High Value-Added Utilization of Waste Hydrodesulfurization Catalysts: Low-Cost Synthesis of Cathode Materials for Lithium-Ion Batteries
Abstract
1. Introduction
2. Experimental
2.1. Materials and Characterization Methods
2.2. Experimental Procedure
2.2.1. Sodium Salt Roasting
2.2.2. Leaching in Deionized Water
2.2.3. Sulfuric Acid Leaching
2.2.4. Titration in Removing Impurity
2.2.5. Precipitation Synthesis and Calcination of NCM523 Precursors
2.2.6. Electrochemical Performance Test
3. Results and Discussion
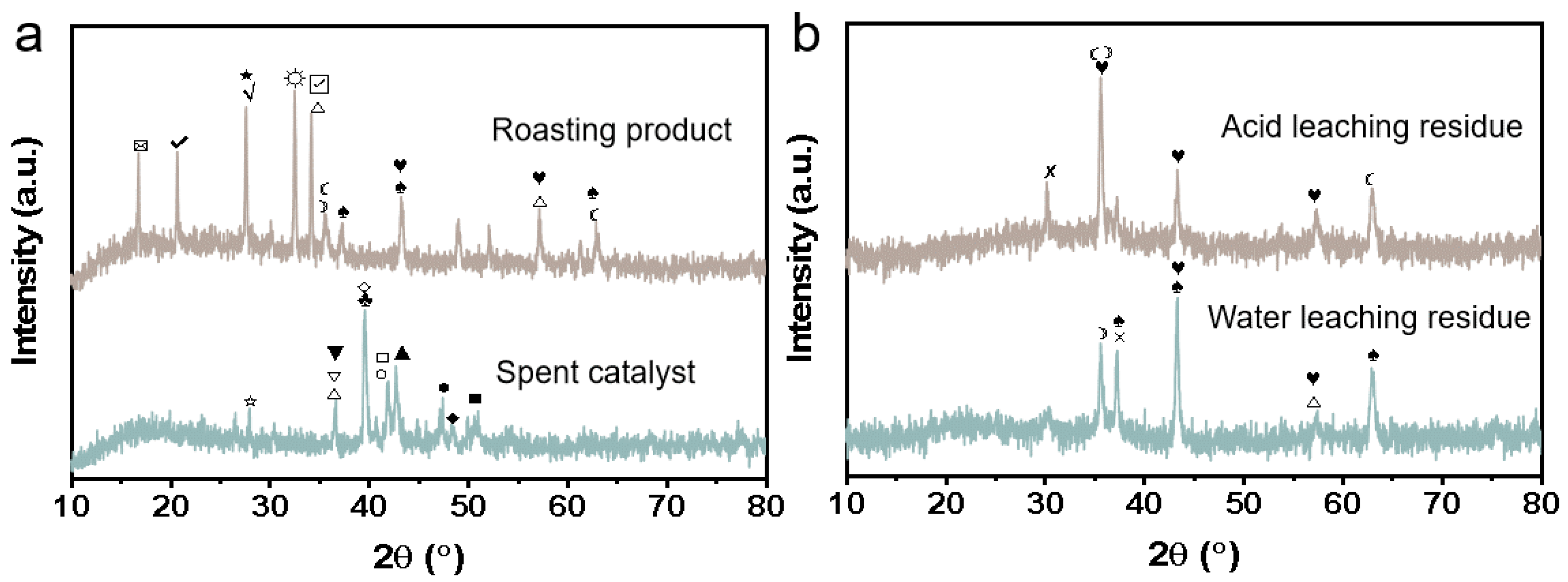
3.1. Mixed Oxidation Roasting of Waste HDS Catalyst with Na2CO3
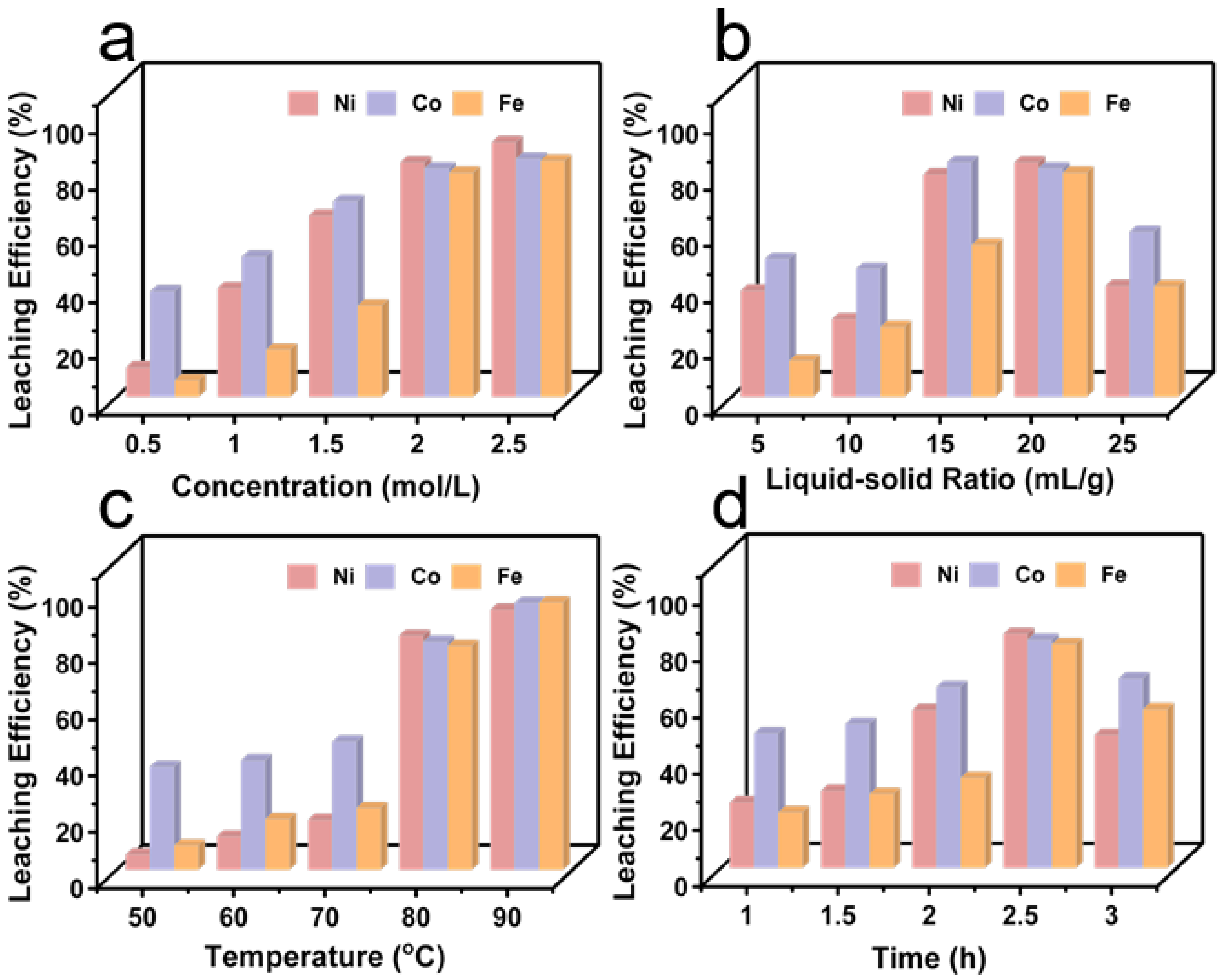
3.2. Sulfuric Acid Leaching and Impurity Removal
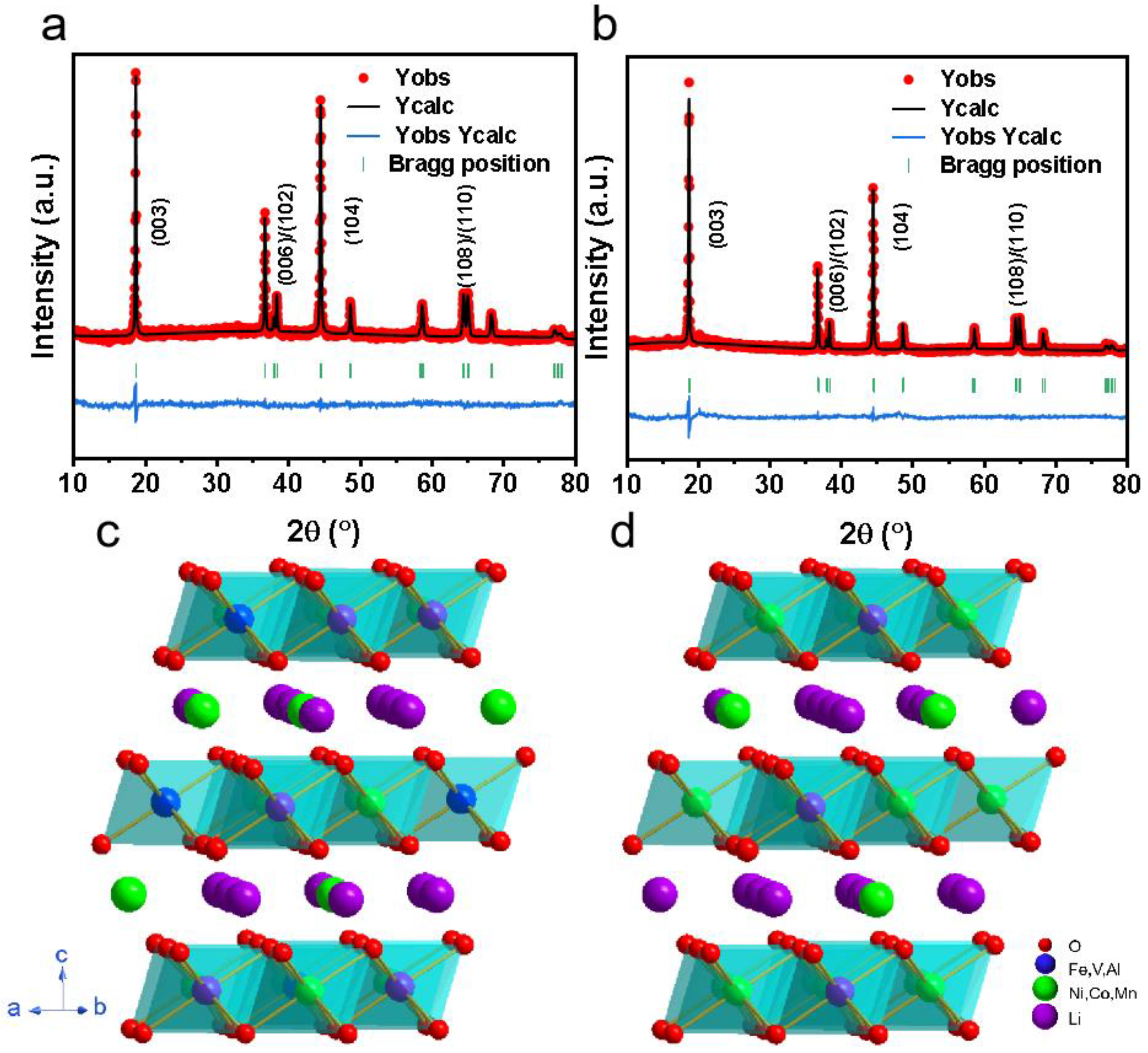
3.3. Characterization of Cathode Materials Prepared by Pickle Liquor
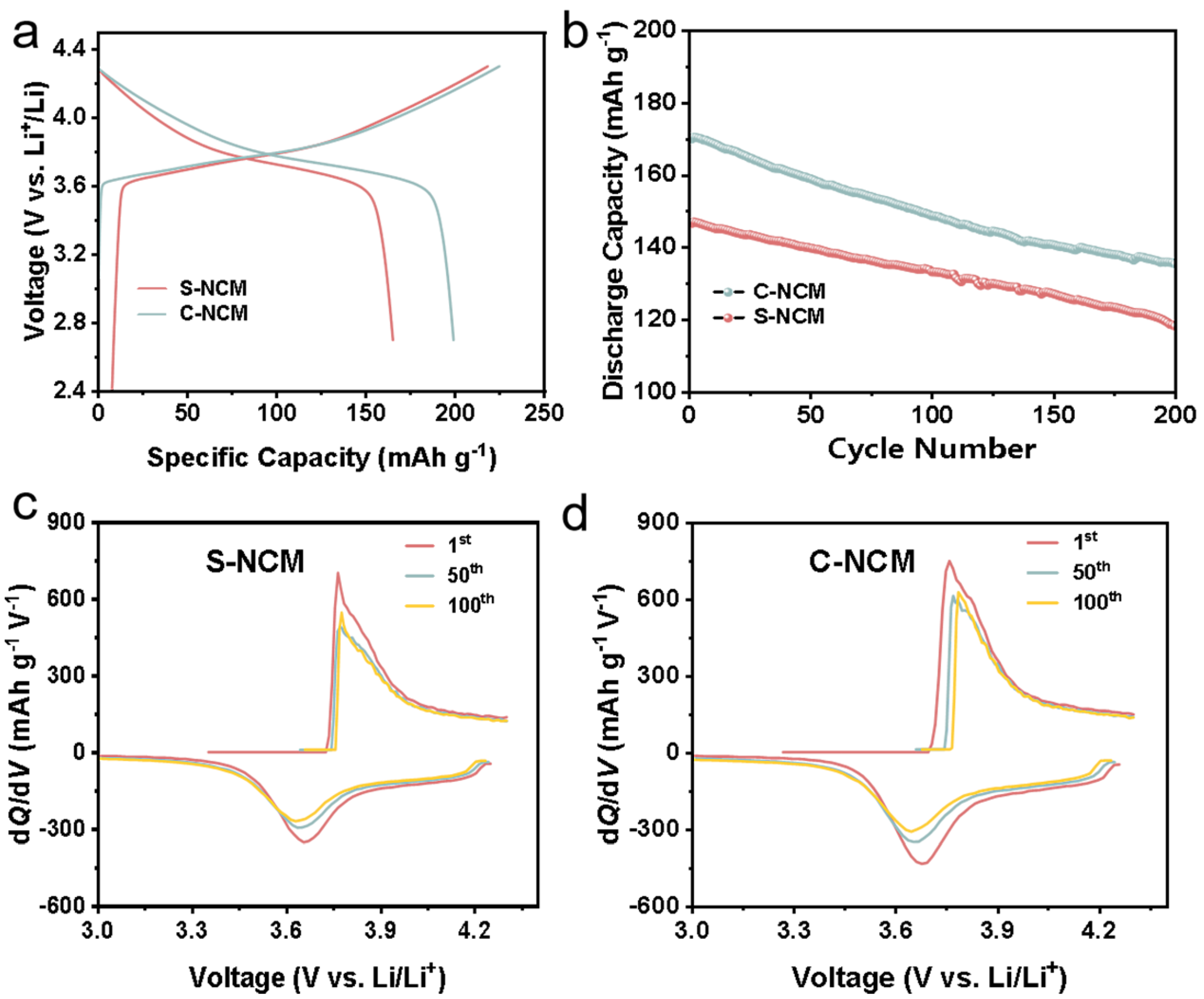
3.4. Electrochemical Performance
3.5. Economic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arslanoğlu, H.; Yaraş, A. Recovery of molybdenum, cobalt and nickel from spent hydrodesulphurization catalyst through oxidizing roast followed by sodium persulfate leaching. Sustain. Mater. Technol. 2021, 28, e00286–e00296. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A. Spent catalyst waste management: A review. Resour. Conserv. Recycl. 2008, 52, 859–873. [Google Scholar] [CrossRef]
- Tran, T.T.; Lee, M.S. Separation of Mo(VI), V(V), Ni(II), Al(III) from synthetic hydrochloric acidic leaching solution of spent catalysts by solvent extraction with ionic liquid. Sep. Purif. Technol. 2020, 247, 117005–117012. [Google Scholar] [CrossRef]
- Kansomket, C.; Chandakhiaw, T.; Ma-ud, N.; Yingnakorn, T.; Patcharawit, T.; Khumkoa, S. Study on Leaching of Molybdenum from a Spent HDS Catalyst. Mater. Sci. Forum 2020, 1009, 143–148. [Google Scholar] [CrossRef]
- Akcil, A.; Veglio, F.; Ferella, F.; Okudan, M.D.; Tuncuk, A. A review of metal recovery from spent petroleum catalysts and ash. Waste Manag. 2015, 45, 420–433. [Google Scholar] [CrossRef]
- Bai, X.; Shang, X.; Wan, H.; Che, Y.; Yang, B.; He, J.; Song, J. Sustainable recycling of titanium from TiO2 in spent SCR denitration catalyst via molten salt electrolysis. J. Energy Chem. 2021, 58, 557–563. [Google Scholar] [CrossRef]
