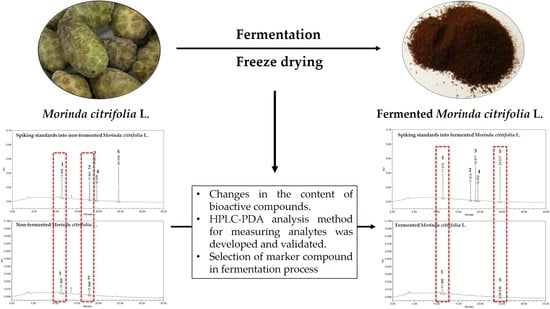
Development and Validation of an Analytical Method for Deacetylasperulosidic Acid, Asperulosidic Acid, Scopolin, Asperuloside and Scopoletin in Fermented Morinda citrifolia L. (Noni)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Sample Preparation
2.3. HPLC Instrument Conditions
2.4. Method Validation
3. Results
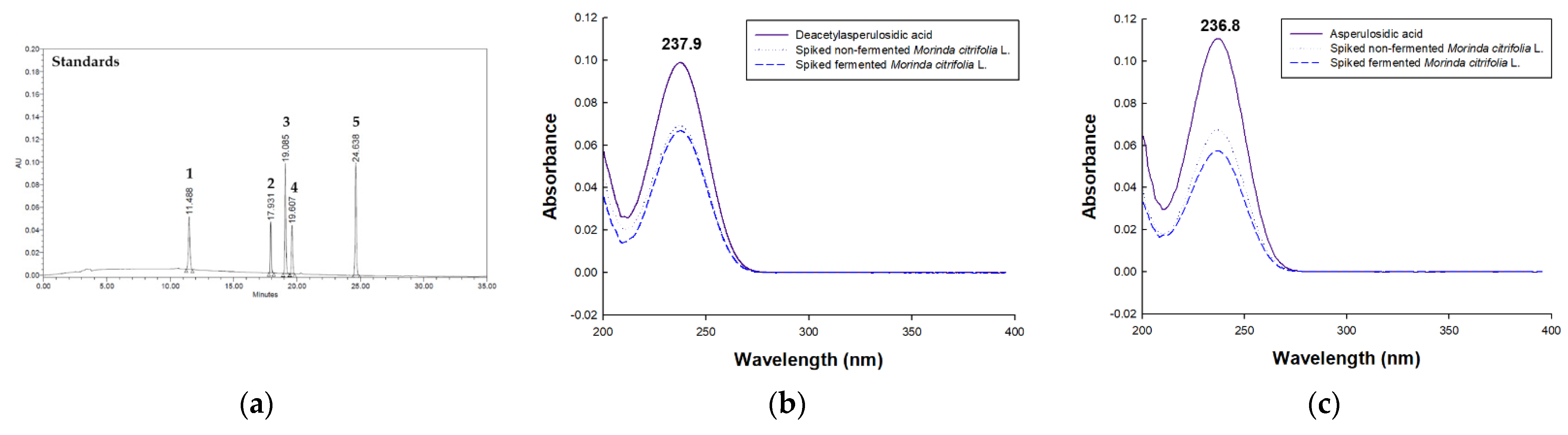
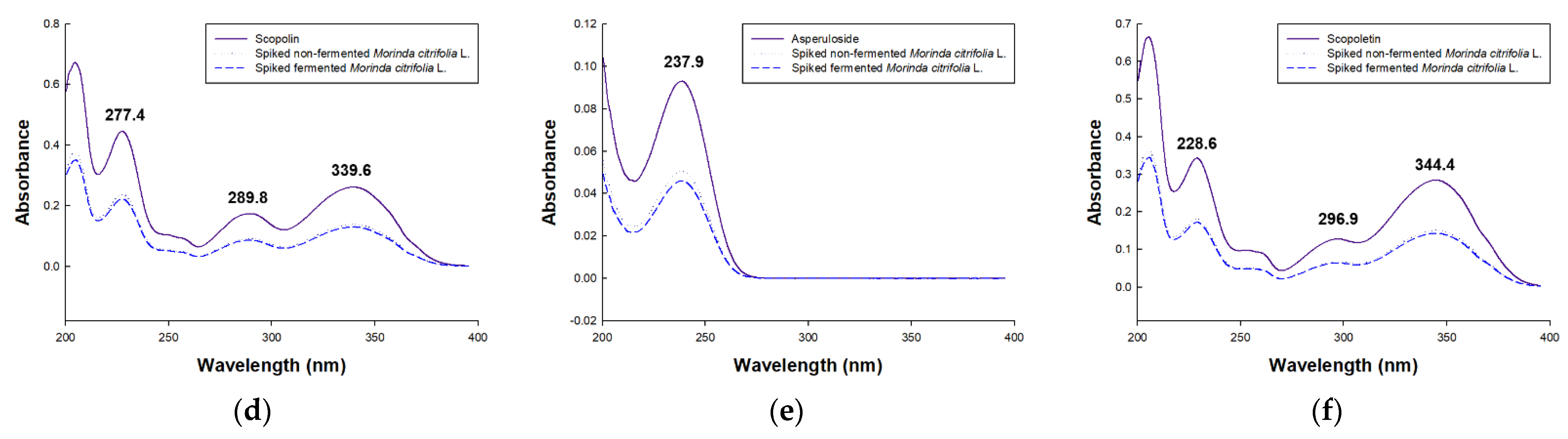
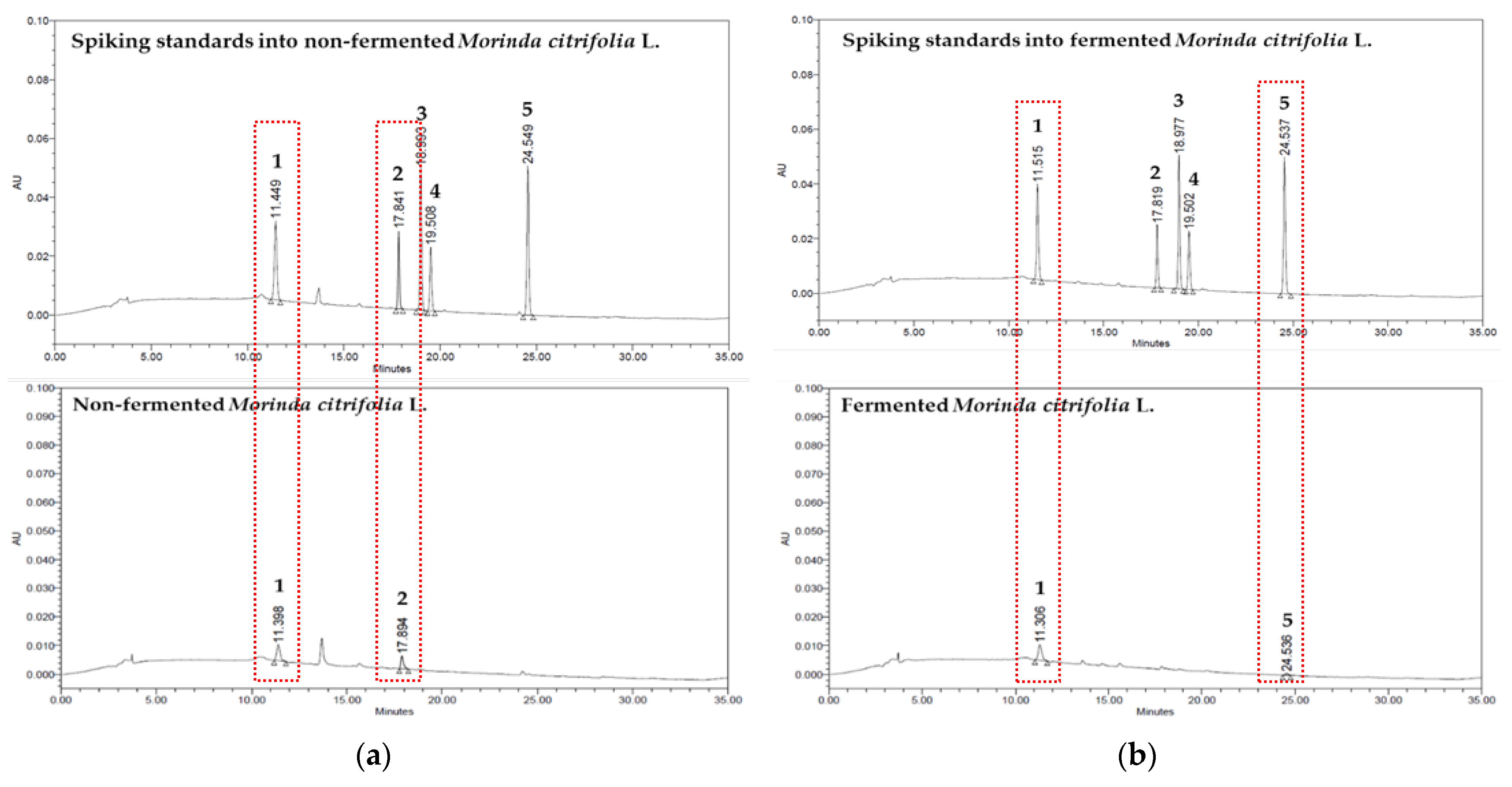
3.1. Method Development
3.2. Method Validation
3.2.1. Selectivity, Linearity, LOD and LOQ
3.2.2. Precision and Accuracy
3.3. Quantitative Analysis of Bioactive Compound in Non-Fermented and Fermented Morinda citrifolia L. Extracts
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.Y.; West, B.J.; Jensen, C.J.; Nowicki, D.; Su, C.; Palu, A.K.; Anderson, G. Morinda citrifolia (noni): A literature review and recent advances in noni research. Acta Pharmacol. Sin. 2002, 23, 1127–1141. [Google Scholar] [PubMed]
- Chan-Blanco, Y.; Vaillant, F.; Mercedes Perez, A.; Reynes, M.; Brillouet, J.M.; Brat, P. The noni fruit (Morinda citrifolia L.): A review of agricultural research, nutritional and therapeutic properties. J. Food Compos. Anal. 2006, 19, 645–654. [Google Scholar] [CrossRef]
- Motshakeri, M.; Ghazali, H.M. Nutritional, phytochemical and commercial quality of noni fruit: A multi-beneficial gift from nature. Trends Food Sci. Technol. 2015, 45, 118–129. [Google Scholar] [CrossRef]
- García-Vilas, J.A.; Quesada, A.R.; Medina, M.A. Damnacanthal, a noni anthraquinone, inhibits c-Met and is a potent antitumor compound against HepG2 human hepatocellular carcinoma cells. Sci. Rep. 2015, 5, 8021. [Google Scholar] [CrossRef]
- Murata, K.; Abe, Y.; Futamura-Masuda, M.; Uwaya, A.; Isami, F.; Deng, S.; Matsuda, H. Effect of Morinda citrifolia fruit extract and its iridoid glycosides on blood fluidity. J. Nat. Med. 2014, 68, 498–504. [Google Scholar] [CrossRef]
- Almeida, É.S.; de Oliveira, D.; Hotza, D. Properties and applications of Morinda citrifolia (noni): A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 883–909. [Google Scholar] [CrossRef]
