3.1. Analytical Parameters of Moravian Wines, Harvest 2015
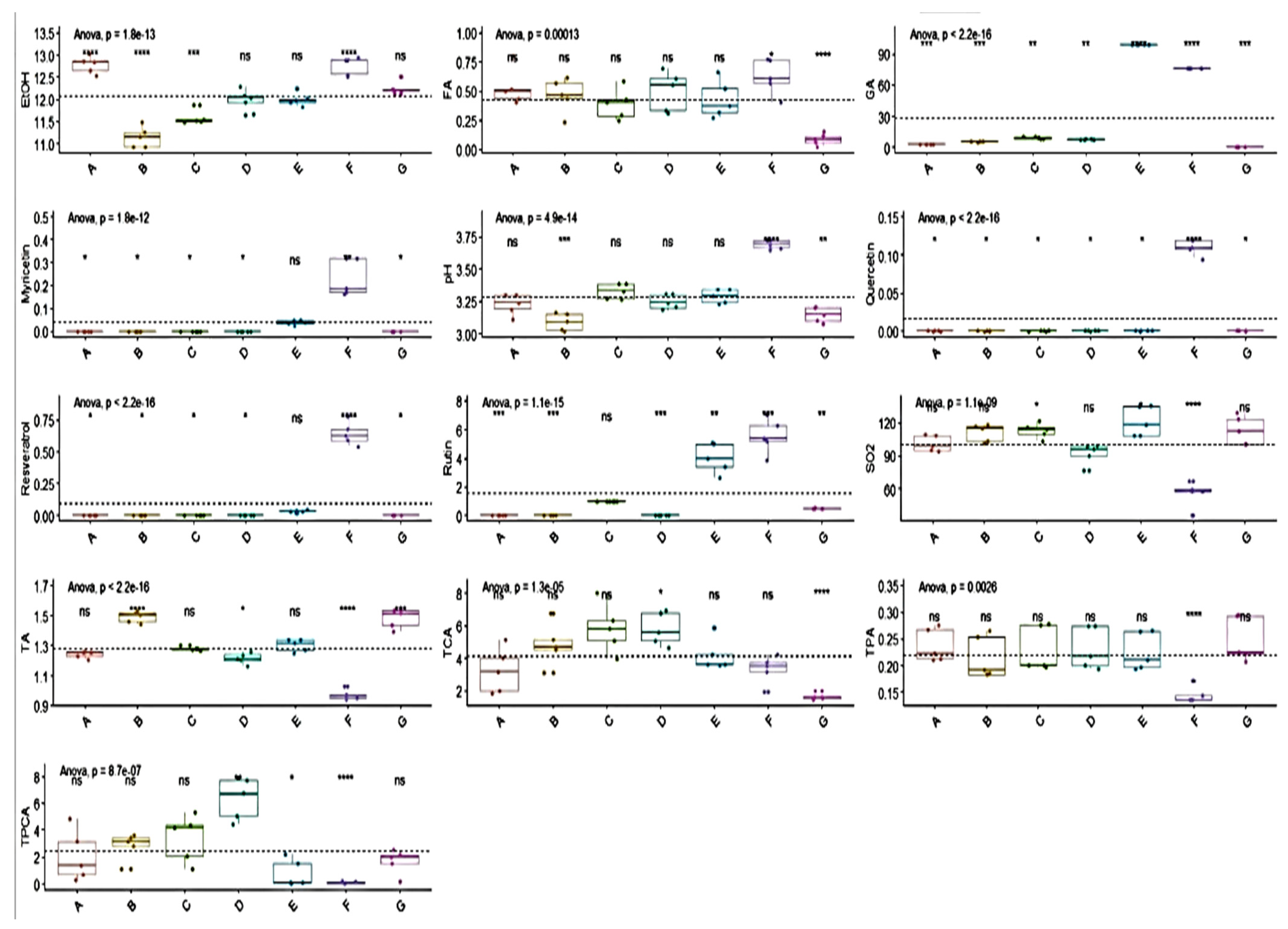
The results of the determination of oenological parameters and biologically active substances are shown in the charts of
Figure 1. The pH values ranged from 3.05 to 3.76, which is consistent with the statement by Robinson [
28] that the pH of wine is usually between 2.9 and 4.2. Chira et al. [
29] measured slightly higher values for Cabernet Sauvignon samples. The ethanol levels in their wine samples ranged between 10.53% and 13.16% and the values measured by us were also within this range. The presence of total SO
2 in our samples is important for preservation. It has been used as a preservative in winemaking for centuries [
30]. The content of total polyphenols is affected by the terrain, processing technique, climate, and harvest year [
31,
32,
33,
34,
35]. The content of total polyphenols in our samples was relatively consistent, with the exception of sample F. The results of the basic analytical values such as total phenolic acid (TPA), total sulfur dioxide (SO
2), pH, actual alcohol content (EtOH), and titratable acidity (TA) were in accordance with generally known values in wines and met the legislative requirements within oenological practices. These results may be due to the fact that all the tested wines were harvested in 2015, from a single producer, as well as from a single region of the South Moravian area. They are associated with properties such as appearance, taste, mouth-feel, fragrance to a certain extent, and antimicrobial activity [
36]. A significant group of phenols is formed by flavonoids. As reported by Ali et al. [
37], the most common flavonoids in wine are flavonols (quercetin, kaempferol, myricetin, etc.), flavan-3-ols (catechin and epicatechin), and anthocyanins. Polymerization of polyhydroxy flavan-3-ol units, (+)-catechin and (−)-epicatechin, and their gallate esters produces oligomers and polymers called proanthocyanidins (often referred to as procyanidins).
In our study, we successfully identified some of the biologically active substances, such as gallic acid, trans-caffeic acid, trans-p-coumaric acid, rutin, ferulic acid, myricetin, resveratrol, and quercetin.
The occurrence of substances, such as myricetin, quercetin, resveratrol, and rutin, in red wines has been described by many authors. The average value of resveratrol in sample F was almost six times higher than those in other wines, where it was found in low concentrations. This parameter attracts a lot of attention for its positive effects on human health. It is generally found in wines in relatively low concentrations compared to other phenols [
