Comparison of Online Comprehensive HILIC × RP and RP × RP with Trapping Modulation Coupled to Mass Spectrometry for Microalgae Peptidomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Columns
2.4. Instrumentation
2.5. LC and HRMS Parameters
3. Results and Discussion
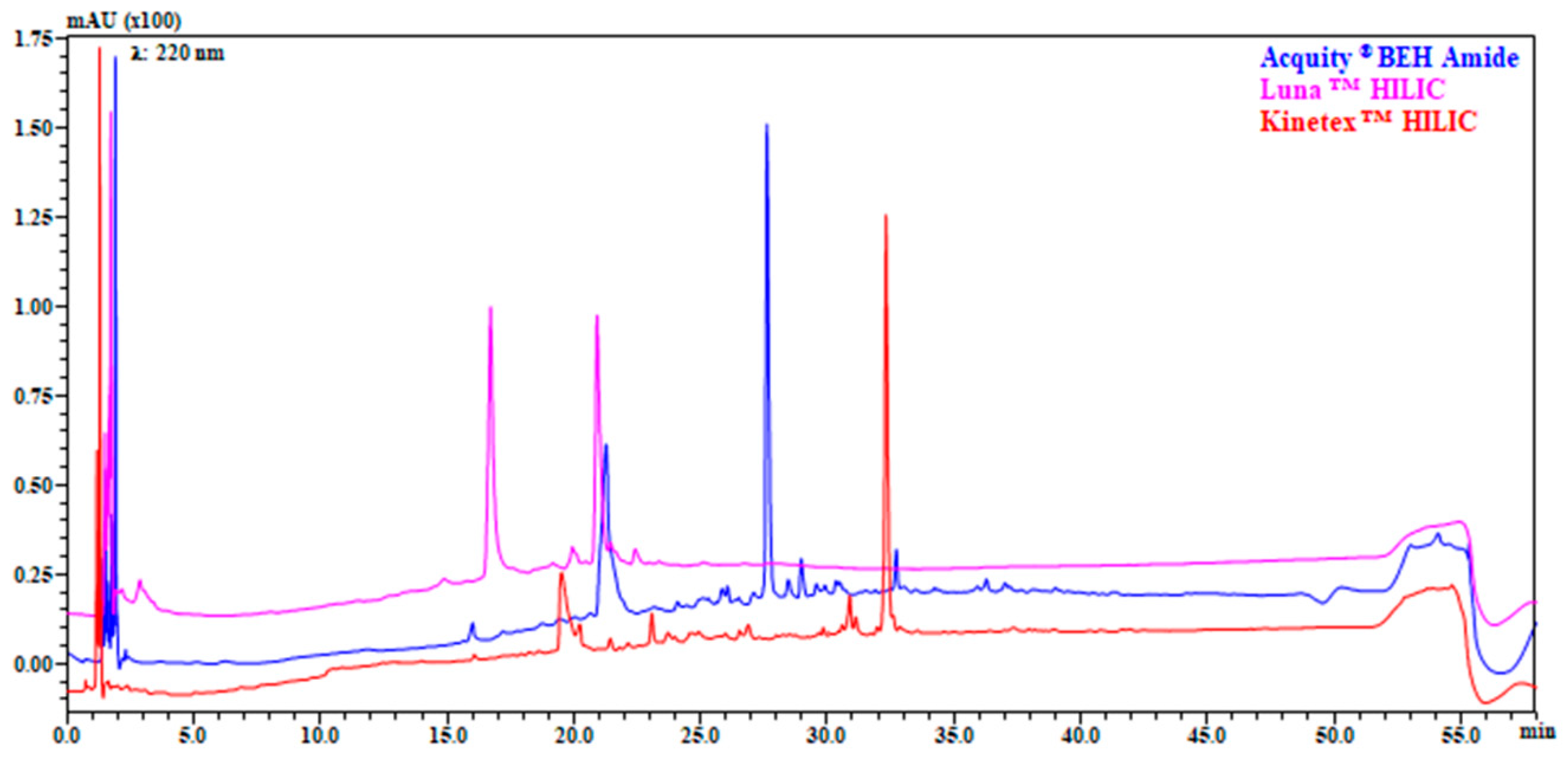
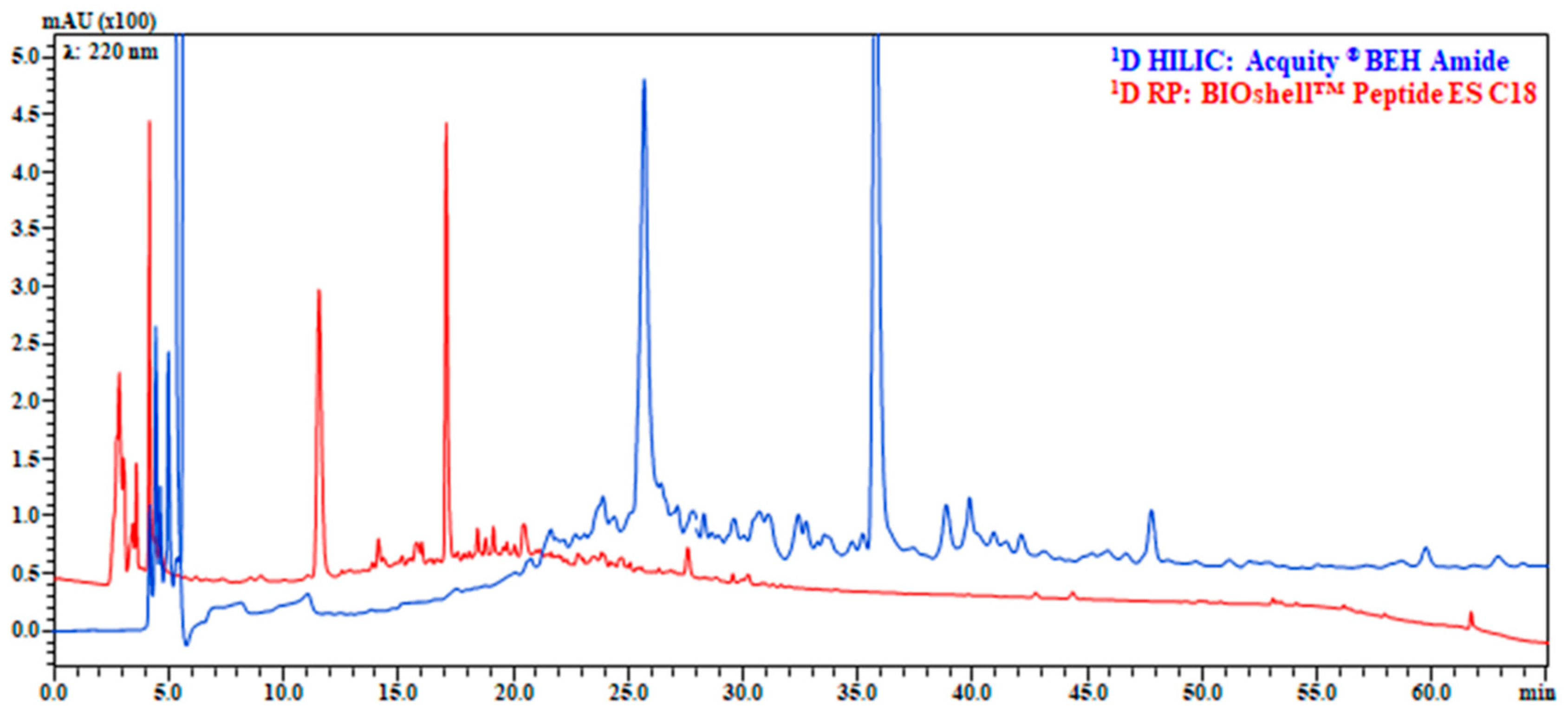
3.1. Evaluation of Chromatographic Conditions in 1D (HILIC and RP) and 2D (RP)
3.2. HILIC × RP and RP × RP with Trapping Modulation: System Performance Evaluation
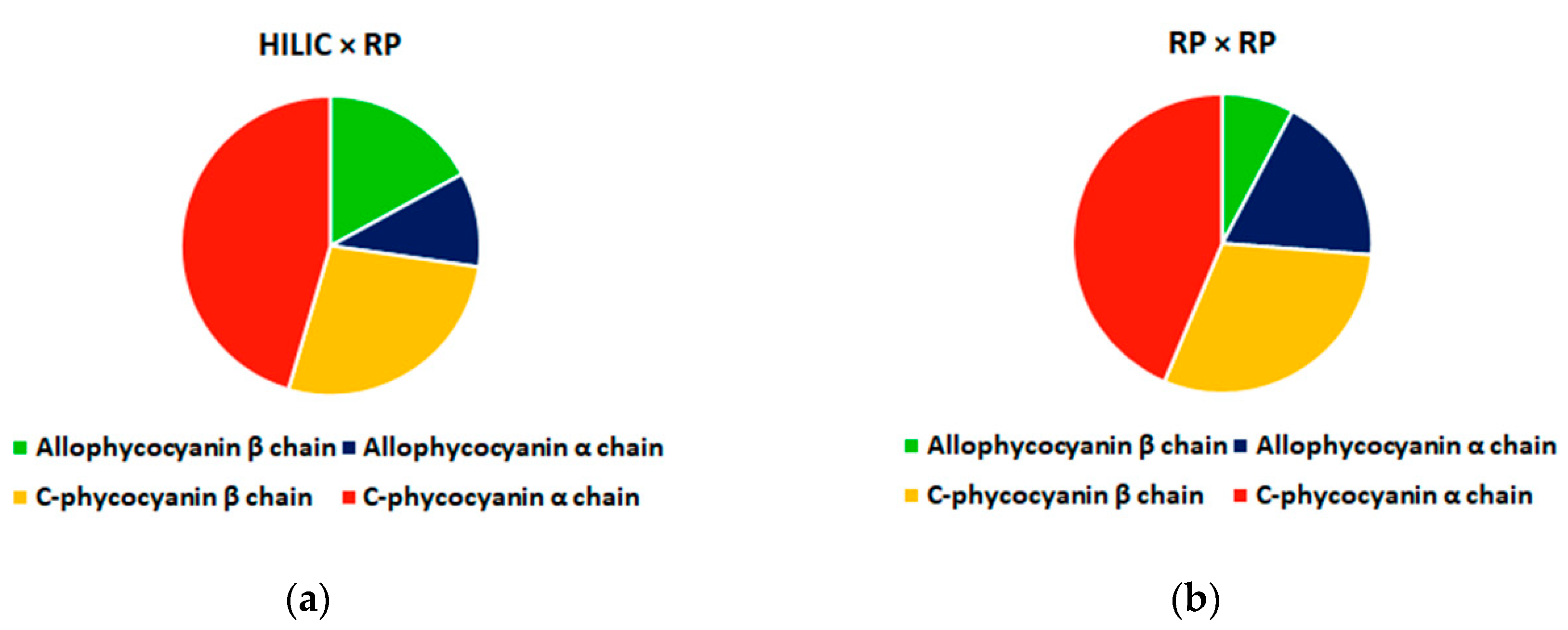
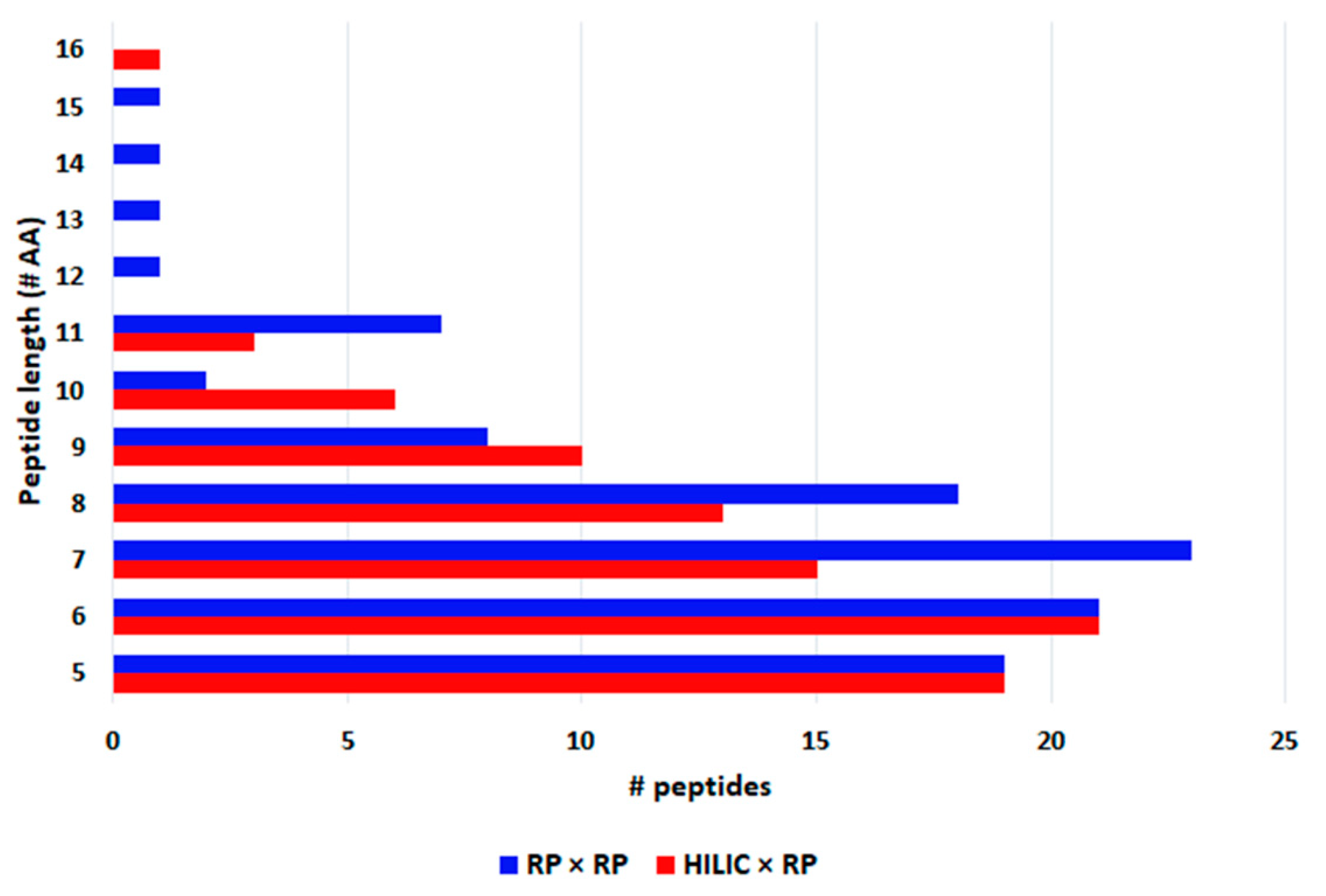
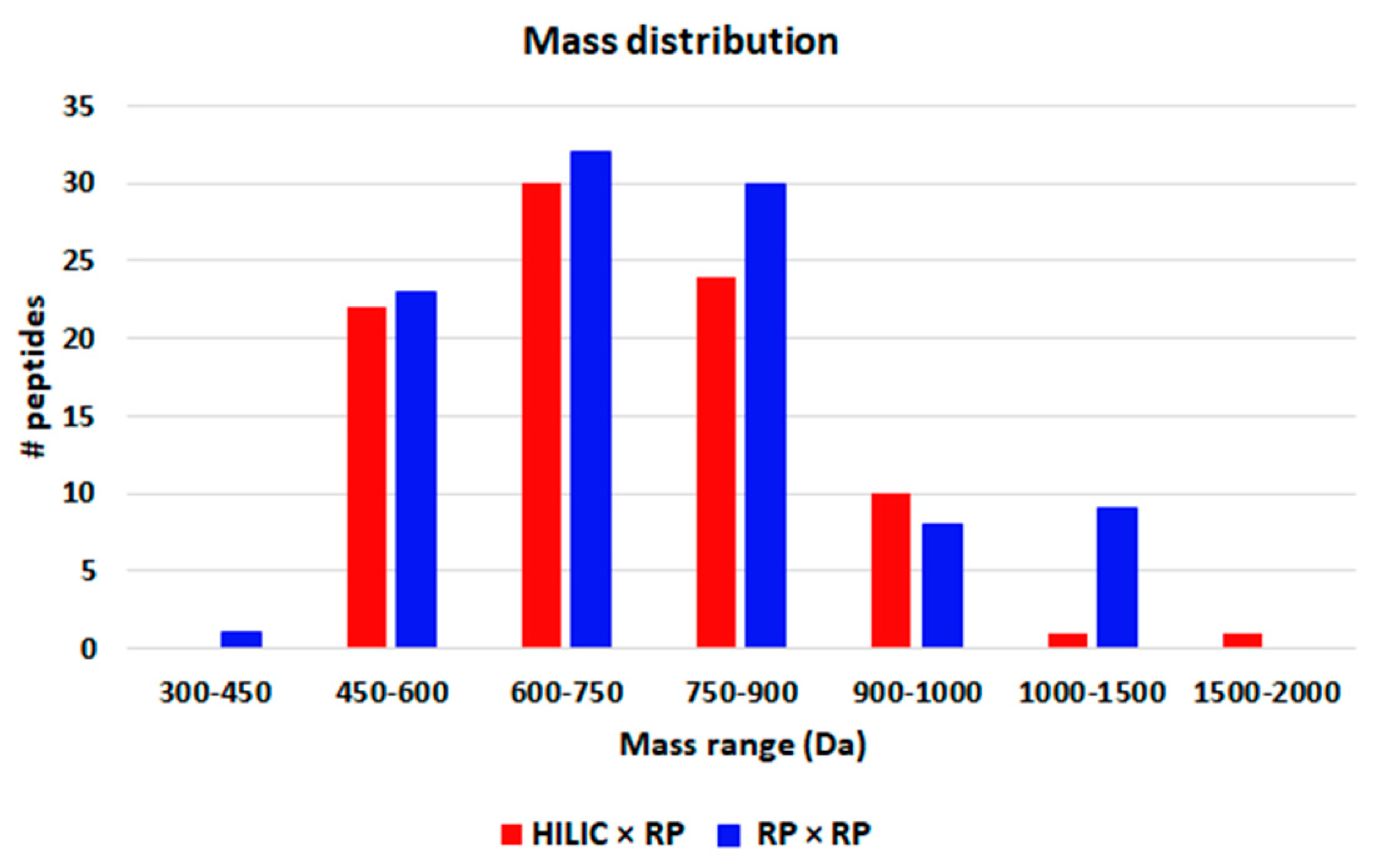
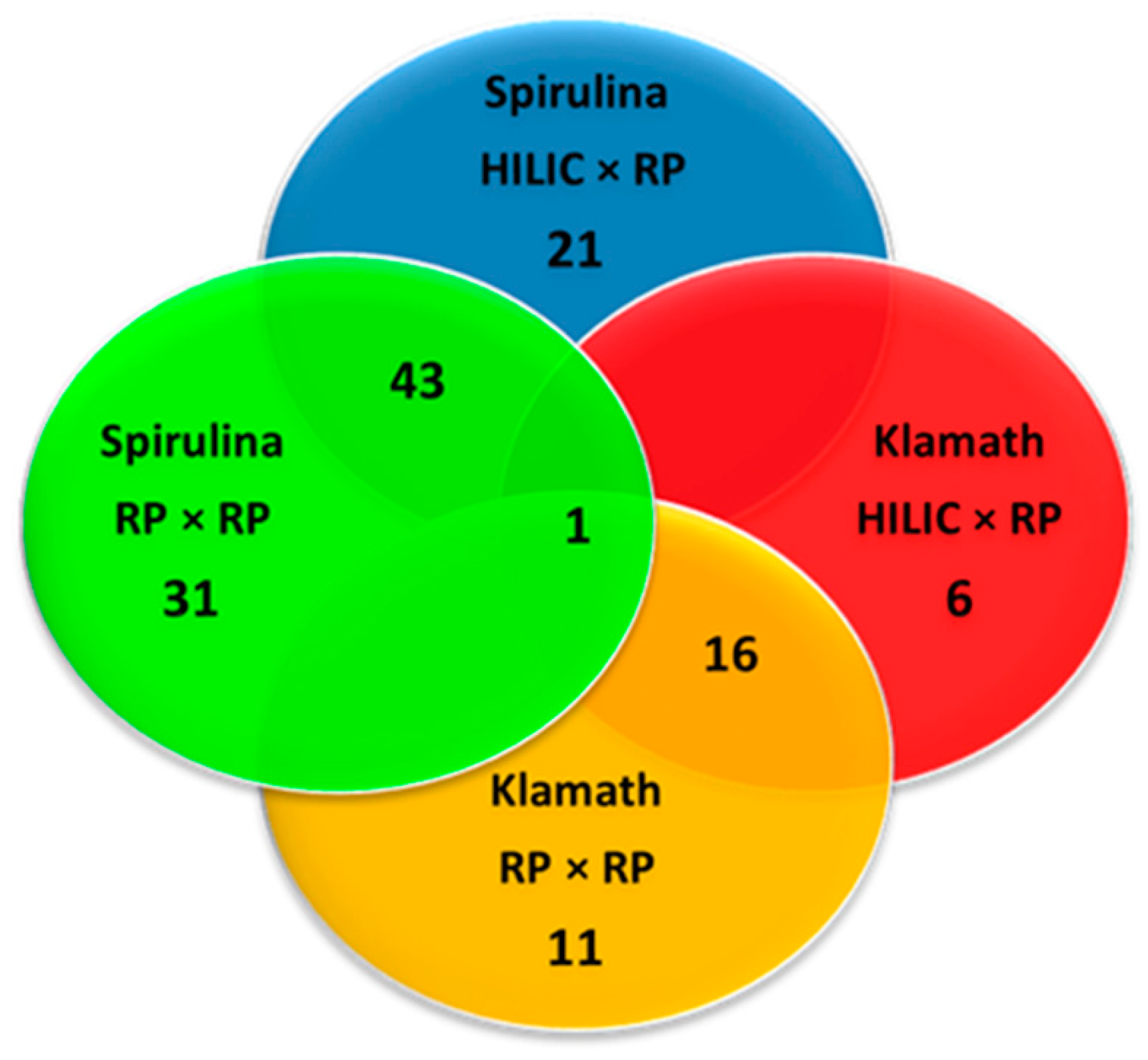
3.3. Comparison of HILIC × RP and RP × RP Coupled to Tandem Mass Spectrometry for Peptide Mapping
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lv, W.; Shi, X.; Wang, S.; Xu, G. Multidimensional liquid chromatography-mass spectrometry for metabolomic and lipidomic analyses. Trends Anal. Chem. 2019, 120, 115302. [Google Scholar] [CrossRef]