- Alvial-Hein, G.; Mahandra, H.; Ghahreman, A. Separation and recovery of cobalt and nickel from end of life products via solvent extraction technique: A review. J. Clean. Prod. 2021, 297, 126592–126617. [Google Scholar] [CrossRef]
- Le, M.N.; Lee, M.S. Separation of Al(III), Mo(VI), Ni(II), and V(V) from model hydrochloric acid leach solutions of spent petroleum catalyst by solvent extraction. J. Chem. Technol. Biotechnol. 2020, 95, 2886–2897. [Google Scholar] [CrossRef]
- Saratale, R.G.; Kim, H.-Y.; Park, Y.; Shin, H.S.; Ghodake, G.; Bharagava, R.N.; Mulla, S.I.; Kim, D.-S.; Saratale, G.D. Hydrometallurgical process for the recovery of yttrium from spent fluorescent lamp: Leaching and crystallization experiments. J. Clean. Prod. 2020, 261, 121009–121023. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. Development of a hydrometallurgical process for the recovery of calcium molybdate and cobalt oxalate powders from spent hydrodesulphurization (HDS) catalyst. J. Clean. Prod. 2015, 90, 388–396. [Google Scholar] [CrossRef]
- Busnardo, R.G.; Busnardo, N.G.; Salvato, G.N.; Afonso, J.C. Processing of spent NiMo and CoMo/Al2O3 catalysts via fusion with KHSO4. J. Hazard. Mater. 2007, 139, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Moosakazemi, F.; Tavakoli Mohammadi, M.R.; Zakeri, M.; Esmaeili, M.J.; Rafiei, H. Development of an environmentally friendly flowsheet for the hydrometallurgical recovery of nickel and aluminum from spent methanation catalyst. J. Clean. Prod. 2020, 244, 118731–118744. [Google Scholar] [CrossRef]
- Li, H.-Y.; Fang, H.-X.; Wang, K.; Zhou, W.; Yang, Z.; Yan, X.-M.; Ge, W.-S.; Li, Q.-W.; Xie, B. Asynchronous extraction of vanadium and chromium from vanadium slag by stepwise sodium roasting–water leaching. Hydrometallurgy 2015, 156, 124–135. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Hu, Q.; Ke, X.; Yuan, S.; Liu, S.; Ji, X.; Hu, J. Sustainable recovery of nickel, molybdenum, and vanadium from spent hydroprocessing catalysts by an integrated selective route. J. Clean. Prod. 2020, 252, 119763–119771. [Google Scholar] [CrossRef]
