Abstract
Lettuce (Lactuca sativa L.) is one of the most popular leafy vegetables, appreciated globally as a low-calorie food with bioactive compounds. The application of a low dose of abiotic stress is considered a sustainable pre-harvest strategy to modify the nutraceutical value of horticultural products. In this work, we explored the response of two differently colored (red or green) baby leaf lettuce varieties to four NaCl concentrations in the nutrient solution (from 1 to 30 mM), using a full factorial design. We focused on leaf morphological parameters and possible phytochemical enhancement of the main polyphenols and anthocyanins, analyzed by LC-MS. The response to low-to-moderate salt stress exposure was affected mainly by salt concentration for leaf traits or by the cultivar for leaf color, with very limited factors’ interactions. Multivariate analysis indicated a predominant role of the genotypic factor in shaping differences in the two weeks growing cycle for baby leaf lettuce. Phytochemically, different dose–response models to sub-optimal saline conditions may be applied to the various compounds. A significant hormetic stimulation was present only for cyanidin-malonyl glucoside, the main anthocyanin present in the red cultivar.
Keywords:
stress response; hormesis; hydroponics; food quality; sodium; chlorine; polyphenols; anthocyanins 1. Introduction
Lettuce (Lactuca sativa L.) is an annual species of the Asteraceae family that has become one of the most popular fresh vegetables worldwide [1]. The leaves are most often eaten fresh and their increasing consumption associates with an urban lifestyle (such as care in meeting daily vegetable recommendations) and preference for fresh, low-calorie, minimally processed fiber-rich food, such as ready-to-eat pre-cut bagged (mixed) salads [2]. The growing appreciation of lettuce is also due to the high level of antioxidants in relation to the low caloric value as well as its vitamin and mineral content [3,4]. The main component of the lettuce antioxidant activity is represented by polyphenols, mainly caffeic acid derivatives (chlorogenic acid) and flavonoids (anthocyanins) [5,6]. Polyphenols share the ability of an antioxidant activity in vitro, with possible wide-ranging protective cellular roles [7]. This large and widespread class of phytochemical also influences relevant commercial features of lettuce, such as the color and the damage-induced leaf browning [8,9].
L. sativa is the only important horticultural species of its genus, but marketed lettuce presents a significant range of morphological variations that are absent in its wild relative (L. serriola) [1]. All cultivated lettuces have leaves (with a small petiole or sessile) that are circularly arranged to form a rosette with a different degree of compactness (i.e., overlap of the upper part of leaves). Leaves considerably vary in shape, color, texture, and glossiness as well as in division and undulation of their margin. Visual features are important determinants of food selection and, especially for leafy vegetables, color interacts with freshness and flavor perception [10,11]. The color is also influenced by anthocyanins, a well-known pigmented class of polyphenolic secondary metabolites mainly involved in plant adaptation and response to the environment [12,13]. As a consequence, the tint, the intensity of coloration, and the leaf area covered by anthocyanins can strongly differ among varieties. According to all these features, breeders and retailers usually distinguish various horticultural types, although a clear taxonomic distinction cannot be always made because of synonyms and genericized trademarks [1]. This classification is further complicated because varieties are also distinguished according to the suitability to specific cultivation techniques. For instance, the so-called “baby leaf” is a fast-growing segment within the lettuce market [14]. A baby leaf crop is any plant whose true leaves and petioles (and, rarely, roots) are harvested before the eight true-leaf stage, typically at a height between 50–120 mm according to the species [15]. Baby leaf crops, also known as “cut leaf vegetables” are routinely grown in high densities in hydroponics [16] and are valued especially because of their high concentration of bioactive phytochemicals [15]. Moreover, because of reduced damage at harvest and better color preservation [17], these vegetables are mostly required by the food industry to be marketed, after minimum processing, as bagged salad [14,18].
Recently, there is growing evidence of the usefulness in horticulture of the so-called “eu-stress” to sustainably promote the accumulation of beneficial, health-related compounds with a minor or negligible yield loss [19]. Biological processes can be characterized by a nonlinear response to the exposure of the increasing amount of substances or to a variation in environmental conditions, a phenomenon long known as hormesis [20]. In short, it has been shown that a low dose of stress may trigger an effect opposite to that recorded for a high dose, yielding a biphasic dose-dependent response [19]. The most evoked explanation is that the plant reaction to a low dose of stress includes a compensatory regulation of various molecules with an adaptive role. This may give rise to a biologically significant positive response that surpasses the inhibitory effect of mild stress [20]. Although shared by virtually all living organisms, hormesis may differ significantly within a species in terms of amplitude and sensitivity [21,22]. It has also been reported that the hormetic increase may be preceded by a so-called sub-hormetic drop, originating a S-shaped curve (a triphasic dose response), although this has been typically associated with ionizing radiation [23,24].
