Occurrence of Selected Known or Suspected Endocrine-Disrupting Pesticides in Portuguese Surface Waters Using SPME-GC-IT/MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Solvents, and Materials
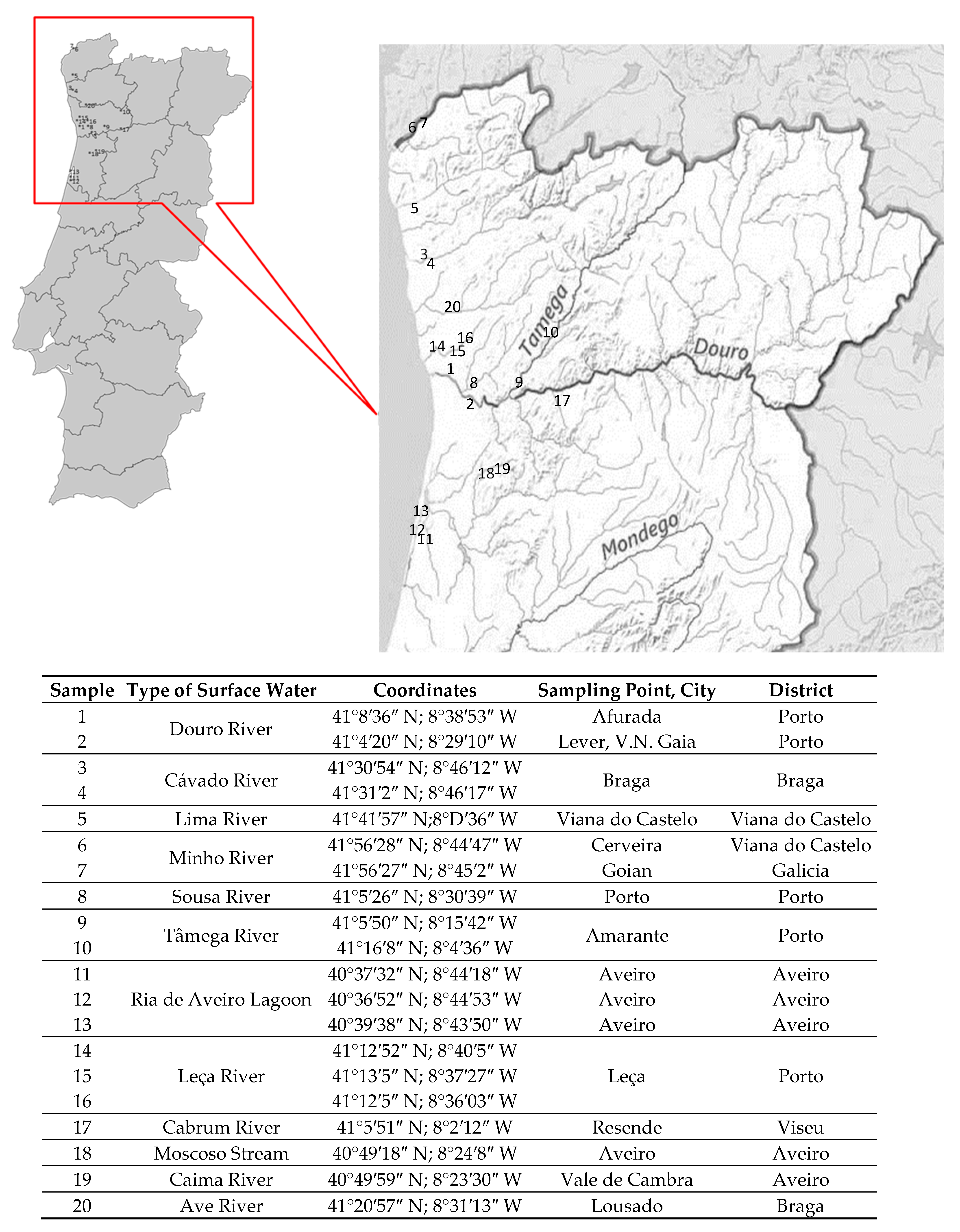
2.2. Site Selection and Sampling
2.3. Description of the Surface Water Samples (Rivers, Lagoon, and Stream)
2.4. Extraction Procedure
2.4.1. Conditioning and Cleaning Procedure
2.4.2. Sample Enrichment
2.4.3. Optimization of the SPME Process
2.5. Gas Chromatography
3. Results and Discussion
3.1. Optimization of the SPME Procedure
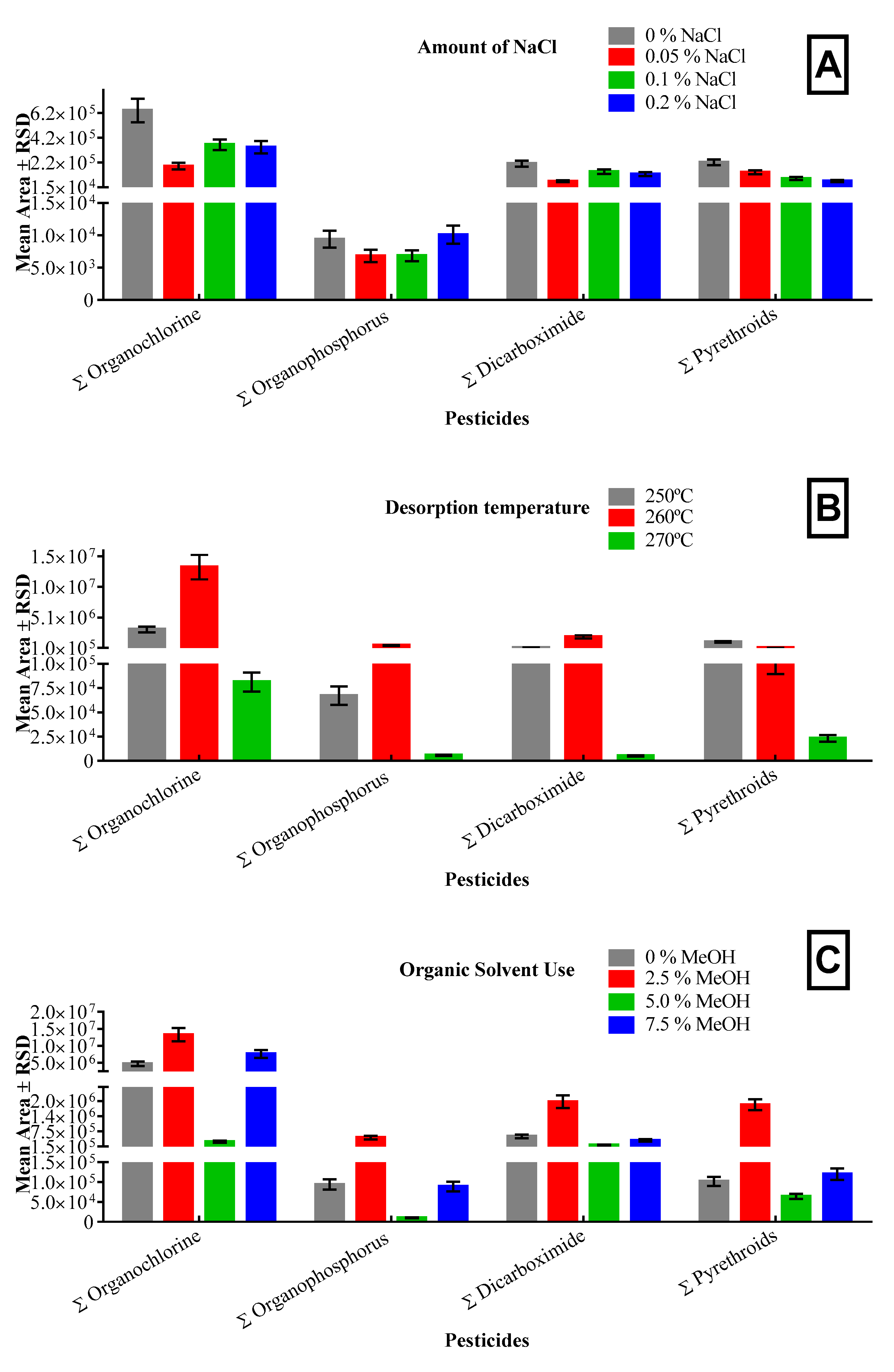
3.1.1. Ionic Strength Adjustments
3.1.2. Desorption Temperature
3.1.3. Organic Solvent
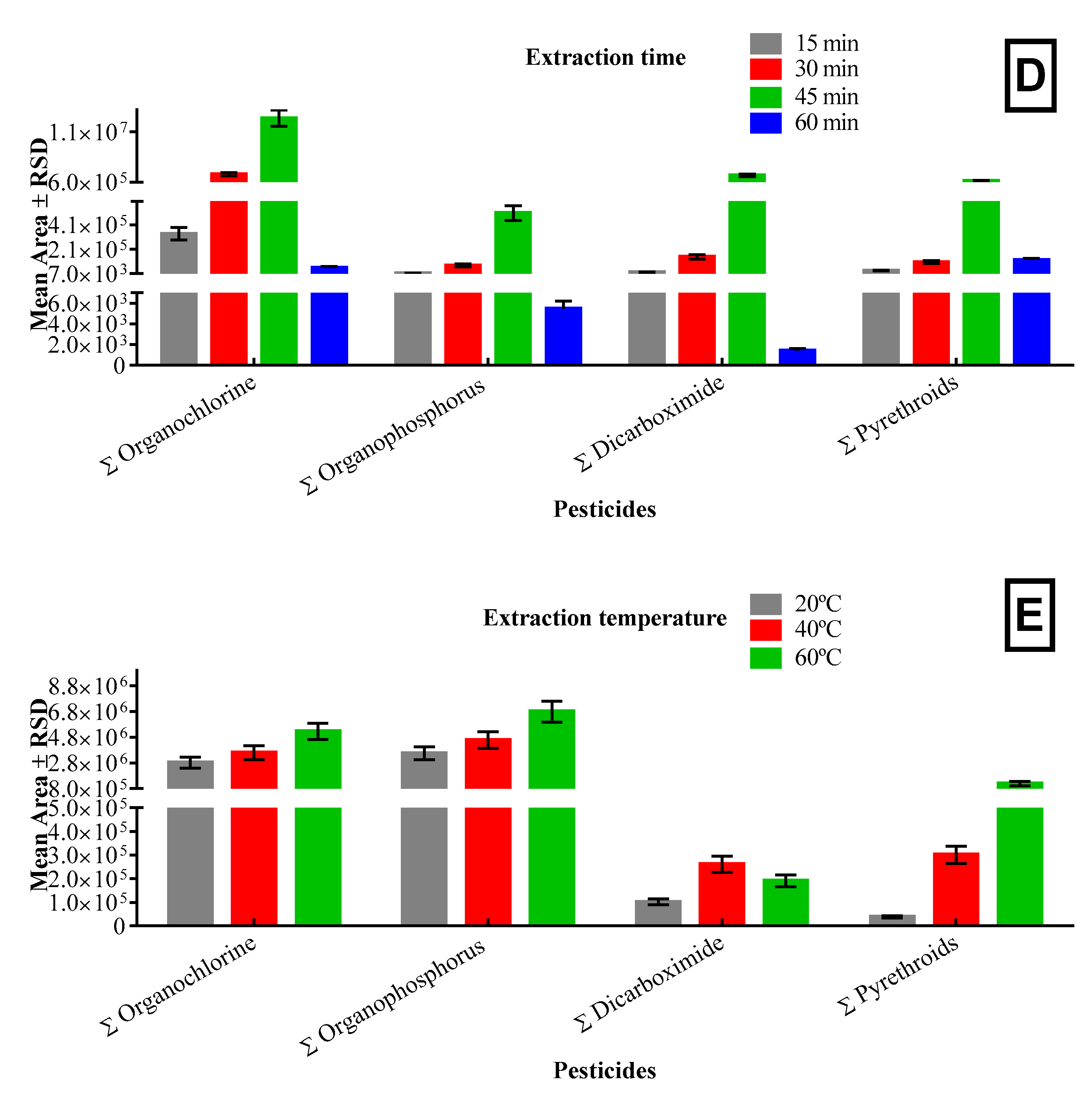
3.1.4. Extraction Temperature and Time
3.2. Method Validation
3.3. Monitoring of 20 Known or Suspected Endocrine-Disrupting Pesticides in Portuguese Surface Water Samples
3.3.1. Organochlorine Pesticides in Surface Water Samples
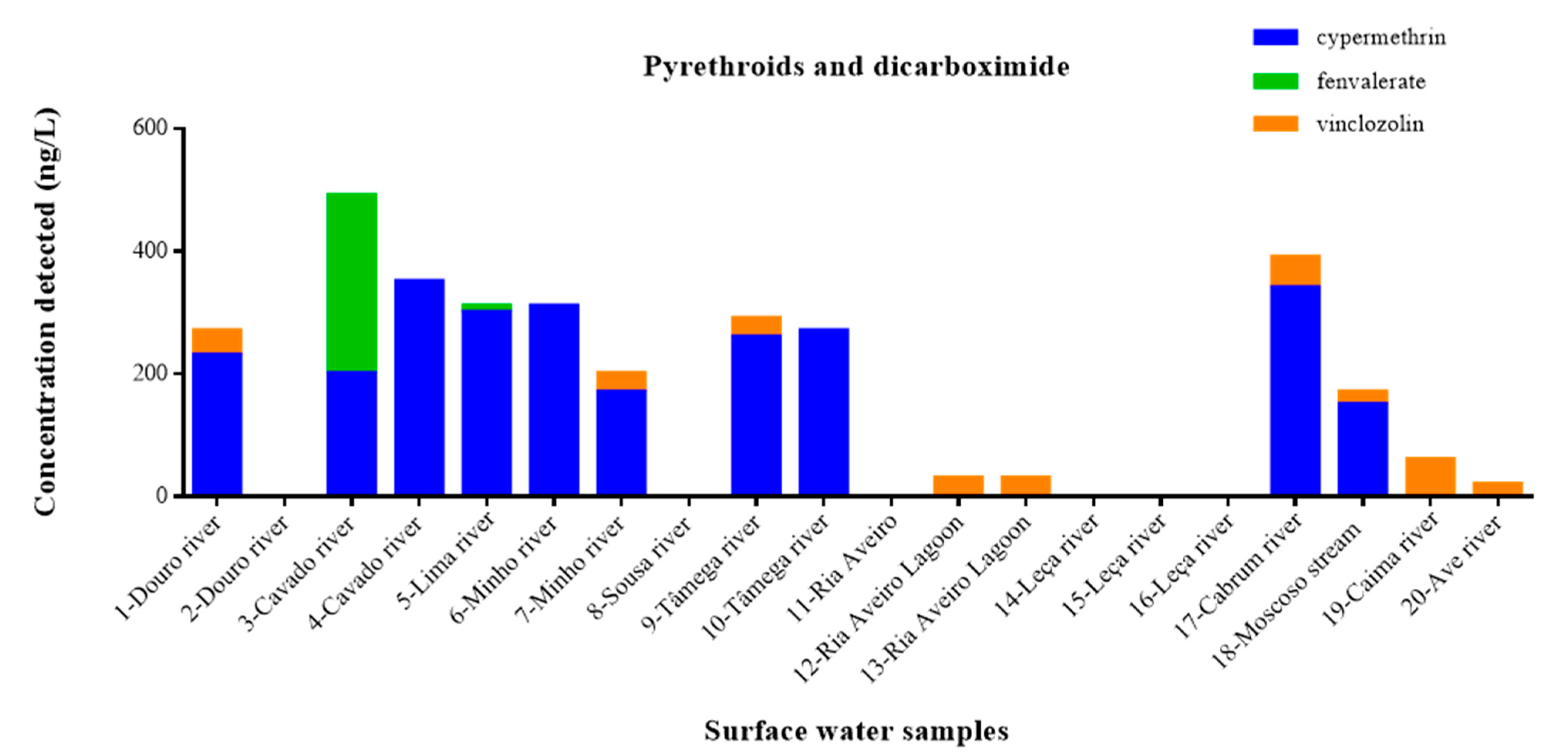
3.3.2. Pyrethroids and Dicarboximide Pesticides in Portuguese Surface Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lauretta, R.; Sansone, A.; Sansone, M.; Romanelli, F.; Appetecchia, M. Endocrine Disrupting Chemicals: Effects on Endocrine Glands. Front. Endocrinol. 2019, 10, 178. [Google Scholar] [CrossRef]
- Kassotis, C.D.; Vandenberg, L.N.; Demeneix, B.A.; Porta, M.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Economic, regulatory, and policy implications. Lancet Diabetes Endocrinol. 2020, 8, 719–730. [Google Scholar] [CrossRef]
- Özkara, A.; Akyil, D.; Konuk, M. Pesticides, Environmental Pollution, and Health; InTech: London, UK, 2016. [Google Scholar]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.C.; de Souza, A.O.; Bernardes, M.F.F.; Pazin, M.; Tasso, M.J.; Pereira, P.H.; Dorta, D.J. A perspective on the potential risks of emerging contaminants to human and environmental health. Environ. Sci. Pollut. Res. 2015, 22, 13800–13823. [Google Scholar] [CrossRef] [PubMed]
- Kasonga, T.K.; Coetzee, M.A.A.; Kamika, I.; Ngole-Jeme, V.M.; Benteke Momba, M.N. Endocrine-disruptive chemicals as contaminants of emerging concern in wastewater and surface water: A review. J. Environ. Manag. 2021, 277, 111485. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Megha, P.; Sreedev, P. Review Article. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef]
