Abstract
The drug 5-fluorouracil (5-FU) is a common cancer chemotherapeutic, presenting toxicity. Mild toxicity is treated with administration of probiotics. The interaction of these probiotics with the drug may have a crucial effect on its therapeutic efficacy. In the present work, a method for the quantification of uracil, 5-FU, and its active metabolite 5-fluorodeoxyuridin monophosphate in cells and culture medium of the probiotic L. lactis is presented. Extraction using H2O containing 0.05% v/v formic acid (1:5 v/v) was followed by ammonium sulphate protein precipitation and SPE. Analysis was conducted in a Nucleosil column using a gradient of water, formic acid, and acetonitrile. Calibration curves were constructed for 5-FU (5–100 μg/mL), uracil (5–20 μg/mL), and 5-fluorodeoxyuridin monophosphate (5–20 μg/mL) using 5-bromouracil as the internal standard (R2 ≥ 0.999). The photodegradation of 5-FU amounted to 36.2% at 96 h. An administration experiment in the dark revealed a decline in 5-FU concentration in the culture media (88.3%) and uptake by the cells, while the uracil and FdUMP levels increased in the cells. The inactive metabolite 5,6 dihydrofluorouracil was detected in the medium. Our results demonstrate that uptake and metabolism of 5-FU in L. lactis cells leads to a decline in the drug levels and in the formation of both the active and the inactive metabolites of the drug.
1. Introduction
Chemotherapy, in combination with radiation and surgery, has been the treatment of choice against cancer for over sixty years [1]. The drug 5-fluorouracil (5-FU) was developed to target increased uracil uptake and use [2,3]. The chemotherapeutic 5-FU is currently used in the treatment of various types of cancers, including breast, head, neck, and digestive tract cancers including colorectal cancer [4,5].
Following its administration, 5-FU is transformed in the cells through anabolism to 5-fluorodeoxyuridin monophosphate (FdUMP) [4], which exerts inhibitory action on thymidylate synthase (TS), resulting in a reduced formation of thymidine precursors for DNA synthesis. Hence, the administration of 5-FU results in the depletion of such precursors and “thymineless cell death” [6]. However, a major part of the administered drug is subjected to catabolism and is converted to 5,6-dihydro-5-fluorouracil (5-FUH2), an inactive metabolite, through a reaction catalyzed by dihydropyrimidine dehydrogenase (DPD) [7]. The activity of this enzyme is highly variable, and its absence results in the life-threatening toxicity of 5-FU [8,9]. As a diagnostic tool for DPD deficiency, uracil or thymine determination in plasma or urine has been proposed [10]. Therefore, the analysis of 5-FU, uracil, and 5-FU metabolites following administration of a test dose is considered essential to achieve both increased efficacy and safety of the drug [11]. The toxicity of 5-FU induces vomiting and diarrhea, which have been associated with an imbalance in the gut microbiome [12]. Such side effects are commonly treated with probiotics, mainly of the genera Lactobacillus or Bifidobacteria [13,14,15], increasing the apoptotic action of chemotherapeutics [16]. Lactococcus lactis has also been successfully employed [15], leading to improved clinical outcomes [17]. However, the need for further studies on the use of probiotics as adjuvant therapy for cancer patients to reduce the side effects of chemotherapy is imperative in order to define exact personalized doses [18]. While chemotherapeutics, including 5-FU, inhibit bacterial growth [19], bacteria have been reported to accumulate or even biotransform drugs and pollutants [20], and the study of such interactions is crucial [21]. A drug’s oral bioavailability, and hence its efficacy, are affected by the biotransformation and elimination of drugs by the microbiome and should be assessed [22]. The bacterial metabolism of 5-FU modified its efficacy in the nematode C. elegans [23]. Moreover, strains of L. lactis resistant to 5-FU were isolated [24], and an enzyme isolated from L. lactis [25] presented great resemblance to DPD, which catalyzed the formation of the 5-FU inactive metabolite [26]. These findings indicate possible effects of the microbiota and its manipulation of the efficacy of chemotherapeutics. The interactions of probiotics with 5-FU have not yet been reported, and require the determination of the drug and its active metabolite FdUMP in cultures and in bacterial cells.
Several methods have been developed for the extraction and determination of 5-FU and its metabolites in biological samples. Both 5-FU and its active metabolite FdUMP present a high aqueous solubility and a low solubility to organic solvents, thus resulting in extraction difficulties [27]. Extraction usually employs the acidification of human serum samples as well as protein precipitation [27,28] followed by liquid-liquid extraction, employing different solvents such as propanol: diethyl ether [29] or ethyl acetate [30,31]. Solid phase extraction has also been employed to ensure improved 5-FU recovery using the C18 column matrix [32,33], ion exchange SPE [34,35,36], or the polymeric matrix SPE [37]. The methods employed for the analysis of 5-FU in human plasma, serum, and urine have been thoroughly reviewed [27,38,39] and employ HPLC-UV analysis [29,40] or GC analysis [41,42], and more recently, LC-MS/MS [43,44,45].
However, extraction from tissue samples is hindered by coeluting matrix components [40], hence fewer methods are specific for tissue in either human cancer [46,47,48,49] or human skin [50], and more elaborate extraction protocols, or even derivatization and column switching, have been proposed [51]. The need for tissue specific extraction methods has been emphasized [40].
Although the methods for the determination of these compounds in human samples are abundant, there is no report on a method specific for bacterial cultures that would allow the study of the possible biotransformation of the drug by the probiotic bacteria which are coadministered with chemotherapy. To this end, in the present study a simple method for the extraction and simultaneous determination of 5-FU, its active metabolite 5-fluorodeoxyuridin monophosphate, and uracil in the cultures of the probiotic L. lactis was developed and validated.
2. Materials and Methods
2.1. Chemicals and Reagents
Acetonitrile, methanol, formic acid, and NaCl were acquired from VWR chemicals (VWR International GmbH, Graumanngasse 7, A-1150 Wien, Austria); n-Hexane was acquired from Labscan (RCI Labscan Limited, 24 Rama 1 Rd., Rongmuang, Pathumwan, Bangkok, Thailand). (NH4)2SO4 was acquired from Lach-ner (Tovární 157, 277 11 Neratovice, Czech Republic); KH2PO4, CaCl2, MgSO4, NH4Cl, and FeCl3 were acquired from Panreac (Panreac Química SLU, Castellar del Vallès, Spain). Brain Heart Infusion broth (BHI broth), liquid culture medium, and yeast extract were acquired from Applichem (AppliChem GmbH, Darmstadt, Germany). Uracil and FdUMP were acquired from Merck (MERCK KGAA, Darmstadt, Germany). 5-fluorouracil (5-FU) and the internal standard 5-bromouracil (5-BrU) were purchased from Alfa Aesar (Ward Hill, Haverhill, MA, USA). Solid phase extraction columns, frits, C18 sorbent material, and 35–75 U were acquired from Agilent (Santa Clara, CA, USA). The composition of Basal Salts Medium (BSM) was KH2PO4 1 g/L, NH4Cl 1 g/L, MgSO4 0.1 g/L, NaCl, and CaCl2, all at 0.05 g/L each, as well as FeCl3 0.01 g/L and yeast extract 1 g/L diluted in double-distilled water (ddH2O).
