Establishing and Verifying a Robust Liquid Chromatography-Tandem Mass Spectrometry Method to Simultaneously Measure Seven Androgens Present in Plasma Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation and Analytical Conditions
2.3. Preparation of the Stock Solutions, Calibration Standards, and Quality Control Samples
2.4. Sample Preparation
2.5. Method Validation
2.6. Clinical Application
2.7. Statistical Analysis
3. Results
3.1. Representative Chromatography
3.2. Method Validation
3.2.1. Linearity
3.2.2. LOD and LOQ
3.2.3. Accuracy and Precision
3.2.4. Matrix Effect
3.2.5. Carryover
3.3. Clinical Application
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, F.C.; Tajar, A.; Beynon, J.M.; Pye, S.R.; Silman, A.J.; Finn, J.D.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; Forti, G.; et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N. Engl. J. Med. 2010, 363, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef] [PubMed]
- Walsh, P.C. American Society of Clinical Oncology recommendations for the initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer. J. Urol. 2005, 173, 1966. [Google Scholar] [CrossRef]
- Kelly, D.M.; Jones, T.H. Testosterone and obesity. Obes. Rev. 2015, 16, 581–606. [Google Scholar] [CrossRef]
- Bienenfeld, A.; Azarchi, S.; Sicco, K.L.; Marchbein, S.; Shapiro, J.; Nagler, A.R. Androgens in women: Androgen-mediated skin disease and patient evaluation. J. Am. Acad. Dermatol. 2019, 80, 1497–1506. [Google Scholar] [CrossRef]
- Wittert, G.; Bracken, K.; Robledo, K.P.; Grossmann, M.; Yeap, B.B.; Handelsman, D.J.; Stuckey, B.; Conway, A.; Inder, W.; McLachlan, R.; et al. Testosterone treatment to prevent or revert type 2 diabetes in men enrolled in a lifestyle programme (T4DM): A randomised, double-blind, placebo-controlled, 2-year, phase 3b trial. Lancet Diabetes Endocrinol. 2021, 9, 32–45. [Google Scholar] [CrossRef]
- Lv, W.; Du, N.; Liu, Y.; Fan, X.; Wang, Y.; Jia, X.; Hou, X.; Wang, B. Low Testosterone Level and Risk of Alzheimer’s Disease in the Elderly Men: A Systematic Review and Meta-Analysis. Mol. Neurobiol. 2016, 53, 2679–2684. [Google Scholar] [CrossRef]
- Kyriazis, J.; Tzanakis, I.; Stylianou, K.; Katsipi, I.; Moisiadis, D.; Papadaki, A.; Mavroeidi, V.; Kagia, S.; Karkavitsas, N.; Daphnis, E. Low serum testosterone, arterial stiffness and mortality in male haemodialysis patients. Nephrol. Dial. Transplant. 2011, 26, 2971–2977. [Google Scholar] [CrossRef]
