In-Line Vis-NIR Spectral Analysis for the Column Chromatographic Processes of the Ginkgo biloba L. Leaves. Part II: Batch-to-Batch Consistency Evaluation of the Elution Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design of NOC and AOC Batches
2.2. In-Line Spectra Acquisition
2.3. Data Processing
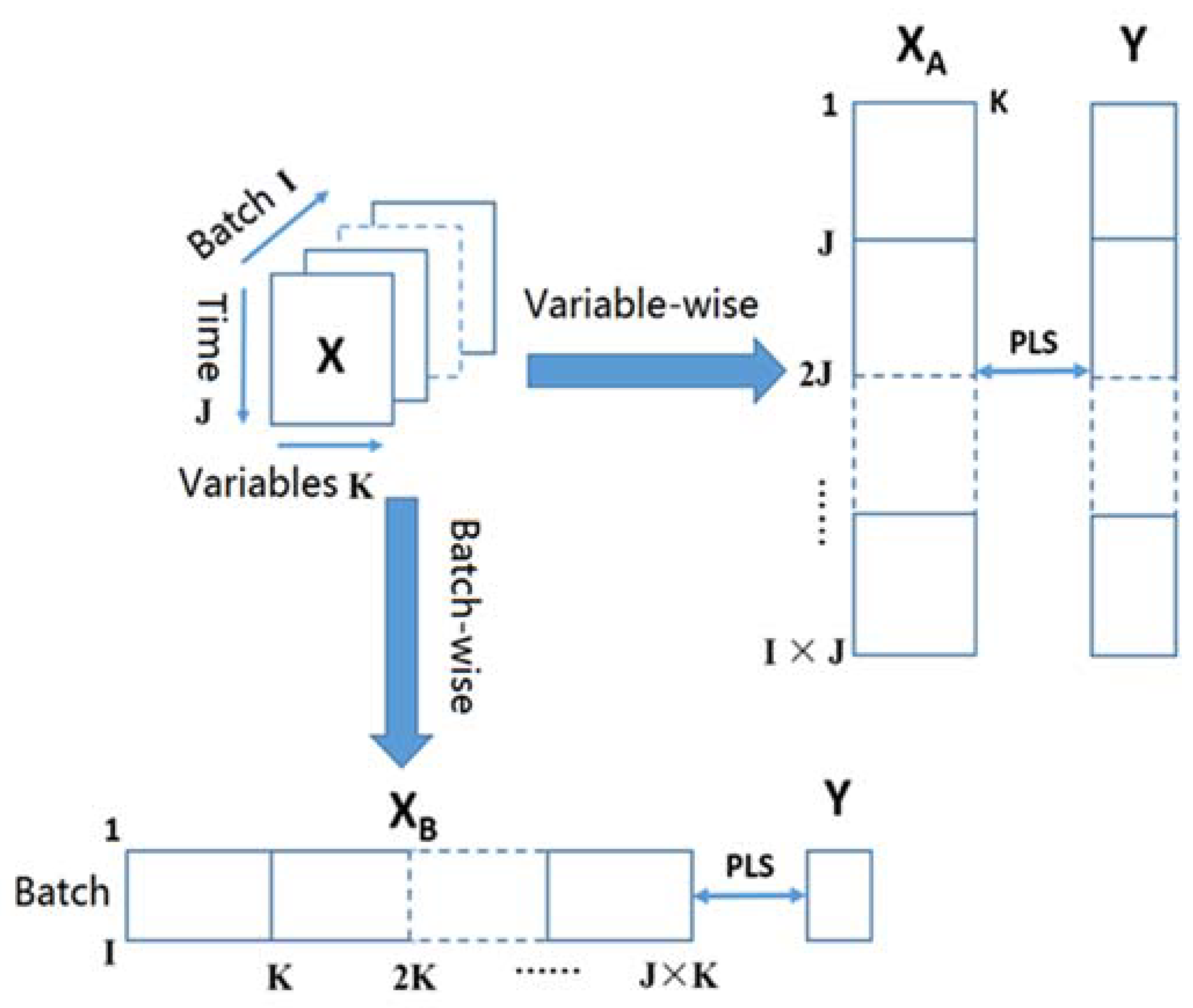
2.4. Unfolding of the Batch Data
2.5. Multivariate Statistical Process Control Charts
3. Results and Discussion
3.1. Spectral Data Pretreatment
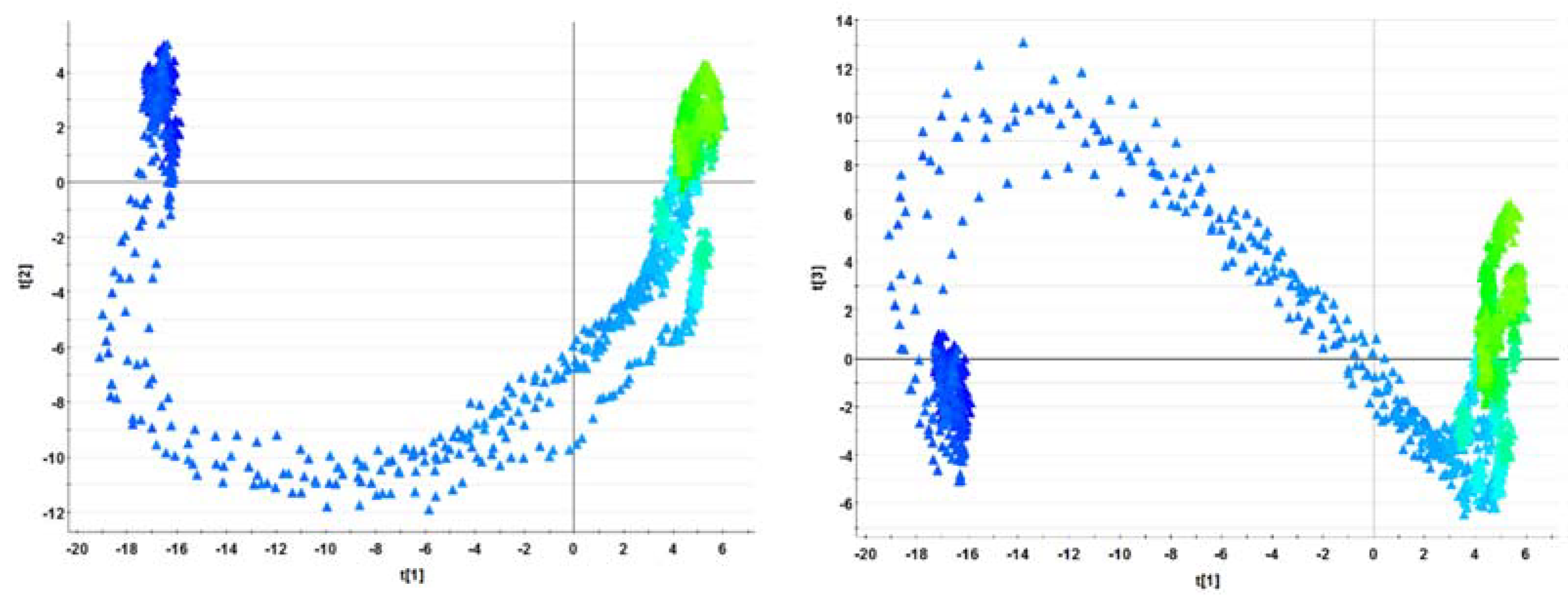