- Dugo, P.; Cacciola, F.; Kumm, T.; Dugo, G.; Mondello, L. Comprehensive multidimensional liquid chromatography: Theory and applications. J. Chromatogr. A 2008, 1184, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Donato, P.; Cacciola, F.; Sommella, E.; Fanali, C.; Dugo, L.; Dachà, M.; Campiglia, P.; Novellino, E.; Dugo, P.; Mondello, L. Online comprehensive RPLC × RPLC with mass spectrometry detection for the analysis of proteome samples. Anal. Chem. 2011, 83, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pepe, G.; Ventre, G.; Pagano, F.; Manfra, M.; Pierri, G.; Ismail, O.H.; Ciogli, A.; Campiglia, P. Evaluation of two sub-2 m stationary phases, core-shell and totally porous monodisperse, in the second dimension of on-line comprehensive two dimensional liquid chromatography, a case study: Separation of milk peptides after expiration date. J. Chromatogr. A 2015, 1375, 54–61. [Google Scholar] [CrossRef]
- Sandra, K.; Steenbeke, M.; Vandenheede, I.; Vanhoenacker, G.; Sandra, P. The versatility of heart-cutting and comprehensive two-dimensional liquid chromatography in monoclonal antibody clone selection. J. Chromatogr. A 2017, 1523, 283–292. [Google Scholar] [CrossRef]
