Abstract
Over the past few years, research studies on the therapeutic benefits of medicinal plants with potent antioxidant activity and few side effects have grown significantly. This has sparked interest in determining whether naturally occurring antioxidants could take the place of synthetic antioxidants, which are currently being constricted because of their toxic and carcinogenic properties. The identification and quantification of phytochemicals in the methanolic extract of Kigelia pinnata fruits was measured using gas chromatography–mass spectrometry (GC-MS) and ultra-high-performance liquid chromatography–mass spectrometry (UPLC-MS/MS) techniques. Additionally, the methanolic extract of fruits was used to determine antioxidant activity. Free radical-scavenging (DPPH) and ferric ion-reducing antioxidant power were measured using spectrophotometry, and total antioxidant capacity (TAC) was compared with two common antioxidants, vitamin C and α-tocopherol. Moreover, mature fruits have high DDPH, ferric ion-reducing antioxidant power and total antioxidant capacity. Furthermore, mature fruits have high levels of total phenolic, flavonoid, and tannin content; these compounds are thought to be the sources of the antioxidant activity. The major constituents of the methanolic extracts from the mature fruits of K. pinnata were found to be larixinic acid, 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one (DMDP), and 5-Hydrxoymethylfurfural. We performed the elemental analysis of the whole fruit. Huh-7 (liver cancer), PANC-1 (pancreatic cancer), Colo-205 (colorectal cancer), HT-29 (colorectal cancer), SNU-16 (gastric carcinoma), SW620 (colorectal adenocarcinoma) and HCT116 (colon carcinoma) were tested in vitro for anticancer activity. Both methanolic and ethyl acetate extracts of mature fruits had a positive effect on all cancer cell lines as compared to the doxorubicin drug. In addition, the methanolic extracts of mature fruits showed more potent cytotoxic effects than the ethyl acetate extracts. Moreover, the most pronounced cytotoxic effects of the methanolic extract were detected in SW620 (colorectal adenocarcinoma), with an IC50 value of 6.79 μg/mL, SNU-16 (gastric carcinoma), with and IC50 value of 8.69 μg/ ml, and in PANC-1 (pancreatic cancer) with an IC50 value of 10.34 μg/mL. Moreover, the results show that the water, ethyl acetate and methanolic extracts of mature fruits have antioxidant capacity, ferric ion-reducing antioxidant power, DPPH scavenging activity and also anticancer activity. Therefore, the present study suggests that the phytochemical profiles of mature fruits of K. pinnata may be used as potential natural antioxidants and anti-cancer cell lines.
1. Introduction
Humans develop both knowledge and technologies. They produce a wide variety of substances to improve their quality of life. Some of these substances, such as drugs or pesticides, harm both consumers and the environment. To address issues related to health and the environment, researchers have focused on using natural alternatives, such as plant extracts and medicinal plants [1]. Plant extracts have the advantage of being biologically derived, biodegradable, and having a positive impact on both the environment and human health [2]. People have been known to treat illnesses with plant extracts since ancient times. The earliest apothecaries were used by the Egyptians, Chinese, and Indians, but they first appeared in Mesopotamian civilizations, particularly the Babylonian (4000–5000 BC) [3]. Antimicrobial, anticancer, or antioxidant activities are considered among the benefits of medicinal plants, vegetables, and fruits [4,5]. Although the human body has an antioxidant defense system, it frequently is not enough to stop all of the daily attacks that the body faces. In order to maintain a balance between oxidants and antioxidants in the body, typically, substances acting on reactive oxygen species are used in the form of food supplements. However, due to their toxicity and cancer-causing properties, the use of some synthetic antioxidants has recently been restricted [1]. Therefore, alternative medicine has been employed, including using fruit and herbal plant extracts as natural antioxidants. More research is being done on these traits by the scientific community. Studies have shown that some medicinal plants are greater sources of natural antioxidants than some fruits and vegetables [6,7]. Consuming antioxidant-rich foods, such as fruits and vegetables and medicinal herbs and plants, can improve human health and nutritional status [8]. This is based on the theory that good nutrition may protect against some chronic diseases that are common in society [8,9]. A significant hurdle to the rise in life expectancy in the twenty-first century is cancer, which is among the world’s most prevalent causes of death [10,11]. According to recent statistics based on GLOBOCAN, there were 19.3 million new cancer cases reported globally in 2020. This led to at least 10 million cancer-related deaths [12]. Breast cancer had the highest incidence during this time, with 2.3 million new cases (11.7%). Other cancers appeared in the following order: lung cancer (11.4%), colorectal cancer (10.0%), stomach cancer (5.6%), and prostate cancer (7.3%). However, lung cancer accounted for about 18% (1.8 million) of all cancer-related deaths, making it the most common cause of death. Breast cancer (6.9%), stomach cancer (7.7%), liver cancer (8.3%), and colorectal cancer (9.4%) were all significant contributors to estimated cancer fatalities [12]. The consumption of contaminated foods containing mycotoxin and heavy metals, genetic factors, and the residential combustion of raw solid fuels such as dung, wood, charcoal, or agricultural wastes, are the primary causes of the global increase in cancer cases [13,14]. More than 80% of people in developing nations receive their care primarily through traditional medicine, according to the World Health Organization [13]. Plants have been used for thousands of years to produce anticancer agents, which are now used in clinical situations to treat various cancers. Anticancer drugs and compounds were discovered as a result of in-depth research into cytotoxic compounds found in plants that were traditionally used in conventional cancer phytotherapy [15,16].
K. africana (Lam.) Benth. Synonym K. pinnata (Jacq.) DC. is a tropical African plant that is widely cultivated or dispersed in South, Central, or West Africa, and that is classified as a medicinal plant. It belongs to the Bignoniaceae family and is referred to as the sausage tree because of its enormous fruit [17]. The tree is deciduous where there is a long dry season but evergreen where there is year-round rainfall. It is a tree that can reach a height of 20 m or higher. The bark is 6 mm thick on branches that are 15 cm long, grey, smooth, and peeling on older trees. Its wood is light brown or golden [17]. The fruit is a woody berry that can reach lengths of 30 to 100 cm and a width of up to 18 cm. It weighs between 5 and 10 kg and hangs from long peduncles that resemble ropes [18]. The fruit is indehiscent, has a woody wall, and its surface is heavily marked with lenticels. When fully grown, it has many grey-brown seeds. The seeds are obovoid, about 10 mm × 7 mm, and have a leathery testa that is encased in fibrous pulp [19]. The tree has in past centuries been used medicinally to treat a variety of skin conditions, including eczema, fungal infections, psoriasis, or boils, as well as more severe issues such as impetigo, syphilis, leprosy, or skin cancer [18]. The powdered mature fruit can also be used to treat conditions such as dysentery, malaria, diabetes, pneumonia, worm infestations, convulsions, venereal diseases, toothaches, or as a snakebite antidote [18].
The fruit is said to have potent purgative characteristics. Immature and unripe fruit are said to be extremely poisonous if consumed orally [18]. The fruit is thought to be a cure-all for almost all gynecological issues [20]. Additionally, internal uses of the mature fruit pulp include the treatment of toothaches, ringworm, tapeworm, postpartum hemorrhage, malaria, diabetes, dysentery, and pneumonia [21]. The most commonly applied plant part in indigenous medicinal preparations is the fruit, which is followed by stems, bark, roots, and leaves [22,23]. Fruit extracts significantly reduce the viability of mouse tumor cells. Bark and fruit extracts have only displayed sporadic effectiveness against melanotic cell lines [24]. Less cytotoxicity was achieved when using dried fruit than fresh fruit, suggesting that the active ingredients may be thermolabile. Some extracts of the bark, wood, fruits, or roots contain naphthoquinones like lapachol and isopinnatal, which have anticancer properties against melanoma cell lines [25]. Various parts of K. africana contain a wide range of phytoconstituents, such as iridoids, flavonoids, naphthoquinones, coumarins, terpenes, and terpenoids [22,26]. Additionally, analyses of fruit extracts of K. africana (ethanol, hexane, butanol, ethyl acetate, or aqueous) have revealed the presence of flavonoids, carbohydrates, tannins, alkaloids, phenols, sterols, glycosides, and saponins [27]. K. pinnata contains large amounts of sterols and iridoids, which may contribute to their capacity to treat melanoma [28]. The anticonvulsant properties of K. africana, which are used to stop epileptic fits, are thought to be attributed to derivatives of cinnamic acid. Flavonoids are present in the leaves and fruits. The antimicrobial components, which are boosted by a high flavonoid concentration, may be the source of the antidiarrheal effects [29].
The aim of this research study is to investigate the phytochemicals in fruit (mature or immature) extracts of K. pinnata and their potential antioxidant and anticancer activities.
2. Materials and Methods
2.1. Plant Materials
Fruits of mature and immature K. pinnata DC. trees were obtained from the botanical garden of the Faculty of Agriculture, Ain Shams University, Cairo, Egypt during April and December. Collected samples (mature or immature whole fruits) were sliced into small pieces. All the samples were bagged in sealed-cap plastic sample bags and kept at −80 °C until used. The complete sliced fruits were dried at reduced pressure at 45 °C (RAYPA-EV 50L, drying oven-Spain). After complete drying the samples were ground into fine powder.
2.2. Determination of N, P, and K
According to the Association of Official Analytical Chemists (AOAC) [30], the amount of elements was identified in both mature (Km) and immature (KI) fruits.
2.3. Determination of Trace Element Levels
2.3.1. Microwave Digestion
Microwave acid digestion of K. pinnata extract samples was carried out using analytically ultra-pure graded nitric acid (65%, for ICP, Sigma-Aldrich, St. Louis, MI, USA) and hydrogen peroxide (30%, ultra-pure for AA, Carlo-Erba, Val de Reuil, France). For sample dilutions, ultrapure water from the Milli-Q system (Millipore, France) was acquired. In a closed microwave digestion/extraction system (SINEO-MDS-6G SMART, Shanghai, China) as described by Marin et al. [31], 1.0 g of powdered sample was digested with 8 mL of nitric acid or 2 mL of hydrogen peroxide. The final mixtures were cooled and then completed to 100 mL with distilled water before being subjected to ICP-OES analysis.
2.3.2. Determination of Trace Element Levels by ICP-OES
ICP-OES (Agilent 700 series coupled with Ultrasonic Nebulizer CETAC U-6000AT-CETAC, CA, USA) was used to measure the levels of trace elements, and an autosampler AS 93-plus Argon (purity > 99.995%) was used to maintain the plasma as well as the carrier gas. To calibrate, a multi-elemental standard solution of 1000 mg/L from Sigma-Aldrich was used, which contained all of the elements that were analyzed (Mg, Na, Ca, Zn, Cu, B, Mn, Cr, Fe, Pb, Al, Si, and Cd).
2.4. Proximate Analysis
The moisture content, total carbohydrates, reducing sugars and crude fat were measured in the dried powder samples of mature (Km) and immature (KI) fruits according to AOAC [32]. The crude fiber was determined using the fully automated system (FOSS—Fibertic 8000, Hilleroed,, Denmark) according to Tobaruela et al. [33]. Free amino acids were determined in the 85% ethanolic extract of powdered samples using ninhydrin reagent (ACS Sigma Aldrich) and the absorbance was read at 570 nm using a spectrophotometer according to Jayaraman [32] and L-Lysine (20–100 µg/mL) as a calibration standard curve.
2.5. Determination of Total Phenolic Content
According to Goffman and Bergman [34], with a few modifications, the total phenolic content of mature (Km) and immature (KI) fruits was determined in an 80% ethanolic extract. In total, 0.1 mL of ethanolic extract was combined with 0.50 mL of deionized water, 0.25 mL of 20% Folin-Ciocalteu reagent, and 0.5 mL of 0.5 M ethanolamine after 5 min and kept for 90 min at room temperature. The optical density was then measured at 760 nm against a reagent blank using a Thermo Scientific Evolution 350 UV-Vis spectrophotometer (Waltham, MA, USA). The total phenolic content was expressed as mg gallic acid (GE)/100 g sample (DW).