- Chrustek, A.; Hołyńska-Iwan, I.; Dziembowska, I.; Bogusiewicz, J.; Wróblewski, M.; Cwynar, A.; Olszewska-Słonina, D. Current Research on the Safety of Pyrethroids Used as Insecticides. Medicina 2018, 54, 61. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101–1136. [Google Scholar] [CrossRef]
- Sagiv, S.K.; Bruno, J.L.; Baker, J.M.; Palzes, V.; Kogut, K.; Rauch, S.; Gunier, R.; Mora, A.M.; Reiss, A.L.; Eskenazi, B. Prenatal exposure to organophosphate pesticides and functional neuroimaging in adolescents living in proximity to pesticide application. Proc. Natl. Acad. Sci. USA 2019, 116, 18347. [Google Scholar] [CrossRef] [PubMed]
- Brander, S.M.; Gabler, M.K.; Fowler, N.L.; Connon, R.E.; Schlenk, D. Pyrethroid pesticides as endocrine disruptors: Molecular mechanisms in vertebrates with a focus on fishes. Environ. Sci. Technol. 2016, 50, 8977–8992. [Google Scholar] [CrossRef] [PubMed]
- Nasuti, C.; Carloni, M.; Fedeli, D.; Gabbianelli, R.; Di Stefano, A.; Serafina, C.L.; Silva, I.; Domingues, V.; Ciccocioppo, R. Effects of early life permethrin exposure on spatial working memory and on monoamine levels in different brain areas of pre-senescent rats. Toxicology 2013, 303, 162–168. [Google Scholar] [CrossRef]
- Yang, C.; Lim, W.; Song, G. Mediation of oxidative stress toxicity induced by pyrethroid pesticides in fish. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2020, 234, 108758. [Google Scholar] [CrossRef]
- Hecker, M.; Hollert, H. Endocrine Disruptor Screening: Regulatory Perspectives and Needs. Environ. Sci. Eur. 2011, 23, 15. [Google Scholar] [CrossRef]
- European Union. Directive 2013/39/EU of the European Parliament and of the Council. Off. J. Eur. Union 2013, 1–17. [Google Scholar]
- European Union. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off. J. 1998, 32–54. [Google Scholar]
- Ouyang, G.; Pawliszyn, J. SPME in environmental analysis. Anal. Bioanal. Chem. 2006, 386, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Albaseer, S.S.; Nageswara Rao, R.; Swamy, Y.V.; Mukkanti, K. An overview of sample preparation and extraction of synthetic pyrethroids from water, sediment and soil. J. Chromatogr. A 2010, 1217, 5537–5554. [Google Scholar] [CrossRef] [PubMed]