38]. As argued by García-Puente Rivas et al. [
39], Monagas et al. [
40], and Parpinello et al. [
41], phenolic compounds are a crucial factor in the quality of red wine, as they are responsible for the color, bitterness, and astringency, which are the key sensory attributes of the consumer’s acceptability of wine.
Higher values of GA, rutin, and total SO
2 were measured in sample E (Pinot Noir—claret, medium dry). As stated by Pozo-Bayon et al. [
42], GA is one of the most common hydroxybenzoic acids and it occurs in wines in lower concentrations. This statement is consistent with the results of our samples with the exception of samples E and F. Interesting results were found for sample F (Pinot Noir—red, dry), where higher values of GA, myricetin, pH, quercetin, resveratrol, and rutin and, on the other hand, lower values of total SO
2, TA, TPA, and TPCA were measured compared to other tested wines. In general, low FA, GA, and TCA values were measured in sample G. Higher TCA and TPCA values were detected in wine sample D.
The oenological parameters and biologically active substances determined in our experiment were statistically evaluated by Levene and Barlett tests. The results of the statistical tests are shown in
Table 3.
Statistically significant differences are highlighted in bold. Using the Levene test (<0.01), statistically significant differences were found between the tested wine varieties for the parameters of TPA, TA, gallic acid, trans-p-coumaric acid, rutin, myricetin, resveratrol, and quercetin. The Barlett test showed statistically significant differences (<0.01) between the tested wines in the parameters of gallic acid, trans-p-coumaric acid, rutin, myricetin, resveratrol, and quercetin.
Despite the diversity of the wines tested, no statistically significant differences were found using both tests for total SO2, pH, EtOH, trans-caffeic acid, and ferulic acid.
The results shown in
Figure 2 clearly demonstrate that the most statistically significant correlations were for the parameters of quercetin, resveratrol, and pH, which correlated with all parameters except trans-caffeic acid. A similar result was observed for myricetin and rutin that did not significantly correlate only with trans-caffeic acid and also with ferulic acid. TA significantly correlated with all the parameters tested except trans-caffeic acid and trans-p-coumaric acid.