2.6. Determination of Total Flavonoid Content
The total flavonoid content was determined using a spectrophotometric method according to Chang et al. [35]. In total, 0.075 mL of 5% NaNO2 (w/v), 1 mL of deionized water, and 0.25 mL of 80% ethanolic extract were combined. The mixture received 0.15 mL of 10% AlCl3 (w/v) after 5 min. Then, 0.5 mL of 1 M NaOH was added after 6 min. The addition of 0.5 mL of deionized water came next. The absorbance of the mixture was read at 510 nm using a Thermo Scientific Evolution 350 UV-Vis spectrophotometer (Waltham, MA, USA) after centrifugation at 8000 g for 4 min at room temperature in comparison to the blank reagent. Total flavonoid content was expressed as mg catechin (CE)/100 g sample (DW).
2.7. Determination of Total Anthocyanin Content
Using the Abdel-Aal et al. [36] method, total anthocyanins were extracted, and their concentrations were determined. Five milliliters of acidified methanol (85% methanol or 15% 1.5 N HCl, v/v) were utilized to extract samples (0.5 g) for 40 min at room temperature. The supernatant was collected in a 10 mL volumetric flask and extraction was carried out once more after centrifugation at 20,000 g for 20 min at 4 °C (HERMEL -Z36HK—Germany) and then the supernatant was completed to a known volume. The absorbance of the supernatant was read at 535 nm utilizing a spectrophotometer via reagent blank. Total anthocyanin content was expressed as mg kuromanin (KE)/100 g sample (DW). The calibration standard curve of kuromanin (Sigma—USA) was made by using the concentration range 2.5–20.0 mg of KE. The average of molar extinction coefficient (Ɛ) was 25,700 ± 110 M−1 cm−1.
2.8. Determination of Total Condensed Tannins Content
Total condensed tannins (content of proanthocyanidins) were measured according to the procedure of Waterman and Mole [37]. In the ethanolic extract of Kigelia fruits, 3 mL of 4% vanillin solution in methanol and 1.5 mL of concentrated hydrochloric acid were mixed with 50 μL of the sample extract. After 15 min of incubation at room temperature, the absorbance of the mixture was read at 500 nm using a spectrophotometer against methanol as a reference. The total content of proanthocyanidins was expressed as mg of catechin (CAE)/ 100 g of extract, (Sigma-Aldrich, Taufkirchen, Germany, 0–150 µg/mL, R2 = 0.9984). All samples were analyzed in triplicate.
2.9. Fe+2 Ion Reducing Power
Different Kigelia fruit extracts’ reducing powers were assessed using the Oyaizu method [38]. The powdered extract of Km and KI was reconstituted in absolute ethanol. A volume of 0.4 mL of ethanolic solution was added to each sample with different concentrations (0.5, 2.0, 4.0 and 8.0 mg/mL for Km and KI extracts) while solvent fractions with water, ethyl acetate, methanol and water were reconstituted in DMSO (dimethyl sulfoxide 99%, Millipore, Merck, Germany). The concentrations used (0.5, 3.0, 4.8, and 6.4 mg/mL for K water, K ethyl acetate, K methanol and K dichloro mathane extracts, respectively) were mixed with 1 mL potassium ferricyanide 1% or 1 mL phosphate buffer 0.2 mol/ L, pH 6.6. After the incubation of the mixture at 50 °C for 20 min, 1 mL of 10% trichloroacetic acid was added. The mixture was then centrifuged at 650 g for 10 min (HERMEL—Z36HK-Germany). The reducing power was measured as μg gallic acid/g extract (ε = 15.23 µg mL−1cm−1) at 700 nm using a Thermo Scientific Evolution 350 UV-Vis spectrophotometer (MA, USA) after 2 mL of supernatant, 2 mL of distilled water, and 0.4 mL of ferric chloride 0.1% were combined.
2.10. Determination of Total Antioxidant Capacity
Using the Prieto et al. [39] protocol, the total antioxidant capacity of various kigelia fruit extracts was assessed. In total, 0.5 mL of ethanolic solution of each sample at different concentrations (2.08, 4.16, 6.25, 8.33 and 10.42 mg/mL for Km and KI extracts, and 1.6, 3.2, 4.8, 6.4 and 8 mg/mL for K water, K ethyl acetate, K methanol and K dichloro mathane extracts) were mixed with 3.5 mL of the molybdate reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate) in screw cap tubes. The tubes were incubated in a thermostatic shaking water bath (Witeg, WB 20, Wertheim, Germany) at 95 °C for 90 min. The tubes were left to cool and then the absorbance of the mixture was read at 695 nm using a spectrophotometer against a blank. The antioxidant capacity was expressed as µg α-tocopherol/g extract from α-tocopherol standard curve (5–100 µg/mL), or as µg L-ascorbic acid/g extract from the L-ascorbic acid standard curve (10–250 µg/mL).
2.11. Scavenging of DPPH Radical
A solution of 5 mM of 2,2-Diphenyl-2-picrylhydrazyl DPPH radical (Analar grade, Sigma-Aldrich, St. Louis, MI, USA) was prepared in 95% ethanol. Utilizing the spectrophotometric analysis of the DPPH radical’s absorption at 515 nm and a molar absorptivity of 1.09 × 104 M−1 cm−1, the DPPH radical concentration was identified. The Hatano et al. [40] protocol was utilized to assess the ability of various kigelia fruit extracts to scavenge DPPH radicals. The reaction mixture included 0.1 mL of DPPH solution (5 mM) as well as various kigelia fruit extracts with concentrations ranging from 0.25 mg/mL to 13.89 mg/mL. The total volume of the mixture was completed to 3 mL. After 10 min, the absorbance of DPPH at 515 nm was read using a spectrophotometer against a blank solution (3 mL of 95% ethanol). The results were calculated as follows:
where
- As = absorbance of sample;
- Ac = absorbance of control (DPPH radical solution in ethanol).
By plotting the percent of scavenging activity against the extract concentration, the kinetic parameter IC50 (effective concentration of antioxidant required to reduce initial DPPH concentration by 50%) was calculated form the line equation of the concentration-dependent curve for each extract.
2.12. Extraction of Samples
The powdered samples of Km and KI were subjected to extraction for antioxidant activity, chromatographic analysis and anticancer assay. The crude methanolic extract was prepared from 250 g of dried powder samples with one liter of methanol absolute (High-Performance Liquid Chromatography (HPLC) grade, Sigma-Aldrich, St. Louis, MI, USA) at 20 °C overnight in dark bottles, then filtered (Whatman 1 filter paper), and the filtrate was pooled in an amber bottle. The residue was re-extracted twice with 500 mL of fresh methanol; the pooled extract was concentrated and evaporated to dryness by a rotary evaporator (BUCHI ROTAVAPOR R-300pro equipped with a vacuum pump, Taufkirchen, Germany) at 40 °C. The crude methanolic extract was subjected to vacuum freeze-drying (LAB1ST–FDL2E-1A, Shanghai, China), and the crude extract yielded 26.05 g. The resulting crude methanolic extract was weighted and kept in sealed flasks under N2 gas at −20 °C for further use (chromatographic analysis, antioxidant assay and anticancer assay). The powdered samples (50 g) were subjected to stepwise solvent extraction with dichloromethane, ethyl acetate, methanol (HPLC grad, Carlo-Erba, Emmendingen, France), and finally with deionized water. The extraction process was carried out twice for each solvent using a 250 mL portion and the pooled extract was concentrated to dryness by a rotary evaporator at reduced pressure and at 40 °C. The dried extracts were freeze-dried, weighted, and stored at −20 °C in sealed flasks under N2. The yields of stepwise extraction were 0.8, 1.04, 3.94, and 1.0 g for chloromethane, ethyl acetate, methanol and water solvents, respectively.
2.13. Gas Chromatography–Mass Spectrometry (GC-MS) Qualitative Identification
A crude methanolic extract powder (10 mg) of Km and KI was reconstituted in 1 mL of methanol:diethyl ether at a 7:3 ratio, and 1µL of sample solution (10 μg extract/µL) was injected for separation into a GC-MS (Shimadzu-QP-2010S plus, Tokyo, Japan) instrument equipped with (AOC-20i+s) an autosampler autoinjector and capillary column (Rtx-1 30 m × 0.32 mm I.D., 0.25 μm). The oven temperature was set to 50 °C, followed by a 5 °C/min temperature ramp to 200 °C that was held for 1 min, then raised to 280 °C at 10 °C/min. The final temperature was maintained for 1 min. The injector and mass interface temperatures were adjusted to 280 °C. The helium carrier gas (He) column flow was 2.62 mL/min with a linear velocity of 58.7 cm/s. The mass parameters were set as follows: ion source temperature of 220 °C, solvent cut time of 4.0 min, and compounds were acquired by scan mode ACQ (start m/z 70 and end m/z 600) on an MS detector (EI-mode). Integration was performed by the Lab Solution software 4.1 and compounds were compared via the NIST 11s library, Mass Finder, and information on related compounds in the literature. The main extract constituents were recognized via AMDIS software (www.amdis.net, accessed on 25 October2022), according to their retention indices (relative to n-alkanes C7–C30). Individual component concentrations were calculated using Lab Solution® software post-run integration and an internal standard (Diisooctyl phthalate ester 15 ng/µL) spiked to the sample, with each sample injected in triplicate.
2.14. Ultra-High-Performance Liquid Chromatography–Mass Spectrometry (UPLC-MS/MS) Identification of Phytochemicals Compounds
The crude methanolic extract powder (25 mg) of mature K. pinnata fruits (Km) was reconstituted in an acetonitrile:water 9:1 ratio (10 µg extract/µL); the extract was pre separated (clean up) using an automated cartridge separator (Smart prep II extractor system 24VDC module, Horizon Technology, Concord, NH, USA) using a 1 g RP-18 cartridge to eliminate the interfering compounds. The elution was carried out with water:acetonitrile (95:5) to remove the impurities, then the adsorbed analytes were removed from RP-18 using 100% acetonitrile. The identification of individual flavonoids, free phenolics and different metabolite profiles was performed by Waters Acquity UPLC –I class coupled with Xevo TQD MS (Milford, MA, USA), Acquity UPLC BEH C18 1.7 um–2.1 × 100 mm, with a column flow rate of 0.2 mL min−1 and an injection volume of 10 µL, using the Masslynix v 4.1 software with Mass library, Argon as the collision cell gas at an inlet of 7 psi, and a nitrogen pressure of 60 psi. The MS was set to an atmospheric pressure electrospray ionization (ESI) source, operated in positive (event MS1) and negative (event MS2) ion modes. The electrospray capillary voltage was set to 3000 V, with a nebulizing gas flow rate of 12 L/h or an interface temperature of 300 °C, and desolvation temperature of 526 °C. Mass spectrometry data were acquired in scan mode (mass range m/z 100–1200 with scan speed 1111 µ/s). To scan the phenolic compounds’ profile, we used a binary gradient of (A) 1% formic acid in water and (B) 100% acetonitrile at 0.2 mL/min at 25 °C. The gradient used was as follows: 0 min, 10% B; 0.01 to 29 min, linear gradient to 80% B; 31 min, linear gradient to 65% A, 35% B; 31 to 40 min, linear gradient to 15% A or 85%; 29 to 35 min, linear return to 10% B; 35.01 to 40 min, isocratic at 100% B to re-equilibrate. Mass spectral data were compared via the mass metabolite spectral library and compared with previously identified metabolites in the literature. The metabolite library uses high mass accuracy MS/MS spectra, retention time (Rt) or isotopic information to identify and confirm compounds.
