Evaluation of New, Sputtered Carbon SPME Fibers with a Multi-Functional Group Test Mixture
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fiber Preparation
2.2.1. Sputtered Carbon Coatings on Fused Silica Fibers
2.2.2. PDMS Deposition on Sputtered Carbon Coatings
2.3. HS/DI–SPME–GC–MS Analysis
3. Results and Discussion
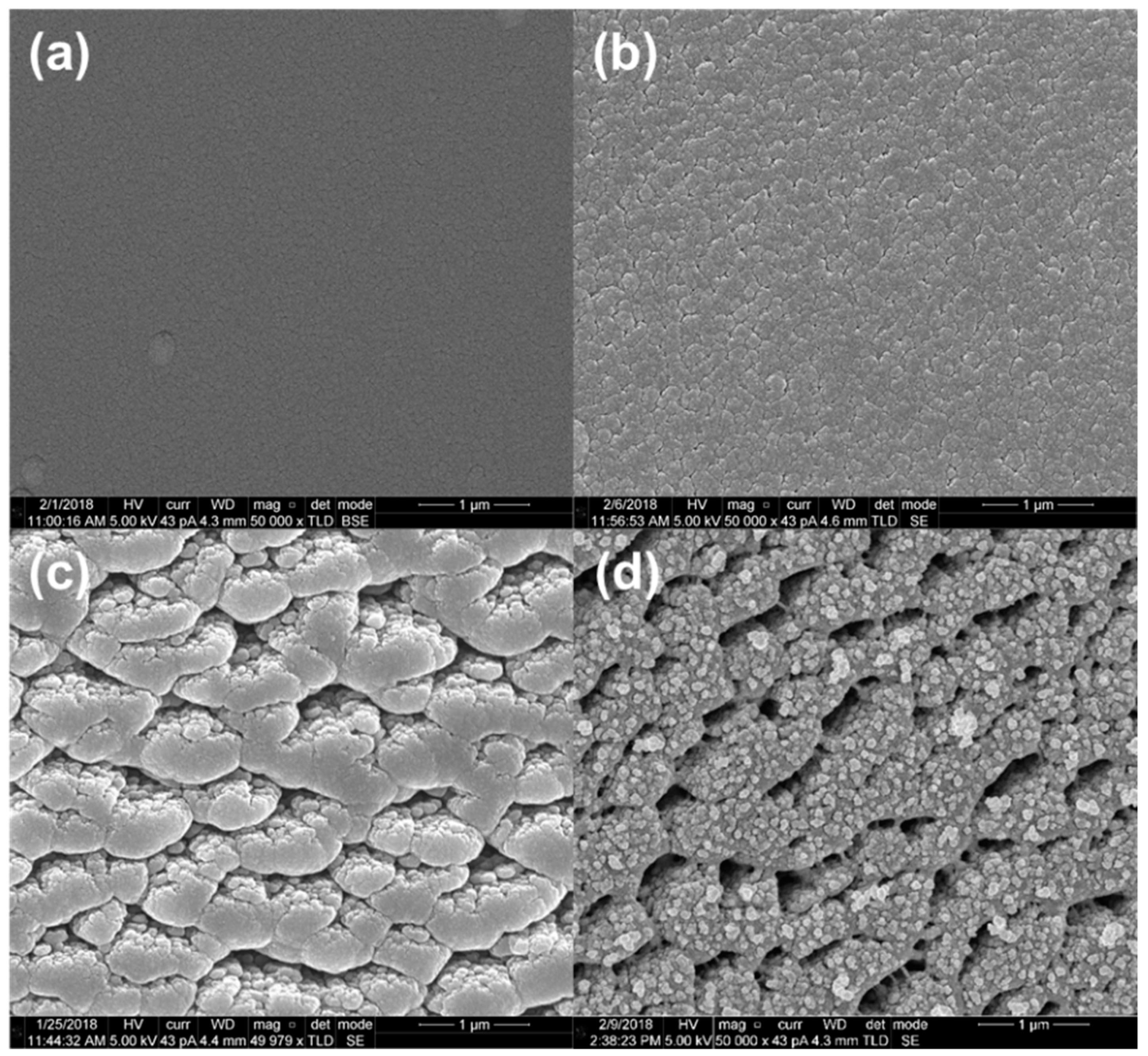
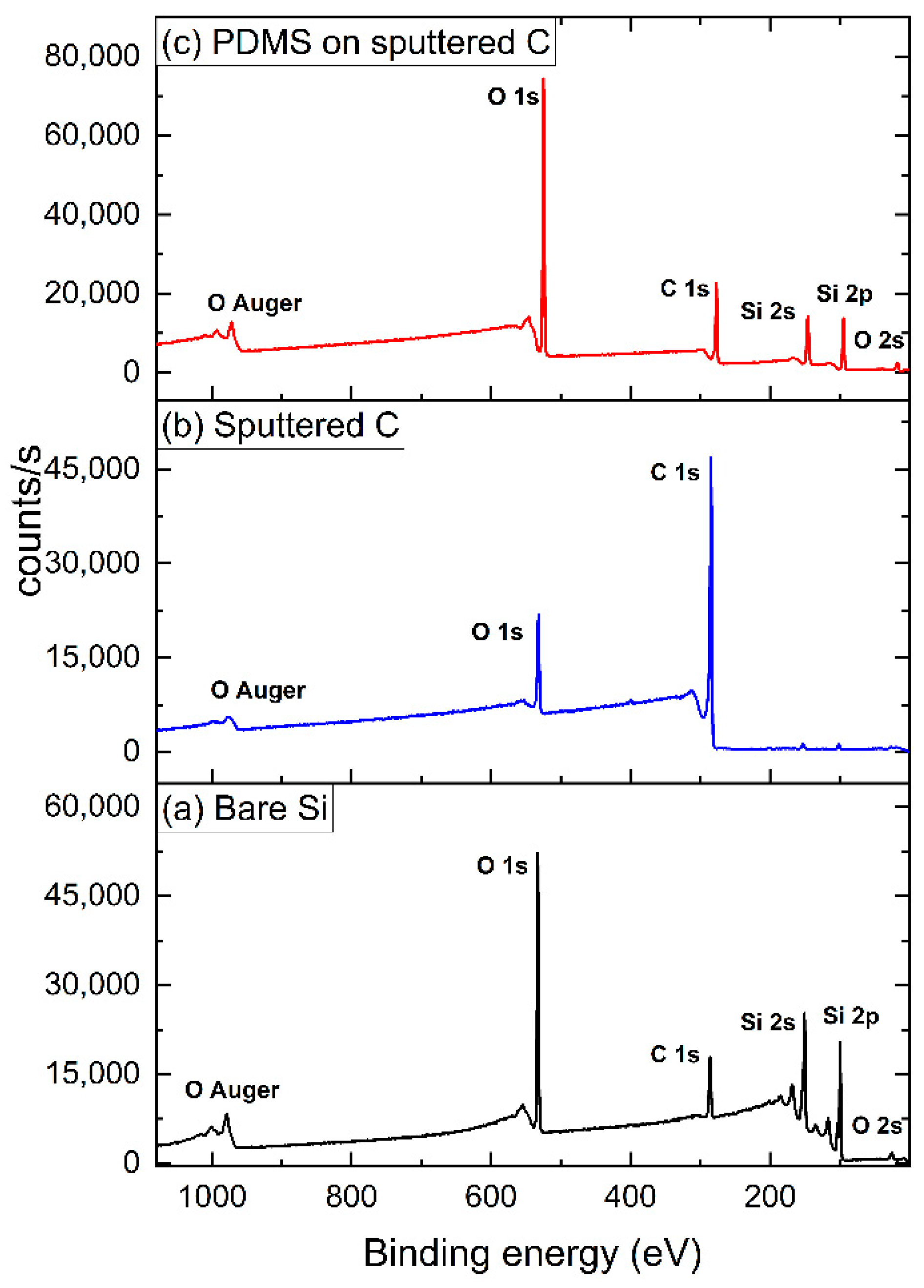
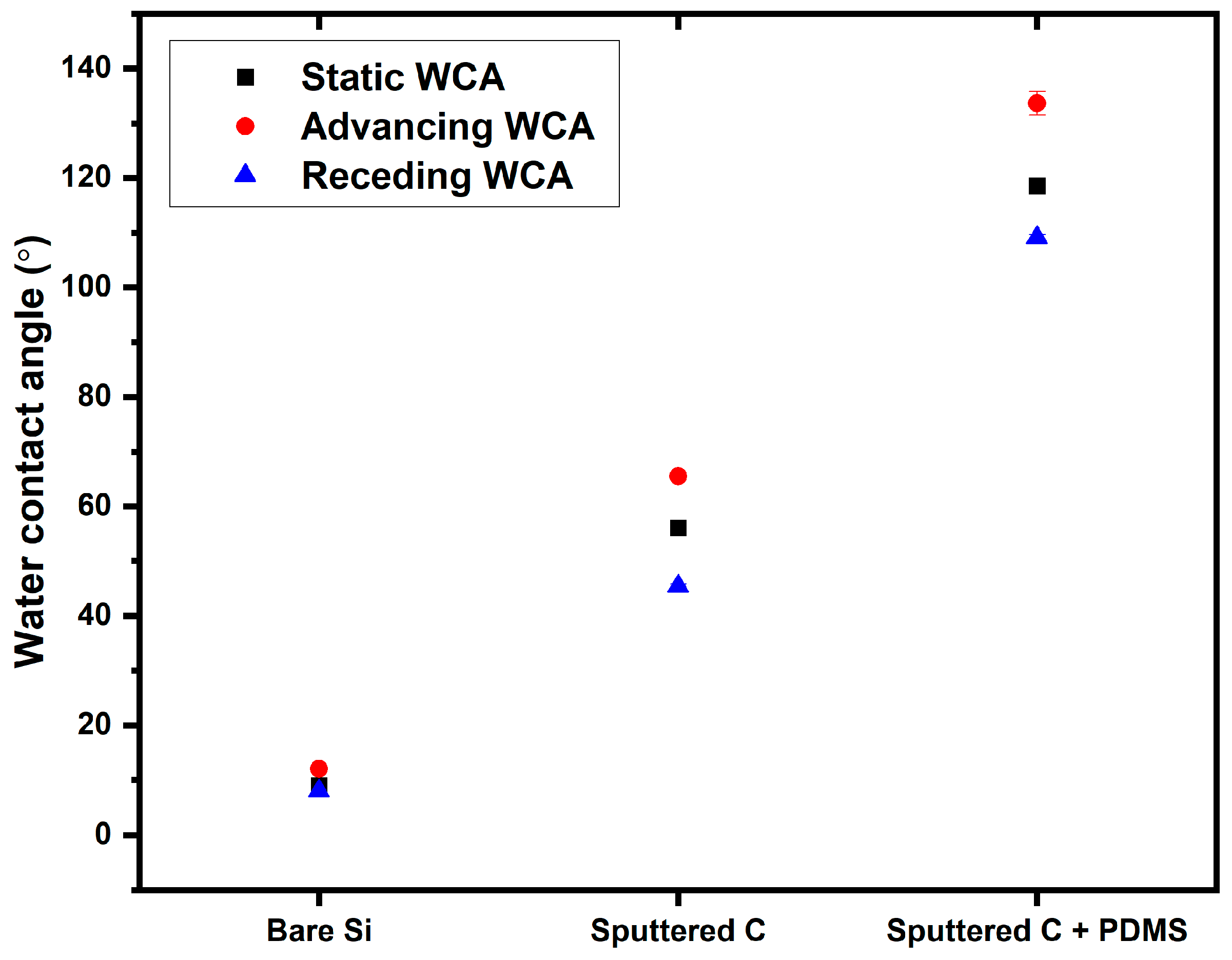
3.1. Material Characterization
3.2. HS–SPME–GC–MS Evaluation of Sputtered Carbon Fibers with Commercially Available Test Mixes
3.3. New SPME Evaluation Mix
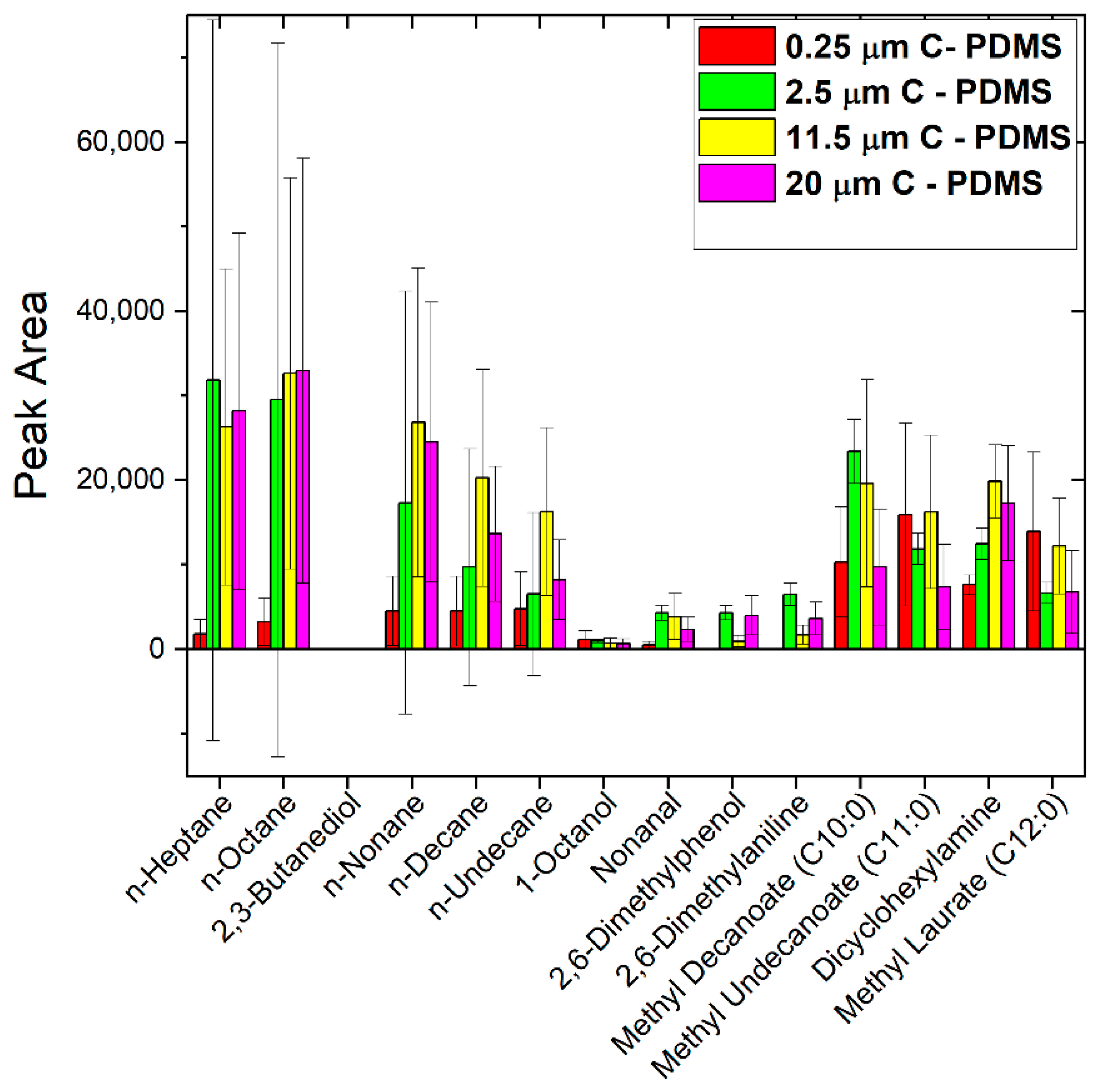
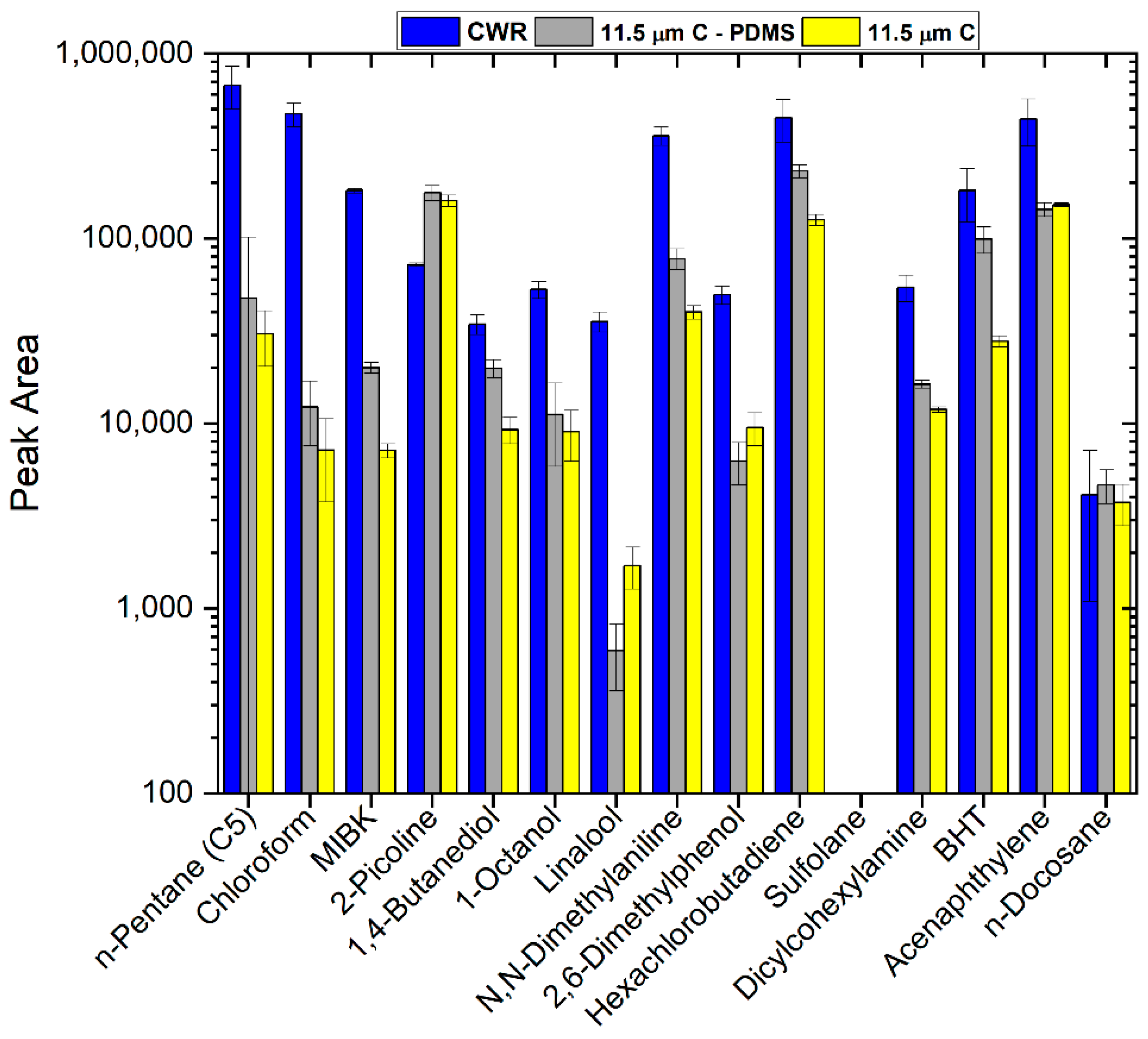
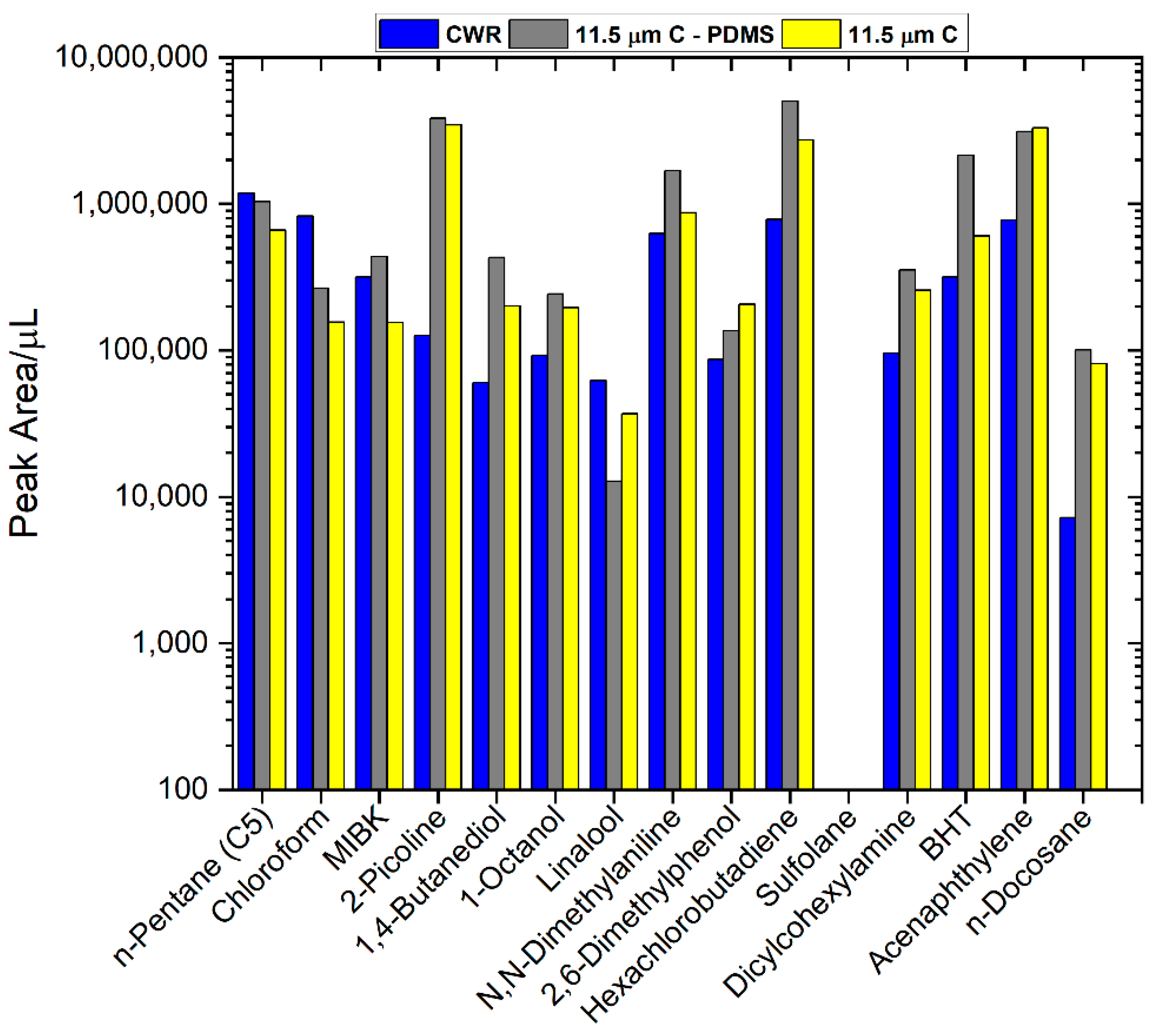
3.4. Comparison of Commercial CWR and Sputtered Carbon SPME Fibers with Our New Test Mix
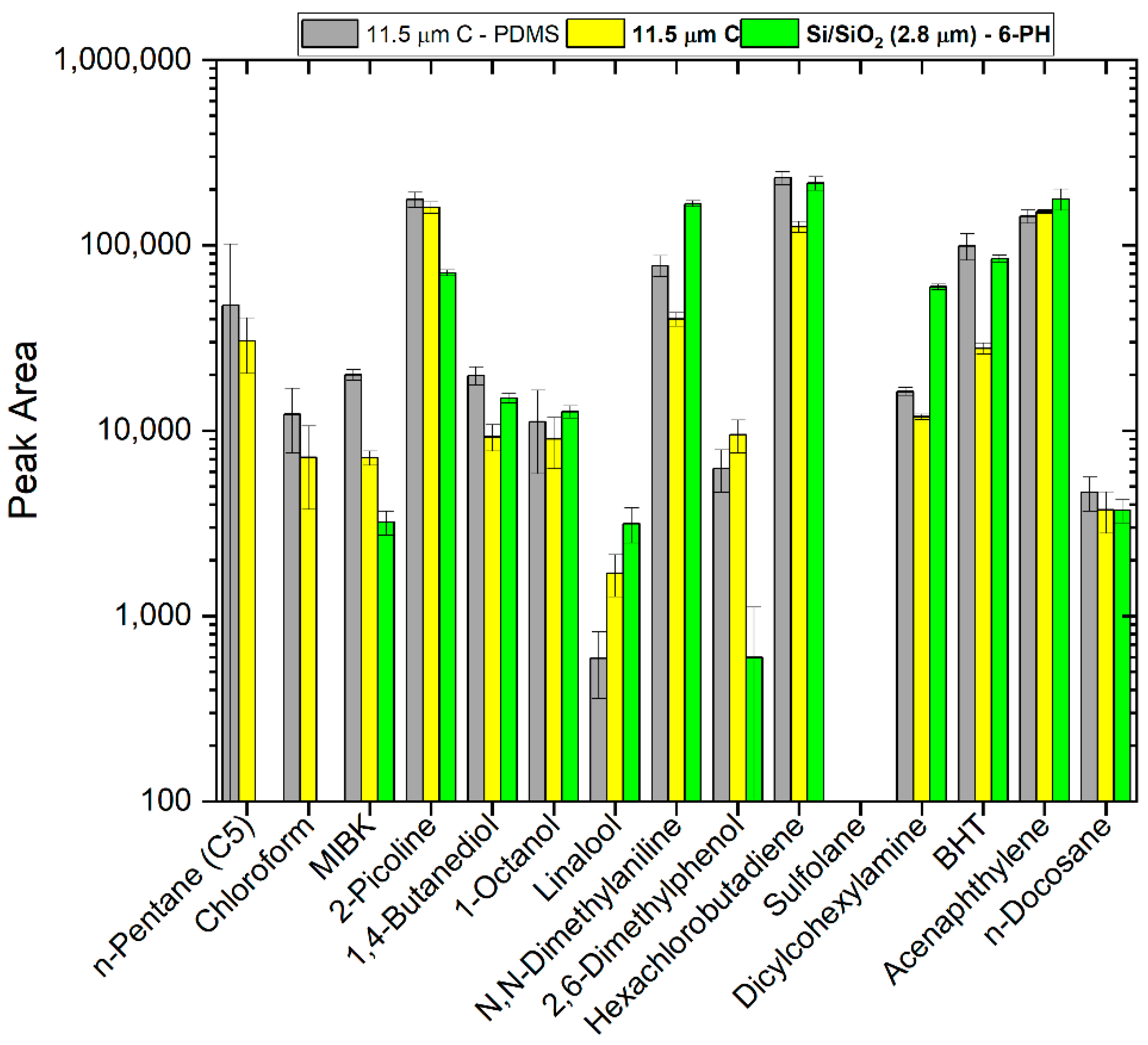
3.5. Comparison of Sputtered Carbon and Silicon SPME Fibers with Our New Test Mix
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Benfenati, E.; Müller, L.; Perani, L.; Pierucci, P. Analysis of industrial pollutants in environmental samples. In Applications of Solid Phase Microextraction; Pawliszyn, J., Ed.; The Royal Society of Chemistry: London, UK, 1999; pp. 333–346. [Google Scholar]
- Khaled, A.; Belinato, J.R.; Pawliszyn, J. Rapid and high-throughput screening of multi-residue pharmaceutical drugs in bovine tissue using solid phase microextraction and direct analysis in real time-tandem mass spectrometry (SPME-DART-MS/MS). Talanta 2020, 217, 121095. [Google Scholar] [CrossRef]
- Vuckovic, D.; Zhang, X.; Cudjoe, E.; Pawliszyn, J. Solid-phase microextraction in bioanalysis: New devices and directions. J. Chrom. A 2010, 1217, 4041–4060. [Google Scholar] [CrossRef]
