Green Stability Indicating Organic Solvent-Free HPLC Determination of Remdesivir in Substances and Pharmaceutical Dosage Forms
Abstract
:1. Introduction
2. Materials and Methods
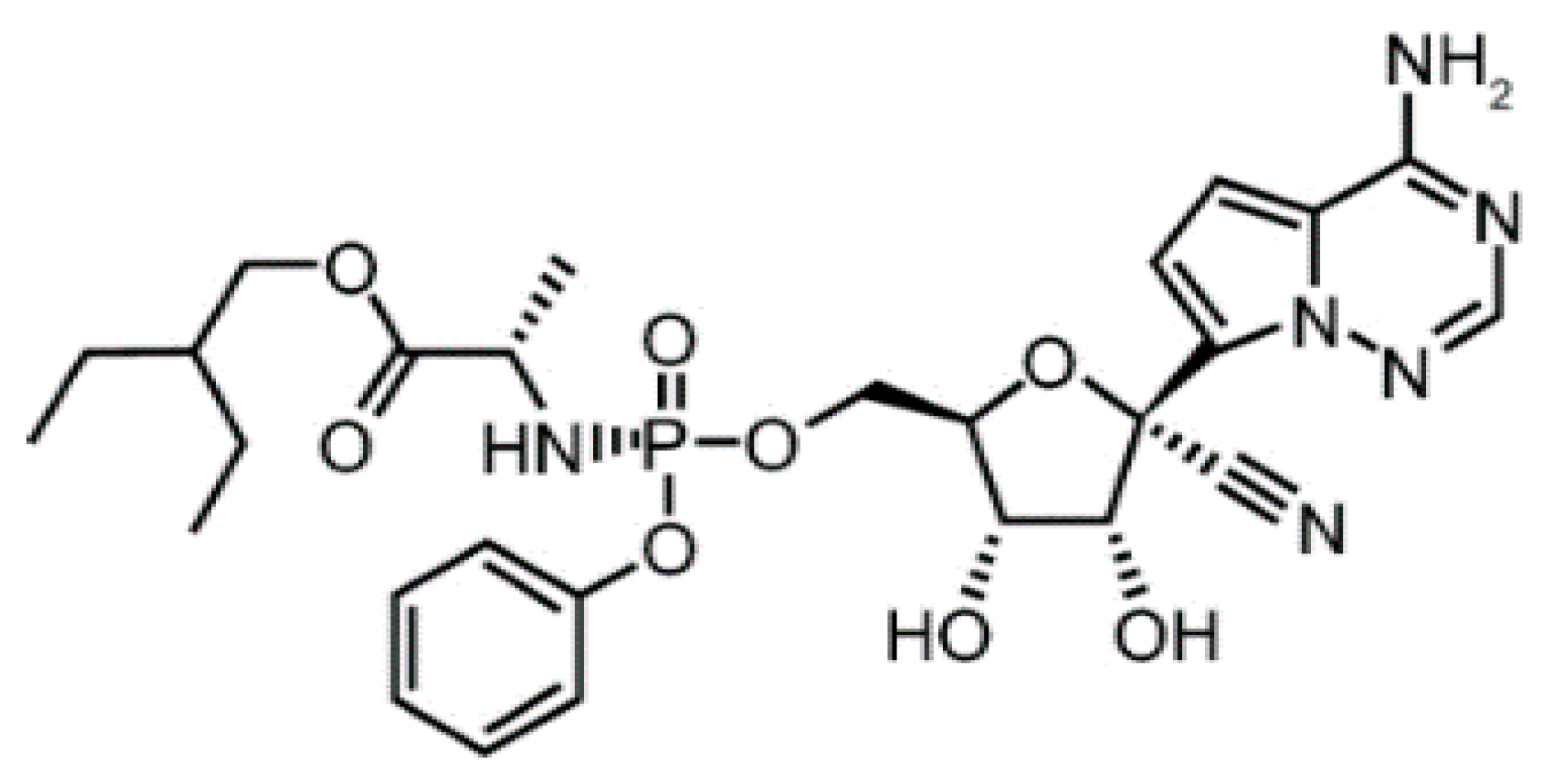
2.1. Materials
2.2. Instrumentation
2.3. Chromatographic Separation Conditions
2.4. Preparation of Stock Solutions, Standard Solutions and Pharmaceutical Dosage Form
2.5. Drug Forced Degradation
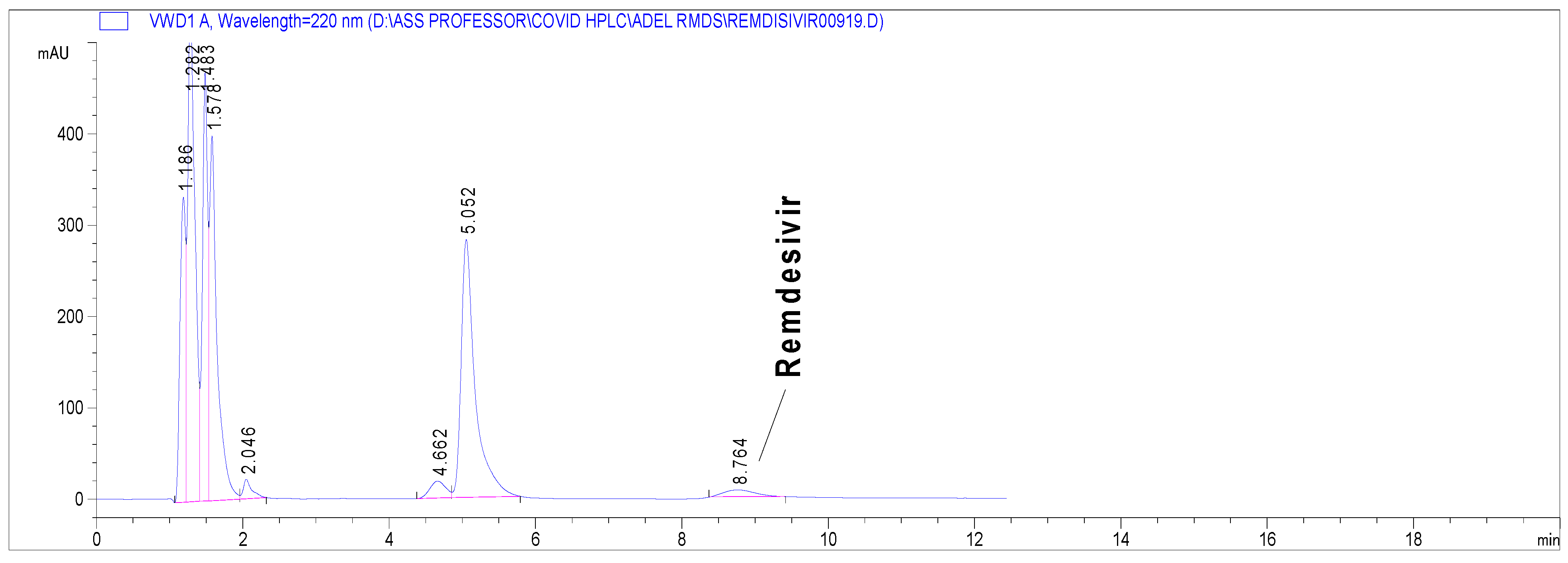
3. Results
3.1. Analytical Method Validation
3.2. Stability Indicating Capability of the Proposed Method
3.3. Method Application
3.4. Comparison and Evaluation to Previously Reported Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Scavone, C.; Brusco, S.; Bertini, M.; Sportiello, L.; Rafaniello, C.; Zoccoli, A.; Berrino, L.; Racagni, G.; Rossi, F.; Capuano, A. Current pharmacological treatments for COVID-19: What’s next? Br. J. Pharmacol. 2020, 177, 4813–4824. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.N.; Feldmann, F.; Schwarz, B.; Meade-White, K.; Porter, D.P.; Schulz, J.; Van Doremalen, N.; Leighton, I.; Yinda, C.K.; Pérez-Pérez, L. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 2020, 585, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Remdesivir: First Approval. Drugs 2020, 80, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Humeniuk, R.; Mathias, A.; Cao, H.; Osinusi, A.; Shen, G.; Chng, E.; Ling, J.; Vu, A.; German, P. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects. Clin. Transl. Sci. 2020, 13, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Górecki, T. Green Analytical Chemistry: Past, Present and Perspectives; Płotka-Wasylka, J., Namieśnik, J., Eds.; Springer: Singapore, 2019; pp. 241–298. [Google Scholar]
- Sayed, R.A.; Ibrahim, A.E.; Sharaf, Y.A. Chemometry-assisted UV-spectrophotmetric methods for the simultaneous determination of paritaprevir, ritonavir, and ombitasvir in their combined tablet dosage forms: A comparative study. J. Chemom. 2021, 35, e3339. [Google Scholar] [CrossRef]
