1. Introduction
Smoking combustible cigarettes (CC) is the number one cause of preventable death [
1] and their prolific use has a dramatic negative impact on public health. While no tobacco product is safe, the FDA established the Comprehensive Plan for Tobacco, which describes nicotine delivery through the perspective of a continuum of risk, with combustible cigarettes at the highest-risk end and nicotine replacement therapies at the lowest-risk end of the continuum. This perspective is based in large part on evidence and understanding that nicotine is not responsible for serious disease and death in cigarette smokers [
2]. The serious disease and premature death that result from cigarette smoking is due to the combination of thousands of other chemical constituents known to be in cigarette smoke, including the chemicals on the FDA’s established list of 93 harmful or potentially harmful constituents (HPHCs) recognized in tobacco products [
3], that are responsible for causing over 8 million deaths each year and 30% of cancer-related deaths overall [
4,
5].
In contrast to CCs, electronic nicotine delivery systems (ENDS) were designed to use a combustion-free delivery mechanism. ENDS generally consist of a battery, a heating element and a reservoir for storing nicotine-containing liquid. The chemical composition of CC smoke has been well studied [
6] and much is known about the harmfulness of smoking CCs [
1]. Studies have indicated that ENDS use is less harmful than CC smoking [
7,
8,
9,
10,
11] but there are still questions about the potential harmfulness of ENDS aerosols. These questions are rooted in the fact that much of our understanding regarding the harmfulness of cigarette smoking is founded on an understanding of the chemical constituents which are produced during combustion in the production of mainstream smoke. The processes associated with combustion produce degradation of the tobacco plant materials, paper, and non-tobacco ingredients. Because of this, the smoke generated from combustion is a complex mixture of more than 5000 chemical constituents, including carcinogenic, mutagenetic and respiratory toxicants [
6,
12,
13,
14,
15,
16,
17,
18,
19]. However, these processes associated with combustion are not found in aerosols from e-liquids, so it is not surprising that ENDS aerosols have been reported to contain greatly reduced amounts of combustion byproducts. Nevertheless, the question of additional potentially harmful constituents in ENDS aerosols warrants further research, as it has been reported that flavorants in e-liquids may impact the chemical toxicants present in the resultant aerosols [
20,
21,
22,
23]. Others are interested in addressing the concern that there may be harmful constituents unique to ENDS aerosols—including regulators and public health organizations. Consistent with this identified interest, on 5 August 2019, the FDA proposed the addition of 19 ENDS-specific chemicals to the HPHC list [
3].
Targeted aerosol analysis methods have provided a valuable understanding of ENDS aerosol chemistry; these targeted chemical constituents in ENDS aerosols have been well studied. However, there are limitations associated with these targeted methods in that such analyses are limited to what is known about the chemical constituents of interest beforehand [