- Meng, L.; Dai, Y.; Chen, C.; Zhang, J. Determination of amphetamines, ketamine and their metabolites in hair with high-speed grinding and solid-phase microextraction followed by LC-MS. Forensic Sci. Res. 2021, 6, 273–280. [Google Scholar] [CrossRef]
- Merkle, S.; Kleeberg, K.K.; Fritsche, J. Recent Developments and Applications of Solid Phase Microextraction (SPME) in Food and Environmental Analysis—A Review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef] [Green Version]
- Łyczko, J.; Jałoszyński, K.; Surma, M.; Masztalerz, K.; Szumny, A. HS-SPME Analysis of True Lavender (Lavandula angustifolia Mill.) Leaves Treated by Various Drying Methods. Molecules 2019, 24, 764. [Google Scholar] [CrossRef] [Green Version]
- Azenha, M.A.; Nogueira, P.J.; Silva, A.F. Unbreakable solid-phase microextraction fibers obtained by sol-gel deposition on titanium wire. Anal. Chem. 2006, 78, 2071–2074. [Google Scholar] [CrossRef]
- Mehdinia, A.; Aziz-Zanjani, M.O. Recent advances in nanomaterials utilized in fiber coatings for solid-phase microextraction. TrAC-Trend Anal. Chem. 2013, 42, 205–215. [Google Scholar] [CrossRef]
- Koster, E.H.M.; Crescenzi, C.; Den Hoedt, W.; Ensing, K.; De Jong, G.J. Fibers coated with molecularly imprinted polymers for solid-phase microextraction. Anal. Chem. 2001, 73, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Rocío-Bautista, P.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Are metal-organic frameworks able to provide a new generation of solid-phase microextraction coatings?—A review. Anal. Chim. Acta 2016, 939, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-F.; Li, N.; Jiang, G.-B.; Liu, J.-M.; Jönsson, J.Å.; Wen, M.-J. Disposable ionic liquid coating for headspace solid-phase microextraction of benzene, toluene, ethylbenzene, and xylenes in paints followed by gas chromatography–flame ionization detection. J. Chrom. A 2005, 1066, 27–32. [Google Scholar] [CrossRef]
