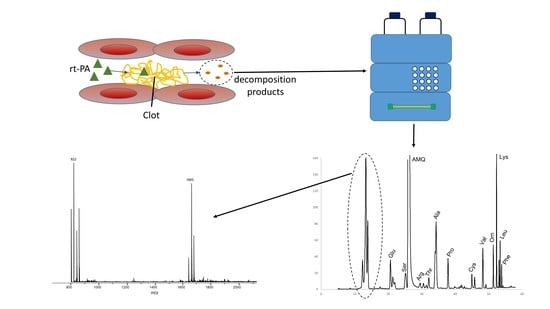
Liquid Chromatography Fingerprint Analysis of Released Compounds in Plasma Samples of Stroke Patients after Thrombolytic Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Blood Sample Collection
2.3. Chromatographic Analysis
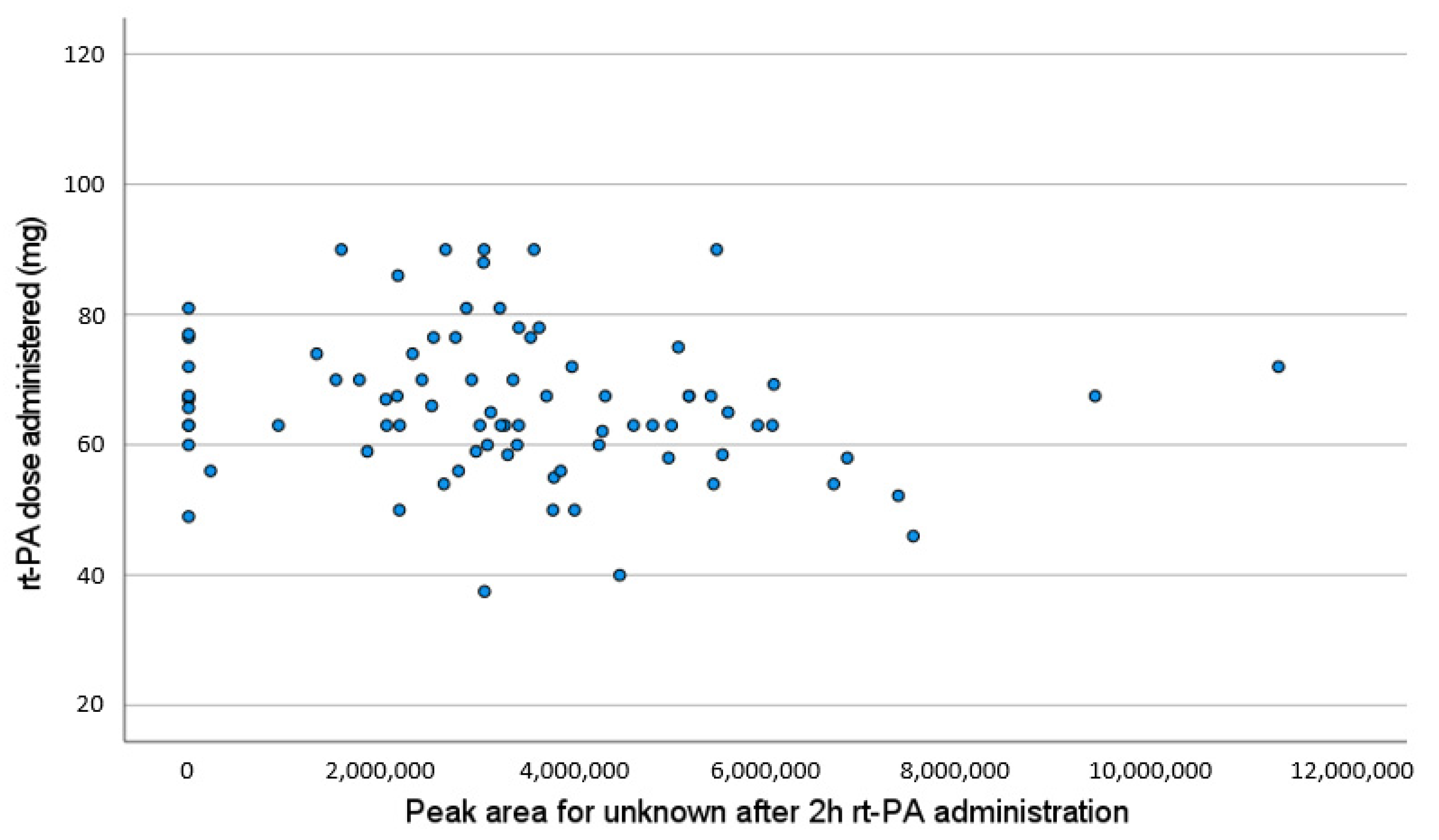
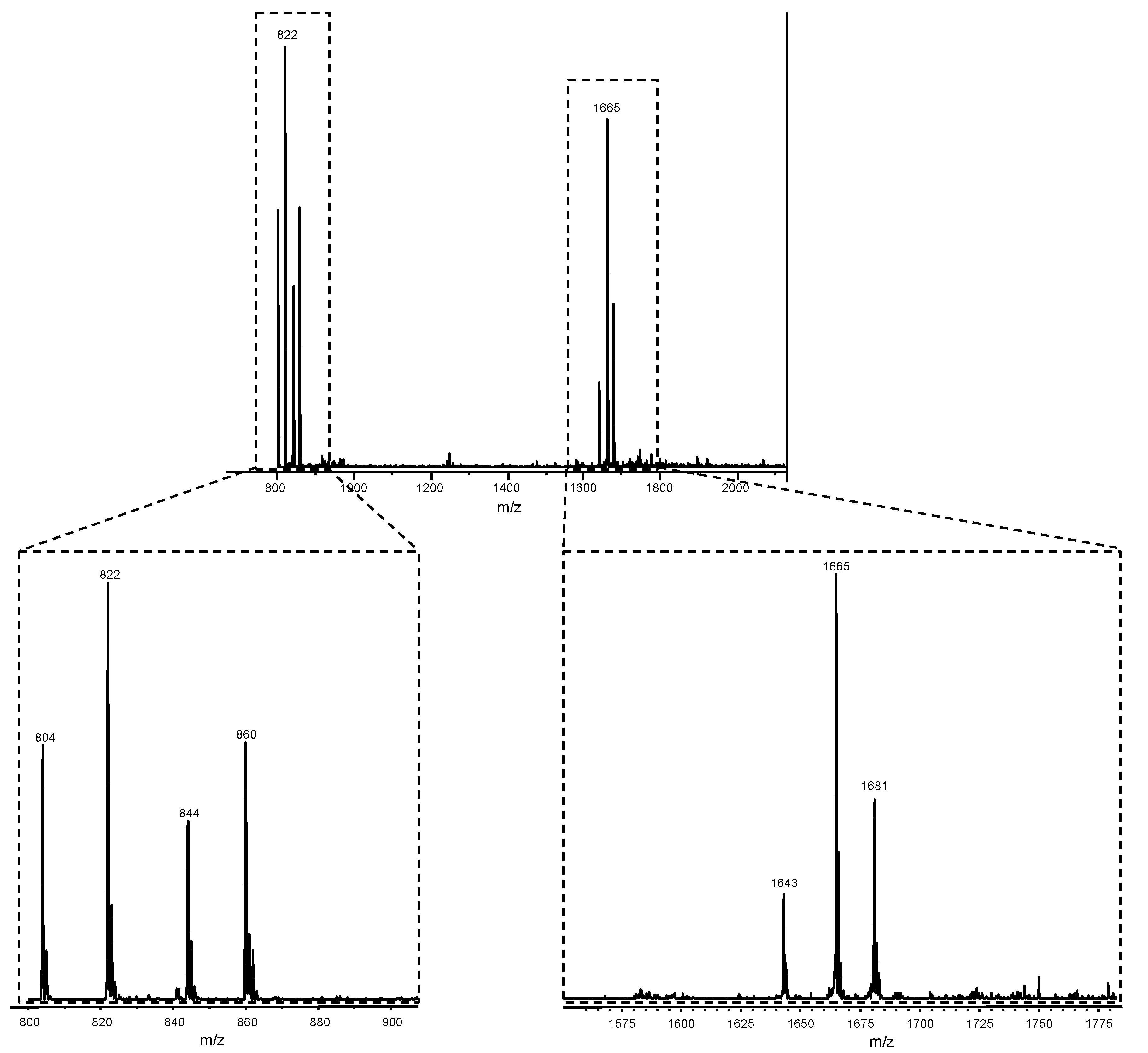
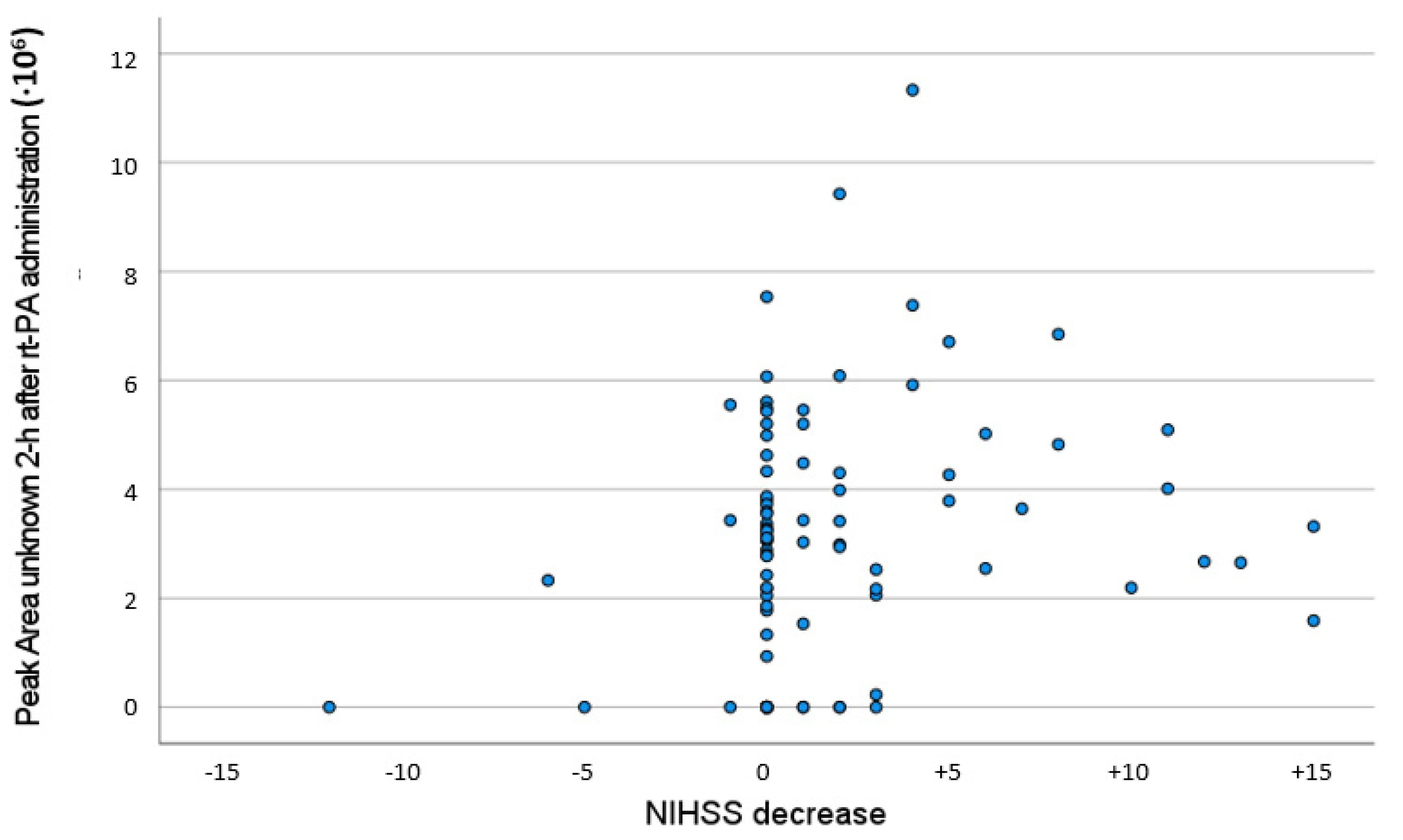
3. Results
4. Discussion
5. Possible Clinical Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chevilley, A.; Lesept, F.; Lenoir, S.; Ali, C.; Parcq, J.; Vivien, D. Impacts of tissue-type plasminogen activator (tPA) on neuronal survival. Front. Cell Neurosci. 2015, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Hacke, W.; Kaste, M.; Bluhmi, E.; Brozman, M.; Dávalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, A.; Machnig, T.; et al. ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [PubMed]
- Leymarie, N.; Zaia, J. Effective use of mass spectrometry for glycan and glycopeptide structural analysis. Anal. Chem. 2012, 84, 3040–3048. [Google Scholar] [CrossRef]