3.2. PCA of the NOC Batches
3.3. MPCA of the NOC Batches
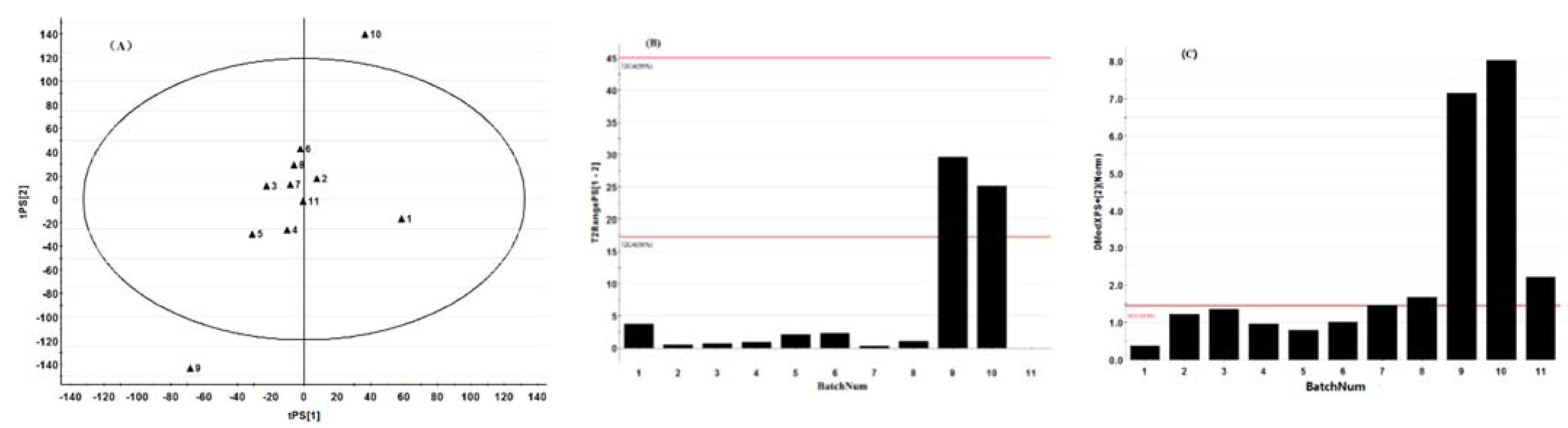
3.4. Establishment of the MSPC Model
3.5. Validation of the Established MSPC Model
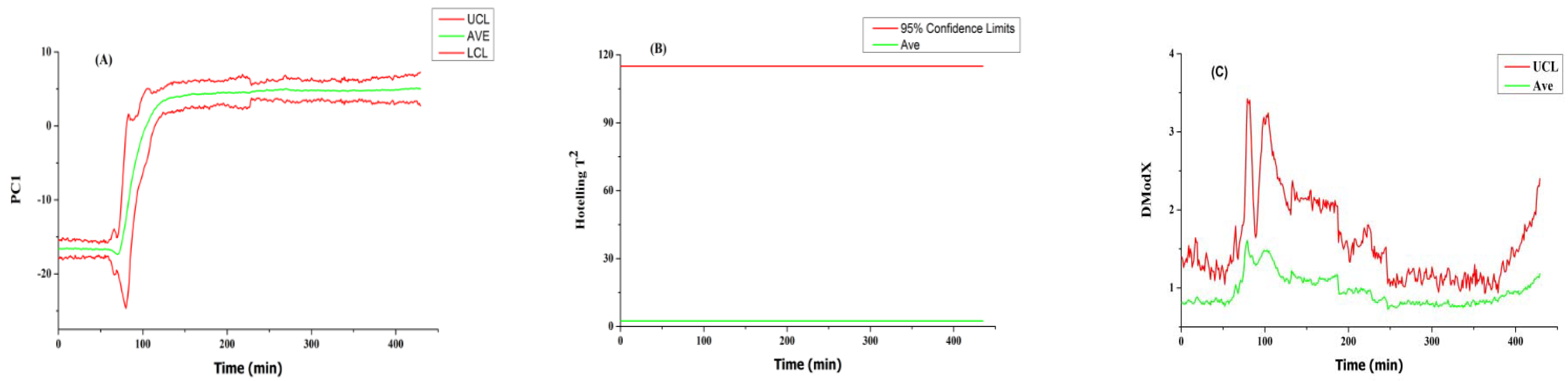
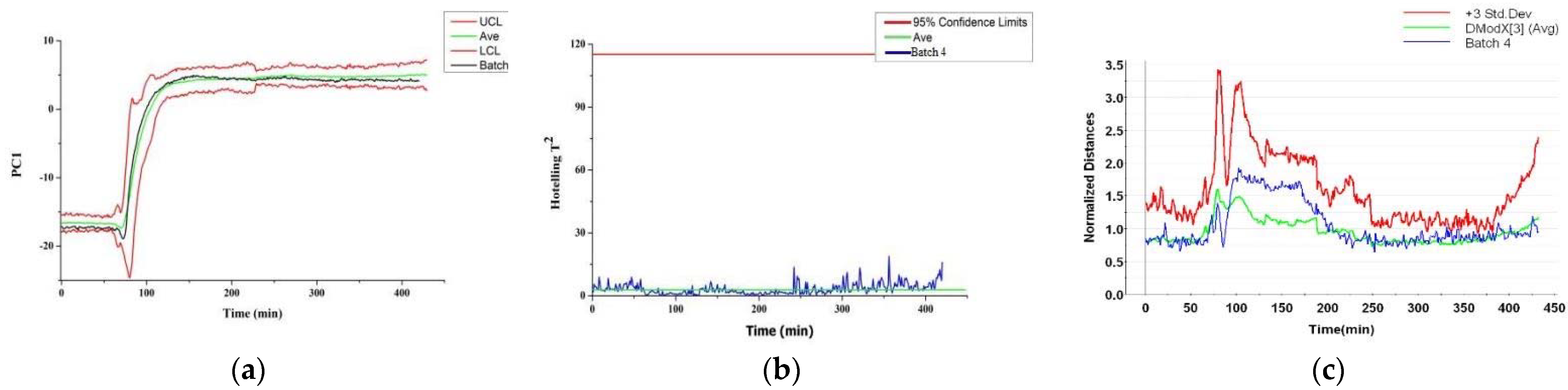