- Duan, X.; Liu, X.; Dong, Y.; Duan, T.; Zhang, J.; He, S.; Yang, F.; Dong, Y. A Mixed Micellar Liquid Chromatography with Direct Injection for Rapid Analysis of Eight Sulfonamides in Milk. Food Anal. Methods 2020, 13, 1148–1158. [Google Scholar] [CrossRef]
- Alwera, V.; Sehlangia, S.; Alwera, S. Micellar liquid chromatographic green enantioseparation of racemic amino alcohols and determination of elution order. Biomed. Chromatogr. 2020, 34, e4954. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.E.; Elmansi, H.; Belal, F. Solvent-free mixed micellar mobile phases; an advanced green chemistry approach for reversed phase HPLC determination of some antihypertensive drugs. J. Sep. Sci. 2020, 43, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chang, L.; Ke, C.; Xie, Y.; Shen, J.; Tan, B.; Liu, J. Challenges and stepwise fit-for-purpose optimization for bioanalyses of remdesivir metabolites nucleotide monophosphate and triphosphate in mouse tissues using LC–MS/MS. J. Pharm. Biomed. Anal. 2021, 194, 113806. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.-C.; Moine, P.; Etting, I.; Annane, D.; Larabi, I.A. Quantification of plasma remdesivir and its metabolite GS-441524 using liquid chromatography coupled to tandem mass spectrometry. Application to a Covid-19 treated patient. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, R.R.; Tsai, P.-C.; Ponnusamy, V.K.; Pugazhendhi, A. Rapid determination of remdesivir (SARS-CoV-2 drug) in human plasma for therapeutic drug monitoring in COVID-19-Patients. Process Biochem. 2021, 102, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Avataneo, V.; de Nicolò, A.; Cusato, J.; Antonucci, M.; Manca, A.; Palermiti, A.; Waitt, C.; Walimbwa, S.; Lamorde, M.; di Perri, G.; et al. Development and validation of a UHPLC-MS/MS method for quantification of the prodrug remdesivir and its metabolite GS-441524: A tool for clinical pharmacokinetics of SARS-CoV-2/COVID-19 and Ebola virus disease. J. Antimicrob. Chemother. 