- Merkle, S.; Kleeberg, K.; Fritsche, J. Recent Developments and Applications of Solid Phase Microextraction (SPME) in Food and Environmental Analysis—A Review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef]
- Locatelli, M.; Sciascia, F.; Cifelli, R.; Malatesta, L.; Bruni, P.; Croce, F. Analytical methods for the endocrine disruptor compounds determination in environmental water samples. J. Chromatogr. A 2016, 1434, 1–18. [Google Scholar] [CrossRef]
- Alam, M.N.; Pawliszyn, J. Effect of Binding Components in Complex Sample Matrices on Recovery in Direct Immersion Solid-Phase Microextraction: Friends or Foe? Anal. Chem. 2018, 90, 2430–2433. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.M.; dos Santos, F.N.; Pereira, P.A.d.P. Development, validation and application of a method based on DI-SPME and GC–MS for determination of pesticides of different chemical groups in surface and groundwater samples. Microchem. J. 2010, 96, 139–145. [Google Scholar] [CrossRef]
- Jabali, Y.; Millet, M.; El-Hoz, M. Optimization of a DI-SPME-GC–MS/MS method for multi-residue analysis of pesticides in waters. Microchem. J. 2019, 147, 83–92. [Google Scholar] [CrossRef]
- Olisah, C.; Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Occurrence and risk evaluation of organochlorine contaminants in surface water along the course of Swartkops and Sundays River Estuaries, Eastern Cape Province, South Africa. Environ. Geochem. Health 2019, 41, 2777–2801. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, T.A.; Qureshi, R.; Tipre, D.; Menon, S. Investigation of pesticide residues in water, sediments and fish samples from Tapi River, India as a case study and its forensic significance. Environ. Forensics 2020, 21, 1–10. [Google Scholar] [CrossRef]
- Fernandes, V.C.; Domingues, V.F.; Mateus, N.; Delerue-Matos, C. Organochlorine Pesticide Residues in Strawberries from Integrated Pest Management and Organic Farming. J. Agric. Food Chem. 2011, 59, 7582–7591. [Google Scholar] [CrossRef]