22,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33]. Resultantly, these methods leave unsampled portions of the aerosol, which causes blind spots in the characterization of the aerosol’s chemical constituents, especially when the composition may change as a result of flavorants. Several research groups [
34,
35,
36] have published studies on the non-targeted analysis (NTA) of aerosols from combustible and heated tobacco products [
34,
35,
36] and ENDS aerosols [
35,
37,
38,
39,
40,
41,
42,
43]. In order to better understand the flavor-dependent chemical composition of menthol flavored ENDS aerosols vs. our previously published work on tobacco-flavored E-liquids [
39], we applied a non-targeted analysis approach developed to capture, detect, identify, and semi-quantitate chemical constituents with a broad range of properties, including polar and non-polar chemical constituents [
44]. Our key objectives in this study were to (1) detect chemical constituents not included in the FDA’s established or proposed list of HPHCs, (2) evaluate chemical constituents present in menthol-flavored JUUL aerosols vs. our previously published work on tobacco-flavored JUUL aerosols [
39], (3) provide semi-quantitative information on the chemical constituents detected, and (4) evaluate the complexity and commonalities of JUUL aerosols compared to cigarette smoke [
6]. Non-targeted analyses have been previously shown to be well-suited, and are widely accepted, for analysis of complex matrices, including tobacco smoke [
45,
46,
47,
48,
49,
50,
51,
52,
53,
54]. Our non-targeted analysis included a set of two complementary non-targeted methods. The GC-EI-MS method [
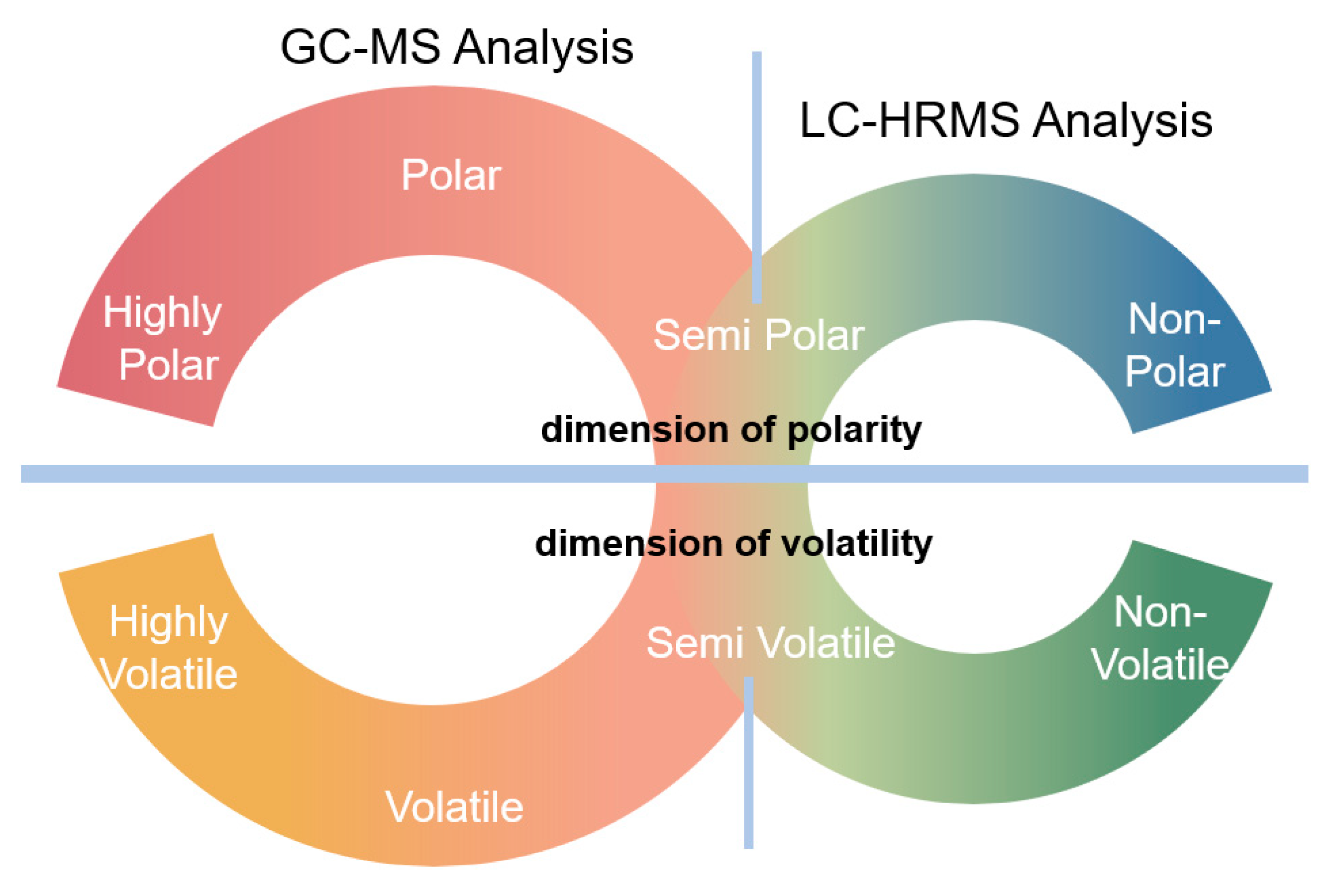
55] was developed to be suitable for volatile and higher-polarity (see
supplemental information for details on the polar column used in the GC-EI-MS analysis) chemical constituents, and the LC-HRMS method [
39] was developed to be suitable for less-volatile and less-polar chemical constituents (see
supplemental information for details on the reversed phase column used in the LC-HRMS analysis).
2. Materials and Methods
As previously described [
39], two semi-quantitative non-targeted analytical methods were implemented to provide a more complete list of aerosol chemical constituents. These NTA methods were developed for the detection and identification of chemical constituents from covering a wide range of chemical space (
Figure 1). The LC-HRMS system consisted of a Q-Exactive Orbitrap mass spectrometer (Thermo, Waltham, MA) coupled to a Vanquish Horizon-Class liquid chromatograph with an autosampler and heated column compartment (Thermo). Sample introduction and chromatographic separation was performed on 100 nL of sample using a Waters BEH C18 (2.1 × 100 mm, 1.7 µm) column with a mobile phase gradient. Full scan data were collected across M/Z 60–800 at resolving power 140,000. Data-dependent MS/MS fragments were generated by collision of 3 arbitrary energies and mass spectra were collected from M/Z 60–800 with a resolving power of 17,500. The GC-MS systems consisted of an Agilent 7890GC/5977MSD gas chromatograph equipped with an electron ionization source. The separation was performed on 1 uL of sample using a Restek Stabilwax (30 m × 0.25 mm × 0.25 μm) column with a Restek (5 m) integra guard column, using an oven program and an inlet split ratio of 5:1. Full scan data were collected across M/Z 35–450 at unit resolving power. Fragments were generated using electron impact ionization at 70 eV. The
Supplemental Tables S1–S3 contain full details on the LC and MS instrument parameters.
