1. Introduction
Citric acid is an important intermediate in metabolic function. Its urinary excretion in mammals varies under different physiological conditions. In humans, 10–35% of filtered citrate is excreted and variations in the acid–base balance, or changes in intracellular pH, influence citrate excretion. Metabolic alkalosis increases citrate excretion, while metabolic acidosis decreases it [
1,
2,
3].
Citrate in urine contributes to the inhibitory potential toward crystallization of calcium salts [
4,
5,
6], acting through both surface-controlled mechanisms [
7,
8,
9] and the formation of stable soluble complexes with calcium [
10,
11]. Therefore, citrate is a relevant component of ionic equilibria in urine and its determination is essential to assess the state of saturation with respect to calcium oxalates and phosphates [
12,
13].
Indeed, low citrate may be crucial in lowering the inhibition potential toward calcium stone formation in the urine environment [
14,
15,
16]. Hypocitraturia has been reported as a common metabolic abnormality in patients with calcium stones, occurring in as many as 15% to 63% of cases. While it is often associated with other metabolic defects, in isolation, it accounts for about 10% of the patients [
5,
17].
There is convincing clinical evidence that the oral administration of alkaline citrate salts significantly reduces the calcium stone recurrence rate, taking into account both calcium complexation and crystal growth, and aggregation inhibition [
18,
19,
20,
21].
The traditional enzymatic spectrophotometric method for the determination of citrate uses citrate lyase (EC 4.1.3.6), malate dehydrogenase (EC 1.1.1.37), lactate dehydrogenase (EC 1.1.1.27), and NADH [
22,
23,
24,
25]. We have previously reported some modifications of earlier methods that eased routine use and improved sensitivity [
26].
Ion chromatographic (IC) methods have been previously described for the determination of urinary citrate [
27,
28,
29,
30]. Other methods have been proposed, including capillary electrophoresis (CE) [
31,
32]. Holmes described a method for measuring urinary oxalate and citrate using CE and indirect UV absorbance detection [
33], while Munoz et al. validated a method for measuring urine oxalate, citrate, uric acid, and creatinine [
34]. In more recent studies, liquid chromatography–tandem mass spectrometry (LC–MS/MS) was employed, in which urine was either pre-treated by solid-phase extraction [
35] or simply diluted with water [
36].
Herein, we describe a simple IC method for the quantitative determination of urinary citrate. As well as using internal standardization, it is fast, cost-effective, and requires low instrumental maintenance. It has been validated as a quantitative method, according to the main guidelines on bioanalytical method validation [
37,
38,
39], and successfully applied to the analysis of thousands of urine samples.
2. Materials and Methods
2.1. Chemicals
All reagents were of analytical grade. Hydrochloric acid 37%, sulfuric acid 98%, sodium hydroxide solution 50% (w/w), 100% w/v trichloroacetic acid, 5.20 mmol/L of IC-grade citric acid (Citrate Standard for IC) certified standard solution, trisodium trimetaphosphate purity >95% (IS), trichloroacetic acid 30% w/v, bis-tris purity ≥99.0%, trichloroacetic acid purity ≥99.0%, synthetic urine (Surine, Negative Urine Control), and citrate lyase from Aerobacter aerogenes (EC 4.1.3.6) lyophilized with a specific activity ≥0.25 U/mg (at 25 °C with citrate as the substrate) were purchased from Merck (Darmstadt, Germany). Hibitane (chlorhexidine gluconate 20%) was purchased from VWR International S.r.l. (Milano, Italy). Ultrapure water (conductivity <0.05 µS/cm; ASTM parameter Type I) was obtained by using a 1720 Deionizer Device (G. Maina, Pecetto Torinese, Torino, Italy).
2.2. Standard and Working Solutions
A certified 5.20 mmol/L citric acid standard solution was stored at 4 °C and used for the two-point, 2.60 and 5.20 mmol/L, routine calibration curve. The 2.60 mmol/L solution was prepared by diluting the concentrated 5.20 mmol/L standard.
A 100 mmol/L IS stock standard solution was prepared in 10 mL of ultrapure water and 0.5 mL aliquots were stored at −20 °C for one year. The operative 0.05 mmol/L IS solution was prepared by dissolving a 0.5 mL aliquot of stock solution in 1000 mL of water in a dark glass bottle with a top dispenser.