- Álvarez-Muñoz, D.; Rodríguez-Mozaz, S.; Jacobs, S.; Serra-Compte, A.; Cáceres, N.; Sioen, I.; Verbeke, W.; Barbosa, V.; Ferrari, F.; Fernández-Tejedor, M.; et al. Pharmaceuticals and endocrine disruptors in raw and cooked seafood from European market: Concentrations and human exposure levels. Environ. Int. 2018, 119, 570–581. [Google Scholar] [CrossRef]
- Correia-Sá, L.; Fernandes, V.C.; Carvalho, M.; Calhau, C.; Domingues, V.F.; Delerue-Matos, C. Optimization of QuEChERS method for the analysis of organochlorine pesticides in soils with diverse organic matter. J. Sep. Sci. 2012, 35, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Bio, A.; Guimarães, L.; Fernandes, V.C.; Delerue-Matos, C.; Vieira, N. Assessing the ecological status of fluvial ecosystems employing a macroinvertebrate multi-taxon and multi-biomarker approach. Environ. Monit. Assess. 2019, 191, 503. [Google Scholar] [CrossRef]
- Carvalho, P.N.; Rodrigues, P.N.R.; Basto, M.C.P.; Vasconcelos, M.T.S.D. Organochlorine pesticides levels in Portuguese coastal areas. Chemosphere 2009, 75, 595–600. [Google Scholar] [CrossRef]
- Rodrigues, E.T.; Alpendurada, M.F.; Guimarães, A.; Avó, R.; Ferreira, B.; Pardal, M.A. The environmental condition of an estuarine ecosystem disturbed by pesticides. Environ. Sci. Pollut. Res. 2019, 26, 24075–24087. [Google Scholar] [CrossRef]
- Pinto, M.I.; Burrows, H.D.; Sontag, G.; Vale, C.; Noronha, J.P. Priority pesticides in sediments of European coastal lagoons: A review. Mar. Pollut. Bull. 2016, 112, 6–16. [Google Scholar] [CrossRef]
- Álvarez-Muñoz, D.; Rodríguez-Mozaz, S.; Maulvault, A.L.; Tediosi, A.; Fernández-Tejedor, M.; van den Heuvel, F.; Kotterman, M.; Marques, A.; Barceló, D. Occurrence of pharmaceuticals and endocrine disrupting compounds in macroalgaes, bivalves, and fish from coastal areas in Europe. Environ. Res. 2015, 143, 56–64. [Google Scholar] [CrossRef]
- Silva, E.; Daam, M.A.; Cerejeira, M.J. Aquatic risk assessment of priority and other river basin specific pesticides in surface waters of Mediterranean river basins. Chemosphere 2015, 135, 394–402. [Google Scholar] [CrossRef]
- Cruzeiro, C.; Amaral, S.; Rocha, E.; Rocha, M.J. Determination of 54 pesticides in waters of the Iberian Douro River estuary and risk assessment of environmentally relevant mixtures using theoretical approaches and Artemia salina and Daphnia magna bioassays. Ecotoxicol. Environ. Saf. 2017, 145, 126–134. [Google Scholar] [CrossRef]