2.2. Standard Preparation
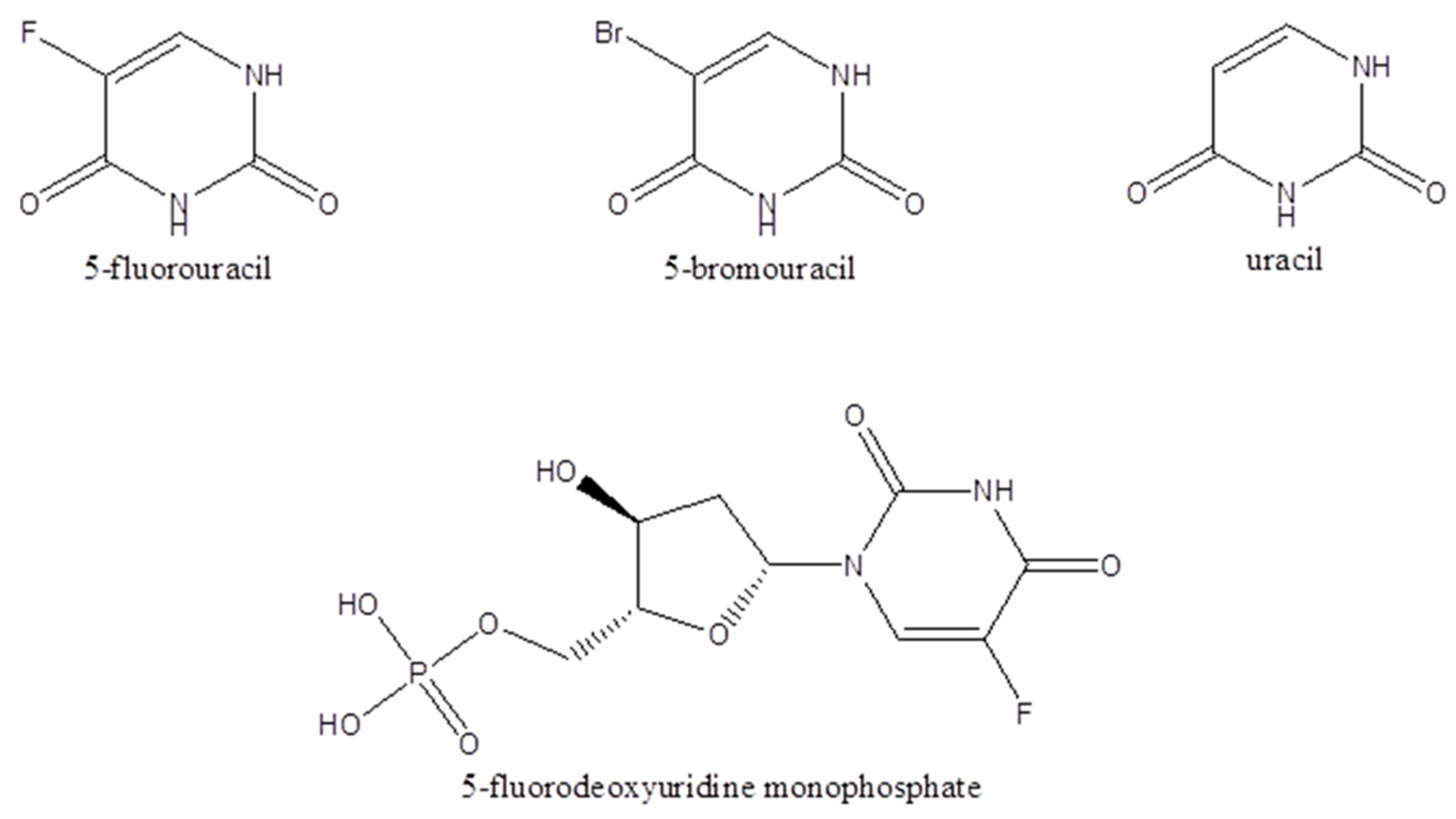
Stock solutions of the analytes 5-FU (1 mg/mL), uracil (1 mg/mL), FdUMP (100 μg/mL), and the internal standard 5-BrU (500 μg/mL) were prepared in 1:1 (v/v) methanol: ddH2O. Calibration curves of 5-FU, FdUMP, and uracil were constructed in BSM for the supernatant. For the 5-FU calibration curve preparation in BSM, working standard solutions were prepared at concentrations of 5, 25, 50, 75, and 100 μg/mL. For uracil and for the FdUMP calibration curves, working standard solutions were prepared at concentrations of 5,10 15, and 20 μg/mL. Calibration curves in the cell precipitates were prepared for 5-FU, FdUMP, and uracil using cells suspended in 0.5 mL of ddH2O containing 0.05% v/v formic acid. Moreover, 5-FU was added at concentrations of 5, 25, 50, 75, and 100 μg/mL, while FdUMP and uracil were added at concentrations of 5, 10, 15, and 20 μg/mL. The chemical structures of the analytes 5-FU, U, FdUMP, and the internal standard 5-BU are shown in Figure 1.
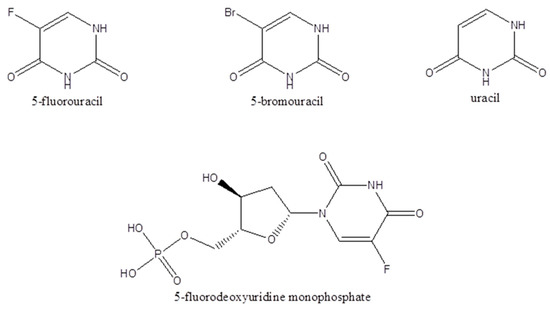
Figure 1.
The chemical structures of 5-fluorouracil, 5-bromouracil, uracil, and 5-fluorodeoxyuridine monophosphate.
2.3. Sample Preparation
To prepare the bacterial pellet for analysis, 0.1 g of wet cell weight L. lactis was suspended in 0.5 mL of ddH2O containing 0.05% v/v formic acid, and the desired amount of 5-FU was added to the sample. The sample was then homogenized while the tube was submerged in ice. The homogenized mixture was then transferred to an Eppendorf tube and centrifuged at 8000 rpm for 10 min. The supernatant was collected and 0.5 g of (NH4)2SO4 was added. After thoroughly stirring the sample for 1 min, it was centrifuged for 20 min at 10,000 rpm, then the supernatant was collected. The sample was then loaded on a solid phase extraction (SPE) cartridge (0.5 g C18 sorbent) following conditioning with 5 mL methanol, 5 mL dH2O, and 5 mL dH2O containing 0.1% v/v formic acid (pH = 3.5–4). A manual flow of 1 mL/min was employed. The cartridge was washed with 2 mL n-Hexane, and 5-FU was eluted using 0.5 mL of dH2O containing 0.05% formic acid and acetonitrile at a ratio of 95:5 v/v. The analytical standard 5-BrU was added in the SPE eluate at a concentration of 50 μg/mL.
Bacterial supernatant samples (0.5 mL) were collected at 0 h and at 96 h and placed in an Eppendorf tube, 0.5 g (NH4)2SO4 was added, and the procedure described for the cell pellet, including centrifuging and SPE, was followed.
All of the above procedures were performed in tubes covered in triplicate and all tubes were kept in the dark to avoid the photodegradation of the 5-FU. All of the samples were either analyzed directly or stored at −20 °C until analysis.
2.4. Sample Analysis Using HPLC-DAD
An LC20AD pump and an SPD-20A photodiode array detector (DAD) (Shimadzu, Kyoto, Japan) was used. The injection volume was 80 μL, and the separation was done at room temperature using a Nucleosil 100 C18 column (250 × 4.6 mm, 5 μm) purchased from Macherey-Nagel GmbH & Co. (Duren, Germany) with a binary mobile phase system. Mobile phase A consisted of ddH2O containing 0.05% formic acid and acetonitrile at a ratio of 95:5 v/v, while mobile phase B consisted of ddH2O containing 0.05% formic acid and acetonitrile at a ratio of 5:95 v/v. A gradient elution was used to separate the analytes, and the flow rate was constant at 0.5 mL/min. The gradient started at 100% during mobile phase A, which was brought down by 90% over 2 min, 80% over 5 min, 70% at 8 min, and lastly at 50% over 20 min, where it was kept until the end of the analyses. All analytes were detected at 260 nm with a bandwidth of 2.0 nm. Each of them was identified by matching the peak retention time (Rt) and peak purity via spectrum overlay with those of the pure standards. The concentration of the analyte was plotted against the peak area ratio of the analyte to the peak area of the internal standard and was used for internal standard calibration.
2.5. Peak Identification
Peaks originating from HPLC analysis for 5-FU and uracil were identified by retention time and spectrum analysis. Unidentified peaks were subjected to ESI-MS analysis on an LC-20AD Shimadzu connected to Shimadzu LCMS-2010EV equipped with a C18 analytical column (Reprospher 100 C18-DE, 5 µm 250 × 4.6 mm, Dr Maisch GmbH, Ammerbuch, Germany) using 1% formic acid in 50:50 v/v acetonitrile: water. The obtained mass spectra of these compounds were compared to the standard spectra of the NIST Mass Spectral Library (NIST Chemistry WebBook, SRD 69, National Institute of Standards and Technology NIST 100 Bureau Drive, Gaithersburg, MD, USA) for 5-FU and uracil, while for 5FdUMP, the mass spectra were compared to the spectra of the pure standard and to the standard spectra of the Human Metabolome Database (HMDB).