The eluent, a 20 mmol/L NaOH aqueous solution, was freshly prepared by mixing 1.0 mL of sodium hydroxide 50% (w/w) into 1000 mL of degassed ultrapure water.
The solution for the suppression of conductivity was prepared by mixing 3.6 mL of sulfuric acid 98% into 5 L of ultrapure water.
Citrate lyase from Aerobacter aerogenes (120 U, 302 mg) was suspended in 9 mL of ultrapure water, and 1 mL aliquots were stored at −20 °C. Suspended enzyme was stable for at least 1 month at +4 °C or 6 months at −20 °C.
2.3. Internal Quality Control
Quality control samples were prepared from true urine samples with a citrate concentration <1.5 mmol/L (low control) and >5.0 mmol/L (high control). An amount of 2 L of urine containing the proper citrate concentration was collected, mixed, acidified by adding 5 mL/L of hydrochloric acid 37%, and subjected to five cycles of subsequent freezing, defrosting, and filtration steps. Once ready, the control samples were subdivided into 10 mL aliquots and stored at −20 °C in the dark for one year. Weekly, one aliquot was defrosted and stored at +4 °C.
2.4. Sample Preparation
2.4.1. Urine
Both second-morning fasting (2 h) and 24 h (24 h) urine samples were analyzed. The 2 h urine specimens were acidified with hydrochloric acid 37% (50 µL of 37% hydrochloric acid/5 mL of urine) and stored at 4 °C. The 24 h urines were collected upon addition of 10 mL of 37% hydrochloric acid and stored at 4 °C. Samples were analyzed within one week of the collection.
Each analytical series included two controls (low and high) and two points of calibration (2.60 and 5.20 mmol/L). Amounts of 25 µL aliquots of the urine sample, standard solution, or control were transferred into 2 mL glass capped autosampler vials. An amount of 1.5 mL of IS working solution was added. The mixtures were vortexed and analyzed.
2.4.2. Plasma
Plasma samples can be analyzed as well using the aforementioned instrumental method. Blood samples were collected into heparinized vials and centrifuged. One 200 µL aliquot of plasma, placed in a 1.5 mL plastic-capped vial, was deproteinized by adding 40 µL of trichloroacetic acid, 30% w/v, vortex-mixed, and centrifuged at 4.000 g for 10 min. The clear supernatant was stored at −20 °C until analysis. An amount of 50 µL of the extract was diluted with 1.5 mL of IS and the mixture was injected into the chromatograph.
2.5. Apparatus and Chromatographic Conditions
Chromatographic separation was performed using a Dionex, Series 4500i, Ion Chromatograph (IC) system (Thermo Fisher Scientific, Waltham, MA, USA), including an eluent degasser, a gradient pump, and a conductivity detector. The system was equipped with a Dionex ACRS 500 conductivity suppressor. A Merck Hitachi AS 2000A (Hitachi Ltd., Tokyo, Japan) autosampler was used. The IC was equipped with a Dionex IonPacTM Fast Anion IIIA column (3 mm × 250 mm) and a Dionex IonPacTM Fast Anion IIIA guard-column (3 mm × 50 mm) (Thermo Fisher Scientific, Waltham, MA, USA). The mobile phase was a 20 mmol/L aqueous sodium hydroxide solution, at a flow rate of 1 mL/min, at a 23 °C temperature. The injection volume was 20 µL. The eluent conductivity background was suppressed by using a Dionex AMMS/LS-ll cation-exchange membrane set in-line after the separator. It was regenerated with 12.5 mmol/L of sulfuric acid flowing countercurrently at a flow rate of 1.5 mL/min.
2.6. Method Validation
All the validation parameters usually required for quantitative bioanalytical procedures were determined [
37,
38,
39], including selectivity, carry-over, lower limit of quantification (LLOQ), limit of detection (LOD), calibration curve, accuracy, precision, matrix effect, and stability. Synthetic urine was used for the validation experiments following the analytical protocol described above.
2.6.1. Selectivity
The synthetic urine was analyzed in order to verify the effective absence of both citrate and other potential interferences at the expected retention time interval of analyte and IS. The correct identification of the analyte was evaluated considering the within- and between-run relative retention time (RRT) precision in urine samples spiked at three concentration levels (0.32, 1.30, and 5.20 mmol/L) and in two sets of internal quality control samples (low and high level, respectively, of 1.06 and 5.93 mmol/L).
