Antioxidant, Anti-Cancer Activity and Phytochemicals Profiling of Kigelia pinnata Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of N, P, and K
2.3. Determination of Trace Element Levels
2.3.1. Microwave Digestion
2.3.2. Determination of Trace Element Levels by ICP-OES
2.4. Proximate Analysis
2.5. Determination of Total Phenolic Content
2.6. Determination of Total Flavonoid Content
2.7. Determination of Total Anthocyanin Content
2.8. Determination of Total Condensed Tannins Content
2.9. Fe+2 Ion Reducing Power
2.10. Determination of Total Antioxidant Capacity
2.11. Scavenging of DPPH Radical
- As = absorbance of sample;
- Ac = absorbance of control (DPPH radical solution in ethanol).
2.12. Extraction of Samples
2.13. Gas Chromatography–Mass Spectrometry (GC-MS) Qualitative Identification
2.14. Ultra-High-Performance Liquid Chromatography–Mass Spectrometry (UPLC-MS/MS) Identification of Phytochemicals Compounds
2.15. Cell Culture
2.16. Cytotoxicity Assay
2.17. Statistical Analysis
3. Results
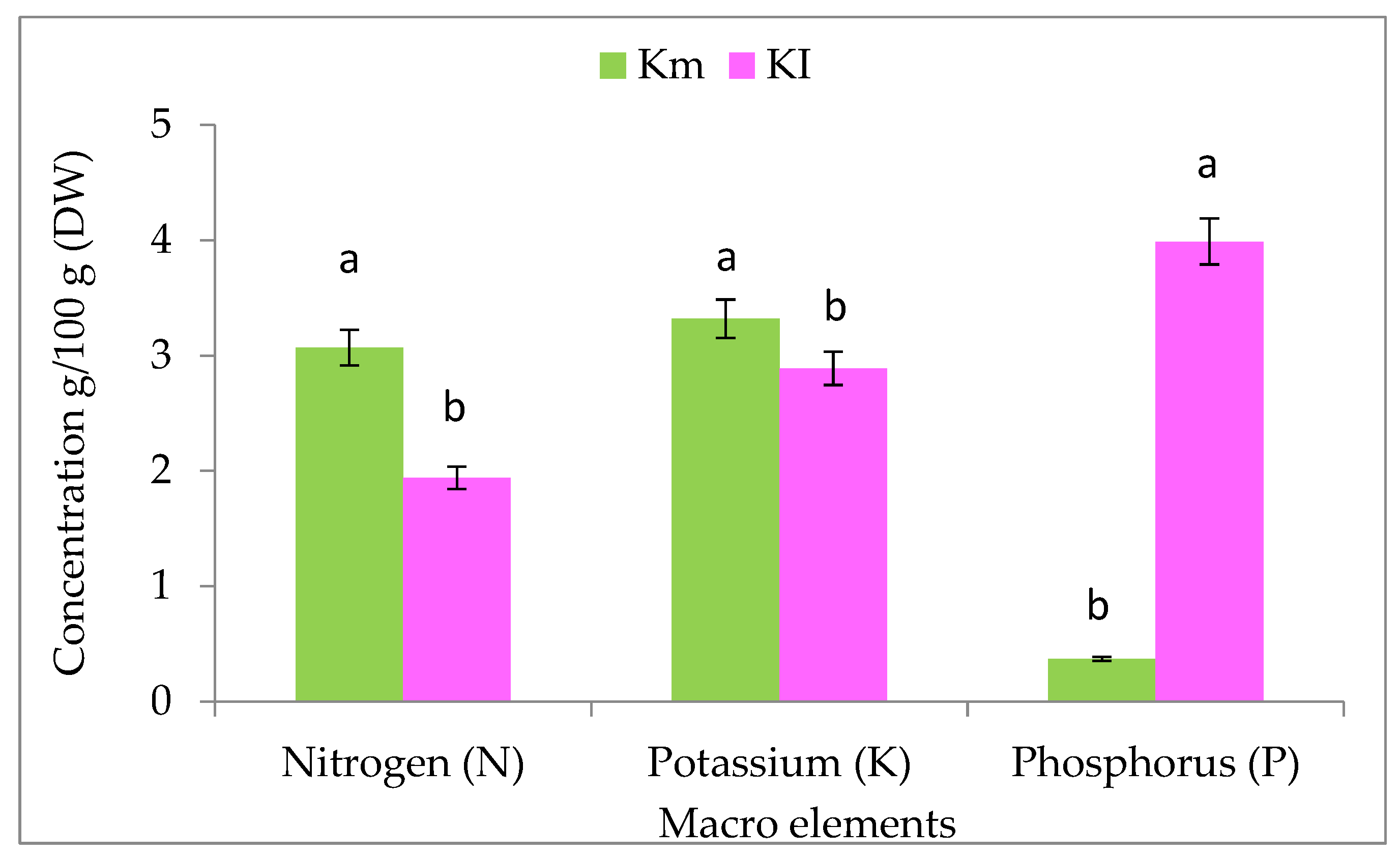
3.1. Macro- and Microelements in K. pinnata Fruits
3.2. Proximate Analysis
3.3. Secondary Metabolites
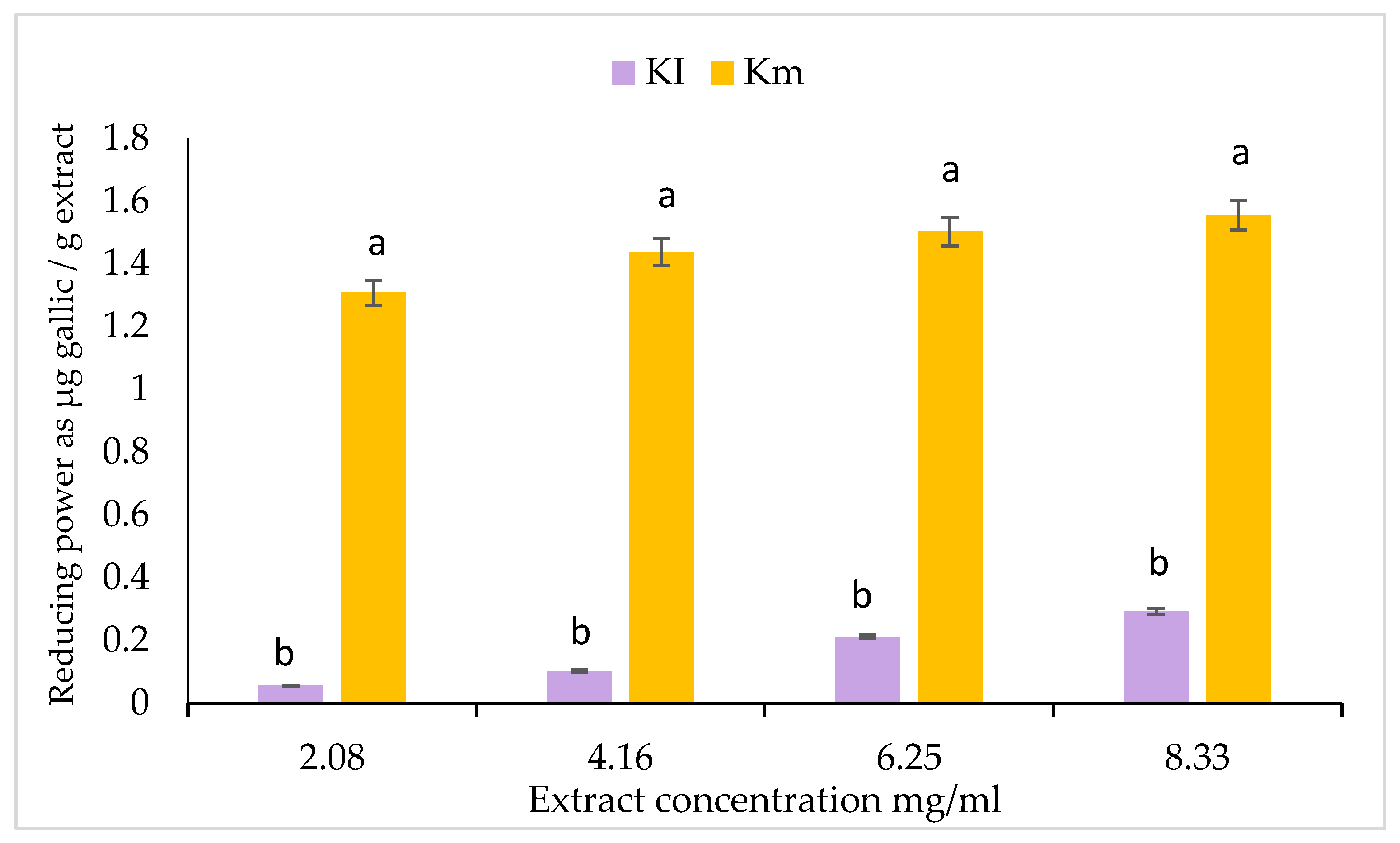
3.4. Fe+2 ion Reducing Power
3.5. Antioxidant Capacity
3.6. DPPH Free Radical Scavenging Activity
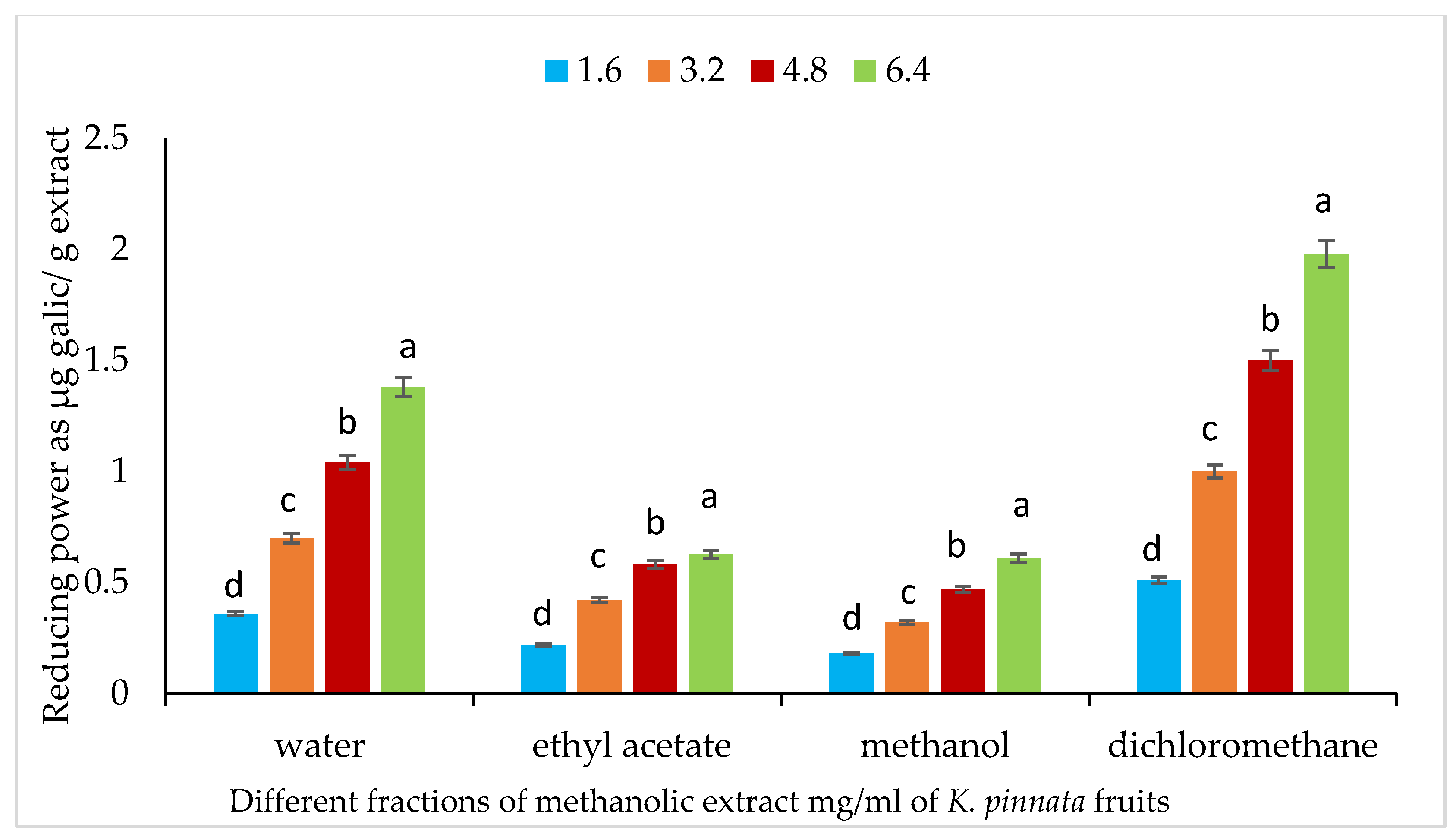
3.7. Fe+2 Ion Reducing Power of Different Fractions
3.8. Antioxidant Capacity of Different Fractions
3.9. DPPH-Scavenging Activity of Different Fractions
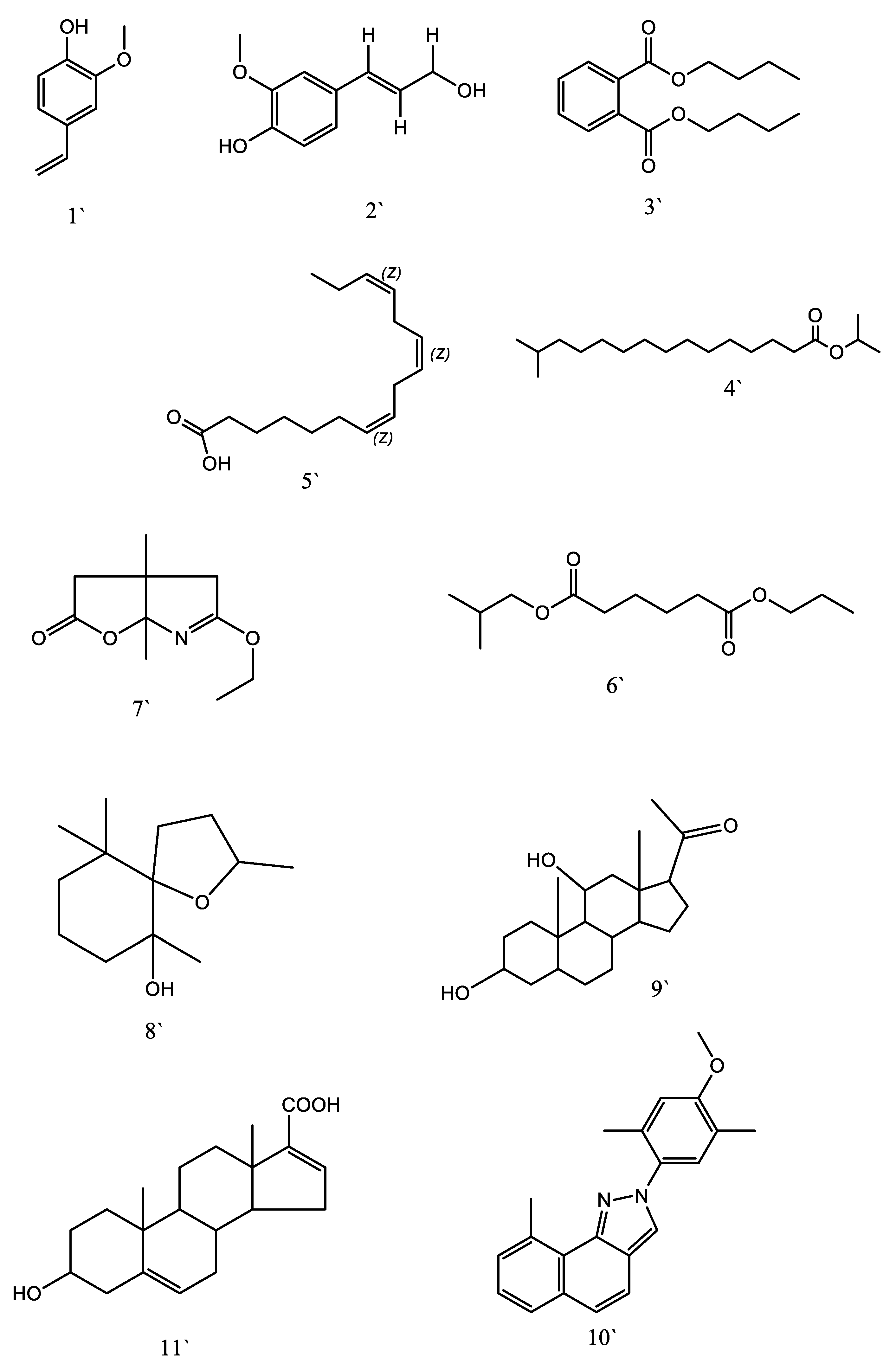
3.10. GC-MS Qualitative Identification
3.11. KI (Kovat’s Index) Determined Experimentally Relative to C7–C30 n-alkanes
3.12. UPLC-MS/MS Identification of Phytochemicals Compounds
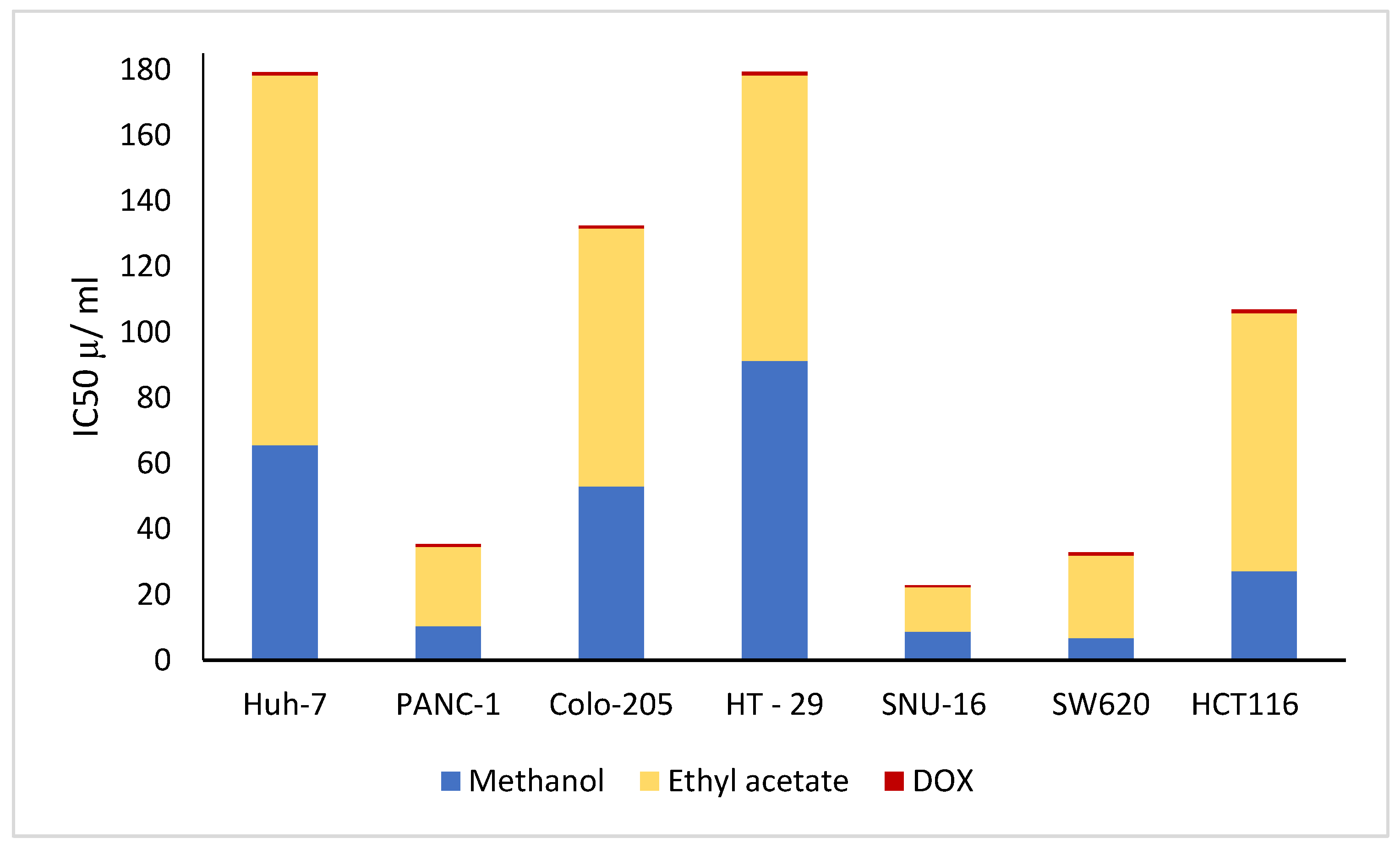
3.13. Anticancer Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reyes-Munguía, A.; Carrillo-Inungaray, M.L.; Carranza-Álvarez, C.; Pimentel-González, D.J.; Alvarado-Sánchez, B. Antioxidant activity, antimicrobial and effects in the immune system of plants and fruits extracts. Front. Life Sci. 2016, 9, 90–98. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Abdelazeem, A.S.; Youssef, R.; Safwat, G. GC-MS analysis, antioxidant, antimicrobial and anticancer activities of extracts from Ficus sycomorus fruits and leaves. Not. Bot. Horti Agrobot. Cluj Napoca 2019, 47, 493–505. [Google Scholar] [CrossRef]