To prevent artifacts and reduce sample bias, samples were analyzed without sample clean-up (absent of any matrix removal steps). This approach minimized sample manipulation and potential analyte loss. This approach was used to capture a full range of diverse chemical constituents. Aerosol-relevant constituents were differentiated from room air controls based on the analysis of three collection replicates from each of the three production batches (
n = 9) of sample and three replicates of room air controls [
39].
Except for those chemical constituents, some of which were HPHCs, which were quantitated using targeted analytical methods and as published by Chen et al. [
56], all chemical constituents detected in the aerosols were identified and assigned an identification confidence and were categorized (
Table 1) as previously described [
39]. The chemical constituents that were detected and were contained in the flavor library of the Flavor Extract Manufacturers Association (FEMA) [
57] were assigned as flavorants. Chemical constituents found in the FDA’s Tobacco Products and Tobacco Smoke: Established List [
3] and were not detected by Chen et al. [
58] were assigned as HPHCs. Chemical constituents known to migrate from packaging materials of consumer products [
59,
60]—such as a siloxane—were assigned as leachables. Chemical constituents proposed to form from chemical reactions, except when the product is an HPHC, were assigned as reaction products (
Figure 2). All other chemical constituents that were not able to be identified or rationalized were assigned to group 5.
In line with the previously published work and in line with recommendations from the FDA in the final rule on premarket tobacco product applications for ENDS, intense and non-intense puffing parameters were used to generate aerosols from the JUUL system as previously described (
Table 2) [
39,
61]. Puffing count was determined from an end-of-life (EOL) study. EOL is the total number of machine puffs required for the depletion of a JUUL pod. Similar to the approach by Belushkin et al. [
62], puff count was set to achieve 85–90% of total aerosol yield for both puffing regimens.
3. Results
All chemical constituents from LC–HRMS with a
p-value less than 0.05 [
63] were reported. As reported previously, for GC–MS NTA results, all chemical constituents determined to be different from the control were reported [
39,
55]. Due to their high concentrations—making amount estimation unreliable—and because these compounds were reported by Chen et al. [
56], nicotine, PG, VG, and benzoic acid amounts are not reported here. Quantitative values for these major constituents can be found in Chen et al. [
56]. Nornicotine, beta nicotyrine, menthol, myosmine, and diethylene glycol, which were detected in the NTA, are not reported because they were quantitated and published in a separate study [
56]. As glycidol is known to form from thermal degradation of glycerol under GC inlet temperatures of 260 °C [
64], and as this compound was reported using targeted methods [
56], it has been excluded from the NTA results. For a complete list of all detected compounds see
Supplemental Tables S4–S11.
3.1. Menthol 3.0% Nicotine (Me3)
In total, 74 chemical constituents were detected in the Me3 aerosol collected using intense puffing parameters, and 68 chemical constituents were detected using non-intense puffing parameters. All detections for Me3 aerosol (using both puffing parameters) using LC–HRMS and GC–MS are shown in
Supplementary Tables S1–S4. Six chemical constituents were detected in both GC-EI-MS- and LC-HRMS-based NTA methods, namely hydroxyacetone, 1-(1-methyl-5-(pyridine-3-yl)-1H-pyrrol-2-yl) propan-2-one isomer 1 and isomer 2, N,2,3-trimethyl-2-isopropylbutamide, caffeine, and (Z)-beta-damascone for both intense and non-intense aerosol samples. Because GC–EI-MS is less susceptible to analyte-specific ionization suppression or enhancement, when compared to LC-ESI-HRMS in instances when both LC–HRMS and GC–MS detected the same chemical constituent, the GC–MS-estimated amounts were used for the calculations in
Table 3,
Table 4, Tables 7 and 8.
