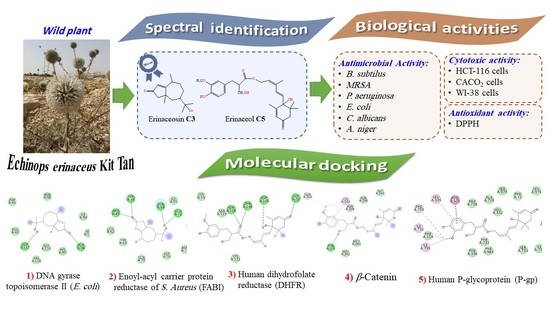
Phytochemical, Antimicrobial, Antioxidant, and In Vitro Cytotoxicity Evaluation of Echinops erinaceus Kit Tan
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. Isolation and Purification of Compounds from the CHCl3 Fraction
2.3. In Vitro Cytotoxicity Assay
2.3.1. Materials and Cell Lines
2.3.2. Cell Culture Condition and Propagation
2.3.3. Cytotoxicity Evaluation Using Viability Assay
2.4. In Vitro Antimicrobial Activity
2.5. In Vitro Antioxidant Effect
2.6. In Silico Studies of the Isolated Compounds
2.6.1. PASS and ADME Predictions
2.6.2. Molecular Docking Analysis

3. Results and Discussion
3.1. Identification of the Isolated Compounds
3.1.1. Identification of Compounds C1 and C2
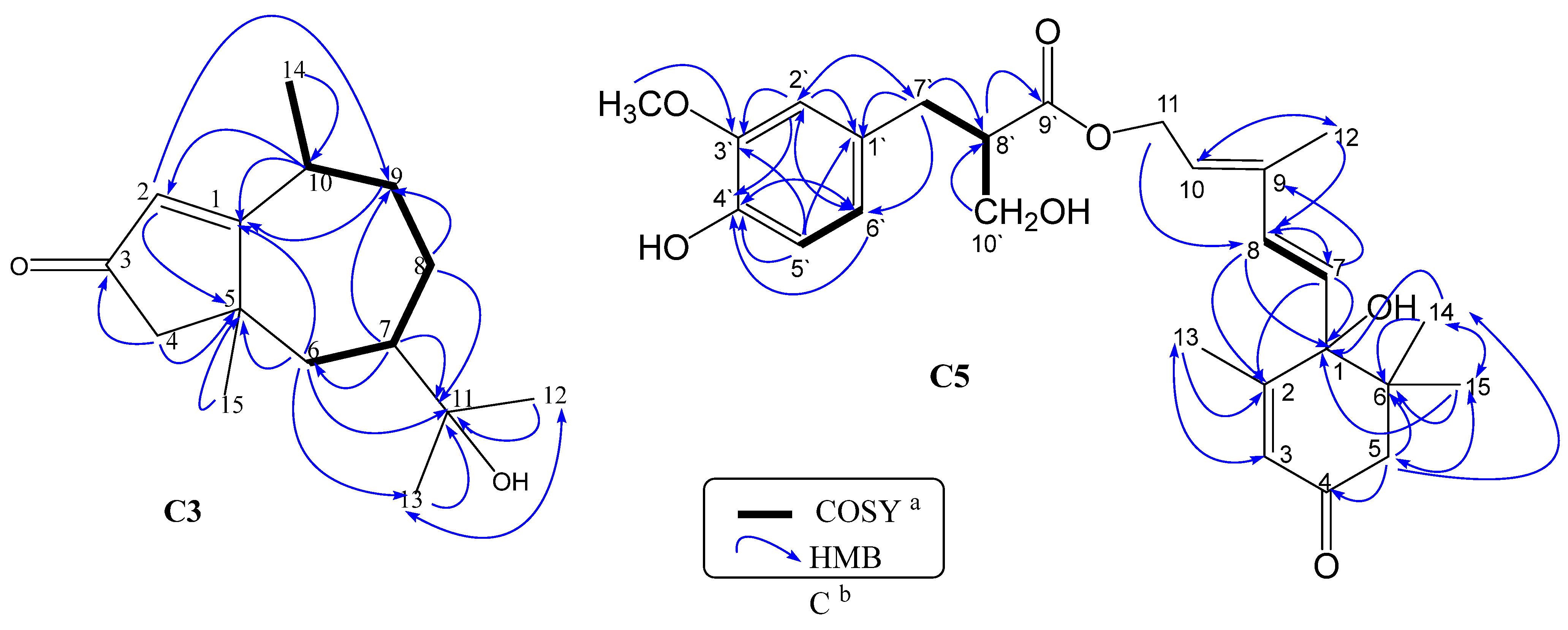
3.1.2. Identification of Compound C3
| HSQC | HMBC (H→C) | COSY | |||||
|---|---|---|---|---|---|---|---|
| Type | δH (J in Hz) | δC | 2JCH | 3JCH | 4JCH | 1H-1H | |
| 1 | C | 179.8 | |||||
| 2 | CH | 5.76, s | 125.9 | C-5 | C-9 | ||
| 3 | C=O | 202.5 | |||||
| 4 | CH2 | 2.27, m; 2.21, m, overlapped | 43.5 | C-3, C-5 | C-1, C-2, C-6 | ||
| 5 | C | 41.9 | |||||
| 6 | CH2 | 1.23, m; 1.88, m | 35.9 | C-5 | C-1, C-8, C-11 | C-10, C-13 | H-7 |
| 7 | CH | 1.36, m | 43.4 | C-6, C-11 | C-9 | C-4 | H-6, H-8 |
| 8 | CH2 | 1.67, m | 27.6 | C-9 | C-11 | C-1 | H-7, H-9 |
| 9 | CH2 | 2.21, m, overlapped; 2.50, m | 30.6 | C-1, | C-2, C-5, C-6 | H-10, H-8 | |
| 10 | CH | 2.22, overlapped | 36.6 | C-1 | C-2, C-5 | C-3, C-7 | H-14 |
| 11 | C | 73.4 | |||||
| 12 | CH3 | 1.07, s | 26.1 | C-11 | C-6, C-8 | ||
| 13 | CH3 | 1.09, s | 28.1 | C-11 | C-12 | ||
| 14 | CH3 | 0.96, d (6.7) | 16.2 | C-10 | C-5 | H-10 | |
| 15 | CH3 | 1.04, s | 20.1 | C-5 | C-1, C-4, C-6 | ||
3.1.3. Identification of Compound C4
3.1.4. Identification of Compound C5
| C/H# | HSQC | HMBC (H→C) | COSY | ||||
|---|---|---|---|---|---|---|---|
| Type | δH (J in Hz) | δC | 2JCH | 3JCH | 4JCH | 1H-1H | |
| Abscisic alcohol moiety | |||||||
| 1 | C | 82.1 | |||||
| 2 | C | 152.2 | |||||
| 3 | CH | 5.85, 1H, s | 128.0 | C-1, C-13 | |||
| 4 | C | 203.0 | |||||
| 5 | CH2 | 2.11, m; 2.45, m | 51.1 | C-4, C-6 | C-1, C-14, C-15 | ||
| 6 | C | 43.4 | |||||
| 7 | CH | 6.13, d (16.1) | 138.2 | C-1, C-8 | C-2, C-9 | H-7 | |
| 8 | CH | 7.66, d (16.1) | 129.9 | C-7 | C-1, C-10, C-12 | C-2 | H-8 |
| 9 | C | 131.0 | |||||
| 10 | CH | 5.67, br.s | 120.4 | C-9 | C-12 | ||
| 11 | CH2 | 3.45, m a | 62.6 b | C-8 | C-8` | ||