2.6. Photodegradation Assay
To investigate the photodegradation of 5-FU in the bacterial cultures, a quantity of 5-FU (1 mg/mL) was added in a falcon containing 50 mL of BSM so that the final concentration would be 50 μg/mL. An identical tube was prepared and kept in the dark, wrapped in aluminum foil. The two falcon tubes were incubated at 28 °C under constant stirring. The 5-FU was quantified using the calibration curves and the methods described above in the beginning (0 h) and at the end of the 96 h period.
2.7. FU Administration
The bacterial strain L. lactis (ATCC 11454) was inoculated in 50 mL of Brain Heart Infusion broth (BHI broth). After a 24 h incubation period at 28 °C under constant stirring, the culture was centrifuged at 3000 rpm for 15 min at 4 °C and the precipitate was resuspended in 50 mL of Basal Salts Medium (BSM). Then, a quantity of 5-FU (1 mg/mL) was added so that the final concentration would be 50 μg/mL, with 5-FU serving as the main carbon source. The culture was left under constant stirring at 28 °C for 96 h. At 96 h the culture was centrifuged at 3000 rpm for 15 min at 4 °C, and a sample (0.5 mL) of the supernatant was collected and analyzed using the method and calibration curves that were described above, while the precipitated cells were harvested and stored at −20 °C until analysis.
3. Results and Discussion
3.1. Optimization of Sample Preparation
Extraction of the analytes from BHI medium, recommended for growth of L. lactis, resulted in matrix peaks that might interfere with the analytes peaks. Hence, the analysis was performed in BSM medium and not in the medium which is recommended for L. lactis growth. The use of BSM also contributed to the biotransformation of 5-FU by increasing its uptake by the cells, since it was their main carbon source. The 5-bromouracil was selected as the internal standard due its structural similarity with 5-FU and because it is not expected to be present in microorganism liquid cultures. The same internal standard has been used previously during the analysis of 5-FU in plasma and tissue samples [48] or human plasma [52], pointing out the superiority of 5-bromouracil and 5-chlorouracil against 5-fluorocytosine. Extraction of 5-FU, uracil, and FdUMP from cells following homogenization was performed in acidic conditions employing formic acid, since 5-FU and uracil analytes are weak organic acids with corresponding pKa values of 8.02 and 9.45, respectively (PubChem Compound Summary for CID 3385, CID 1174), while 5-fluorodeoxyuridine monophosphate is acidic, with a pKa of 1.2 (Drugbank). Protein precipitation followed since proteins expected to be present in analytical samples such as bacterial cells comprise interfering matrix components [27,28]. Further purification of the samples and removal of salts from the preceding ammonium sulfate step employed solid phase extraction. In our preliminary experiments, following sample addition the column was washed with 5 mL of ddH2O solution containing 0.05% NH3, aiming at the ionization of 5-FU so that it would remain attached to the column during the washing step, to be eluted later. However, the analytes were washed out due to high water solubility, resulting in poor recoveries, and hence, washing with hexane, a nonpolar solvent, was employed. The recovery of our method ranged between 99.25–107.02% for the culture supernatant and between 98.93–105.42% for the cell precipitate (Table 1). Representative chromatograms of the analytes are presented in Figure 2 for the cells and in Figure 3 for the culture supernatant.

Table 1.
Validation parameters for the determination of 5-FU, 5-FUMP, and uracil. Results for the Mean Calculated Concentrations and Recoveries are expressed as mean ± SD (n = 3). In calculations for Intraday Precision n = 3, and for Interday Precision n = 6 over three days.
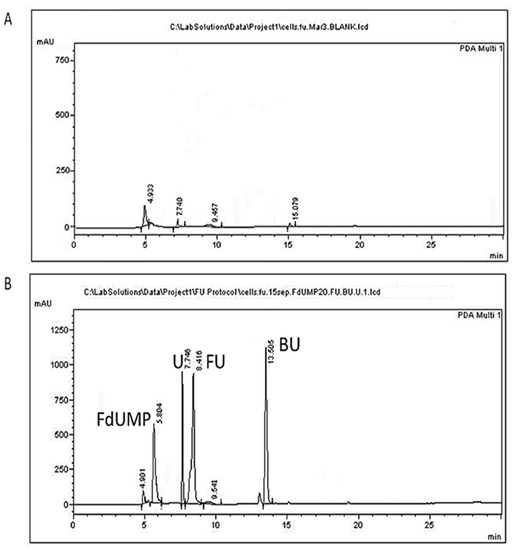
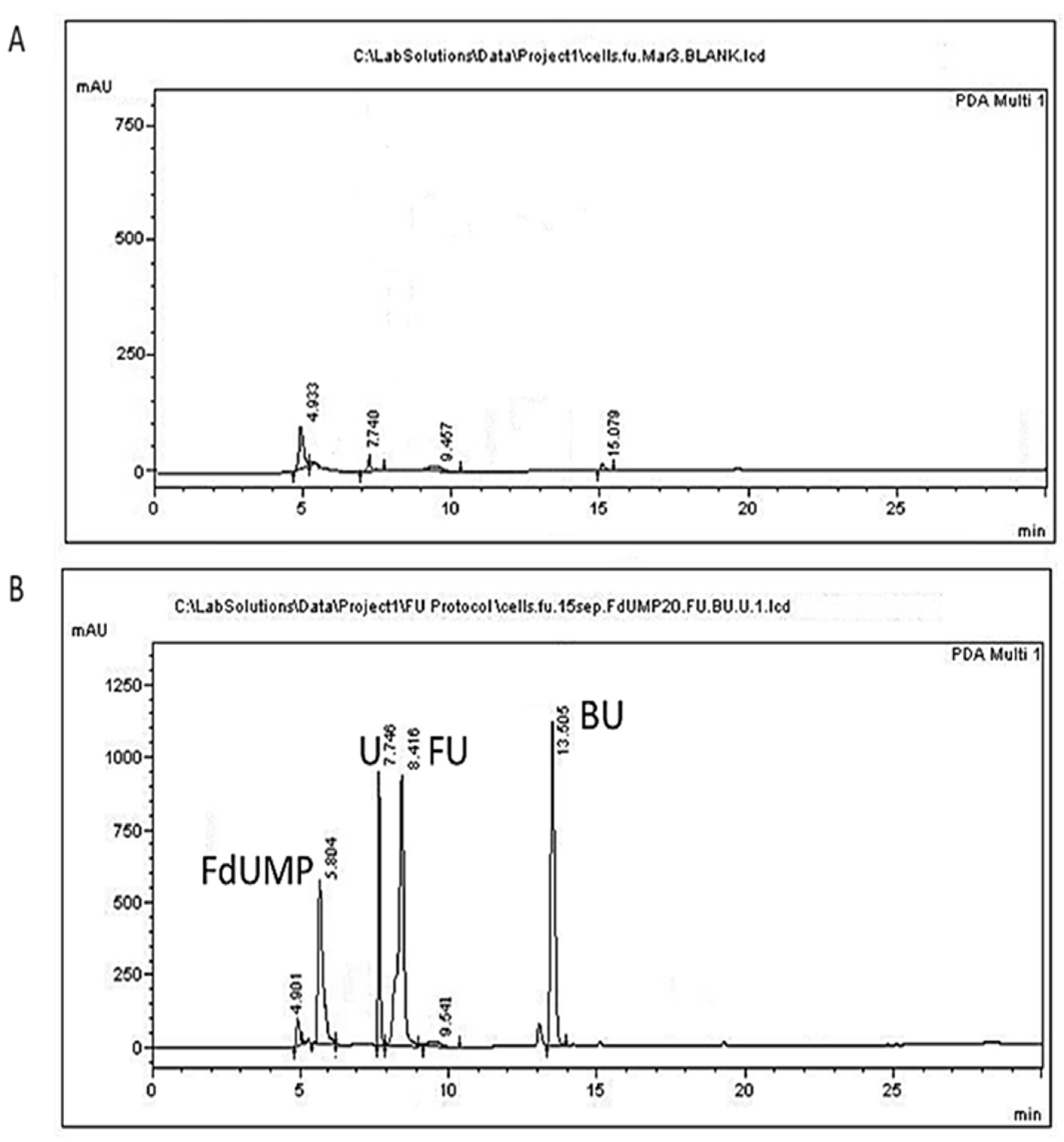
Figure 2.
Representative chromatograms of L. lactis cells. (A) A blank sample; (B) cells spiked with 50 μg/mL of 5-FU (FU), 20 μg/mL uracil (U), 20 μg/mL FdUMP (FdUMP), and 50 μg/mL of the internal standard 5-BU (BU).