2.15. Cell Culture
Huh-7 (liver cancer), PANC-1 (pancreatic cancer), Colo-205 (colorectal cancer), HT-29 (colorectal cancer), SNU-16 (gastric carcinoma), SW620 (colorectal adenocarcinoma), HCT116 (colon carcinoma) and THLE2 (immortalized, normal liver) cells were used in this investigation, obtained from American Type Culture Collection (ATCC, Rockville, MD, USA), and maintained in the VACSERA Cell Culture Unit, Cairo, Egypt. Cells were maintained in DMEM media supplemented with 100 mg/mL of streptomycin, 100 units/mL of penicillin and 10% heat-inactivated fetal bovine serum in a humidified, 5% (v/v) CO2 atmosphere at 37 °C.
2.16. Cytotoxicity Assay
According to Skehan et al. [41] and Allam et al. [42], the sulforhodamine B (SRB) assay was utilized to measure the viability of cells. In total, 5 × 103 cells suspended in 100 μL aliquots were placed in 96-well plates and incubated for 24 h in complete media. Another aliquot of 100 µL of media containing various concentrations of drugs was used to treat cells. The cells were fixed by replacing the media with 150 µL of 10% TCA and incubating at 4 °C for 1 h after 72 h of drug exposure. After the TCA solution was removed, distilled water was utilized to wash the cells five times. In total, 70 µL of 0.4% w/v SRB solution was added to the aliquots, and they were then incubated at room temperature for 10 min in a dark area. The plates were cleaned with 1% acetic acid three times before being left to air dry overnight. Then, the protein-bound SRB stain was dissolved using 150 µL of TRIS (10 mM), and the absorbance was determined at 540 nm using a BMGLABTECH®—FLUO star Omega microplate reader (Ortenberg, Germany). The data are expressed as the mean percentage of viable cells as compared to the respective control cultures treated with the solvent. The half maximal growth inhibitory concentration (IC50 values) was calculated from the line equation of the dose-dependent curve of each compound.
2.17. Statistical Analysis
Each treatment involved three replications. SPSS statistics version 22.0 was utilized to analyze the data using analysis of variance (ANOVA). To separate means, the p ≤ 0.05 levels of Duncan’s multiple range tests were used.
3. Results
3.1. Macro- and Microelements in K. pinnata Fruits
The results in Figure 1 show that the mature fruits of K. pinnata contain higher amounts of nitrogen and potassium than immature fruits, and lower amounts of phosphorus than immature fruits. On the other hand, immature fruits contained higher amounts of magnesium than mature fruits. In addition, higher concentrations of magnesium, sodium, calcium, iron, manganese, zinc, and copper were detected in immature fruits than in mature fruits. The mature fruits exhibited higher concentrations of boron, molybdenum, cadmium, chromium, lead, aluminum, and silicon than immature fruits (Table 1).
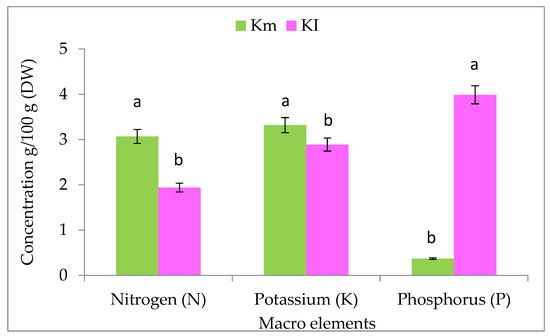
Figure 1.
Quantification of macro-elements of mature (Km) and immature (KI) fruits of K. pinnata. The values are the means of three replicates with standard deviation (±SD). Duncan’s multiple tests (p ≤ 0.05) found no statistically significant differences between the values in each bar with the same letter.

Table 1.
ICP-OES of trace elements of mature (Km) and immature (KI) fruits of K. pinnata.
3.2. Proximate Analysis
The results of the proximate analysis of mature and immature fruits of K. pinnata are shown in Table 2. The findings indicate that reducing sugars (5.03%), total carbohydrates (28.05%), crude fiber (15.4%), and crude protein (15.25%) were all higher in mature fruits than in immature ones. Additionally, immature fruits showed higher levels of total free amino acids FAA (1.48%) and moisture (28.78%). In addition, there was a non-significant difference in crude fat percentage between mature and immature fruits.

Table 2.
Moisture, reducing sugars, total carbohydrate, fibers, crude protein, total free amino acids (FAA) and crude fat content (dry weight bases) of mature (Km) and immature (KI) fruits of K. pinnata.
3.3. Secondary Metabolites
Table 3 illustrates the contents of secondary metabolites (total phenolics, anthocyanin, flavonoids, and tannin content) in the methanolic extracts of mature and immature fruits of K. pinnata. The results show that mature fruits exhibited higher amounts of total phenolics (171 mg/100 g), total flavonoids (398.7 mg/100 g) and total tannins (35.17 mg/100 g) than immature fruits. In addition, there were non-significant differences in total anthocyanin content.

Table 3.
Total phenolics, anthocyanin, flavonoids and tannins contents (dry weight bases) in methanol extracts of mature (Km) and immature (KI) fruits of K. pinnata.
3.4. Fe+2 ion Reducing Power
A simple direct test of antioxidant power involves determining the ferric reducing antioxidant power. After we did this, the Fe2+ was monitored by measuring the formation of blue-colored ferrous at 700 nm. The results show that the extracts of mature fruits of K. pinnata had greater reducing power than the extracts of immature fruits. As obtained in Figure 2, the reducing power of the fruit extract may serve as a significant indicator of its potential antioxidant activity.
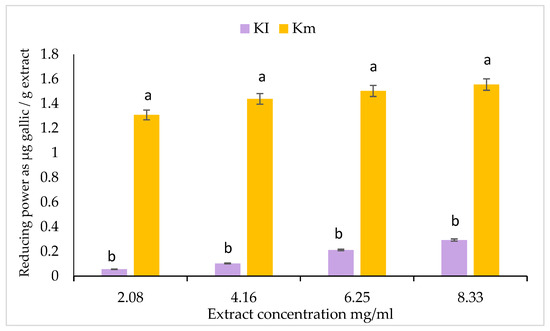
Figure 2.
Reducing powers of mature (Km) and immature (KI) fruit extracts of K. pinnata. The values are the means of three replicates with standard deviation (±SD). Duncan’s multiple tests (p ≤ 0.05) found no statistically significant differences between the values in each bar with the same letter.
3.5. Antioxidant Capacity
The antioxidant capacities of mature and immature fruit extracts of K. pinnata are illustrated in Figure 3. The extracts of mature fruits exhibited antioxidant capacities that were higher by about 103.79 and 69.42 µg, in relation to vitamin E equivalent and vitamin C equivalent, respectively, than the extracts of immature fruits.
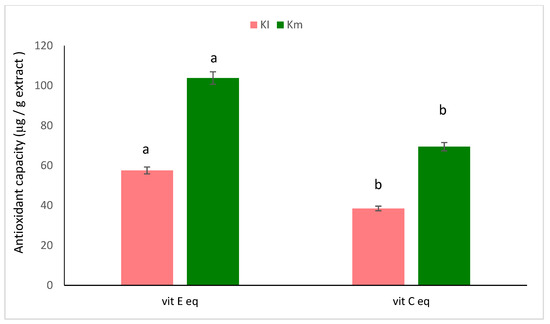
Figure 3.
Antioxidant capacity of extracts of mature (Km) and immature (KI) fruits of K. pinnata. The values are the means of three replicates with standard deviation (±SD). Duncan’s multiple tests (p ≤ 0.05) found no statistically significant differences between the values in each bar with the same letter. Vit E eq (vitamin E equivalent) and vit C eq (vitamin C equivalent).
3.6. DPPH Free Radical Scavenging Activity
DPPH radical is used for the quick evaluation of free radical-scavenging assays. Antioxidants’ ability to donate hydrogen is thought to be what causes them to have an impact on DPPH radical-scavenging. The results in Table 4 show that the DPPH-scavenging activity of mature fruit extracts was higher (75.41%) than immature fruits (22.32%). Moreover, a lower IC50 value was detected in mature fruits compared to immature fruits (about 2.56% lower), which shows this substance is more potent in scavenging DPPH, and this implies a higher antioxidant activity.

Table 4.
DPPH-scavenging activities of extracts of mature (Km) and immature (KI) fruits of K. pinnata.
3.7. Fe+2 Ion Reducing Power of Different Fractions
Different fractions (water, ethyl acetate, methanol, and dichloromethane) at various concentrations (1.6, 3.2, 4.8, and 6.4 mg/mL) of the crude methanolic extracts of mature fruits of K. pinnata were used to determine reducing power (Figure 4). The results show that the reducing power was increased significantly by increasing the extract concentrations in all fractions. The most pronounced increase in reducing power was detected at a concentration of 6.4 mg/mL. In addition, the dichloromethane fraction exhibited a higher reducing power than the water fraction.
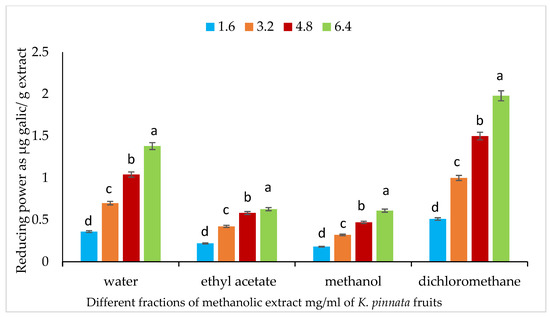
Figure 4.
Reducing power of different fractions (water, ethyl acetate, methanol and dichloromethane) of mature (Km) fruits of K. pinnata. The values are the means of three replicates with standard deviation (± SD). Duncan’s multiple tests (p ≤ 0.05) found no statistically significant differences between the values in each bar with the same letter.
3.8. Antioxidant Capacity of Different Fractions
Figure 5 shows the antioxidant capacities of different fractions (water, ethyl acetate, methanol, and dichloromethane) of crude methanolic extracts of mature fruits of K. pinnata. The water and dichloromethane fractions of mature fruits exhibited antioxidant capacities that were higher by about 95.63, 94.13 and 63.96, 62.94 ppm equivalent of vit E and vit C, respectively, compared to the other fractions.
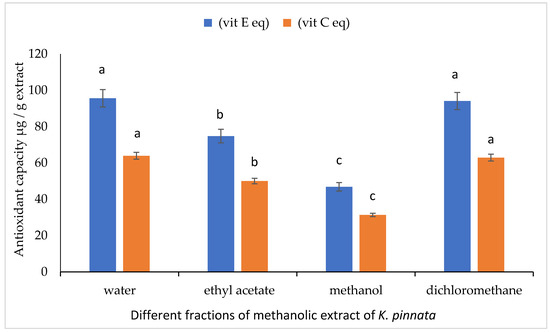
Figure 5.
Antioxidant capacity of different fractions (water, ethyl acetate, methanol and dichloromethane) of mature (Km) fruits of K. pinnata. The values are the means of three replicates with standard deviation (±SD). Duncan’s multiple tests (p ≤ 0.05) found no statistically significant differences between the values in each bar with the same letter. Vit E eq (vitamin E equivalent) and vit C eq (vitamin C equivalent).
3.9. DPPH-Scavenging Activity of Different Fractions
The effects of different fractions (water, ethyl acetate, methanol, and dichloromethane) of crude methanolic extracts (1.0–6.0 mg/mL) of mature K. pinnata fruits on the scavenging of DPPH radicals are shown in Table 5. The results show that the rate of scavenging of DPPH radicals was greater in the methanolic fraction of mature fruits compared to ethyl acetate. Additionally, lower IC50 values were found in the ethyl acetate fraction and the methanolic fraction of mature fruits, with values lower by about 1.91% and 0.77%, respectively.

Table 5.
