Effect of Electrofiltration on the Dewatering Kinetics of Arthrospira platensis and Biocompound Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Arthrospira platensis Suspensions
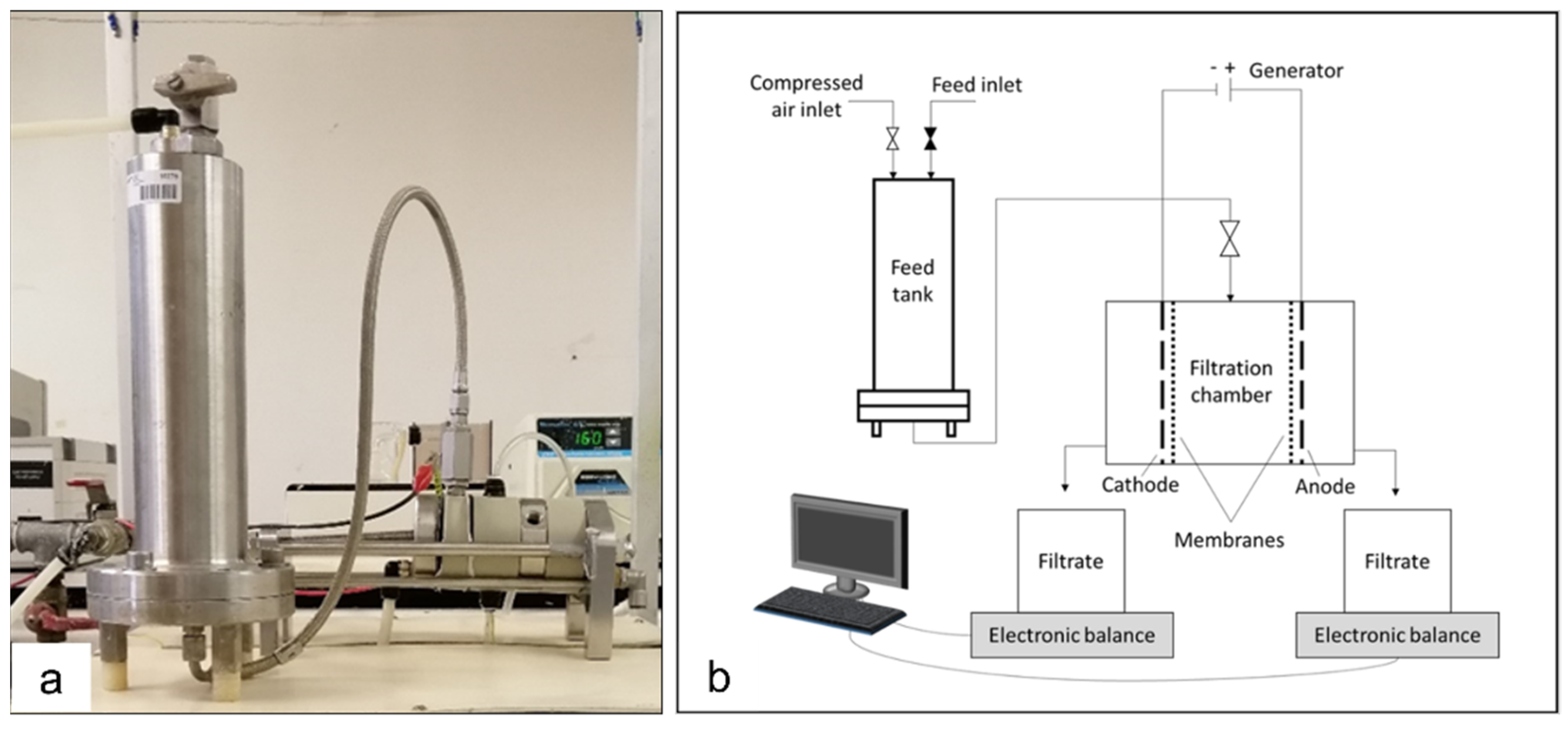
2.2. Dead-End Filtration and Electrofiltration
2.3. Freeze Drying the Filter Cake
2.4. Filtrate and Filter Cake Characterization
2.4.1. Protein Characterization
2.4.2. Pigment Characterization
2.4.3. Filter Cake Dryness
2.5. Energy Consumption
2.6. Statistical Analyses
3. Results and Discussion
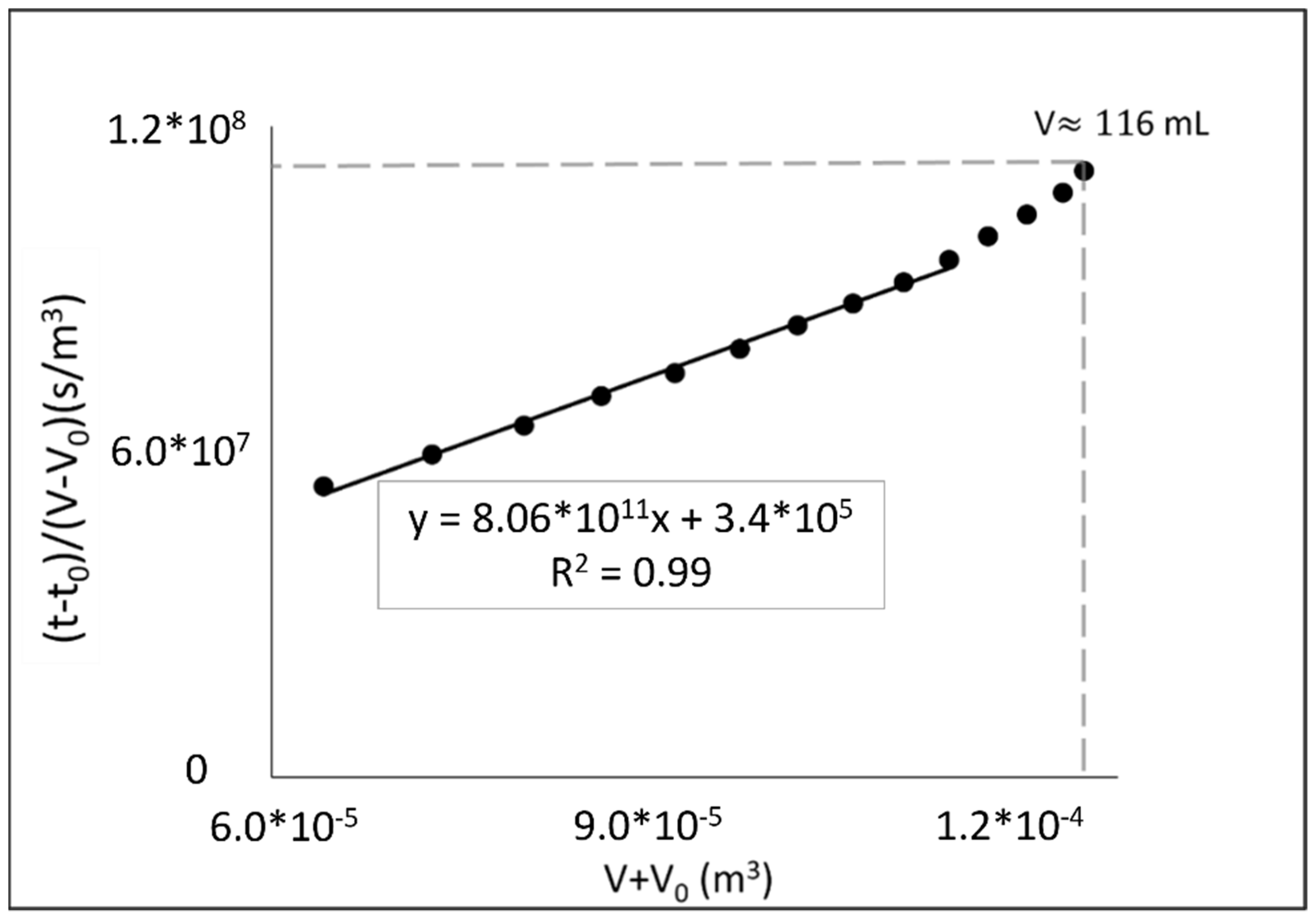
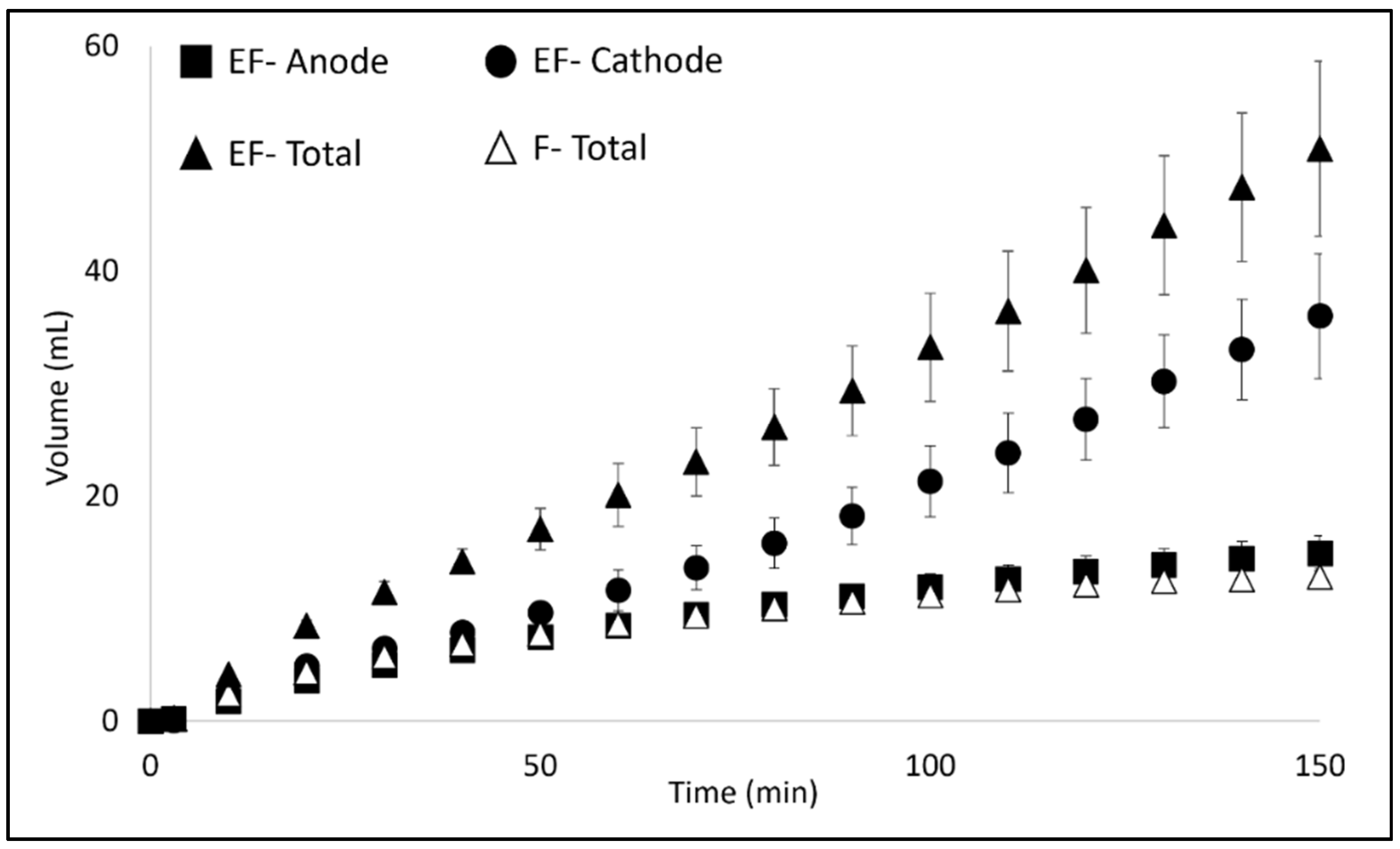
3.1. Filtration and Electrofiltration Behavior
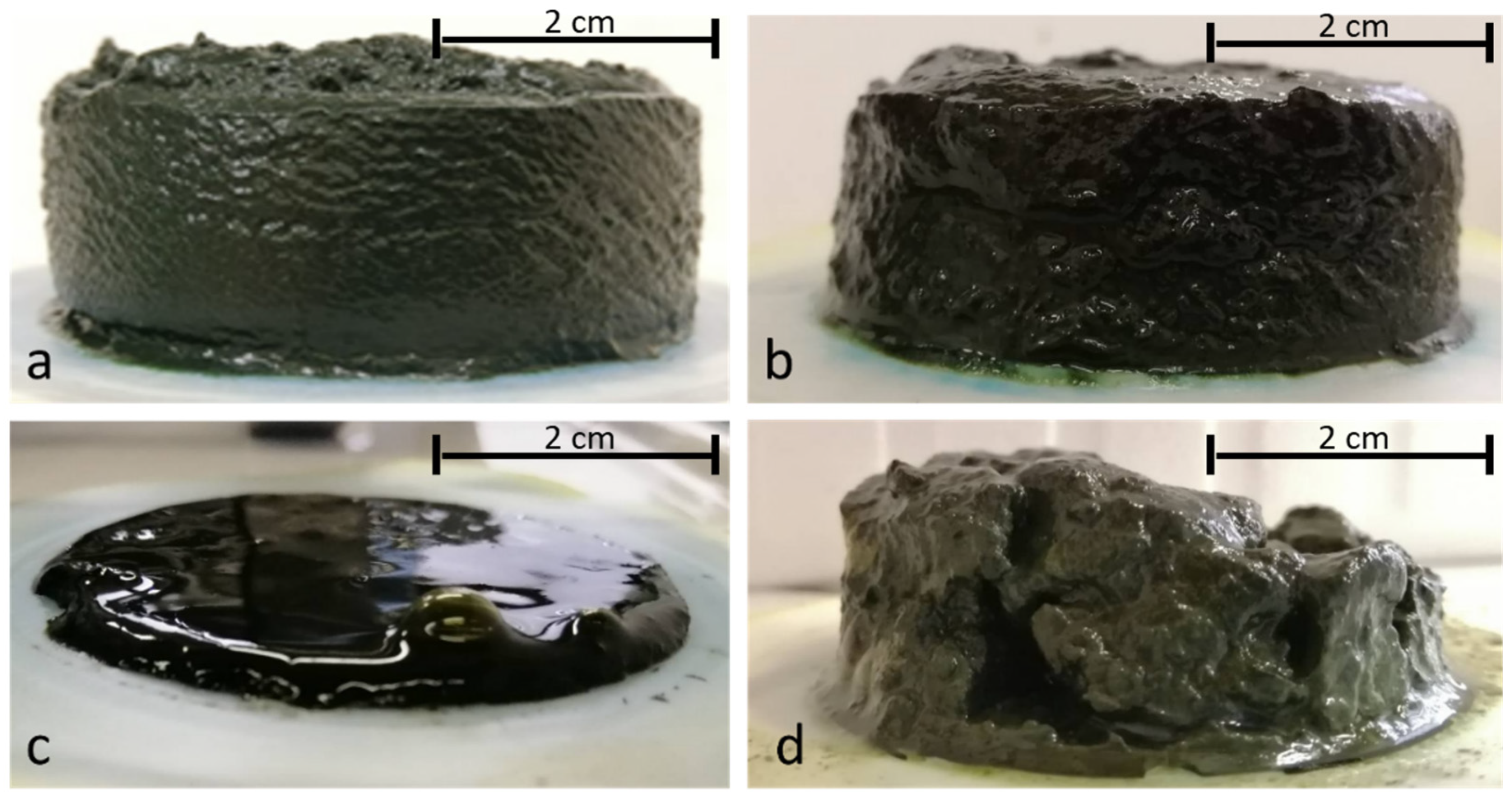
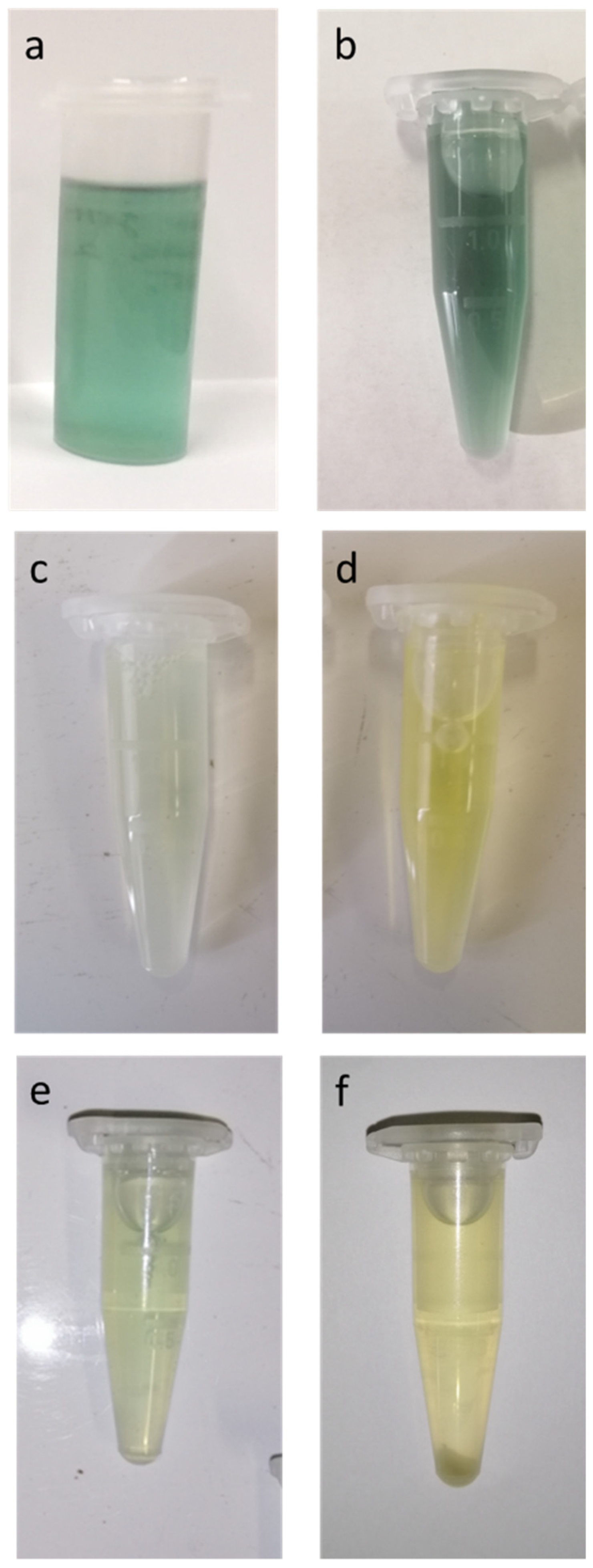
3.2. Qualitative Characteristics of Extract Solutions and Filter Cakes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae Biomass as an Alternative Ingredient in Cookies: Sensory, Physical and Chemical Properties, Antioxidant Activity and in Vitro Digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Sahin, S.C. The Potential of Arthrospira Platensis Extract as a Tyrosinase Inhibitor for Pharmaceutical or Cosmetic Applications. S. Afr. J. Bot. 2018, 119, 236–243. [Google Scholar] [CrossRef]
- Singh, U.; Singh, P.; Singh, A.K.; Laxmi; Kumar, D.; Tilak, R.; Shrivastava, S.K.; Asthana, R.K. Identification of Antifungal and Antibacterial Biomolecules from a Cyanobacterium, Arthrospira Platensis. Algal Res. 2021, 54, 102215. [Google Scholar] [CrossRef]
- Çelekli, A.; Alslibi, Z.A.; Bozkurt, H. üseyin Influence of Incorporated Spirulina Platensis on the Growth of Microflora and Physicochemical Properties of Ayran as a Functional Food. Algal Res. 2019, 44, 101710. [Google Scholar] [CrossRef]
- Han, P.; Li, J.; Zhong, H.; Xie, J.; Zhang, P.; Lu, Q.; Li, J.; Xu, P.; Chen, P.; Leng, L.; et al. Anti-Oxidation Properties and Therapeutic Potentials of Spirulina. Algal Res. 2021, 55, 102240. [Google Scholar] [CrossRef]
- Almeida, L.M.R.; Cruz, L.F.d.S.; Machado, B.A.S.; Nunes, I.L.; Costa, J.A.V.; Ferreira, E.d.S.; Lemos, P.V.F.; Druzian, J.I.; de Souza, C.O. Effect of the Addition of Spirulina sp. Biomass on the Development and Characterization of Functional Food. Algal Res. 2021, 58, 102387. [Google Scholar] [CrossRef]
- Adjali, A.; Clarot, I.; Chen, Z.; Marchioni, E.; Boudier, A. Physicochemical Degradation of Phycocyanin and Means to Improve Its Stability: A Short Review. J. Pharm. Anal. 2021, 12, 406–414. [Google Scholar] [CrossRef]