Selectivity was confirmed by analyzing citrate standard solutions and true urine samples that were incubated either in the presence of citrate lyase or without it.
An amount of 25 µL of each sample or standard solution was placed in double into a/s glass vials. Amounts of 50 µL aliquots of Bis-Tris buffer (200 mmol/L, pH 8.0) were added. Each series of mixtures were added with 25 µL of suspended citrate lyase (0.33 Units) or 25 µL of water.
After 60 min of incubation at 23 °C, 1.5 mL of IS solution was added to all samples and the mixtures were injected into the chromatograph.
2.6.2. Carry-Over
Carry-over was evaluated by injecting an alternate sequence of high-internal-quality control samples and ultra-pure water. To ensure the absence of any carry-over effect, no analytical signals had to be present at the attended citrate and IS retention times in ultrapure water chromatograms.
2.6.3. Calibration Curve
The linear calibration model was checked by analyzing synthetic urine samples spiked at six concentration levels (0.08, 0.32, 1.30, 2.60, 5.20, 10.4 mmol/L). Each concentration level was measured in triplicate. A calibration curve was obtained by plotting the relative peak area (citrate/IS) against concentration of citrate. In addition, a blank sample (processed matrix sample without analyte and without IS) and a zero sample (processed matrix with IS) were processed, but not considered among the calibration curve parameters.
2.6.4. LOD and LLOQ
An estimation of the LOD and LLOQ values was obtained by analyzing 10 replicates of blank samples (synthetic urine), as the concentrations corresponding to the average signal plus 3 and 10 times the standard deviation, respectively. A three-level and two-replicates calibration curve, in the range of LLOQ (0.04–0.08–0.16 µmol/L), was used to calculate the LOD and LLOQ concentrations.
LOD and LLOQ values were then experimentally verified. At a LLOQ concentration, the accuracy (expressed as percentage) should be within ±20% of the nominal value and the precision (expressed as percent variation coefficient, CV%) should be below 20% [
39]. The analyte signal of samples at the LLOQ level should be at least 5 times higher than the signal of blanks [
37].
2.6.5. Accuracy and Precision
Accuracy (%) was evaluated on synthetic urine samples spiked at four concentration levels (0.08, 0.32, 1.30, and 5.20 mmol/L; n = 10).
The within- and between-run precisions (CV%) were evaluated on quality control samples (low and high levels; n = 10) and analyzed in the same batch and across different days. For the between-run precision estimation, the last ten analytical sessions were considered. Within- and between-run precisions were evaluated also on spiked urine samples for accuracy determination.
2.6.6. Matrix Effect
The matrix effect was evaluated by comparing peak areas of citrate (mean value from five replicates) obtained by analyzing both synthetic urine and ultrapure water at three concentration levels (0.32, 1.30, and 5.20 mmol/L). Differences highlight matrix suppression (values below 100%) or signal enhancement (values above 100%).
2.6.7. Urinary Citrate Stability
We collected spot urine samples from 8 male and 4 female volunteers. These samples were pooled (about 2 L collected), mixed, analyzed immediately for citric acid, and subdivided into five aliquots. Each aliquot was spiked with: (i) Hibitane (chlorhexidine gluconate 20%)—2 mL/L of urine; (ii) HCl 37% (w/w; 10 mol/L)—10 mL/L of urine; (iii) acetic acid 100% (w/w; 10 mol/L)—10 mL/L of urine; (iv) trichloroacetic acid (solid)—10 g/L of urine; (v) no preservative. Each differently preserved urine was subdivided into five 5 mL aliquots, using 10 mL capped plastic vials (25 vials in total). Vials were stored at +4 °C. Analyses of each of the differently preserved urine were performed in the following 1, 2, 5, 10, 30 days.
2.7. Application to Real Samples
Citrate and creatinine were analyzed in both 2 h and 24 h urine samples. Urines were from 16 healthy subjects, 7 females, aged 28 through 58 years, on a free home diet and with normal renal function. The subjects provided written informed consent before attending the study, and an anonymous code was attributed to each subject participating in the study to adhere to privacy regulations.