DPPH-scavenging activity of different fractions (water, ethyl acetate, methanol, and dichloromethane) of mature (Km) fruits of K. pinnata.
3.10. GC-MS Qualitative Identification
The components of the mature and immature K. pinnata fruits’ methanolic extracts were determined by GC-MS analysis, and the results are shown in Table 6 and Table 7 and Supplementary Figure S1a,b as the relative peak area of every constituent. As shown in Table 6 and Supplementary Figure S1a, the GC-MS analysis of mature fruits of K. pinnata resulted in the identification of 25 components. 5-Hydrxoymethylfurfural was considered the predominant and principal component (148.96 µg/g), followed by 2-Hydroxy-2-methylsuccinic acid (97.47 µg/g), 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one (DMDP) (74.29 µg/g), Larixinic acid (64.55 µg/g), 5-Ethoxy-3a,6a-dimethyl-3,3a,4,6a-tetrahydro-furo [2,3-b]pyrrol-2-one (49.81 µg/g), 1,6-Hexanedioic acid, bis(DMOX) derivative (32.53 µg/g), and 4-(3,5-Dimethyl-1-benzofuran-2-yl)-2-butanone (29.03 µg/g).

Table 6.
Phytochemical compounds identified by GC-MS in methanolic extracts of mature (Km) fruits of K. pinnata.

Table 7.
Phytochemical compounds identified by GC-MS in methanolic extracts of immature (KI) fruits of K. pinnata.
3.11. KI (Kovat’s Index) Determined Experimentally Relative to C7–C30 n-alkanes
As shown in Table 7 and Supplementary Figure S1b, the GC-MS analysis of immature fruits of K. pinnata resulted in the identification of 19 components. The most abundant components were (3.α.,5.α.,11.β.)-3,11-dihydroxy-Pregnan-20-one (58.45 µg/g), 1,2-Benzenedicarboxylic acid, diisooctyl ester (54.64 µg/g), gamma-sitosterol (53.87 µg/g), i-propyl 14-methyl-pentadecanoate (35.62 µg/g), benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl ester (33.92 µg/g), 2,6,10,10-Tetramethyl-1-oxaspiro[4.5]decan-6-ol (28.87 µg/g), oxolinic acid (20.93 µg/g), 3.α.-Hydroxy-5,16-androstadiene-17-carboxylic acid (18.45 µg/g), and 5-Ethoxy-3a,6a-dimethyl-3,3a,4,6a-tetrahydro-furo[2,3-b]pyrrol-2-one (15.36 µg/g). In addition, the methanolic extracts of immature fruits contained small amounts of previously identified phytochemical compounds. Figure 6 and Figure 7 represent the structures of the phytochemicals identified in the methanolic extracts of mature (Km) and immature (KI) fruits of K. pinnata, respectively.

Figure 6.
Structure of phytochemicals identified in methanol extracts of mature (Km) fruits of K. pinnata.
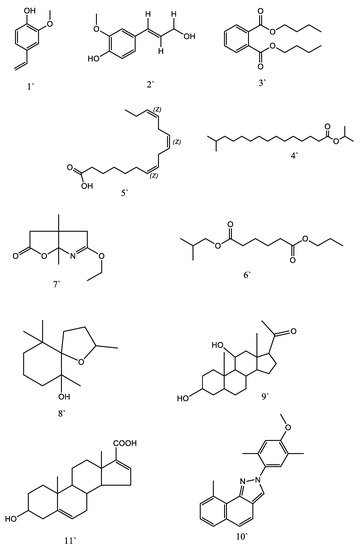
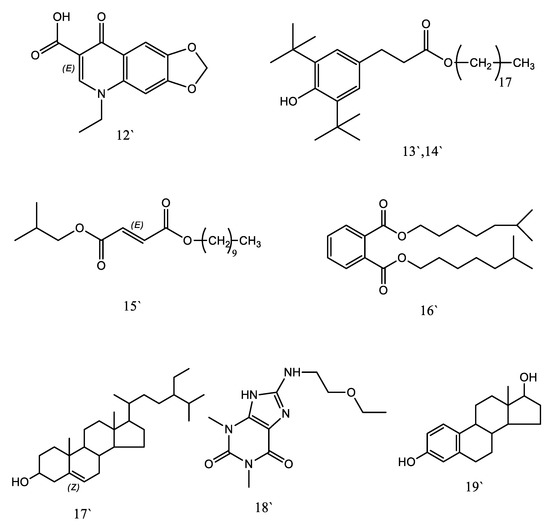
Figure 7.
Structure of phytochemicals identified in methanol extracts of immature (KI) fruits of K. pinnata.
3.12. UPLC-MS/MS Identification of Phytochemicals Compounds
The data in Table 8 and Supplementary Figure S2 show that sixteen peaks in total were separated and detected in negative and positive mode by UPLC-MS/MS. In the positive mode, only five compounds were detected at the base peak (259–383 m/z). The most abundant compounds were pinnatal (21.39%), kigelinol (20.52%), kigelinone (14.23%) and isokigelinol (12.58%). These compounds belong to the iridoid and quinone classes. Using the negative mode, we detected eleven compounds at the base peak (81–387.15 m/z). The most abundant compounds were isopinnatal (36.28%), coffeic acid (14.06%) and ferulic acid (12.91%). These compounds belong to the iridoid, phenolic, sterol, and quinone classes. Figure 8 and Figure 9 show the structures of the phytochemicals determined by LC-MSMS in crude methanolic extracts of mature fruits (Km) of K. pinnata in positive (TIC 1) and negative (TIC2) ion modes.

Table 8.
Phytochemical compounds identified by LC-MS/MS in crude methanolic extracts of mature (Km) fruits of K. pinnata in positive (TIC 1) and negative (TIC2) ion modes.
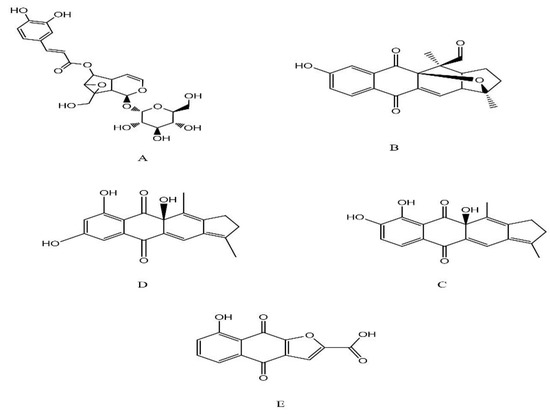
Figure 8.
Structure of the phytochemicals identified by LC-MSMS in crude methanolic extracts of mature fruits (Km) of K. pinnata in positive ion mode (TIC 1): (A) verminoside, (B) pinnatal, (C) kigelinol, (D) isokigelinol, (E) kigelinone.
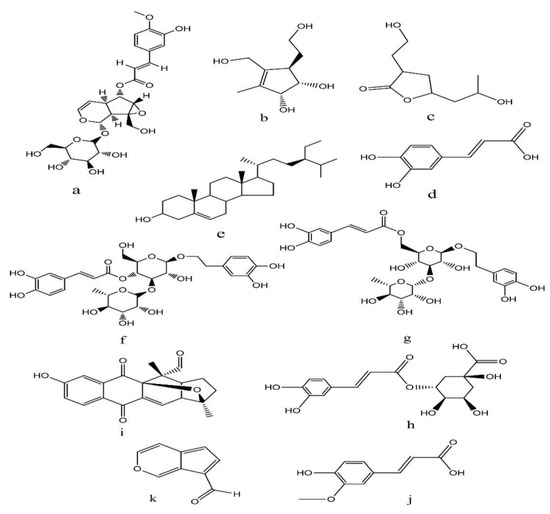
Figure 9.
Structure of the phytochemicals identified by LC-MSMS in crude methanolic extracts of mature fruits (Km) of K. pinnata in negative ion mode (TIC 2): (a) minecoside, (b) 7-hydroxy-10-deoxyeucommiol, (c) 3-(2-hydroxyethyl)-5-(2-hydroxypropyl)-4,5-dihydrofuran-2(3H)-one, (d) coffeic acid, (e) sitosterol, (f) verbascoside, (g) isoverbascoside, (h) chlorogenic acid, (i) isopinnatal, (j) ferulic acid and (k) norviburtinal.
3.13. Anticancer Activity
We used five concentrations each of methanol and ethyl acetate extracts (50, 1000, 200, 400 and 800 µg /mL) and five concentrations of DOX drug (5, 10, 20, 40 and 80 µg/mL), in three replicates measured at 700 nm; DMSO was used as the solvent and blank. The data in Table 9 show the cytotoxic activity of crude methanolic and ethyl acetate extracts of mature fruits used in vitro against Huh-7 (liver cancer), PANC-1 (pancreatic cancer), Colo-205 (colorectal cancer), HT-29 (colorectal cancer), SNU-16 (gastric carcinoma), SW620 (colorectal adenocarcinoma), and HCT116 (colon carcinoma), in a viability test compared with THLE2 (normal liver cells).

Table 9.
IC50 of ethyl acetate and methanolic extracts of mature fruits (Km) of K. pinnata.
The results in Table 9 and Figure 10 show that both methanolic and ethyl acetate extracts of the mature fruits had a positive effect on all cancer cell lines as compared to the doxorubicin drug. In addition, the methanolic extract of mature fruits showed more potent cytotoxic effects than the ethyl acetate extract. Moreover, the most pronounced cytotoxic effects of the methanolic extract were detected against SW620 (colorectal adenocarcinoma), with an IC50 value of 6.79 μg/mL, SNU-16 (gastric carcinoma), with an IC50 value of 8.69 μg/mL, and against PANC-1 (pancreatic cancer), with an IC50 value of 10.34 μg/mL.
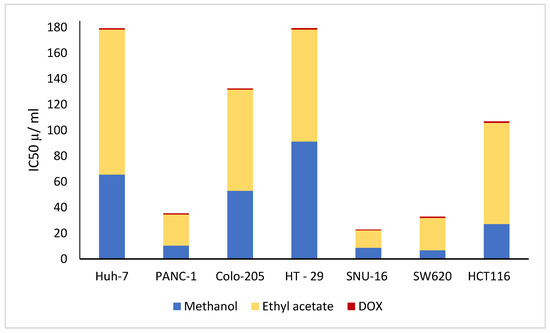
Figure 10.
IC50 of K. pinnata mature fruit methanolic and ethyl acetate extracts and the DOX drug on a dose–response graph.
4. Discussion
The mature fruits of K. pinnata have higher amounts of nitrogen, potassium, boron, molybdenum, cadmium, chromium, lead, aluminum, and silicon than immature fruits. On the other hand, immature fruits have higher amounts of magnesium, phosphorus, sodium, calcium, iron, manganese, zinc, and copper (Figure 1 and Table 2). These findings are in line with those of Fagbohun et al. [75], who reported that trace elements such as zinc, nickel, iron, copper, cobalt, cadmium, lead, manganese, and chrome are present in the various solvent extracts of K. africana fruit; these increase the antioxidant activity, and their concentrations vary depending on the extract. A crucial mineral and an important electrolyte in the human body is potassium. It is crucial for controlling blood pressure, nerve function, and electrolyte balance. Calcium is necessary for healthy skeletal development, muscle function, and growth [75]. Zinc, manganese, and copper are elements that are associated with the strategies of cellular antioxidant resistance. In addition, it is known that the metals copper and manganese play a role in the generation of reactive oxygen species (ROS) [76,77,78,79,80,81,82,83,84]. Hemoglobin contains zinc, which supports blood vessel and lung function. Studies have shown that zinc has positive effects on people with atherosclerosis [85]. In addition to aiding in wound healing, it is crucial for the immune system, taste, smell, growth, vision, blood clothing, healthy insulin and thyroid function. It has also shown significant antitumor activity via human carcinoma of the nasopharynx [86]. Additionally, zinc plays a crucial part in the production of insulin and the catalysis of multiple enzymatic processes [87]. Manganese is both a necessary element and a strong neurotoxin [76]. A small amount of copper is necessary for the proper operation of the body’s organs and systems, but an excessive amount can be toxic. According to studies, deficiencies in copper may cause anemia, abnormalities of the bones, growth retardation, and abnormalities of the metabolism of glucose and cholesterol [88,89].