- Nersisyan, H.H.; Kwon, S.C.; Re, V.; Lee, Y.J.; Yoo, B.U.; Lee, J.H. Recovery of Ti and Zr metals from spent leaching solutions by a precipitation-reduction pathway. Mater. Res. Bull. 2020, 122, 110687–110694. [Google Scholar] [CrossRef]
- Wu, H.; Duan, S.; Liu, D.; Guo, X.; Yi, A.; Li, H. Recovery of nickel and molybdate from ammoniacal leach liquor of spent hydrodesulfurization catalyst using LIX84 extraction. Sep. Purif. Technol. 2021, 269, 118750–118757. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Sharifi-Asl, S.; Soto, F.A.; Foroozan, T.; Asadi, M.; Yuan, Y.; Deivanayagam, R.; Rojaee, R.; Song, B.; Bi, X.; Amine, K.; et al. Anti-Oxygen Leaking LiCoO2. Adv. Funct. Mater. 2019, 29, 1901110–1901121. [Google Scholar] [CrossRef]
- Tsai, F.-Y.; Jhang, J.-H.; Hsieh, H.-W.; Li, C.-C. Dispersion, agglomeration, and gelation of LiFePO4 in water-based slurry. J. Power Sources 2016, 310, 47–53. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Li, H.; Liu, A.; Huang, Q.; Ma, L.; Li, Y.; Dahn, J.R. Structural, Electrochemical, and Thermal Properties of Nickel-Rich LiNixMnyCozO2 Materials. Chem. Mater. 2018, 30, 8852–8860. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Ni, Y.; Zhang, K.; Cheng, F.; Chen, J. Recent breakthroughs and perspectives of high-energy layered oxide cathode materials for lithium ion batteries. Mater. Today 2020, 43, 132–165. [Google Scholar] [CrossRef]
- Park, K.H.; Mohapatra, D.; Reddy, B.R. Selective recovery of molybdenum from spent HDS catalyst using oxidative soda ash leach/carbon adsorption method. J. Hazard. Mater. 2006, 138, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ming, Y.; Xiang, W.; Xu, C.; Feng, G.; Li, Y.; Chen, Y.; Wu, Z.; Zhong, B.; Guo, X. Structure and electrochemical performance modulation of a LiNi0.8Co0.1Mn0.1O2 cathode material by anion and cation co-doping for lithium ion batteries. RSC Adv. 2019, 9, 36849–36857. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Luo, S.-H.; Wang, X.; Wang, L.; Wang, J.; Teng, F.; Wang, Q.; Zhang, Y.; Liu, X.; Zhang, H.; et al. Study on the high-efficiency separation of Fe and Mn from low-grade pyrolusite and the preparation of LiMn2O4 materials for lithium-ion batteries. Sep. Purif. Technol. 2021, 278, 119611–119621. [Google Scholar] [CrossRef]
