Nutritional Value of Sea Urchin Roe (Strongylocentrotidae)—Study of Composition and Storage Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Examined Material
2.2. Initial Procedure for Preparing Sea Urchins for Study
2.3. Sea Urchin Gonad Pretreatment Methods for Long-Term Storage
2.4. Sensory Evaluation of Roe
2.5. Water Content Determination
2.6. Determination of Bioactive Compounds
2.7. Statistics
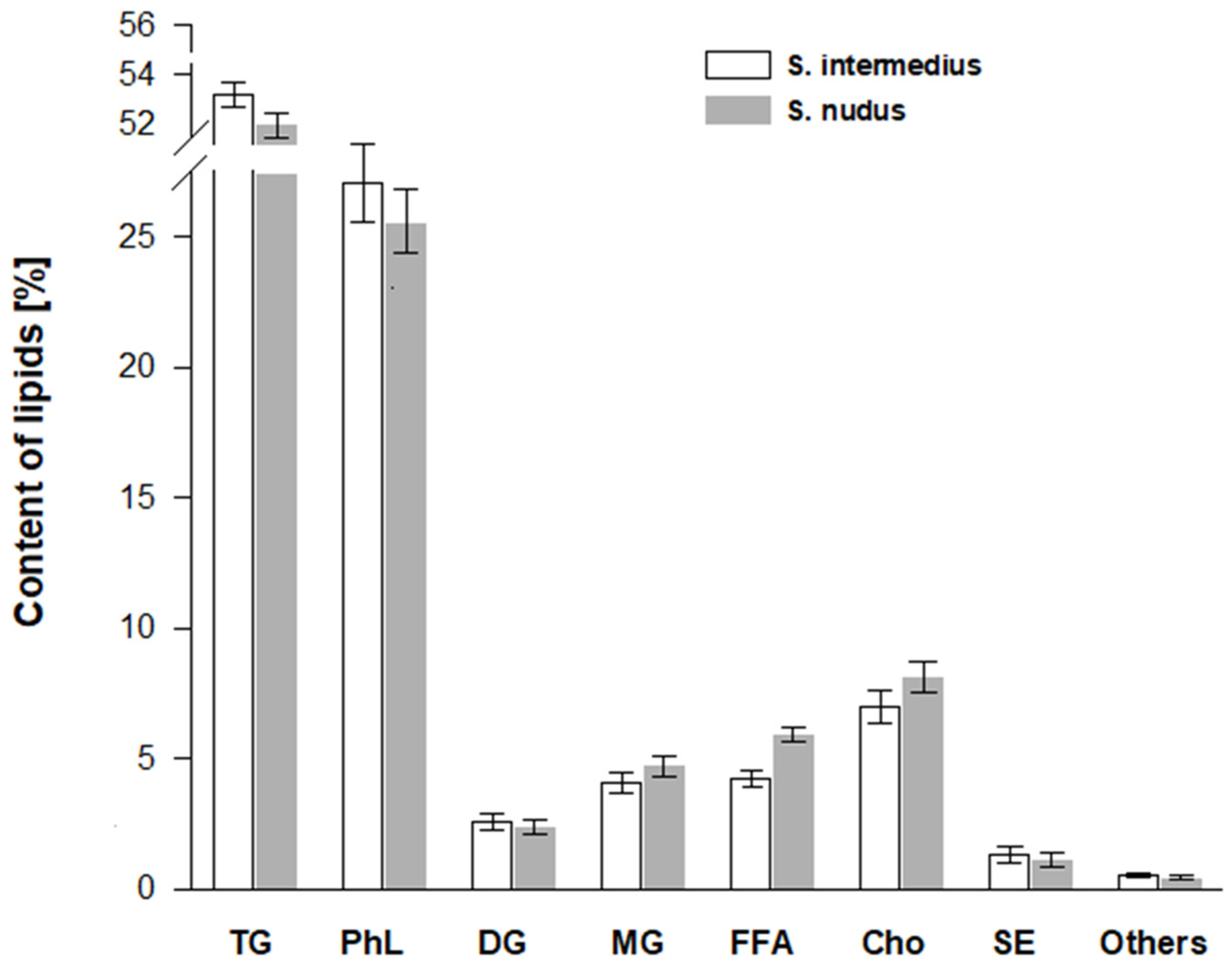
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bazhin, A.G.; Stepanov, V.G. Sea urchins of the Strongylocentrotidae family of the seas of Russia. In Monograph; Kamchatka Research Institute of Fisheries and Oceanography Press: Petropavlovsk-Kamchatsky, Russia, 2012; ISBN 978-5-902210-34-4. [Google Scholar]
- Mottet, M.G. The Fishery Biology of Sea Urchin in the Family Strongylocentrotidae; Technical Report 20; Washington Department of fisheries: Seattle, WA, USA, 1976. [Google Scholar]
- Kalogeropoulos, N.; Mikellidi, A.; Nomikos, T.; Chiou, A. Screening of macro-and bioactive microconstituents of commercial finfish and sea urchin eggs. LWT Food Sci. Technol. 2012, 46, 525–531. [Google Scholar] [CrossRef]
- Kostetskiy, E.Y.; Velanskiy, P.V.; Sanina, N.M. Sites phospholipids of echinoderms and tunicates from the Peter the Great Bay (The Sea of Japan). Russ. J. Mar. Biol. 2012, 38, 65–71. [Google Scholar]
- Kovalev, N.N.; Kryzhanovsky, S.P.; Kostetsky, E.Y.; Kuznetsova, T.A.; Besednova, N.N. Sea Urchins: Biomedical Aspects of Practical Application; Dalnauka Publishing House: Vladivostok, Russia, 2016; Volume 128, ISBN 978-5-8044-1590-8. [Google Scholar]
- Mamelona, J.; Pelletier, E.; Girard-Lalancette, K.; Legault, J.; Karboune, S.; Kermasha, S. Antioxidants in digestive tracts and gonads of green urchin (Strongylocentrotus droebachiensis). J. Food Compos. Anal. 2011, 24, 179–183. [Google Scholar] [CrossRef]
- Qin, L.; Zhu, B.W.; Zhou, D.Y.; Wu, H.T.; Tan, H.; Yang, J.F.; Li, D.M.; Dong, X.P.; Murata, Y. Preparation and antioxidant activity of enzymatic hydrolysates from purple sea urchin (Strongylocentrotus nudus) gonad. LWT Food Sci. Technol. 2011, 44, 1113–1118. [Google Scholar] [CrossRef]