Chromium is a vital trace element with a biological function that is necessary for maintaining insulin sensitivity and glucose metabolism; this finding is considered a significant development in recent diabetes studies [90]. A crucial component of human nutrition is iron. It is a component of hemoglobin that supports the immune system, in addition to carrying oxygen throughout the body. About 600–700 million individuals worldwide suffer from acute iron deficiency anemia, with developing nations comprising the majority of cases [91]. Cadmium and lead have no nutritional value, and when levels of cadmium and lead are too high, they can lead to a number of illnesses such as cancer and cardiovascular issues [92]. The maximum acceptable concentrations of cadmium and lead in fruits are 0.05 mg/kg wet weight and 0.10 mg/kg wet weight, respectively, as per European legislation (1881/2006/EC) [93]. The amounts of cadmium and lead found in mature and immature K. pinnata fruit extracts were lower than what is recommended by European law. This suggests that both mature and immature fruit extracts of K. pinnata have no negative effects on human health.
The proximate analyses showed that the mature fruits have high amounts of reducing sugars, total carbohydrates, crude fiber and crude protein, while immature fruits have high amounts of moisture and total free amino acids. In addition, there is a non-significant difference in crude fat percentage between mature and immature fruits (Table 3). Similar results have been reported by Oseni and Williams [94], who found that the carbohydrate content is 36.10%, the crude fiber content is 21.09%, the crude protein content is 16.31%, the moisture content is 9.70%, fat is 4.23% and ash is 7.56% in K. africana fruit. The fruits with high protein contents were found to be beneficial for maintaining healthy adult, child, and fetal growth and development, which all require a sufficient amount of protein each day [95]. Fibers are necessary for digestion and waste removal, and they can lower serum cholesterol, the risk of coronary heart disease, constipation, diabetes, colon, hypertension and breast cancer, among other conditions [96]. As a result, K. pinnata fruit can be regarded as an important source of dietary fiber with nutritive value. With regard to carbohydrates, the K. pinnata fruit has the highest value. It is well known that carbohydrates play a significant role in many foods, and that they are a crucial energy source when they are digestible [75]. The study’s findings demonstrate that K. pinnata fruit is a good source of carbohydrates that provide the necessary energy for both humans and animals.
The mature fruits exhibited higher amounts of total phenolics, total flavonoids and total tannins than immature fruits. In addition, there were non-significant differences in total anthocyanin content (Table 4). Owing to their ability to scavenge free radicals due to the presence of hydroxyl groups [97], phenols are crucial plant components that help stabilize lipid oxidation [98]. The high concentrations of phenolic compounds in K. africana extracts are related to the fruit’s capacity to scavenge free radicals [75,99]. Inhibiting the growth of tumors, fighting off parasites, and treating depression are all benefits of flavonoids [100]. Flavonoids are known to have a variety of antioxidant activities. The phenolic structures of these antioxidants serve as their foundation. It is also believed that phenolic compounds can restore endogenous α-tocopherol, which is found in the lipoprotein or bilayer of phospholipids, to its active antioxidant state [75]. Tannins are generally recognized as being beneficial for treating inflamed or ulcerated tissues and possessing remarkable anticancer properties [101]. Tannins have antibacterial, insecticidal, antiviral, and antitumor properties [102,103]. The traditional use of K. pinnata in the treatment of cancer is thus supported by the presence of tannins in fruit extracts.
The number of studies on the therapeutic potential of different plants with potent antioxidant characteristics and few side effects [104] has increased considerably. This has sparked interest in determining whether naturally occurring antioxidants could take the place of synthetic antioxidants, which are currently being restricted due to their toxic and carcinogenic properties [104]. The antioxidant activities of mature (Km) and immature (KI) fruit ethanolic extract have also been examined against DPPH radicals. The reducing power and antioxidant capacity were also evaluated for both extracts (Figure 2 and Figure 3, Table 5). The results obtained show that the mature fruit methanolic extract (Km) has a higher antioxidant activity than KI, leading to greater reducing power, antioxidant capacity, and DPPH activity. The high contents of antioxidant compounds in these extracts, such as total phenolics and flavonoids, may contribute to their greater total antioxidant capacity. Due to their high redox properties, phenolic compounds are crucial for quenching singlet or triplet oxygen, as well as for the breakdown of peroxides. They also absorb and neutralize free radicals, which helps to prevent oxidative chain reactions [98]. Antioxidants’ ability to donate hydrogen is thought to be what causes them to have an impact on DPPH radical-scavenging. The findings of this study demonstrate that K. africana fruit extracts function as primary antioxidants and free radical-inhibitors [75]. The reduction of ferric to ferrous reductants (antioxidants) in the sample material serves as the basis for the assay of reducing activity in this study. The outcomes demonstrate the significant reducing power of K. africana fruit extract. The fruit’s potential antioxidant activity may be significantly indicated by its reducing power [75].
In addition, the reducing power and total antioxidant capacity were higher in the dichloromethane fraction compared to the water fraction of fruits, while the DPPH activity was higher in the methanolic fraction than in the other fractions. These findings demonstrate the capacity of the methanolic fraction to function as a hydrogen atom donor or electron donor in the conversion of stable, purple-colored DPPH to reduced, yellow-colored DPPH-H, and the ability of the dichloromethane fraction (Fe3+) to reduce to Fe2+ via electron transfer. These findings are in line with those reported by Fagbohun et al. [75], who found that various solvent extracts of the K. africana fruit showed hydroxyl ion radical-scavenging activity, confirming the presence of primary antioxidants that regulate the anti-lipid peroxidation potential. According to this study, the solvent extracts of K. africana fruit derived using butanol, ethyl acetate, ethanol, and hexane have a good chance of neutralizing the potential hydrogen peroxide hazard.
Additionally, the antioxidant abilities of fruits could be explained by the presence of different metabolite profiles of both types, and also the quantities of the antioxidant compounds. The GC-MS analysis of mature fruits showed the presence of remarkable antioxidant compounds compared with immature fruit extracts. Larixinic acid (maltol 9.94%), 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one (11.44%), 5-Hydrxoymethylfurfural (22.94%), 2-Methoxy-4-vinylphenol (2.4%), and 4-((1E)-3-Hydoxy-1-propenyl)-2-methoxyphenol (2.01%) were found in the mature extracts.
In addition, the immature extracts contained pregnan-20-one and 3,11-dihydroxy-(3.beta.,5.alpha.,11.beta.) as the principal components (11.34%), as well as 1,2-Benzenedicarboxylic acid, diisooctyl ester (10.6%), gamma-sitosterol (10.45%), i-Propyl 14-methyl-pentadecanoate (6.91%), benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl ester (6.85%), and 2,6,10,10-Tetramethyl-1-oxaspiro[4,5]decan-6-ol (5.40%). Our investigation has identified, for the first time, the profile of novel constituents in mature fruits, even though several investigations have already sought to characterize the metabolites of different parts of the K. pinnata tree. The detected compounds are an intriguing heterogeneous mixture of various chemical structure families, including furanes, sterols, phthalate esters, fatty acid esters, phenolic acid derivatives, and phthalate esters. Maltol, 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one and 5-Hydrxoymethylfurfural were formed as products of the Maillard reaction. These compounds may also be responsible for the brown color of mature fruits, and their antioxidant activity [105]. For example, 5-hydroxymethyl-2-furfural (5-HMF) had the strongest scavenging activity against the hydroxyl radical, DPPH radicals, and superoxide anion radicals compared with the relatively weak alkyl radical [106]. Additionally, 5-HMF demonstrated a novel antioxidant activity by neutralizing ABTS and DPPH free radicals, or by reducing AAPH-induced hemolysis in a dose-dependent way [107]. 2-methoxy-4-vinylphenol showed the strongest antioxidant activity [108].
The electron-deficient structure of maltol, with an unshared pair of electrons on the oxygen atom, is closely related to its enhanced oxidation of Fe2+ ion as a pro-oxidant, and can explain the strong antioxidant properties of maltol. The 3-hydroxy group in the maltol structure participates in the formation of chelation complexes with iron. Additionally, the pyrone ring is a rather potent base, and maltol comprises a resonance hybrid of carbonium and oxonium (pyrylium) forms. The 3-hydroxy group can bind with iron and attract electrons from Fe2+ to Fe3+ ions, resulting in the scavenging of the pro-oxidant Fe2+ ions [109].
3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one (DDMP) was identified as a strong antioxidant in glucose–histidine Maillard reaction products (Glc–His MRPs). DDMP was also responsible for the increase in DPPH radical-scavenging activity, ABTS radical-scavenging activity and reducing power [110]. The hydroxyl group at the olefin position had a more remarkable impact than the C-3 hydroxyl group of DDMP, indicating that the unstable enol structure in the DDMP moiety can be considered the source of its antioxidant activity [111].
The data in Table 9 and Supplementary Figure S2 show that sixteen peaks in total were separated and detected in the negative and positive modes of UPLC-MS/MS. These include pinnatal (21.39%), kigelinol (20.52%), kigelinone (14.23%), isokigelinol (12.58%), isopinnatal (36.28%), coffeic acid (14.06%) and ferulic acid (12.91%). These compounds belong to the iridoid, phenolic, sterol, and quinone classes. According to this study, K. pinnata fruit extracts contain a variety of bioactive molecules that have a variety of pharmacological effects. Similar studies have found that several K. africana phytochemicals, including demethylkigelin, kigelin, ferulic acid and 2-(1-hydroxyethyl)-naphtho[2,3-b]furan-4,9-dione, can inhibit cancer cell proliferation [112]. Norviburtinal and isopinnatal, two compounds in K. pinnata, have been shown to be cytotoxic in vitro against cancer cell lines, and against human melanoma cells that may have growth-inhibitory properties [74].
The results in Table 9 and Figure 10 show that both methanolic and ethyl acetate extracts of mature fruits have a positive effect on all cancer cell lines as compared to the drug doxorubicin. In addition, the methanolic extracts of mature fruits showed stronger cytotoxic effects than the ethyl acetate extract. Moreover, the most pronounced cytotoxic effects of the methanolic extract were detected in SW620 (colorectal adenocarcinoma), SNU-16 (gastric carcinoma) and PANC-1 (pancreatic cancer). The fruit extracts may have these effects because they contain bioactive substances. For example, maltol demonstrated good anti-inflammatory and antioxidative properties. Maltol’s potent antioxidant properties have been suggested to be responsible for its hepatoprotective and anti-liver cancer effects on an alcohol-induced liver oxidative injury model [113,114,115]. In PC12 cells, maltol treatment also promotes PINK1/Parkin-mediated mitophagy, which aids in the restoration of mitochondrial functions. As a result of our research, maltol can be suggested as a potential therapeutic candidate for the treatment and management of spinal cord injury (SCI) [116]. Maltol also has a strong anti-inflammatory and anti-apoptotic effect, which may be partially attributed to its regulation of the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway [117,118]. On human melanoma A375 cells, 5-HMF demonstrated greater antiproliferative activity than other cell lines. The action mechanisms show that 5-HMF could cause G0/G1 cell cycle arrest and apoptosis in A375 cells [119]. Moreover, Panc-1 and SNU-213 pancreatic cancer cell lines are resistant to the effects of cancer related to 2-Methoxy-4-vinyl phenol (2M4VP). By suppressing the expression of the cell nuclear antigen (PCNA) protein, 2M4VP lessens the viability of Panc-1 cells. Both cell lines’ migratory activities were reduced by 2M4VP. Additionally, 2M4VP treatment successfully reduced the phosphorylation of AKT and Focal Adhesion Kinase (FAK). 2M4VP, a supplement, may be used to treat pancreatic cancer [120,121,122,123].