3. Results
3.1. Method Development
We analyzed citrate in urine by using an enzyme-spectrophotometric method, based on citrate lyase-catalyzed and phenylhydrazine reactions [
26]. A suppressed conductivity IC method operating with a weak anion exchanger and a diluted alkaline mobile phase was subsequently developed. Under these conditions, the complete dissociation of citrate, its chromatographic handling, and efficient suppression of conductivity could be achieved.
We tested two different weak anion exchangers that showed useful performances (DIONEXTM IonPacTM Fast Anion IIIA and IonPacTM AS22 Fast). The flow of the mobile phase (1 mL/min) was selected in agreement with the suggested operative conditions, which are compatible with a “low-pressure” chromatographic system.
For both columns, the main interference for citrate was isocitrate, which is excreted in urine at a lower extent (5–10%).
Different concentrations of NaOH in aqueous solution (10, 20, 30, and 40 mmol/L) were used as the mobile phase. The chromatographic performances of the columns were compared by evaluating the logarithm capacity factors (log k′) and resolution (R) for the two citrate-isocitrate peaks. The data obtained for each column are reported in
Table 1.
By decreasing the eluent concentration, the resolution generally increased, except for the IonPacTM AS22 fast column that, with NaOH 10 mmol/L as the mobile phase, yielded excessive peaks widening. Furthermore, with this column, peaks were quite asymmetrical. Thus, we chose to proceed with IonPacTM Fast Anion IIIA.
Complete separation (R ≥ 1.5) was achieved already at 30 mmol/L. Notwithstanding, in the routine, we preferred to operate with 20 mmol/L. Under this condition, despite the longer run time, satisfactory separations were obtained even after thousands of analyzed samples.
Thanks to the higher specificity of the chromatographic method and the relatively high urinary citrate concentrations, a simple urine dilution was applied, making further sample pre-treatments unnecessary.
3.2. Method Validation
3.2.1. Selectivity
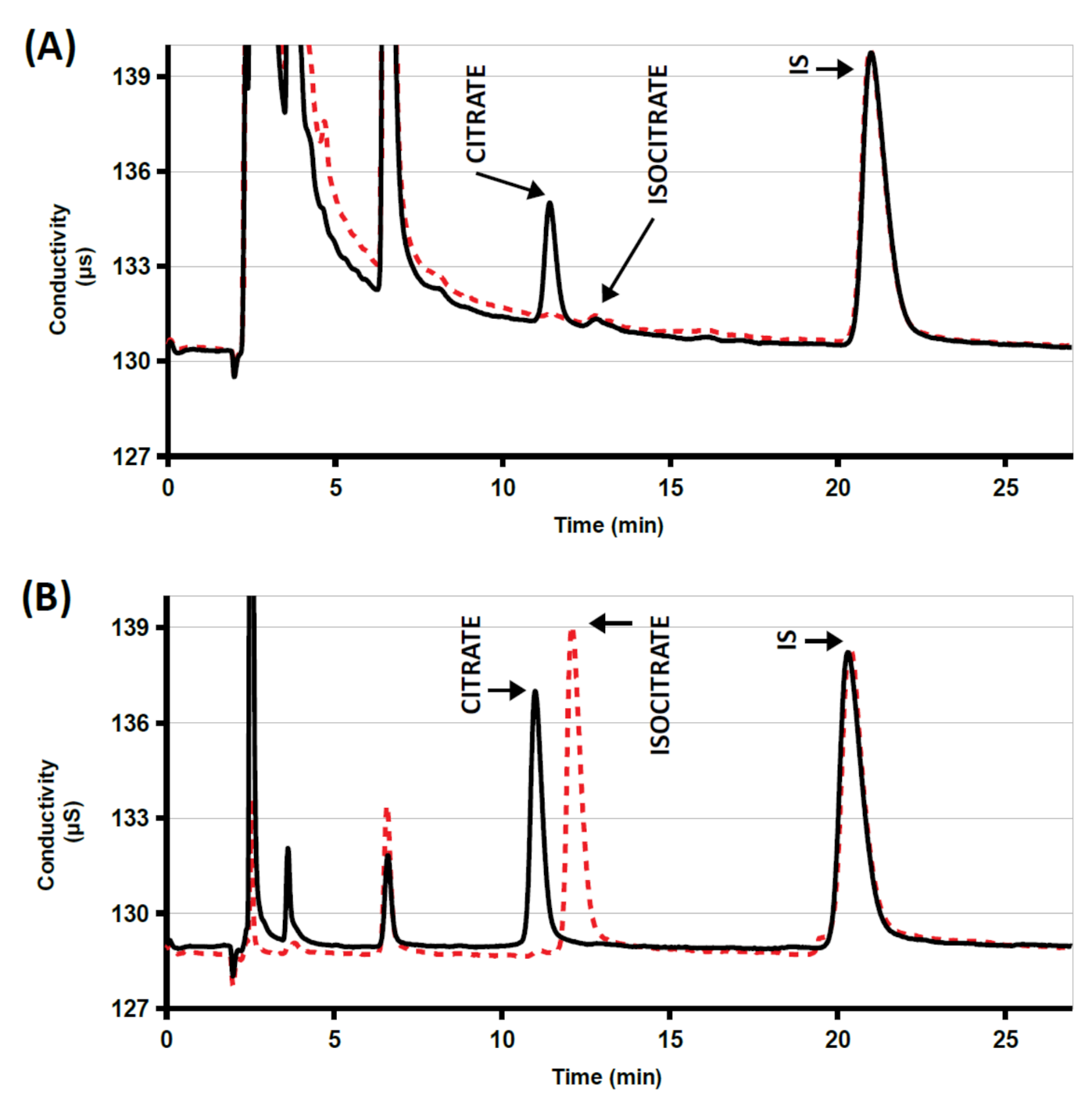
The chromatographic profiles of the synthetic urine sample did not show significant signals at retention times of citrate and IS. The analyte was clearly identified in all the spiked samples and quality control samples, with RRT within- and between-run imprecisions under 1%. Retention times gradually decreased over time as a function of the number of analyzed samples with the same column.
The high specificity of citrate lyase toward citrate is known [
22]. A standard solution and three urine samples were incubated in the presence of citrate lyase at pH 8.