| 12 | CH3 | 1.85, s | 20.2 | C-8, C-10 | C-9` | ||
| 13 | CH3 | 1.95, s | 21.7 | C-2 | C-1, C-3 | ||
| 14 | CH3 | 0.98, s | 24.1 | C-6 | C-1, C-5, C-15 | ||
| 15 | CH3 | 0.94, s | 25.1 | C-6 | C-1, C-5, C-14 | C-4 | |
| 4-(3-Hydroxypropyl)-2-methoxyphenol moiety | |||||||
| 1` | C | 134.4 | |||||
| 2` | CH | 6.49, br. s | 113.7 | C-3` | C-4`, C-7`, C-6` | ||
| 3` | C | 149.3 | |||||
| 4` | C | 146.4 | |||||
| 5` | CH | 6.70, d (7.8) | 116.2 | C-4` | C-3`, C-1` | H-6` | |
| 6` | CH | 6.45, br. d (7.9) | 123.2 | C-4`, C-2` | H-5` | ||
| 7` | CH2 | 2.49, m; 2.65, m | 36.5 | C-1`, C-8` | C-2`, C-6` | H-8` | |
| 8` | CH | 1.84, m | 44.5 | C-9` | H-7`, H-10` | ||
| 9` | C | 169.4 | |||||
| 10` | CH2 | 3.45, dd (8.8, 4.5) a | 62.6 b | C-8` | |||
| O-CH3 | 3.64, s | 56.6 | C-3` | ||||
3.1.5. Identification of Compound C6
3.1.6. Identification of Compound C7
3.2. Biological Activities of Main Fractions and Isolates from E. erinaceus
3.2.1. In Vitro Cytotoxic Activity
3.2.2. In Vitro Antimicrobial Activity
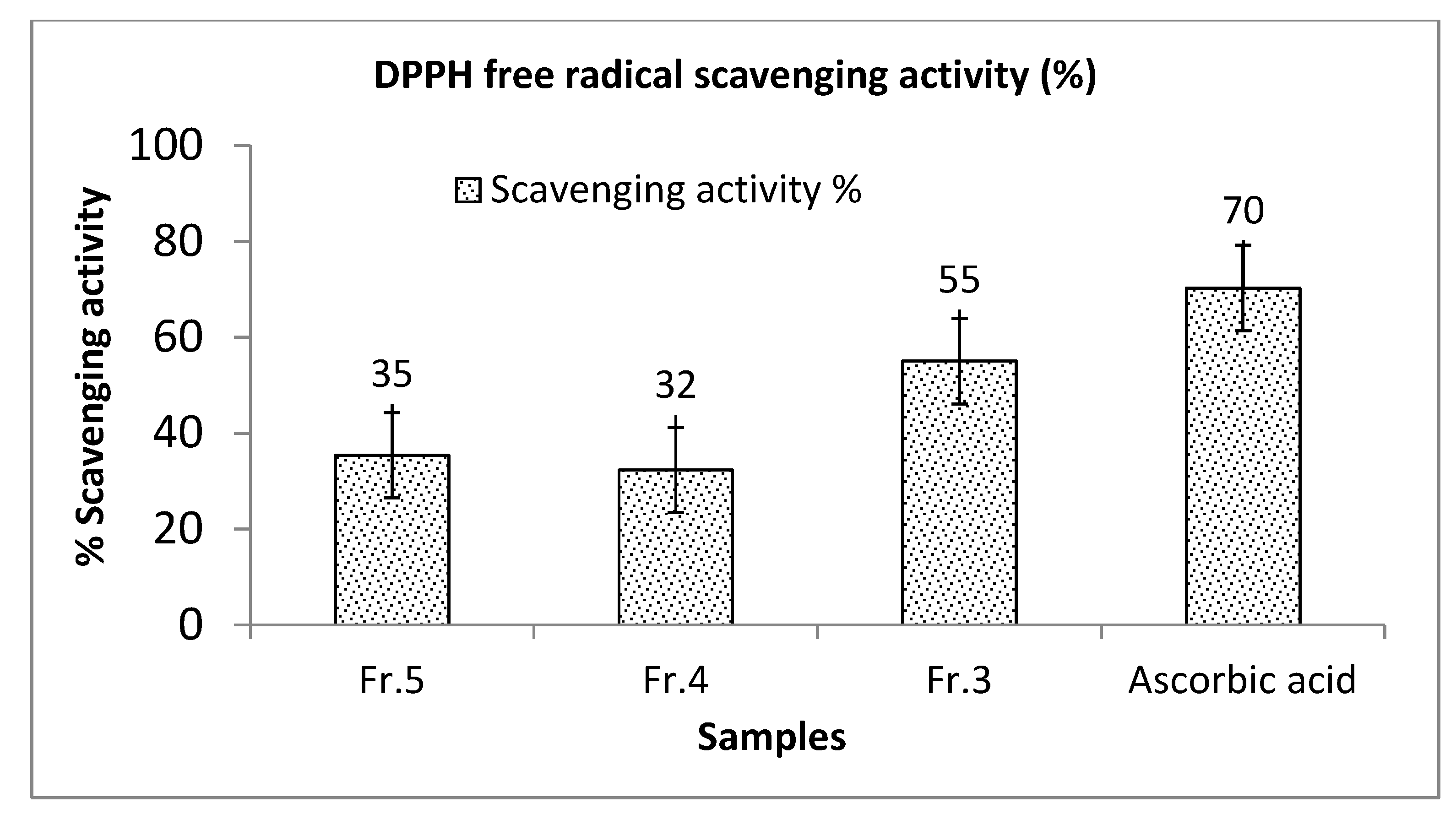
3.2.3. Antioxidant Activity
3.3. PASS and ADME Predictions of the Isolated Compounds
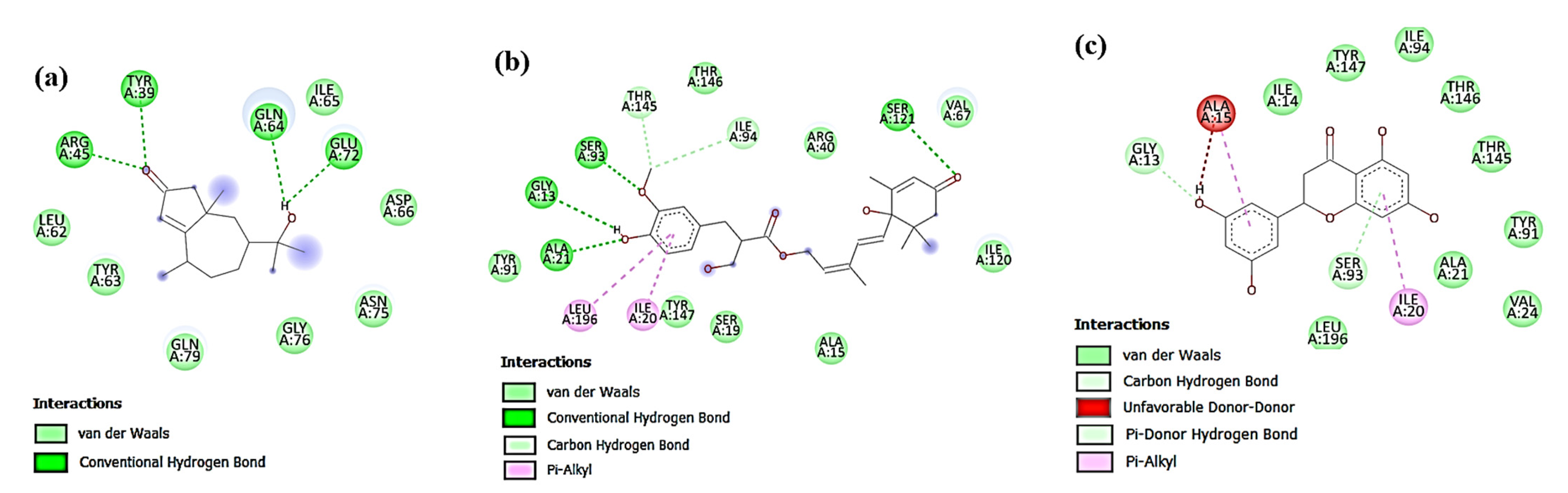
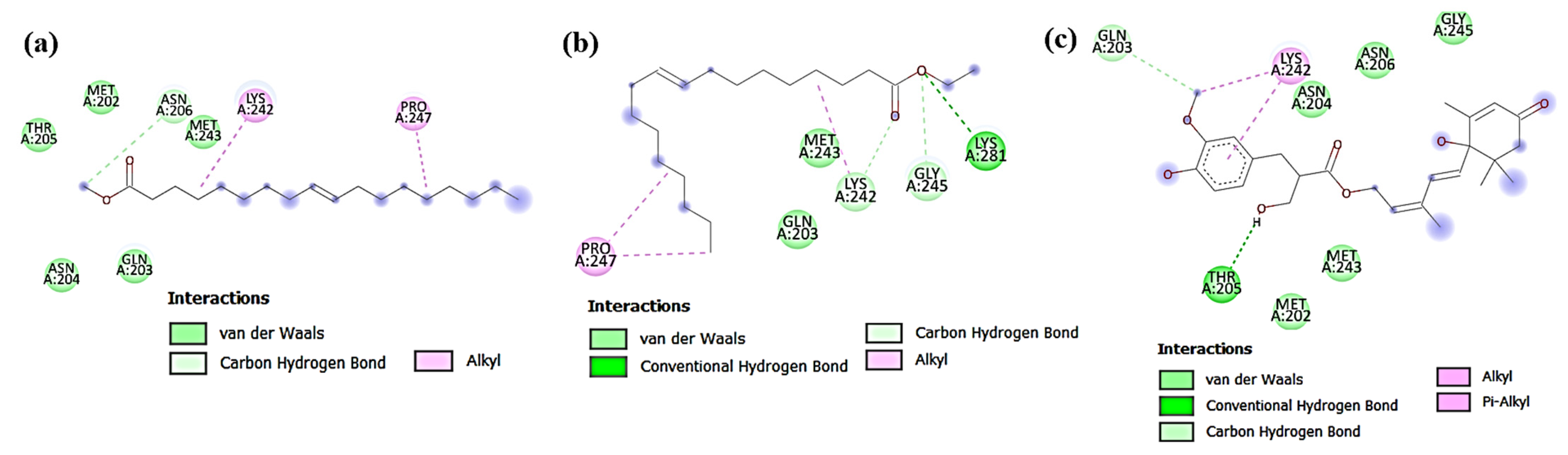
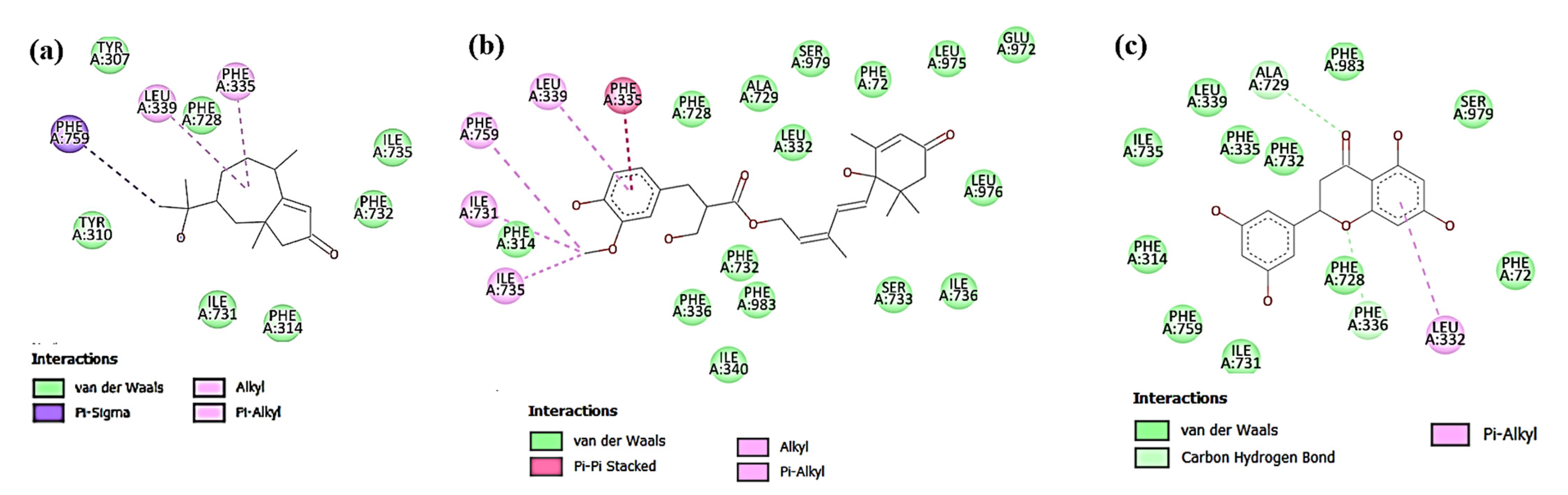
3.4. In Silico Docking Study of the Isolated Compounds
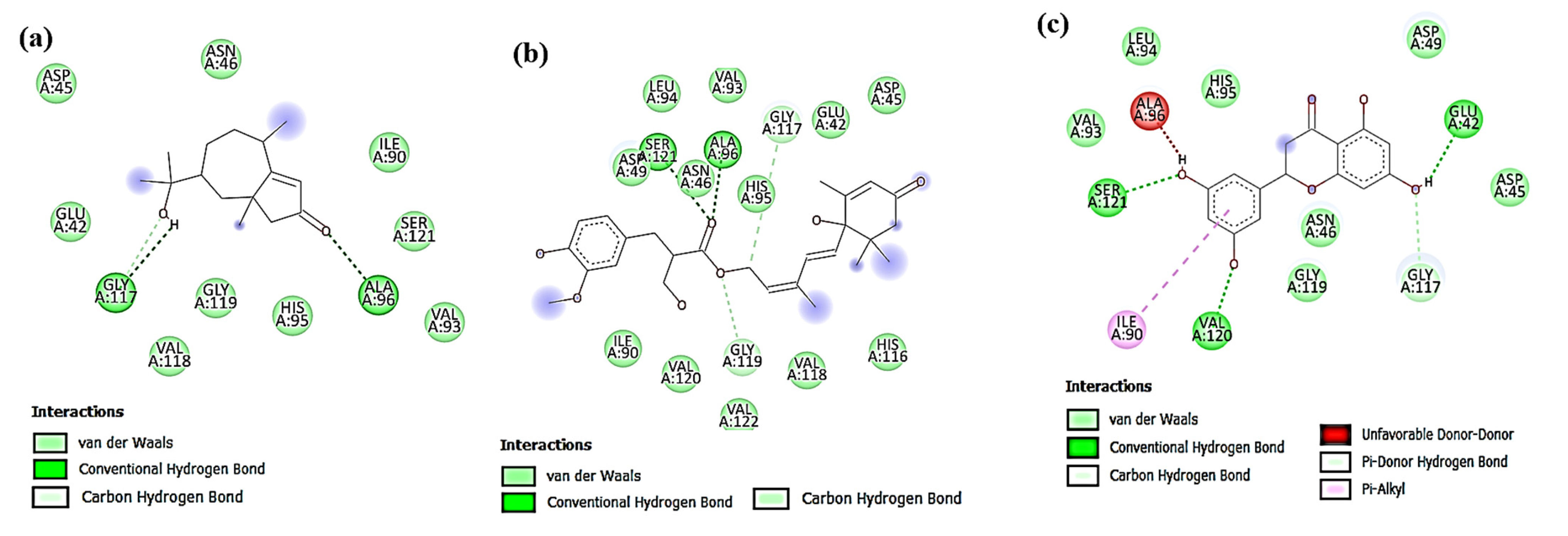
3.4.1. Docking against Antimicrobial Molecular Targets
Interactions with DNA Gyrase Topoisomerase II
Interactions with Enoyl-Acyl Carrier Protein Reductase (FabI)
3.4.2. Docking against Cytotoxic Molecular Targets
Interactions with β-Catenin
Interactions with Human Dihydrofolate Reductase (DHFR)
Interactions with Human P-gp
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senejoux, F.; Demougeot, C.; Karimov, U.; Muyard, F.; Kerram, P.; Aisa, H.A.; Girard-Thernier, C. Chemical constituents from Echinops integrifolius. Biochem. Syst. Ecol. 2013, 47, 42–44. [Google Scholar] [CrossRef]
- Dong, M.; Cong, B.; Yu, S.-H.; Sauriol, F.; Huo, C.-H.; Shi, Q.-W.; Gu, Y.-C.; Zamir, L.O.; Kiyota, H. Echinopines A and B: Sesquiterpenoids Possessing an Unprecedented Skeleton from Echinops spinosus. Org. Lett. 2008, 10, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Khadim, E.J.; Abdulrasool, A.A.; Awad, Z.J. Phytochemical Investigation of Alkaloids in the Iraqi Echinops heterophyllus (Compositae). Iraqi J. Pharm. Sci. 2014, 23, 26–34. [Google Scholar] [CrossRef]