5. Conclusions
The methanolic extract of the K. pinnata fruit exhibits antioxidant activity, which may be useful in preventing or delaying the onset of various oxidative stress-related diseases. The findings of the current study also suggest that the fruit contains a variety of phytochemicals, including minerals, carbohydrates, proteins, terpenoids, flavonoids, and phenols, all of which have positive pharmacological effects and are essential for human and animal nutrition. Therefore, the methanolic extracts of K. pinnata fruits may be useful as a feed additive or as a medicine to enhance humans’ and domestic animals’ health and growth. The research also suggests that the methanolic extract of fruits can be used as a cancer treatment, and is a good source of natural antioxidants. In this study, mature fruits were analyzed using GC-MS and UPLC-MS/MS to identify bioactive compounds with antioxidant and anticancer properties. The fruits of K. pinnata are considered inedible by people. Understanding the antioxidant properties of K. pinnata fruit extracts requires further research into their cytotoxicity towards the human body.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations9110379/s1, Figure S1a,b: GC-MS total ion chromatogram (TIC) of methanolic extract of (a) mature (Km) and (b) immature (KI) fruits of K. pinnata.; Figure S2a,b: LC-MS/MS total ion chromatogram (TIC) in positive (up) or negative ion modes (down) of crude methanolic extract of mature (Km) fruits of K. pinnata. Table S1. Qualitative phytochemical screening of water and methanol extracts of mature (Km) and Immature (KI) fruits of K. pinnata. Refs. [124,125,126,127,128,129].
Author Contributions
Conceptualization, K.M.A.R., H.S.E.-B., H.I.M., T.A.S., A.T.M., A.G., M.M.A.F. and E.S.A.B.; methodology, K.M.A.R., M.M.A.F., A.G. and E.S.A.B.; software, K.M.A.R. and H.S.E.-B.; validation, K.M.A.R., H.S.E.-B., H.I.M., T.A.S., A.T.M., A.G., M.M.A.F. and E.S.A.B.; formal analysis, K.M.A.R., M.M.A.F., A.G.E and E.S.A.B.; investigation, K.M.A.R., H.S.E.-B. and H.I.M., resources, K.M.A.R., H.S.E.-B., T.A.S. and A.T.M.; data curation, K.M.A.R., H.I.M., M.M.A.F., A.G. and E.S.A.B.; writing—original draft preparation, H.S.E.-B., H.I.M. and K.M.A.R.; writing—review and editing, K.M.A.R., H.S.E.-B., H.I.M. and E.S.A.B.; visualization, K.M.A.R., H.S.E.-B., H.I.M., T.A.S., A.T.M., A.G., M.M.A.F. and E.S.A.B.; supervision, K.M.A.R., H.S.E.-B. and H.I.M.; project administration, K.M.A.R. and H.S.E.-B.; funding acquisition, K.M.A.R., H.S.E.-B., T.A.S. and A.T.M., All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Al Bilad Bank Scholarly Chair for Food Security in Saudi Arabia, the Deanship of Scientific Research, the Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Grant No. CHAIR 10).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors acknowledge the Al Bilad Bank Scholarly Chair for Food Security in Saudi Arabia, the Deanship of Scientific Research, and the Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reyes-Munguía, A.; Carrillo-Inungaray, M.L.; Carranza-Álvarez, C.; Pimentel-González, D.J.; Alvarado-Sánchez, B. Antioxidant activity, antimicrobial and effects in the immune system of plants and fruits extracts. Front. Life Sci. 2016, 9, 90–98. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Abdelazeem, A.S.; Youssef, R.; Safwat, G. GC-MS analysis, antioxidant, antimicrobial and anticancer activities of extracts from Ficus sycomorus fruits and leaves. Not. Bot. Horti Agrobot. Cluj Napoca 2019, 47, 493–505. [Google Scholar] [CrossRef]
- Hamed, M.M.; Abd El-Mobdy, M.A.; Kamel, M.T.; Mohamed, H.I.; Bayoumi, A.E. Phytochemical and biological activities of two asteraceae plants Senecio vulgaris and Pluchea dioscoridis L. PharmacologyOnline 2019, 2, 101–121. [Google Scholar]
- Romeilah, R.M.; El-Beltagi, H.S.; Shalaby, E.A.; Younes, K.M.; El Moll, H.; Rajendrasozhan, S.; Mohamed, H.I. Antioxidant and cytotoxic activities of Artemisia monosperma L. and Tamarix aphylla essential oils. Not. Bot. Horti Agrobot. Cluj Napoca 2021, 9, 12233. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Aldaej, M.I.; Al-Khayri, J.M.; Rezk, A.A.; Al-Mssallem, M.Q.; Sattar, M.N.; Ramadan, K.M.A. Production and antioxidant activity of secondary metabolites in Hassawi rice (Oryza sativa L.) cell suspension under salicylic acid, yeast extract, and pectin elicitation. Vitr. Cell. Dev. Biol. Plant 2022, 58, 615–629. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Eshak, N.S.; Mohamed, H.I.; Bendary, E.S.A.; Danial, A.W. Physical characteristics, minerals content, antioxidants and antibacterial activities of Punica granatum or Citrus sinensis peel extracts and their applications to improve cake quality. Plants 2022, 11, 1740. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.R.; El-Beltagi, H.S.; Aly, A.A.; El-Ansary, A.E. Antioxidant enzyme activities and lipid peroxidation as biomarker for potato tuber stored by two essential oils from Caraway and Clove and its main component carvone and eugenol. Asian Pac. J. Trop. Biomed. 2012, 2, S772–S780. [Google Scholar] [CrossRef]
- Elkatry, H.O.; Ahmed, A.R.; El-Beltagi, H.S.; Mohamed, H.I.; Eshak, N.S. Biological activities of grape seed by-products and their potential use as natural sources of food additives in the production of Balady bread. Foods 2022, 11, 1948. [Google Scholar] [CrossRef]
- Abdel-Rahim, E.A.; El-Beltagi, H.S. Constituents of apple, parsley and lentil edible plants and their therapy treatments for blood picture as well as liver and kidney functions against lipidemic disease. Elec. J. Environ. Agricult. Food Chem. 2010, 9, 1117–1127. [Google Scholar]
- Dalmartello, M.; La Vecchia, C.; Bertuccio, P.; Boffetta, P.; Levi, F.; Negri, E.; Malvezzi, M. European cancer mortality predictions for the year 2022 with focus on ovarian cancer. Ann. Oncol. 2021, 33, 330–339. [Google Scholar] [CrossRef]
- Wekha, G.; Ssewante, N.; Iradukunda, A.; Jurua, M.; Nalwoga, S.; Lanyero, S.; Olum, R.; Bongomin, F. Colorectal cancer in Uganda: A 10-year, facility-based, retrospective study. Cancer Manag. Res. 2021, 13, 7697–7707. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Omara, T.; Odero, M.P.; Obakiro, S.B. Medicinal plants used for treating cancer in Kenya: An ethnopharmacological overview. Bull. Natl. Res. Cent. 2022, 46, 1–33. [Google Scholar] [CrossRef]
- Mohamed, A.A.; El-Beltagi, H.S.; Rashed, M.M. Cadmium stress induced change in some hydrolytic enzymes, free radical formation and ultrastructural disorders in radish plant. Elec. J. Environ. Agric. Food Chem. 2009, 8, 969–983. [Google Scholar]
- Omara, T.; Kiprop, A.K.; Ramkat, R.C.; Cherutoi, J.; Kagoya, S.; Moraa Nyangena, D.; Chepkemoi Koske, M. Medicinal plants used in traditional management of cancer in Uganda: Ethnobotanical surveys, phytochemistry and anticancer studies. Evid. Based Complement. Altern. Med. 2020, 2020, 3529081. [Google Scholar] [CrossRef]
- El-desoky, A.H.; Abdel-Rahman, R.F.; Ahmed, O.K.; El-Beltagi, H.S.; Hattori, M. Anti-inflammatory and antioxidant activities of naringin isolated from Carissa carandas L.: In vitro and in vivo evidence. Phytomedicine 2018, 42, 126–134. [Google Scholar] [CrossRef]
- Joffe, P. Kigelia africana (Lam) Benth. Pretoria National Botanical Garden. Available online: www.plantzafrica.com (accessed on 19 June 2009).
- Jackson, S.; Beckett, K. Sausage Tree Kigelia pinnta: An Ethnobotanical and Scientific Review. Am. Bot. Counc. United States 2012, 94, 48–59. Available online: https://www.herbalgram.org/resources/herbalgram/issues/94/table-of-contents/feat_sausagetree/ (accessed on 19 June 2009).