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Ribeiro, C.; Tiritan, M.E.; Pereira, M.F.R.; Silva, A.M.T. Monitoring of the 17 EU Watch List contaminants of emerging concern in the Ave and the Sousa Rivers. Sci. Total Environ. 2019, 649, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Cruzeiro, C.; Pardal, M.; Rocha, E.; Rocha, M.J. Occurrence and seasonal loads of pesticides in surface water and suspended particulate matter from a wetland of worldwide interest—The Ria Formosa Lagoon, Portugal. Environ. Monit. Assess. 2015, 187, 669. [Google Scholar] [CrossRef]
- Cruzeiro, C.; Rocha, E.; Pardal, M.Â.; Rocha, M.J. Uncovering seasonal patterns of 56 pesticides in surface coastal waters of the Ria Formosa lagoon (Portugal), using a GC-MS method. Int. J. Environ. Anal. Chem. 2015, 95, 1370–1384. [Google Scholar] [CrossRef]
- Mansilha, C.; Melo, A.; Ferreira, I.M.P.L.V.O.; Pinho, O.; Domingues, V.; Pinho, C.; Gameiro, P. Groundwater from Infiltration Galleries Used for Small Public Water Supply Systems: Contamination with Pesticides and Endocrine Disruptors. Bull. Environ. Contam. Toxicol. 2011, 87, 312–318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vieira, M.E.C.; Bordalo, A.A. The Douro estuary (Portugal): A mesotidal salt wedge. Oceanol. Acta 2000, 23, 585–594. [Google Scholar] [CrossRef]
- Gomes, A.I.; Pires, J.C.M.; Figueiredo, S.A.; Boaventura, R.A.R. Optimization of River Water Quality Surveys by Multivariate Analysis of Physicochemical, Bacteriological and Ecotoxicological Data. Water Resour. Manag. 2014, 28, 1345–1361. [Google Scholar] [CrossRef]
- Costa-Dias, S.; Freitas, V.; Sousa, R.; Antunes, C. Factors influencing epibenthic assemblages in the Minho Estuary (NW Iberian Peninsula). Mar. Pollut. Bull. 2010, 61, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Vilar, V.J.P.; Alves, P.; Boaventura, R.A.R.; Botelho, C. Water quality in Minho/Miño River (Portugal/Spain). Environ. Monit. Assess. 2013, 185, 3269–3281. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.M.R.; Mucha, A.P.; Teresa Vasconcelos, M. Role of different salt marsh plants on metal retention in an urban estuary (Lima estuary, NW Portugal). Estuar. Coast. Shelf Sci. 2011, 91, 243–249. [Google Scholar] [CrossRef]