The profile of the bioactive compounds in lettuce can be easily varied according to the growing conditions [25,26] and the genotype [27,28], with significant interactions between these two factors [29]. The aim of this paper is to provide insights on possible different accumulation models of bioactive compounds in lettuce within a gradient of low-to-moderate saline stress. This work was carried out with two differently colored baby leaf varieties, either green or full red [30]. While several studies have focused on green leaf lettuce [31,32], recent evidence indicated that not only the phytochemical constituents but also the morpho-physiological and metabolomic response to stress may differ between the lettuce cultivars with differently pigmented leaves [29,33,34,35]. Although severe NaCl stress causes detrimental effects on plant growth and yield, more limited salt concentrations can be deliberately employed to improve the content of beneficial compounds and crop adaptation to stress [36,37,38]. Compared to other environmental factors that influence plant secondary metabolites (e.g., light and temperature), the saline stress can be tightly controlled in hydroponics with limited effort. Moreover, the management of the electric conductivity of the nutrient solution is economically affordable and easy to implement in already available cultivation systems. We focused on polyphenols because they are the most abundant classes of lettuce phytochemicals that contribute to the in vitro antioxidant properties of the edible product [39,40]. Moreover, polyphenols are both highly responsive and essential components of the cellular response to stress [41,42]. The present study constitutes a continuation of our previously published works [34,43], where the physiological traits and (bio)chemical composition of two differently pigmented baby lettuce varieties indicated that, in certain conditions, salinity can increase total phenols, total ascorbic acids, and macrocations’ concentration in lettuce leaves. Nonetheless, it remained unaddressed if saline eustress modulates the accumulation of single bioactive compounds of a chemical class differently as well as if this phenomenon includes some cultivar specific interaction. To address these points, in this work we investigated the effect of a range of saline concentrations, from 1 to 30 mM, in two different baby leaf varieties that differ in leaf coloration. Using a full factorial design, we analyzed polyphenol composition (by LC-MS and HPLC), and leaf morphological parameters and color, as main determinants of the quality of the edible product and appeal for consumers.
2. Materials and Methods
2.1. Plant Material
We used two lettuce (Lactuca sativa L. var. acephala) varieties, ‘Green Salad Bowl’ (GSB) and ‘Red Salad Bowl’ (RSB) both from SAIS (Cesena, Italy) (Supplementary Figure S1). Plants were sown in March in 2015 in a glasshouse at the experimental farm of the Department of Agricultural Sciences (University of Naples Federico II), in Bellizzi (SA). Seeds were placed in polystyrene trays filled with vermiculite and eleven days later (at the two true-leaf stage), transferred to a floating raft system (150 l capacity) with a density of 1025 plants per square meter. Minimum and maximum daily air temperatures and relative humidity inside the glasshouse during the spring growing season are presented in the Supplementary Figure S2. The glasshouse experiment was conducted using a randomized complete-block design with two cultivars (C; GSB and RSB) and four salinity concentrations (S; 1, 10, 20, or 30 mM NaCl) in the nutrient solution (NS), and three replicates (R), resulting in 24 experimental blocks (2C × 4S ×3R). The composition of the NSs was equal for all treatments regarding the other macro and microelements namely, 12.0 mM nitrate, 1.0 mM ammonium, 1.5 mM phosphorus, 5.0 mM potassium, 4.0 mM calcium, 1.75 mM sulphur, 1.5 mM magnesium, 20 µM iron, 20 µM boron, 9 µM manganese, 1.6 µM zinc, 0.3 µM copper, and 0.3 µM molybdenum as reported [34]. The electric conductivity of the demineralized water was 0.03 dS/m and that of the four NSs was 1.9, 2.8, 4.0, and 5.1 dS/m. The saline treatments were initialized three days after transferring the polystyrene trays to the floating raft system. The half maximal effective concentration (EC50) at 120 h for lettuce (at germination) has been estimated in 5.73 ± 0.56 g/L, corresponding to 98 mM [44]. The pH was set at 6.0 ± 0.2. To prevent large fluctuations in EC, pH, and ionic concentrations, the nutrient solutions were weekly renewed in all tanks.
2.2. Morphological Analysis
We measured ten plants per block for the morphological analysis. Leaves were harvested 26 days following sowing and fresh (FW) and dry weight (DW), leaf area, and yield were measured as reported [34]. Leaf colour was quantified with a portable chlorophyll meter SPAD-502 (Konica Minolta, Tokyo, Japan), on twenty leaves per block (two leaves per plant) and expressed using the CIELAB colour parameters, L* (lightness, from 0 to 100, i.e., black to white), a* and b* (the two chroma components, from −60 to +60; from green to red for a*, and from blue to yellow for b*). The hue angle and the colour difference were calculated as reported [45]. Chroma (the amount of saturation of the colour) was computed as square root of the sum of the squared a* and b* values. For subsequent analysis, leaves were immediately frozen in liquid nitrogen and stored at −80 °C.
2.3. Micro Mineral Content Analysis
The amount of sodium and potassium was analyzed in finely ground leaves (0.25 g by ion chromatography (ICS-3000, Dionex, Sunnyvale, CA, USA), as already reported [34]. Results were expressed as g/kg dw.
2.4. Polyphenol Analysis
The extraction of total polyphenols was carried out at harvest (26 days after sowing) starting from 0.2 g of freeze-dried leaves as reported, with some modifications [39]. Samples were homogenized in 8 mL of a methanol/water/formic acid solution (25/24/3 v/v/v), thoroughly mixed, and sonicated for 30 min in an ultrasonic cleaning bath at room temperature (Q500; Qsonica, Newtown, CT, USA). After a 10 min mixing on a rocking shaker (SSL4; Stuart Equipment, UK), samples were centrifuged (4000 rpm for 30 min at 4 °C, using the F34-6-38 rotor) in a Eppendorf 5810 R centrifuge (Eppendorf, Hamburg, Germany) and then the supernatant was collected, spun again (14,800 rpm for 15 min at 4 °C, using the FA-45-30-11 rotor), and passed through a cellulose-based filter (0.22 mm gauge, Phenomenex, CA, USA). For HPLC analysis, the clean supernatant was eluted according to the previously described procedures with slight modifications [39]. A total of 20 μL of sample was injected twice onto a Prodigy ODS3 250 mm × 4.6 reversed phase C-18 (Phenomenex) column, with a flow rate of 1 mL/min, installed into LC-10A apparatus (Shimadzu, Kyoto, Japan) equipped with an SPD-M10A DA detector (Shimadzu) and a Series 200 Autosampler (Perkin Elmer, Waltham, MA, USA). The mobile phases were made of 5% (v/v) of formic acid in water and methanol (MeOH) to create the following conditions (exact time in min–% of MeOH) during the run: 0–5, 25–40, 32–40. Within the same LC-DAD runs, chromatograms were obtained by reading at 330 nm for phenolic acids and at 520 nm for anthocyanins. Six phenolic acids were identified by LC-MS and quantified on calibration curves built with chlorogenic acid and cichoric acid (with HPLC). For anthocyanins, the cyanidin-malonyl glucoside was identified by LC-MS and quantified using the oenin to build a standard curve. Three replicate samples (i.e., one leaf from three plants) per experimental block were analyzed. Data are reported as mean ± standard error of the mean (s.e.). HPLC grade solvents and standards were purchased from Sigma-Aldrich (Milan, Italy).