- Gabriel, O.A.; Olubunmi, A. Comprehensive scientific demystification of Kigelia africana: A review. Afr. J. Pure Appl. Chem. 2009, 3, 158–164. [Google Scholar]
- Oyelami, O.A.; Yusuf, K.O.; Oyelami, A.O. The use of Kigelia africana in the management of polycysticovary syndrome (PCOS). Chin. Med. 2012, 3, 1–3. [Google Scholar] [CrossRef]
- Kumawat, R.B.; Mali, P.C.; Sharma, R.A. Ethanomedicine and pharmacological activities of five traditionally used in Indian medicinal plants: A review. Adv. Pharmacol. Toxicol. 2015, 16, 45–56. [Google Scholar]
- Bello, I.; Shehu, M.; Musa, M.; Asmawi, M.; Mahmud, R. Kigelia africana (Lam.) Benth. (Sausage tree): Phytochemistry and pharmacological review of a quintessential African traditional medicinal plant. J. Ethnopharmacol. 2016, 189, 253–276. [Google Scholar] [CrossRef]
- Nabatanzi, A.; Nkadimeng, S.M.; Lall, N.; Kabasa, J.D.; McGaw, L.J. Antioxidant and anti-inflammatory activities of Kigelia Africana (Lam.) Benth. Evid. Based Complement. Altern. Med. 2020, 2020, 4352084. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.G.; Ali, Z.; Chittiboyina, A.G.; Khan, I.A. Kigelia africana fruit: Constituents, bioactivity, and reflection on composition disparities. World J. Tradit. Chin. Med. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Atawodi, S.E.O.; Olowoniyi, O.D. Pharmacological and Therapeutic Activities of Kigelia africana (Lam.) Benth. Ann.Res. Rev. Biol. 2015, 5, 1–17. [Google Scholar] [CrossRef]
- Imran, I.Z.; Elusiyan, C.A.; Agbedahunsi, J.M.; Omisore, N.O. Bioactivity-directed evaluation of fruit of Kigelia africana (Lam.) Benth. Used in treatment of malaria in Iwo, Nigeria. J. Ethnopharmacol. 2021, 268, 113680. [Google Scholar] [CrossRef] [PubMed]
- Fagbohun, O.F.; Joseph, J.S.; Salami, O.A.; Msagati, T.A.M. Exploration of modern chromatographic methods coupled to mass spectrometric techniques for trace element and chemical composition analyses in the leaf extracts of Kigelia africana. Biol. Trace Elem. Res. 2021, 199, 1633–1648. [Google Scholar] [CrossRef]
- Gouda, Y.G.; Abdel-baky, A.M.; Darwish, F.M.; Mohamed, K.M.; Kasai, R.; Yamasaki, K. Iridoids from Kigelia pinnata DC. fruits. Phytochemistry 2003, 63, 887–892. [Google Scholar] [CrossRef]
- Costa, R.; Albergamo, A.; Pellizzeri, V.; Dugo, G. Phytochemical screening by LC-MS and LC-PDA of ethanolic extracts from the fruits of Kigelia africana (Lam.) benth. Nat. Prod. Res. 2017, 31, 1397–1402. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Marin, B.; Chopin, E.I.B.; Jupinet, B.; Gauthier, D. Comparison of microwave-assisted digestion procedures for total trace element content determination in calcareous soils. Talanta 2008, 77, 282–288. [Google Scholar] [CrossRef]
- Jayaraman, J. Postharvest Biological Control; Wiely Eastern Limited: New Delhi, India, 1985. [Google Scholar]
- Tobaruela, E.D.C.; Santos, A.D.O.; de Almeida-Muradian, L.B.; Araujo, E.D.S.; Lajolo, F.M.; Menezes, E.W. Application of dietary fiber method AOAC 2011.25 in fruit and comparison with AOAC 991.43 method. Food Chem. 2018, 238, 87–93. [Google Scholar] [CrossRef]
- Goffman, F.D.; Bergman, C.J. Rice kernel phenolic content and its relationship with antiradical efficiency. J. Sci. Food Agric. 2004, 84, 1235–1240. [Google Scholar] [CrossRef]
- Chang, C.; Yang, M.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in Propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites. In Methods in Ecology; Blackwell Scientific Publications: Oxford, UK, 1994. [Google Scholar]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Allam, R.M.; Al-Abd, A.M.; Khedr, A.; Sharaf, O.A.; Nofal, S.M.; Khalifa, A.E.; Mosli, H.A.; Abdel-Naim, A.B. Fingolimod interrupts the cross talk between estrogen metabolism and sphingolipid metabolism within prostate cancer cells. Toxicol. Lett. 2018, 291, 77–85. [Google Scholar] [CrossRef]
- Hamad, Y.K.; Abobakr, Y.; Salem, M.Z.; Ali, H.M.; Al-Sarar, A.S.; Al-Zabib, A.A. Activity of plant extracts/essential oils against three plant pathogenic fungi and mosquito larvae: GC/MS analysis of bioactive compounds. BioResources 2019, 14, 4489–4511. [Google Scholar]
- Uchegbu, R.I.; Ahuchaogu, A.A.; Amanze, K.O.; Ibe, C.O. Chemical constituents analysis of the leaves of Bryophyllum pinnatum by GC-MS. AASCIT J. Chem. 2017, 3, 19–22. [Google Scholar]
- Purushothaman, A.; Nandhakumar, E.; Shanthi, P.; Sachidanandam, T.P. Shemamruthaa, a Herbal Formulation Induces Apoptosis in Breast Cancer Cells and Inhibits Tumor Progression in Rats. J. Evid. Based Complement. Altern. Med. 2016, 21, NP1–NP10. [Google Scholar] [CrossRef]
- Song, L.; Wang, R.; Che, L.; Jiang, Y.; Zhou, M.; Zhao, Y.; Pang, J.; Jiang, M.; Zhou, G.; Zheng, M.; et al. Catalytic aerobic oxidation of lignocellulose-derived levulinic acid in aqueous solution: A novel route to synthesize dicarboxylic acids for bio-based polymers. ACS Catal. 2021, 11, 11588–11596. [Google Scholar] [CrossRef]
- Rubab, M.; Chelliah, R.; Saravanakumar, K.; Barathikannan, K.; Wei, S.; Kim, J.R.; Yoo, D.; Wang, H.W.; Oh, D.H. Bioactive potential of 2-methoxy-4-vinylphenol and benzofuran from Brassica oleracea L. var. capitate f, rubra (Red Cabbage) on oxidative and microbiological stability of beef meat. Foods 2020, 9, 568. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, E.; Gopalakrishnan, S. GC-MS analysis of some bioactive constituents of Mussaenda frondosa Linn. Int. J. Pharma Bio. Sci. 2011, 2, 313–320. [Google Scholar]
- Smith, P.M. Approaches to the Synthesis of Galbonolide Macrolides. PhD. Thesis, The University of Manchester, Manchester, UK, 1994. [Google Scholar]
- Khatiwora, E.; Adsul, V.B.; Kulkarni, M.; Deshpande, N.R.; Kashalkar, R.V. Antibacterial activity of Dibutyl Phthalate: A secondary metabolite isolated from Ipomoea carnea stem. J. Pharm. Res. 2012, 5, 150–152. [Google Scholar]
- Jebastella, J.; Appavoo, R. Bioactive components of Cynodon dactylon using ethanol extract. World J. Pharm. Res. 2015, 3, 2388–2391. [Google Scholar]
- Tinikul, R.; Chenprakhon, P.; Maenpuen, S.; Chaiyen, P. Biotransformation of plant-derived phenolic acids. Biotechnol. J. 2018, 13, 1700632. [Google Scholar] [CrossRef]
- D’Auria, M.; Fascetti, S.; Racioppi, R.; Romano, V.A.; Rosati, L. Orchids from Basilicata: The scent. In Orchids Phytochemistry, Biology and Horticulture; Reference Series in Phytochemistry; Merillon, J.M., Kodja, H., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Brown, A. Cutin and Suberin in Mixed-Wood Boreal Forest Plants and Their Use as Markers for Origin of Soil Organic Matter.Ms.C. Thesis. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2020. [Google Scholar] [CrossRef]
- Doshi, G.M.; Nalawade, V.V.; Mukadam, A.S.; Chaskar, P.K.; Zine, S.P.; Somani, R.R.; Une, H.D. Structural elucidation of chemical constituents from Benincasa hispida seeds and Carissa congesta roots by gas chromatography: Mass spectroscopy. Pharmacogn. Res. 2015, 7, 282. [Google Scholar] [CrossRef]
- Hou, C.T. A novel compound, 12, 13, 17-trihydroxy-9 (Z)-Octadecenoic acid, from linoleic acid by a new microbial isolateClavibacter sp. ALAJ. Am. Oil Chem. Soc. 1996, 73, 1359–1362. [Google Scholar] [CrossRef]
- Nakai, S.; Yamada, S.; Hosomi, M. Anti-cyanobacterial fatty acids released from Myriophyllum Spicatum. Hydrobiol. 2005, 543, 71–78. [Google Scholar] [CrossRef]
- Sivagnanam, S.; Valliammai, R. Determination of Bioactive and Pharmaceutical Components of Croton bonplandianus by GC-MS Analysis. Int. J. Pharm. Sci. Nanotechnol. 2016, 9, 3488–3493. [Google Scholar] [CrossRef]
- Romano, V.A.; Rosati, L.; Fascetti, S.; Cittadini, A.M.R.; Racioppi, R.; Lorenz, R.; D’Auria, M. Spatial and temporal Variability of the floral scent emitted by Barlia robertiana (Loisel.) Greuter, a Mediterranean food-deceptive orchid. Compounds 2022, 2, 37–53. [Google Scholar] [CrossRef]
- Dong, M.; Oda, Y.; Hirota, M. (10E, 12Z, 15Z)-9-hydroxy-10, 12, 15-octadecatrienoic acid methyl ester as an anti-inflammatory compound from Ehretia dicksonii. Biosci. Biotechnol. Biochem. 2000, 64, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Abdulhafiz, F.; Mohammed, A.; Kayat, F.; Bhaskar, M.; Hamzah, Z.; Podapati, S.K.; Reddy, L.V. Xanthine oxidase inhibitory activity, chemical composition, antioxidant properties and GC-MS Analysis of Keladi Candik (Alocasia longiloba Miq). Molecules 2020, 25, 2658. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, D.; Meltzer, R.I. Quinoline antibacterial agents. Oxolinic acid and related compounds. J. Med. Chem. 1968, 11, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Veilumuthu, P. Characterization of Secondary Metabolites Derived from Tomato Endophyte–Streptomyces sp. Shanivit. Curr. Trends Biotechnol. Pharm. 2022, 16 (Suppl. 1), 141–152. [Google Scholar] [CrossRef]
- Fadipe, L.A.; Haruna, A.K.; Mohammed, I. Antibacterial activity of 1, 2-benzenedicarboxylic acid, dioctyl ester isolated from the ethyl acetate soluble sub-portion of the unripe fruits of Nauclea Latifolia. Int. J. Pure Appl. Biosci. 2014, 2, 223–230. [Google Scholar]
- Ji, M.; Yu, Z.; Chen, G.; Masood, T.; Ma, F. Chemical Constituents and Biological Functions of Different Extracts of Millettia speciosa Leaves. J. Food Nutr. Res. 2020, 8, 506–515. [Google Scholar] [CrossRef]
- Balami, V.M.; Yakubu, J.; Mamza, U.T.; Dawa, S.I.; Abdulrahman, F.I.; Sodipo, O.A. Isolation and Analysis of Methanol Extract of Leaf of Senna siamea. J. Chem. Soc. Nigeria 2020, 45, 1110–1119. [Google Scholar] [CrossRef]
- Janeczko, A.; Skoczowski, A. Mammalian sex hormones in plants. Folia Histochem. Cytobiol. 2005, 43, 71–79. [Google Scholar]
- Picerno, P.; Autore, G.; Marzocco, S.; Meloni, M.; Sanogo, R.; Aquino, R.P. Anti-inflammatory activity of verminoside from kigelia a fricana and evaluation of cutaneous irritation in cell cultures and reconstituted human epidermis. J. Nat. Prod. 2005, 68, 1610–1614. [Google Scholar] [CrossRef]
- Joshi, K.C.; Singh, P.; Taneja, S.; Cox, P.J.; Howie, R.A.; Thomson, R.H. New terpenoid aldehydes from Kigelia pinnata: Crystal structure of pinnatal. Tetrahedron 1982, 38, 2703–2708. [Google Scholar] [CrossRef]
- Weiss, C.R.; Moideen, S.V.; Croft, S.L.; Houghton, P.J. Activity of extracts and isolated naphthoquinones from Kigelia pinnata against Plasmodium falciparum. J. Nat. Prod. 2000, 63, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Bharti, N.; Singh, S.; Naqvi, F.; Azam, A. Isolation and in vitro antiamoebic activity of iridoids isolated from Kigelia pinnata. Arkivoc 2006, 10, 69–79. [Google Scholar] [CrossRef]
- Afify, A.E.M.M.; El-Beltagi, H.S. Effect of insecticide cyanophos on liver function in adult male rats. Fresenius Environ. Bull. 2011, 20, 1084–1088. [Google Scholar]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio) synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.J.; Houghton, P.J.; Retsas, S.; Photiou, A. In vitro cytotoxicity of norviburtinal and isopinnatal from Kigelia pinnata against cancer cell lines. Planta Med. 66 2000, 8, 758–761. [Google Scholar] [CrossRef]
- Fagbohun, O.F.; Babalola, O.O.; Agboola, F.K.; Joseph, J.S.; Malindisa, S.; Msagati, T.A.M. Evaluation of phytochemicals, antioxidants, trace elements in Kigelia africana fruit extracts and chemical profiling analysis using UHPLC-qTOF-MS2 spectrometry. Biol. Trace Elem. Res. 2020, 195, 679–695. [Google Scholar] [CrossRef]