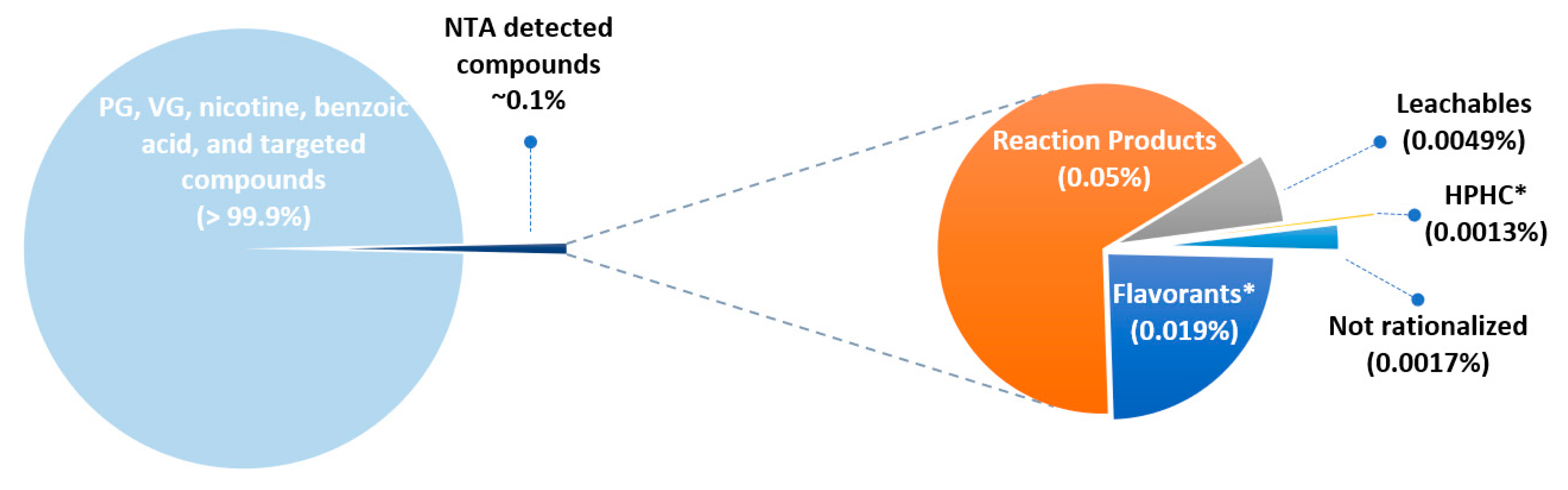
Figure 3 presents a summary of the constituents in the Me3 intense and non-intense aerosol detected by NTA on a percent basis for each group described in
Table 1. In generating
Figure 3, the higher concentration of all duplicate chemical constituents detected in both puffing regimes was used, and any unique chemical constituents from each puffing regime were reported. The five groups described in
Table 1 represent about 0.07% of the total aerosol mass. The remaining mass consisted of the major components PG, VG, nicotine, benzoic acid, and the chemical constituents, some of which were HPHCs, which were targeted and published by Chen et al. [
56].
Table 3 presents a summary of the number of constituents and their mass percent for the Me3 intense puffing regimen. Reaction products made up 55% of the number of chemical constituents identified. However, reaction products contributed to less than one-tenth of one percent (0.05%) of the total aerosol mass. Flavorants(excluding methanol)made up 30% of the total number of chemical constituents and 0.018% of the aerosol. Leachables contributed 10% of the total number of chemical constituents and a small percentage of the total aerosol mass (0.0049%). HPHCs in intense Me3 aerosols were not detected by this NTA methodology, but have been reported by our group using targeted analysis elsewhere [
56]. There was much less chemical complexity detected for M3 aerosols vs. CC smoke. This is mostly because many chemical constituents are byproducts which are formed during the combustion of tobacco plant materials and paper. Only 0.0005% of the total aerosol mass was unable to be identified or rationalized (group 5).
Table 4 presents a summary of the number of constituents and their mass percent for the Me3 non-intense puffing regimen data. Reaction products made up 59% of the number of chemical constituents identified. However, reaction products contributed to less than one percent (0.05%) of the total aerosol mass. Flavorants made up about 30% of the total number of chemical constituents and 0.019% of the aerosol mass. Leachables contributed 9% of the chemical constituents by count and a small percentage by mass (0.0037%). HPHCs in Me3 non-intense aerosols were not detected by this NTA methodology, but have been reported by our group using targeted analysis of Me3 elsewhere [
56]. Only 0.0017% of the total aerosol mass was unable to be identified or rationalized (group 5).
As summarized in
Table 5, 53 common chemical constituents were determined using the LC and/or GC NTA methods for each group under both puffing regimes’ chemical constituent. There were 21 chemical constituents identified only in the intense puffing regimen (30% of 74 chemical constituents), and there were 15 chemical constituents identified only in the non-intense puffing regimen (21% of 68 chemical constituents). Therefore, the total number of chemical constituents in Me3 was determined to be 89 (53 common identifications + 21 only detected in intense + 15 only detected in non-intense). For a list of chemical constituents used to generate
Table 5 organized by group, see
Supplemental Tables S11 and S12. Excluding those compounds targeted and published by Chen et al. [
56] and nicotine, benzoic acid, PG, and VG, the chemical constituents reported here make up approximately 0.073% and 0.075% of the aerosol mass under intense and non-intense puffing conditions, respectively.
The number of chemical constituents detected using the LC and/or GC NTA methods from non-intense puffing was slightly lower than the number of chemical constituents detected using the intense regimen. However, the average mass under non-intense puffing was higher than the intense regimen. Approximately 25% of the mass was attributed to flavorants, making this category represent the second highest percentage by mass and by number of chemical constituents. Considering both puffing regimens, there were nine chemical constituents categorized as leachable. Most of the chemical constituents determined to be reaction products were associated with PG-, VG- or nicotine-related degradation.