- Ji, J.; Liu, H.; Chen, J.; Zeng, J.; Huang, J.; Gao, L.; Wang, Y.; Chen, X. ZnO nanorod coating for solid phase microextraction and its applications for the analysis of aldehydes in instant noodle samples. J. Chrom. A 2012, 1246, 22–27. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Li, G. Fabrication of novel nanoporous array anodic alumina solid-phase microextraction fiber coating and its potential application for headspace sampling of biological volatile organic compounds. Anal. Chim. Acta 2012, 727, 13–19. [Google Scholar] [CrossRef]
- Ghaemi, F.; Amiri, A.; Yunus, R. Methods for coating solid-phase microextraction fibers with carbon nanotubes. TrAC Trends Anal. Chem. 2014, 59, 133–143. [Google Scholar] [CrossRef]
- Chen, J.; Zou, J.; Zeng, J.; Song, X.; Ji, J.; Wang, Y.; Ha, J.; Chen, X. Preparation and evaluation of graphene-coated solid-phase microextraction fiber. Anal. Chim. Acta 2010, 678, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Z.; Yang, X.; Wang, C.; Wang, Z. Fabrication of a three-dimensional graphene coating for solid-phase microextraction of polycyclic aromatic hydrocarbons. RSC Adv. 2015, 5, 54329–54337. [Google Scholar] [CrossRef]
- Diwan, A.; Singh, B.; Roychowdhury, T.; Yan, D.; Tedone, L.; Nesterenko, P.N.; Paull, B.; Sevy, E.T.; Shellie, R.A.; Kaykhaii, M.; et al. Porous, high capacity coatings for solid phase microextraction by sputtering. Anal. Chem. 2016, 88, 1593–1600. [Google Scholar] [CrossRef]
- Patel, D.I.; Roychowdhury, T.; Shah, D.; Jacobsen, C.; Herrington, J.S.; Hoisington, J.; Myers, C.; Salazar, B.G.; Walker, A.V.; Bell, D.S.; et al. 6-Phenylhexyl silane derivatized, sputtered silicon solid phase microextraction fiber for the parts-per-trillion detection of polyaromatic hydrocarbons in water and baby formula. J. Sep. Sci. 2021, 44, 2824–2836. [Google Scholar] [CrossRef]