- Boersema, P.J.; Mohammed, S.; Heck, A.J.R. Hydrophilic interaction liquid chromatography (HILIC) in proteomics. Anal. Bioanal. Chem. 2008, 391, 151–159. [Google Scholar] [CrossRef]
- Gilar, M.; Olivova, P.; Daly, A.E.; Gebler, J.C. Orthogonality of separation in two-dimensional liquid chromatography. Anal. Chem. 2005, 77, 6426–6434. [Google Scholar] [CrossRef]
- Stoll, D.R.; Harmes, D.C.; Staples, G.O.; Potter, O.G.; Dammann, C.T.; Guillarme, D.; Beck, A. Development of Comprehensive Online Two-Dimensional Liquid Chromatography/Mass Spectrometry Using Hydrophilic Interaction and Reversed-Phase Separations for Rapid and Deep Profiling of Therapeutic Antibodies. Anal. Chem. 2018, 90, 5923–5929. [Google Scholar] [CrossRef]
- Gargano, A.F.G.; Shaw, J.B.; Zhou, M.; Wilkins, C.S.; Fillmore, T.L.; Moore, R.J.; Somsen, G.W.; Paša-Tolić, L. Increasing the Separation Capacity of Intact Histone Proteoforms Chromatography Coupling Online Weak Cation Exchange-HILIC to Reversed Phase LC UVPD-HRMS. J. Proteome Res. 2018, 11, 3791–3800. [Google Scholar] [CrossRef]