Figure 1 shows analytical traces of a sample either incubated or not in the presence of the enzyme: all citrate was destroyed within 60 min of incubation. Complete disappearance of the citrate peaks indicates that the method was selective and not affected by interfering substances. The isocitrate peak, eluting 1 min later, did not interfere (see
Figure 1). It normally represents 5–10% of the citrate peak and could be taken into account in the estimation of citrate contribution to supersaturation [
13].
3.2.2. Carry-Over
The chromatographic profiles monitored during the analysis of ultra-pure water injected after a high-level quality control sample did not show the presence of significant signals at the peak retention times expected for both analyte and IS.
3.2.3. Calibration Curve
Quantitative data resulting from area counts were corrected using the respective IS areas. The results were linearly correlated according to the equation y = 0.0687x − 0.0100; R2 = 0.9999 (y = relative peak area; x = concentration of citrate in mmol/L).
3.2.4. LOD and LLOQ
LOD and LLOQ estimated values were 0.03 mmol/L and 0.07 mmol/L respectively. The equation used to convert the analytical signal into concentration is y = 0.0772x − 0.0003. In addition, 10 replicates of synthetic urine spiked with citrate at 0.04 mmol/L and 0.08 mmol/L were analyzed. At 0.08 mmol/L, the CV% was 11%, the accuracy was 85%, and the citrate signal was more than 5 times the signal of the blank samples. At 0.04 mmol/L, the citrate peak was clearly detectable (S/N ≥ 3).
3.2.5. Accuracy and Precision
Within- and between-run data on accuracy are reported in
Table 2. The results were satisfactory, as the percentage (%) ranged from 85% to 103%.
Within- and between-run data on precision are reported in
Table 3. The results show satisfactory repeatability, as the CV% is lower than 10% at all levels, both in spiked urines and internal quality controls, except for concentrations at the LLOQ level (CV = 11%).
3.2.6. Matrix Effect
Matrix effect values are given in
Table 2. It was acceptable at all concentrations, allowing the correct determination of the analyte.
3.2.7. Urinary Citrate Stability
Citrate in pooled urine was stable at +4 °C using any preservative over the 30-day storing period. Without preservative, the citrate concentration was stable for 4 days, disappearing afterward within ten days (see
Table 4). Our results are in contrast with data reported by Saude et al., who observed only a slight decrease (−7%) in citrate concentration of raw female urine samples stored at +4 °C for 4 weeks [
40].