3.5.1. NOC Batch
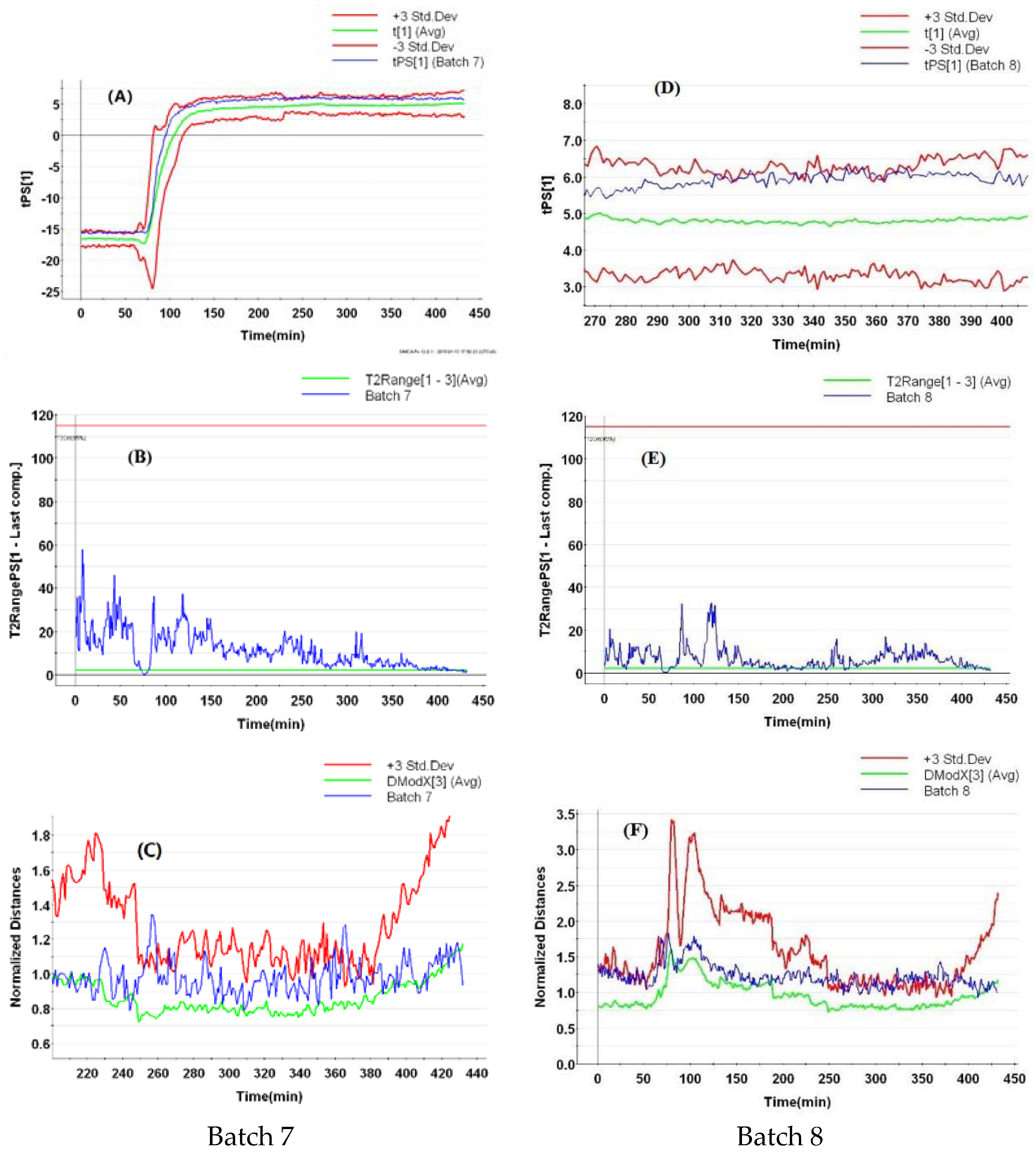
3.5.2. AOC Batches with Abnormal Starting Materials
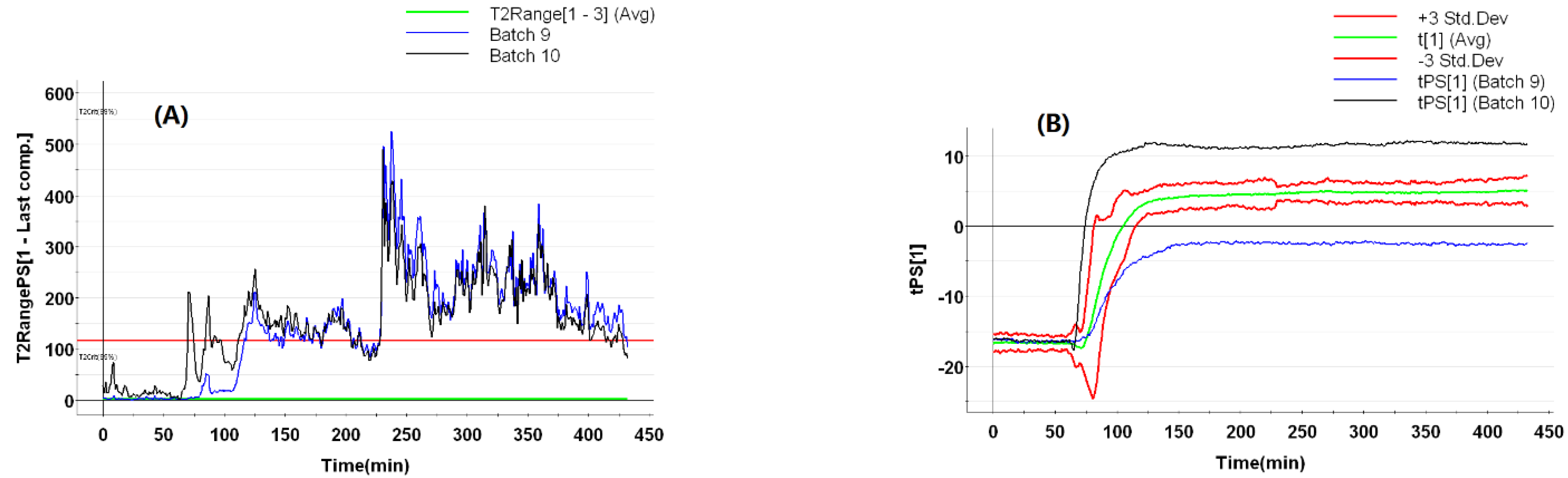
3.5.3. AOC Batches with Abnormal Ethanol Concentrations
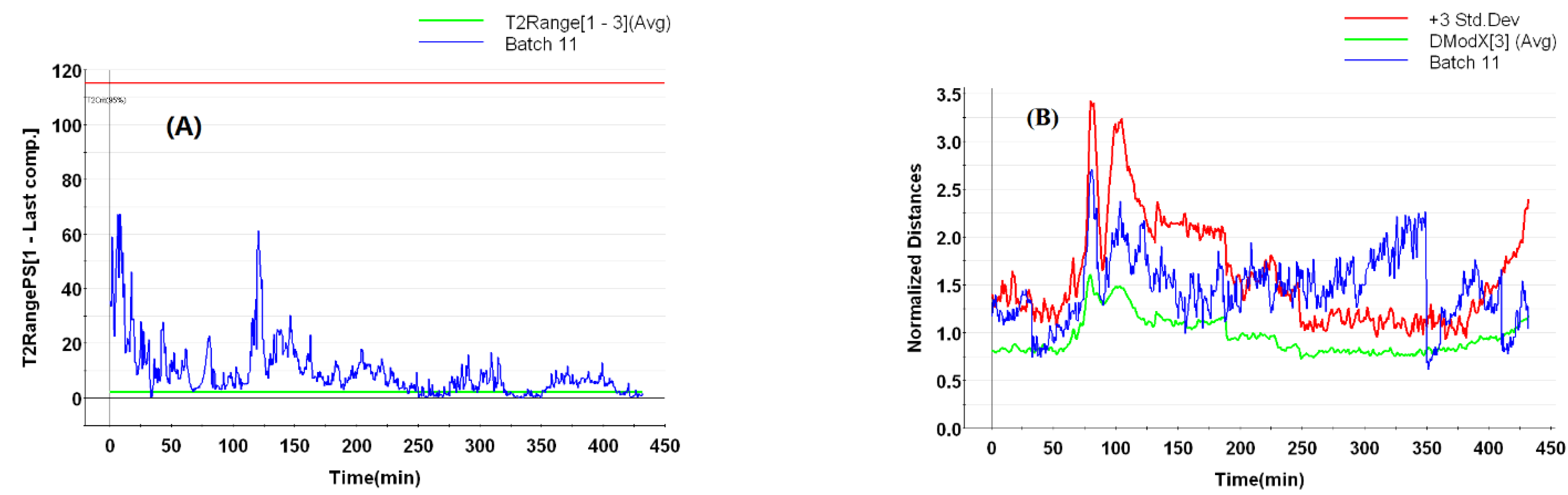
3.5.4. AOC Batches with Abnormal Flow Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Drug Administration. Guidance for Industry: PAT—A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance; Food and Drug Administration: Washington, DC, USA, 2004.
