Characterization of Turmeric and Curry Samples by Liquid Chromatography with Spectroscopic Detection Based on Polyphenolic and Curcuminoid Contents
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Samples and Sample Treatment
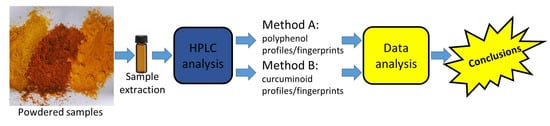
2.3. Chromatographic Analysis
2.4. Data Analysis
3. Results and Discussion
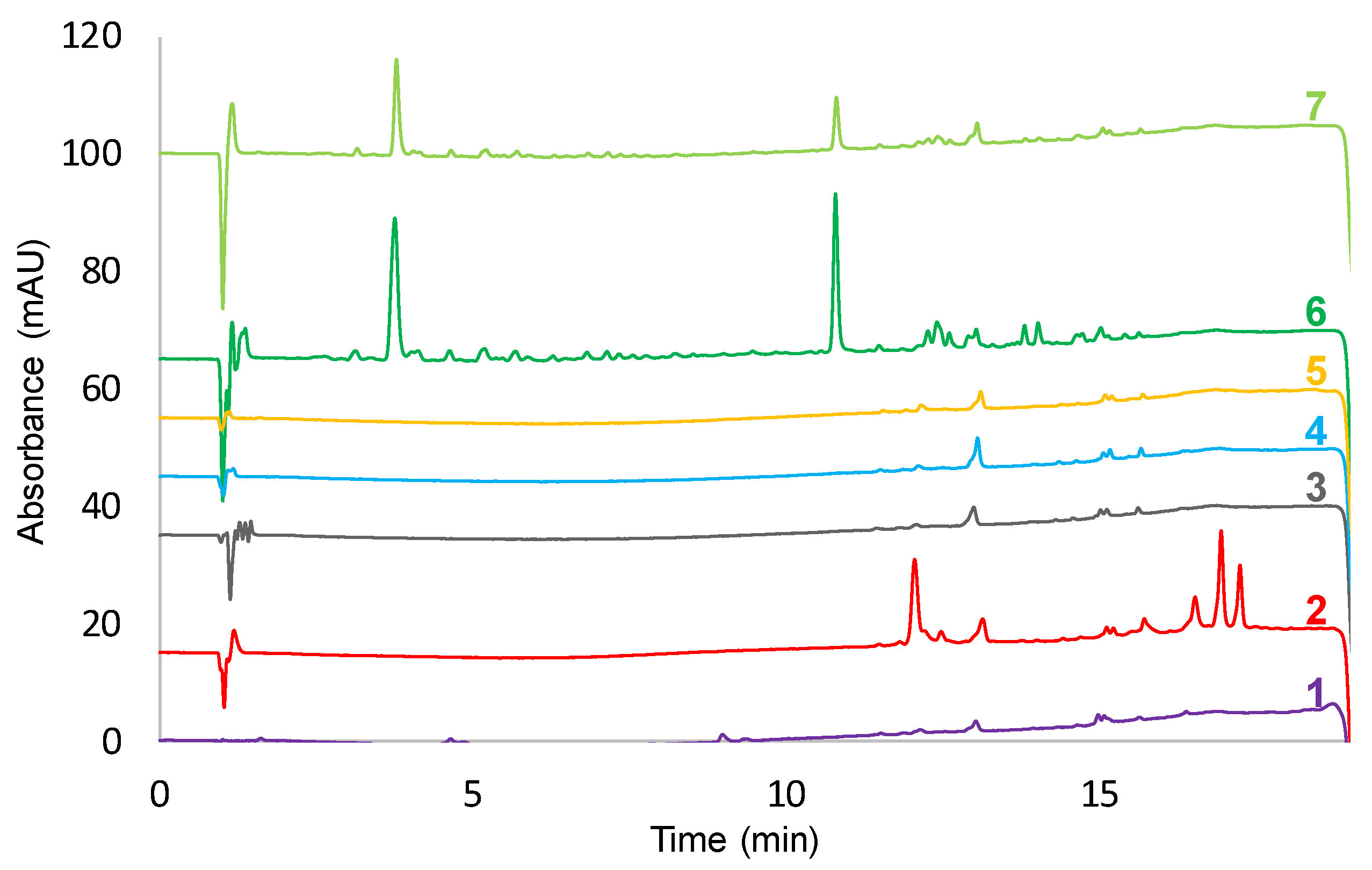
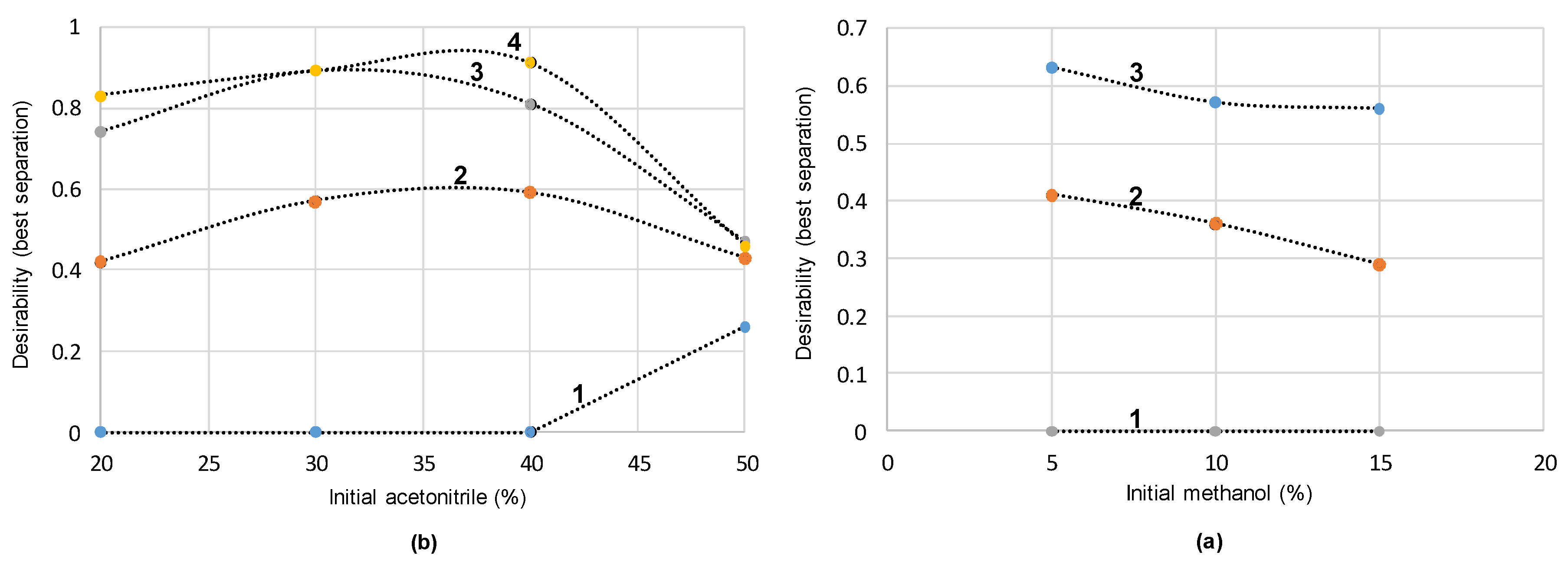
3.1. Optimization of the Extraction
3.2. Optimization of the Separation Conditions
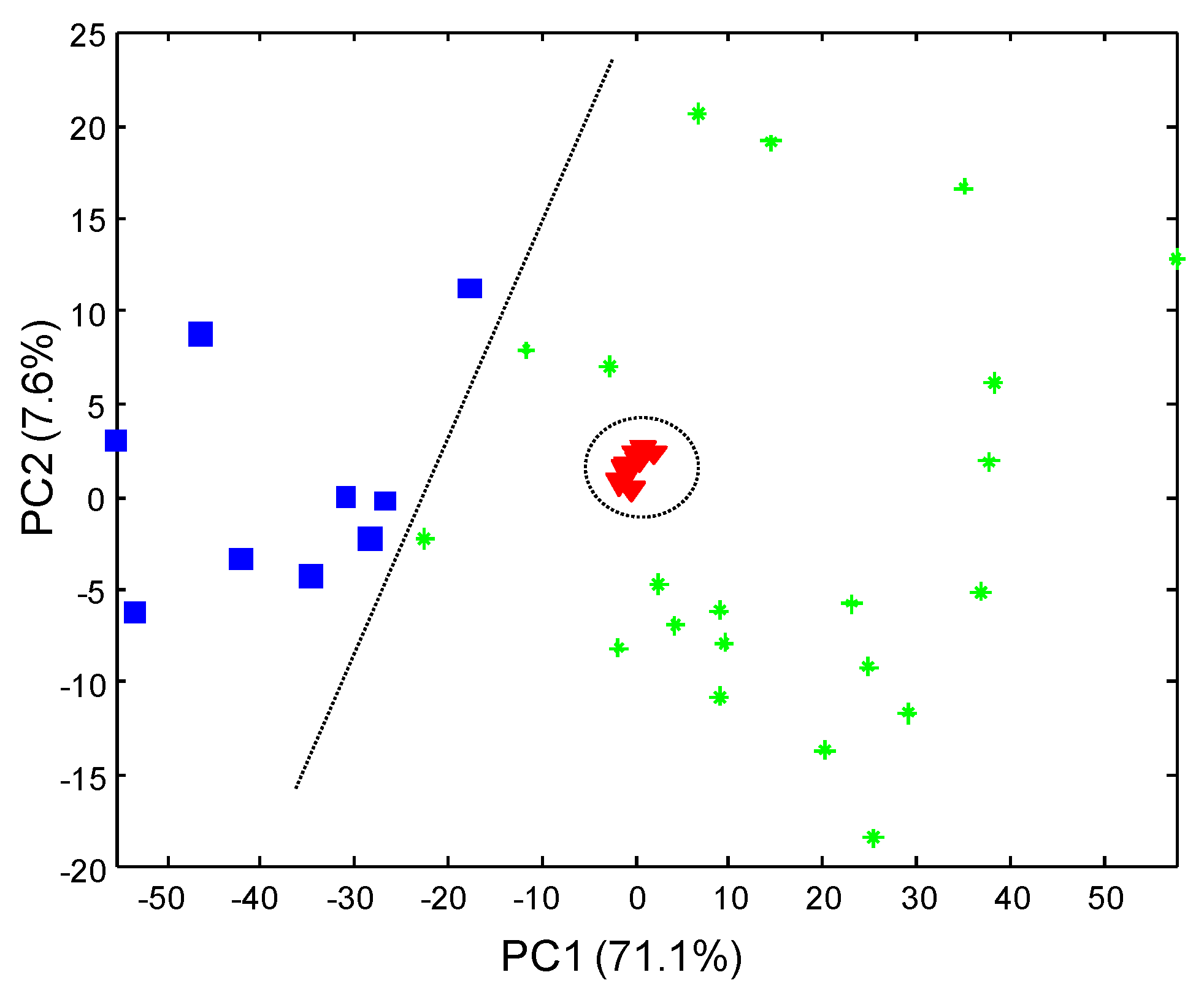
3.3. Sample Characterization by PCA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, B. Genetic Resources of Curcuma: Diversity, Characterization and Utilization. Plant Genet. Resour. 2005, 3, 230–251. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Roselin, P.; Singh, K.K.; Zachariah, J.; Saxena, S.N. Postharvest Processing and Benefits of Black Pepper, Coriander, Cinnamon, Fenugreek, and Turmeric Spices. Crit. Rev. Food Sci. Nutr. 2016, 56, 1585–1607. [Google Scholar] [CrossRef]
- Nair, K.L.; Thulasidasan, A.K.T.; Deepa, G.; Anto, R.J.; Kumar, G.S.V. Purely Aqueous PLGA Nanoparticulate Formulations of Curcumin Exhibit Enhanced Anticancer Activity with Dependence on the Combination of the Carrier. Int. J. Pharm. 2012, 425, 44–52. [Google Scholar] [CrossRef]
- Bhawana; Basniwal, R.K.; Buttar, H.S.; Jain, V.K.; Jain, N. Curcumin Nanoparticles: Preparation, Characterization, and Antimicrobial Study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar] [CrossRef]