- Jespersen, L.; Strømdahl, L.D.; Olsen, K.; Skibsted, L.H. Heat and Light Stability of Three Natural Blue Colorants for Use in Confectionery and Beverages. Eur. Food Res. Technol. 2005, 220, 261–266. [Google Scholar] [CrossRef]
- Sarada, R.; Pillai, M.G.; Ravishankar, G. Phycocyanin from Spirulina Sp: Influence of Processing of Biomass on Phycocyanin Yield, Analysis of Efficacy of Extraction Methods and Stability Studies on Phycocyanin. Process Biochem. 1999, 34, 795–801. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and Phycoerythrin: Strategies to Improve Production Yield and Chemical Stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Srinorasing, T.; Attasat, S.; Nopharatana, A.; Bunnag, B. Enhanced Biomass and Phycocyanin Production of Arthrospira (Spirulina) Platensis by a Cultivation Management Strategy: Light Intensity and Cell Concentration. Bioresour. Technol. 2022, 343, 126077. [Google Scholar] [CrossRef]
- Yu, J.; Hu, H.; Wu, X.; Wang, C.; Zhou, T.; Liu, Y.; Ruan, R.; Zheng, H. Continuous Cultivation of Arthrospira Platensis for Phycocyanin Production in Large-Scale Outdoor Raceway Ponds Using Microfiltered Culture Medium. Bioresour. Technol. 2019, 287, 121420. [Google Scholar] [CrossRef]
- Rosero-Chasoy, G.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Buitrón, G.; Chairez, I.; Ruiz, H.A. Growth Kinetics and Quantification of Carbohydrate, Protein, Lipids, and Chlorophyll of Spirulina Platensis under Aqueous Conditions Using Different Carbon and Nitrogen Sources. Bioresour. Technol. 2021, 346, 126456. [Google Scholar] [CrossRef] [PubMed]
- Dixon, C.; Wilken, L.R. Green Microalgae Biomolecule Separations and Recovery. Bioresour. Bioprocess. 2018, 5, 1–24. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and Microalgae as a Potential Source for Commercial Applications along with Biofuels Production: A Biorefinery Approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Mehariya, S.; Fratini, F.; Lavecchia, R.; Zuorro, A. Green Extraction of Value-Added Compounds Form Microalgae: A Short Review on Natural Deep Eutectic Solvents (NaDES) and Related Pre-Treatments. J. Environ. Chem. Eng. 2021, 9, 105989. [Google Scholar] [CrossRef]
- Soto-Sierra, L.; Stoykova, P.; Nikolov, Z.L. Extraction and Fractionation of Microalgae-Based Protein Products. Algal Res. 2018, 36, 175–192. [Google Scholar] [CrossRef]