- Wood, C.L.; Maxon, M.; Desaire, H. Software for automated interpretation of mass spectrometry data from glycans and glycopeptides. Analyst 2013, 138, 2793–2803. [Google Scholar]
- Itoh, S.; Kawasaki, N.; Ohta, M.; Hyuga, M.; Hyuga, S.; Hayakawa, T. Simultaneous microanalysis of N-linked oligosaccharides in a glycoprotein using microbore graphitized carbon column liquid chromatography-mass spectrometry. J. Chromatogr. A 2002, 968, 89–100. [Google Scholar] [CrossRef]
- Papac, D.I.; Briggs, J.B.; Chin, E.T.; Jones, A.J.S. A high-throughput microscale method to release N-linked oligosaccharides from glycoproteins from matrix-assisted laser desorption/ionization time-of-flight mass spectrometric analysis. Glycobiology 1998, 8, 445–454. [Google Scholar] [CrossRef]
- Miller, J.J.; Bohnsack, R.N.; Olson, L.J.; Ishihara, M.; Aoki, K.; Tiemeyer, M.; Dahms, N.M. Tissue plasminogen activator is a ligand of cation-independent mannose 6-phosphate receptor and consists of glycoforms that contain mannose 6-phospate. Sci. Rep. 2021, 11, 8213. [Google Scholar] [CrossRef]