- Kiyekbayeva, L.; Mohamed, N.M.; Yerkebulan, O.; Mohamed, E.I.; Ubaidilla, D.; Nursulu, A.; Assem, M.; Srivedavyasasri, R.; Ross, S.A. Phytochemical constituents and antioxidant activity of Echinops albicaulis. Nat. Prod. Res. 2018, 32, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Qi-Rong, C.; Rong, L.; Guo-Qiang, L.; Hao, H. A new pentacyclic triterpene, gmeliniin A, from Echinops gmelinii Turcz. Chin. J. Chem. 2000, 18, 112–114. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef]
- Bulut, G.; Haznedaroğlu, M.Z.; Doğan, A.; Koyu, H.; Tuzlacı, E. An ethnobotanical study of medicinal plants in Acipayam (Denizli-Turkey). J. Herb. Med. 2017, 10, 64–81. [Google Scholar] [CrossRef]
- Menut, C.; Lamaty, G.; Weyerstahl, P.; Marschall, H.; Seelmann, I.; Amvam Zollo, P.H. Aromatic plants of tropical Central Africa. Part XXXI. Tricyclic sesquiterpenes from the root essential oil of Echinops giganteus var. lelyi C. D. Adams. Flavour Fragr. J. 1997, 12, 415–421. [Google Scholar] [CrossRef]
- Bitew, H.; Hymete, A. The Genus Echinops: Phytochemistry and Biological Activities: A Review. Front. Pharmacol. 2019, 10, 1234. [Google Scholar] [CrossRef]
- Mustafa, B.; Hajdari, A.; Krasniqi, F.; Hoxha, E.; Ademi, H.; Quave, C.L.; Pieroni, A. Medical ethnobotany of the Albanian Alps in Kosovo. J. Ethnobiol. Ethnomed. 2012, 8, 6. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Ezzat, S.M.; El Dine, R.S.; Abdel-Sattar, E.; Abdel-Naim, A.B. Protective effect of Echinops galalensis against CCl4-induced injury on the human hepatoma cell line (Huh7). Phytochem. Lett. 2013, 6, 73–78. [Google Scholar] [CrossRef]
- Sharma, K.S.; Mishra, S.; Mehta, B.K. Antifertility activity of Echinops echinatus in albino rats. Indian J. Med. Sci. 1988, 42, 23–26. [Google Scholar]
- Jin, Q.; Lee, J.W.; Jang, H.; Choi, J.E.; Kim, H.S.; Lee, D.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Dimeric sesquiterpene and thiophenes from the roots of Echinops latifolius. Bioorg. Med. Chem. Lett. 2016, 26, 5995–5998. [Google Scholar] [CrossRef]
- Alam, M.K.; Ahmed, S.; Anjum, S.; Akram, M.; Shah, S.M.; Wariss, H.M.; Usmanghani, K. Evaluation of antipyretic activity of some medicinal plants from Cholistan desert Pakistan. Pak. J. Pharm. Sci. 2016, 29, 529–533. [Google Scholar]
- Nakano, H.; Ali, A.; Ur Rehman, J.; Mamonov, L.K.; Cantrell, C.L.; Khan, I.A. Toxicity of thiophenes from Echinops transiliensis (Asteraceae) against Aedes aegypti (Diptera: Culicidae) larvae. Chem. Biodivers. 2014, 11, 1001–1009. [Google Scholar] [CrossRef]
