulata | Whole plant → EtOH-extr. (20 °C) → LLE PE/H2O, EtOAc/H2O, BuOH/H2O → EtOAc-extr. NP-APC (SiO2) | [21] |
| K. flavescens subsp. cichoracea | Flowers → 80% EtOH-extr. (20 °C) → LLE PE/H2O, CHCl3/H2O, EtOAc/H2O → EtOAc-extr. prep. TLC (SiO2) | [68] |
| K. lyratifolia | Leaves → MeOH-extr. (20 °C) → MeOH-extr. PC, NP-APC (Sephadex LH-20) | [63] |
| K. radiata subsp. gmelinii | Roots → Et2O-PE-extr. (1:2) → pecip. | [14] |
| Sorbent, Column | Eluent | Ref. |
|---|---|---|
| Atmospheric Pressure Chromatography | ||
| Cellulose | EtOH-H2O (90:10 → 70:30) | [11] |
| SiO2 | Et2O-PE (5:1) | [21] |
| CHCl3-Me2CO, Hexane-EtOAc | [68] | |
| CCl4-EtOH (100:0 → 0:100) | [69,71] | |
| Sephadex LH-20 | 70% EtOH | [63] |
| MeOH; CH2Cl2:MeOH (8:2) | [66] | |
| Polyamide | H2O-MeOH (100:0 → 0:100); EtOAc-MeOH (100:0 → 50:50) | [66] |
| Thin Layer Chromatography | ||
| Cellulose | BuOH-AcOH-H2O (4:1:5); 15% AcOH; BuOH-EtOH-H2O (4:1:2.2); AcOH-conc.HCl-H2O (30:3:10) | [63] |
| Analytic RP-HPLC | ||
| GLC Mastro C18 (150 × 2.1 mm, 3 μm; Shimadzu, Kyoto, Japan) | 0.5% HCOOH in water (A), 0.5% HCOOH in MeCN (B); gradient: 0–2 min 5–6% B, 2–9 min 6–11% B, 9–15 min 11–25% B, 15–20 min 25–55% B, 20–25 min 55–5% B | [59] |
| Kromasil 100-5-C18 (250 × 4.6 mm, 5 μm; Kromasil, Göteborg, Sweden) | MeOH-H2O-H3PO4 (400:600:5) | [70] |
| PerfectSil Target ODS-3 (250 × 4.6 mm, 5 μm; MZ-Analysentechnik GmbH, Mainz, Germany) | 0.1% TFA (A), MeCN-iPrOH (B); gradient: 0–45 min 15–35% B | [47] |
| Simpak CLC-ODS (150 × 6 mm, 5 μm; Shimadzu, Columbia, MA, USA) | MeCN-H2O-H3PO4 (22:78:0.1), (35:65:0.2) | [63] |
| Supelco Discovery C18 (250 × 4.6 mm, 5 μm; Thermo Fisher Scientific, Waltham, MA, USA) | 1% AcOH (A), MeOH (B); gradient: 0–10 min 20% B, 10–56 min 80% B, 56–60 min 20% B | [67,69,71] |
| Symmetry C18 (150 × 2.1 mm, 5 μm; Waters, Milford, MA, USA) | H2O (A), MeCN (B); gradient: 0–30 min 15–45% B | [63] |
| Zorbax SB-Phenyl (150 × 4.6 mm, 3.5 μm; Agilent Technologies, Santa-Clara, CA, USA) | 0.15% HCOOH in H2O (A), MeOH (B); gradient: 0–15 min 40–60% B, 15–20 min 60–70% B, 20–27 min 70–100% B, 27–30 min 100% B | [65] |
| Extract, Compound | Assay, Model | Dose * | Positive Control | Result | Ref. |
|---|---|---|---|---|---|
| Serratula species | |||||
| 50% MeOH extracts of S. coronata, S. coronata subsp. coronata, S. tinctoria herb | In vitro: enzyme-independent lipid peroxidation of brain homogenate, enzyme-dependent lipid peroxidation of rat liver microsomes | 0–20 μg/mL | Vitamin E | Antioxidant effect | [66] |
| Methanol extract of S. coronata herb | In vivo: seborrheic dermatitis of human | 8 mg/human | Lekobaza® (Fagron, Kraków, Poland) | Antiseborrheic dermatitis effect | [78] |
| Water extract of S. coronata herb | In vivo: electric shock of Wistar rats | 5–15 mg/kg; i.p. | - | Adaptogenic effect | [85] |
| Methanol extract of S. coronata flowers | In vitro: rat lens aldose reductase inhibition | 0.1–10 μg/mL | 3,3′-Tetramethy-leneglutaric acid | Aldose reductase inhibition | [86] |
| Ecdysteroid fraction of S. coronata herb | In vivo: normal pressure hypoxia of rats | 20 mg/kg; i.p. | Rhaponticum carthamoides extract | Antihypoxic effect | [12] |
| In vivo: swimming duration, immobilization stress of rats | 20 mg/kg; i.p. | Adaptogenic effect | [12] | ||
| In vivo: hypo- and hyperthermia of rats | 20 mg/kg; i.p. | Termoprotective effect | [12] | ||