- Alves, C.M.; Boaventura, R.R.A.R.; Soares, H.M.V.M. Evaluation of heavy metals pollution loadings in the sediments of the Ave river basin (Portugal). Soil Sediment. Contam. 2009, 18, 603–618. [Google Scholar] [CrossRef]
- Pato, P.; Lopes, C.; Válega, M.; Lillebø, A.I.; Dias, J.M.; Pereira, E.; Duarte, A.C. Mercury fluxes between an impacted coastal lagoon and the Atlantic Ocean. Estuar. Coast. Shelf Sci. 2008, 76, 787–796. [Google Scholar] [CrossRef]
- Nunes, M.L.; Ferreira Da Silva, E.; de Almeida, S.F.P. Assessment of water quality in the Caima and Mau River basins (Portugal) using geochemical and biological Indices. Water Air Soil Pollut. 2003, 149, 227–250. [Google Scholar] [CrossRef]
- Cruzeiro, C.; Rocha, E.; Pardal, M.Â.; Rocha, M.J. Environmental assessment of pesticides in the Mondego River Estuary (Portugal). Mar. Pollut. Bull. 2016, 103, 240–246. [Google Scholar] [CrossRef]
- Waszak, I.; Dabrowska, H.; Komar-Szymczak, K. Comparison of common persistent organic pollutants (POPs) in flounder (Platichthys flesus) from the Vistula (Poland) and Douro (Portugal) River estuaries. Mar. Pollut. Bull. 2014, 81, 225–233. [Google Scholar] [CrossRef]
- Yang, D.; Qi, S.; Zhang, J.; Wu, C.; Xing, X. Organochlorine pesticides in soil, water and sediment along the Jinjiang River mainstream to Quanzhou Bay, southeast China. Ecotoxicol. Environ. Saf. 2013, 89, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, J.; Hildebrandt, A.; Martínez, E.; Lacorte, S.; Morillo, E.; Maqueda, C.; Viana, P.; Barceló, D. Priority pesticides and their degradation products in river sediments from Portugal. Sci. Total Environ. 2008, 390, 507–513. [Google Scholar] [CrossRef]
- Fernandes, V.C.; Lehotay, S.J.; Geis-Asteggiante, L.; Kwon, H.; Mol, H.G.; van der Kamp, H.; Mateus, N.; Domingues, V.F.; Delerue-Matos, C. Analysis of pesticide residues in strawberries and soils by GC-MS/MS, LC-MS/MS and two-dimensional GC-time-of-flight MS comparing organic and integrated pest management farming. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2014, 31, 262–270. [Google Scholar] [CrossRef]