- Käferböck, A.; Smetana, S.; de Vos, R.; Schwarz, C.; Toepfl, S.; Parniakov, O. Sustainable Extraction of Valuable Components from Spirulina Assisted by Pulsed Electric Fields Technology. Algal Res. 2020, 48, 101914. [Google Scholar] [CrossRef]
- Garg, S.; Wang, L.; Schenk, P.M. Flotation Separation of Marine Microalgae from Aqueous Medium. Sep. Purif. Technol. 2015, 156, 636–641. [Google Scholar] [CrossRef]
- Demir, I.; Besson, A.; Guiraud, P.; Formosa-Dague, C. Towards a Better Understanding of Microalgae Natural Flocculation Mechanisms to Enhance Flotation Harvesting Efficiency. Water Sci. Technol. 2020, 82, 1009–1024. [Google Scholar] [CrossRef]
- Kurniawati, H.A.; Ismadji, S.; Liu, J.C. Microalgae Harvesting by Flotation Using Natural Saponin and Chitosan. Bioresour. Technol. 2014, 166, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zou, X.; Chang, W.; Qu, Y.; Li, Y. Microalgae Harvesting Technique Using Ballasted Flotation: A Review. Sep. Purif. Technol. 2021, 276, 119439. [Google Scholar] [CrossRef]
- Laamanen, C.A.; Ross, G.M.; Scott, J.A. Flotation Harvesting of Microalgae. Renew. Sustain. Energy Rev. 2016, 58, 75–86. [Google Scholar] [CrossRef]
- Whitelam, G.C.; Lanaras, T.; Codd, G.A. Rapid Separation of Microalgae by Density Gradient Centrifugation in Percoll. Br. Phycol. J. 1983, 18, 23–28. [Google Scholar] [CrossRef]
- Dassey, A.J.; Theegala, C.S. Harvesting Economics and Strategies Using Centrifugation for Cost Effective Separation of Microalgae Cells for Biodiesel Applications. Bioresour. Technol. 2013, 128, 241–245. [Google Scholar] [CrossRef]
- Najjar, Y.S.H.; Abu-Shamleh, A. Harvesting of Microalgae by Centrifugation for Biodiesel Production: A Review. Algal Res. 2020, 51, 102046. [Google Scholar] [CrossRef]
- Japar, S.A.; Azis, N.M.; Sobri, M.; Haiza, N.; Yasin, M. Application of Different Techniques to Harvest Microalgae. Trans. Sci. Technol. 2017, 4, 98–108. [Google Scholar]
- Hung, M.T.; Liu, J.C. Microfiltration of Microalgae in the Presence of Rigid Particles. Sep. Purif. Technol. 2018, 198, 10–15. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Mat Yasin, N.H.; Derek, C.J.C.; Lim, J.K. Crossflow Microfiltration of Microalgae Biomass for Biofuel Production. Desalination 2012, 302, 65–70. [Google Scholar] [CrossRef]
- Ríos, S.D.; Salvadó, J.; Farriol, X.; Torras, C. Antifouling Microfiltration Strategies to Harvest Microalgae for Biofuel. Bioresour. Technol. 2012, 119, 406–418. [Google Scholar] [CrossRef]