- Hamed, M.M.; Abd El-Mobdy, M.A.; Kamel, M.T.; Mohamed, H.I.; Bayoumi, A.E. Phytochemical and biological activities of two asteraceae plants Senecio vulgaris and Pluchea dioscoridis L. PharmacologyOnline 2019, 2, 101–121. [Google Scholar]
- Romeilah, R.M.; El-Beltagi, H.S.; Shalaby, E.A.; Younes, K.M.; El Moll, H.; Rajendrasozhan, S.; Mohamed, H.I. Antioxidant and cytotoxic activities of Artemisia monosperma L. and Tamarix aphylla essential oils. Not. Bot. Horti Agrobot. Cluj Napoca 2021, 9, 12233. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Aldaej, M.I.; Al-Khayri, J.M.; Rezk, A.A.; Al-Mssallem, M.Q.; Sattar, M.N.; Ramadan, K.M.A. Production and antioxidant activity of secondary metabolites in Hassawi rice (Oryza sativa L.) cell suspension under salicylic acid, yeast extract, and pectin elicitation. Vitr. Cell. Dev. Biol. Plant 2022, 58, 615–629. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Eshak, N.S.; Mohamed, H.I.; Bendary, E.S.A.; Danial, A.W. Physical characteristics, minerals content, antioxidants and antibacterial activities of Punica granatum or Citrus sinensis peel extracts and their applications to improve cake quality. Plants 2022, 11, 1740. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.R.; El-Beltagi, H.S.; Aly, A.A.; El-Ansary, A.E. Antioxidant enzyme activities and lipid peroxidation as biomarker for potato tuber stored by two essential oils from Caraway and Clove and its main component carvone and eugenol. Asian Pac. J. Trop. Biomed. 2012, 2, S772–S780. [Google Scholar] [CrossRef]
- Elkatry, H.O.; Ahmed, A.R.; El-Beltagi, H.S.; Mohamed, H.I.; Eshak, N.S. Biological activities of grape seed by-products and their potential use as natural sources of food additives in the production of Balady bread. Foods 2022, 11, 1948. [Google Scholar] [CrossRef]