- Wang, M.Y.; Su, C. Cancer preventive effect of Morinda citrifolia (Noni). Ann. N. Y. Acad. Sci. 2001, 952, 161–168. [Google Scholar] [CrossRef]
- Heinicke, R.M. The pharmacologically active ingredient of noni. Pac. Trop. Bot. Gard. Bull. 1985, 15, 10–14. [Google Scholar]
- Sanni, D.M.; Fatoki, T.H.; Kolawole, A.O.; Akinmoladun, A.C. Xeronine structure and function: Computational comparative mastery of its mystery. In Silico Pharmacol. 2017, 5, 1–7. [Google Scholar] [CrossRef]
- Israili, Z.H.; Lyoussi, B. Ethnopharmacology of the plants of genus Ajuga. Pak. J. Pharm. Sci. 2009, 22, 425–462. [Google Scholar]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Statti, G.A.; Menichini, F. Biological and pharmacological activities of iridoids: Recent developments. Mini Rev. Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Szumny, A.; Sokół-Łętowska, A.; Piórecki, N.; Klymenko, S.V. Iridoids and anthocyanins in cornelian cherry (Cornus mas L.) cultivars. J. Food Compost. Anal. 2015, 40, 95–102. [Google Scholar] [CrossRef]
- Deng, S.; West, B.J.; Palu, A.K.; Jensen, C.J. Determination and comparative analysis of major iridoids in different parts and cultivation sources of Morinda citrifolia. Phytochem. Anal. 2011, 22, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Abou Assi, R.; Darwis, Y.; Abdulbaqi, I.M.; Vuanghao, L.; Laghari, M.H. Morinda citrifolia (Noni): A comprehensive review on its industrial uses, pharmacological activities, and clinical trials. Arab. J. Chem. 2017, 10, 691–707. [Google Scholar] [CrossRef]
- Robe, K.; Izquierdo, E.; Vignols, F.; Rouached, H.; Dubos, C. The Coumarins: Secondary metabolites playing a primary role in plant nutrition and health. Trends Plant Sci. 2020, 26, 248–259. [Google Scholar] [CrossRef]
- Bansal, Y.; Sethi, P.; Bansal, G. Coumarin: A potential nucleus for anti-inflammatory molecules. Med. Chem. Res. 2013, 22, 3049–3060. [Google Scholar] [CrossRef]