- Food and Drug Administration. Guidance for Industry: Botanical Drug Products; Food and Drug Administration: Washington, DC, USA, 2004.
- Food and Drug Administration. Botanical Drug Development Guidance for Industry; Food and Drug Administration: Washington, DC, USA, 2015.
- Biagi, D.; Nencioni, P.; Valleri, M.; Calamassi, N.; Mura, P. Development of a Near Infrared Spectroscopy method for the in-line quantitative bilastine drug determination during pharmaceutical powders blending. J. Pharm. Biom. Anal. 2021, 204, 114277. [Google Scholar] [CrossRef] [PubMed]
- Khatiwada, B.P.; Subedi, P.P.; Hayes, C.; Jnr, L.C.C.C.; Walsh, K.B. Assessment of internal flesh browning in intact apple using visible-short wave near infrared spectroscopy. Postharvest Biol. Technol. 2016, 120, 103–111. [Google Scholar] [CrossRef]
- Cecchini, C.; Antonucci, F.; Costa, C.; Marti, A.; Menesatti, P. Application of near-infrared handheld spectrometers to predict semolina quality. J. Sci. Food. Agric. 2021, 101, 151–157. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, H.; Yang, H. Rapid determination of major bioactive isoflavonoid compounds during the extraction process of kudzu (Pueraria lobata) by near-infrared transmission spectroscopy. Spectrochim. Acta A 2015, 137, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Herrmann, I.; Angileri, M. Improved prediction of potassium and nitrogen in dried bell pepper leaves with visible and near-infrared spectroscopy utilising wavelength selection techniques. Talanta 2021, 225, 121971. [Google Scholar] [CrossRef]
- Giannuzzi, D.; Mota, L.F.M.; Pegolo, S.; Gallo, L.; Schiavon, S.; Tagliapietra, F.; Katz, G.; Fainboym, D.; Minuti, A.; Trevisi, E.; et al. In-line near-infrared analysis of milk coupled with machine learning methods for the daily prediction of blood metabolic profile in dairy cattle. Sci. Rep. 2022, 12, 8058. [Google Scholar] [CrossRef]
- Assi, S.; Arafat, B.; Lawson-Wood, K.; Robertson, I. Authentication of Antibiotics Using Portable Near-Infrared Spectroscopy and Multivariate Data Analysis. Appl. Spectrosc. 2021, 75, 434–444. [Google Scholar] [CrossRef]
- Li, W.; Han, H.; Cheng, Z.; Zhang, Y.; Liu, S.; Qu, H. A Feasibility Research on the Monitoring of Traditional Chinese medicine Production Process Using NIR-based Multivariate Process Trajectories. Sens. Actuators B Chem. 2016, 231, 313–323. [Google Scholar] [CrossRef]
- Xue, J.T.; Yang, Q.W.; Li, C.Y.; Liu, X.L.; Niu, B.X. Rapid and simultaneous quality analysis of the three active components in Lonicerae Japonicae Flos by near-infrared spectroscopy. Food. Chem. 2021, 342, 128386. [Google Scholar]
- Wu, H.L.; Chen, G.C.; Zhang, G.B.; Dai, M.H. Application of Multimodal Fusion Technology in Image Analysis of Pretreatment Examination of Patients with Spinal Injury. J. Healthc. Eng. 2022, 2022, 4326638. [Google Scholar] [CrossRef]
- Mishra, V.; Thakur, S.; Patil, A.; Shukla, A. Quality by design (QbD) approaches in current pharmaceutical set-up. Expert Opin. Drug Deliv. 2018, 15, 737–758. [Google Scholar] [CrossRef] [PubMed]