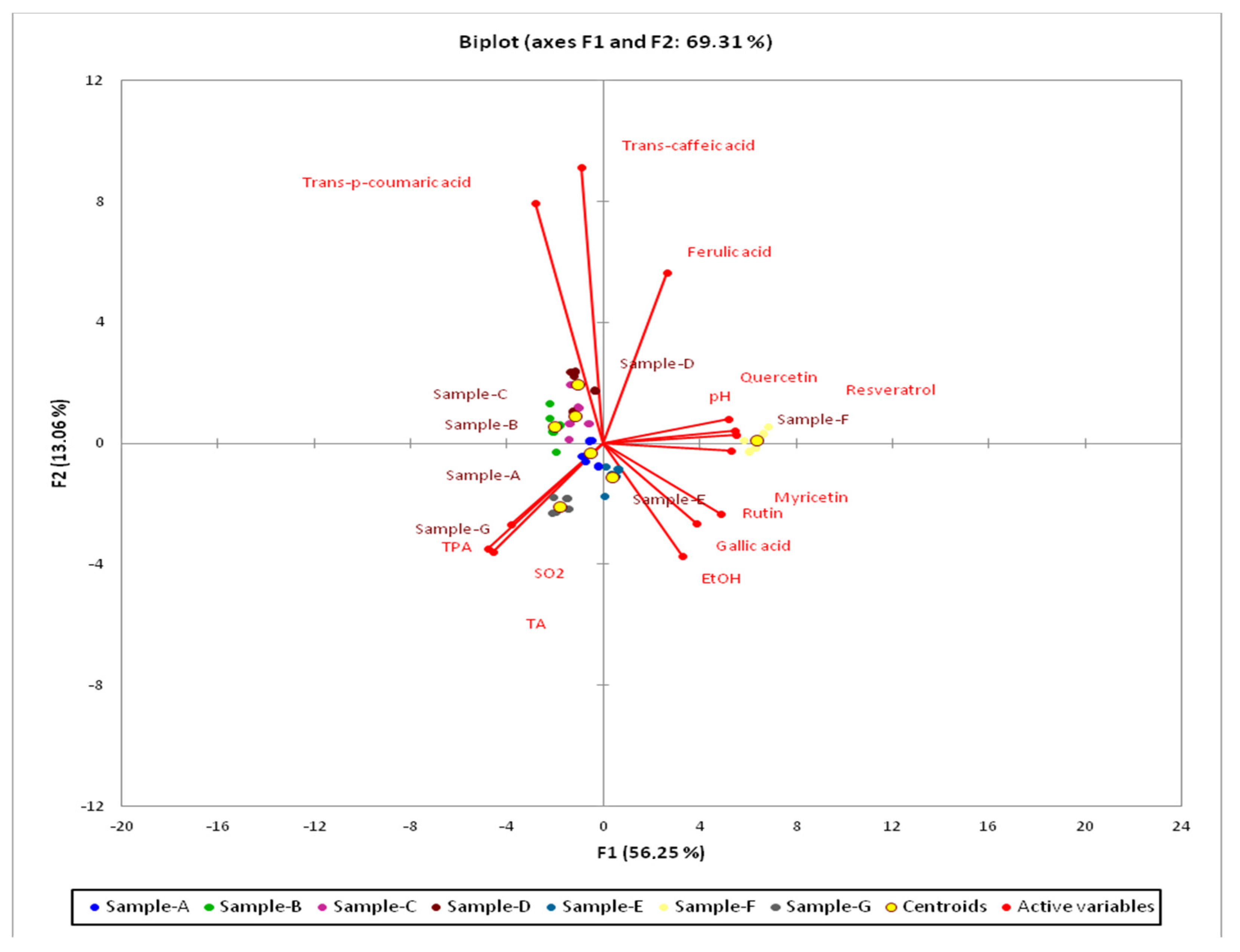
The Kaiser–Meyer–Olkin (KMO) test of sampling adequacy showed middling suitability of the data for the complete model (KMO = 0.67). Bartlett’s test of sphericity was significant (chi-square (observed) = 574, chi-square (critical) = 99.6,
p ≤ 0.0001), indicating that the data were likely factorizable. The PCA revealed that 69.31% of the total variation embodied in 13 variables could be effectively condensed into and explained by the first two principal components (PCs), with eigenvalues of 7.31 and 1.69, respectively. PC1, accounting for 56.25% of the inertia, and PC2, explaining 13.06% of the inertia, clearly reflected the content of trans-caffeic acid and trans-p-coumaric acid.