- Zhu, J.J.; Jiang, J.G. Pharmacological and nutritional effects of natural coumarins and their structure activity relationships. Mol. Nutr. Food Res. 2018, 62, 1701073. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, X.; Xu, W.; Farzaneh, F.; Xu, R. The structure and pharmacological functions of coumarins and their derivatives. Curr. Med. Chem. 2009, 16, 4236–4260. [Google Scholar] [CrossRef]
- Ikeda, R.; Wada, M.; Nishigaki, T.; Nakashima, K. Quantification of coumarin derivatives in Noni (Morinda citrifolia) and their contribution of quenching effect on reactive oxygen species. Food Chem. 2009, 113, 1169–1172. [Google Scholar] [CrossRef]
- Shah, N.P. Functional cultures and health benefits. Int. Dairy J. 2007, 17, 1262–1277. [Google Scholar] [CrossRef]
- Hasler, C.M. Functional foods: Benefits, concerns and challenges—A position paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Jain, S.; Rastamanesh, R.; Bomba, A.; Catanzaro, R.; Marotta, F. Fermentation technology in the development of functional foods for human health: Where we should head. Ferment. Technol. 2011, 1, 1–2. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Joana Gil-Chávez, G.; Villa, J.A.; Fernando Ayala-Zavala, J.; Basilio Heredia, J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Cho, B.Y.; Park, M.R.; Lee, J.H.; Ra, M.J.; Han, K.C.; Kang, I.J.; Lee, O.H. Standardized Cirsium setidens Nakai ethanolic extract suppresses adipogenesis and regulates lipid metabolisms in 3T3-L1 adipocytes and C57BL/6J mice fed high-fat diets. J. Med. Food 2017, 20, 763–776. [Google Scholar] [CrossRef]
- Yadav, N.P.; Dixit, V.K. Recent approaches in herbal drug standardization. Int. J. Integr. Biol. 2008, 2, 195–203. [Google Scholar]
- Ong, E.S. Extraction methods and chemical standardization of botanicals and herbal preparations. J. Chromatogr. B. 2004, 812, 23–33. [Google Scholar] [CrossRef]
- Swartz, M. HPLC detectors: A brief review. J. Liq. Chrom. Relat. Technol. 2010, 33, 1130–1150. [Google Scholar] [CrossRef]
- Scotter, M.J.; Roberts, D.P.; Rees, G.O. Development and single-laboratory validation of an HPLC method for the determination of coumarin in foodstuffs using internal standardization and solid-phase extraction cleanup. Anal. Methods 2011, 3, 414–419. [Google Scholar] [CrossRef]