- Bamba, B.S.B.; Tranchant, C.C.; Ouattara, A.; Lozano, P. Harvesting of Microalgae Biomass Using Ceramic Microfiltration at High Cross-Flow Velocity. Appl. Biochem. Biotechnol. 2021, 193, 1147–1169. [Google Scholar] [CrossRef] [PubMed]
- Babel, S.; Takizawa, S. Microfiltration Membrane Fouling and Cake Behavior during Algal Filtration. Desalination 2010, 261, 46–51. [Google Scholar] [CrossRef]
- Sarkar, S.; Manna, M.S.; Bhowmick, T.K.; Gayen, K. Extraction of Chlorophylls and Carotenoids from Dry and Wet Biomass of Isolated Chlorella Thermophila: Optimization of Process Parameters and Modelling by Artificial Neural Network. Process Biochem. 2020, 96, 58–72. [Google Scholar] [CrossRef]
- Xu, L.; Wim Brilman, D.W.F.; Withag, J.A.M.; Brem, G.; Kersten, S. Assessment of a Dry and a Wet Route for the Production of Biofuels from Microalgae: Energy Balance Analysis. Bioresour. Technol. 2011, 102, 5113–5122. [Google Scholar] [CrossRef] [PubMed]
- Aslam, A.; Bahadar, A.; Liaquat, R.; Saleem, M.; Waqas, A.; Zwawi, M. Algae as an Attractive Source for Cosmetics to Counter Environmental Stress. Sci. Total Environ. 2021, 772, 144905. [Google Scholar] [CrossRef]
- Corrêa, P.S.; Morais Júnior, W.G.; Martins, A.A.; Caetano, N.S.; Mata, T.M. Microalgae Biomolecules: Extraction, Separation and Purification Methods. Processes 2021, 9, 10. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Chen, G.; Zhang, J.; Wang, C.; Liu, B. Extraction and Purification of Eicosapentaenoic Acid and Docosahexaenoic Acid from Microalgae: A Critical Review. Algal Res. 2019, 43, 101619. [Google Scholar] [CrossRef]
- Barton, W.A.; Miller, S.A.; Veal, C.J. The Electrodewatering of Sewage Sludges. Dry. Technol. 1999, 17, 498–522. [Google Scholar] [CrossRef]
- Citeau, M.; Olivier, J.; Mahmoud, A.; Vaxelaire, J.; Larue, O.; Vorobiev, E. Pressurised Electro-Osmotic Dewatering of Activated and Anaerobically Digested Sludges: Electrical Variables Analysis. Water Res. 2012, 46, 4405–4416. [Google Scholar] [CrossRef]
- Loginov, M.; Citeau, M.; Lebovka, N.; Vorobiev, E. Electro-Dewatering of Drilling Sludge with Liming and Electrode Heating. Sep. Purif. Technol. 2013, 104, 89–99. [Google Scholar] [CrossRef]
- Patel, R.; Shah, D.; Prajapti, B.G.; Patel, M. Overview of Industrial Filtration Technology and Its Applications. Indian J. Sci. Technol. 2010, 3, 1121–1127. [Google Scholar] [CrossRef]
- Warring, R.H. Filters and Filtration Handbook; Trade & Technical Press Ltd.: Morden, UK, 1981. [Google Scholar] [CrossRef]