- Spellman, M.W.; Basa, L.J.; Leonard, C.K.; Chakel, J.A.; O’Connor, J.V.; Wilson, S.; van Halbeek, H. Carbohydrate structures of human tissue plasminogen activator expressed in Chinese hamster ovary cells. J. Biol. Chem. 1989, 264, 14100–14111. [Google Scholar] [CrossRef]
- Zhuo, S.; Veillon, L.; Dong, X.; Huan, Y.; Mechref, Y. Direct comparison of derivatization strategies for LC-MS/MS analysis of N-glycans. Analyst 2017, 142, 4446–4455. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Song, X. Preparation of complex glycans from natural sources for functional study. Front. Chem. 2020, 8, 508. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, H. Glycomic analysis of glycans released from glycoproteins using chemical immobilization and mass spectrometry. Curr. Protoc. Chem. Biol. 2015, 6, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.; Saldova, R. Current methods for the characterization of O-glycans. J. Proteom. Res. 2020, 19, 3890–3905. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wooding, K.; Mechref, Y. Analysis of permethylated glycan by liquid chromatography (LC) and mass spectrometry (MS). Methods Mol. Bio. 2017, 1503, 83–96. [Google Scholar]
- Parker, N.H.; Lawson, M.A.; Jardine, D.R.; Redmond, J.W. A general approach to desalting oligosaccharides released from glycoproteins. Glycoconj. J. 1998, 15, 737–747. [Google Scholar]
- Wu, Y.; Sha, Q.; Du, J.; Wang, C.; Zang, L.; Liu, B.F.; Lin, Y.; Liu, X. Determination of N-glycans by high performance liquid chromatography using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate as the glycosylamine labeling reagent. J. Chromatogr. A 2018, 1535, 114–122. [Google Scholar] [CrossRef]
- Vreeker, G.C.M.; Wuhrer, M. Reversed-phase separation methods for glycan analysis. Anal. Bioanal. Chem. 2017, 409, 359–378. [Google Scholar] [CrossRef]
- Kolarich, D.; Jensen, P.H.; Altmann, F.; Packer, N.H. Determination of site-specific glycan heterogeneity on glycoproteins. Nat. Protoc. 2012, 7, 1285–1289. [Google Scholar] [CrossRef]
- Gurman, P.; Miranda, O.R.; Nathan, A.; Washington, C.; Rosen, Y.; Elman, N.M. Recombinant tissue plasminogen activators (rtPA): A review. Clin. Pharmacol. Ther. 2015, 97, 274–285. [Google Scholar] [CrossRef]
- Jilani, T.N.; Siddiqui, A.H. Tissue plasminogen activator. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507917/ (accessed on 28 November 2022).