- Radulović, N.S.; Denić, M.S. Essential oils from the roots of Echinops bannaticus Rochel ex Schrad. and Echinops sphaerocephalus L. (Asteraceae): Chemotaxonomic and biosynthetic aspects. Chem. Biodivers. 2013, 10, 658–676. [Google Scholar] [CrossRef]
- Sweilam, S.H.; Abdel Bar, F.M.; ElGindi, O.D.; El- Sherei, M.M.; Abdel-Sattar, E.A. Chemical and In Vitro Anti-inflammatory Assessment of Echinops erinaceus. Trop. J. Nat. Prod. Res. 2021, 5, 715–719. [Google Scholar]
- Gillet, J.-P.; Gottesman, M.M. Mechanisms of multidrug resistance in cancer. In Multi-Drug Resistance in Cancer; Springer: Berlin/Heidelberg, Germany, 2010; pp. 47–76. [Google Scholar]
- Wróbel, A.; Eklund, P.; Bobrowska-Hägerstrand, M.; Hägerstrand, H. Lignans and norlignans inhibit multidrug resistance protein 1 (MRP1/ABCC1)-mediated transport. Anticancer Res. 2010, 30, 4423–4428. [Google Scholar]
- Luo, L.; Yang, J.; Wang, C.; Wu, J.; Li, Y.; Zhang, X.; Li, H.; Zhang, H.; Zhou, Y.; Lu, A.; et al. Natural products for infectious microbes and diseases: An overview of sources, compounds, and chemical diversities. Sci. China Life Sci. 2022, 65, 1123–1145. [Google Scholar] [CrossRef]
- Marques, S.M.; Šupolíková, L.; Molčanová, L.; Šmejkal, K.; Bednar, D.; Slaninová, I. Screening of Natural Compounds as P-Glycoprotein Inhibitors against Multidrug Resistance. Biomedicines 2021, 9, 357. [Google Scholar] [CrossRef]
- Bhabha, G.; Ekiert, D.C.; Jennewein, M.; Zmasek, C.M.; Tuttle, L.M.; Kroon, G.; Dyson, H.J.; Godzik, A.; Wilson, I.A.; Wright, P.E. Divergent evolution of protein conformational dynamics in dihydrofolate reductase. Nat. Struct. Mol. Biol 2013, 20, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Aziz, A.S.; El-Ghezlani, E.G.; Elaasser, M.M.; Afifi, T.H.; Okasha, R.M. First example of cationic cyclopentadienyliron based chromene complexes and polymers: Synthesis, characterization, and biological applications. J. Inorg. Organomet. Polym. Mater. 2020, 30, 131–146. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and Anticancer Activities of Thiazoles, 1,3-Thiazines, and Thiazolidine Using Chitosan-Grafted-Poly(vinylpyridine) as Basic Catalyst. Heterocycles 2015, 91, 1227–1243. [Google Scholar]