2020, 75, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Habler, K.; Brügel, M.; Teupser, D.; Liebchen, U.; Scharf, C.; Schönermarck, U.; Vogeser, M.; Paal, M. Simultaneous quantification of seven repurposed COVID-19 drugs remdesivir (plus metabolite GS-441524), chloroquine, hydroxychloroquine, lopinavir, ritonavir, favipiravir and azithromycin by a two-dimensional isotope dilution LC–MS/MS method in human serum. J. Pharm. Biomed. Anal. 2021, 196, 113935. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; John Ling, K.H.; Tarnowski, T.; Humeniuk, R.; German, P.; Mathias, A.; Chu, J.; Chen, Y.-S.; van Ingen, E. Validation of LC-MS/MS methods for determination of remdesivir and its metabolites GS-441524 and GS-704277 in acidified human plasma and their application in COVID-19 related clinical studies. Anal. Biochem. 2021, 617, 114118. [Google Scholar] [CrossRef] [PubMed]
- Tkach, V.V.; Kushnir, M.; de Oliveira, S.C.; Ivanushko, J.; Velyka, A.V.; Molodianu, A.F.; Yagodynets, P.I.; Kormosh, Z.O.; Vaz dos Reis, L.; Luganska, O.V. Theoretical Description for Anti-COVID-19 Drug Remdesivir Electrochemical Determination, Assisted by Squaraine Dye–Ag2O2 Composite. Biointerface Res. Appl. Chem. 2021, 11, 9201–9208. [Google Scholar]
- Blessy, M.; Patel, R.D.; Prajapati, P.N.; Agrawal, Y.K. Development of forced degradation and stability indicating studies of drugs—A review. J. Pharm. Anal. 2014, 4, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guideline. International Conference on Harmonization; Validation of Analytical Procedures, ICH Q2 (R1); ICH: Geneva, Switzerland, 2005; pp. 11–12. [Google Scholar]
- Guideline. International Conference on Harmonisation of Technical Requirements for the Registration of Pharmaceuticals for Human Use; ICH: Geneva, Switzerland, 2003. [Google Scholar]
- Taleuzzaman, M.; Ali, S.; Gilani, S.J.; Imam, S.S.; Hafeez, A. Ultra performance liquid chromatography (UPLC)-a review. Austin. J. Anal. Pharm. Chem. 2015, 2, 1056–1060. [Google Scholar]
- Ibrahim, A.E.; Elmaaty, A.A.; El-Sayed, H.M. Determination of six drugs used for treatment of common cold by micellar liquid chromatography. Anal. Bioanal. Chem. 2021, 413, 5051–5065. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Result |