2.5. Statistical Analysis
Data were analyzed by two-way analysis of variance (ANOVA) considering as fixed factors (i.e., categorical variables) the cultivar (C) and the NaCl concentration (S). The factor C had two levels represented by the GSB and the RSB cultivated varieties. The factor S had four levels, namely, 1, 10, 20, and 30 mM NaCl concentration in the NS. The Duncan multiple range test (respectively, the two-tailed Student’s t-test) was employed as a post-hoc procedure for mean separations for the NaCl concentration (resp., for the cultivar). Data are presented as mean ± standard error of the mean. Calculations were performed with SPSS 26 (IBM, Arkon, NY, USA). Principal component analysis was carried out and plotted in R as described [46].
3. Results
3.1. Effects on Leaf Biometric Parameters
The evaluation of leaf traits at harvest indicated that the fresh weight of the harvested leaves per plant (LFW), water content (WTC), leaf area (LA) and number (LN), specific leaf area (SLA), and leaf succulence (LSH) were significantly affected by the genotype (Table 1). For this reason, for each trait data are also graphically reported per cultivar as a function of the salt concentration (Supplementary Figure S3).

Table 1.
Main effect of the cultivar (C), salt concentration (S; in mM NaCl), and their interaction on the leaf morphological parameters. The significance of the factors and their interactions was evaluated with a two-way ANOVA (ns: not significant; *: p < 0.05; ***: p < 0.001), using Duncan’s multiple range test as post-hoc test for mean separation among conditions. In case of simple main effect of C or S, different letters indicate, for each variable, different homogenous groups according to Duncan’s test for S, or the Student’s t-test for C. GSB: ‘Green Salad Bowl’; RSB: ‘Red Salad Bowl’. Salt concentration is in mM NaCl. LFW: harvested leaves per plant (LFW); WTC: water content: LA: leaf area per plant; LN: leaf number per plant; SLA: specific leaf area; LSH: leaf succulence.
The NaCl treatment significantly influenced the water content and the average leaf area, although variations were limited. Factor interactions were not present and differences among single experimental conditions were statistically evaluated using a one-way ANOVA (Table 1). Differences in yield between the two varieties were limited and the data suggest that saline levels higher than those employed in this study may have incurred in higher yield penalties mainly by a reduction of leaf surface (Supplementary Figure S4), also considering that the modest main effect on the water content was mainly due to the 30 mM NaCl treatment. Leaves of the GBS were also more succulent because the (significant) difference in water content percentage was very limited (less than 1%). On the other hand, the RSB cultivar was more efficient in producing larger leaves per dry biomass.
3.2. Effects on Leaf Coloration
Not surprisingly, the colour of the leaves was strongly dependent on the cultivar (Table 2). The colour parameters of the two varieties statistically differed not only for greenness/redness, but also for lightness, saturation, and blueness/yellowness. The colour difference (ΔE), calculated for each variety considering as reference the 1 mM NaCl concentration, was not statistically affected by the salt treatment (not shown). Moreover, the saline treatment, and its interaction with the genotype, did not significantly influence any visual parameters under investigation and with a constant linear pattern in the salt interval tested (Supplementary Figure S4).

Table 2.
Main effect of the cultivar (C), salt concentration (S; in mM NaCl), and their interaction on the colour parameters of the leaves. The significance of the factors and their interactions was evaluated with a two-way ANOVA (ns: not significant; *: p < 0.05; ***: p < 0.001), using Duncan’s multiple range test as post-hoc test for mean separation among conditions. In case of simple main effect of C or S, different letters indicate, for each variable, different homogenous groups according to Duncan’s test for S, or the Student’s t-test for C. GSB: ‘Green Salad Bowl’; RSB: ‘Red Salad Bowl’. Salt concentration is in mM NaCl. L* (lightness), a* (redness), b* (yellowness), and chroma (relative saturation) and hue indicate the color dimensions according to the CIELAB color space as defined by the International Commission on Illumination.
3.3. Effects on the K/Na Ratio in Leaves
Considering that in saline conditions Na interferes with K homeostasis in plants, we measured the Na and K accumulation in leaves in the different experimental conditions (Table S1). The presence of suboptimal conditions (e.g., any saline concentration higher than the control condition) significantly lowered the K/Na ratio (Table 3). Moreover, the cultivar factor has a significance influence with a positive main effect for the red cultivar. Moreover, there was a significant interaction between the experimental factors. Although for both varieties an increased NaCl concentration in the NS reduced the K/Na, the effect was more pronounced for the RSB variety. While the K/Na ratio was higher in RSB at 1 and 10 mM NaCl, at 20 mM and above there was no difference between the two cultivars.

Table 3.