- Mishra, V.; Prasad, D.N. Application of in vitro methods for selection of Lactobacillus casei strains as potential probiotics. Int. J. Food Microbiol. 2005, 103, 109–115. [Google Scholar] [CrossRef]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Foudah, A.I.; Alqarni, M.H.; Alam, A.; Salkini, M.A.; Ross, S.A.; Yusufoglu, H.S. Phytochemical Screening, In Vitro and In Silico Studies of Volatile Compounds from Petroselinum crispum (Mill) Leaves Grown in Saudi Arabia. Molecules 2022, 27, 934. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lafitte, D.; Lamour, V.; Tsvetkov, P.O.; Makarov, A.A.; Klich, M.; Deprez, P.; Moras, D.; Briand, C.; Gilli, R. DNA gyrase interaction with coumarin-based inhibitors: The role of the hydroxybenzoate isopentenyl moiety and the 5′-methyl group of the noviose. Biochemistry 2002, 41, 7217–7223. [Google Scholar] [CrossRef]
- Priyadarshi, A.; Kim, E.E.; Hwang, K.Y. Structural insights into Staphylococcus aureus enoyl-ACP reductase (FabI), in complex with NADP and triclosan. Proteins 2010, 78, 480–486. [Google Scholar] [CrossRef]
- Kessler, D.; Mayer, M.; Zahn, S.K.; Zeeb, M.; Wöhrle, S.; Bergner, A.; Bruchhaus, J.; Ciftci, T.; Dahmann, G.; Dettling, M.; et al. Getting a Grip on the Undrugged: Targeting β-Catenin with Fragment-Based Methods. ChemMedChem 2021, 16, 1420–1424. [Google Scholar] [CrossRef]
- Khatun, M.; Muhit, M.; Hossain, M.J.; Al-Mansur, M.; Rahman, S.M. Isolation of phytochemical constituents from Stevia rebaudiana (Bert.) and evaluation of their anticancer, antimicrobial and antioxidant properties via in vitro and in silico approaches. Heliyon 2021, 7, e08475. [Google Scholar] [CrossRef]
- Chira, N.; Nicolescu, A.; Raluca, S.; Rosca, S. Fatty Acid Composition of Vegetable Oils Determined from 13C-NMR Spectra. Rev. Chim. (Bucharest) 2016, 67, 1257–1263. [Google Scholar]
- Di Pietro, M.E.; Mannu, A.; Mele, A. NMR Determination of Free Fatty Acids in Vegetable Oils. Processes 2020, 8, 410. [Google Scholar] [CrossRef]
- Hymete, A.; Rohloff, J.; Kjøsen, H.; Iversen, T.-H. Acetylenic thiophenes from the roots of Echinops ellenbeckii from Ethiopia. Nat. Prod. Res. 2005, 19, 755–761. [Google Scholar] [CrossRef]
- Lao, A.; Fujimoto, Y.; Tatsuno, T. Studies on the Constituents of Artemisia argyi LEVL et VANT. Chem. Pharm. Bull. 1984, 32, 723–727. [Google Scholar] [CrossRef]
- Atta-Ur-Rahman; Ahmad, V.U. 13C-NMR of Natural Prodacts: Volume 1 Monoterpenes and Sesquiterpenes, 1st ed.; Springer: Boston, MA, USA, 1992; Volume 1, pp. X–966. [Google Scholar]