- Liu, C.H.; Lin, Q.; Gao, Y.; Ye, L.; Xing, Y.; Xi, T. Characterization and antitumor activity of a polysaccharide from Strongylocentrotus nudus eggs. Carbohydr. Polym. 2007, 67, 313–318. [Google Scholar] [CrossRef]
- Ke, M.Y.; Wang, H.; Zhang, M.; Tian, Y.W.; Wang, Y.Z.; Li, B.; Yu, J.; Dou, J.; Xi, T. The antilung cancer activity of SEP is mediated by the activation and cytotoxicity of NK cells via TLR2/4 in vivo. Biochem. Pharmacol. 2014, 89, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Sheean, P.D.; Hodges, L.D.; Kalafatis, N.; Wright, P.F.; Wynne, P.M.; Whitehouse, M.W.; Margides, T.A. Bioactivity of extracts from gonadal tissue of the edible Australia purple sea urchin Heliocidaris erythrogramma. J. Sci. Food Agric. 2007, 87, 694–701. [Google Scholar] [CrossRef]
- Solovyev, A.Y.; Morozova, P.Y.; Chernova, I.A.; Chalisova, N.I.; Shataeva, L.K.; Zakutskiy, A.N.; Khavinson, V.H. Extraction and activity of the sea urchins roe regulatory peptides. J. Pharm. Chem. 2010, 44, 14–17. [Google Scholar]
- Abubakar, L.; Mwangi, C.; Uku, J.; Ndirangu, S. Antimicrobial activity of various extracts of the sea urchin Tripneustes gratilla (Echinoidea). Afr. J. Pharmacol. Ther. 2012, 1, 19–23. [Google Scholar]
- Liu, C.H.; Xi, T.; Lin, Q.; Gao, Y.; Xing, Y. Extraction, purification and immunological activity assay of a polysaccharide eggs of sea urchin Strongylocentrotus nudus. Chin. J. Mar. Drugs 2006, 25, 7–11. [Google Scholar]
- Pozharitskaya, O.N.; Shikov, A.N.; Laakso, I.; Seppanen-Laakso, T.; Makarenko, I.E.; Faustova, N.M.; Makarova, M.N.; Makarov, V.G. Bioactivity and chemical characterization of gonads of green sea urchin Strongylocentrotus droebachiensis from Barents Sea. J. Funct. Foods 2015, 7, 227–234. [Google Scholar] [CrossRef]
- Artukov, A.A.; Popov, A.M.; Tsibulskiy, A.V.; Krivoshapko, O.N.; Polyakova, N.V. Echinochrome A pharmacological activity separately and in the dietary supplement “Timarin” composition. Biomed. Chem. 2012, 58, 281–290. [Google Scholar]
- Guseva, M.R.; Beslaneeva, M.B. Clinical reasoning for domestic antioxidant medication “Histochrom” application efficiency. Ophthalmol. Bull. 2010, 126, 37–40. [Google Scholar]