- Zhao, X.J.; Larkin, T.M.; Lauver, M.A.; Ahmad, S.; Ducruet, A.F. Tissue plasminogen activator mediates deleterious complement cascade activation in stroke. PLoS ONE 2017, 12, e0180822. [Google Scholar] [CrossRef]
- Ning, M.M.; Sarracino, D.A.; Buonano, F.S.; Krastins, B.; Chou, S.; McMullin, D.; Wang, X.; Lopez, M.; Lo, E.H. Proteomic protease substrate profiling of tPA treatment in acute ischemic stroke patients: A step toward individualizing thrombolytic therapy at the bedside. Transl. Stroke Res. 2010, 1, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Dagonnier, M.; Cooke, I.R.; Faou, P.; Sidon, T.K.; Dewey, H.M.; Donnan, G.A.; Howells, D.W. Discovery and longitudinal evaluation of candidate biomarkers for ischaemic stroke by mass spectrometry-based proteomics. Biomark. Insights 2017, 12, 1177271917749216. [Google Scholar] [CrossRef] [PubMed]
- Yakolev, S.; Makogonenko, E.; Kurochkina, N.; Nieuwwnhuizen, W.; Ingham, K.; Medved, L. Conversion of fibrinogen to fibrin: Mechanism of exposure of tPA- and plasminogen-binding sites. Biochemistry 2000, 39, 15730–15741. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I.; Leslie, B.; Ginsberg, J. Soluble fibrin degradation products potentiate tissue plasminogen activator-induced fibrinogen proteolysis. J. Clin. Investig. 1991, 87, 1082–1090. [Google Scholar] [CrossRef]
- Kmiécik, D.; Herman, V.; Stroop, C.J.M.; Michalski, J.C.; Mir, A.M.; Labiau, O.; Verbert, A.; Cacan, R. Catabolism of glycan moieties of lipid intermediates leads to a single Man5GLcNAc oligosaccharide isomer: A study with permeabilized CHO cells. Glycobiology 1995, 5, 483–494. [Google Scholar] [CrossRef]
- Yanagida, K.; Natsuka, S.; Hase, S. Structural diversity of cytosolic free oligosaccharides in the human hepatoma cell line, HepG2. Glycobiology 2006, 16, 294–304. [Google Scholar] [CrossRef]
- Iwatsuka, K.; Watanabe, S.; Kinoshita, M.; Mamisue, K.; Yamada, D.; Hayakawa, T.; Suzuki, T.; Kakehi, K. Free glycans derived from glycoproteins present in human sera. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 928, 16–21. [Google Scholar] [CrossRef]
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995, 333, 1581–1587. [Google Scholar] [CrossRef]
- Castellanos, M.; van Eendeburg, C.; Gubern, C.; Sanchez, J.M. Ethyl-bridged hybrid column as an efficient alternative for HPLC analysis of plasma amino acids by pre-column derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 2016, 1029, 137–144. [Google Scholar] [CrossRef]
- Liu, H.; Worthen, H.G. Measurement of free amino acid level in ultrafiltrates of blood plasma by high-performance liquid chromatography with automated pre-column derivatization. J. Chromatogr. B 1992, 579, 215–224. [Google Scholar] [CrossRef]
- Ralston, P.B.; Strein, T.G. A study of deproteinization methods for subsequent serum analysis with capillary electrophoresis. Microchem. J. 1997, 55, 270–283. [Google Scholar] [CrossRef]