- Nahata, A.; Dixit, V.K. Spectrofluorimetric estimation of scopoletin in Evolvulus alsinoides Linn. and Convulvulus pluricaulis choisy. Indian J. Pharm. Sci. 2008, 70, 834–837. [Google Scholar]
- Raters, M.; Matissek, R. Analysis of coumarin in various foods using liquid chromatography with tandem mass spectrometric detection. Eur. Food Res. Technol. 2008, 227, 637–642. [Google Scholar] [CrossRef]
- Liu, H.W. Extraction and isolation of compounds from herbal medicines. In Traditional Herbal Medicine Research Methods: Identification, Analysis, Bioassay, and Pharmaceutical and Clinical Studies; Liu, W.J.H., Ed.; John Wiley & Sons: New York, NY, USA, 2011; pp. 81–138. [Google Scholar]
- International Conference on Harmonization (ICH). International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guidline. Validation of Analytical Procedures: Text and Methodology. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1_Guideline.pdf (accessed on 11 March 2019).
- Marın, A.; Garcıa, E.; Garcıa, A.; Barbas, C. Validation of a HPLC quantification of acetaminophen, phenylephrine and chlorpheniramine in pharmaceutical formulations: Capsules and sachets. J. Pharm. Biomed. Anal. 2002, 29, 701–714. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Chen, Y.; Guo, J.; Li, C.; Zhang, J. Metatranscriptomic approach reveals the functional and enzyme dynamics of core microbes during noni fruit fermentation. Food Res. Int. 2021, 141, 109999. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dou, R.; Yang, R.; Cai, K.; Li, C.; Li, W. Changes in Phenols, Polysaccharides and Volatile Profiles of Noni (Morinda citrifolia L.) Juice during Fermentation. Molecules 2021, 26, 2604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Meng, J.; Li, X.; Tang, X.; Ma, S.; Lv, Y.; Yang, S. Noni (Morinda citrifolia L.) wine prevents the oxidative stress and obesity in mice induced by high-fat diet. J. Food Biochem. 2020, 44, e13460. [Google Scholar] [CrossRef]
- Liang, Y.Z.; Xie, P.; Chan, K. Quality control of herbal medicines. J. Chromatogr. B 2004, 812, 53–70. [Google Scholar] [CrossRef]