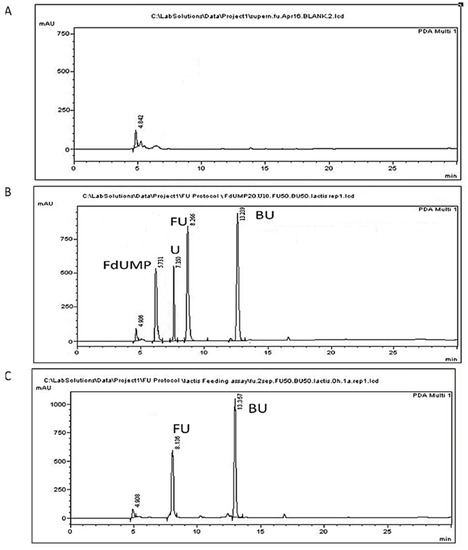
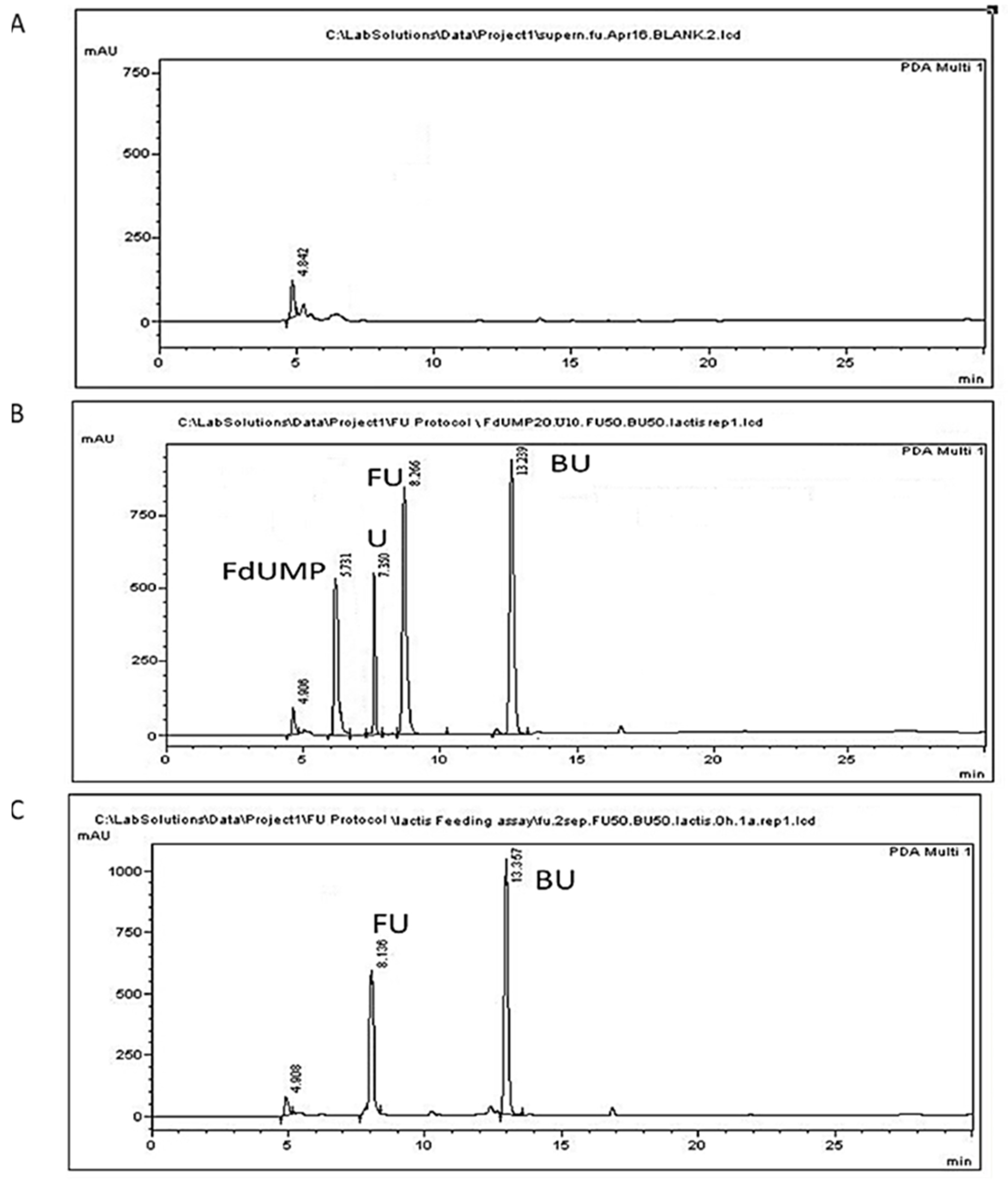
Figure 3.
Representative chromatograms of L. lactis cell-free culture supernatants. (A) A blank sample; (B) a BSM sample spiked with 50 μg/mL of 5-FU (FU), 10 μg/mL uracil (U), 30 μg/mL FdUMP (FdUMP), and 50 μg/mL of the internal standard 5-BU (BU); (C) culture supernatant collected at 0 h following administration of 50 μg/mL 5-FU at 0 h.
The peak appearing at 4.9 min does not correspond to any of the studied analytes and could be attributed to a BSM compound since it is present in all chromatograms originating from all samples of L. lactis cultures (Figure 2 and Figure 3). The peak appearing in blank cells at 7.74 min (Figure 2A) might correspond to endogenous free uracil. This peak, however, corresponded to about 0.53 μg/mL, which is below our LOQ and was not taken into account in our calculations. The retention times of 5-FU, FdUMP, uracil, and of the internal standard 5-BU in cells were 8.37 ± 0.17, 5.81 ± 0.11, 7.76 ± 0.11, and 13.29 ± 0.12 min, respectively (Table 2). Similar retention times were acquired for supernatant samples, except for uracil, which was eluted at 7.252 ± 0.11 min.

Table 2.
LOD, LOQ, and RT values for 5-FU, uracil, and 5FdUMP. Values are provided for both the culture supernatant (BSM) and the bacterial precipitate (L. lactis cells). Retention time values are means ± SD (n = 6).
3.2. Method Validation
The standard curves of all analytes, both for the culture supernatant and for the L. lactis precipitate, presented adequate coefficients of determination and acceptable F-test results (Table 1). Relative bias, estimated as the percentage of the difference of the mean calculated concentration to the nominal concentration divided by the nominal concentration of the analyte, was below the accepted range of ±10% in all cases. A total of three injections of the nominal concentration were performed on the same day to calculate the intraday precision, while the interday precision was calculated from a total of six injections of the nominal concentration performed over a two-day period; both are expressed as % R.S.D.
The limit of detection ((3.3 × SE Intercept)/slope of the standard curve), the limit of quantitation ((10 × SE Intercept)/ slope of the standard curve), and the retention time of the analytes in the two samples, namely the cells and cell-free culture supernatant, are shown in Table 2. Although lower values of 3.2 ng/mL [8] or 12.5 ng/mL [48] have been reported in human plasma in 5-FU determinations, their calculations were based on a signal-to-noise ratio. In the present study, calculations were based on the residual standard error of the intercept of the calibration line, which is recommended, in order to obtain more accurate estimates [53]. Moreover, these estimations largely depend on the analyte concentrations employed. In the present study, the calibration curve was constructed in a manner such that the concentration of 50 μg/mL 5-FU employed in the administration experiment falls within the range of the curve while being slightly below the 50% minimum inhibitory concentration (MIC50). The estimated recovery rates, all above 94.6%, were comparable to the value of 96.2% reported for human serum [35], further supporting the efficacy of our method.