A global compilation of the approximately 5000 chemical constituents in CC smoke catalogued by Rodgman and Perfetti [
6] was compared to the 89 chemical constituents (53 common identifications + 21 unique identifications in intense + 15 unique identifications in non-intense) detected in the Me3 aerosol. This comparison was carried out using CAS numbers, which means that (1) unless a chemical constituent in the JUUL aerosols was fully identified and had a CAS number, it was treated as if it were exclusive to JUUL aerosol, and (2) we make no claim as to their concentration in CCs and only report their estimated amount in the JUUL aerosols. Of the 89 chemical constituents detected in Me3, 34 were common with CC smoke and 55 were exclusive to Me3 (
Supplemental Table S9). Of the 55 chemical constituents, 24 were termed exclusive to Me3 due to lack of CAS number and 13 were classified as nicotine degradants.
Table 6 summarizes the total number and aerosol mass represented by each group of the 55 exclusive chemical constituents in Me3 aerosol. The highest number of chemical constituents exclusive to Me3 aerosols were reaction products. A complete list of all chemical constituents either common with or exclusive from CC smoke is presented in
Supplementary Table S9.
3.2. Menthol 5.0% Nicotine (Me5)
Eighty-three chemical constituents were detected using the LC and/or GC NTA methods in intense Me5 aerosols and seventy-four were detected in non-intense aerosols. The complete list of aerosol constituents detected in Me5 aerosols (intense and non-intense) using LC–HRMS and GC–MS are shown in
Supplementary Tables S5–S8.
Considering both intense and non-intense data, a comparison of LC–HRMS and GC–MS results indicates that six chemical constituents were detected in both analyses, namely hydroxyacetone, N,2,3-trimethyl-2-isopropylbutamide, caffeine, 1-(1-methyl-5-(pyridin-3-yl)-1H-pyrrol-2-yl) propan-2-one isomer 1&2, and (Z)-beta-damascone.
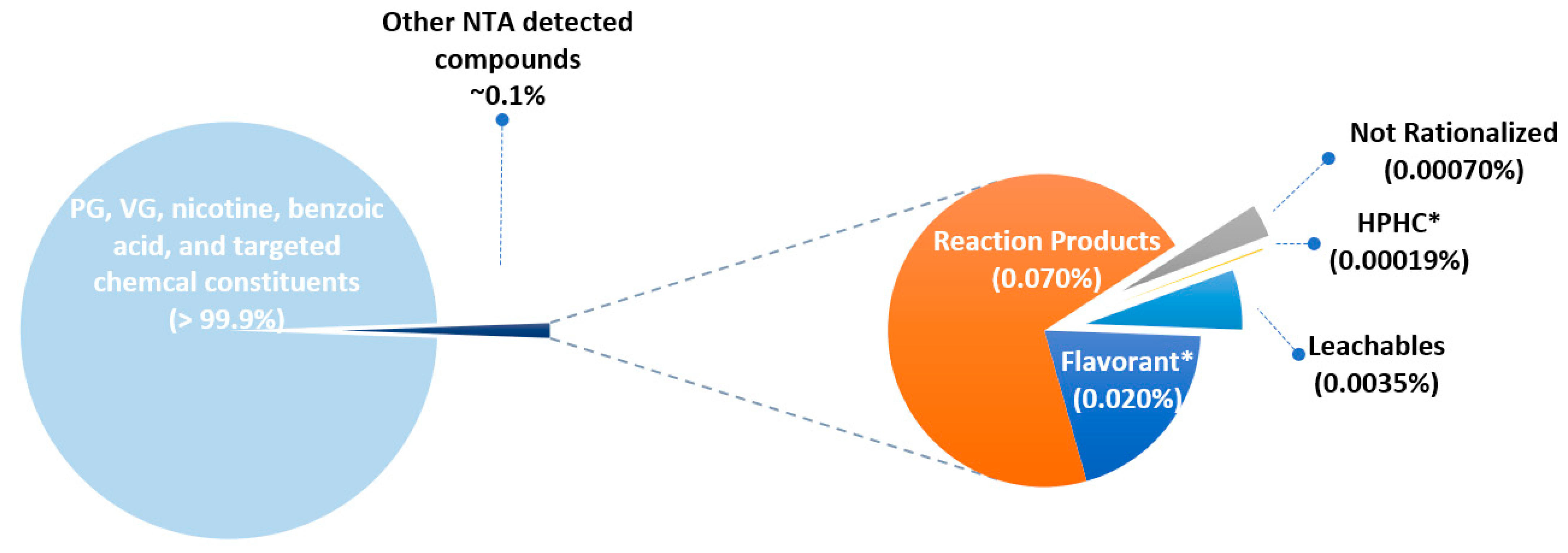
Table 7 presents a summary of the number of constituents and their mass percent for the Me5 intense puffing regimen. Reaction products made up 58% of the number of chemical constituents identified. However, reaction products contributed to less than one-tenth of one percent (0.07%) of the total aerosol mass. Flavorants—excluding methanol—made up 25% of the total number of chemical constituents and 0.02% of the aerosol. Leachables contributed to 10% of the total number of chemical constituents and a small percentage of the total aerosol mass (0.0036%). There was one HPHC (phenol) detected for the intense Me5 aerosol using these NTA methods which was not reported by Chen et al. [
56]. Only 0.0007% of the total aerosol mass was placed into group 5. There was much less chemical complexity detected for M3 aerosols vs. CC smoke. This is mostly because many chemical constituents are byproducts which are formed during the combustion of tobacco plant materials and paper. Only 0.0007% of the total aerosol mass was unable to be identified or rationalized (group 5).