3.3. Application to Real Samples
Citrate-to-creatinine ratios from males and females were compared for both 24 h and 2 h samples. Female values were significantly higher (unpaired t-test: p < 0.05 for both 24 h and 2 h samples). These differences disappeared when net citrate excretions were compared.
Comparing 24 h and 2 h citrate-to-creatinine ratios in individual subjects (paired
t-test) failed to find significant differences. The two sets of data showed a close correlation (
p = 0.146; R
2 = 0.733) (
Table 5).
4. Discussion
We aimed to develop a simple, sensitive, and rugged method, useful for high throughput. Among the current analytical techniques, IC appears to be the most adequate in fulfilling our requirements. Although this technique has already been widely used for citrate determination, the availability of new separators could improve its performance.
We tested different separators and Dionex IonPacTM Fast Anion IIIA performed better in terms of column efficiency. Specifically, it was observed that Dionex IonPacTM Fast Anion IIIA easily separated citrate from other polycarboxylic acids, and particularly from isocitrate, its main interferer.
The following necessary step was the choice of an appropriate exogenous substance to be used as an IS. Among the eligible ones, trimetaphosphate was chosen because of its chromatographic behavior and its stability. It is remarkable that up to now, no other published studies about citrate determination with internal standardization were found, except for a recent LC–MS/MS method [
35].
This fully validated method is currently operating in our laboratory for monitoring citraturia in patients with calcium nephrolithiasis, and in line with previous works, the urine analysis from healthy subjects showed a higher ratio of citric acid rather than creatinine in females [
41,
42].
5. Conclusions
The method described here is suitable for the laboratory routines and is effective in detecting hypocitraturia (LLOQ = 0.08 mmol/L), which is the main concern for renal stone laboratories when undertaking citrate determination.
It is easy, cost-effective, and requires little maintenance. The chromatographic run is completed within twenty-five minutes and the sample preparation only requires a simple dilution with an aqueous IS solution. Internal standardization allows us to improve the precision and to prevent any variations in injection volumes.
The validated method appears adequate to measure the real concentration of this analyte in patients’ urine samples. High sensitivity referring to the relatively high citrate concentration in urine allows this test to be performed using only some fractions of a microliter of urine, which ensures no system perturbations, a long column life, and, as a consequence, cost-effectiveness. It has shown easy suitability in our laboratory where it is currently adopted to analyze over 1000 urine samples per year.
Author Contributions
Conceptualization, M.P.; data curation, M.L.; formal analysis, F.P., M.F. and V.N.; resources, D.C.; methodology, M.P.; supervision, M.P., M.M. and D.C.; visualization, M.L.; writing—original draft, M.L.; writing—review & editing, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the AO Ordine Mauriziano Laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CE | capillary electrophoresis |
| CV% | coefficient of variation expressed as percentage |
| IC | ion chromatography |
| IS | internal standard |
| LC–MS/MS | liquid chromatography-tandem mass spectrometry |
| LLOQ | lower limit of quantification |
| LOD | limit of detection |
| S/N | signal to noise ratio |
References
- Simpson, D.P. Citrate excretion: A window on renal metabolism. Am. J. Physiol. 1983, 244, F223–F234. [Google Scholar] [CrossRef] [PubMed]
- Marangella, M.; Vitale, C.; Manganaro, M.; Cosseddu, D.; Martini, C.; Petrarulo, M.; Linari, F. Renal handling of citrate in chronic renal insufficiency. Nephron 1991, 57, 439–443. [Google Scholar] [CrossRef]