- Cao, Z.; Lu, Y.; Cong, Y.; Liu, Y.; Li, Y.; Wang, H.; Zhang, Q.; Huang, W.; Liu, J.; Dong, Y.; et al. Simultaneous quantitation of four androgens and 17-hydroxyprogesterone in polycystic ovarian syndrome patients by LC-MS/MS. J. Clin. Lab. Anal. 2020, 34, e23539. [Google Scholar] [CrossRef]
- Janse, F.; Eijkemans, M.J.; Goverde, A.J.; Lentjes, E.G.; Hoek, A.; Lambalk, C.B.; Hickey, T.E.; Fauser, B.C.; Norman, R.J. Assessment of androgen concentration in women: Liquid chromatography-tandem mass spectrometry and extraction RIA show comparable results. Eur. J. Endocrinol. 2011, 165, 925–933. [Google Scholar] [CrossRef]
- Turcu, A.F.; Auchus, R.J. Clinical significance of 11-oxygenated androgens. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Bloem, L.M.; Storbeck, K.H.; Swart, P.; Toit, T.d.; Schloms, L.; Swart, A.C. Advances in the analytical methodologies: Profiling steroids in familiar pathways-challenging dogmas. J. Steroid. Biochem. Mol. Biol. 2015, 153, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, S. A robust LC-MS/MS assay with online cleanup for measurement of serum testosterone. J. Sep. Sci. 2019, 42, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Peng, Y.; Chen, F.; Zhao, L.; Li, X.; Qin, J.; Li, Q.; Wang, B.; Pan, B.; et al. A liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based assay to profile 20 plasma steroids in endocrine disorders. Clin. Chem. Lab. Med. 2020, 58, 1477–1487. [Google Scholar] [CrossRef]
- Rege, J.; Turcu, A.F.; Kasa-Vubu, J.Z.; Lerario, A.M.; Auchus, G.C.; Auchus, R.J.; Smith, J.M.; White, P.C.; Rainey, W.E. 11-Ketotestosterone Is the Dominant Circulating Bioactive Androgen During Normal and Premature Adrenarche. J. Clin. Endocrinol. Metab. 2018, 103, 4589–4598. [Google Scholar] [CrossRef]
- Imamichi, Y.; Yuhki, K.I.; Orisaka, M.; Kitano, T.; Mukai, K.; Ushikubi, F.; Taniguchi, T.; Umezawa, A.; Miyamoto, K.; Yazawa, T. 11-Ketotestosterone Is a Major Androgen Produced in Human Gonads. J. Clin. Endocrinol. Metab. 2016, 101, 3582–3591. [Google Scholar] [CrossRef]
- Barnard, L.; Schiffer, L.; du-Toit, R.L.; Tamblyn, J.A.; Chen, S.; Africander, D.; Arlt, W.; Foster, P.A.; Storbeck, K.H. 11-Oxygenated Estrogens Are a Novel Class of Human Estrogens but Do not Contribute to the Circulating Estrogen Pool. Endocrinology 2021, 162, bqaa231. [Google Scholar] [CrossRef]