- Yuan, Z.; Zheng, X.; Zhao, Y.; Liu, Y.; Zhou, S.; Wei, C.; Hu, Y.; Shao, H. Phytotoxic Compounds Isolated from Leaves of the Invasive Weed Xanthium spinosum. Molecules 2018, 23, 2840. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Martins, A.; Susano, P.; Simões, M.; Guedes, M.; Rehfeldt, S.; Pinteus, S.; Gaspar, H.; Rodrigues, A.; et al. Loliolide, a New Therapeutic Option for Neurological Diseases? In Vitro Neuroprotective and Anti-Inflammatory Activities of a Monoterpenoid Lactone Isolated from Codium tomentosum. Int. J. Mol. Sci. 2021, 22, 1888. [Google Scholar] [CrossRef]
- Milborrow, B.V. The conformation of abscisic acid by NMR. and a revision of the proposed mechanism for cyclization during its biosynthesis. Biochem. J. 1984, 220, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Andrade, P.; Tomás-Barberán, F.A. Natural Occurrence of Abscisic Acid in Heather Honey and Floral Nectar. J. Agric. Food Chem. 1996, 44, 2053–2056. [Google Scholar] [CrossRef]
- Frackenpohl, J.; Grill, E.; Bojack, G.; Baltz, R.; Busch, M.; Dittgen, J.; Franke, J.; Freigang, J.; Gonzalez, S.; Heinemann, I.; et al. Insights into the in Vitro and in Vivo SAR of Abscisic Acid—Exploring Unprecedented Variations of the Side Chain via Cross-Coupling-Mediated Syntheses. Eur. J. Org. Chem. 2018, 2018, 1403–1415. [Google Scholar] [CrossRef]
- Jo, M.S.; Lee, S.; Yu, J.S.; Baek, S.C.; Cho, Y.-C.; Kim, K.H. Megastigmane Derivatives from the Cladodes of Opuntia humifusa and Their Nitric Oxide Inhibitory Activities in Macrophages. J. Nat. Prod. 2020, 83, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.A.; Agoo, E.M.; Shen, C.-C.; Ragasa, C. Chemical Constituents of Cycas aenigma. J. Appl. Pharm. Sci 2015, 5, 32–36. [Google Scholar] [CrossRef]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2016, 25, 631–641. [Google Scholar] [CrossRef]
- Nessa, F.; Ismail, Z.; Mohamed, N. Xanthine oxidase inhibitory activities of extracts and flavonoids of the leaves of Blumea balsamifera. Pharm. Biol. 2010, 48, 1405–1412. [Google Scholar] [CrossRef]
- Ayyad, S.E.; Abdel-Lateff, A.; Alarif, W.M.; Patacchioli, F.R.; Badria, F.A.; Ezmirly, S.T. In vitro and in vivo study of cucurbitacins-type triterpene glucoside from Citrullus colocynthis growing in Saudi Arabia against hepatocellular carcinoma. Environ. Toxicol. Pharmacol. 2012, 33, 245–251. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Bojarska, J.; Remko, M.; Breza, M.; Madura, I.D.; Kaczmarek, K.; Zabrocki, J.; Wolf, W.M. A Supramolecular Approach to Structure-Based Design with A Focus on Synthons Hierarchy in Ornithine-Derived Ligands: Review, Synthesis, Experimental and in Silico Studies. Molecules 2020, 25, 1135. [Google Scholar] [CrossRef]