| In vivo: chronic cardiac failure of rats | 20 mg/kg; i.p. | Cardioprotective effect | [87] | ||
| In vivo: survival of Drosophila melanogaster | 0.2–1 μM | Fenugreek extract, dioscin | Adaptogenic, geroprotective effects | [88] | |
| Klasea species | |||||
| Ethanol extract of K. centauroides herb | In vivo: elevated plus maze test, light/dark test, Vogel test | 50–150 mg/kg; p.o. | Rhaponticum carthamoides extract | Anxyolitic effect | [70] |
| In vivo: acute/chromic emotional stress of Wistar rats | 25–200 mg/kg; p.o. | Stress-protective effect | [59] | ||
| Ethanol extract of K. centauroides leaves | In vivo: bilateral occlusion of the carotid arteries of Wistar rats | 50–200 mg/kg; p.o. | Anti-ishemic effect | [89] | |
| In vivo: hypobaric hypoxia/reoxigenation of Wistar rats | 50–100 mg/kg; p.o. | Antihypoxic, neuroprotective effect | [90] | ||
| In vivo: positive reinforcement of Wistar rats | 50–200 mg/kg; p.o. | Anxyolitic effect | [91] | ||
| Ecdysteroids | |||||
| 20-Hydroxyecdysone | In vivo: pulse heat stress of housefly larvae | 2 × 10–7 M | - | Stress-protective effect | [92] |
| 20-Hydroxyecdysone, 25-deoxy-11,20-dihydroxyecdysone, 20-hydroxyecdysone-20,22-monoacetonide, 24-(2-hydroxyethyl)-20-hydroxyecdysone | In vitro: human eritrocite oxidation hemolysis induced by AAPH; Fe2+- cysteine-induced lipid peroxidation of liver microsome | 0–3.2 mM | Glutatione reduced | Medium antioxidant effect | [42] |
| Ajugasterone C, 22-epi-ajugasterone C | In vitro: DPPH test | 0–300 μg/mL | Myricetin | Low antioxidant effect | [31] |
| Ecdysone, 20-hydroxyecdysone, 20-hydroxyecdysone 2-O-acetate, inokosterone | In vitro: serial dilutions method, Staphylococcus aureus, Escherichia coli, Proteus rettgeri, P. morgani, P. vulgaris, Bacillus cereus, B. subtilis, Micrococcus luteus, Pseudomonas aeruginosa, Candida tropicalis, C. utilis, C. pelliculosa, C. albicans, C. rugosa, Saccharomyces cerevisiae, Rhodotorula dracilis, Erwinia caratovora, Alternaria alternata, Fusarium solani, Aspergillus niger, Penicillium expansum | 0–1000 μg/mL | - | Low or null antibacterial effect | [93] |
| Ajugasterone C, 22-epi-ajugasterone C | In vitro: serial dilutions method, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Serratia sp., Pseudomonas aurogenosa, Candida albicans | 0–1000 μg/mL | Gentamicin, streptomycin, nystatin | Medium antibacterial effect | [31] |
| Flavonoids | |||||
| 3-Methylquercetin, apigenin, acacetin, luteolin, genkwanin | In vitro: DPPH test | 0–50 μg/mL | Quercetin | Antioxidant effect | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olennikov, D.N. Metabolites of Serratula L. and Klasea Cass. (Asteraceae): Diversity, Separation Methods, and Bioactivity. Separations 2022, 9, 448. https://doi.org/10.3390/separations9120448
Olennikov DN. Metabolites of Serratula L. and Klasea Cass. (Asteraceae): Diversity, Separation Methods, and Bioactivity. Separations. 2022; 9(12):448. https://doi.org/10.3390/separations9120448
Chicago/Turabian StyleOlennikov, Daniil N. 2022. "Metabolites of Serratula L. and Klasea Cass. (Asteraceae): Diversity, Separation Methods, and Bioactivity" Separations 9, no. 12: 448. https://doi.org/10.3390/separations9120448
APA StyleOlennikov, D. N. (2022). Metabolites of Serratula L. and Klasea Cass. (Asteraceae): Diversity, Separation Methods, and Bioactivity. Separations, 9(12), 448. https://doi.org/10.3390/separations9120448