- Häkkinen, M.R.; Heinosalo, T.; Saarinen, N.; Linnanen, T.; Voutilainen, R.; Lakka, T.; Jääskeläinen, J.; Poutanen, M.; Auriola, S. Analysis by LC-MS/MS of endogenous steroids from human serum, plasma, endometrium and endometriotic tissue. J. Pharm. Biomed. Anal. 2018, 152, 165–172. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Peitzsch, M.; Kaden, D.; Langton, K.; Pamporaki, C.; Masjkur, J.; Tsatsaronis, G.; Mangelis, A.; Williams, T.A.; Reincke, M.; et al. Reference intervals for plasma concentrations of adrenal steroids measured by LC-MS/MS: Impact of gender, age, oral contraceptives, body mass index and blood pressure status. Clin. Chim. Acta 2017, 470, 115–124. [Google Scholar] [CrossRef]
- Desai, R.; Harwood, D.T.; Handelsman, D.J. Simultaneous measurement of 18 steroids in human and mouse serum by liquid chromatography-mass spectrometry without derivatization to profile the classical and alternate pathways of androgen synthesis and metabolism. Clin. Mass. Spectrom. 2019, 11, 42–51. [Google Scholar] [CrossRef]
- Büttler, R.M.; Martens, F.; Kushnir, M.M.; Ackermans, M.T.; Blankenstein, M.A.; Heijboer, A.C. Simultaneous measurement of testosterone, androstenedione and dehydroepiandrosterone (DHEA) in serum and plasma using Isotope-Dilution 2-Dimension Ultra High Performance Liquid-Chromatography Tandem Mass Spectrometry (ID-LC-MS/MS). Clin. Chim. Acta 2015, 438, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, M.M.; Blamires, T.; Rockwood, A.L.; Roberts, W.L.; Yue, B.; Erdogan, E.; Bunker, A.M.; Meikle, A.W. Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin. Chem. 2010, 56, 1138–1147. [Google Scholar] [CrossRef]
- Gaudl, A.; Kratzsch, J.; Bae, Y.J.; Kiess, W.; Thiery, J.; Ceglarek, U. Liquid chromatography quadrupole linear ion trap mass spectrometry for quantitative steroid hormone analysis in plasma, urine, saliva and hair. J. Chromatogr. A 2016, 1464, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, M.R.; Murtola, T.; Voutilainen, R.; Poutanen, M.; Linnanen, T.; Koskivuori, J.; Lakka, T.; Jääskeläinen, J.; Auriola, S. Simultaneous analysis by LC-MS/MS of 22 ketosteroids with hydroxylamine derivatization and underivatized estradiol from human plasma, serum and prostate tissue. J. Pharm. Biomed. Anal. 2019, 164, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Davio, A.; Woolcock, H.; Nanba, A.T.; Rege, J.; O’Day, P.; Ren, J.; Zhao, L.; Ebina, H.; Auchus, R.; Rainey, W.E.; et al. Sex Differences in 11-Oxygenated Androgen Patterns Across Adulthood. J. Clin. Endocrinol. Metab. 2020, 105, e2921–e2929. [Google Scholar] [CrossRef]
- Koseki, J.; Matsumoto, T.; Matsubara, Y.; Tsuchiya, K.; Mizuhara, Y.; Sekiguchi, K.; Nishimura, H.; Watanabe, J.; Kaneko, A.; Hattori, T.; et al. Inhibition of Rat 5α-Reductase Activity and Testosterone-Induced Sebum Synthesis in Hamster Sebocytes by an Extract of Quercus acutissima Cortex. Evid.-Based Complement. Alternat. Med. 2015, 2015, 853846. [Google Scholar] [CrossRef]