Table 8 presents a summary of the number of constituents and their mass percent for Me5 non-intense puffing regimen data. Reaction products contributed to 64% of the number of chemical constituents identified. However, reaction products contribute less than one percent (0.06%) of the total aerosol mass. Flavorants contributed to about 25% of the total number of chemical constituents and 0.018% of the aerosol mass. Leachables contributed to 5% of the chemical constituents by count and a small percentage by mass (0.0015%). There was one HPHC (phenol) detected for the non-intense Me5 aerosol using these NTA methods. Only 0.0001% of the total aerosol mass was placed into group 5.
Figure 4 presents a summary of the constituents in the intense and non-intense Me5 aerosol detected by NTA on a percent basis for each group described in
Table 1. In generating
Figure 3, the higher concentration of all duplicate chemical constituents detected in both puffing regimes was used and any unique chemical constituents from each puffing regime were reported. The five groups described in
Table 1 represent about 0.1% of the total aerosol mass. The remainder consisted of the major components PG, VG, nicotine, benzoic acid, and the chemical constituents, some of which were HPHCs, which were targeted and published by Chen et al. [
56].
As summarized in
Table 9, a comparison of intense and non-intense puffing shows the effect of puffing regime on the generation of chemical constituents not reported by Chen et al. [
56]. A total of 83 chemical constituents were identified using the LC and/or GC NTA methods in the intense aerosol data, while fewer (75) constituents were identified in the non-intense aerosol data. The decrease in the number of chemical constituents correlates with a decrease in the total mass of chemical constituents identified in the NTA, 957.7 µg/g versus 823.2 µg/g, in the intense versus non-intense aerosol results. A decrease in the generation of reaction products, which includes unknown nicotine-related chemical constituents, accounts for the reduction in the number and mass of the chemical constituents in the non-intense compared to the intense puffing regime. Sixty-two common chemical constituents were determined for each group under both puffing regimes’ chemical constituents. There were 21 unique identifications (25% of 83 chemical constituents) for intense and 13 unique identifications (17% of 75 chemical constituents) for non-intense aerosol data. Therefore, the total number of chemical constituents in Me5% at T = 0 was determined to be 97 (61 common identifications + 22 unique identifications in intense + 14 unique identifications in non-intense). For a list of chemical constituents detected in each analysis, see the
Supplemental Materials.
In summary, the chemical constituents detected using the LC and/or GC NTA methods accounted for less than 0.1% of the total aerosol mass in both intense and non-intense aerosols, as determined by this NTA methodology. The total number of chemical constituents detected using non-intense collections was lower than that of the intense collections. Most of the mass detected was comprised of flavorants and reaction products. Additionally, the majority of reaction products were involved in either PG-, VG-, or nicotine-related degradation. There were four chemical constituents categorized as leachable under either puffing regimen. The small number of remaining chemical constituents could not be placed into one of these categories.
As with Me3, the 97 chemical constituents detected using the LC and/or GC NTA methods in the aerosol of Me5 were compared to the approximate 5000 chemical constituents in CC smoke catalogued by Rodgman and Perfetti. This comparison was performed using CAS numbers, which means that (1) unless a chemical constituent from the NTA was fully identified, it was labeled to be exclusive to JUUL aerosol, and (2) we make no claim as to their concentration in CCs and only report their estimated amount in the JUUL aerosols. Of the 97 chemical constituents detected in Me5, 36 were found to be in common with cigarette smoke and 61 were labeled as exclusive to Me5.
Table 10 summarizes the total number represented by each group of the 61 exclusive chemical constituents in Me5 aerosol (
Supplemental Table S10). Of the 43 reaction products exclusive to JUUL aerosols, 22 were nicotine degradants and 18 were without known structures. A complete list of all chemical constituents either in common with or exclusive from CC smoke is presented in
Supplemental Tables S12 and S13.