- Mercier, S.M.; Diepenbroek, B.; Wijffels, R.H.; Streefland, M. Multivariate PAT solutions for biopharmaceutical cultivation: Current progress and limitations. Trends Biotechnol. 2014, 32, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, J.H.; Kim, M.S.; Jeong, S.H.; Choi, D.H. Process Analytical Technology Tools for Monitoring Pharmaceutical Unit Operations: A Control Strategy for Continuous Process Verification. Pharmaceutics 2021, 13, 919. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.M.; Wang, Y.T.; Zhang, J.C.; Wang, S.F. Advances in the chemical constituents and chemical analysis of Ginkgo biloba leaf, extract, and phytopharmaceuticals. J. Pharm. Biomed. Anal. 2021, 193, 113704. [Google Scholar] [CrossRef]
- Noor-E-Tabassum; Das, R.; Lami, M.S.; Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Idroes, R.; Mohamed, A.A.; Hossain, M.J.; Dhama, K.; et al. Ginkgo biloba: A Treasure of Functional Phytochemicals with Multimedicinal Applications. Evid. Based Complement. Altern. Med. 2022, 2022, 8288818. [Google Scholar] [CrossRef]
- Liu, Y.X.; Xin, H.W.; Zhang, Y.C.; Che, F.; Shen, N.; Cui, Y. Leaves, seeds and exocarp of Ginkgo biloba L. (Ginkgoaceae): A Comprehensive Review of Traditional Uses, phytochemistry, pharmacology, resource utilization and toxicity. J. Ethnopharmacol. 2022, 298, 115645. [Google Scholar] [CrossRef]
- Blumberg, L.M. Practical limits to column performance in liquid chromatography-Optimal operations. J. Chromatogr. A 2020, 1629, 461482. [Google Scholar] [CrossRef]
- Li, W.; Yan, X.; Chen, H.; Qu, H. In-line Vis-NIR spectral analysis for the column chromatographic processes of Ginkgo biloba part I: End-point determination of the elution process. Chemom. Intell. Lab. Syst. 2018, 172, 159–166. [Google Scholar] [CrossRef]
- Kim, B.; Woo, Y.A. Optimization of in-line near-infrared measurement for practical real time monitoring of coating weight gain using design of experiments. Drug Dev. Ind. Pharm. 2021, 47, 72–82. [Google Scholar] [CrossRef]
- Zhang, D.J.; Sun, L.L.; Mao, B.B.; Zhao, D.S.; Cui, Y.L.; Sun, L.; Zhang, Y.X.; Zhao, X.; Zhao, P.; Zhang, X.L. Analysis of chemical variations between raw and wine-processed Ligustri Lucidi Fructus by ultra-high-performance liquid chromatography-Q-Exactive Orbitrap/MS combined with multivariate statistical analysis approach. Biomed. Chromatogr. 2021, 35, e5025. [Google Scholar] [CrossRef]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N. Multi-and Megavariate Data Analysis: Principles and Applications; Umetrics: Umea, Sweden, 2006. [Google Scholar]
- Sheng, X.C.; Xiong, W.L. Soft sensor design based on phase partition ensemble of LSSVR models for nonlinear batch processes. Math. Biosci. Eng. 2019, 17, 1901–1921. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.M.; Li, S.N.; Hu, Y.F.; Zeng, X.Y.; Qiu, P.; Li, Y.X.; Li, W.L.; Li, Z. Quality assessment of Succus Bambusae oral liquids based on gas chromatography/mass spectrometry fingerprints and chemometrics. Rapid Commun. Mass Spectrom. 2021, 35, e9200. [Google Scholar] [CrossRef] [PubMed]
- Clavaud, M.; Lema-Martinez, C.; Roggo, Y.; Bigalke, M.; Guillemain, A.; Hubert, P.; Ziemons, E.; Allmendinger, A. Near-Infrared Spectroscopy to Determine Residual Moisture in Freeze-Dried Products: Model Generation by Statistical Design of Experiments. J. Pharm. Sci. 2020, 109, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.R.; Avila, C.; Bourne, R.; Muller, F.; Juan, A. Data fusion strategies to combine sensor and multivariate model outputs for multivariate statistical process control. Anal. Bioanal. Chem. 2020, 412, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Cui, T.C.; Zhang, Z.Y.; Li, Z.; Yang, M.; Zang, Z.Z.; Li, W.L. Real-time monitoring of the column chromatographic process of Phellodendri Chinensis Cortex part II: Multivariate statistical process control based on nearinfrared spectroscopy. New J. Chem. 2022, 46, 10690–10699. [Google Scholar] [CrossRef]