- Roychowdhury, T.; Patel, D.I.; Shah, D.; Diwan, A.; Kaykhaii, M.; Herrington, J.S.; Bell, D.S.; Linford, M.R. Sputtered silicon solid phase microextraction fibers with a polydimethylsiloxane stationary phase with negligible carry-over and phase bleed. J. Chrom. A 2020, 1623, 461065. [Google Scholar] [CrossRef]
- Harris, K.D.; Brett, M.J.; Smy, T.J.; Backhouse, C. Microchannel surface area enhancement using porous thin films. J. Electrochem. Soc. 2000, 147, 2002–2006. [Google Scholar] [CrossRef]
- Wu, A.T.; Brett, M.J. Sensing humidity using nanostructured SiO posts: Mechanism and optimization. Sens. Mater. 2001, 13, 399–431. [Google Scholar]
- Smith, D.O.; Cohen, M.S.; Weiss, G.P. Oblique-Incidence Anisotropy in Evaporated Permalloy Films. J. Appl. Phys. 1960, 31, 1755–1762. [Google Scholar] [CrossRef]
- Dick, B.; Brett, M.J.; Smy, T. Controlled growth of periodic pillars by glancing angle deposition. J. Vac. Sci. Technol. B: Microelectron. Nanometer Struct. Process. Meas. Phenom. 2003, 21, 23–28. [Google Scholar] [CrossRef]
- Robbie, K.; Brett, M.J. Sculptured thin films and glancing angle deposition: Growth mechanics and applications. J. Vac. Sci. Technol. A 1997, 15, 1460–1465. [Google Scholar] [CrossRef]
- Zhu, F.; Guo, J.; Zeng, F.; Fu, R.; Wu, D.; Luan, T.; Tong, Y.; Lu, T.; Ouyang, G. Preparation and characterization of porous carbon material-coated solid-phase microextraction metal fibers. J. Chrom. A 2010, 1217, 7848–7854. [Google Scholar] [CrossRef]
- Wang, X.; Lu, M.; Wang, H.; Huang, P.; Ma, X.; Cao, C.; Du, X. Three-dimensional graphene aerogel–mesoporous carbon composites as novel coatings for solid-phase microextraction for the efficient enrichment of brominated flame retardants. New J. Chem. 2016, 40, 6308–6314. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, M.; Zhang, G.; Zang, X.; Li, H.; Zhang, S.; Wang, C.; Wang, Z. Carbon nanospheres as solid-phase microextraction coating for the extraction of polycyclic aromatic hydrocarbons from water and soil samples. J. Sep. Sci. 2020, 43, 2594–2601. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, C.; Li, J.; Luo, R.; Jiang, H.; Sun, X.; Shen, J.; Han, W.; Wang, L. Hollow mesoporous carbon spheres-based fiber coating for solid-phase microextraction of polycyclic aromatic hydrocarbons. J. Chrom. A 2017, 1520, 58–64. [Google Scholar] [CrossRef]
- Kovats, E. Characterization of organic compounds by gas chromatography. Part 1. Retention indices of aliphatic halides, alcohols, aldehydes and ketones. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Kováts, E. Gas-chromatographische Charakterisierung organischer Verbindungen. Teil 1: Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Mc Reynolds, W.O. Characterization of Some Liquid Phases. J. Chrom. Sci. 1970, 8, 685–691. [Google Scholar] [CrossRef]
- Grob, K.; Grob, K., Jr. Splitless injection and the solvent effect. J. High. Res. Chrom. 1978, 156, 1–20. [Google Scholar] [CrossRef]
- Khaled, A.; Pawliszyn, J. Time-weighted average sampling of volatile and semi-volatile airborne organic compounds by the solid-phase microextraction device. J. Chrom. A 2000, 892, 445–467. [Google Scholar] [CrossRef]
- Kremser, A.; Jochmann, M.A.; Schmidt, T.C. PAL SPME Arrow—Evaluation of a novel solid-phase microextraction device for freely dissolved PAHs in water. Anal. Bioanal. Chem. 2016, 408, 943–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, C.; Herrington, J.S.; Hamrah, P.; Anderson, K. Accelerated Solvent Extraction of Terpenes in Cannabis Coupled With Various Injection Techniques for GC-MS Analysis. Front. Chem. 2021, 9, 2. [Google Scholar] [CrossRef]