- Kandsi, F.; Elbouzidi, A.; Lafdil, F.Z.; Meskali, N.; Azghar, A.; Addi, M.; Hano, C.; Maleb, A.; Gseyra, N. Antibacterial and Antioxidant Activity of Dysphania ambrosioides (L.) Mosyakin and Clemants Essential Oils: Experimental and Computational Approaches. Antibiotics 2022, 11, 482. [Google Scholar] [CrossRef]
- He, S.; Tang, S. WNT/β-catenin signaling in the development of liver cancers. Biomed. Pharmacother. 2020, 132, 110851. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.H.; Chen, Z.S. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist. Updates 2016, 27, 14–29. [Google Scholar] [CrossRef]
- Guzel, A.; Aksit, H.; Elmastas, M.; Erenler, R. Bioassay-guided isolation and identification of antioxidant flavonoids from Cyclotrichium origanifolium (Labill.) Manden and Scheng. Pharmacogn. Mag. 2017, 13, 316–320. [Google Scholar] [CrossRef]
- Hussein, N.; Amen, Y.; Abdel Bar, F.; Halim, A.; Saad, H.-E. Antioxidants and α-Glucosidase Inhibitors from Lactuca serriola L. Rec. Nat. Prod. 2020, 14, 410–415. [Google Scholar] [CrossRef]

| Test Sample | IC50 (µg/mL) | CC50 (µg/mL) | Selectivity Index (SI) | ||
|---|---|---|---|---|---|
| HCT-116 a | CACO2 a | WI-38 a | HCT-116 | CACO2 | |
| Extract | |||||
| Total MeOH | 165.92 ± 9.82 | 192.82 ± 12.86 | 226.14 ± 11.82 | 1.36 | 1.17 |
| n-Hex | 88.91 ± 5.42 | 87.93 ± 4.89 | 110.79 ± 7.43 | 1.25 | 1.26 |
| CHCl3 | 67.30 ± 4.87 ʺ | 81.95 ± 4.63 ʺ | 116.53 ± 9.27 | 1.73 | 1.42 |
| EtOAc | 170.84 ± 10.29 | 218.72 ± 11.04 | 246.41 ± 14.23 | 1.44 | 1.13 |
| Re. Aq | 323.25 ± 15.83 | 361.08 ± 18.24 | 449.72 ± 21.34 | 1.39 | 1.25 |
| * CHCl3 Fractions | |||||
| * Fr.1 | 14.93 ± 1.28 ʺ | 10.50 ± 0.61 ʺ | 50.36 ± 3.80 | 3.37 | 4.80 |
| * Fr.3 | 30.94 ± 1.78 ʺ | 38.9 ± 1.89 ʺ | 60.62 ± 3.42 | 1.96 | 1.56 |
| * Fr.4 | 24.93 ± 1.29 ʺ | 12.95 ± 0.61 ʺ | 53.24 ± 3.08 | 2.14 | 4.11 |
| * Fr.5 | 83.41 ± 4.03 | 101.78 ± 4.08 | 117.64 ± 6.72 | 1.41 | 1.16 |
| * Fr.6 | 54.43 ± 2.19 | 59.85 ± 2.73 | 105.31 ± 4.93 | 1.93 | 1.76 |
| Compounds | |||||
| C1/C2 | 24.95 ± 1.23 ʺ | 19.74 ± 1.94 ʺ | 48.75 ± 3.91 | 1.95 | 2.47 |
| C3 | 82.82 ± 3.94 ʺ | 99.09 ± 5.84 ʺ | 178.02 ± 8.74 | 2.15 | 1.80 |
| C4 | 173.12± 9.74 | 217.25 ± 8.73 | 272.93 ± 16.25 | 1.58 | 1.26 |
| C5 | 76.70 ± 3.71 ʺ | 87.27 ± 4.67 ʺ | 162.84 ± 7.08 | 2.12 | 1.87 |
| C6 | 179.81 ± 14.08 | 425.48 ± 16.71 | 416.52 ± 18.96 | 2.32 | 0.98 |