- Sansone, G.; Reisner, R.M. Differential rates of conversion of testosterone to dihydrotestosterone in acne and in normal human skin—A possible pathogenic factor in acne. J. Investig. Dermatol. 1971, 56, 366–372. [Google Scholar] [CrossRef]
- Da Cunha, M.G.; Fonseca, F.L.; Machado, C.D. Androgenic hormone profile of adult women with acne. Dermatology 2013, 226, 167–171. [Google Scholar] [CrossRef]
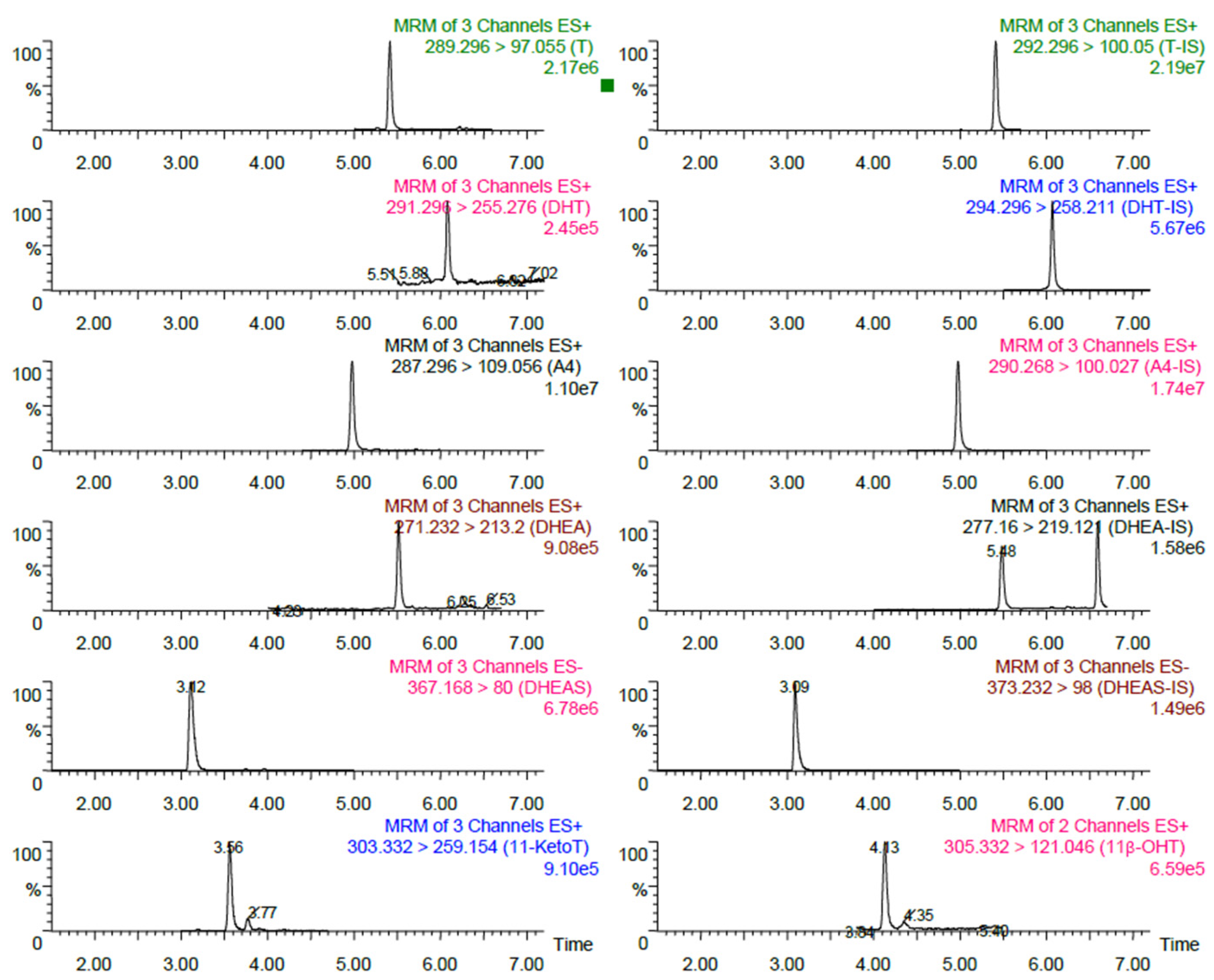

| Analytes | MRM (m/z) | Retention Time (min) | Cone (V) | CE (eV) |
|---|---|---|---|---|
| T | 289.30 > 97.06 * 289.30 > 109.08 | 5.47 | 50 | 10 20 |
| T-IS | 292.30 > 100.05 | 5.47 | 30 | 14 |
| DTH | 291.30 > 159.10 291.30 > 255.28 * | 6.12 | 50 | 16 16 |
| DTH-IS | 294.30 > 258.21 | 6.12 | 50 | 14 |
| A4 | 287.30 > 97.03 * 287.30 > 109.06 | 5.02 | 50 | 10 15 |
| A4-IS | 290.27 > 100.03 | 5.02 | 50 | 20 |
| DHEA | 271.23 > 197.20 271.23 > 213.20 * | 5.52 | 40 | 18 15 |
| DHEA-IS | 277.16 > 219.12 | 5.48 | 40 | 16 |
| DHEAS | 367.17 > 80.00 * 367.17 > 97.00 | 3.12 | 50 | 62 10 |
| DHEAS-IS | 373.23 > 98.00 | 3.09 | 50 | 32 |
| 11-KetoT | 303.33 > 121.05 * 303.33 > 259.15 | 3.56 | 46 | 22 22 |
| 11β-OHT | 305.33 > 121.05 305.33 > 269.16 * | 4.13 | 40 | 22 14 |
| Expected Concentration (ng/mL) | Mean Measured Concentration (ng/mL) | Recovery (%) | |
|---|---|---|---|
| T | 0.050 | 0.049 | 94.2–105.8 |
| 0.500 | 0.483 | 94.8–98.2 | |
| 5.000 | 4.721 | 90.3–96.1 | |
| DHT | 0.100 | 0.095 | 91.7–98.1 |
| 1.000 | 0.909 | 89.4–92.1 | |
| 5.000 | 4.501 | 88.7–91.0 | |
| A4 | 0.200 | 0.195 | 94.6–100.0 |
| 2.000 | 1.978 | 96.3–102.5 | |
| 10.000 | 9.502 | 92.4–98.3 | |
| DHEA | 0.500 | 0.496 | 94.9–106.7 |
| 5.000 | 4.780 | 90.5–99.3 | |
| 25.000 | 24.476 | 92.8–103.0 | |
| DHEAS | 125.000 | 112.632 | 88.6–93.0 |
| 500.000 | 467.756 | 91.4–94.3 | |
| 2500.000 | 2368.597 | 87.6–99.9 | |
| 11-KetoT | 0.050 | 0.049 | 93.4–104.0 |
| 0.500 | 0.479 | 93.3–98.0 | |
| 10.000 | 10.372 | 102.9–105.3 | |
| 11β-OHT | 0.050 | 0.047 | 90.2–96.2 |
| 0.500 | 0.504 | 97.7–104.4 | |
| 10.000 | 9.986 | 95.9–104.2 |
| Concentration (ng/mL) | Intra-Assay CV (%) | Total CV (%) | |
|---|---|---|---|
| T | 0.050 | 3.3 | 3.7 |
| 0.500 | 3.4 | 3.9 | |
| 4.921 | 2.7 | 4.1 | |
| DHT | 0.024 | 5.4 | 7.7 |
| 0.239 | 3.3 | 4.5 | |
| 2.394 | 2.5 | 5.8 | |
| A4 | 0.053 | 4.9 | 5.1 |
| 0.497 | 2.0 | 2.7 | |
| 4.933 | 2.8 | 3.0 | |
| DHEA | 0.513 | 5.6 | 6.4 |