- Koziel, J.A.; Martos, P.A.; Pawliszyn, J. System for the generation of standard gas mixtures of volatile and semi-volatile organic compounds for calibrations of solid-phase microextraction and other sampling devices. J. Chrom. A 2004, 1025, 3–9. [Google Scholar] [CrossRef]
- Wang, Y.; O’Reilly, J.; Chen, Y.; Pawliszyn, J. Equilibrium in-fibre standardisation technique for solid-phase microextraction. J. Chrom. A 2005, 1072, 13–17. [Google Scholar] [CrossRef]
- Stefanuto, P.-H.; Perrault, K.A.; Dubois, L.M.; L’Homme, B.; Allen, C.; Loughnane, C.; Ochiai, N.; Focant, J.-F. Advanced method optimization for volatile aroma profiling of beer. J. Chrom. A 2017, 1507, 45–52. [Google Scholar] [CrossRef]
- Eckert, K.E.; Carter, D.O.; Perrault, K.A. Sampling Dynamics for Volatile Organic Compounds. Separations 2018, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Grandy, J.J.; Murtada, K.; Belinato, J.R.; Suarez, P.A.O.; Pawliszyn, J. Development and validation of an improved, thin film solid phase microextraction based, standard gas generating vial for the repeatable generation of gaseous standards. J. Chrom. A 2020, 1632, 461541. [Google Scholar] [CrossRef]
- Shah, D.; Patel, D.I.; Roychowdhury, T.; Rayner, G.B.; O’Toole, N.; Baer, D.R.; Linford, M.R. Tutorial on interpreting x-ray photoelectron spectroscopy survey spectra: Questions and answers on spectra from the atomic layer deposition of Al2O3 on silicon. J. Vac. Sci. Technol. B 2018, 36, 062902. [Google Scholar] [CrossRef]
- Herrington, J.S.; Gómez-Ríos, G.A.; Myers, C.; Stidsen, G.; Bell, D.S. Hunting Molecules in Complex Matrices with SPME Arrows: A Review. Separations 2020, 7, 12. [Google Scholar] [CrossRef] [Green Version]

| Compounds | Structure | Functional Groups | Properties |
|---|---|---|---|
| Dicyclohexylamine | 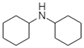 | Cycloalkane, Amine | Irreversible adsorption |