- Abdel-Rahim, E.A.; El-Beltagi, H.S. Constituents of apple, parsley and lentil edible plants and their therapy treatments for blood picture as well as liver and kidney functions against lipidemic disease. Elec. J. Environ. Agricult. Food Chem. 2010, 9, 1117–1127. [Google Scholar]
- Dalmartello, M.; La Vecchia, C.; Bertuccio, P.; Boffetta, P.; Levi, F.; Negri, E.; Malvezzi, M. European cancer mortality predictions for the year 2022 with focus on ovarian cancer. Ann. Oncol. 2021, 33, 330–339. [Google Scholar] [CrossRef]
- Wekha, G.; Ssewante, N.; Iradukunda, A.; Jurua, M.; Nalwoga, S.; Lanyero, S.; Olum, R.; Bongomin, F. Colorectal cancer in Uganda: A 10-year, facility-based, retrospective study. Cancer Manag. Res. 2021, 13, 7697–7707. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Omara, T.; Odero, M.P.; Obakiro, S.B. Medicinal plants used for treating cancer in Kenya: An ethnopharmacological overview. Bull. Natl. Res. Cent. 2022, 46, 1–33. [Google Scholar] [CrossRef]
- Mohamed, A.A.; El-Beltagi, H.S.; Rashed, M.M. Cadmium stress induced change in some hydrolytic enzymes, free radical formation and ultrastructural disorders in radish plant. Elec. J. Environ. Agric. Food Chem. 2009, 8, 969–983. [Google Scholar]
- Omara, T.; Kiprop, A.K.; Ramkat, R.C.; Cherutoi, J.; Kagoya, S.; Moraa Nyangena, D.; Chepkemoi Koske, M. Medicinal plants used in traditional management of cancer in Uganda: Ethnobotanical surveys, phytochemistry and anticancer studies. Evid. Based Complement. Altern. Med. 2020, 2020, 3529081. [Google Scholar] [CrossRef]
- El-desoky, A.H.; Abdel-Rahman, R.F.; Ahmed, O.K.; El-Beltagi, H.S.; Hattori, M. Anti-inflammatory and antioxidant activities of naringin isolated from Carissa carandas L.: In vitro and in vivo evidence. Phytomedicine 2018, 42, 126–134. [Google Scholar] [CrossRef]
- Joffe, P. Kigelia africana (Lam) Benth. Pretoria National Botanical Garden. Available online: www.plantzafrica.com (accessed on 19 June 2009).
- Jackson, S.; Beckett, K. Sausage Tree Kigelia pinnta: An Ethnobotanical and Scientific Review. Am. Bot. Counc. United States 2012, 94, 48–59. Available online: https://www.herbalgram.org/resources/herbalgram/issues/94/table-of-contents/feat_sausagetree/ (accessed on 19 June 2009).