Main effect of the cultivar (C), salt concentration (S; in mM NaCl), and their interaction on the K/Na. The significance of the factors and their interactions was evaluated with a two-way ANOVA (ns: not significant; **: p < 0.01; ***: p < 0.001), using Duncan’s multiple range test as post-hoc test for mean separation among conditions. Different letters indicate, for each variable, different homogenous groups according to Duncan’s test for S, or the Student’s t-test for C. GSB: ‘Green Salad Bowl’; RSB: ‘Red Salad Bowl’. Salt concentration is in mM NaCl.
3.4. Effects on Phytochemicals
The analysis of the polyphenols indicated that the 5-O-Caffeoylquinic acid (5-CQA) (aka chlorogenic acid) and the D-cichoric acid (DCTA) were the most abundant compounds in both varieties, found at a concentration around 10 × and 5 × higher than other phenolic compounds, respectively (Table 4).

Table 4.
Main effect of the cultivar (C), salt concentration (S; in mM NaCl), and their interaction on the leaf polyphenols. The significance of the factors and their interactions was evaluated with a two-way ANOVA (ns: not significant; **: p < 0.01; ***: p < 0.001), using Duncan’s multiple range test as post-hoc test for mean separation among conditions. In case of simple main effect of C or S, different letters indicate, for each variable, different homogenous groups according to Duncan’s test for S, or the Student’s t-test for C. GSB: ‘Green Salad Bowl’; RSB: ‘Red Salad Bowl’. Salt concentration is in mM NaCl. 3,5-CQA: isochlorogenic acid; 5-CQA: chlorogenic acid; CMA: caffeoylmalic acid; CTA: caffeoyltartaric acid; DCTA: cichoric acid; m-DCTA: meso-di-O-caffeoyltartaric acid.
While the amount of DCTA was not influenced by the genotype, the cultivar with red leaves had almost double the amount of 5-CQA. A main effect of the genotype was present for two other compounds (Table 3), with caffeoylmalic acid (CMA) present in a higher quantity in RSB and caffeoyltartaric acid (CTA) in GSB. The saline concentration in the NS had a strong main effect on all the analyzed compounds (Table 3), with an average reduction of 34% considering the maximum and minimum amount of the polyphenols. On the other hand, CMA was the least variable compound according to the coefficient of variation (Table S2), even though it was affected by the genotype or the salt concentration. Interestingly, a (threshold or non-threshold) linear response was not present for all the polyphenols but 3,5-Dicaffeoylquinic acid (3,5-CQA; aka isochlorogenic acid) (Table 3), which decreased constantly with increasing NaCl concentrations (see also Supplementary Figure S5). For the other compounds, the higher (respectively, lower) amount was present at the lowest (resp., higher) saline concentration. However, an inverse dose–response relationship was recorded between 10 and 20 mM NaCl in the nutrient solution. Factor interactions had a significant impact only for 5-CQA. Its amount linearly decreased in the GSB variety while a non-linear response was present in the RSB.
Finally, we quantified cyanidin-malonyl glucoside because anthocyanins are abundant pigments in coloured lettuce leaves as well as being contributors of the antioxidant activities. This cyanidin conjugate was undetectable in GSB and therefore data were analyzed by a one-way (between groups) ANOVA (Table 5). This is consistent with the failure to develop a perceptible red colour in the leaves of the GSB variety under the salt concentrations and the duration of stress employed.

Table 5.
Main effect of the salt concentration on cyanidin-malonyl glucoside (CMG) content of the ‘Red Salad Bowl’ cultivar. The significance was evaluated with a one-way ANOVA (*: p < 0.05). Different letters indicate different homogenous groups according to Duncan’s multiple range test.
The saline concentration had a significant effect on CMG accumulation. Interestingly, the dose response is most consistent with a biological response at low doses of saline stress (10 and 20 mM) that has the opposite direction from the response at the highest dose (30 mM) (Supplementary Figure S6). In relative terms, the increase in CMG was around 50% compared to the control condition (1 mM NaCl).
3.5. Multivariate Analysis of the Lettuce Responses
To visualize the relationship of the multivariate cultivar profiles in the different stress conditions, we performed a principal component analysis (PCA) on all the standardized variables but CMG (n = 16). The first (respectively, the second) principal component (PC) explained 62.0% (resp., 30.5) of the total, an indication of the collinearity, especially in the morphometric variables. PC1 and PC2 were therefore used to graphically represent samples’ relations (Figure 1).
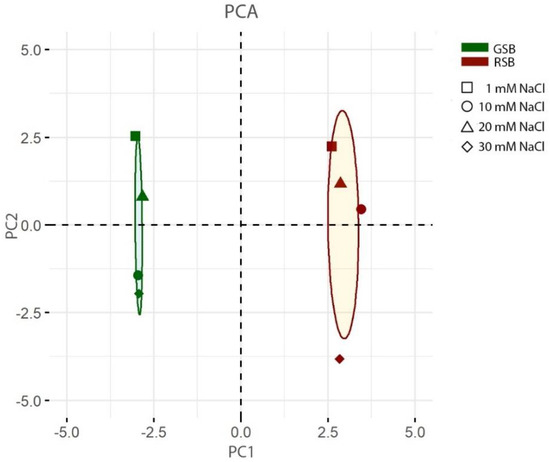
Figure 1.
Principal component analysis (PCA) of the lettuce response to salt stress. The variance explained by the PC1 and PC2 is 62.0 and 30.5, respectively. The plot displays, in a two-dimensional space, clusters of samples based on their response to saline conditions. Red colored symbols refer to the RSB variety, while green symbols to GSB. Shapes indicate different NaCl concentrations in the nutrient solution—square: 1 mM; circle: 10 mM; triangle: 20 mM; diamond: 30 mM. For each variety, the plot displays a confidence ellipse (confidence level: 0.95) around the mean point (not shown).