3.3. Photodegradation of 5-FU
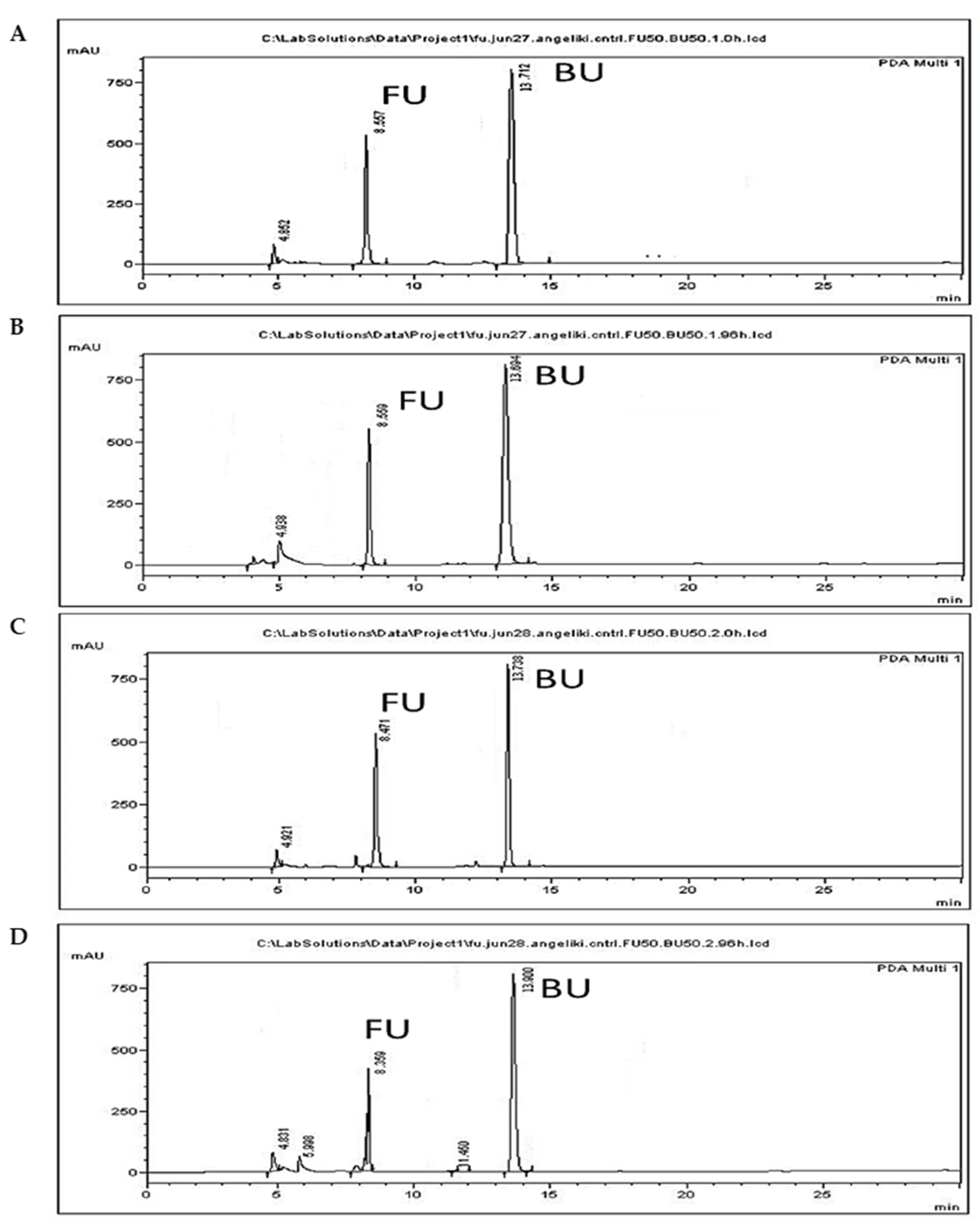
The reduction in 5-FU levels observed when incubations were performed in the dark amounted to the negligible amount of 0.2% (Figure 4A,B), indicating that abiotic factors other than light do not result in the degradation of 5-FU. However, a reduction of 36.2% was observed following incubation under normal light for 96 h in combination with the occurrence of a peak with a retention time of 6.9 min. Similar reduction and two extra peaks were observed when 5-FU was incubated under UVB light [54], and the peaks were identified as photoproducts formed following the addition of water to one double bond of the molecule. Since the parent compound was reported to present improved toxicity against cancer cells compared to the products of transformation by light, care was taken to avoid photodecomposition of the drug in the present study, and all experiments were conducted in the dark.
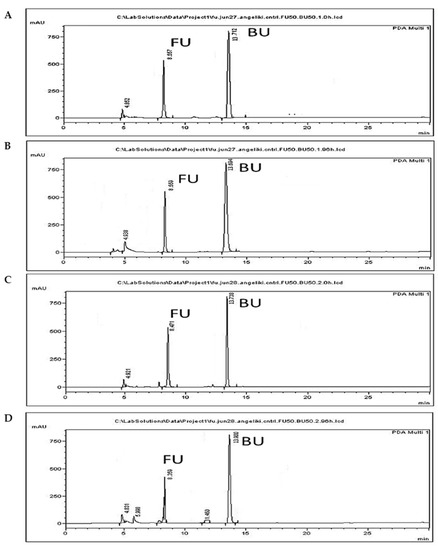
Figure 4.
Photodegradation of 5-FU. Representative chromatograms from 5-FU (FU) (50 μg/mL) incubation in the dark at 0 h (A) and 96 h (B), and under normal light at 0 h (C) and 96 h (D).
3.4. 5-FU Administration
In preliminary experiments on the toxicity of 5-FU against L. lactis (data not shown), the MIC50 was determined at about 65 μg/mL. The 5-FU concentration selected to be used in the administration experiments was 50 μg/mL, which is below that of MIC50. Representative chromatograms of the 5-FU administration assay are presented for L. lactis cell precipitate and culture supernatant in Figure 4B and Figure 5A, respectively. The analyte 5-FU was detected in cell samples even after 96 h of incubation (Figure 5A), amounting to 10.5 μg/mL originating from a 0.1 g sample or to 26.2 μg in total cell weight (0.25 g) of the culture (Table 3).
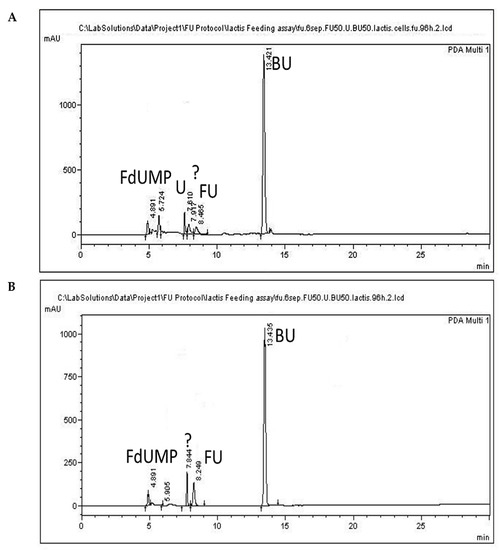
Figure 5.
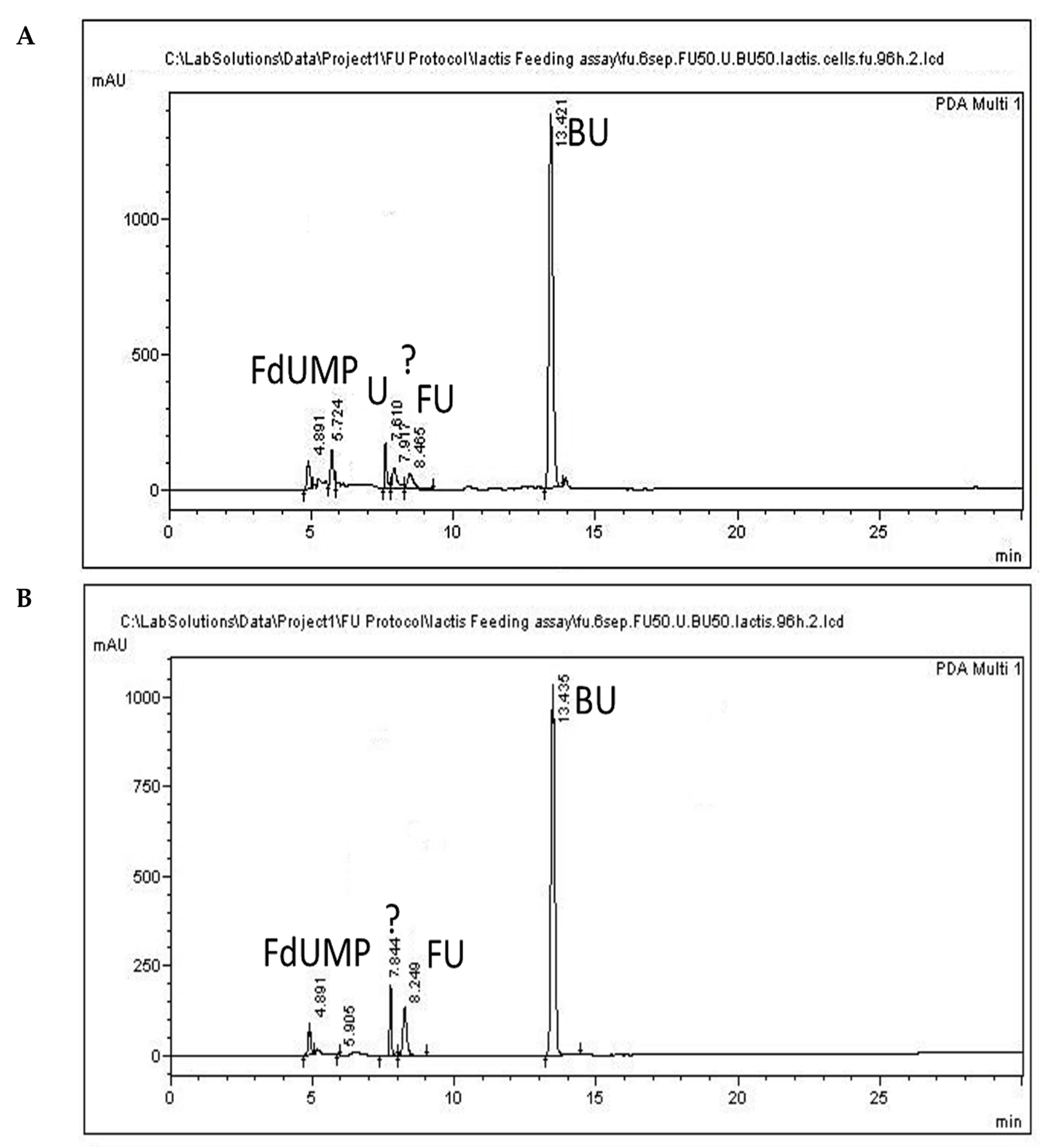
Representative chromatograms of cell precipitate (A) and cell-free culture supernatant (B) collected at 96 h, following administration of 5-FU (FU) (50 μg/mL) at 0 h. Uracil and FdUMP are indicated as (U) and (FdUMP), respectively. An unidentified compound eluting at 7.9 min is indicated with a question mark.