- Seo, Y. Characterization of Industrial Filtration Systems for Fine Particle. ARPN J. Eng. Appl. Sci. 2015, 10, 10605–10610. [Google Scholar]
- Wakeman, R.J.; Tarleton, E.S. Solid/Liquid Separation: Principles of Industrial Filtration. In Solid/Liquid Sep. Equip. Sel. Process Des., 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Citeau, M.; Larue, O.; Vorobiev, E. Electric (Electro/Dielectro-Phoretic)—Force Field Assisted Separators. In Progress in Filtration and Separation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 325–397. [Google Scholar]
- Discart, V.; Bilad, M.R.; Vandamme, D.; Foubert, I.; Muylaert, K.; Vankelecom, I.F.J. Role of Transparent Exopolymeric Particles in Membrane Fouling: Chlorella Vulgaris Broth Filtration. Bioresour. Technol. 2013, 129, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, N.M.; Kleijn, J.M.; van Loosdrecht, M.C.M.; Kemperman, A.J.B. Formation and Ripening of Alginate-like Exopolymer Gel Layers during and after Membrane Filtration. Water Res. 2021, 195, 116959. [Google Scholar] [CrossRef] [PubMed]
- De Gouvion Saint Cyr, D.; Wisniewski, C.; Schrive, L.; Farhi, E.; Rivasseau, C. Feasibility Study of Microfiltration for Algae Separation in an Innovative Nuclear Effluents Decontamination Process. Sep. Purif. Technol. 2014, 125, 126–135. [Google Scholar] [CrossRef]
- Mo, W.; Soh, L.; Werber, J.R.; Elimelech, M.; Zimmerman, J.B. Application of Membrane Dewatering for Algal Biofuel. Algal Res. 2015, 11, 1–12. [Google Scholar] [CrossRef]
- Desabres, J.; Equisoain, C.; Loginov, M.; Gésan-Guiziou, G.; Vorobiev, E. Improvement of Anode Lifetime by Flushing during Electrofiltration. Dry. Technol. 2018, 36, 1145–1157. [Google Scholar] [CrossRef]
- Sorensen, P.B.; Moldrup, P.; Hansen, J.A.A. Filtration and Expression of Compressible Cakes. Chem. Eng. Sci. 1996, 51, 967–979. [Google Scholar] [CrossRef]
- Mahmoud, A.; Olivier, J.; Vaxelaire, J.; Hoadley, A.F.A. Electrical Field: A Historical Review of Its Application and Contributions in Wastewater Sludge Dewatering. Water Res. 2010, 44, 2381–2407. [Google Scholar] [CrossRef]
- Khosravanipour Mostafazadeh, A.; Zolfaghari, M.; Drogui, P. Electrofiltration Technique for Water and Wastewater Treatment and Bio-Products Management: A Review. J. Water Process Eng. 2016, 14, 28–40. [Google Scholar] [CrossRef]
- Aoude, C.; Grimi, N.; El Zakhem, H.; Vorobiev, E. Dewatering of Arthrospira Platensis Microalgae Suspension by Electrofiltration. Dry. Technol. 2022, 1–13. [Google Scholar] [CrossRef]