| C7 | 219.35 ± 9.76 | 284.73 ± 14.93 | 382.53 ± 17.21 | 1.74 | 1.34 |
| Vin b | 2.35 ± 0.41 | 2.62 ± 0.44 | 13.98 ± 1.34 | 5.95 | 5.34 |
| Bacterial Isolates | Gram-Positive | Gram-Negative | Fungi and Yeast | |||
|---|---|---|---|---|---|---|
| B. subtilus (a) | MRSA(a) | P. aeruginosa (a) | E. coli (a) | C. albicans (b) | A. niger (b) | |
| ATCC6633 | ATCC25923 | ATCC27953 | ATCC25922 | NRRLY477 | NRRL599 | |
| MeOH ext. | 27.5 ± 0.7 | - | 23.5± 0.7 | 24 ± 1.41 | 26 ± 1.41 | 16 ± 1.41 |
| n-hex ext. | 22.5 ± 0.7 | - | 22 ± 2.82 | 22.25 ± 1.76 | 22.5 ± 2.82 | - |
| CHCl3 ext. | 20.5 ± 1.41 | - | 17.5 ± 1.41 | 18 ± 1.41 | - | - |
| EtOAc ext. | 20.0 ± 1.41 | - | 22 ± 1.41 | 21.5 ± 0.7 | 22 ± 1.41 | 12.5 ± 0.7 |
| Re. Aq. ext. | 18.5 ± 2.12 | - | 17 ± 1.41 | 20.5 ± 2.12 | 18 ± 1.41 | - |
| * Fr.3 | 17.5± 0.7 | - | 16 ± 1.41 | 17.25 ± 2.82 | - | - |
| * Fr.4 * | 14.5 ± 2.12 | - | 14 ± 0.71 | 16 ± 0.7 | - | - |
| * Fr.5 * | 17 ± 1.41 | - | 14 ± 1.41 | 14.5 ± 0.7 | - | -- |
| Streptomycin a | 18 ± 1.41 | 20 ± 1.41 | 27 ± 1.41 | 25 ± 2.82 | - | - |
| Clotrimazole b | - | - | - | - | 28 ± 2.82 | 26 ± 1.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sweilam, S.H.; Abdel Bar, F.M.; Foudah, A.I.; Alqarni, M.H.; Elattal, N.A.; El-Gindi, O.D.; El-Sherei, M.M.; Abdel-Sattar, E. Phytochemical, Antimicrobial, Antioxidant, and In Vitro Cytotoxicity Evaluation of Echinops erinaceus Kit Tan. Separations 2022, 9, 447. https://doi.org/10.3390/separations9120447
Sweilam SH, Abdel Bar FM, Foudah AI, Alqarni MH, Elattal NA, El-Gindi OD, El-Sherei MM, Abdel-Sattar E. Phytochemical, Antimicrobial, Antioxidant, and In Vitro Cytotoxicity Evaluation of Echinops erinaceus Kit Tan. Separations. 2022; 9(12):447. https://doi.org/10.3390/separations9120447
Chicago/Turabian StyleSweilam, Sherouk Hussein, Fatma M. Abdel Bar, Ahmed I. Foudah, Mohammed H. Alqarni, Nouran A. Elattal, Omayma D. El-Gindi, Moshera M. El-Sherei, and Essam Abdel-Sattar. 2022. "Phytochemical, Antimicrobial, Antioxidant, and In Vitro Cytotoxicity Evaluation of Echinops erinaceus Kit Tan" Separations 9, no. 12: 447. https://doi.org/10.3390/separations9120447
APA StyleSweilam, S. H., Abdel Bar, F. M., Foudah, A. I., Alqarni, M. H., Elattal, N. A., El-Gindi, O. D., El-Sherei, M. M., & Abdel-Sattar, E. (2022). Phytochemical, Antimicrobial, Antioxidant, and In Vitro Cytotoxicity Evaluation of Echinops erinaceus Kit Tan. Separations, 9(12), 447. https://doi.org/10.3390/separations9120447