- Gabriel, O.A.; Olubunmi, A. Comprehensive scientific demystification of Kigelia africana: A review. Afr. J. Pure Appl. Chem. 2009, 3, 158–164. [Google Scholar]
- Oyelami, O.A.; Yusuf, K.O.; Oyelami, A.O. The use of Kigelia africana in the management of polycysticovary syndrome (PCOS). Chin. Med. 2012, 3, 1–3. [Google Scholar] [CrossRef]
- Kumawat, R.B.; Mali, P.C.; Sharma, R.A. Ethanomedicine and pharmacological activities of five traditionally used in Indian medicinal plants: A review. Adv. Pharmacol. Toxicol. 2015, 16, 45–56. [Google Scholar]
- Bello, I.; Shehu, M.; Musa, M.; Asmawi, M.; Mahmud, R. Kigelia africana (Lam.) Benth. (Sausage tree): Phytochemistry and pharmacological review of a quintessential African traditional medicinal plant. J. Ethnopharmacol. 2016, 189, 253–276. [Google Scholar] [CrossRef]
- Nabatanzi, A.; Nkadimeng, S.M.; Lall, N.; Kabasa, J.D.; McGaw, L.J. Antioxidant and anti-inflammatory activities of Kigelia Africana (Lam.) Benth. Evid. Based Complement. Altern. Med. 2020, 2020, 4352084. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.G.; Ali, Z.; Chittiboyina, A.G.; Khan, I.A. Kigelia africana fruit: Constituents, bioactivity, and reflection on composition disparities. World J. Tradit. Chin. Med. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Atawodi, S.E.O.; Olowoniyi, O.D. Pharmacological and Therapeutic Activities of Kigelia africana (Lam.) Benth. Ann.Res. Rev. Biol. 2015, 5, 1–17. [Google Scholar] [CrossRef]
- Imran, I.Z.; Elusiyan, C.A.; Agbedahunsi, J.M.; Omisore, N.O. Bioactivity-directed evaluation of fruit of Kigelia africana (Lam.) Benth. Used in treatment of malaria in Iwo, Nigeria. J. Ethnopharmacol. 2021, 268, 113680. [Google Scholar] [CrossRef] [PubMed]
- Fagbohun, O.F.; Joseph, J.S.; Salami, O.A.; Msagati, T.A.M. Exploration of modern chromatographic methods coupled to mass spectrometric techniques for trace element and chemical composition analyses in the leaf extracts of Kigelia africana. Biol. Trace Elem. Res. 2021, 199, 1633–1648. [Google Scholar] [CrossRef]
- Gouda, Y.G.; Abdel-baky, A.M.; Darwish, F.M.; Mohamed, K.M.; Kasai, R.; Yamasaki, K. Iridoids from Kigelia pinnata DC. fruits. Phytochemistry 2003, 63, 887–892. [Google Scholar] [CrossRef]
- Costa, R.; Albergamo, A.; Pellizzeri, V.; Dugo, G. Phytochemical screening by LC-MS and LC-PDA of ethanolic extracts from the fruits of Kigelia africana (Lam.) benth. Nat. Prod. Res. 2017, 31, 1397–1402. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Marin, B.; Chopin, E.I.B.; Jupinet, B.; Gauthier, D. Comparison of microwave-assisted digestion procedures for total trace element content determination in calcareous soils. Talanta 2008, 77, 282–288. [Google Scholar] [CrossRef]
- Jayaraman, J. Postharvest Biological Control; Wiely Eastern Limited: New Delhi, India, 1985. [Google Scholar]
- Tobaruela, E.D.C.; Santos, A.D.O.; de Almeida-Muradian, L.B.; Araujo, E.D.S.; Lajolo, F.M.; Menezes, E.W. Application of dietary fiber method AOAC 2011.25 in fruit and comparison with AOAC 991.43 method. Food Chem. 2018, 238, 87–93. [Google Scholar] [CrossRef]
- Goffman, F.D.; Bergman, C.J. Rice kernel phenolic content and its relationship with antiradical efficiency. J. Sci. Food Agric. 2004, 84, 1235–1240. [Google Scholar] [CrossRef]
- Chang, C.; Yang, M.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in Propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites. In Methods in Ecology; Blackwell Scientific Publications: Oxford, UK, 1994. [Google Scholar]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Allam, R.M.; Al-Abd, A.M.; Khedr, A.; Sharaf, O.A.; Nofal, S.M.; Khalifa, A.E.; Mosli, H.A.; Abdel-Naim, A.B. Fingolimod interrupts the cross talk between estrogen metabolism and sphingolipid metabolism within prostate cancer cells. Toxicol. Lett. 2018, 291, 77–85. [Google Scholar] [CrossRef]
- Hamad, Y.K.; Abobakr, Y.; Salem, M.Z.; Ali, H.M.; Al-Sarar, A.S.; Al-Zabib, A.A. Activity of plant extracts/essential oils against three plant pathogenic fungi and mosquito larvae: GC/MS analysis of bioactive compounds. BioResources 2019, 14, 4489–4511. [Google Scholar]
- Uchegbu, R.I.; Ahuchaogu, A.A.; Amanze, K.O.; Ibe, C.O. Chemical constituents analysis of the leaves of Bryophyllum pinnatum by GC-MS. AASCIT J. Chem. 2017, 3, 19–22. [Google Scholar]
- Purushothaman, A.; Nandhakumar, E.; Shanthi, P.; Sachidanandam, T.P. Shemamruthaa, a Herbal Formulation Induces Apoptosis in Breast Cancer Cells and Inhibits Tumor Progression in Rats. J. Evid. Based Complement. Altern. Med. 2016, 21, NP1–NP10. [Google Scholar] [CrossRef]
- Song, L.; Wang, R.; Che, L.; Jiang, Y.; Zhou, M.; Zhao, Y.; Pang, J.; Jiang, M.; Zhou, G.; Zheng, M.; et al. Catalytic aerobic oxidation of lignocellulose-derived levulinic acid in aqueous solution: A novel route to synthesize dicarboxylic acids for bio-based polymers. ACS Catal. 2021, 11, 11588–11596. [Google Scholar] [CrossRef]
- Rubab, M.; Chelliah, R.; Saravanakumar, K.; Barathikannan, K.; Wei, S.; Kim, J.R.; Yoo, D.; Wang, H.W.; Oh, D.H. Bioactive potential of 2-methoxy-4-vinylphenol and benzofuran from Brassica oleracea L. var. capitate f, rubra (Red Cabbage) on oxidative and microbiological stability of beef meat. Foods 2020, 9, 568. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, E.; Gopalakrishnan, S. GC-MS analysis of some bioactive constituents of Mussaenda frondosa Linn. Int. J. Pharma Bio. Sci. 2011, 2, 313–320. [Google Scholar]
- Smith, P.M. Approaches to the Synthesis of Galbonolide Macrolides. PhD. Thesis, The University of Manchester, Manchester, UK, 1994. [Google Scholar]
- Khatiwora, E.; Adsul, V.B.; Kulkarni, M.; Deshpande, N.R.; Kashalkar, R.V. Antibacterial activity of Dibutyl Phthalate: A secondary metabolite isolated from Ipomoea carnea stem. J. Pharm. Res. 2012, 5, 150–152. [Google Scholar]
- Jebastella, J.; Appavoo, R. Bioactive components of Cynodon dactylon using ethanol extract. World J. Pharm. Res. 2015, 3, 2388–2391. [Google Scholar]
- Tinikul, R.; Chenprakhon, P.; Maenpuen, S.; Chaiyen, P. Biotransformation of plant-derived phenolic acids. Biotechnol. J. 2018, 13, 1700632. [Google Scholar] [CrossRef]
- D’Auria, M.; Fascetti, S.; Racioppi, R.; Romano, V.A.; Rosati, L. Orchids from Basilicata: The scent. In Orchids Phytochemistry, Biology and Horticulture; Reference Series in Phytochemistry; Merillon, J.M., Kodja, H., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Brown, A. Cutin and Suberin in Mixed-Wood Boreal Forest Plants and Their Use as Markers for Origin of Soil Organic Matter.Ms.C. Thesis. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2020. [Google Scholar] [CrossRef]