- Pestana, D.; Fernandes, V.; Faria, G.; Sa, C.; Meireles, M.; Monteiro, R.; Domingues, V.; Calhau, C. Persistent organic pollutant (POPs) levels in human visceral and subcutaneous adipose tissue on an obese Portuguese population-Metabolic improvement after bariatric surgery versus POPs burden. Toxicol. Lett. 2011, 205, S75–S76. [Google Scholar] [CrossRef]
- Martins, J.; Esteves, C.; Limpo-Faria, A.; Barros, P.; Ribeiro, N.; Simões, T.; Correia, M.; Delerue-Matos, C. Analysis of six fungicides and one acaricide in still and fortified wines using solid-phase microextraction-gas chromatography/tandem mass spectrometry. Food Chem. 2012, 132, 630–636. [Google Scholar] [CrossRef]


| Compounds * | Time Retention (min) | SIM Ions (Q1, Q2 and Q3) (m/z) | GC Segment (min) |
|---|---|---|---|
| α-HCH | 9.66 | 181, 219, 109 | 8.50–9.90 |
| HCB | 9.82 | 284, 142, 249 | 8.50–9.90 |
| diazinon | 10.04 | 179, 199, 304 | 9.90–10.20 |
| β-HCH | 10.36 | 181, 219, 109 | 10.20–11.20 |
| lindane | 11.04 | 181, 219, 109 | 10.20–11.20 |
| vinclozolin | 11.38 | 212, 124, 187 | 11.20–11.85 |
| aldrin | 12.47 | 263, 293, 66 | 11.85–13.00 |
| α-endosulfan | 14.89 | 241, 195, 209 | 14.10–15.00 |
| p,p′-DDE | 15.54 | 246, 176, 318 | 15.00–16.00 |
| dieldrin | 15.68 | 79, 263, 277 | 15.00–16.00 |
| endrin | 16.35 | 244, 263, 317 | 16.00–17.00 |
| o,p′-DDT | 16.74 | 235, 165, 81 | 16.00–17.00 |
| p,p′-DDD | 17.21 | 235, 165, 199 | 17.00–18.00 |
| β-endosulfan | 17.30 | 195, 335, 339 | 17.00–18.00 |
| bifenthrin | 19.51 | 165, 166, 141 | 18.00–24.00 |
| methoxychlor | 20.19 | 227, 152, 165 | 18.00–24.00 |
| iprodione | 20.32 | 187, 244 | 18.00–24.00 |
| cypermethrin | 31.72, 31.98, 32.44 | 163, 181, 91 | 24.00–35.00 |
| fenvalerate | 38.59, 40.63 | 125, 167, 419 | 35.00–43.00 |
| deltamethrin | 46.62 | 181, 253 | 43.00–50.00 |
| Groups | Pesticides | Coefficient of Determination | LOD ng/L | LOQ ng/L |
|---|---|---|---|---|
| Organochlorines | α-HCH | 0.9912 | 25.0 | 75.0 |
| β-HCH | 0.9991 | 60.0 | 180.0 | |
| Lindane | 0.9991 | 75.0 | 225.0 | |
| HCB | 0.9978 | 2.5 | 7.5 | |
| aldrin | 0.9983 | 2.4 | 7.2 | |
| dieldrin | 0.9976 | 0.7 | 2.1 | |
| endrin | 0.9978 | 12.0 | 36.0 | |
| p,p′-DDE | 0.9957 | 0.3 | 0.9 | |
| o,p′-DDT | 0.9904 | 1.8 | 5.4 | |
| p,p′-DDD | 0.9935 | 5.3 | 15.9 | |
| α-endosulfan | 0.9991 | 5.9 | 17.7 | |
| β-endosulfan | 0.9977 | 3.0 | 9.0 | |