4. Discussion
Overall, the two non-targeted methods employed in these experiments detected 89 (Me3) and 97 (Me5) chemical constituents (excluding PG, VG, nicotine, benzoic acid, and the chemical constituents which were targeted and published by Chen et al. [
56]). Flavorants contributed approximately 25%, and reaction products, including nicotine-related degradants, contributed about 75% of the aerosol mass detected. A small amount of remaining mass consisted of E&L, HPHCs, and group 5 chemical constituents. Overall, the total mass of the five groups accounted for less than 0.1% of the total aerosol mass, with flavorants and reaction being higher in number mass. More chemical constituents were present in the 5.0% nicotine concentration product than the 3.0% nicotine product, and more chemical constituents were identified in aerosols generated with the intense puffing regimen than the non-intense puffing regimen. There was, however, very little distinction between the various groups. The consistency observed across nicotine strengths and puffing parameters in the JUUL system aerosol is in part due to the temperature regulation [
65,
66], which makes performing a detailed and reproducible characterization of the aerosols achievable.
In a collaborative study to address the effectiveness of various approaches to non-targeted methods, the US EPA [
67] performed a study using 1269 unique substances from EPA’s ToxCast library. Samples spiked with these substances were sent to a cross-section of government, university and private labs to be tested using various NTA methods, namely GC-EI-MS, LC-MS with positive mode (+ESI) and LC-MS with negative mode (−ESI). The outcome of these studies showed that 195 of the chemical constituents were not detected by any methods and only 75 were detected exclusively by LC-MS −ESI. The other 999 chemical constituents were detected by either LC-MS +ESI or GC-EI-MS. This study strongly indicates that the two most robust non-targeted approaches are GC-EI-MS and LC-MS (+ESI). Resultantly, we employed the two complimentary techniques of GC-EI-MS [
55] and LC-HRMS +ESI [
39]. These techniques were developed to be orthogonal to each other in order to detect the largest range of chemical constituents possible. The GC–MS method uses a polar column for retention of volatile and polar chemical constituents [
55]. The LC–HRMS method uses a reversed-phase column for the retention of non/semi-polar chemical constituents and is amenable to detection of larger-molecular-weight chemical constituents, including extractable and leachable compounds (
Figure 1). These approaches estimate the amount of each chemical constituent detected by comparison to an internal standard in a semi-quantitative fashion. As is the case with all methods, there are limitations with these non-targeted methods, and not all chemical constituents present in the aerosol (e.g., metals and chemicals that require specialized detection approaches, such as carbonyls) are detectable under these methods. For example, to cover a broad range of chemical properties, the GC NTA method was developed to be complimentary to the LC NTA. Therefore, the GC method employed a Stabilwax column. The use of a Stabilwax column precludes the characterization of some low-molecular-weight, low-polarity compounds. One such low-molecular-weight and non-polar compound, which is known to be present in these JUUL aerosols but was not detected using these NTA methods, is formaldehyde. However, formaldehyde has been quantified using targeted methods and these results have been published as part of a separate study by Chen et al. [
56]. Other such low-molecular-weight and low-polarity compounds, such as 1,3-butadiene, benzene, acrolein, acetaldehyde, and toluene, were not detected in the NTA analysis and correspondingly not detected in the targeted work by Chen et al. [
56], but have been detected in ENDS products not addressed in this work.
A challenging aspect of NTA comes from defensibly and reliably distinguishing between background and sample-relevant peaks. This owes to the fact that relying only on background subtraction to determine which chemical constituents are sample-relevant in non-targeted analyses where molecular ions are monitored, as is the case for LC-HRMS approaches [
68], is not sufficient. In these cases, robust approaches are necessary to extract the relevant information from the vast amount of data generated from non-targeted analyses [
69], and sample-relevant chemical constituents must be determined using advanced approaches, such as multivariant techniques. This is necessary because chemical contaminants that are captured by blanks vary across data sets, which can lead to misassignment as sample-relevant chemical constituents [
68].
There is still significant debate about background subtraction and/or control differentiation [
39,
70,
71,
72] and how it should or should not be applied to the NTA of ENDS aerosols. It should be noted that background subtraction in GC-EI-MS analyses is substantially different from background subtraction in LC-HRMS. In approaches where multi-ion spectra are undergoing deconvolution, as is the case in GC-EI-MS, software packages use peak picking algorithms (e.g., Mass Hunter) which require that a minimum number of associated ions in the mass spectrum rise and fall in intensity and with the same retention times to form a peak together. This alone eliminates many of the sources of false positives, which result from several causes including instrument and random noise. These intermittent signals often do not meet the deconvolution criteria for GC-EI-MS. However, for LC-HRMS +ESI, when molecular parent ions are being measured under soft ionization conditions (e.g., LC-HRMS), there is no deconvolution; the detection of a
m/
z alone is sufficient to be reported by the software. This is relevant because even without any sample being introduced to a high-resolving-power mass spectrometer, there are hundreds (if not thousands) of ions detected. This can be further complicated when work is performed in urban areas with high levels of air pollution, where a wide range of chemicals, including chemicals that overlap with the FDA’s HPHC list, are commonly found in ambient air [
73,
74]. Therefore, special consideration was given to possible experimental artefacts often present in non-targeted LC-HRMS data sets, many of which are introduced as part of the sample preparation process itself [
68]. Aerosol collection controls are necessary to identify which chemical constituents arise from the ENDS aerosols as opposed to those derived from the sample preparation process (e.g., pipette tips, vials, tubing, room air, etc.) or room air. Because of this contamination from ambient air, the routine collection of puffing machine room air blanks are needed to distinguish between collection artifacts and sample-related detections.