- Doshi, G.M.; Nalawade, V.V.; Mukadam, A.S.; Chaskar, P.K.; Zine, S.P.; Somani, R.R.; Une, H.D. Structural elucidation of chemical constituents from Benincasa hispida seeds and Carissa congesta roots by gas chromatography: Mass spectroscopy. Pharmacogn. Res. 2015, 7, 282. [Google Scholar] [CrossRef]
- Hou, C.T. A novel compound, 12, 13, 17-trihydroxy-9 (Z)-Octadecenoic acid, from linoleic acid by a new microbial isolateClavibacter sp. ALAJ. Am. Oil Chem. Soc. 1996, 73, 1359–1362. [Google Scholar] [CrossRef]
- Nakai, S.; Yamada, S.; Hosomi, M. Anti-cyanobacterial fatty acids released from Myriophyllum Spicatum. Hydrobiol. 2005, 543, 71–78. [Google Scholar] [CrossRef]
- Sivagnanam, S.; Valliammai, R. Determination of Bioactive and Pharmaceutical Components of Croton bonplandianus by GC-MS Analysis. Int. J. Pharm. Sci. Nanotechnol. 2016, 9, 3488–3493. [Google Scholar] [CrossRef]
- Romano, V.A.; Rosati, L.; Fascetti, S.; Cittadini, A.M.R.; Racioppi, R.; Lorenz, R.; D’Auria, M. Spatial and temporal Variability of the floral scent emitted by Barlia robertiana (Loisel.) Greuter, a Mediterranean food-deceptive orchid. Compounds 2022, 2, 37–53. [Google Scholar] [CrossRef]
- Dong, M.; Oda, Y.; Hirota, M. (10E, 12Z, 15Z)-9-hydroxy-10, 12, 15-octadecatrienoic acid methyl ester as an anti-inflammatory compound from Ehretia dicksonii. Biosci. Biotechnol. Biochem. 2000, 64, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Abdulhafiz, F.; Mohammed, A.; Kayat, F.; Bhaskar, M.; Hamzah, Z.; Podapati, S.K.; Reddy, L.V. Xanthine oxidase inhibitory activity, chemical composition, antioxidant properties and GC-MS Analysis of Keladi Candik (Alocasia longiloba Miq). Molecules 2020, 25, 2658. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, D.; Meltzer, R.I. Quinoline antibacterial agents. Oxolinic acid and related compounds. J. Med. Chem. 1968, 11, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Veilumuthu, P. Characterization of Secondary Metabolites Derived from Tomato Endophyte–Streptomyces sp. Shanivit. Curr. Trends Biotechnol. Pharm. 2022, 16 (Suppl. 1), 141–152. [Google Scholar] [CrossRef]
- Fadipe, L.A.; Haruna, A.K.; Mohammed, I. Antibacterial activity of 1, 2-benzenedicarboxylic acid, dioctyl ester isolated from the ethyl acetate soluble sub-portion of the unripe fruits of Nauclea Latifolia. Int. J. Pure Appl. Biosci. 2014, 2, 223–230. [Google Scholar]
- Ji, M.; Yu, Z.; Chen, G.; Masood, T.; Ma, F. Chemical Constituents and Biological Functions of Different Extracts of Millettia speciosa Leaves. J. Food Nutr. Res. 2020, 8, 506–515. [Google Scholar] [CrossRef]
- Balami, V.M.; Yakubu, J.; Mamza, U.T.; Dawa, S.I.; Abdulrahman, F.I.; Sodipo, O.A. Isolation and Analysis of Methanol Extract of Leaf of Senna siamea. J. Chem. Soc. Nigeria 2020, 45, 1110–1119. [Google Scholar] [CrossRef]
- Janeczko, A.; Skoczowski, A. Mammalian sex hormones in plants. Folia Histochem. Cytobiol. 2005, 43, 71–79. [Google Scholar]
- Picerno, P.; Autore, G.; Marzocco, S.; Meloni, M.; Sanogo, R.; Aquino, R.P. Anti-inflammatory activity of verminoside from kigelia a fricana and evaluation of cutaneous irritation in cell cultures and reconstituted human epidermis. J. Nat. Prod. 2005, 68, 1610–1614. [Google Scholar] [CrossRef]
- Joshi, K.C.; Singh, P.; Taneja, S.; Cox, P.J.; Howie, R.A.; Thomson, R.H. New terpenoid aldehydes from Kigelia pinnata: Crystal structure of pinnatal. Tetrahedron 1982, 38, 2703–2708. [Google Scholar] [CrossRef]
- Weiss, C.R.; Moideen, S.V.; Croft, S.L.; Houghton, P.J. Activity of extracts and isolated naphthoquinones from Kigelia pinnata against Plasmodium falciparum. J. Nat. Prod. 2000, 63, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Bharti, N.; Singh, S.; Naqvi, F.; Azam, A. Isolation and in vitro antiamoebic activity of iridoids isolated from Kigelia pinnata. Arkivoc 2006, 10, 69–79. [Google Scholar] [CrossRef]
- Afify, A.E.M.M.; El-Beltagi, H.S. Effect of insecticide cyanophos on liver function in adult male rats. Fresenius Environ. Bull. 2011, 20, 1084–1088. [Google Scholar]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio) synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.J.; Houghton, P.J.; Retsas, S.; Photiou, A. In vitro cytotoxicity of norviburtinal and isopinnatal from Kigelia pinnata against cancer cell lines. Planta Med. 66 2000, 8, 758–761. [Google Scholar] [CrossRef]
- Fagbohun, O.F.; Babalola, O.O.; Agboola, F.K.; Joseph, J.S.; Malindisa, S.; Msagati, T.A.M. Evaluation of phytochemicals, antioxidants, trace elements in Kigelia africana fruit extracts and chemical profiling analysis using UHPLC-qTOF-MS2 spectrometry. Biol. Trace Elem. Res. 2020, 195, 679–695. [Google Scholar] [CrossRef]
- Elekofehinti, O.O.; Iwaloye, O.; Olawale, F.; Ariyo, E.O. Saponins in cancer treatment: Current progress and future prospects. Pathophysiology 2021, 28, 250–272. [Google Scholar] [CrossRef]
- Ramadan, K.M.A.; El-Beltagi, H.S.; Shanab, S.M.M.; El-fayoumy, E.A.; Shalaby, E.A.; Bendary, E.S.A. Potential antioxidant and anticancer activities of secondary metabolites of Nostoc linckia cultivated under Zn and Cu stress conditions. Processes 2021, 9, 1972. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Dawi, F.; Ashoush, I.S.; Ramadan, K.M.A. Antioxidant, anticancer and ameliorative activities of Spirulina platensis and pomegranate juice against hepatic damage induced by CClNot. Bot. Hort. Agrobot. Cluj Napoca 2020, 48, 1941–1956. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Dawi, F.; Aly, A.A.; El-Ansary, A.E. Chemical compositions and biological activities of the essential oils from gamma irradiated celery (Apium graveolens L.) seeds. Not. Bot. Hort. Agrobot. Cluj Napoca 2020, 48, 2114–2133. [Google Scholar] [CrossRef]
- Rajendrasozhan, S.; Moll, H.E.; Snoussi, M.; Romeilah, R.M.; El-Beltagi, H.S.; Shalaby, E.A.; Younes, K.M.; El-Beltagi, H.S. Phytochemical screening and antimicrobial activity of various extracts of aerial parts of Rhanterium epapposum. Processes 2021, 9, 1351. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Elmelegy, A.A.; Eldesoky, S.E.; Safwat, G. Phytochemical screening, antimicrobial, antioxidant, anticancer activities and nutritional values of cactus (Opuntia ficus Indicia) pulp and peel. Fresenius Environ. Bull. 2019, 28, 1534–1551. [Google Scholar]