- Kamiya, K.; Tabnaka, Y.; Endgang, H.; Umar, M.; Satake, T. New anthraquinone and iridoid from the fruits of Morinda citrifolia. Chem. Pharm. Bull. 2005, 53, 1597–1599. [Google Scholar] [CrossRef] [PubMed]
- Levand, O.; Larson, H.O. Some chemical constituents of Morinda citrifolia. Planta Med. 1979, 36, 186–187. [Google Scholar] [CrossRef]
- Manuele, M.G.; Ferraro, G.; Arcos, M.L.B.; López, P.; Cremaschi, G.; Anesini, C. Comparative immunomodulatory effect of scopoletin on tumoral and normal lymphocytes. Life Sci. 2006, 79, 2043–2048. [Google Scholar] [CrossRef]
- Pawlus, A.D.; Su, B.N.; Keller, W.J.; Kinghorn, A.D. An anthraquinone with potent quinone reductase-inducing activity and other constituents of the fruits of Morinda citrifolia (noni). J. Nat. Prod. 2005, 68, 1720–1722. [Google Scholar] [CrossRef]
- Ma, D.L.; Chen, M.; Su, C.X.; West, B.J. In vivo antioxidant activity of deacetylasperulosidic acid in noni. J. Anal. Methods Chem. 2013, 2013, 804504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, M.; Zhang, J.; Wu, L.; Liu, J.; Si, J. Identification and evaluation of antioxidant components in the flowers of five Chimonanthus species. Ind. Crops Prod. 2017, 102, 164–172. [Google Scholar] [CrossRef]
- Leema, G.; Tamizhselvi, R. Protective effect of scopoletin against cerulein-induced acute pancreatitis and associated lung injury in mice. Pancreas 2018, 47, 557–585. [Google Scholar] [CrossRef] [PubMed]
- Jamuna, S.; Karthika, K.; Paulsamy, S.; Thenmozhi, K.; Kathiravan, S.; Venkatesh, R. Confertin and scopoletin from leaf and root extracts of Hypochaeris radicata have anti-inflammatory and antioxidant activities. Ind. Crops Prod. 2015, 70, 221–230. [Google Scholar] [CrossRef]
- Pinto, D.; Silva, A.M.S. Anticancer natural coumarins as lead compounds for the discovery of new drugs. Curr. Top. Med. Chem. 2017, 17, 3190–3198. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A. Evaluation of the antithyroid, antioxidative and antihyperglycemic activity of scopoletin from Aegle marmelos leaves in hyperthyroid rats. Phytother. Res. 2006, 20, 1103–1105. [Google Scholar] [CrossRef]