Figure 3 and
Figure 4 show 2-D maps for PC1 and PC2 in which the tested variables are clustered around the centroids. The centroids present factor scores and squared cosines that are the coordinates and representation qualities, respectively. The most important variables for F1 were resveratrol, quercetin, myricetin, pH, and TA. The most important variables for F2 were trans-caffeic acid and trans-p-coumaric acid. Only the F variety was clearly separated from the other localities (
Figure 3). The F variety was characterized by pH, myricetin, quercetin, and resveratrol. The B, C, and D varieties were characterized by trans-caffeic acid and trans-p-coumaric acid, while the A and G samples were characterized by total SO
2, TPA, and TA. The E sample was characterized mostly by EtOH (
Figure 4).
3.2. Waste Treatment in the Production of Wines Harvest 2015
A partial study of the wines of the year 2015 was also the monitoring of the grape pomace produced during the production of wine with the indication of the possibilities of their further use. Due to the dates from the evidence book of the producer, it is clear that, in the production of wine, there are losses of around 30% from the process of processing grapes to the production of wine intended for bottling. Losses in production are mostly grape pomace. According to the literature data, the amount of grape pomace accumulated during wine production is about 20–30% [
20,
43].
Grape pomace includes the skins and the pulp, usually the seeds, and, in some cases, the stems. Leaving the stems with the grapes during crushing, pressing and fermentation used to be the traditional practice. The tannin-rich stems give the wine a more bitter, astringent, and herbaceous taste. However, as this type of wine has become less popular, current wine-making practices favor destemming, and many wines are made with grapes crushed after the removal of the stems [
44]. The producer supplies grape marc originated during wine production to another company in the South Moravian Region. Grape marc is processed in such a way that the present grape seeds stays intact. Grape seeds are dried, cleaned, and crushed, and grape oil is produced from them by cold pressing. The oil produced in this way is 100% extra virgin, without direct and preservative substances.
Grape seed represents the valuable byproduct of the wine production. Recently, the use of grape seeds has been increasing worldwide. They are used to produce nutritionally valuable oil with a high proportion of polyunsaturated fatty acids [
45]. Compared with other vegetable oils, grape seed oil has low quantities of linolenic acid, which increases the oxidative stability and can represent an advantage in terms of human consumption and shelf life of the oil [
46]. The oil represents a valuable source of bioactive substances beneficial to the human body. However, although consumers perceive grape seed oil as a healthier alternative than other oils, this product is not widely used, probably because of its high price, and it is not extensively utilized by the food industry [
43].
Wine lees is a sludge material mainly composed of dead yeast precipitated at the bottom of wine tanks. Along with grape pomace and grape stalks, it is one of the main by-products of the winemaking industry [
47]. Yeast as another waste generated during wine production is taken from another company in the South Moravian Region, where it is processed for isolation and subsequent sale in order to increase the sensory properties of wine for use in the production.
Other wastes from wine production are used, where necessary, to enrich the soil in the vineyards of the producer.
Most wine producers in the Czech Republic use marc, sludge, pomace, and other grape byproducts in other productions, especially to enrich the soil.
Fresh marc from ripe grapes is suitable for the production of distillate, often so-called pomace brandy. White varieties are usually used in the process of pomace brandy production; the marc is not pressed too much. The process of pomace brandy includes the squeezing of grape pomace, and about 20% water is added. It is important that the material is fermented rapidly and distilled immediately after fermentation [
48].
It is also possible to produce flour (gluten-free alternative) from seeds. Grape seed flour (GSF), a byproduct of grape seed in the wine-making process, contains 60–70% of the extractable flavonoids of the grape, including catechin, epicatechin, and epigallocatechin, as well as procyanidin dimers and trimers. Studies have shown that the antioxidant activities of GSF play major roles in the attenuation of high-fat diet-induced oxidative stress, resulting in beneficial health effects on hepatic steatosis [
49].