- Elekofehinti, O.O.; Iwaloye, O.; Olawale, F.; Ariyo, E.O. Saponins in cancer treatment: Current progress and future prospects. Pathophysiology 2021, 28, 250–272. [Google Scholar] [CrossRef]
- Ramadan, K.M.A.; El-Beltagi, H.S.; Shanab, S.M.M.; El-fayoumy, E.A.; Shalaby, E.A.; Bendary, E.S.A. Potential antioxidant and anticancer activities of secondary metabolites of Nostoc linckia cultivated under Zn and Cu stress conditions. Processes 2021, 9, 1972. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Dawi, F.; Ashoush, I.S.; Ramadan, K.M.A. Antioxidant, anticancer and ameliorative activities of Spirulina platensis and pomegranate juice against hepatic damage induced by CClNot. Bot. Hort. Agrobot. Cluj Napoca 2020, 48, 1941–1956. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Dawi, F.; Aly, A.A.; El-Ansary, A.E. Chemical compositions and biological activities of the essential oils from gamma irradiated celery (Apium graveolens L.) seeds. Not. Bot. Hort. Agrobot. Cluj Napoca 2020, 48, 2114–2133. [Google Scholar] [CrossRef]
- Rajendrasozhan, S.; Moll, H.E.; Snoussi, M.; Romeilah, R.M.; El-Beltagi, H.S.; Shalaby, E.A.; Younes, K.M.; El-Beltagi, H.S. Phytochemical screening and antimicrobial activity of various extracts of aerial parts of Rhanterium epapposum. Processes 2021, 9, 1351. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Elmelegy, A.A.; Eldesoky, S.E.; Safwat, G. Phytochemical screening, antimicrobial, antioxidant, anticancer activities and nutritional values of cactus (Opuntia ficus Indicia) pulp and peel. Fresenius Environ. Bull. 2019, 28, 1534–1551. [Google Scholar]
- El-Beltagi, H.S.; Mohamed, H.I.; Safwat, G.; Gamal, M.; Megahed, B.M.H. Chemical composition and biological activity of Physalis peruviana L. Gesunde Pflanz. 2019, 71, 113–122. [Google Scholar] [CrossRef]
- Rizvi, A.; Ahmed, B.; Khan, M.S.; El-Beltagi, H.S.; Umar, S.; Lee, J. Bioprospecting plant growth promoting rhizobacteria for enhancing the biological properties and phytochemical composition of medicinally important crops. Molecules 2022, 27, 1407. [Google Scholar] [CrossRef]
- Valko, M. .; Morris, H.; Cronin, M.T. Metals, toxicity, and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Knez, M.; Glibetic, M. Zinc as a biomarker of cardiovascular health. Front. Nutr. 2021, 8, 686078. [Google Scholar] [CrossRef]
- Porchia, M.; Pellei, M.; Del Bello, F.; Santini, C. Zinc complexes with nitrogen donor ligands as anticancer agents. Molecules 2020, 25, 5814. [Google Scholar] [CrossRef]
- Maret, W. Zinc in pancreatic islet biology, insulin sensitivity, and diabetes. Prev. Nutr. Food Sci. 2017, 22, 1–8. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Popiołek, Ł.; Kocot, J. The many “faces” of copper in medicine and treatment. Biometals 2014, 27, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahim, E.A.; El-Beltagi, H.S.; Romela, R.M. White Bean seeds and Pomegranate peel and fruit seeds as hypercholesterolemic and hypolipidemic agents in albino rats. Grasas Y Aceites 2013, 64, 50–58. [Google Scholar] [CrossRef]
- Guimarães, M.M.; Carvalho, A.C.; Silva, M.S. Effect of chromium supplementation on the glucose homeostasis and anthropometry of type 2 diabetic patients: Double blind, randomized clinical trial. J. Trace Elem. Med. Biol. 2016, 36, 65–72. [Google Scholar] [CrossRef]
- Petry, N.; Olofin, I.; Hurrell, R.F.; Boy, E.; Wirth, J.P.; Moursi, M.; Donahue, A.M.; Rohner, F. The proportion of anemia associated with Iron deficiency in low, medium, and high human development index countries: A systematic analysis of national surveys. Nutrients 2016, 8, 693. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- European Commission. (Regulation 1881/2006/EC) Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. 2006, 364, 5–24. [Google Scholar]
- Oseni, O.A.; Williams, O.D. In-vitro compositional investigations of antioxidants, phytochemicals, nutritional, and minerals in the fruit of Kigelia africana (Lam.) Benth. Int. J. Contemp. Res. Rev. 2018, 9, 20259–20268. [Google Scholar] [CrossRef]
- Elango, R.; Ball, R.O. Protein and Amino Acid Requirements during Pregnancy. Adv. Nutr. 2016, 7, 839S–844S. [Google Scholar] [CrossRef] [PubMed]
- Ioniță-Mîndrican, C.-B.; Ziani, K.; Mititelu, M.; Oprea, E.; Neacșu, S.M.; Moroșan, E.; Dumitrescu, D.-E.; Roșca, A.C.; Drăgănescu, D.; Negrei, C. Therapeutic benefits and dietary restrictions of fiber intake: A state of the art review. Nutrients 2022, 14, 2641. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical scavenging mechanisms of phenolic compounds: A Quantitative structure-property relationship (QSPR) study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef] [PubMed]
- Assanti, G.; Kaur, R.; Nizard, S.; Pollack, E.; Rafferty, B.; Priano, C.; Fernández Romero, J.A.; Koroch, A.R. Biology, chemistry, and pharmacological activity of Kigelia africana (Bignoniaceae) and Garcinia kola (Clusiaceae)-a Review. J. Med. Act. Plants 2022, 11, 1–21. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Otero, P.; Cassani, L.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Chamorro, F.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Traditional applications of tannin rich extracts supported by scientific data: Chemical composition, bioavailability and bioaccessibility. Foods 2021, 10, 251. [Google Scholar] [CrossRef]
- Kumari, M.; Jain, S. Tannins: An antinutrient with positive effect to manage diabetes. Res. J. Recent. Sci. 2012, 1, 1–8. [Google Scholar]
- Mohamed, H.I.; Abd–El Hameed, A.G. Molecular and biochemical markers of some Vicia faba L. genotype in response to storage insect pests infestation. J. Plant Interact. 2014, 9, 618–626. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: An Updated Review. Oxid. Med. Cell Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.; Gan, S. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef]
- Li, Y.X.; Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. In vitro antioxidant activity of 5-HMF isolated from marine red alga Laurencia undulata in free radical mediated oxidative systems. J. Microbiol. Biotechnol. 2009, 19, 1319–1327. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Su, J.; Li, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agric. Food Chem. 2013, 61, 10604–10611. [Google Scholar] [CrossRef]
- Kamiyama, M.; Moon, J.K.; Jang, H.W.; Shibamoto, T. Role of degradation products of chlorogenic acid in the antioxidant activity of roasted coffee. J. Agric. Food Chem. 2015, 63, 1996–2005. [Google Scholar] [CrossRef]
- Murakami, K.; Ito, M.; Tanemura, Y.; Yoshino, M. Maltol as an antioxidant: Inhibition of lipid peroxidation and protection of NADP-isocitrate dehydrogenase from the iron-mediated inactivation. Biomed. Res. 2001, 22, 183–186. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, M.; Liu, F.; Zeng, S.; Hu, J. Identification of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one as a strong antioxidant in glucose-histidine Maillard reaction products. Food Res. Inter. 2013, 51, 397–403. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Q.; Zhao, Z.; Bai, B.; Sun, Z.; Cai, L.; Fu, Y.; Ma, Y.; Wang, Q.; Xi, G. Effect of hydroxyl on antioxidant properties of 2,3-dihydro-3,5-dihydroxy-6-methyl-4: H -pyran-4-one to scavenge free radicals. RSC Adv. 2021, 11, 34456–34461. [Google Scholar] [CrossRef]
- Higgins, C.A.; Bell, T.; Delbederi, Z.; Feutren-Burton, S.; McClean, B.; O’Dowd, C.; Watters, W.; Armstrong, P.; Waugh, D.; van den Berg, H. Growth inhibitory activity of extracted material and isolated compounds from the fruits of Kigelia pinnata. Planta Med. 2010, 76, 1840–1846. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Q.; Hu, J.N.; Han, X.Y.; Li, W.; Zhao, L.C. Maltol, a food flavoring agent, attenuates acute alcohol-induced oxidative damage in mice. Nutrients 2015, 7, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Geran, R.I.; Greenberg, N.H.; Macdonald, M.M.; Shumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother. Rep. Part. III 1972, 3, 1–103. [Google Scholar]
- Srisawat, T.; Chumkaew, P.; Heed-Chim, W.; Sukpondma, Y.; Kanokwiroon, K. Phytochemical Screening and Cytotoxicity of Crude Extracts of Vatica diospyroides Symington Type LS. Trop. J. Pharm. Res. 2013, 12, 71–76. [Google Scholar] [CrossRef]
- Mao, Y.; Du, J.; Chen, X.; Al Mamun, A.; Cao, L.; Yang, Y.; Mubwandarikwa, J.; Zaeem, M.; Zhang, W.; Chen, Y.; et al. Maltol promotes mitophagy and inhibits oxidative stress via the Nrf2/PINK1/Parkin pathway after spinal cord injury. Oxid. Med. Cell. Longev. 2022, 2022, 1337630. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, W.; Hu, J.; Mi, X.; Han, Y.; Ren, S.; Jiang, S.; Wang, Y.; Li, X.; Li, W. Maltol improves APAP-induced hepatotoxicity by inhibiting oxidative stress and inflammation response via NF-κB and PI3K/Akt signal pathways. Antioxidants 2019, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Thienthiti, K.; Tuchind, P.; Wongnoppavich, A.; Anantachoke, N.; Soonthornchareonnon, N. Cytotoxic effect of compounds isolated from Goniothalamus marcanii Craib stem barks. Pharm. Sci. Asia 2017, 44, 86–95. [Google Scholar] [CrossRef]
- Jiménez-Estrada, M.; Velázquez-Contreras, C.; Garibay-Escobar, A.; Sierras-Canchola, D.; Lapizco-Vázquez, R.; Ortiz-Sandoval, C.; Burgos-Hernández, A.; Robles-Zepeda, R.E. In vitro antioxidant and antiproliferative activities of plants of the ethnopharmacopeia from northwest of Mexico. BMC Complement. Altern. Med 2013, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; El-Mahdy, O.M.; Mohamed, H.I.; El-Ansary, A.E. Antioxidants, antimicrobial, and anticancer activities of purified chitinase of Talaromyces funiculosus strain CBS 129594 biosynthesized using crustacean bio-wastes. Agronomy 2022, 12, 2818. [Google Scholar] [CrossRef]
- Sofy, M.R.; Mohamed, H.I.; Dawood, M.F.A.; Abu-Elsaoud, A.M.; Soliman, M.H. Integrated usage of Trichoderma harzianum and biochar to ameliorate salt stress on spinach plants. Arch. Agr. Soil Sci. 2021, 68, 2005–2026. [Google Scholar] [CrossRef]
- Ghonaim, M.M.; Mohamed, H.I.; Omran, A.A.A. Evaluation of wheat salt stress tolerance using physiological parameters and retrotransposon-based markers. Genet Resour. Crop Evol. 2021, 68, 227–242. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, S.I.; Go, B.; Oh, U.H.; Kim, C.S.; Jung, Y.H.; Lee, J.; Kim, J.H. 2-methoxy-4-vinylphenol attenuates migration of human pancreatic cancer cells via blockade of fak and akt signaling. Anticancer Res. 2019, 39, 6685–6691. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Phenolic compounds. In Phytochemical Methods; Springer: Dordrecht, The Netherlands, 1973; pp. 33–88. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Trease, G.E.; Evans, W.C. Pharmacognosy, 13th ed.; J & A. Churchill Ltd.: London, UK, 1989; pp. 234–492. [Google Scholar]
- Sofowora, A. Recent trends in research into African medicinal plants. J. Ethnopharmacol. 1993, 38, 209–214. [Google Scholar] [CrossRef]
- Alexei, Y.B.; Joseph, I.S.; Olga, V.F. Endogenous cardiotonic steroids: Physiology, pharmacology and novel therapeutic targets. Pharmacol Rev. 2009, 61, 9–38. [Google Scholar] [CrossRef]
- El Aziz, M.M.A.; Ashour, A.S.; Melad, A.S.G. A review on saponins from medicinal plants: Chemistry, isolation, and determination. J. Nanomed. Res. 2019, 8, 282–288. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).