| 5.221 | 3.1 | 6.3 | |
| 25.001 | 4.0 | 5.6 | |
| DHEAS | 48.374 | 3.8 | 6.4 |
| 478.842 | 3.5 | 4.0 | |
| 2390.420 | 4.2 | 5.6 | |
| 11-KetoT | 0.051 | 5.9 | 7.3 |
| 0.498 | 2.7 | 4.8 | |
| 10.377 | 2.4 | 3.2 | |
| 11β-OHT | 0.047 | 6.4 | 6.9 |
| 0.502 | 2.9 | 3.0 | |
| 10.128 | 2.5 | 4.2 |
| Add (ng/mL) | Plasma1 | Plasma2 | Plasma3 | Plasma4 | Plasma5 | Plasma6 | ||
|---|---|---|---|---|---|---|---|---|
| T (ng/mL) | 0.173 | without IS | 82.95% | 65.27% | 74.06% | 78.33% | 56.66% | 70.00% |
| with IS | 116.18% | 116.18% | 106.36% | 109.83% | 108.67% | 110.40% | ||
| 0.654 | without IS | 88.40% | 70.36% | 78.72% | 87.41% | 66.54% | 74.76% | |
| with IS | 117.43% | 114.68% | 111.62% | 115.29% | 113.76% | 117.13% | ||
| 3.440 | without IS | 73.39% | 69.05% | 80.76% | 87.70% | 67.71% | 77.83% | |
| with IS | 110.41% | 109.04% | 112.21% | 111.31% | 112.15% | 114.59% | ||
| DHT (ng/mL) | 0.134 | without IS | 41.08% | 66.93% | 57.86% | 58.97% | 42.35% | 46.13% |
| with IS | 85.82% | 93.28% | 94.03% | 85.82% | 87.31% | 82.84% | ||
| 0.507 | without IS | 43.52% | 61.85% | 55.15% | 63.98% | 46.07% | 50.51% | |
| with IS | 92.31% | 95.86% | 95.86% | 96.84% | 96.06% | 93.29% | ||
| 2.728 | without IS | 51.02% | 56.60% | 51.93% | 63.28% | 47.27% | 52.07% | |
| with IS | 91.17% | 86.73% | 88.49% | 89.37% | 89.96% | 88.86% | ||
| A4 (ng/mL) | 0.164 | without IS | 66.61% | 91.28% | 83.03% | 89.20% | 52.14% | 82.38% |
| with IS | 106.10% | 112.80% | 112.20% | 103.05% | 112.80% | 110.37% | ||
| 0.623 | without IS | 76.28% | 96.62% | 89.82% | 95.62% | 78.30% | 87.48% | |
| with IS | 112.95% | 117.50% | 114.61% | 114.93% | 112.52% | 117.34% | ||
| 3.358 | without IS | 73.84% | 94.50% | 86.13% | 93.17% | 78.84% | 90.27% | |
| with IS | 113.73% | 112.81% | 111.08% | 109.83% | 110.84% | 112.54% | ||
| DHEA (ng/mL) | 0.198 | without IS | 67.29% | 49.40% | 66.77% | 63.07% | 58.42% | 73.14% |
| with IS | 112.22% | 82.83% | 112.12% | 86.36% | 113.23% | 114.65% | ||
| 0.768 | without IS | 67.26% | 69.71% | 69.61% | 70.29% | 60.64% | 65.88% | |
| with IS | 106.33% | 116.15% | 118.36% | 103.26% | 118.10% | 111.07% | ||
| 4.171 | without IS | 61.30% | 66.89% | 67.95% | 74.76% | 64.22% | 69.97% | |
| with IS | 97.03% | 103.96% | 105.75% | 104.58% | 113.14% | 104.12% | ||
| DHEAS (ng/mL) | 0.795 | without IS | 173.74% | 77.13% | 27.34% | 103.10% | 76.21% | 84.61% |
| with IS | 95.97% | 98.11% | 109.31% | 93.58% | 92.58% | 98.87% | ||
| 3.034 | without IS | 90.52% | 91.30% | 9.71% | 108.36% | 74.81% | 74.50% | |