Table 3.
Levels of 5-FU, FdUMP, and uracil in cells and in the culture medium following administration of 50 μg/mL 5-FU for 96 h. The estimated values are presented as mean ± SDp (n = 3) in the first column, and the values in parentheses indicate the confidence interval (CI) at a = 0.025, df = 2, and t value = 4.303. Values in the second column are estimated by multiplying the average appearing in the first column with either the total weight of the cells or the total volume of the culture, as appropriate.
The levels of 5-FU in the medium (Figure 3C) were determined to be 49.5 μg/mL at the onset of the experiment and 5.2 μg/mL at the end (Figure 5B); that is a reduction of 89.5%. These values correspond to 156 μg in total culture volume (30 mL), and added to the 5-FU levels in the cells (Table 2), they produce a sum of 182.2 μg 5-FU, a value which is quite lower than the originally added nominal amount of 50μg/mL of 5-FU or the determined amount of 1485 μg in total culture volume.
Uracil was not detected in the culture medium, and although it was detected in cells, its levels amounted to 1.15 μg/mL, a value below the LOQ (Table 2). The levels of the active metabolite of 5-FU, namely FdUMP, in the cells amounted to 21.25 μg/mL (Table 3), which corresponds to only 26% of the determined 5-FU in the 96 h sample and to about only 3% of the originally added 5-FU (1500 μg/mL). FdUMP was also detected in the supernatant; however, its levels amounted to 0.9 μg/mL and were below the LOQ. The determined 5-FU levels and the detection of the active metabolite in the cells indicate an uptake of 5-FU by L. lactis cells.
Moreover, our results presented in Table 3 suggest that only a small portion of 5-FU is transformed to its active metabolite, while the other part may be excreted or may lead to the formation of other products. The resistance of certain bacteria to 5-FU has been attributed to mutations in the uracil phosphotransferase (upp) or to the protein/uracil phosphoribosyl transferase (pyrR) genes, either by inhibiting the formation of FdUMP or by de novo production of UMP [19]. Although strain-specific differences occur, the activity of upp has been documented in L. lactis MG1363 [55]. However, both upp and pyrR mutations lead to decreased uracil metabolism [56]. Uracil concentrations in cells at 96 h following 5-FU administration amounted to 0.94 μg/mL and presented a distinct increase compared to control cells in which free uracil levels were below LOQ, indicating a decreased metabolism of uracil. These increased uracil levels agree with accumulating uracil due to its decreased metabolism. All peaks showed increased similarities in absorbance data comparisons (Figure S1A–C). The extra peak appearing at 7.8 min in culture supernatant, and at 7.9 min in the cells and the culture supernatant indicated by a question mark in Figure 5, showed a similarity of only 75% in the UV spectrum to commercial uracil or to commercial 5-FU (Figure S1D,E). This led us to further examinations of this peak using ESI-MS analysis, which revealed that it does not correspond either to uracil or to 5-FU, but that it presents close resemblance to the inactive metabolite 5,6-dihydro-5-fluorouracil (FUH2) (Figure S1F). Although this metabolite was not quantified in the present study, its presence indicates that a considerable part of 5-FU is transformed into this inactive metabolite. This finding agrees with previous reports of inactivation of the majority of the administered 5-FU as FUH2 [57]. A low 5-FU degradation rate in patients with metastatic colon cancer was correlated with an increased efficacy of the drug and improved chances of survival [58]. Further research on other bacterial strains and their combinations is needed to determine the levels of 5-FU that are inactivated through metabolism by the probiotic bacteria coadministered with the drug, possibly leading to a decreased efficacy of treatment.
4. Conclusions
The RP-HPLC-DAD method for the determination of uracil, 5-FU, and its possible metabolite 5FdUMP in bacterial culture media and in bacterial cells was developed and validated. The estimated validation parameters indicate that it is accurate, precise, specific, and sensitive, with similar limits of detection for all analytes and both biological samples. This renders the method suitable for the analysis of these compounds in bacterial cultures. The method was applied to the bacterial culture of the probiotic L. lactis, and the analytes were determined in the cells as well as in the culture medium, following administration of 5-FU to the bacterium for 96 h. All analytes were identified by retention time and peak purity analysis. The formation and presence of the inactive metabolite of 5-FU in both culture media and bacterial cells were verified by MS analysis. To the best of our knowledge, this is the first quantitative determination of free uracil, 5-FU, and 5-FU metabolites originating from bacterial metabolism, facilitating studies on 5-FU biotransformation. Further research towards the quantification of 5-FU, 5,6-dihydro-5-fluorouracil, uracil, and FdUMP at time points of culture time will provide evidence on the mechanism of 5-FU biotransformation by probiotic bacteria, contributing to a personalized use of probiotics in chemotherapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations9110376/s1, Figure S1: Absorbance spectrum analysis of peak similarity for (A) 5-FU, (B) uracil, (C) 5FdUMP, (D) mismatch of peak with a retention time of 7.8 to 5-FU, and (E) mismatch of peak with a retention time of 7.8 to Uracil and (F) MS analysis.