The PCA analysis indicated that two varieties were separated along PC1 while the different salt concentrations mainly accounted for the distribution along PC2. This signifies that the two experimental factors were associated with different variances of the measured traits. The analysis also indicated that in the measured variables there are strong genotypic differences between the varieties which were not rearranged by the saline stress. Moreover, the two extreme NaCl concentrations (1 and 30 mM) were far apart for both varieties. However, samples were not progressively separated along PC2 according to the salt stress, consistent with an overall response of both varieties that is not linearly dependent on the NaCl concentration in the NS. Finally, the PCA indicated that the larger response of the RBS variety is mainly due to the 30 mM condition, the only one that lied well outside the 95% confidence interval (of the mean condition).
4. Discussion
Previous works investigated the effect of cultural conditions such as salinity or light on the mineral and/or phytochemical composition of differently colored baby lettuces [34,47,48]. For instance, it was shown that a relatively low saline concentration (20 mM NaCl) was associated with a minimal loss of yield per cultivated surface and diminished the phytonutrients and beneficial minerals (e.g., K, Ca, and Mg) per dry matter [34]. The nutritional value of lettuce mainly depends on compounds with known antioxidant activities, especially because baby leaf lettuce is almost exclusively eaten raw. In this work, we analyzed the dose response to four different NaCl concentrations of the most important polyphenols and flavonoids in two varieties. The result indicated that different dose–response models to sub-optimal conditions should be considered for the various compounds.
The factor “salt stress” had a largely predominant role on lettuce, while a main effect of the genotypes was present in a more limited number of variables. A main effect on leaf weight and number was not significant but the observed trends suggest that higher saline concentrations may have caused an agronomically relevant inhibitory effect. It should be added that in our experimental system, fresh cut lettuce experienced a two-week period of stress, being the commercial product mechanically harvested at the 5–6 true leaf stage. While the non-significant effects imply that low levels of salt stress could be applied in horticulture to manipulate the fresh cut lettuce phytochemical profile, it remains untested if longer periods of growth under the employed levels of saline stress could have caused more conspicuous effects. The short growing period may also account for the fact that factor interaction (salinity × cultivar) was very limited, observed for only one variable, the chlorogenic acid. Besides this exception, the lack of interactions indicates that these two genotypes did not employ different stress-response strategies. In particular, leaf color parameters appeared to be fixed by the genotype and little altered by salt concentration. Wild lettuce has green leaves that accumulate anthocyanins at the edge of the lamina, and the red color in the cultivated lettuce germplasm was fixed by breeding [49]. A metabolomics comparison between red and green butterhead varieties also implied that breeding for leaf color selected and fixed compounds that vary little according to mineral nutrition [35]. Green cultivars may have different responses because various green varieties, such as GSB, do not develop red pigments when stressed [49]. While the effect on leaf color was negligible, the salt concentration affected the K/Na ratio in leaves, considered a good indicator of the ability of glycophytes to cope with saline stress. Under the control condition, the red cultivar had a higher capacity of potassium uptake and translocation to leaves. Nonetheless, based on the absolute amount of Na and K and their ratio, both cultivars should be considered sodium includers. Specifically, at the higher saline concentrations (20 mM and above), the two genotypes did not show a selectivity or a preferential accumulation of K over Na, thus suggesting similar sensitivity and adaptive strategies to salt stress with respect to the maintenance of high cytosolic K/Na.
Salt concentration significantly altered the analyzed polyphenols. Our data confirmed that the chlorogenic and cichoric acids are the predominant products of cinnamates (and derivatives) biosynthesis in both varieties [40]. The predominant main effect of salt was not monophasic, with only isochlorogenic acid progressively decreasing with increasing NaCl concentration. The concentration of the other five compounds at 20 mM was comparable to that at the control condition (1 mM), while at 30 mM there was a reduced accumulation of all the compounds implying an S-shaped dose response. The red and green variety differed in the quantitative reaction to salt stress. In particular, the chlorogenic acid, present in double the amount in the RSB, gradually decreased at higher NaCl concentration in the GSB while a triphasic response was present in RSB. S × C interaction was not observed for the other compounds, including those present in different amounts between the two cultivars (i.e., caffeoylmalic and caffeoyltartaric acid). Although a stimulatory peak (e.g., an increase in compounds at low dose of stress compared to the control condition) under salt stress was not detected, it is significant that the 20 mM dose was indistinguishable from a no-observed adverse-effect level for most polyphenols. A study of six toxins in lettuce indicated that while more than half of the dose responses were monophasic, triphasic responses accounted for around a third of the 12 studied relationships [50]. In that work, the inhibitory peak at low doses ranged between 6 and 25% of control and it is noteworthy that inhibition at very low doses was very often followed by a subsequent growth that did not surpass the control condition [50].
Due to the lack of red pigmentation in the GSB also under stress, the anthocyanin cyanidin-malonyl glucoside could be only studied in the RSB. The presence of an hormetic response in the 10–20 mM NaCl range is noteworthy. The relative improvement was consistent with the values previously reported in the literature, commonly below a 60% increase over the control [20]. Flavonoids and phenolic acids are both connected to the shikimic acid/phenylpropanoid pathway but, also in strawberry fruits, a mild stress (40 mM NaCl) induced the accumulation anthocyanins while polyphenols were increased at a higher concentration (80 mM) [51]. Even if limited to one variety, the observed larger accumulation of anthocyanins increases its dietary value, considering that these compounds are potentially useful for human health because of their antioxidant activities [52,53].