- Norman, P.; Curthoys, N.P.; Moe, O.W. Proximal Tubule Function and Response to Acidosis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1627–1638. [Google Scholar]
- Rudman, D.; Kutner, M.H.; Red, S.C.; Waters, W.C.; Garron, G.G.; Bleier, J. Hypocitraturia in calcium nephrolithiasis. J. Clin. Endocrinol. Metab. 1982, 55, 1052–1057. [Google Scholar] [CrossRef]
- Marangella, M.; Bianco, O.; Grande, M.L.; Petrarulo, M.; Valente, D.; Vitale, C.; Linari, F. Patterns of citrate excretion in healthy subjects and patients with idiopathic stone disease. Contrib. Nephrol. 1987, 58, 34–38. [Google Scholar]
- Schwille, P.O.; Scholl, D.; Schwille, K.; Leutschaft, R.; Goldberg, I.; Sigel, A. Citrate in Urine and Serum and Associated Variables in Subgroups of Urolithiasis. Results from an Outpatient Stone Clinic. Nephron 1982, 31, 194–202. [Google Scholar] [CrossRef]
- Kok, D.J.; Papapoulos, S.E.; Bijovet, O.L.M. Excessive crystal agglomeration with low citrate excretion in recurrent stone-formers. Lancet 1986, 10, 1056–1058. [Google Scholar] [CrossRef]
- Tiselius, H.; Fornander, A.; Nilsson, M. The effects of citrate and urine on calcium oxalate crystal aggregation. Urol. Res. 1993, 21, 363–366. [Google Scholar] [CrossRef]
- Ryall, R.L.; Harnett, R.M.; Marshall, V.R. The effect of urine, pyrophosphate, citrate, magnesium and glycosaminoglycans on the growth and aggregation of calcium oxalate crystals in vitro. Clin. Chim. Acta 1981, 112, 349–356. [Google Scholar] [CrossRef]
- Meier, J.L. Formation constants for interaction of citrate with calcium and magnesium ions. Anal. Biochem. 1974, 62, 295–300. [Google Scholar] [CrossRef]
- Hallson, P.C.; Rose, G.A.; Sulaiman, S. Raising Urinary Citrate Lowers Calcium Oxalate and Calcium Phosphate Crystal Formation in Whole Urine. Urol. Int. 1983, 38, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Fleish, H. Inhibitors and promoters of stone formation. Kidney Int. 1978, 13, 361–371. [Google Scholar] [CrossRef]
- Marangella, M.; Daniele, P.G.; Sonego, S.; Linari, F. Urine saturation with calcium salts in normal subjects and idiopathic calcium stone-formers estimated by an improved computer model system. Urol. Res. 1985, 13, 189–193. [Google Scholar] [CrossRef]
- Nicar, M.; Skurla, C.; Sakhaee, K.; Pak, C.Y. Low urinary citrate excretion in nephrolithiasis. Urology 1983, 21, 8–14. [Google Scholar] [CrossRef]
- Zuckerman, J.M.; Dean, B.S.; Assimos, G. Hypocitraturia: Pathophysiology and Medical Management. Rev. Urol. 2009, 11, 134–144. [Google Scholar]
- Hamm, L.L.; Hering-Smith, K.S. Pathophysiology of hypocitraturic nephrolithiasis. Endocrinol. Metab. Clin. N. Am. 2002, 31, 885–893. [Google Scholar] [CrossRef]
- Koff, S.G.; Paquette, E.L.; Cullen, J.; Gancarczyk, K.K.; Tucciarone, P.R.; Schenkman, N.S. Comparison between lemonade and potassium citrate and impact on urine pH and 24-hour urine parameters in patients with kidney stone formation. Urology 2007, 69, 1013–1016. [Google Scholar] [CrossRef]
- Ashby, R.A.; Sleet, R.J. The role of citrate complexes in preventing urolithiasis. Clin. Chim. Acta 1992, 210, 157–165. [Google Scholar] [CrossRef]
- Leumann, E.; Hoppe, B.; Neuhaus, T. Management of primary hyperoxaluria: Efficacy of oral citrate administration. Pediatr. Nephrol. 1993, 7, 207–211. [Google Scholar] [CrossRef]
- Kato, Y.; Yamaguchi, S.; Yachiku, S.; Nakazono, S.; Hori, J.; Wada, N.; Hou, K. Changes in urinary parameters after oral administration of potassium-sodium citrate and magnesium oxide to prevent urolithiasis. Urology 2004, 63, 7–12. [Google Scholar] [CrossRef]
- Khanniazi, M.K.; Khanam, A.; Jaffer Naqvi, S.A.; Sheikh, M.A. Study of potassium citrate treatment of crystalluric nephrolithiasis. Biomed. Pharmacother. 1993, 47, 25–28. [Google Scholar] [CrossRef]
- Moellering, H.; Gruber, W. Determination of citrate with citrate lyase. Anal. Biochem. 1966, 17, 369–376. [Google Scholar] [CrossRef]