- Stefansson, G.; Olafsdottir, A. The Keeping Quality of Chilled Sea Urchin Roe and Whole Urchins; Matís: Reykjavik, Iceland, 2017; ISSN 1670-7192. Available online: https://matis.is/wp-content/uploads/skyrslur/08-17-Report-shelf-life-of-sea-urchin-roe-and-live-urchinsfinal.pdf (accessed on 1 June 2021).
- ISO 8586:2012. Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors; The International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Fresenius J. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef] [Green Version]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Christie, W.W. Equivalent chain lengths of methyl ester derivatives of fatty acids on gas-chromatography a reappraisal. J. Chromatogr. A 1988, 447, 305–314. [Google Scholar] [CrossRef]
- Tsushima, M.; Matsuno, T. Comparative biochemical studies of carotenoids in sea-urchins—I. Comp. Biochem. Physiol. Part B 1999, 96, 801–810. [Google Scholar] [CrossRef]
- Dincer, T.; Cakli, S. Chemical composition and biometrical measurements of the Turkish Sea Urchin (Paracentrotus Lividus, Lamarck, 1816). Crit. Rev. Food Sci. Nutr. 2007, 47, 21–26. [Google Scholar] [CrossRef]
- Cuevas-Acuña, D.A.; Valenzuela, M.H.; Santacruz-Ortega, H.C.; Melchor, R.G.V.; Arias-Moscoso, J.L. Sea Urchin (Strongylocentrotus Franciscanus) gonads chemical composition, protein and amino acid contents and morphology. Biotecnia 2019, 21, 86–91. [Google Scholar] [CrossRef]
- Murzina, S.A.; Pekkoeva, S.N.; Voronin, V.P.; Dgebuadze, P.Y.; Mekhova, E.S.; Thanh, N.T.H. Lipids and fatty acids of the gonads of Sea Urchin Diadema Setosum (Echinodermata) from the coastal area of the Nha Trang Bay, Central Vietnam. Eur. J. Lipid Sci. Technol. 2021, 123, 2000321. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, D.-Y.; Lu, T.; Liu, Z.-Y.; Zhao, Q.; Liu, Y.-X.; Hu, X.-P.; Zhang, J.-H.; Shahidi, F. Characterization of lipids in three species of sea urchin. Food Chem. 2018, 241, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.S.; Owen, P.V.; Pantazis, P. The commercial potential of the common sea urchin Echinus esculentus from the west coast of Scotland. Hydrobiologia 2001, 46, 85–94. [Google Scholar] [CrossRef]