- Guo, M.; Mao, B.; Sadiq, F.A.; Hao, Y.; Cui, S.; Yi, M.; Hong, Q.; Lee, Y.H.; Zhao, J. Effects of noni fruit and fermented noni juice against acute alcohol induced liver injury in mice. J. Funct. Foods 2020, 70, 103995. [Google Scholar] [CrossRef]
- Kim, S.H.; Seong, G.S.; Choung, S.Y. Fermented Morinda citrifolia (Noni) alleviates DNCB-induced atopic dermatitis in NC/Nga mice through modulating immune balance and skin barrier function. Nutrients 2020, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Yin, L.J.; Tai, H.M.; Jiang, S.T. Facilitating the release of bionutrients from Morinda citrifolia (noni) by cellulase hydrolysis and lactic acid bacteria fermentation and their effects on α-amylase and α-glucosidase activities. J. Mar. Sci. Technol. 2016, 24, 648–655. [Google Scholar]
| Analytes | Range (μg/mL) | Slope | Intercept | Coefficient of Determination (R2) | LOD 1 (μg/mL) | LOQ 2 (μg/mL) |
|---|---|---|---|---|---|---|
| Deacetylasperulosidic acid | 1.56–100 | 4081.36 | 360.71 | 1.0000 | 0.76 | 2.30 |
| Asperulosidic acid | 1.56–100 | 2716.59 | 736.72 | 0.9999 | 0.26 | 0.77 |
| Scopolin | 1.56–100 | 6052.10 | 1032.58 | 1.0000 | 0.44 | 1.34 |
| Asperuloside | 1.56–100 | 2797.46 | −167.51 | 1.0000 | 0.97 | 2.95 |
| Scopoletin | 1.56–100 | 7441.30 | 619.61 | 1.0000 | 0.04 | 0.13 |
| Matrix | Analytes | Concentration (μg/mL) | Mean ± SD (μg/mL) | RSD 1 (%) | Recovery (%) | |
|---|---|---|---|---|---|---|
| Non-fermented Morinda citrifolia L. | Deacetylasperulosidic acid | Intraday | 12.5 | 12.87 ± 0.20 | 1.53 | 103.0 |
| 25 | 27.15 ± 0.72 | 2.66 | 110.5 | |||
| 50 | 60.95 ± 2.08 | 3.41 | 117.7 | |||
| Interday | 12.5 | 13.30 ± 0.03 | 0.21 | 106.4 | ||
| 25 | 27.62 ± 0.51 | 1.85 | 110.5 | |||
| 50 | 58.83 ± 2.32 | 3.95 | 117.7 | |||
| Asperulosidic acid | Intraday | 12.5 | 14.27 ± 0.13 | 0.93 | 114.1 | |
| 25 | 28.23 ± 0.26 | 0.93 | 112.9 | |||
| 50 | 58.75 ± 2.10 | 3.58 | 117.5 | |||
| Interday | 12.5 | 14.15 ± 0.12 | 0.83 | 113.2 | ||
| 25 | 28.49 ± 0.54 | 1.91 | 114.0 | |||
| 50 | 59.05 ± 1.92 | 3.26 | 118.1 | |||
| Scopolin | Intraday | 12.5 | 12.18 ± 0.05 | 0.40 | 97.5 | |
| 25 | 24.43 ± 0.05 | 0.20 | 97.7 | |||