- El-Beltagi, H.S.; Mohamed, H.I.; Safwat, G.; Gamal, M.; Megahed, B.M.H. Chemical composition and biological activity of Physalis peruviana L. Gesunde Pflanz. 2019, 71, 113–122. [Google Scholar] [CrossRef]
- Rizvi, A.; Ahmed, B.; Khan, M.S.; El-Beltagi, H.S.; Umar, S.; Lee, J. Bioprospecting plant growth promoting rhizobacteria for enhancing the biological properties and phytochemical composition of medicinally important crops. Molecules 2022, 27, 1407. [Google Scholar] [CrossRef]
- Valko, M. .; Morris, H.; Cronin, M.T. Metals, toxicity, and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Knez, M.; Glibetic, M. Zinc as a biomarker of cardiovascular health. Front. Nutr. 2021, 8, 686078. [Google Scholar] [CrossRef]
- Porchia, M.; Pellei, M.; Del Bello, F.; Santini, C. Zinc complexes with nitrogen donor ligands as anticancer agents. Molecules 2020, 25, 5814. [Google Scholar] [CrossRef]
- Maret, W. Zinc in pancreatic islet biology, insulin sensitivity, and diabetes. Prev. Nutr. Food Sci. 2017, 22, 1–8. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Popiołek, Ł.; Kocot, J. The many “faces” of copper in medicine and treatment. Biometals 2014, 27, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahim, E.A.; El-Beltagi, H.S.; Romela, R.M. White Bean seeds and Pomegranate peel and fruit seeds as hypercholesterolemic and hypolipidemic agents in albino rats. Grasas Y Aceites 2013, 64, 50–58. [Google Scholar] [CrossRef]
- Guimarães, M.M.; Carvalho, A.C.; Silva, M.S. Effect of chromium supplementation on the glucose homeostasis and anthropometry of type 2 diabetic patients: Double blind, randomized clinical trial. J. Trace Elem. Med. Biol. 2016, 36, 65–72. [Google Scholar] [CrossRef]
- Petry, N.; Olofin, I.; Hurrell, R.F.; Boy, E.; Wirth, J.P.; Moursi, M.; Donahue, A.M.; Rohner, F. The proportion of anemia associated with Iron deficiency in low, medium, and high human development index countries: A systematic analysis of national surveys. Nutrients 2016, 8, 693. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- European Commission. (Regulation 1881/2006/EC) Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. 2006, 364, 5–24. [Google Scholar]
- Oseni, O.A.; Williams, O.D. In-vitro compositional investigations of antioxidants, phytochemicals, nutritional, and minerals in the fruit of Kigelia africana (Lam.) Benth. Int. J. Contemp. Res. Rev. 2018, 9, 20259–20268. [Google Scholar] [CrossRef]
- Elango, R.; Ball, R.O. Protein and Amino Acid Requirements during Pregnancy. Adv. Nutr. 2016, 7, 839S–844S. [Google Scholar] [CrossRef] [PubMed]
- Ioniță-Mîndrican, C.-B.; Ziani, K.; Mititelu, M.; Oprea, E.; Neacșu, S.M.; Moroșan, E.; Dumitrescu, D.-E.; Roșca, A.C.; Drăgănescu, D.; Negrei, C. Therapeutic benefits and dietary restrictions of fiber intake: A state of the art review. Nutrients 2022, 14, 2641. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical scavenging mechanisms of phenolic compounds: A Quantitative structure-property relationship (QSPR) study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef] [PubMed]
- Assanti, G.; Kaur, R.; Nizard, S.; Pollack, E.; Rafferty, B.; Priano, C.; Fernández Romero, J.A.; Koroch, A.R. Biology, chemistry, and pharmacological activity of Kigelia africana (Bignoniaceae) and Garcinia kola (Clusiaceae)-a Review. J. Med. Act. Plants 2022, 11, 1–21. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Otero, P.; Cassani, L.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Chamorro, F.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Traditional applications of tannin rich extracts supported by scientific data: Chemical composition, bioavailability and bioaccessibility. Foods 2021, 10, 251. [Google Scholar] [CrossRef]
- Kumari, M.; Jain, S. Tannins: An antinutrient with positive effect to manage diabetes. Res. J. Recent. Sci. 2012, 1, 1–8. [Google Scholar]
- Mohamed, H.I.; Abd–El Hameed, A.G. Molecular and biochemical markers of some Vicia faba L. genotype in response to storage insect pests infestation. J. Plant Interact. 2014, 9, 618–626. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: An Updated Review. Oxid. Med. Cell Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.; Gan, S. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef]
- Li, Y.X.; Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. In vitro antioxidant activity of 5-HMF isolated from marine red alga Laurencia undulata in free radical mediated oxidative systems. J. Microbiol. Biotechnol. 2009, 19, 1319–1327. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Su, J.; Li, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agric. Food Chem. 2013, 61, 10604–10611. [Google Scholar] [CrossRef]
- Kamiyama, M.; Moon, J.K.; Jang, H.W.; Shibamoto, T. Role of degradation products of chlorogenic acid in the antioxidant activity of roasted coffee. J. Agric. Food Chem. 2015, 63, 1996–2005. [Google Scholar] [CrossRef]
- Murakami, K.; Ito, M.; Tanemura, Y.; Yoshino, M. Maltol as an antioxidant: Inhibition of lipid peroxidation and protection of NADP-isocitrate dehydrogenase from the iron-mediated inactivation. Biomed. Res. 2001, 22, 183–186. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, M.; Liu, F.; Zeng, S.; Hu, J. Identification of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one as a strong antioxidant in glucose-histidine Maillard reaction products. Food Res. Inter. 2013, 51, 397–403. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Q.; Zhao, Z.; Bai, B.; Sun, Z.; Cai, L.; Fu, Y.; Ma, Y.; Wang, Q.; Xi, G. Effect of hydroxyl on antioxidant properties of 2,3-dihydro-3,5-dihydroxy-6-methyl-4: H -pyran-4-one to scavenge free radicals. RSC Adv. 2021, 11, 34456–34461. [Google Scholar] [CrossRef]
- Higgins, C.A.; Bell, T.; Delbederi, Z.; Feutren-Burton, S.; McClean, B.; O’Dowd, C.; Watters, W.; Armstrong, P.; Waugh, D.; van den Berg, H. Growth inhibitory activity of extracted material and isolated compounds from the fruits of Kigelia pinnata. Planta Med. 2010, 76, 1840–1846. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Q.; Hu, J.N.; Han, X.Y.; Li, W.; Zhao, L.C. Maltol, a food flavoring agent, attenuates acute alcohol-induced oxidative damage in mice. Nutrients 2015, 7, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Geran, R.I.; Greenberg, N.H.; Macdonald, M.M.; Shumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother. Rep. Part. III 1972, 3, 1–103. [Google Scholar]