- Nielsen, T.T. A method for enzymatic determination of citrate in serum and urine. Scand. J. Clin. Lab. Investig. 1976, 36, 513–519. [Google Scholar] [CrossRef]
- Welshman, S.G.; McCambridge, H. The estimation of citrate in serum and urine using a citrate lyase technique. Clin. Chim. Acta 1973, 46, 243–246. [Google Scholar] [CrossRef]
- Tomisek, A.J.; Winkler, E.M.; Natelson, S. Fluorometry of citrate in serum, with use of citrate (pro-3S)-lyase. Clin. Chem. 1975, 21, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Petrarulo, M.; Facchini, P.; Cerelli, E.; Marangella, M.; Linari, F. Citrate in Urine Determined with a New Citrate Lyase Method. Clin. Chem. 1995, 41, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Nancollas, G.H. Determination of urinary citrate by high performance ion chromatography. Kidney Int. 1985, 28, 985–987. [Google Scholar] [CrossRef][Green Version]
- Petrarulo, M.; Bianco, O.; Marangella, M.; Pellegrino, S.; Linari, F. Improved ion chromatographic determination of urine citrate and isocitrate. G. Ital. Chim. Clin. 1991, 16, 17–22. [Google Scholar] [CrossRef]
- Ogawa, Y.; Morozumi, M.; Tanaka, T.; Yamaguchi, K. Determination of urinary citrate by ion chromatography. J. Urol. 1985, 135, 178–181. [Google Scholar] [CrossRef]
- Jenke, D.R. Quantitation of oxalate and citrate by ion chromatography with a buffered, strong acid eluent. J. Chrom. A 1988, 437, 231–237. [Google Scholar] [CrossRef]
- Wildman, D.R.; Jackson, P.E.; Jones, W.R.; Alden, P.G. Analysis of anion constituents of urine by inorganic capillary electrophoresis. J. Chromatogr. 1991, 546, 459–466. [Google Scholar] [CrossRef]
- Zhao, S.; Yin, D.; Du, H.; Tian, X.; Chen, Y.; Zhang, W.; Yu, A.; Zhang, S. Determination of oxalate and citrate in urine by capillary electrophoresis using solid-phase extraction and capacitively coupled contactless conductivity based on an improved mini-cell. J. Sep. Sci. 2018, 41, 2623–2631. [Google Scholar] [CrossRef]
- Holmes, R. Measurement of urinary oxalate and citrate by capillary electrophoresis and indirect ultraviolet absorbance. Clin. Chem. 1995, 41, 1297–1301. [Google Scholar] [CrossRef]
- Munoz, J.A.; López-Mesas, M.; Valiente, M. Development and validation of a simple determination of urine metabolites (oxalate, citrate, uric acid and creatinine) by capillary zone electrophoresis. Talanta 2010, 81, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.; Adaway, J.; Keevil, B. A combined liquid chromatography tandem mass spectrometry assay for the quantification of urinary oxalate and citrate for diagnosis and management of nephrolithiasis. Ann. Clin. Biochem. 2017, 55, 461–468. [Google Scholar] [CrossRef]
- Keevil, B.G.; Owen, L.; Thornton, S.; Kavanagh, J. Measurement of citrate in urine using liquid chromatography tandem mass spectrometry: Comparison with an enzymatic method. Ann. Clin. Biochem. 2005, 42, 357–363. [Google Scholar] [CrossRef]
- Guideline on Bioanalytical Method Validation; EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2**; European Medicines Agency: Amsterdam, The Netherlands, 2011.
- Food and Drug Administration. FDA Guidance for Industry: Bioanalytical Method Validation; US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research: Rockville, MD, USA, 2001.
- Peters, F.T.; Drummer, O.H.; Musshoff, F. Validation of new methods. For. Sci. Int. 2007, 165, 216–224. [Google Scholar] [CrossRef]
- Saude, E.J.; Sykes, B.D. Urine stability for metabolomic studies: Effects of preparation and storage. Metabolomics 2007, 3, 19–27. [Google Scholar] [CrossRef]
- Shorr, E.; Bernheim, A.; Tbussky, H. The relation of urinary citric acid excretion to the menstrual cycle and the steroidal reproductive hormones. Science 1942, 95, 606–607. [Google Scholar] [CrossRef]
- Welshman, S.G.; Mcgeown, M.G. Urinary Citrate Excretion in Stone-Formers and Normal Controls. Br. J. Urol. 1976, 48, 7–11. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).