- Jaworska, M.; Stanczyk, M.; Wilk, M.; Klaczkow, G.; Anuszewska, E.; Barzal, J.; Rzepecki, P. New approach for amino acid profiling in human plasma by selective fluorescence derivatization. Amino Acids 2012, 43, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Laubert, M.A.; Yu, Y.Q.; Brousmiche, D.W.; Hua, Z.; Koza, S.M.; Magnelli, P.; Guthrie, E.; Taron, C.H.; Fountain, K.J. Rapid preparation of released N-glycans for HILIC analysis using a labelling reagent that facilitates sensitive fluorescence and ESI-MS detection. Anal. Chem. 2015, 87, 5401–5409. [Google Scholar] [CrossRef] [PubMed]
- Ullmer, R.; Plemati, A.; Rizzi, A. Derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate for enhancing the ionization yield of small peptides and glycopeptides in matrix-assisted laser desorption/ionization and electrospray ionization mass spectrometry. Rapid. Commun. Mass. Spectrom. 2006, 20, 1469–1479. [Google Scholar] [CrossRef]
- Chen, J.L.; Lee, C.; Lu, I.C.; Chien, C.L.; Lee, Y.T.; Hu, W.P.; Ni, C.K. Theoretical investigation of low detection sensitivity for underivatized carbohydrates in ESI and MALDI. J. Mass. Spectrom. 2016, 51, 1180–1186. [Google Scholar] [CrossRef]
- Hovest, A.S.; Horne, M.K. The effect of arginine on coagulation and fibrinolysis in vitro. Fibrinolysis Proteol. 1999, 13, 31–34. [Google Scholar] [CrossRef]
- Arakawa, T.; Tsumoto, K.; Kita, Y.; Chang, B.; Ejima, D. Biotechnology applications of amino acids in protein purification and formulations. Amino Acids 2007, 33, 587–605. [Google Scholar] [CrossRef]
- Lareau, N.M.; May, J.C.; McLean, J.A. Non-derivatized glycan analysis by reverse phase liquid chromatography and ion mobility-mass spectrometry. Analyst 2015, 140, 3335–3388. [Google Scholar] [CrossRef]
- Kawasaki, N.; Ohta, M.; Hyuga, S.; Hashimoto, O.; Hayakawa, T. Analysis of carbohydrate heterogeneity in a glycoprotein using liquid chromatography/mass spectrometry and liquid chromatography with tandem mass spectrometry. Anal. Biochem. 1999, 269, 297–303. [Google Scholar] [CrossRef]
- Pabst, M.; Bondili, J.S.; Stadlmann, J.; Mach, L.; Altmann, F. Mass + retention time = structure: A strategy for the analysis of N-glycans by carbon LC-ESI-MS and its application to fibrin N-glycans. Anal. Chem. 2007, 79, 5051–5057. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.D.; Flynn, G.C. The effect of Fc glycan forms on human IgG2 antibody clearance in humans. Glycobiology 2009, 19, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Goetze, A.M.; Liu, Y.D.; Zhang, Z.; Shah, B.; Lee, E.; Bondarendi, P.V.; Flynn, G.C. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology 2011, 21, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Brown, D.; Reed, C.; Chung, S.; Lutman, J.; Stefanich, E.; Wong, A.; Stephan, J.P.; Bayer, R. Production, characterization and pharmacokinetic properties of antibodies with N-linked Mannose-5 glycans. MAbs 2012, 4, 475–487. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Miyamoto, S.; Lancaster, K.S.; Kirmiz, C.; Li, B.; Lam, K.S.; Leiserowitx, G.S.; Lebrilla, C.B. Profiling of glycans in serum for the discovery of potential biomarkers for ovarian cancer. J. Proteome Res. 2006, 5, 1626–1635. [Google Scholar] [CrossRef]