- Chapel, S.; Rouvière, F.; Heinisch, S. Pushing the limits of resolving power and analysis time in on-line comprehensive hydrophilic interaction × reversed phase liquid chromatography for the analysis of complex peptide samples. J. Chromatogr. A 2019. [Google Scholar] [CrossRef]
- Sommella, E.; Salviati, E.; Merciai, F.; Manfra, M.; Bertamino, A.; Gasparrini, F.; Novellino, E.; Campiglia, P. Online comprehensive hydrophilic interaction chromatography × reversed phase liquid chromatography coupled to mass spectrometry for in depth peptidomic profile of microalgae gastro-Intestinal digests. J. Pharm. Biomed. Anal. 2019, 175, 112783. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Conte, G.M.; Salviati, E.; Pepe, G.; Bertamino, A.; Ostacolo, C.; Sansone, F.; Del Prete, F.; Aquino, R.P.; Campiglia, P. Fast profiling of natural pigments in different Spirulina (Arthrospira platensis) dietary supplements by DI-FT-ICR and evaluation of their antioxidant potential by pre-column DPPH-UHPLC assay. Molecules 2018, 23, 1152. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Sommella, E.; Ventre, G.; Scala, M.C.; Adesso, S.; Ostacolo, C.; Marzocco, S.; Novellino, E.; Campiglia, P. Antioxidant peptides released from gastrointestinal digestion of “Stracchino” soft cheese: Characterization, in vitro intestinal protection and bioavailability. J. Func. Foods 2016, 26, 494–505. [Google Scholar] [CrossRef]
- Carrizzo, A.; Conte, G.M.; Sommella, E.; Damato, A.; Ambrosio, M.; Sala, M.; Scala, M.C.; Aquino, R.P.; De Lucia, M.; Madonna, M.; et al. Novel potent decameric peptide of Spirulina platensis reduces blood pressure levels through a PI3K/AKT/eNOS-Dependent mechanism. Hypertension 2019, 73, 449–457. [Google Scholar] [CrossRef]
- Donato, P.; Cacciola, F.; Mondello, L.; Dugo, P. Comprehensive chromatographic separations in proteomics. J. Chromatogr. A 2011, 1218, 8777–8790. [Google Scholar] [CrossRef]
- Stoll, D.R.; Lhotka, H.R.; Harmes, D.C.; Madigan, B.; Hsiao, J.J.; Staples, G.O. High resolution two-Dimensional liquid chromatography coupled with mass spectrometry for robust and sensitive characterization of therapeutic antibodies at the peptide level. J. Chromatogr. B 2019, 121832, 1134–1135. [Google Scholar] [CrossRef]