- Srisawat, T.; Chumkaew, P.; Heed-Chim, W.; Sukpondma, Y.; Kanokwiroon, K. Phytochemical Screening and Cytotoxicity of Crude Extracts of Vatica diospyroides Symington Type LS. Trop. J. Pharm. Res. 2013, 12, 71–76. [Google Scholar] [CrossRef]
- Mao, Y.; Du, J.; Chen, X.; Al Mamun, A.; Cao, L.; Yang, Y.; Mubwandarikwa, J.; Zaeem, M.; Zhang, W.; Chen, Y.; et al. Maltol promotes mitophagy and inhibits oxidative stress via the Nrf2/PINK1/Parkin pathway after spinal cord injury. Oxid. Med. Cell. Longev. 2022, 2022, 1337630. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, W.; Hu, J.; Mi, X.; Han, Y.; Ren, S.; Jiang, S.; Wang, Y.; Li, X.; Li, W. Maltol improves APAP-induced hepatotoxicity by inhibiting oxidative stress and inflammation response via NF-κB and PI3K/Akt signal pathways. Antioxidants 2019, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Thienthiti, K.; Tuchind, P.; Wongnoppavich, A.; Anantachoke, N.; Soonthornchareonnon, N. Cytotoxic effect of compounds isolated from Goniothalamus marcanii Craib stem barks. Pharm. Sci. Asia 2017, 44, 86–95. [Google Scholar] [CrossRef]
- Jiménez-Estrada, M.; Velázquez-Contreras, C.; Garibay-Escobar, A.; Sierras-Canchola, D.; Lapizco-Vázquez, R.; Ortiz-Sandoval, C.; Burgos-Hernández, A.; Robles-Zepeda, R.E. In vitro antioxidant and antiproliferative activities of plants of the ethnopharmacopeia from northwest of Mexico. BMC Complement. Altern. Med 2013, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; El-Mahdy, O.M.; Mohamed, H.I.; El-Ansary, A.E. Antioxidants, antimicrobial, and anticancer activities of purified chitinase of Talaromyces funiculosus strain CBS 129594 biosynthesized using crustacean bio-wastes. Agronomy 2022, 12, 2818. [Google Scholar] [CrossRef]
- Sofy, M.R.; Mohamed, H.I.; Dawood, M.F.A.; Abu-Elsaoud, A.M.; Soliman, M.H. Integrated usage of Trichoderma harzianum and biochar to ameliorate salt stress on spinach plants. Arch. Agr. Soil Sci. 2021, 68, 2005–2026. [Google Scholar] [CrossRef]
- Ghonaim, M.M.; Mohamed, H.I.; Omran, A.A.A. Evaluation of wheat salt stress tolerance using physiological parameters and retrotransposon-based markers. Genet Resour. Crop Evol. 2021, 68, 227–242. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, S.I.; Go, B.; Oh, U.H.; Kim, C.S.; Jung, Y.H.; Lee, J.; Kim, J.H. 2-methoxy-4-vinylphenol attenuates migration of human pancreatic cancer cells via blockade of fak and akt signaling. Anticancer Res. 2019, 39, 6685–6691. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Phenolic compounds. In Phytochemical Methods; Springer: Dordrecht, The Netherlands, 1973; pp. 33–88. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Trease, G.E.; Evans, W.C. Pharmacognosy, 13th ed.; J & A. Churchill Ltd.: London, UK, 1989; pp. 234–492. [Google Scholar]
- Sofowora, A. Recent trends in research into African medicinal plants. J. Ethnopharmacol. 1993, 38, 209–214. [Google Scholar] [CrossRef]
- Alexei, Y.B.; Joseph, I.S.; Olga, V.F. Endogenous cardiotonic steroids: Physiology, pharmacology and novel therapeutic targets. Pharmacol Rev. 2009, 61, 9–38. [Google Scholar] [CrossRef]
- El Aziz, M.M.A.; Ashour, A.S.; Melad, A.S.G. A review on saponins from medicinal plants: Chemistry, isolation, and determination. J. Nanomed. Res. 2019, 8, 282–288. [Google Scholar] [CrossRef]




| Element | Concentration mg/Kg (DW) | |
|---|---|---|
| Km | KI | |
| Magnesium (Mg) | 194.17 ± 0.14 b | 472.22 ± 0.28 a |
| Sodium (Na) | 6.04 ± 0.27 b | 10.20 ± 0.57 a |
| Calcium (Ca) | 112.24 ± 0.86 b | 167.60 ± 0.34 a |
| Iron (Fe) | 499.6 ± 0.42 b | 530.1 ± 0.13 a |
| Manganese (Mn) | 99.47 ± 0.56 b | 272.5 ± 0.19 a |
| Zinc (Zn) | 152.93 ± 0.34 b | 230.9 ± 0.26 a |
| Copper (Cu) | 8.60 ± 0.24 b | 12.40 ± 0.52 a |
| Boron(B) | 52.33 ± 0.18 a | 45.72 ± 0.3 b |
| Molybdenum (Mo) | 2.57 ± 0.54 a | 1.76 ± 0.15 b |
| Cadmium (Cd) | 0.048 ± 0.12 a | 0.033 ± 0.17 b |
| Chromium (Cr) | 16.32 ± 0.69 a | 4.68 ± 0.59 b |
| Lead (Pb) | 0.048 ± 0.34 a | 0.020 ± 0.04 b |
| Aluminum (Al) | 1.74 ± 0.50 a | 0.99 ± 0.31 b |
| Silicon (Si) | 16.29 ± 0.53 a | 11.85 ± 0.12 b |
| Sample | %Moisture | %Reducing Sugars | %Total Carbohydrates | %Crude Fiber | %Crude Protein | %FAA | %Crude Fat |
|---|---|---|---|---|---|---|---|
| Km | 26.89 ± 0.04 b | 5.03 ± 0.12 a | 28.05 ± 0.02 a | 15.4 ± 0.04 a | 15.25 ± 0.01 a | 1.12 ± 0.94 b | 6.87 ± 0.45 a |
| KI | 28.78 ± 0.09 a | 1.47 ± 0.1 b | 22.61± 0.5 b | 11.7 ± 0.87 b | 9.43 ± 0.9 b | 1.48 ± 0.85 a | 6.31 ± 0.55 a |
| Sample | T. Phenolics GAE mg/100 g | T. Anthocyanin KE mg/100 g | T. Flavonoids QE mg/100 g | T. Tannins CAE mg/100 g |
|---|---|---|---|---|
| Km | 171 ± 0.34 a | 0.61 ± 0.04 a | 398.7 ± 0.98 a | 35.17 ± 0.41 a |
| KI | 134 ± 0.15 b | 0.52 ± 0.03 a | 353.2 ± 0.71 b | 33.01 ± 0.30 b |
| Range of Crude Methanol Extract (mg/mL) | DPPH Scavenging Activity% Median | |
|---|---|---|
| KI | Km | |
| 2. 0–12.0 | 22.32 ± 1.57 b | 75.41 ± 0.65 a |
| IC50 | ||
| 15.67 ± 0.48 a | 2.56 ± 0.19 b | |
| Range Extract (mg/mL) | DPPH-Scavenging Activity% Median | |||
|---|---|---|---|---|
| K Water | K ethyl acetate | K methanol | K dichloromethane | |
| 1.0- 6.0 | 30.42 ± 0.54 c | 77.81 ± 0.65 b | 85.23 ± 0.69 a | 26.36 ± 0.36 d |
| IC50 | ||||
| 2.91 ± 0.21 b | 1.91 ± 0.15 c | 0.77 ± 0.08 d | 6.76 ± 0.39 a | |
| Peak No | Compound Name | M. Ion | Base Peak m/z | Retention Time (min) | KI | µg/g Crude Extract | References |
|---|---|---|---|---|---|---|---|
| 1 | Larixinic acid | 126 | 126 | 5.15 | 1092.2 | 64.55 | [43] |
| 2 | 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one(DMDP) | 144 | 144 | 6.229 | 1124 | 74.29 | [44] |
| 3 | 5-Hydrxoymethylfurfural | 126 | 126 | 7.65 | 1195 | 148.96 | [45] |
| 4 | 2-Hydroxy-2-methylsuccinic acid | 148 | 103 | 7.914 | 1217 | 97.47 | [46] |
| 5 | 2-Methoxy-4-vinylphenol | 150 | 135 | 9.366 | 1289 | 15.58 | [47] |
| 6 | 4-((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol | 180 | 137 | 16.499 | 1689 | 13.05 | [48] |
| 7 | (2E)-5-([tert-Butyl(dimethyl)silyl]oxy)-2-hexena | 228 | 127 | 18.677 | 1986 | 4.29 | [49] |
| 8 | Dibutyl phthalate | 278 | 149 | 19.422 | 1923 | 3.64 | [50] |
| 9 | Benzenemethanol, 2,5-dimethoxy-, acetate | 210 | 210 | 19.702 | 1996 | 3.57 | [51] |
| 10 | Adipic acid, butyl hexyl ester | 286 | 129 | 19.768 | 1596 | 11.95 | [52] |
| 11 | i-Propyl 14-methyl-pentadecanoate | 298 | 256 | 20.559 | 1892 | 2.47 | [53] |
| 12 | 9-Hydroxypentadecanoic acid, methyl ester | 272 | 155 | 21.02 | 2455 | 3.90 | [54] |
| 13 | Butyl 9,12-octadecadienoate | 336 | 106 | 21.247 | 2631 | 2.27 | [55] |
| 14 | 9,12-(Z,Z)-Octadecadienoic acid | 280 | 109 | 21.675 | 2092 | 3.12 | [56] |