- Zhang, Q.; Cui, G.; He, X.; Wang, Z.; Tang, T.; Zhao, Q.; Liu, Y. Effects of Voltage and Pressure on Sludge Electro-Dewatering Process and the Dewatering Mechanisms Investigation. Environ. Res. 2022, 212, 113490. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Chen, Y.; Wei, X.; Dong, C.; Cai, M.; Song, Z.; Shi, Y.; Jin, M.; Xu, T. Understanding Mechanism of Improved-Dewatering of Waste Activated Sludge by Multi-Stage Pressurized Vertical Electro-Osmotic. Process Saf. Environ. Prot. 2022, 164, 846–856. [Google Scholar] [CrossRef]
- Li, Q.; Zhi, S.; Yu, X.; Li, Y.; Guo, H.; Yang, Z.; Zhang, S. Biodegradation of Volatile Solids and Water Mass Balance of Bio-Drying Sewage Sludge after Electro-Dewatering Pretreatment. Waste Manag. 2019, 91, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, S.; Lee, J.; Zhang, X.; Lee, S.H. Roles of Soluble Microbial Products and Extracellular Polymeric Substances in Membrane Fouling. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 45–79. [Google Scholar]
- Chen, G. Electrochemical Technologies in Wastewater Treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Käppler, T.; Posten, C. Fractionation of Proteins with Two-Sided Electro-Ultrafiltration. J. Biotechnol. 2007, 128, 895–907. [Google Scholar] [CrossRef]
- Hofmann, R.; Posten, C. Improvement of Dead-End Filtration of Biopolymers with Pressure Electrofiltration. Chem. Eng. Sci. 2003, 58, 3847–3858. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Hwang, T.; Oh, Y.-K.; Han, J.-I. Harvesting Chlorella Sp. KR-1 Using Cross-Flow Electro-Filtration. Algal Res. 2014, 6, 170–174. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, Y.; Shi, Y.; Ren, R.; Wu, H.; Zhang, W.; Wang, D.; Zhang, T.; Xiong, J. Extracellular Organic Matter (EOM) Distribution Characteristic in Algae Electro-Dewatering Process. J. Environ. Manag. 2020, 265, 110541. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bennett, A.; Bogobad, L. Complementary Chromatic Adaptation in a Filamentous Blue-Green Alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Stahl, W. Improvement of Filtration Kinetics by Pressure Electrofiltration. Sep. Purif. Technol. 2002, 26, 69–80. [Google Scholar] [CrossRef]
- Vorobiev, E.; Jany, S. Étapes de Filtration Frontale Assistée Par Un Champ Électrique. Entropie 1999, 16, 23–28. [Google Scholar]