| 4-Methyl-2-pentanone (MIBK) |  | Alkane, Ketone | Proton acceptor |
| 2-Picoline | 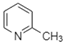 | Arene, Amine | Acid-base interactions |
| n-Pentane (C5) |  | Alkane | Nonpolar, short chain, volatile alkane |
| 1,4-Butanediol | 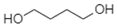 | Alkane, Alcohol | Short chain, volatile alkane, hydrogen bonding |
| 1-Octanol | 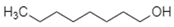 | Alkane, Alcohol | Fatty alcohol, hydrogen bonding |
| N,N-Dimethylaniline | 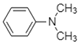 | Arene, Amine | Aromatic pi-pi interactions, irreversible adsorption |
| Sulfolane |  | Sulfonyl | Polar aprotic solvent |
| 2,6-Di-tert-butyl-4-methylphenol (BHT) | 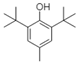 | Arene, Alcohol | Aromatic pi-pi interactions, hydrogen bonding |
| Acenaphthylene | 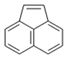 | Arene | PAH of interest, aromatic pi-pi interactions |
| 2,6-Dimethylphenol | 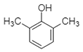 | Phenol | Aromatic pi-pi interactions, acid-base interactions |
| n-Docosane (C22) | 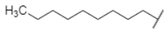 | Alkane | Nonpolar, long chain, semi volatile alkane |
| Chloroform |  | Haloalkane | Very volatile alkyl chloride |
| Linalool | 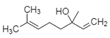 | Alkane, Alcohol | Monoterpene alcohol of interest, hydrogen bonding |
| Hexachlorobutadiene | 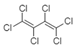 | Haloalkane | Highly halogenated, unsaturated alkane |
| CTC PAL Parameters | |
|---|---|
| HS/DI Modes | |
| Conditioning temperature | 280 °C |
| Conditioning time | 60 s |
| Preconditioning | Yes |
| Post conditioning | No |
| Vial penetration depth | 35 mm |
| Incubation/extraction temperature | 300 s |
| Incubation/extraction time | 120 s |
| Injector penetration depth | 50 mm |