| metoxyclhor | 0.9923 | 1.6 | 4.8 | |
| Pyrethroids | bifenthrin | 0.9925 | 7.0 | 21.0 |
| cypermethrin | 0.9976 | 30.0 | 900.0 | |
| fenvalerate | 0.9917 | 3.3 | 9.9 | |
| deltamethrin | 0.9939 | 150.0 | 450.0 | |
| Organophosphorus | diazinon | 0.9990 | 3.1 | 9.3 |
| Dicarboximide | Iprodione | 0.9990 | 27.3 | 91.0 |
| vinclozolin | 0.9994 | 0.2 | 0.6 |
| Group | Compounds | Frequency (%) | Min ng/L | Max ng/L | Mean ng/L |
|---|---|---|---|---|---|
| Organochlorines | Σ(α-HCH + β-HCH + lindane) | 30 | <LOQ | 1800 | 460 |
| HCB | 45 | <LOQ | 912 | 201 | |
| Σ(aldrin + dieldrin + endrin) | 45 | 10 | 430 | 142 | |
| Σ(p,p′-DDE + o,p′-DDT + p,p′-DDD) | 40 | <LOQ | 70 | 63 | |
| Σ(α-endosulfan + β-endosulfan) | 75 | <LOQ | 30 | 11 | |
| methoxychlor | 5 | nd | 31 | 31 | |
| Pyrethroids | bifenthrin | 10 | nd | <LOQ | - |
| cypermethrin | 55 | <LOQ | 351 | 261 | |
| fenvalerate | 10 | 10 | 294 | 150 | |
| deltamethrin | 5 | nd | <LOQ | - | |
| Dicarboximide | vinclozolin | 45 | 20 | 62 | 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera, J.; Fernandes, V.C.; Correia-Sá, L.; Mansilha, C.; Delerue-Matos, C.; Domingues, V.F. Occurrence of Selected Known or Suspected Endocrine-Disrupting Pesticides in Portuguese Surface Waters Using SPME-GC-IT/MS. Separations 2021, 8, 81. https://doi.org/10.3390/separations8060081
Vera J, Fernandes VC, Correia-Sá L, Mansilha C, Delerue-Matos C, Domingues VF. Occurrence of Selected Known or Suspected Endocrine-Disrupting Pesticides in Portuguese Surface Waters Using SPME-GC-IT/MS. Separations. 2021; 8(6):81. https://doi.org/10.3390/separations8060081
Chicago/Turabian StyleVera, José, Virgínia Cruz Fernandes, Luísa Correia-Sá, Catarina Mansilha, Cristina Delerue-Matos, and Valentina F. Domingues. 2021. "Occurrence of Selected Known or Suspected Endocrine-Disrupting Pesticides in Portuguese Surface Waters Using SPME-GC-IT/MS" Separations 8, no. 6: 81. https://doi.org/10.3390/separations8060081
APA StyleVera, J., Fernandes, V. C., Correia-Sá, L., Mansilha, C., Delerue-Matos, C., & Domingues, V. F. (2021). Occurrence of Selected Known or Suspected Endocrine-Disrupting Pesticides in Portuguese Surface Waters Using SPME-GC-IT/MS. Separations, 8(6), 81. https://doi.org/10.3390/separations8060081