- Sommella, E.; Pagano, F.; Salviati, E.; Chieppa, M.; Bertamino, A.; Manfra, M.; Sala, M.; Novellino, E.; Campiglia, P. Chemical profiling of bioactive constituents in hop cones and pellets extracts by online comprehensive two-dimensional liquid chromatography with tandem mass spectrometry and direct infusion Fourier transform ion cyclotron resonance mass spectrometry. J. Sep. Sci. 2018, 41, 1548–1557. [Google Scholar] [CrossRef]
- Filgueira, M.R.; Huang, Y.; Witt, K.; Castells, C.; Carr, P.W. Improving peak capacity in fast on line comprehensive two-dimensional liquid chromatography with post first dimension flow splitting. Anal. Chem. 2011, 83, 9531–9539. [Google Scholar] [CrossRef]
- Camenzuli, M.; Schoenmakers, P.J. A new measure of orthogonality for multidimensional chromatography. Anal. Chim. Acta 2014, 838, 93–101. [Google Scholar] [CrossRef]
- Mihailova, A.; Malerød, H.; Wilson, S.R.; Karaszewski, B.; Hauser, R.; Lundanes, E.; Greibrokk, T. Improving the resolution of neuropeptides in rat brain with on-line HILIC-RP compared to on-Line SCX-RP. J. Sep. Sci. 2008, 31, 459–467. [Google Scholar] [CrossRef]