5. Conclusions
The optimal management of nutrient supply in hydroponic systems is critical for sustaining prime crop physiological and productive attributes, besides being crucial for configuring desirable nutritive and phytochemical attributes in fresh vegetables. Our study indicated that a short (two weeks) exposure to low-to-moderate salt stress caused divergent responses among phytochemicals in fresh cut lettuce growing at high density. Furthermore, our work indicated that the two varieties in some cases modulate the accumulation of specific polyphenols differently in controlled conditions. An adequate description of hormesis can be obtained with four to five doses (that are less than 25% of the EC50 concentration) [54]. However, modelling of the here described responses would require in-depth investigations and further studies will have to clarify whether the phytochemical responses are part of a (threshold dependent) compensatory mechanism with an adaptive role to low doses of salt stress. Compared to time-demanding improvement of key plant traits through breeding or the establishment of costly cultivation infrastructure, combining the selection of optimal genotypes with a tailored management, the electrical conductivity of the nutrient solution represents an effective, low-cost alternative to modulate the phytochemical profile in lettuce. Finally, the significant differences of the two cultivars and of their response also highlight the importance of the genetic factor in the short-growing cycle of baby lettuce.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/separations8100175/s1, Table S1: Amount of K and Na in leaf. Data are mean ± standard error of the mean. Table S2: Coefficient of variation (CV) of the polyphenols across conditions. 3,5-CQA: isochlorogenic acid; 5-CQA: chlorogenic acid; CMA: caffeoylmalic acid; CTA: caffeoyltartaric acid; DCTA: cichoric acid; m-DCTA: meso-di-O-caffeoyltartaric acid; TP: total polyphenols. Figure S1: Amount of K and Na in leaf. Data are mean ± standard error of the mean. Figure S2: Minimum and maximum air temperature (°C) and mean relative humidity (%) during the growing cycle (DAS: days after sowing). Figure S3: Biometric analysis of the leaves of the two lettuce cultivars, GSB (green) and RSB (red). For each plot, the graph displays the mean and the standard error of the mean (n = 3) at the four different NaCl concentrations. A: Fresh weight; B: average leaf area; C: water content; D: leaf number per plant; E: specific leaf area (SLA); F: leaf succulence at harvest (LSH). The statistical analysis is reported in Table 1. Figure S4: Leaf coloration analysis of the two lettuce cultivars, GSB (green) and RSB (red). For each plot, the graph displays the mean and the standard error of the mean (n = 3) at the four different NaCl concentrations. A: lightness (L*); B: degree of redness (a*); C: degree of yellowness (b*); D: chroma. The statistical analysis is reported in Table 2. Figure S5: Analysis of the leaf polyphenols of the two lettuce cultivars GSB (green) and RSB (red). For each plot, the graph displays the mean and the standard error of the mean (n = 3) at the four different NaCl concentrations. 3,5-CQA: isochlorogenic acid; 5-CQA: chlorogenic acid; CMA: caffeoylmalic acid; CTA: caffeoyltartaric acid; DCTA: cichoric acid; m-DCTA: meso-di-O-caffeoyltartaric acid. The statistical analysis is reported in Table 3. Figure S6: Analysis of the cyanidin-malonyl glucoside (CMG) in the RSB variety. The graph displays the mean and the standard error of the mean (n = 3) at the four different NaCl concentration. The statistical analysis is reported in Table 4.
Author Contributions
Conceptualization, Y.R.; methodology, G.C.; software, G.C. and Y.R.; validation, G.C. and Y.R.; formal analysis, M.G., G.R., F.N., E.D.S., I.D.M. and M.M.; investigation, G.C., P.V. and Y.R.; resources, Y.R.; data curation, G.C. and Y.R.; writing—original draft preparation, G.C.; writing—review and editing, G.C., P.V. and Y.R.; visualization, G.C. and Y.R.; supervision, G.C. and Y.R.; project administration, G.C. and Y.R.; funding acquisition, Y.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or supplementary material. The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mou, B. Lettuce. In Vegetables I: Asteraceae, Brassicaceae, Chenopodicaceae, and Cucurbitaceae; Prohens, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; pp. 75–116. [Google Scholar]
- De Corato, U. Improving the shelf-life and quality of fresh and minimally-processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. Nutr. 2020, 60, 940–975. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A. Pubchem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Galardi, C.; Sani, G.; Cimato, A.; Heimler, D. Polyphenols in greenhouse and open-air-grown lettuce. Food Chem. 2002, 79, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Materska, M.; Olszówka, K.; Chilczuk, B.; Stochmal, A.; Pecio, Ł.; Pacholczyk-Sienicka, B.; Piacente, S.; Pizza, C.; Masullo, M. Polyphenolic profiles in lettuce (Lactuca sativa L.) after CaCl2 treatment and cold storage. Eur. Food Res. Technol. 2019, 245, 733–744. [Google Scholar] [CrossRef] [Green Version]