- Othman, R.; Abdurasid, M.A.; Mahmad, N.; Ahmad Fadzillah, N. Alkaline-Based Curcumin Extraction from Selected Zingiberaceae for Antimicrobial and Antioxidant Activities. Pigment Resin Technol. 2019, 48, 289–296. [Google Scholar] [CrossRef]
- Da Silva, A.C.; de Santos, P.D.F.; do Silva, J.T.P.; Leimann, F.V.; Bracht, L.; Gonçalves, O.H. Impact of Curcumin Nanoformulation on Its Antimicrobial Activity. Trends Food Sci. Technol. 2018, 72, 74–82. [Google Scholar] [CrossRef]
- Kotra, V.S.R.; Satyabanta, L.; Goswami, T.K. A Critical Review of Analytical Methods for Determination of Curcuminoids in Turmeric. J. Food Sci. Technol. 2019, 56, 5153–5166. [Google Scholar] [CrossRef]
- Carolina Alves, R.; Perosa Fernandes, R.; Fonseca-Santos, B.; Damiani Victorelli, F.; Chorilli, M. A Critical Review of the Properties and Analytical Methods for the Determination of Curcumin in Biological and Pharmaceutical Matrices. Crit. Rev. Anal. Chem. 2019, 49, 138–149. [Google Scholar] [CrossRef]
- Chao, I.C.; Wang, C.M.; Li, S.P.; Lin, L.G.; Ye, W.C.; Zhang, Q.W. Simultaneous Quantification of Three Curcuminoids and Three Volatile Components of Curcuma Longa Using Pressurized Liquid Extraction and High-Performance Liquid Chromatography. Molecules 2018, 23, 1568. [Google Scholar] [CrossRef] [PubMed]
- Peram, M.R.; Jalalpure, S.S.; Joshi, S.A.; Palkar, M.B.; Diwan, P.V. Single Robust RP-HPLC Analytical Method for Quantification of Curcuminoids in Commercial Turmeric Products, Ayurvedic Medicines, and Nanovesicular Systems. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 487–498. [Google Scholar] [CrossRef]