- Li, P.; Luo, S.-H.; Wang, J.; Wang, Y.; Wang, Q.; Zhang, Y.; Liu, X.; Gao, D.; Lv, F.; Mu, W.; et al. Extraction and separation of Fe and Ti from extracted vanadium residue by enhanced ammonium sulfate leaching and synthesis of LiFePO4/C for lithium-ion batteries. Sep. Purif. Technol. 2022, 282, 120065–120075. [Google Scholar] [CrossRef]
- Li, H.-Y.; Wang, C.-J.; Yuan, Y.-H.; Guo, Y.; Diao, J.; Xie, B. Magnesiation roasting-acid leaching: A zero-discharge method for vanadium extraction from vanadium slag. J. Clean. Prod. 2020, 260, 121091–121100. [Google Scholar] [CrossRef]
- Nie, W.; Wen, S.; Feng, Q.; Liu, D.; Zhou, Y. Mechanism and kinetics study of sulfuric acid leaching of titanium from titanium-bearing electric furnace slag. J. Mater. Res. Technol. 2020, 9, 1750–1758. [Google Scholar] [CrossRef]
- Aarabi-Karasgani, M.; Rashchi, F.; Mostoufi, N.; Vahidi, E. Leaching of vanadium from LD converter slag using sulfuric acid. Hydrometallurgy 2010, 102, 14–21. [Google Scholar] [CrossRef]
- Muralidharan, N.; Essehli, R.; Hermann, R.P.; Parejiya, A.; Amin, R.; Bai, Y.; Du, Z.; Belharouak, I. LiNixFeyAlzO2, a new cobalt-free layered cathode material for advanced Li-ion batteries. J. Power Sources 2020, 471, 228389–228398. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Tang, J.; Liu, X.; Gan, H.; Yang, J. One-step selective recovery and cyclic utilization of valuable metals from spent lithium-ion batteries via low-temperature chlorination pyrolysis. Resour. Conserv. Recycl. 2021, 175, 105840–105849. [Google Scholar] [CrossRef]
- Xu, S.; Wang, X.; Zhang, W.; Xu, K.; Zhou, X.; Zhang, Y.; Wang, H.; Zhao, J. The effects of washing on LiNi0.83Co0.13Mn0.04O2 cathode materials. Solid State Ion. 2019, 334, 105–110. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.H. Surface modification of LiNi0.5Co0.2Mn0.3O2 cathode materials with Li2O-B2O3-LiBr for lithium-ion batteries. Int. J. Energy Res. 2019, 43, 4644–4651. [Google Scholar] [CrossRef]
- Tang, M.; Yang, J.; Chen, N.; Zhu, S.; Wang, X.; Wang, T.; Zhang, C.; Xia, Y. Overall structural modification of a layered Ni-rich cathode for enhanced cycling stability and rate capability at high voltage. J. Mater. Chem. A 2019, 7, 6080–6089. [Google Scholar] [CrossRef]