- Liu, Y.; Su, Y.; Cao, J.; Guan, J.; Zhang, R.; He, M.; Fan, L.; Zhang, Q.; Jiang, Z. Antifouling, High-Flux Oil/Water Separation Carbon Nanotube Membranes by Polymer-Mediated Surface Charging and Hydrophilization. J. Memb. Sci. 2017, 542, 254–263. [Google Scholar] [CrossRef]
- Chong, W.C.; Mahmoudi, E.; Chung, Y.T.; Koo, C.H.; Mohammad, A.W.; Kamarudin, K.F. Improving Performance in Algal Organic Matter Filtration Using Polyvinylidene Fluoride–Graphene Oxide Nanohybrid Membranes. Algal Res. 2017, 27, 32–42. [Google Scholar] [CrossRef]
- Teow, Y.H.; Wong, Z.H.; Takriff, M.S.; Mohammad, A.W. Fouling Behaviours of Two Stages Microalgae/Membrane Filtration System Applied to Palm Oil Mill Effluent Treatment. Membr. Water Treat. 2018, 9, 373–383. [Google Scholar] [CrossRef]
- Rossi, N.; Jaouen, P.; Legentilhomme, P.; Petit, I. Harvesting of Cyanobacterium Arthrospira Platensis Using Organic Filtration Membranes. Food Bioprod. Process. 2004, 82, 244–250. [Google Scholar] [CrossRef]
- Rossi, N.; Derouiniotchaplain, M.; Jaouen, P.; Legentilhomme, P.; Petit, I. Arthrospira Platensis Harvesting with Membranes: Fouling Phenomenon with Limiting and Critical Flux. Bioresour. Technol. 2008, 99, 6162–6167. [Google Scholar] [CrossRef]
- Wakeman, R. The Influence of Particle Properties on Filtration. Sep. Purif. Technol. 2007, 58, 234–241. [Google Scholar] [CrossRef]
- Bourcier, D.; Féraud, J.P.; Colson, D.; Mandrick, K.; Ode, D.; Brackx, E.; Puel, F. Influence of Particle Size and Shape Properties on Cake Resistance and Compressibility during Pressure Filtration. Chem. Eng. Sci. 2016, 144, 176–187. [Google Scholar] [CrossRef]
- Boskovic, L.; Altman, I.S.; Agranovski, I.E.; Braddock, R.D.; Myojo, T.; Choi, M. Influence of Particle Shape on Filtration Processes. Aerosol Sci. Technol. 2005, 39, 1184–1190. [Google Scholar] [CrossRef]
- Larue, O.; Wakeman, R.J.; Tarleton, E.S.; Vorobiev, E. Pressure Electroosmotic Dewatering with Continuous Removal of Electrolysis Products. Chem. Eng. Sci. 2006, 61, 4732–4740. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Freitas, B.C.B.; Rosa, G.M.; Moraes, L.; Morais, M.G.; Mitchell, B.G. Operational and Economic Aspects of Spirulina-Based Biorefinery. Bioresour. Technol. 2019, 292, 121946. [Google Scholar] [CrossRef] [PubMed]
- Hagglund, J.K.; Insley, T.I.; Johnson, T.W. Electrofiltration Process. U.S. Patent US 6,471,746 B2, 2002. Available online: https://patentimages.storage.googleapis.com/63/03/93/47c02b9043c337/US6471746.pdf (accessed on 27 September 2022).