- Angioni, A.; Addis, P. Characterization of the lipid fraction of Wild Sea Urchin from the Sardinian Sea (Western Mediterranean). J. Food Sci. 2014, 79, C156–C162. [Google Scholar] [CrossRef] [PubMed]
| Components | Per 100 g of Raw Sea Urchins Roe | |
|---|---|---|
| S. intermedius | S. nudus | |
| Water, g | 73.0 3.2 | 75.7 2.7 |
| Protein, g | 13.9 1.1 | 13.8 0.7 |
| Fat, g | 7.3 1.2 | 6.3 1.5 |
| Carbohydrades, g | 3.5 0.7 | 2.1 0.4 |
| Minerals, g | 2.3 0.2 | 2.1 0.2 |
| Energy value, kcal | 117.3–153.3 | 102.4–138.2 |
| Amino Acids | Amino Acid Standard FAO/WHO g/100 g Protein | Sea Urchins Roe | |||
|---|---|---|---|---|---|
| S. intermedius | S. nudus | ||||
| A | S | A | S | ||
| Leu | 7.0 | 7.1 0.5 | 101.4 | 7.0 0.6 | 100.0 |
| Phe + Tyr | 6.0 | 7.2 0.3 | 120.0 | 7.8 0.8 | 130.0 |
| Lys | 5.5 | 6.9 0.3 | 125.4 | 6.2 0.5 | 112.7 |
| Val | 5.0 | 5.8 0.6 | 116.0 | 5.5 0.6 | 110.0 |
| Ile | 4.0 | 4.7 0.4 | 117.5 | 4.60.5 | 115.0 |
| Thr | 4.0 | 6.6 0.4 | 165.0 | 6.9 0.3 | 172.5 |
| Met + Cys | 3.5 | 4.6 0.3 | 131.4 | 4.8 0.6 | 137.1 |
| Σessential | 35.0 | 42.9 3.1 | 42.8 3.6 | ||
| Ala | 5.7 0.6 | 5.8 0.4 | |||
| Arg | 5.5 0.4 | 5.2 0.3 | |||
| Asp | 11.6 0.7 | 12.1 0.5 | |||
| His | 7.3 0.5 | 6.8 0.3 | |||
| Gly | 4.2 0.1 | 3.8 0.2 | |||
| Glu | 13.2 0.6 | 12.9 0.7 | |||
| Pro | 2.9 0.2 | 3.3 0.1 | |||
| Ser | 5.2 0.3 | 5.4 0.4 | |||
| Σnonessential | 55.63.0 | 55.33.4 | |||
| Fatty Acid | Content, % of Total Fatty Acid | |
|---|---|---|
| S. intermedius | S. nudus | |
| 14:0 | 6.65 0.83 | 12.59 2.1 a |
| 14:1 | 0.10 0.02 | 0.32 0.06 a |
| 15:0-i | 0.53 0.04 | 2.76 0.43 a |
| 15:0 | 0.46 0.02 | 0.51 0.06 |
| 16:0-i | 0.24 0.01 | 0.10 0.02 a |
| 16:0 | 16.72 2.31 | 13.10 1.42 a |
| 16:1(n-11) | - | 0.20 0.04 |
| 16:1(n-7) | 6.94 0.65 | 4.23 0.39 |
| 16:1(n-5) | 1.21 0.13 | 7.74 0.75 a |
| 17:0-ai | 1.42 0.21 | 0.10 0.01 a |
| 16:2(n-4) | - | 0.23 0.03 |
| 3,7,11,15-tetramethyl hexadecanoic acid (Phytanic) | 0.18 0.01 | 0.27 0.04 a |
| 17:0 | 0.16 0.02 | 0.11 0.02 a |
| 17:1 | 0.10 0.01 | 0.13 0.03 |
| 16:4(n-1) | - | 0.10 0.02 |
| 18:0 | 1.93 0.13 | 1.21 0.23 a |
| 18:1(n-11) | 0.54 0.03 | 0.86 0.10 a |
| 18:1(n-9) | 4.57 0.34 | 2.13 0.14 a |
| 18:1(n-7) | 3.38 0.28 | 3.71 0.52 |