- Jia, X.; Yan, M.; Zhou, Z.; Chen, X.; Yao, C.; Li, D.; Chen, D.; Chen, Y. Nd-doped LiNi0.5Co0.2Mn0.3O2 as a cathode material for better rate capability in high voltage cycling of Li-ion batteries. Electrochim. Acta 2017, 254, 50–58. [Google Scholar] [CrossRef]
- Moon, S.-H.; Kim, E.-S.; Lee, J.-E.; Shin, Y.-K.; Kim, M.-C.; Park, K.-W. Improved electrochemical properties of LiNi0.8Co0.15Al0.05O2 cathode materials synthesized using micelle structures. J. Solid State Electrochem. 2020, 24, 2233–2240. [Google Scholar] [CrossRef]
- Mo, Y.; Guo, L.; Cao, B.; Wang, Y.; Zhang, L.; Jia, X.; Chen, Y. Correlating structural changes of the improved cyclability upon Nd-substitution in LiNi0.5Co0.2Mn0.3O2 cathode materials. Energy Storage Mater. 2019, 18, 260–268. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, F.; Wei, G.; Lei, Z.; Qu, J. Improvement of electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode material via Li2.09W0.9Nb0.1O4 Li-ion conductive coating layer. J. Solid State Electrochem. 2020, 24, 2301–2313. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; Zhang, T.; Zhang, Y.; Lian, Y.; Zhao, W. Improving electrochemical performance and thermal stability of LiNi0.8Co0.1Mn0.1O2 via a concentration gradient Nb doping. Ionics 2020, 26, 5971–5979. [Google Scholar] [CrossRef]
- Yang, R.-K.; Wu, Z.-G.; Li, Y.-C.; Li, R.; Qiu, L.; Wang, D.; Yang, L.; Guo, X.-D. Optimization of the electrochemical properties of LiNi0.8Co0.1Mn0.1O2 cathode material by titanium doping. Ionics 2020, 26, 3223–3230. [Google Scholar] [CrossRef]
- Tian, L.; Yuan, H.; Shao, Q.; Zaidi, S.D.A.; Wang, C.; Chen, J. Synergistic effect of Li2MgTi3O8 coating layer with dual ionic surface doping to improve electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode materials. Ionics 2020, 26, 4937–4948. [Google Scholar] [CrossRef]
- Gao, H.; Cai, J.; Xu, G.-L.; Li, L.; Ren, Y.; Meng, X.; Amine, K.; Chen, Z. Surface Modification for Suppressing Interfacial Parasitic Reactions of a Nickel-Rich Lithium-Ion Cathode. Chem. Mater. 2019, 31, 2723–2730. [Google Scholar] [CrossRef]
- Li, L.; Han, E.; Zhu, L.; Qiao, S.; Du, C. Effect of Nb5+ doping on LiNi0.5Co0.25Mn0.25O2 cathode material. Ionics 2020, 26, 2655–2664. [Google Scholar] [CrossRef]