- Moghadam, M.T.; Lesage, G.; Mohammadi, T.; Mericq, J.P.; Mendret, J.; Heran, M.; Faur, C.; Brosillon, S.; Hemmati, M.; Naeimpoor, F. Improved Antifouling Properties of TiO2/PVDF Nanocomposite Membranes in UV-Coupled Ultrafiltration. J. Appl. Polym. Sci. 2015, 132, 1–13. [Google Scholar] [CrossRef]
- Mozdzanowski, J.; Speicher, D.W. Microsequence Analysis of Electroblotted Proteins. I. Comparison of Electroblotting Recoveries Using Different Types of PVDF Membranes. Anal. Biochem. 1992, 207, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Mische, S.M. Enzymatic Digestion of Proteins on PVDF Membranes. Curr. Protoc. Protein Sci. 1995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, Y.; Zang, X.; Zhang, X. Harvesting of Microcystis Aeruginosa Using Membrane Filtration: Influence of Pore Structure on Fouling Kinetics, Algogenic Organic Matter Retention and Cake Formation. Algal Res. 2020, 52, 102112. [Google Scholar] [CrossRef]
- Rodrigues, R.D.P.; de Lima, P.F.; de Santiago-Aguiar, R.S.; Rocha, M.V.P. Evaluation of Protic Ionic Liquids as Potential Solvents for the Heating Extraction of Phycobiliproteins from Spirulina (Arthrospira) Platensis. Algal Res. 2019, 38, 101391. [Google Scholar] [CrossRef]
- Böcker, L.; Ortmann, S.; Surber, J.; Leeb, E.; Reineke, K.; Mathys, A. Biphasic Short Time Heat Degradation of the Blue Microalgae Protein Phycocyanin from Arthrospira Platensis. Innov. Food Sci. Emerg. Technol. 2019, 52, 116–121. [Google Scholar] [CrossRef]
- Buecker, S.; Grossmann, L.; Loeffler, M.; Leeb, E.; Weiss, J. Thermal and Acidic Denaturation of Phycocyanin from Arthrospira Platensis: Effects of Complexation with λ-Carrageenan on Blue Color Stability. Food Chem. 2022, 380, 132157. [Google Scholar] [CrossRef]
- Patel, A.; Pawar, R.; Mishra, S.; Sonawane, S.; Ghosh, P.K. Kinetic Studies on Thermal Denaturation of C-Phycocyanin. Indian J. Biochem. Biophys. 2004, 41, 254–257. [Google Scholar] [PubMed]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of Phycocyanin Extracted from Spirulina Sp.: Influence of Temperature, PH and Preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Galatanu, A.N.; Nylander, T.; Lindman, B. The Behaviour of Protein Preparations from Blue-Green Algae (Spirulina Platensis Strain Pacifica) at the Air/Water Interface. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 181–192. [Google Scholar] [CrossRef]



| Feed Solution | Untreated | US Treated |
|---|---|---|
| pH | 7.87 ± 0.06 | 7.38 ± 0.13 |
| Conductivity (mS/cm) | 1.03 ± 0.12 | 2.59 ± 0.3 |
| Protein concentration (mg/g d.m.) | 15.69 ± 0.79 | 27.18 ± 2.38 |
| C-PC concentration (mg/g d.m.) | 2.65 ± 0.96 | 3.17 ± 0.3 |
| APC concentration (mg/g d.m.) | 1.07 ± 0.3 | 2.12 ± 0.33 |
| PE concentration (mg/g d.m.) | 0.12 ± 0.08 | 1.25 ± 0.07 |
| Untreated | US Treated | ||||
|---|---|---|---|---|---|
| F | EF | F | EF | ||
| pH | Anode | 7.43 ± 0.19 | 2.71 ± 0.02 | 7.73 ± 0.25 | 4.05 ± 0.09 |
| Cathode | 12.3 ± 0.02 | 12.1 ± 0.08 | |||
| Conductivity (mS/cm) | Anode | 2.19 ± 0.21 | 5.66 ± 0.76 | 2.98 ± 0.14 | 10.37 ± 4.24 |
| Cathode | 6.71 ± 0.27 | 16.2 ± 1 | |||
| Protein concentration (mg/g d.m.) | Anode | 1.37 ± 0.27 | 0.28 ± 0.01 | 15.56 ± 1.5 | Negligible |
| Cathode | 4.88 ± 0.05 | 2.71 ± 0.2 | |||
| C-PC concentration (mg/g d.m.) | Anode | 0.43 ± 0.04 | Negligible | 0.21 ±0.02 | Negligible |
| Cathode | Negligible | Negligible | |||
| APC concentration (mg/g d.m.) | Anode | Negligible | Negligible | 0.17 ± 0.03 | Negligible |
| Cathode | Negligible | Negligible | |||
| PE concentration (mg/g d.m.) | Anode | Negligible | Negligible | Negligible | Negligible |
| Cathode | Negligible | Negligible | |||
| Cake | Filtration | Electrofiltration |
|---|---|---|
| Protein concentration (mg/g d.m.) | 164.5 ± 0.57 | 1.81 ± 0.02 |
| C-PC concentration (mg/g d.m.) | 43.2 ± 0.12 | 0.33 ± 0.01 |
| APC concentration (mg/g d.m.) | 18.14 ± 0.06 | 0.28 ± 0 |
| PE concentration (mg/g d.m.) | 0.6 ± 0.41 | 0.09 ± 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoude, C.; Grimi, N.; El Zakhem, H.; Vorobiev, E. Effect of Electrofiltration on the Dewatering Kinetics of Arthrospira platensis and Biocompound Recovery. Separations 2022, 9, 410. https://doi.org/10.3390/separations9120410
Aoude C, Grimi N, El Zakhem H, Vorobiev E. Effect of Electrofiltration on the Dewatering Kinetics of Arthrospira platensis and Biocompound Recovery. Separations. 2022; 9(12):410. https://doi.org/10.3390/separations9120410
Chicago/Turabian StyleAoude, Christa, Nabil Grimi, Henri El Zakhem, and Eugène Vorobiev. 2022. "Effect of Electrofiltration on the Dewatering Kinetics of Arthrospira platensis and Biocompound Recovery" Separations 9, no. 12: 410. https://doi.org/10.3390/separations9120410
APA StyleAoude, C., Grimi, N., El Zakhem, H., & Vorobiev, E. (2022). Effect of Electrofiltration on the Dewatering Kinetics of Arthrospira platensis and Biocompound Recovery. Separations, 9(12), 410. https://doi.org/10.3390/separations9120410