| with IS | 101.38% | 100.13% | 108.51% | 96.80% | 100.49% | 102.67% | ||
| 16.667 | without IS | 132.42% | 154.51% | 168.23% | 183.76% | 128.87% | 141.04% | |
| with IS | 95.07% | 93.02% | 99.05% | 94.22% | 99.65% | 98.78% | ||
| 11-KetoT (ng/mL) | 0.207 | without IS | 76.69% | 72.91% | 60.46% | 80.68% | 65.92% | 68.34% |
| with IS | 115.27% | 118.84% | 83.09% | 114.49% | 107.05% | 108.21% | ||
| 0.820 | without IS | 72.45% | 75.45% | 66.18% | 77.90% | 71.11% | 68.89% | |
| with IS | 113.05% | 114.51% | 92.80% | 102.80% | 91.59% | 107.80% | ||
| 4.031 | without IS | 67.44% | 83.34% | 67.68% | 82.54% | 73.43% | 72.95% | |
| with IS | 106.65% | 105.68% | 93.92% | 104.84% | 101.71% | 107.44% | ||
| 11β-OHT (ng/mL) | 0.235 | without IS | 69.10% | 72.80% | 70.64% | 71.98% | 67.99% | 63.40% |
| with IS | 111.70% | 114.47% | 100.85% | 100.00% | 113.40% | 100.43% | ||
| 0.867 | without IS | 69.72% | 73.95% | 83.36% | 80.78% | 76.84% | 71.17% | |
| with IS | 118.57% | 114.42% | 118.11% | 106.23% | 109.87% | 111.53% | ||
| 4.661 | without IS | 62.11% | 73.11% | 79.28% | 78.83% | 73.37% | 72.28% | |
| with IS | 98.22% | 112.38% | 110.13% | 99.98% | 101.13% | 106.41% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Zou, Y.; Yin, Y.; Yu, J.; Li, Q.; Xie, S.; Luo, W.; Ma, X.; Wang, D.; Qiu, L. Establishing and Verifying a Robust Liquid Chromatography-Tandem Mass Spectrometry Method to Simultaneously Measure Seven Androgens Present in Plasma Samples. Separations 2022, 9, 377. https://doi.org/10.3390/separations9110377
Yu S, Zou Y, Yin Y, Yu J, Li Q, Xie S, Luo W, Ma X, Wang D, Qiu L. Establishing and Verifying a Robust Liquid Chromatography-Tandem Mass Spectrometry Method to Simultaneously Measure Seven Androgens Present in Plasma Samples. Separations. 2022; 9(11):377. https://doi.org/10.3390/separations9110377
Chicago/Turabian StyleYu, Songlin, Yutong Zou, Yicong Yin, Jialei Yu, Qianqian Li, Shaowei Xie, Wei Luo, Xiaoli Ma, Danchen Wang, and Ling Qiu. 2022. "Establishing and Verifying a Robust Liquid Chromatography-Tandem Mass Spectrometry Method to Simultaneously Measure Seven Androgens Present in Plasma Samples" Separations 9, no. 11: 377. https://doi.org/10.3390/separations9110377
APA StyleYu, S., Zou, Y., Yin, Y., Yu, J., Li, Q., Xie, S., Luo, W., Ma, X., Wang, D., & Qiu, L. (2022). Establishing and Verifying a Robust Liquid Chromatography-Tandem Mass Spectrometry Method to Simultaneously Measure Seven Androgens Present in Plasma Samples. Separations, 9(11), 377. https://doi.org/10.3390/separations9110377