- Liu, S.; Wu, H.; Huang, L.; Xiang, M.; Liu, H.; Zhang, Y. Synthesis of Li2Si2O5-coated LiNi0.6Co0.2Mn0.2O2 cathode materials with enhanced high-voltage electrochemical properties for lithium-ion batteries. J. Alloys Compd. 2016, 674, 447–454. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, P.; Chang, D.; Jie, Y.; Yang, S.; Wu, G.; Chen, H.; Zhu, J.; Hu, F.; Wilson, B.P.; et al. Selective extraction of valuable metals from spent EV power batteries using sulfation roasting and two stage leaching process. Sep. Purif. Technol. 2021, 258, 118078–118087. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Qin, Z.; Zhang, G.; Yue, H.; Liang, B.; Luo, D. Investigation of the selective oxidation roasting of vanadium-iron spinel. Powder Technol. 2021, 387, 434–443. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, M.; Zhang, M. Extraction of molybdenum and vanadium from the spent diesel exhaust catalyst by ammonia leaching method. J. Hazard. Mater. 2015, 286, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, Y.; Yuan, H. Recycling strategies of spent V2O5-WO3/TiO2 catalyst: A review. Resour. Conserv. Recycl. 2020, 161, 104983–104998. [Google Scholar] [CrossRef]
- Zhu, Z.; Liang, Y.; Hu, H.; Gao, A.; Meng, T.; Shu, D.; Yi, F.; Ling, J. Enhanced structural and electrochemical stability of LiNi0.83Co0.11Mn0.06O2 cathodes by zirconium and aluminum Co-doping for lithium-ion battery. J. Power Sources 2021, 498, 229857–229866. [Google Scholar] [CrossRef]
- Cao, G.; Jin, Z.; Zhu, J.; Li, Y.; Xu, B.; Xiong, Y.; Yang, J. A green Al2O3 metal oxide coating method for LiNi0.5Co0.2Mn0.3O2 cathode material to improve the high voltage performance. J. Alloys Compd. 2020, 832, 153788–153795. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, C.; Sun, G.; Kong, X.; Lai, S.; Li, J.; Zhou, R.; Wang, J.; Zhao, J. Electrochemical Degradation Mechanism and Thermal Behaviors of the Stored LiNi0.5Co0.2Mn0.3O2 Cathode Materials. ACS Appl. Mater. Interfaces 2018, 10, 25454–25464. [Google Scholar] [CrossRef]
- Li, H.; Dai, Y.; Li, X.; Shao, Z. Preparation of LiNi0.5Co0.2Mn0.3O2 by freeze-drying method. Colloids Surf. A: Physicochem. Eng. Asp. 2021, 629, 127486–127493. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Zhang, X.; Wu, F.; Chen, R. Compound-Hierarchical-Sphere LiNi0.5Co0.2Mn0.3O2: Synthesis, Structure, and Electrochemical Characterization. ACS Appl. Mater. Interfaces 2018, 10, 32120–32127. [Google Scholar] [CrossRef]