| 15 | 6-Octadecenoic acid | 282 | 111 | 21.769 | 2161 | 9.42 | [57] |
| 16 | N,N-dibutyl-Octanamide | 255 | 128 | 22.009 | 1839 | 3.25 | |
| 17 | Phenyl 6-hydroxy-8-phenyloctanoate | 312 | 183 | 22.361 | 2155 | 4.61 | |
| 18 | S-[2-((2-[(4-Methyl-2-quinolinyl)oxy]ethyl)amino)ethyl] hydrogen thiosulfate | 342 | 183 | 23.111 | 2651 | 18.57 | |
| 19 | 4-(3,5-Dimethyl-1-benzofuran-2-yl)-2-butanone | 159 | 216 | 23.176 | 1478 | 29.03 | [58] |
| 20 | 5-Ethoxy-3a,6a-dimethyl-3,3a,4,6a-tetrahydro-furo[2,3-b]pyrrol-2-one | 197 | 126 | 23.899 | 1894 | 49.81 | |
| 21 | 1,6-Hexanedioic acid, bis(DMOX) | 252 | 139 | 23.964 | 2398 | 32.53 | |
| 22 | 3-Methyl-5-[(2-methyl-1H-indol-3-ylmethylene)-amino]thiophene-2,4-dicarboxylic acid,2-ethyl ester 4-methyl-ester | 384 | 158 | 25.114 | 2215 | 3.38 | |
| 23 | 1,3-diaceto-2-Myristin, | 386 | 159 | 25.174 | 2562 | 8.25 | |
| 24 | Diisooctyl phthalate ester (internal standard) | 390 | 149 | 25.765 | 2525 | 15.00 | |
| 25 | (3.α.,5.α.,11.β.)- 3,11-dihydroxy-Pregnan-20-one | 334 | 316 | 26.208 | 2489 | 26.43 |
| Peak No | Compound Name | M. Ion | Base Peak m/z | Retention Time (min) | KI | µg/g Crude Extract | References |
|---|---|---|---|---|---|---|---|
| 1 | 2-Methoxy-4-vinylphenol | 150 | 135 | 9.36 | 1289 | 14.59 | [47] |
| 2 | 4-((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol | 180 | 137 | 16.48 | 1689 | 9.18 | [48] |
| 3 | Dibutyl phthalate | 278 | 149 | 18.26 | 1923 | 9.59 | [50] |
| 4 | i-Propyl 14-methyl-pentadecanoate | 298 | 256 | 19.77 | 1892 | 35.62 | [59] |
| 5 | 9,12,15-(Z,Z,Z)-Octadecatrienoic acid | 278 | 79 | 21.72 | 2092 | 9.85 | [60] |
| 6 | Adipic acid, isobutyl propyl ester | 244 | 129 | 23.38 | 2283 | 10.98 | [52] |
| 7 | 5-Ethoxy-3a,6a-dimethyl-3,3a,4,6a-tetrahydro-furo[2,3-b]pyrrol-2-one | 197 | 126 | 23.89 | 1894 | 15.36 | |
| 8 | 2,6,10,10-Tetramethyl-1-oxaspiro[4.5]decan-6-ol | 212 | 126 | 23.96 | 1298 | 28.87 | [61] |
| 9 | (3.α.,5.α.,11.β.)- 3,11-dihydroxy-Pregnan-20-one | 334 | 316 | 24.18 | 2489 | 58.45 | |
| 10 | 2-(4-Methoxy-2,5-dimethyl-phenyl)-9-methyl-2H-benzo[g]indazole | 316 | 316 | 24.40 | 2491 | 9.69 | |
| 11 | 3.α.-Hydroxy-5,16-androstadiene-17-carboxylic acid | 316 | 316 | 24.43 | 2491 | 18.45 | |
| 12 | Oxolinic acid | 261 | 202 | 24.60 | 1940 | 20.93 | [62] |
| 13 | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl ester | 530 | 530 | 24.74 | 3991 | 33.92 | |
| 14 | Fumaric acid, decyl isobutyl ester | 312 | 117 | 25.42 | 1741 | 14.79 | [63] |
| 15 | 1,2-Benzenedicarboxylic acid, diisooctyl ester | 390 | 149 | 25.76 | 2525 | 54.64 | [64] |
| 16 | gamma-Sitosterol | 414 | 140 | 25.84 | 3790 | 53.87 | [65] |
| 17 | 2,6-dione, 8-(3-ethoxypropylamino)-1,3-dimethyl-3,9-dihydro purine | 281 | 281 | 26.19 | 2159.4 | 10.67 | [66] |
| 18 | Estradiol | 272 | 272 | 26.54 | 2320 | 8.40 | [67] |
| 19 | Diisooctyl phthalate ester (internal standard) | 390 | 149 | 25.765 | 2525 | 14.99 |
| Peak No. | Compound Name (TIC 1) | ID Ret. Time | [M-H]+ | Base Peak m/z | Area% | Compound Class | References |
|---|---|---|---|---|---|---|---|
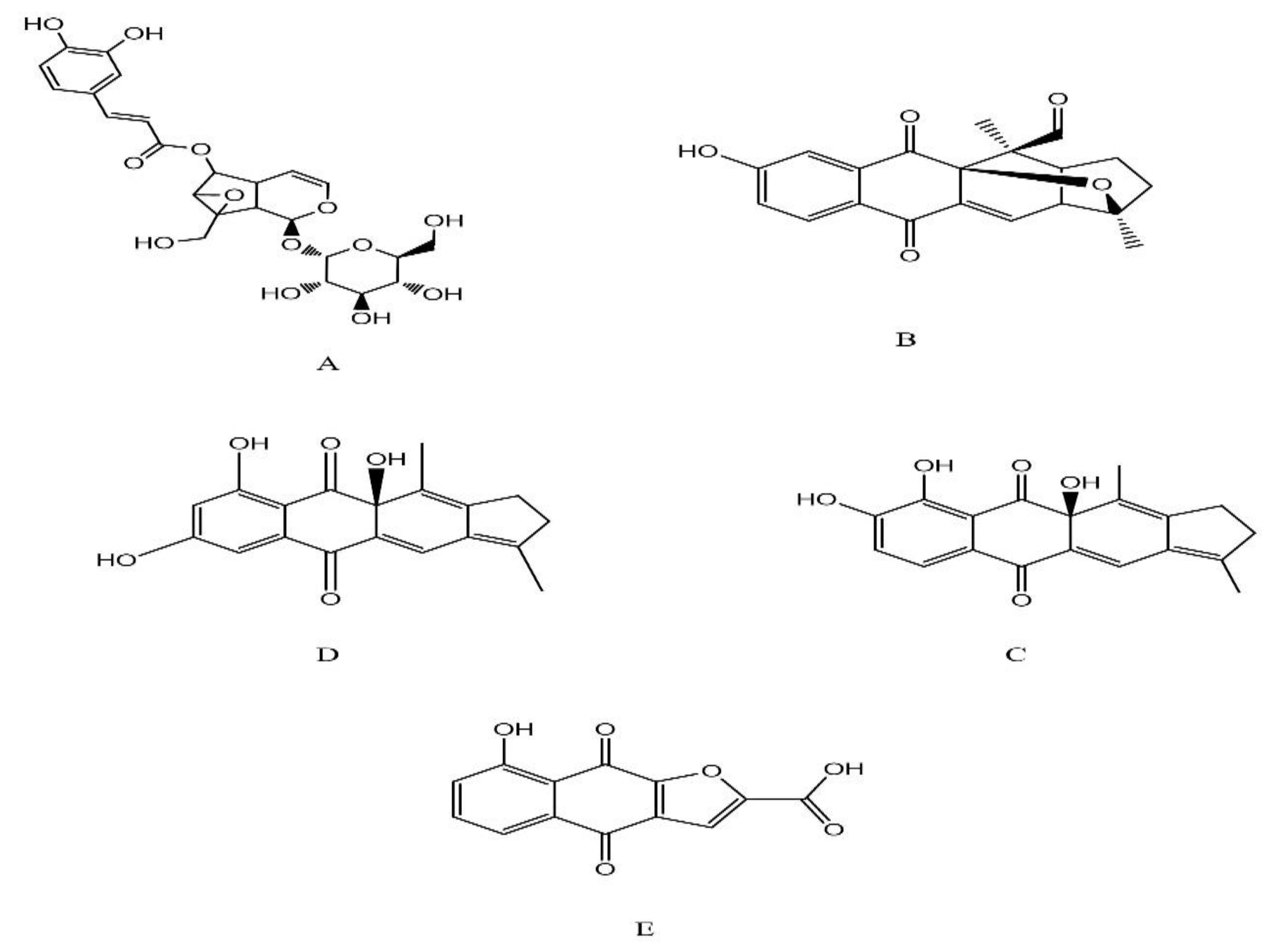
| 1 | Verminoside | 0.71 | 525 | 383 | 8.744 | Iridoid | [68] |
| 2 | Pinnatal | 12.09 | 339 | 339 | 21.394 | Quinones | [69] |
| 3 | Kigelinol | 19.42 | 325 | 281 | 20.522 | Quinones | [70] |
| 4 | Isokigelinol | 22.01 | 325 | 281 | 12.576 | Quinones | [70] |
| 5 | Kigelinone | 21.37 | 259 | 259 | 14.226 | Quinones | [70] |
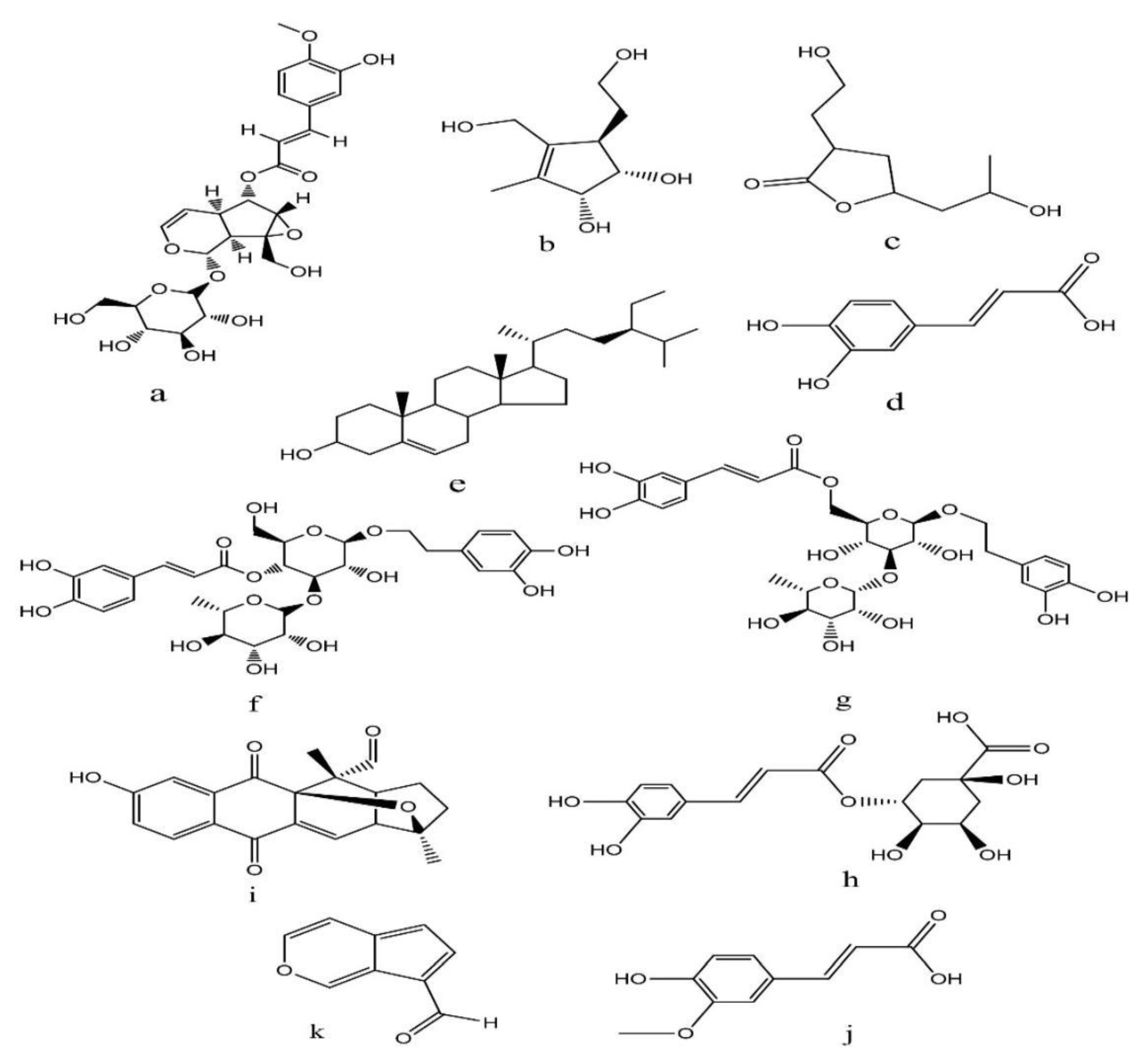
| Peak No | Compound name (TIC 2) | ID Ret. Time | [M-H]- | Base Peak m/z | Area % | Compound class | |
| 1 | Minecoside | 0.70 | 536 | 387.15 | 8.429 | Iridoid | [71] |
| 2 | 7-hydroxy-10-deoxyeucommiol | 0.90 | 187 | 187.00 | 8.651 | Iridoid | [72] |
| 3 | 3-(2-hydroxyethyl)-5-(2-hydroxypropyl)-4,5-dihydrofuran-2(3H)-one | 1.89 | 187 | 187.00 | 7.389 | Iridoid | [72] |
| 4 | Coffeic acid | 3.51 | 179 | 179.10 | 14.063 | Phenolic acid | [71,72] |
| 5 | Sitosterol | 6.33 | 413 | 81.10 | 9.179 | sterol | [71,72] |
| 6 | Verbascoside | 7.07 | 623 | 623.30 | 7.320 | Iridoid | [73] |
| 7 | Isoverbascoside | 7.30 | 623 | 623.25 | 7.341 | Iridoid | [73] |
| 8 | Chlorogenic acid | 7.92 | 353 | 193.15 | 5.645 | Phenolic acid | [71,72] |
| 9 | Isopinnatal | 12.35 | 337 | 293.20 | 36.284 | Quinone | [73,74] |
| 10 | Ferulic acid | 13.84 | 193 | 193.10 | 12.910 | Phenolic acid | [72] |
| 11 | Norviburtinal | 22.00 | 145 | 145.95 | 8.625 | Iridoid | [74] |
| Cell Line | IC50 (µg/mL) | ||
|---|---|---|---|
| Methanolic Extract | Ethyl Acetate Extract | Doxorubicin Drug (DOX) | |
| Huh-7 (liver cancer) | 65.55 ± 0.19 b | 112.74 ± 0.37 a | 1.04 ± 1.01 c |
| PANC-1 (pancreatic cancer) | 10.34 ± 0.91 b | 24.25 ± 2.08 a | 0.74 ± 0.31 c |
| Colo-205 (colorectal cancer) | 52.92 ± 0.24 b | 78.70 ± 3.30 a | 0.9 ± 0.01 c |
| HT-29 (colorectal cancer) | 91.32 ± 2.41 b | 87.02 ± 1.19 a | 1.12 ± 0.04 c |
| SNU-16 (gastric carcinoma) | 8.69 ± 3.79 b | 13.56 ± 0.04 a | 0.59 ± 0.31 c |
| SW620 (colorectal adenocarcinoma) | 6.79 ± 1.37 b | 25.15 ± 1.97 a | 0.88 ± 0.24 c |
| HCT116 (colon carcinoma) | 27.1 ± 0.31 b | 78.66 ± 0.86 a | 1.15 ± 0.07 c |
| THLE2 (ormal liver cells) | 546.75 ± 4.90 a | 443.87 ± 1.01 b | 128.14 ± 0.53 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramadan, K.M.A.; El-Beltagi, H.S.; Mohamed, H.I.; Shalaby, T.A.; Galal, A.; Mansour, A.T.; Aboul Fotouh, M.M.; Bendary, E.S.A. Antioxidant, Anti-Cancer Activity and Phytochemicals Profiling of Kigelia pinnata Fruits. Separations 2022, 9, 379. https://doi.org/10.3390/separations9110379
Ramadan KMA, El-Beltagi HS, Mohamed HI, Shalaby TA, Galal A, Mansour AT, Aboul Fotouh MM, Bendary ESA. Antioxidant, Anti-Cancer Activity and Phytochemicals Profiling of Kigelia pinnata Fruits. Separations. 2022; 9(11):379. https://doi.org/10.3390/separations9110379
Chicago/Turabian StyleRamadan, Khaled M. A., Hossam S. El-Beltagi, Heba I. Mohamed, Tarek A. Shalaby, Ahmed Galal, Abdallah Tageldein Mansour, Mohamed M. Aboul Fotouh, and Eslam S. A. Bendary. 2022. "Antioxidant, Anti-Cancer Activity and Phytochemicals Profiling of Kigelia pinnata Fruits" Separations 9, no. 11: 379. https://doi.org/10.3390/separations9110379
APA StyleRamadan, K. M. A., El-Beltagi, H. S., Mohamed, H. I., Shalaby, T. A., Galal, A., Mansour, A. T., Aboul Fotouh, M. M., & Bendary, E. S. A. (2022). Antioxidant, Anti-Cancer Activity and Phytochemicals Profiling of Kigelia pinnata Fruits. Separations, 9(11), 379. https://doi.org/10.3390/separations9110379