Author Contributions
Conceptualization, M.T.; methodology, M.T. and V.S.; formal analysis, P.M., K.S., A.V. and A.M.; writing—original draft preparation, M.T. and P.M.; writing—review and editing, M.T. and V.S.; supervision, M.T. and V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to thank Vasiliki Sarli for her support on ESI-MS analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- DeVita, V.T., Jr.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Dushinsky, R.; Pleven, E.; Heidelberger, C. The synthesis of 5-fluoropyrimidines. J. Am. Chem. Soc. 1957, 79, 4559–4560. [Google Scholar] [CrossRef]
- Shirasaka, T. Development history and concept of an oral anticancer agent S-1 (TS-1): Its clinical usefulness and future vistas. Jpn. J. Clin. Oncol. 2009, 39, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Cho, Y.H.; Ro, E.J.; Yoon, J.S.; Mizutani, T.; Kang, D.W.; Park, J.C.; Kim, T.I.; Clevers, H.; Choi, K.Y. 5-FU promotes stemness of colorectal cancer via p53-mediated WNT/β-catenin pathway activation. Nat. Commun. 2020, 11, 5321. [Google Scholar] [CrossRef]
- Houghton, J.A.; Harwood, F.G.; Tillman, D.M. Thymineless death in colon carcinoma cells is mediated via fas signaling. Proc. Natl. Acad. Sci. USA 1997, 94, 8144–8149. [Google Scholar] [CrossRef]
- Diasio, R.B.; Harris, B.E. Clinical Pharmacology of 5-Fluorouracil. Clin. Pharmacokinet. 1989, 16, 215–237. [Google Scholar] [CrossRef]
- Di Paolo, A.; Danesi, R.; Ciofi, L.; Vannozzi, F.; Bocci, G.; Lastella, M.; Amatori, F.; Martelloni, B.M.; Ibrahim, T.; Amadori, D.; et al. Improved analysis of 5-Fluorouracil and 5,6-dihydro-5-Fluorouracil by HPLC with diode array detection for determination of cellular dihydropyrimidine dehydrogenase activity and pharmacokinetic profiling. Ther. Drug Monit. 2005, 27, 362–368. [Google Scholar] [CrossRef]
- Lampropoulou, D.I.; Laschos, K.; Amylidi, A.L.; Angelaki, A.; Soupos, N.; Boumpoucheropoulos, S.; Papadopoulou, E.; Nanou, E.; Zidianakis, V.; Nasioulas, G.; et al. Fluoropyrimidine-induced toxicity and DPD deficiency. A case report of early onset, lethal capecitabine-induced toxicity and mini review of the literature. Uridine triacetate: Efficacy and safety as an antidote. Is it accessible outside USA? J. Oncol. Pharm. Pract. 2020, 26, 747–753. [Google Scholar] [CrossRef]
- Ezzeldin, H.; Diasio, R. Dihydropyrimidine dehydrogenase deficiency, a pharmacogenetic syndrome associated with potentially life-threatening toxicity following 5-fluorouracil administration. Clin. Color. Cancer 2004, 4, 181–189. [Google Scholar] [CrossRef]
- Saif, M.W.; Choma, A.; Salamone, S.J.; Chu, E. Pharmacokinetically guided dose adjustment of 5-fluorouracil: A rational approach to improving therapeutic outcomes. J. Natl. Cancer Inst. 2009, 101, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, H.; Zhang, R.; Liu, Y.; Kong, N.; Guo, Y.; Xu, M. 5-Fluorouracil induced dysregulation of the microbiome-gut-brain axis manifesting as depressive like behaviors in rats. Biochim. Et Biophys. Acta Mol. Basis Dis. 2020, 1866, 165884. [Google Scholar] [CrossRef] [PubMed]
- Österlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br. J. Cancer 2007, 97, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Arrastia, M.; Martinez-Ortigosa, A.; Rueda-Ruzafa, L.; Folch Ayora, A.; Ropero-Padilla, C. Probiotic Supplements on Oncology Patients’ Treatment-Related Side Effects: A Systematic Review of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 4265. [Google Scholar] [CrossRef]
- Feng, J.; Gao, M.; Zhao, C.; Yang, J.; Gao, H.; Lu, X.; Ju, R.; Zhang, X.; Zhang, Y. Oral Administration of Probiotics Reduces Chemotherapy-Induced Diarrhea and Oral Mucositis: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 823288. [Google Scholar] [CrossRef]
- Baldwin, C.; Millette, M.; Oth, D.; Ruiz, M.T.; Luquet, F.-M.; Lacroix, M. Probiotic Lactobacillus acidophilus and L. casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutr. Cancer 2010, 62, 371–378. [Google Scholar] [CrossRef]
- Darbandi, A.; Mirshekar, M.; Shariati, A.; Moghadam, M.T.; Lohrasbi, V.; Asadolahi, P.; Talebi, M. The effects of probiotics on reducing the colorectal cancer surgery complications: A periodic review during 2007–2017. Clin. Nutr. 2020, 39, 2358–2367. [Google Scholar] [CrossRef]
- Agraib, L.M.A.-S.A.; Salah, S.; Abu-hijlih, R.; Abuhijla, F. The effect of probiotics supplementation on the side effects of chemo radiotherapy for colorectal cancer: A literature review. Oncol. Radiother. 2020, 1, 1–9. Available online: https://www.oncologyradiotherapy.com/articles/the-effect-of-probiotics-supplementation-on-the-side-effects-of-chemo-radiotherapy-for-colorectal-cancer-a-literature-review-54551.htmL (accessed on 31 March 2022).
- Singh, V.; Brecik, M.; Mukherjee, R.; Evans, J.C.; Svetlíková, Z.; Blaško, J.; Surade, S.; Blackburn, J.; Warner, D.F.; Mikušová, K.; et al. The complex mechanism of antimycobacterial action of 5-fluorouracil. Chem. Biol. 2015, 22, 63–75. [Google Scholar] [CrossRef]
- Kyrila, G.; Katsoulas, A.; Schoretsaniti, V.; Rigopoulos, A.; Rizou, E.; Doulgeridou, S.; Sarli, V.; Samanidou, V.; Touraki, M. Bisphenol A removal and degradation pathways in microorganisms with probiotic properties. J. Hazard. Mater. 2021, 413, 125363. [Google Scholar] [CrossRef]
- Lindell, A.E.; Zimmermann-Kogadeeva, M.; Patil, K.R. Multimodal interactions of drugs, natural compounds and pollutants with the gut microbiota. Nat. Rev. Microbiol. 2022, 20, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Sousa, T.; Paterson, R.; Moore, V.; Carlsson, A.; Abrahamsson, B.; Basit, A.W. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int. J. Pharm. 2008, 363, 1–25. [Google Scholar] [CrossRef] [PubMed]
- García-González, A.P.; Ritter, A.D.; Shrestha, S.; Andersen, E.C.; Yilmaz, L.S.; Walhout, A. Bacterial Metabolism Affects the C. elegans Response to Cancer Chemotherapeutics. Cell 2017, 169, 431–441.e8. [Google Scholar] [CrossRef] [PubMed]
- Martinussen, J.; Hammer, K. Cloning and characterization of upp, a gene encoding uracil phosphoribosyltransferase from Lactococcus lactis. J. Bacteriol. 1994, 176, 6457–6463. [Google Scholar] [CrossRef] [PubMed]
- Björnberg, O.; Rowland, P.; Larsen, S.; Jensen, K.F. Active site of dihydroorotate dehydrogenase A from Lactococcus lactis investigated by chemical modification and mutagenesis. Biochemistry 1997, 36, 16197–16205. [Google Scholar] [CrossRef]
- Dobritzsch, D.; Schneider, G.; Schnackerz, K.D.; Lindqvist, Y. Crystal structure of dihydropyrimidine dehydrogenase, a major determinant of the pharmacokinetics of the anti-cancer drug 5-fluorouracil. EMBO J. 2001, 20, 650–660. [Google Scholar] [CrossRef]
- Breda, M.; Barattè, S. A review of analytical methods for the determination of 5-fluorouracil in biological matrices. Anal. Bioanal. Chem. 2010, 397, 1191–1201. [Google Scholar] [CrossRef]
- Escoriaza, J.; Aldaz, A.; Calvo, E.; Giráldez, J. Simple and sensitive determination of 5-fluorouracil in plasma by high-performance liquid chromatography. Application to clinical pharmacokinetic studies. J. Chromatogr. B Biomed. Sci. Appl. 1999, 736, 97–102. [Google Scholar] [CrossRef]
- Casale, F.; Canaparo, R.; Muntoni, E.; Serpe, L.; Zara, G.P.; Della Pepa, C.; Berno, E.; Costa, M.; Eandi, M. Simultaneous HPLC determination of 5-fluorouracil and its metabolites in plasma of cancer patients. Biomedical chromatography: BMC 2002, 16, 446–452. [Google Scholar] [CrossRef]
- Ackland, S.P.; Garg, M.B.; Dunstan, R.H. Simultaneous Determination of Dihydrofluorouracil and 5-Fluorouracil in Plasma by High-Performance Liquid Chromatography. Anal. Biochem. 1997, 246, 79–85. [Google Scholar] [CrossRef]