| 50 | 50.70 ± 1.68 | 3.31 | 101.4 | |||
| Interday | 12.5 | 12.39 ± 0.17 | 1.35 | 99.1 | ||
| 25 | 24.69 ± 0.51 | 2.08 | 98.8 | |||
| 50 | 50.73 ± 1.75 | 3.44 | 101.5 | |||
| Asperuloside | Intraday | 12.5 | 12.46 ± 0.11 | 0.87 | 99.7 | |
| 25 | 25.06 ± 0.23 | 0.91 | 100.2 | |||
| 50 | 51.73 ± 1.96 | 3.60 | 103.1 | |||
| Interday | 12.5 | 12.46 ± 0.35 | 2.83 | 99.7 | ||
| 25 | 25.13 ± 0.61 | 2.42 | 100.5 | |||
| 50 | 51.57 ± 1.86 | 3.60 | 103.1 | |||
| Scopoletin | Intraday | 12.5 | 12.42 ± 0.07 | 0.57 | 99.4 | |
| 25 | 24.95 ± 0.01 | 0.03 | 99.8 | |||
| 50 | 51.11 ± 1.07 | 2.09 | 102.2 | |||
| Interday | 12.5 | 12.42 ± 0.18 | 1.42 | 99.3 | ||
| 25 | 25.06 ± 0.33 | 1.31 | 100.2 | |||
| 50 | 50.95 ± 1.03 | 2.03 | 101.9 | |||
| Fermented Morinda citrifolia L. | Deacetylasperulosidic acid | Intraday | 12.5 | 13.15 ± 0.28 | 2.13 | 105.2 |
| 25 | 27.82 ± 0.80 | 2.87 | 108.6 | |||
| 50 | 57.80 ± 0.89 | 1.54 | 121.9 | |||
| Interday | 12.5 | 13.37 ± 0.15 | 1.10 | 107.0 | ||
| 25 | 28.13 ± 0.51 | 2.06 | 112.5 | |||
| 50 | 57.59 ± 1.05 | 1.82 | 115.2 | |||
| Asperulosidic acid | Intraday | 12.5 | 12.71 ± 0.30 | 2.38 | 101.7 | |
| 25 | 25.22 ± 0.21 | 0.82 | 100.9 | |||
| 50 | 51.10 ± 0.28 | 0.54 | 102.2 | |||
| Interday | 12.5 | 12.52 ± 0.33 | 2.60 | 100.2 | ||
| 25 | 25.44 ± 0.73 | 2.89 | 101.7 | |||
| 50 | 51.30 ± 0.81 | 1.57 | 102.6 | |||
| Scopolin | Intraday | 12.5 | 12.19 ± 0.08 | 0.66 | 97.5 | |
| 25 | 24.45 ± 0.04 | 0.15 | 97.8 | |||
| 50 | 49.33 ± 0.30 | 0.61 | 98.7 | |||
| Interday | 12.5 | 12.40 ± 0.24 | 1.90 | 99.1 | ||
| 25 | 24.69 ± 0.51 | 2.08 | 98.8 | |||
| 50 | 50.73 ± 1.75 | 3.44 | 99.0 | |||
| Asperuloside | Intraday | 12.5 | 12.47 ± 0.03 | 0.27 | 99.8 | |
| 25 | 24.98 ± 0.24 | 0.97 | 99.9 | |||
| 50 | 49.77 ± 0.38 | 0.76 | 99.5 | |||
| Interday | 12.5 | 12.56 ± 0.22 | 1.78 | 100.5 | ||
| 25 | 25.14 ± 0.45 | 1.80 | 100.6 | |||
| 50 | 49.86 ± 0.87 | 1.74 | 99.7 | |||
| Scopoletin | Intraday | 12.5 | 12.57 ± 0.09 | 0.74 | 100.6 | |
| 25 | 25.12 ± 0.06 | 0.23 | 100.5 | |||
| 50 | 50.47 ± 0.29 | 0.58 | 100.9 | |||
| Interday | 12.5 | 12.55 ± 0.18 | 1.42 | 100.4 | ||