- Dang, R.; Qu, Y.; Ma, Z.; Yu, L.; Duan, L.; Lü, W. The Effect of Elemental Doping on Nickel-Rich NCM Cathode Materials of Lithium Ion Batteries. J. Phys. Chem. C 2021, 126, 151–159. [Google Scholar] [CrossRef]
- He, H.; Zan, L.; Zhang, Y. Effects of amorphous V2O5 coating on the electrochemical properties of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 as cathode material for Li-ion batteries. J. Alloys Compd. 2016, 680, 95–104. [Google Scholar] [CrossRef]
- Kaddami, A.; Ouzaouit, K.; Lamsayety, I.; Faqir, H.; Benzakour, I.; Saadoune, I. Effect of low Al3+ doping on the structural, electrochemical performances, and thermal stability of the LiNi1/3Co1/3Co1/3O2 electrode material for lithium-ion batteries. Int. J. Energy Res. 2021, 45, 13925–13935. [Google Scholar] [CrossRef]
- Levartovsky, Y.; Wu, X.; Erk, C.; Maiti, S.; Grinblat, J.; Talianker, M.; Aurbach, D. Enhancement of Structural, Electrochemical, and Thermal Properties of Ni-Rich LiNi0.85Co0.1Mn0.05O2 Cathode Materials for Li-Ion Batteries by Al and Ti Doping. Batter. Supercaps 2020, 4, 221–231. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Bao, S.; He, H.; Li, Y.; Sun, W.; Yue, B.; Huang, Y.; Zhang, P. Electrochemical properties of LiNi0.6Co0.12Mn0.2V0.08O2 as the tetrad cathode material of lithium-ion battery under high cut-off voltages. J. Solid State Electrochem. 2019, 23, 2009–2019. [Google Scholar] [CrossRef]
- Liu, X.; He, P.; Li, H.; Ishida, M.; Zhou, H. Improvement of electrochemical properties of LiNi1/3Co1/3Mn1/3O2 by coating with V2O5 layer. J. Alloys Compd. 2013, 552, 76–82. [Google Scholar] [CrossRef]
- Lu, Y.; Mo, Y.; Chen, Y.; Yu, F. Effects of Various Elements Doping on LiNi0.6Co0.2Mn0.2O2 Layered Materials for Lithium-Ion Batteries. Energy Technol. 2021, 9, 2100074–2100080. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Reissig, F.; Frankenstein, L.; Heidbüchel, M.; Winter, M.; Placke, T.; Schmuch, R. Magnesium Substitution in Ni-Rich NMC Layered Cathodes for High-Energy Lithium Ion Batteries. Adv. Energy Mater. 2022, 12, 2103045–2103059. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Yang, L.; Shen, X.; Liu, Q.; Hu, Z.; Kong, Q.; Ma, J.; Li, J.; Lin, H.-J.; et al. Anionic redox reaction and structural evolution of Ni-rich layered oxide cathode material. Nano Energy 2022, 98, 107335–107342. [Google Scholar] [CrossRef]