- Nazzaro, F.; Fratianni, F.; De Feo, V.; Battistelli, A.; Cruz, A.; Coppola, R. Polyphenols, the new frontiers of prebiotics. Adv. Food Nutr. Res. 2020, 94, 35–38. [Google Scholar] [PubMed]
- Cheng, D.M.; Pogrebnyak, N.; Kuhn, P.; Poulev, A.; Waterman, C.; Rojas-Silva, P.; Johnson, W.D.; Raskin, I. Polyphenol-rich rutgers scarlet lettuce improves glucose metabolism and liver lipid accumulation in diet-induced obese c57bl/6 mice. Nutrition 2014, 30, S52–S58. [Google Scholar] [CrossRef] [Green Version]
- Chon, S.-U.; Boo, H.-O.; Heo, B.-G.; Gorinstein, S. Anthocyanin content and the activities of polyphenol oxidase, peroxidase and phenylalanine ammonia-lyase in lettuce cultivars. Int. J. Food Sci. Nutr. 2012, 63, 45–48. [Google Scholar] [CrossRef]
- Spence, C.; Levitan, C.A.; Shankar, M.U.; Zampini, M. Does food color influence taste and flavor perception in humans? Chemosens. Percept. 2010, 3, 68–84. [Google Scholar] [CrossRef]
- Arce-Lopera, C.; Masuda, T.; Kimura, A.; Wada, Y.; Okajima, K. Model of vegetable freshness perception using luminance cues. Food Qual. Prefer. 2015, 40, 279–286. [Google Scholar] [CrossRef]
- Gazula, A.; Kleinhenz, M.D.; Scheerens, J.C.; Ling, P.P. Anthocyanin levels in nine lettuce (Lactuca sativa) cultivars: Influence of planting date and relations among analytic, instrumented, and visual assessments of color. HortScience 2007, 42, 232–238. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, microgreens and “baby leaf” vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Springer: Berlin/Heidelberg, Germany, 2017; pp. 403–432. [Google Scholar]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Choe, U.; Yu, L.L.; Wang, T.T. The science behind microgreens as an exciting new food for the 21st century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Luna, M.C.; Selma, M.V.; Tudela, J.A.; Abad, J.; Gil, M.I. Baby-leaf and multi-leaf of green and red lettuces are suitable raw materials for the fresh-cut industry. Postharvest Biol. Technol. 2012, 63, 1–10. [Google Scholar] [CrossRef]
- Subhasree, B.; Baskar, R.; Keerthana, R.L.; Susan, R.L.; Rajasekaran, P. Evaluation of antioxidant potential in selected green leafy vegetables. Food Chem. 2009, 115, 1213–1220. [Google Scholar] [CrossRef]
- Vázquez-Hernández, M.; Parola-Contreras, I.; Montoya-Gómez, L.; Torres-Pacheco, I.; Schwarz, D.; Guevara-González, R. Eustressors: Chemical and physical stress factors used to enhance vegetables production. Sci. Hortic. 2019, 250, 223–229. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: Highly generalizable and beyond laboratory. Trends Plant Sci. 2020, 25, 1076–1086. [Google Scholar] [CrossRef]
- Belz, R.G.; Sinkkonen, A. Low toxin doses change plant size distribution in dense populations–glyphosate exposed Hordeum vulgare as a greenhouse case study. Environ. Int. 2019, 132, 105072. [Google Scholar] [CrossRef] [PubMed]
- Belz, R.G.; Farooq, M.B.; Wagner, J. Does selective hormesis impact herbicide resistance evolution in weeds? Accase-resistant populations of Alopecurus myosuroides huds. As a case study. Pest Manag. Sci. 2018, 74, 1880–1891. [Google Scholar] [CrossRef] [PubMed]
- Hooker, A.M.; Bhat, M.; Day, T.K.; Lane, J.M.; Swinburne, S.J.; Morley, A.A.; Sykes, P.J. The linear no-threshold model does not hold for low-dose ionizing radiation. Radiat. Res. 2004, 162, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Kong, E.Y.; Cheng, S.H.; Yu, K.N. Biphasic and triphasic dose responses in zebrafish embryos to low-dose 150 kv x-rays with different levels of hardness. J. Radiat. Res. 2016, 57, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Riga, P.; Benedicto, L.; Gil-Izquierdo, Á.; Collado-González, J.; Ferreres, F.; Medina, S. Diffuse light affects the contents of vitamin C, phenolic compounds and free amino acids in lettuce plants. Food Chem. 2019, 272, 227–234. [Google Scholar] [CrossRef]
- Carillo, P.; Cirillo, C.; De Micco, V.; Arena, C.; De Pascale, S.; Rouphael, Y. Morpho-anatomical, physiological and biochemical adaptive responses to saline water of Bougainvillea spectabilis Willd. trained to different canopy shapes. Agric. Water Manag. 2019, 212, 12–22. [Google Scholar] [CrossRef]
- Yang, X.; Wei, S.; Liu, B.; Guo, D.; Zheng, B.; Feng, L.; Liu, Y.; Tomás-Barberán, F.A.; Luo, L.; Huang, D. A novel integrated non-targeted metabolomic analysis reveals significant metabolite variations between different lettuce (Lactuca sativa L) varieties. Hortic. Res. 2018, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mampholo, B.M.; Maboko, M.M.; Soundy, P.; Sivakumar, D. Phytochemicals and overall quality of leafy lettuce (Lactuca sativa L.) varieties grown in closed hydroponic system. J. Food Qual. 2016, 39, 805–815. [Google Scholar] [CrossRef]
- Miras-Moreno, B.; Corrado, G.; Zhang, L.; Senizza, B.; Righetti, L.; Bruni, R.; El-Nakhel, C.; Sifola, M.I.; Pannico, A.; Pascale, S.D. The metabolic reprogramming induced by sub-optimal nutritional and light inputs in soilless cultivated green and red butterhead lettuce. Int. J. Mol. Sci. 2020, 21, 6381. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.; Grieve, C. Tolerance of vegetable crops to salinity. Sci. Hortic. 1998, 78, 5–38. [Google Scholar] [CrossRef]