|---|---|
| Retention time (min ± SD) | 8.5 ± 0.4 |
| Number of theoretical plates | 9550 m−1 |
| Symmetry factor | 0.83 |
| Linearity range (μg mL−1) | 5.0–100.0 |
| Linearity equation | Y = 64.4 X − 42.5 |
| Correlation coefficient (r) | 0.999 |
| LOD (S/N ratio = 3) | 0.5 μg mL−1 |
| LOQ (S/N ratio = 10) | 2.0 μg mL−1 |
| Concentration (µg mL−1) | Accuracy * | Inter-Day Precision * | Intra-Day Precision * |
|---|---|---|---|
| 10.0 | 102.6 ± 0.4 | 101.8 ± 1.4 | 101.3 ± 1.7 |
| 25.0 | 100.3 ± 1.7 | 100.2 ± 0.9 | 100.1 ± 0.6 |
| 50.0 | 97.8 ± 0.6 | 98.4 ± 1.3 | 98.5 ± 1.1 |
| 75.0 | 101.9 ± 2.4 Mean; 100.7 ± 2.0 | 102.5 ± 1.4 | 101.6 ± 1.9 |
| Proposed Method * | Reference Method * | t-Test | F-Test |
|---|---|---|---|
| 100.8 ± 1.4 | 101.4 ± 0.8 | 0.531 (2.776) | 3.213 (19) |
| Proposed Method | Reported Method [11] | Reported Method [12] | Reported Method [14] | Reported Method [13] | Reported Method [15] | |
|---|---|---|---|---|---|---|
| Technique | HPLC-UV | UHPLC-MS/MS | HPLC-MS/MS | UHPLC-MS/MS | UHPLC-MS/MS | UHPLC-MS/MS |
| Matrix; Application | Bulk powder; Drug QC | Mouse tissue, Clinical studies | Plasma; Clinical studies | Plasma; Clinical studies | Plasma; Clinical studies | Plasma; Clinical studies |
| Linearity range | 5–100 µg mL−1 | 6.0–60260 ng mL−1 | 1–5000 ng mL−1 | 1–1000 ng mL−1 | 1–5000 ng mL−1 | 85–5450 ng mL−1 |
| LOD | 500 ng mL−1 | 0.3 ng mL−1 | 0.3 ng mL−1 | Not available | 0.24 ng mL−1 | Not available |
| LOQ | 2000 ng mL−1 | 1.0 ng mL−1 | 1.0 ng mL−1 | Not available | 0.98 ng mL−1 | 4.0 ng mL−1 |
| Organic phase | Nil | ACN | ACN | ACN | ACN | Methanol-based |
| Run time (min) | 9.0 | 6.5 | 5.0 | 4.0 | 10.0 | 5.0 |
| Column | C18 (5 μm, 150 × 4.6 mm) | Anion exchange BioBasic AX column (2.1 μm, 50 × 4.6 mm) | C18 (2.6 μm, 100 × 2.1 mm) | C18 (1.8 μm, 50 × 2.1 mm) | C18 (3 μm, 150 × 4.6 mm) | Separation on C18 (MassTox® TDM column) after solid phase extraction |