- Kirmiz, B.C.; Li, H.J.; An, B.H.; Clowers, H.K.; Chew, K.S.; Lam, A.; Ferrige, R.; Alecio, A.D.; Borowsky, S.; Sulaimon, C.B.; et al. Miyamoto. A serum glycomics approach to breast cancer biomarkers. Mol. Cell Proteom. 2007, 6, 43–55. [Google Scholar] [CrossRef]
- Kyselova, Z.; Mechref, Y.; Kang, P.; Goetz, J.A.; Dobrolecki, L.E.; Sledge, G.W.; Schnaper, L.; Hickey, R.J.; Malkas, L.H.; Novotny, M.V. Breast cancer diagnosis and prognosis through quantitative measurements of serum glycan profiles. Clin. Chem. 2008, 54, 1166–1175. [Google Scholar] [CrossRef]
- Isailovic, D.; Kurulugama, R.T.; Plasencia, M.D.; Stokes, S.T.; Kyselova, Z.; Godman, R.; Mechref, Y.; Novotny, M.V.; Clemmer, D.E. Profiling of human serum glycans associated with liver cancer and cirrhosis by IMS-MS. J. Proteome Res. 2008, 7, 1109–1117. [Google Scholar] [CrossRef]
- Bereman, M.S.; Williams, T.I.; Muddlman, D.C. Development of a nanoLC LTQ Orbitrap mass spectrometric method for profiling glycans derived from plasma from healthy, benign tumor control, and epithelial ovarian cancer patients. Anal. Chem. 2009, 81, 1130–1136. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Wang, C.; Wang, R.; Wu, Y.; Zhang, L.; Liu, B.F.; Cheng, K.; Liu, X. Isomer-specific profiling of N-glycans derived from human serum for potential biomarker discovery in pancreatic cancer. J. Proteom. 2018, 181, 160–169. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellanos, M.; Fernández-Couto, D.; Da Silva-Candal, A.; Feal-Painceiras, M.J.; Rodríguez-Yáñez, M.; Gubern-Mérida, C.; Sanchez, J.M. Liquid Chromatography Fingerprint Analysis of Released Compounds in Plasma Samples of Stroke Patients after Thrombolytic Treatment. Separations 2023, 10, 34. https://doi.org/10.3390/separations10010034
Castellanos M, Fernández-Couto D, Da Silva-Candal A, Feal-Painceiras MJ, Rodríguez-Yáñez M, Gubern-Mérida C, Sanchez JM. Liquid Chromatography Fingerprint Analysis of Released Compounds in Plasma Samples of Stroke Patients after Thrombolytic Treatment. Separations. 2023; 10(1):34. https://doi.org/10.3390/separations10010034
Chicago/Turabian StyleCastellanos, Mar, Dolores Fernández-Couto, Andrés Da Silva-Candal, Maria J. Feal-Painceiras, Manuel Rodríguez-Yáñez, Carme Gubern-Mérida, and Juan M. Sanchez. 2023. "Liquid Chromatography Fingerprint Analysis of Released Compounds in Plasma Samples of Stroke Patients after Thrombolytic Treatment" Separations 10, no. 1: 34. https://doi.org/10.3390/separations10010034
APA StyleCastellanos, M., Fernández-Couto, D., Da Silva-Candal, A., Feal-Painceiras, M. J., Rodríguez-Yáñez, M., Gubern-Mérida, C., & Sanchez, J. M. (2023). Liquid Chromatography Fingerprint Analysis of Released Compounds in Plasma Samples of Stroke Patients after Thrombolytic Treatment. Separations, 10(1), 34. https://doi.org/10.3390/separations10010034