- Ozgen, S.; Sekerci, S. Effect of leaf position on the distribution of phytochemicals and antioxidant capacity among green and red lettuce cultivars. Span. J. Agric. Res. 2011, 3, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, N.D.; Simko, I.; Mou, B. Phenomic and physiological analysis of salinity effects on lettuce. Sensors 2019, 19, 4814. [Google Scholar] [CrossRef] [Green Version]
- Senizza, B.; Zhang, L.; Miras-Moreno, B.; Righetti, L.; Zengin, G.; Ak, G.; Bruni, R.; Lucini, L.; Sifola, M.I.; El-Nakhel, C. The strength of the nutrient solution modulates the functional profile of hydroponically grown lettuce in a genotype-dependent manner. Foods 2020, 9, 1156. [Google Scholar] [CrossRef]
- Carillo, P.; Giordano, M.; Raimondi, G.; Napolitano, F.; Di Stasio, E.; Kyriacou, M.C.; Sifola, M.I.; Rouphael, Y. Physiological and nutraceutical quality of green and red pigmented lettuce in response to NaCl concentration in two successive harvests. Agronomy 2020, 10, 1358. [Google Scholar] [CrossRef]
- Corrado, G.; Lucini, L.; Miras-Moreno, B.; Zhang, L.; El-Nakhel, C.; Colla, G.; Rouphael, Y. Intraspecific variability largely affects the leaf metabolomics response to isosmotic macrocation variations in two divergent lettuce (Lactuca sativa L.) varieties. Plants 2021, 10, 91. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Ripoll, J.; Urban, L.; Staudt, M.; Lopez-Lauri, F.; Bidel, L.P.; Bertin, N. Water shortage and quality of fleshy fruits—making the most of the unavoidable. J. Exp. Bot. 2014, 65, 4097–4117. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Kyriacou, M.C. Enhancing quality of fresh vegetables through salinity eustress and biofortification applications facilitated by soilless cultivation. Front. Plant Sci. 2018, 9, 1254. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- Nicolle, C.; Carnat, A.; Fraisse, D.; Lamaison, J.L.; Rock, E.; Michel, H.; Amouroux, P.; Remesy, C. Characterisation and variation of antioxidant micronutrients in lettuce (Lactuca sativa folium). J. Sci. Food Agric. 2004, 84, 2061–2069. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Muzolf-Panek, M.; Goliński, P. Phenolic content changes in plants under salt stress. In Ecophysiology and Responses of Plants under Salt Stress; Springer: Berlin/Heidelberg, Germany, 2013; pp. 283–314. [Google Scholar]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Carillo, P.; Soteriou, G.A.; Kyriacou, M.C.; Giordano, M.; Raimondi, G.; Napolitano, F.; Di Stasio, E.; Mola, I.D.; Mori, M.; Rouphael, Y. Regulated salinity eustress in a floating hydroponic module of sequentially harvested lettuce modulates phytochemical constitution, plant resilience, and post-harvest nutraceutical quality. Agronomy 2021, 11, 1040. [Google Scholar] [CrossRef]
- Campagna-Fernandes, A.F.; Marin, E.B.; Penha, T.H.F.L. Application of root growth endpoint in toxicity tests with lettuce (Lactuca sativa). Ecotoxicol. Environ. Contam. 2016, 11, 27–32. [Google Scholar]
- Lisanti, M.T.; Mataffo, A.; Scognamiglio, P.; Teobaldelli, M.; Iovane, M.; Piombino, P.; Rouphael, Y.; Kyriacou, M.C.; Corrado, G.; Basile, B. 1-methylcyclopropene improves postharvest performances and sensorial attributes of annurca-type apples exposed to the traditional reddening in open-field melaio. Agronomy 2021, 11, 1056. [Google Scholar] [CrossRef]
- Kassambara, A. Practical Guide to Principal Component Methods in R; Datanovia: Montpellier, FR, USA, 2017; Volume 2, p. 264. [Google Scholar]
- Neocleous, D.; Koukounaras, A.; Siomos, A.; Vasilakakis, M. Assessing the salinity effects on mineral composition and nutritional quality of green and red “baby” lettuce. J. Food Qual. 2014, 37, 1–8. [Google Scholar] [CrossRef]
- Zhang, M.; Whitman, C.M.; Runkle, E.S. Manipulating growth, color, and taste attributes of fresh cut lettuce by greenhouse supplemental lighting. Sci. Hortic. 2019, 252, 274–282. [Google Scholar] [CrossRef]
- Su, W.; Tao, R.; Liu, W.; Yu, C.; Yue, Z.; He, S.; Lavelle, D.; Zhang, W.; Zhang, L.; An, G. Characterization of four polymorphic genes controlling red leaf colour in lettuce that have undergone disruptive selection since domestication. Plant Biotechnol. J. 2020, 18, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Belz, R.G.; Patama, M.; Sinkkonen, A. Low doses of six toxicants change plant size distribution in dense populations of lactuca sativa. Sci. Total. Environ. 2018, 631, 510–523. [Google Scholar] [CrossRef]
- Galli, V.; da Silva Messias, R.; Perin, E.C.; Borowski, J.M.; Bamberg, A.L.; Rombaldi, C.V. Mild salt stress improves strawberry fruit quality. LWT 2016, 73, 693–699. [Google Scholar] [CrossRef]
- Liobikas, J.; Skemiene, K.; Trumbeckaite, S.; Borutaite, V. Anthocyanins in cardioprotection: A path through mitochondria. Pharmacol. Res. 2016, 113, 808–815. [Google Scholar] [CrossRef]
- Bendokas, V.; Skemiene, K.; Trumbeckaite, S.; Stanys, V.; Passamonti, S.; Borutaite, V.; Liobikas, J. Anthocyanins: From plant pigments to health benefits at mitochondrial level. Crit. Rev. Food Sci. Nutr. 2020, 60, 3352–3365. [Google Scholar] [CrossRef]
- Cedergreen, N.; Ritz, C.; Streibig, J.C. Improved empirical models describing hormesis. Environ. Toxicol. Chem. Int. J. 2005, 24, 3166–3172. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).