Pressed products of grapes can be a starting material for the extraction of anthocyanin dyes, used as a natural variant for dyeing in the food industry and natural textiles. From the growing popularity of waste-free management, the processing of various parts of the wine production byproducts as a bioenergy raw material is an interesting issue too from an ecological point of view. Wood chips or even pulp can be used as an alternative source of heating. Waste composting, if done correctly, produces material very rich in organic matter and is completely reusable in accordance with the ecology of the processing. Subsequently, it is also possible to use other parts of plants, such as wine leaves, where extracts from them are used in the production of cosmetic or medicinal preparations [
45].
Within the production of wine harvest 2015 used in our research, according to the information obtained from the producer, the yield of grape pomace and information about samples are shown in
Table 4 and
Table 5.
3.3. Evaluation of Selected Biological Compounds of Moravian Wines, Harvest 2016
Wine aging is the period that starts at the end of winemaking (after different processing depending on the wine variety and the vineries common practice) and continues after bottling until consumption. The aging of wine in the bottle, where contact with oxygen is minimal, consists of its evolution under reduced conditions and results, besides the color change, in an improvement of the sensory (olfactory and tasteful) characteristics. The speed of such a transformation is not the same for all wines: it depends on their initial composition and cellar conditions. Redox potential, pH, and humidity, together with temperature, determine the evolutionary conditions during bottle aging. During this process, wine is particularly sensitive to storage temperature and light radiation [
50]. The predominant changes occurring during bottle-ageing involve the transformation of volatile constituents as wines re-establish a chemical equilibrium between acids, alcohols, and corresponding esters—reactions that have temperature-dependent rates [
51].
The research also evaluated the wines from the year 2016 stored under two different storage conditions. Part of the wines were stored in a climatic chamber at the temperature of 12 °C; the same part of the wines was stored at room temperature (21 ± 3 °C). Basic analytical parameters such as total phenolic acid (TPA) of total sulfur dioxide (SO2), pH, actual alcohol content (EtOH), and titratable acidity (TA) were evaluated. Further, we identified and monitored biologically active substances, such as gallic acid, trans-caffeic acid (TCA), trans-p-coumaric acid (TPCA), rutin, ferulic acid (FA), myricetin, resveratrol, and quercetin.
Due to the measured values, a slight increase in TPA under both storage conditions can be observed in
Table 6 and
Table 7 (Basic oenological parameters) for white wines with a lower sugar content. The development of total SO
2 is insignificant due to the measurement in individual stages of storage also due to the permitted limits according to part B Regulation (EU) No. 2019/934. Especially for white wines (
Table 6,
Table 7 and
Table 8) and rosé wine (
Table 9) we can observe a decrease in alcohol content depending on the length of storage. At the end of the storage, TA values were lower for white wines with a lower sugar content (
Table 6 and
Table 7) and also for rosé wine (
Table 9). Oenological parameters of red wines (
Table 10 and
Table 11) were within the normal range for the given types of wines. In
Table 12 and
Table 13 (Biologically active substances) we can observe higher values of biologically active substances measured in dry white wine (Sauvignon: sample A) compared to semi-dry wine (Chardonnay: sample B). The development of gallic acid was not significant for any wine under the given storage conditions. TPCA values had a decreasing tendency especially at room temperature except for white wine with a higher sugar content after 14 months of storage, as can be seen from
Table 14 (Biologically active substances). For sample C and sample D in
Table 14 and
Table 15 (Biologically active substances), white wine with a higher sugar content and rosé wine with a higher sugar content, a slight decrease in ferulic acid was observed after 14 months of storage. In contrast, for sample F (red sweet wine), a slight increase in ferulic acid can be observed from
Table 16 (Biologically active substances) after storage at climatic chamber. The development of rutin had a slight decrease after 14 months of storage at room temperature in sample E (red dry wine,
Table 17); however, in sample F (red sweet wine,
Table 16), a slight increase in rutin was observed under the same conditions. Quercetin and myricetin, two flavonoids belonging to the flavonol class [
52], were detected in red dry wine.