| Desorption time | 60 s |
| Agilent 7890B/5977B GC–MS Parameters | |
|---|---|
| Column | Rxi-624Sil MS—30 m × 0.25 mm × 1.4 µm (Cat# 13868) |
| Injection | See CTC PAL Parameters |
| Mode | Split (50:1) |
| Liner | Topaz 0.75 mm ID Straight/SPME Inlet Liner (Cat# 23434) |
| Injector temperature | 280 °C |
| Purge flow | 3 mL/min |
| Oven | 50 °C (hold 1 min) to 150 °C by 15 °C/min to 280 °C (hold 5 min) by 25 °C/min |
| Carrier gas | He |
| Flow rate | 1.4 mL/min (Constant Flow) |
| Detector | HES-MS |
| Mode | Full Scan |
| Transfer line temp. | 300 °C |
| Source temp. | 325 °C |
| Quad temp. | 200 °C |
| Solvent delay | 1 min |
| Acquisition range | 35–350 m/z |
| Frequency | 4.5 scans/sec |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, D.I.; Roychowdhury, T.; Jacobsen, C.; Myers, C.; Herrington, J.S.; Linford, M.R. Evaluation of New, Sputtered Carbon SPME Fibers with a Multi-Functional Group Test Mixture. Separations 2021, 8, 228. https://doi.org/10.3390/separations8120228
Patel DI, Roychowdhury T, Jacobsen C, Myers C, Herrington JS, Linford MR. Evaluation of New, Sputtered Carbon SPME Fibers with a Multi-Functional Group Test Mixture. Separations. 2021; 8(12):228. https://doi.org/10.3390/separations8120228
Chicago/Turabian StylePatel, Dhananjay I., Tuhin Roychowdhury, Collin Jacobsen, Colton Myers, Jason S. Herrington, and Matthew R. Linford. 2021. "Evaluation of New, Sputtered Carbon SPME Fibers with a Multi-Functional Group Test Mixture" Separations 8, no. 12: 228. https://doi.org/10.3390/separations8120228
APA StylePatel, D. I., Roychowdhury, T., Jacobsen, C., Myers, C., Herrington, J. S., & Linford, M. R. (2021). Evaluation of New, Sputtered Carbon SPME Fibers with a Multi-Functional Group Test Mixture. Separations, 8(12), 228. https://doi.org/10.3390/separations8120228