| 18:1(n-5) | 1.50 0.09 | 0.82 0.11 a |
| 19:0-i | 0.15 0.01 | 0.12 0.03 |
| 18:2(n-6) | 1.42 0.21 | 0.72 0.06 a |
| 18:2(n-4) | 0.37 0.02 | 0.19 0.03 a |
| 18:3(n-6) | - | 0.16 0.02 |
| 18:3(n-3) | 1.96 0.31 | 0.88 0.07 a |
| 18:4(n-3) | 3.75 0.44 | 2.29 0.32 |
| 20:0 | 0.22 0.04 | 0.27 0.04 |
| 20:1(n-11) | 3.08 0.36 | 4.91 0.61 a |
| 20:1(n-9) | 3.38 0.50 | 3.17 0.27 |
| 20:1(n-7) | 1.05 0.22 | 0.85 0.06 |
| 20:2, Δ5,11 | 4.88 0.51 | 5.17 0.43 |
| 20:2, Δ5,13 | 3.12 0.28 | 2.37 0.30 a |
| 20:2(n-6) | 0.98 0.11 | 0.64 0.04 a |
| 20:3, Δ5,11,14 | 0.86 0.07 | 1.04 0.18 |
| 20:3(n-6) | 1.21 0.15 | 0.73 0.06 a |
| 20:4(n-6) | 10.43 2.0 | 8.62 1.05 |
| 20:3(n-3) | 0.76 0.09 | 0.86 0.07 |
| 20:4(n-3) | 0.22 0.03 | 1.05 0.09 a |
| 20:5(n-3) | 6.34 0.66 | 5.85 0.49 |
| 22:1(n-9) | 0.12 0.02 | 0.72 0.08 a |
| 22:2, Δ7,13 | 0.34 0.02 | 0.21 0.03 a |
| 22:2, Δ7,15 | 2.56 0.34 | 1.17 0.12 a |
| 22:6(n-3) | 0.53 0.07 | 1.17 0.16 a |
| 24:1(n-9) | 0.11 0.01 | 0.50 0.04 a |
| Others | 5.53 0.67 | 5.12 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matveeva, V.A.; Shulgina, L.V.; Prikhodko, Y.V.; Shulgin, Y.P.; Madej, K.; Piekoszewski, W. Nutritional Value of Sea Urchin Roe (Strongylocentrotidae)—Study of Composition and Storage Conditions. Separations 2021, 8, 174. https://doi.org/10.3390/separations8100174
Matveeva VA, Shulgina LV, Prikhodko YV, Shulgin YP, Madej K, Piekoszewski W. Nutritional Value of Sea Urchin Roe (Strongylocentrotidae)—Study of Composition and Storage Conditions. Separations. 2021; 8(10):174. https://doi.org/10.3390/separations8100174
Chicago/Turabian StyleMatveeva, Victoria A., Lidia V. Shulgina, Yury V. Prikhodko, Yury P. Shulgin, Katarzyna Madej, and Wojciech Piekoszewski. 2021. "Nutritional Value of Sea Urchin Roe (Strongylocentrotidae)—Study of Composition and Storage Conditions" Separations 8, no. 10: 174. https://doi.org/10.3390/separations8100174
APA StyleMatveeva, V. A., Shulgina, L. V., Prikhodko, Y. V., Shulgin, Y. P., Madej, K., & Piekoszewski, W. (2021). Nutritional Value of Sea Urchin Roe (Strongylocentrotidae)—Study of Composition and Storage Conditions. Separations, 8(10), 174. https://doi.org/10.3390/separations8100174