- Di Palma, S.; Mohammed, S.; Heck, A.J.R. ZIC-cHILIC as a fractionation method for sensitive and powerful shotgun proteomics. Nat. Protoc. 2012, 7, 2041–2055. [Google Scholar] [CrossRef] [PubMed]

| Parameters | HILIC × RP-UHPLC | RP × RP-UHPLC |
|---|---|---|
| 1D column geometry | Acquity UPLCTM BEH Amide | Bioshell® Peptide ES C18 |
| 150 mm × 2.1 mm, 1.7 μm (130 Å) | 150 × 2.1 mm, 2.0 µm (160 Å) | |
| 1D flow rate | 0.1 mL/min | |
| 1D column temperature | 40 °C | |
| 2D column geometry | BIOshellTM Peptide ES C18 | |
| 50 mm × 3.0 mm, 2.7 μm (160 Å) | ||
| 2D Flow rate | 2.2 mL/min | |
| 2D column temperature | 50 °C. | |
| 2D gradient | multi-segment shifting mode | |
| Analysis time | 60 min | 60 min |
| Modulation time | 45 s | |
| Post 1D Dilution flow | 1 mL/min | 0.2 mL/min |
| Theoretical 2Dnc | 1951 | 1613 |
| Undersampling | 1.43 | 1.36 |
| Theoretical 2Dnc/undersampling | 1369 | 1190 |
| Ortogonality A0 | 0.70 | 0.59 |
| Practical 2Dnc | 932 | 701 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommella, E.; Salviati, E.; Musella, S.; Di Sarno, V.; Gasparrini, F.; Campiglia, P. Comparison of Online Comprehensive HILIC × RP and RP × RP with Trapping Modulation Coupled to Mass Spectrometry for Microalgae Peptidomics. Separations 2020, 7, 25. https://doi.org/10.3390/separations7020025
Sommella E, Salviati E, Musella S, Di Sarno V, Gasparrini F, Campiglia P. Comparison of Online Comprehensive HILIC × RP and RP × RP with Trapping Modulation Coupled to Mass Spectrometry for Microalgae Peptidomics. Separations. 2020; 7(2):25. https://doi.org/10.3390/separations7020025
Chicago/Turabian StyleSommella, Eduardo, Emanuela Salviati, Simona Musella, Veronica Di Sarno, Francesco Gasparrini, and Pietro Campiglia. 2020. "Comparison of Online Comprehensive HILIC × RP and RP × RP with Trapping Modulation Coupled to Mass Spectrometry for Microalgae Peptidomics" Separations 7, no. 2: 25. https://doi.org/10.3390/separations7020025
APA StyleSommella, E., Salviati, E., Musella, S., Di Sarno, V., Gasparrini, F., & Campiglia, P. (2020). Comparison of Online Comprehensive HILIC × RP and RP × RP with Trapping Modulation Coupled to Mass Spectrometry for Microalgae Peptidomics. Separations, 7(2), 25. https://doi.org/10.3390/separations7020025