- Maring, J.G.; Schouten, L.; Greijdanus, B.; de Vries, E.G.; Uges, D.R. A simple and sensitive fully validated HPLC-UV method for the determination of 5-fluorouracil and its metabolite 5,6-dihydrofluorouracil in plasma. Ther. Drug Monit. 2005, 27, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Barberi-Heyob, M.; Merlin, J.L.; Weber, B. Determination of 5-Fluorouracil and Its Main Metabolites in Plasma by High-Performance Liquid Chromatography. J. Chromatogr. 1992, 573, 241–252. [Google Scholar] [CrossRef]
- Joulia, J.M.; Pinguet, F.; Grosse, P.Y.; Astre, C.; Bressolle, F. Determination of 5-fluorouracil and its main metabolites in plasma by high-performance liquid chromatography: Application to a pharmacokinetic study. J. Chromatogr. B Biomed. Sci. Appl. 1997, 692, 427–435. [Google Scholar] [CrossRef]
- Guerrieri, A.; Palmisano, F.; Zambonin, P.G.; De Lena, M.; Lorusso, V. Solid-phase extraction of fluoropyrimidine derivatives on a copper-modified strong cation exchanger: Determination of doxifluridine, 5-fluorouracil and its main metabolites in serum by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. 1993, 617, 71–77. [Google Scholar] [CrossRef]
- Nassim, M.A.; Shirazi, F.H.; Cripps, C.M.; Veerasinghan, S.; Molepo, M.J.; Obrocea, M.; Redmond, D.; Bates, S.; Fry, D.; Stewart, D.J.; et al. An HPLC method for the measurement of 5-fluorouracil in human plasma with a low detection limit and a high extraction yield. Int. J. Mol. Med. 2002, 10, 513–516. [Google Scholar] [CrossRef]
- Wickremsinhe, E.R.; Lee, L.B.; Schmalz, C.A.; Torchia, J.; Ruterbories, K.J. High sensitive assay employing column switching chromatography to enable simultaneous quantification of an amide prodrug of gemcitabine (LY2334737), gemcitabine, and its metabolite dFdU in human plasma by LC-MS/MS. J. Chromatogr. B Biomed. Sci. Appl. 2013, 932, 117–122. [Google Scholar] [CrossRef]
- Salvador, A.; Millérioux, L.; Renou, A. Simultaneous LC-MS-MS Analysis of Capecitabine and its Metabolites (5′-deoxy-5-fluorocytidine, 5′-deoxy-5-fluorouridine, 5-fluorouracil) After Off-Line SPE from Human Plasma. Chromatographia 2006, 63, 609–615. [Google Scholar] [CrossRef]
- Pandey, K.; Dubey, R.S.; Prasad, B.B. A Critical Review on Clinical Application of Separation Techniques for Selective Recognition of Uracil and 5-Fluorouracil. Indian J. Clin. Biochem. IJCB 2016, 31, 3–12. [Google Scholar] [CrossRef]
- Semail, N.F.; Abdul Keyon, A.S.; Saad, B.; Noordin, S.S.; Nik Mohamed Kamal, N.N.S.; Mohamad Zain, N.N.; Azizi, J.; Kamaruzaman, S.; Yahaya, N. Analytical method development and validation of anticancer agent, 5-fluorouracil, and its metabolites in biological matrices: An updated review. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 562–579. [Google Scholar] [CrossRef]
- Anderson, L.W.; Parker, R.J.; Collins, J.M.; Ahlgren, J.D.; Wilkinson, D.; Strong, J.M. Gas Chromatographic—Mass Spectrometric Method for Routine Monitoring of 5-Fluorouracil in Plasma of Patients Receiving Low-Level Protracted Infusions. J. Chromatogr. B Biomed. Sci. Appl. 1992, 581, 195–201. [Google Scholar] [CrossRef]
- Marunaka, T.; Umeno, Y. Determination of 5-Fluorouracil and Pyrimidine Bases in Plasma by Gas Chromatography-Chemical Ionization-Mass Fragmentography. J. Chromatogr. B Biomed. Sci. Appl. 1980, 221, 382–386. [Google Scholar] [CrossRef]
- Remaud, G.; Boisdron-Celle, M.; Morel, A.; Gamelin, A. Sensitive MS/MS-liquid chromatography assay for simultaneous determination of tegafur, 5-fluorouracil and 5-fluorodihydrouracil in plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 824, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Licea-Perez, H.; Wang, S.; Bowen, C. Development of a sensitive and selective LC-MS/MS method for the determination of alpha-fluoro-beta-alanine, 5-fluorouracil and capecitabine in human plasma. J. Chromatogr. B Biomed. Sci. Appl. 2009, 877, 1040–1046. [Google Scholar] [CrossRef]
- Holleran, J.L.; Eiseman, J.L.; Parise, R.A.; Kummar, S.; Beumer, J.H. LC–MS/MS Assay for the Quantitation of FdCyd and Its Metabolites FdUrd and FU in Human Plasma. J. Pharm. Biomed. Anal. 2016, 129, 359–366. [Google Scholar] [CrossRef]
- Del Nozal, M.; Bernal, J.; Marenero, P.; Pampliega, A. Extraction Procedures for the HPLC determination of 5-fluorouracil in biological samples. J. Liq. Chromatogr. Relat. Technol. 1994, 17, 1621–1636. [Google Scholar] [CrossRef]
- Odagiri, H.; Ichihara, S.; Semura, E.; Utoh, M.; Tateishi, M.; Kuruma, I. Determination of 5-Fluorouracil in Plasma and Liver after Oral Administration of 5’-Deoxy-5-Fluorouridine using Gas Chromatography-Mass Spectrometry. J. Pharmacobio-Dyn. 1988, 11, 234–240. [Google Scholar] [CrossRef]
- Ozawa, S.; Hamada, M.; Murayama, N.; Nakajima, Y.; Kaniwa, N.; Matsumoto, Y.; Fukuoka, M.; Sawada, J.-I.; Ohno, Y. Cytosolic and microsomal activation of doxifluridine and tegafur to produce 5-fluorouracil in human liver. Cancer Chemother. Pharm. 2002, 50, 454–458. [Google Scholar] [CrossRef]
- Zufía, L.; Aldaz, A.; Castellanos, C.; Giráldez, J. Determination of 5-fluorouracil and its prodrug tegafur in plasma and tissue by high-performance liquid chromatography in a single injection: Validation for application in clinical pharmacokinetic studies. Ther. Drug Monit. 2003, 25, 221–228. [Google Scholar] [CrossRef]
- Wrightson, W.R.; Myers, S.R.; Galandiuk, S. HPLC analysis of 5-FU and FdUMP in tissue and serum. Biochem. Biophys. Res. Commun. 1995, 216, 808–813. [Google Scholar] [CrossRef]
- Petrilli, R.; Eloy, J.O.; Paschoal, J.; Lopez, R. Quantification of 5-FU in skin samples for the development of new delivery systems for topical cancer treatment. Die Pharm. 2018, 73, 133–138. [Google Scholar] [CrossRef]
- Jochheim, C.; Janning, P.; Marggraf, U.; Löffler, T.M.; Hasse, F.; Linscheid, M. A procedure for the determination of 5-fluorouracil in tissue using microbore HPLC and fluorescence detection. Anal. Biochem. 1994, 217, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Gamelin, E.; Boisdron-Celle, M.; Larra, F.; Robert, J. A Simple Chromatographic Method for the Analysis of Pyrimidines and their Dihydrogenated Metabolites. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 3155–3172. [Google Scholar] [CrossRef]
- Uhrovčík, J. Strategy for determination of LOD and LOQ values—Some basic aspects. Talanta 2014, 119, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Miolo, G.; Marzano, C.; Gandin, V.; Palozzo, A.C.; Dalzoppo, D.; Salvador, A.; Caffieri, S. Photoreactivity of 5-fluorouracil under UVB light: Photolysis and cytotoxicity studies. Chem. Res. Toxicol. 2011, 24, 1319–1326. [Google Scholar] [CrossRef]
- Martinussen, J.; Andersen, P.S.; Hammer, K. Nucleotide metabolism in Lactococcus lactis: Salvage pathways of exogenous pyrimidines. J. Bacteriol. 1994, 176, 1514–1516. [Google Scholar] [CrossRef][Green Version]
- Martinussen, J.; Glaser, P.; Andersen, P.S.; Saxild, H.H. Two genes encoding uracil phosphoribosyltransferase are present in Bacillus subtilis. J. Bacteriol. 1995, 177, 271–274. [Google Scholar] [CrossRef]
- Deenen, M.J.; Rosing, H.; Hillebrand, M.J.; Schellens, J.H.; Beijnen, J.H. Quantitative determination of capecitabine and its six metabolites in human plasma using liquid chromatography coupled to electrospray tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 913–914, 30–40. [Google Scholar] [CrossRef]
- Botticelli, A.; Borro, M.; Onesti, C.E.; Strigari, L.; Gentile, G.; Cerbelli, B.; Romiti, A.; Occhipinti, M.; Sebastiani, C.; Lionetto, L.; et al. Degradation Rate of 5-Fluorouracil in Metastatic Colorectal Cancer: A New Predictive Outcome Biomarker? PLoS ONE 2016, 11, e0163105. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).