| Element | Mo | V | Ni | Co | Fe | Si | P | Others |
|---|---|---|---|---|---|---|---|---|
| Catalyst (wt%) | 24.29 | 5.89 | 26.34 | 9.70 | 9.00 | 2.30 | 11.00 | 11.48 |
| Raw Materials | Purity (wt%) | Price (RMB kg−1) | Consumption (kg/kg S–NCM Precursor) | Consumption (kg/kg C–NCM Precursor) |
|---|---|---|---|---|
| Waste HDS catalyst | 0 | 0 | 1.295 | |
| NiSO4 | 99.00 | 42 | 0.853 | 0 |
| CoSO4 | 99.00 | 63.5 | 0.342 | 0.008 |
| MnSO4 | 98.00 | 5.8 | 0.504 | 0.504 |
| Na2CO3 | 99.98 | 3 | 0 | 1.295 |
| H2SO4 | 98.00 | 0.29 | 0 | 2.306 |
| Product cost (RMB kg−1) | 60.466 | 7.985 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Qiu, L.; Li, Y.; Deng, Y.; Zhao, Q.; Hu, Y.; Guo, F.; Zhu, C.; Zhong, B.; Song, Y.; et al. High Value-Added Utilization of Waste Hydrodesulfurization Catalysts: Low-Cost Synthesis of Cathode Materials for Lithium-Ion Batteries. Separations 2022, 9, 449. https://doi.org/10.3390/separations9120449
Zhou J, Qiu L, Li Y, Deng Y, Zhao Q, Hu Y, Guo F, Zhu C, Zhong B, Song Y, et al. High Value-Added Utilization of Waste Hydrodesulfurization Catalysts: Low-Cost Synthesis of Cathode Materials for Lithium-Ion Batteries. Separations. 2022; 9(12):449. https://doi.org/10.3390/separations9120449
Chicago/Turabian StyleZhou, Junbo, Lang Qiu, Yao Li, Yuting Deng, Qing Zhao, Yang Hu, Fuqiren Guo, Chaoqiong Zhu, Benhe Zhong, Yang Song, and et al. 2022. "High Value-Added Utilization of Waste Hydrodesulfurization Catalysts: Low-Cost Synthesis of Cathode Materials for Lithium-Ion Batteries" Separations 9, no. 12: 449. https://doi.org/10.3390/separations9120449
APA StyleZhou, J., Qiu, L., Li, Y., Deng, Y., Zhao, Q., Hu, Y., Guo, F., Zhu, C., Zhong, B., Song, Y., & Guo, X. (2022). High Value-Added Utilization of Waste Hydrodesulfurization Catalysts: Low-Cost Synthesis of Cathode Materials for Lithium-Ion Batteries. Separations, 9(12), 449. https://doi.org/10.3390/separations9120449