Erroneous detections are often reported when care is not taken to eliminate them in a defensible manner. This contributes to the need for advanced data extraction techniques when molecular ions are being monitored, such as in the case of LC-HRMS ESI. To address these known challenges, differential analyses based on nine (
n = 9) collection replicates of each of the nicotine strengths (3.0% and 5.0%) and each collection condition (intense and non-intense) were used to characterize chemical constituents differing from collection blanks. This method relies on the application of statistical tools to extract the relevant information from a large and highly complex dataset [
67,
68]. Due to the large number of variables in non-targeted analyses relative to the number of samples, these tools are imperative to avoid misinterpretation of instrument and collection artifacts as sample-relevant chemical constituents [
75]. LC–HRMS and GC-EI-MS data were collected and processed according to previously reported methods [
39,
55]. An internal standard was used to provide an estimation of the amount of each chemical constituent detected in these non-targeted methods. However, these non-targeted methods are semi-quantitative. For non-targeted methods, the addition of more internal standards, unless they are analogues (e.g., isotopologues) of the chemical constituent of interest, will not permit precise quantitation of the chemical constituents detected. This is due to the fact that there are many factors that impact chemical constituent ionization efficiency [
76]. This is an important limitation of NTA to be understood; otherwise, non-targeted data are at risk of being over-interpretated.
Low injection volumes are helpful in preserving peak shape and alleviating situations where the analyte would be competing for ionization with the matrix [
77]. This is especially important in non-targeted methods where there is no matrix removal step in the sample preparation. Low injection volume also allows a method to be applied to a range of matrices, e.g., Virginia Tobacco- and Menthol-flavored aerosols. There is a balance to find in non-targeted methods with respect to injection volume. Too large a volume will cause peak broadening and instrument contamination, while too low a volume will reduce sensitivity. In this LC-HRMS method, we found that our chosen 100 nL injection volume was suitable for a wide range of e-liquids and aerosol flavor systems.
Evaluating aerosols from e-liquids with different flavor systems is important in gaining a better understanding of the chemical composition from the resulting aerosol. Likewise, measuring only chemical constituents that are found in cigarette smoke leaves a gap in the assessment of ENDS aerosols [
78,
79]. Therefore, an important way to use non-targeted data is to leverage them so that a global comparison can be performed; in this case, the relevant comparison is against CC smoke. In addition to the specific links between HPHCs and tobacco related diseases, it is known that there are additional risks related to the chemical complexity of CC smoke [
19]. Furthermore, it is well established that the chemical complexity of CC smoke makes it difficult to determine the active constituents responsible for all the tobacco-related health risks of smoking. An important component of the negative health effects of CC smoke is related to the combined effect of these chemical constituents through multiple mechanisms rather than just the effects of a single smoke constituent, indicating that the chemical complexity by itself contributes to the harmfulness of cigarette smoke [
80]. Therefore, in addition to holistic evaluation for reasons associated with the flavor components (in this case menthol), understanding the chemical complexity of JUUL aerosols in relation to CC smoke may aid in determining the relative potential health risks of using JUUL as an alternative to smoking for smokers who have not yet quit. Given that (1) JUUL aerosols are fundamentally different to cigarette smoke in part because of the difference in the regulated electronic heating of the JUUL device [
65,
81,
82,
83] versus the high-temperature combustion of a CC, and (2) there is no tobacco plant material—excluding tobacco-derived [pharmaceutical grade] nicotine—nor paper in JUUL products, much less chemical complexity is observed.
The comparison of aerosol constituents detected using NTA to the list of chemical constituents in cigarette smoke catalogued by Perfetti and Rodgman [
6] resulted in 55 unique identifications out of 89 total chemical constituents in Me3 and 61/97 in Me5. Although the NTA methods applied here were developed to be effective and comprehensive based on the results of the EPA collaborative study to evaluate non-targeted method performance [
67], no methodology can detect all chemicals. Nevertheless, based on the results of the non-targeted methods used in this study, it was determined that the JUUL Menthol aerosols studied here are approximately 50-fold less complex when compared to cigarette smoke. The present study helps construct a more complete appraisal of the full chemical space of JUUL Menthol aerosols to complement the previous non-targeted and targeted analyses of JUUL Virginia Tobacco aerosols [
56].