| 25 | 25.06 ± 0.33 | 1.31 | 100.2 | |||
| 50 | 50.40 ± 0.54 | 1.06 | 100.8 | |||
| Sample | Analytes | Mean ± SD (mg/g) | RSD (%) |
|---|---|---|---|
| Non-fermented Morinda citrifolia L. | Deacetylasperulosidic acid | 15.71 ± 0.74 | 4.71 |
| Asperulosidic acid | 15.16 ± 0.38 | 2.51 | |
| Scopolin | N.D. 1 | - | |
| Asperuloside | N.D. | - | |
| scopoletin | N.D. | - | |
| Fermented Morinda citrifolia L. | Deacetylasperulosidic acid | 18.52 ± 0.71 | 3.83 |
| Asperulosidic acid | N.D. | - | |
| Scopolin | N.D. | - | |
| Asperuloside | N.D. | - | |
| scopoletin | 1.01 ± 0.07 | 7.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-I.; Kwon, H.-Y.; La, I.-J.; Jo, Y.-H.; Han, X.; Men, X.; Lee, S.-J.; Kim, Y.-D.; Seong, G.-S.; Lee, O.-H. Development and Validation of an Analytical Method for Deacetylasperulosidic Acid, Asperulosidic Acid, Scopolin, Asperuloside and Scopoletin in Fermented Morinda citrifolia L. (Noni). Separations 2021, 8, 80. https://doi.org/10.3390/separations8060080
Choi S-I, Kwon H-Y, La I-J, Jo Y-H, Han X, Men X, Lee S-J, Kim Y-D, Seong G-S, Lee O-H. Development and Validation of an Analytical Method for Deacetylasperulosidic Acid, Asperulosidic Acid, Scopolin, Asperuloside and Scopoletin in Fermented Morinda citrifolia L. (Noni). Separations. 2021; 8(6):80. https://doi.org/10.3390/separations8060080
Chicago/Turabian StyleChoi, Sun-Il, Hee-Yeon Kwon, Im-Joung La, Yeon-Hui Jo, Xionggao Han, Xiao Men, Se-Jeong Lee, Yong-Deok Kim, Geum-Su Seong, and Ok-Hwan Lee. 2021. "Development and Validation of an Analytical Method for Deacetylasperulosidic Acid, Asperulosidic Acid, Scopolin, Asperuloside and Scopoletin in Fermented Morinda citrifolia L. (Noni)" Separations 8, no. 6: 80. https://doi.org/10.3390/separations8060080
APA StyleChoi, S.-I., Kwon, H.-Y., La, I.-J., Jo, Y.-H., Han, X., Men, X., Lee, S.-J., Kim, Y.-D., Seong, G.-S., & Lee, O.-H. (2021). Development and Validation of an Analytical Method for Deacetylasperulosidic Acid, Asperulosidic Acid, Scopolin, Asperuloside and Scopoletin in Fermented Morinda citrifolia L. (Noni). Separations, 8(6), 80. https://doi.org/10.3390/separations8060080