- Wang, X.J.; Xie, Q.; Liu, Y.; Jiang, S.; Li, W.; Li, B.; Wang, W.; Liu, C.X. Panax japonicus and chikusetsusaponins: A review of diverse biological activities and pharmacology mechanism. Chin. Herb. Med. 2021, 13, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, Y.Z.; Li, B.; Peng, C.Y.; Yang, Y.P.; Wang, W.; Liu, C.X. A review of lignans from genus Kadsura and their spectrum characteristics. Chin. Herb. Med. 2021, 13, 157–166. [Google Scholar] [CrossRef]
- Wang, N.N.; Chen, T.; Yang, X.; Shen, C.; Li, H.M.; Wang, S.; Zhao, J.Y.; Chen, J.L.; Chen, Z.; Li, Y.L. A practicable strategy for enrichment and separation of four minor flavonoids including two isomers from barley seedlings by macroporous resin column chromatography, medium-pressure LC, and high-speed countercurrent chromatography. J. Sep. Sci. 2019, 42, 1717–1724. [Google Scholar] [CrossRef]
- Wang, R.; Li, W.; Chen, Z. Solid phase microextraction with poly(deep eutectic solvent) monolithic column online coupled to HPLC for determination of non-steroidal anti-inflammatory drugs. Anal. Chim. Acta 2018, 1018, 111–118. [Google Scholar] [CrossRef]
- Choudhary, S.; Herdt, D.; Spoor, E.; García Molina, J.F.; Nachtmann, M.; Rädle, M. Incremental Learning in Modelling Process Analysis Technology (PAT)—An Important Tool in the Measuring and Control Circuit on the Way to the Smart Factory. Sensors 2021, 21, 3144. [Google Scholar] [CrossRef]


| Batch No. | Volumes of Concentrated Solution Used in a Batch (L) | Flow Rate of Elution Solvent (mL·min−1) | Ethanol Concentrations in the Elution Solvent (v/v, %) |
|---|---|---|---|
| 1–6 | 6.66 | 25 | 70 |
| 7 | 4.50 | 25 | 70 |
| 8 | 9.00 | 25 | 70 |
| 9 | 6.66 | 25 | 50 |
| 10 | 6.66 | 25 | 90 |
| 11 | 6.66 | 15 | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, X.; Chen, H.; Yan, X.; Qu, H. In-Line Vis-NIR Spectral Analysis for the Column Chromatographic Processes of the Ginkgo biloba L. Leaves. Part II: Batch-to-Batch Consistency Evaluation of the Elution Process. Separations 2022, 9, 378. https://doi.org/10.3390/separations9110378
Li W, Wang X, Chen H, Yan X, Qu H. In-Line Vis-NIR Spectral Analysis for the Column Chromatographic Processes of the Ginkgo biloba L. Leaves. Part II: Batch-to-Batch Consistency Evaluation of the Elution Process. Separations. 2022; 9(11):378. https://doi.org/10.3390/separations9110378
Chicago/Turabian StyleLi, Wenlong, Xi Wang, Houliu Chen, Xu Yan, and Haibin Qu. 2022. "In-Line Vis-NIR Spectral Analysis for the Column Chromatographic Processes of the Ginkgo biloba L. Leaves. Part II: Batch-to-Batch Consistency Evaluation of the Elution Process" Separations 9, no. 11: 378. https://doi.org/10.3390/separations9110378
APA StyleLi, W., Wang, X., Chen, H., Yan, X., & Qu, H. (2022). In-Line Vis-NIR Spectral Analysis for the Column Chromatographic Processes of the Ginkgo biloba L. Leaves. Part II: Batch-to-Batch Consistency Evaluation of the Elution Process. Separations, 9(11), 378. https://doi.org/10.3390/separations9110378