| Mobile phase | Isocratic; | Gradient; | Gradient; | Gradient; | Isocratic; | Gradient; |
| Mixed mode of Brij and SLS. | Ammonium acetate buffer and ACN | Sodium formate buffer and ACN | 0.05% formic acid and ACN | 0.05% formic acid: ACN (52:48) | MassTox® series A mobile phase | |
| Flow; 1 mL min−1 | Flow: 0.6 mL min−1 | Flow; 0.5 mL min−1 | Flow; 0.4 mL min−1 | Flow; 0.5 mL min−1 | Flow; 0.6 mL min−1 | |
| GAPI assessment | 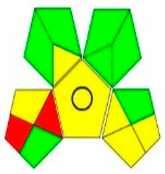 | 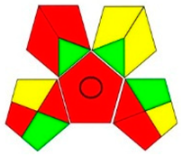 | 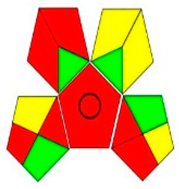 | 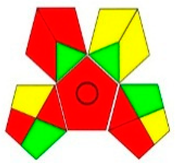 | 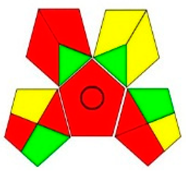 | 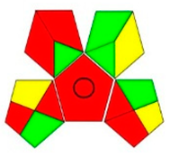 |
| AGREE assessment | 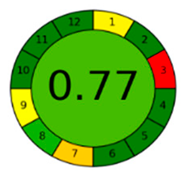 | 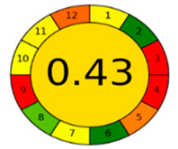 |  | 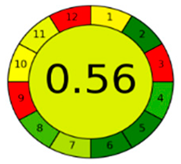 |  | 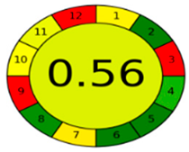 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.E.; Deeb, S.E.; Abdelhalim, E.M.; Al-Harrasi, A.; Sayed, R.A. Green Stability Indicating Organic Solvent-Free HPLC Determination of Remdesivir in Substances and Pharmaceutical Dosage Forms. Separations 2021, 8, 243. https://doi.org/10.3390/separations8120243
Ibrahim AE, Deeb SE, Abdelhalim EM, Al-Harrasi A, Sayed RA. Green Stability Indicating Organic Solvent-Free HPLC Determination of Remdesivir in Substances and Pharmaceutical Dosage Forms. Separations. 2021; 8(12):243. https://doi.org/10.3390/separations8120243
Chicago/Turabian StyleIbrahim, Adel Ehab, Sami El Deeb, Emad Mahmoud Abdelhalim, Ahmed Al-Harrasi, and Rania Adel Sayed. 2021. "Green Stability Indicating Organic Solvent-Free HPLC Determination of Remdesivir in Substances and Pharmaceutical Dosage Forms" Separations 8, no. 12: 243. https://doi.org/10.3390/separations8120243
APA StyleIbrahim, A. E., Deeb, S. E., Abdelhalim, E. M., Al-Harrasi, A., & Sayed, R. A. (2021). Green Stability Indicating Organic Solvent-Free HPLC Determination of Remdesivir in Substances and Pharmaceutical Dosage Forms. Separations, 8(12), 243. https://doi.org/10.3390/separations8120243