- Ali, I.; Haque, A.; Saleem, K. Separation and identification of curcuminoids in turmeric powder by HPLC using phenyl column. Anal. Methods 2014, 6, 2526–2536. [Google Scholar] [CrossRef]
- Hiserodt, R.; Hartman, T.G.; Ho, C.T.; Rosen, R.T. Characterization of Powdered Turmeric by Liquid Chromatography-Mass Spectrometry and Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 1996, 740, 51–63. [Google Scholar] [CrossRef]
- Ashraf, K.; Mujeeb, M.; Ahmad, A.; Ahmad, N.; Amir, M. Determination of Curcuminoids in Curcuma Longa Linn. by UPLC/Q-TOF-MS: An Application in Turmeric Cultivation. J. Chromatogr. Sci. 2015, 53, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Timmermann, B.N.; Gang, D.R. Characterization and Identification of Diarylheptanoids in Ginger (Zingiber Officinale Rosc.) Using High-Performance Liquid Chromatography/Electrospray Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 509–518. [Google Scholar] [CrossRef]
- Jia, S.; Du, Z.; Song, C.; Jin, S.; Zhang, Y.; Feng, Y.; Xiong, C.; Jiang, H. Identification and Characterization of Curcuminoids in Turmeric Using Ultra-High Performance Liquid Chromatography-Quadrupole Time of Flight Tandem Mass Spectrometry. J. Chromatogr. A 2017, 1521, 110–122. [Google Scholar] [CrossRef]
- Qiao, X.; Lin, X.H.; Ji, S.; Zhang, Z.X.; Bo, T.; Guo, D.A.; Ye, M. Global Profiling and Novel Structure Discovery Using Multiple Neutral Loss/Precursor Ion Scanning Combined with Substructure Recognition and Statistical Analysis (MNPSS): Characterization of Terpene-Conjugated Curcuminoids in Curcuma Longa as a Case Study. Anal. Chem. 2016, 88, 703–710. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Nagana Gowda, G.A.; Marquez, S.; Patil, B.S. Rapid Separation and Quantitation of Curcuminoids Combining Pseudo Two-Dimensional Liquid Flash Chromatography and NMR Spectroscopy. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 937, 25–32. [Google Scholar] [CrossRef]
- Shi, M.; Gao, T.; Zhang, T.; Han, H. Characterization of Curcumin Metabolites in Rats by Ultra-High-Performance Liquid Chromatography with Electrospray Ionization Quadrupole Time-of-Flight Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2019, 33, 1114–1121. [Google Scholar] [CrossRef]
- Jude, S.; Amalraj, A.; Kunnumakkara, A.B.; Divya, C.; Löffler, B.M.; Gopi, S. Development of Validated Methods and Quantification of Curcuminoids and Curcumin Metabolites and Their Pharmacokinetic Study of Oral Administration of Complete Natural Turmeric Formulation (CureitTM) in Human Plasma via UPLC/ESI-Q-TOF-MS Spectrometry. Molecules 2018, 23, 2415. [Google Scholar] [CrossRef]
- Wu, C.; Wang, W.; Quan, F.; Chen, P.; Qian, J.; Zhou, L.; Pu, Q. Sensitive Analysis of Curcuminoids via Micellar Electrokinetic Chromatography with Laser-Induced Native Fluorescence Detection and Mixed Micelles-Induced Fluorescence Synergism. J. Chromatogr. A 2018, 1564, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Anubala, S.; Sekar, R.; Nagaiah, K. Determination of Curcuminoids and Their Degradation Products in Turmeric (Curcuma Longa) Rhizome Herbal Products by Non-Aqueous Capillary Electrophoresis with Photodiode Array Detection. Food Anal. Methods 2016, 9, 2567–2578. [Google Scholar] [CrossRef]
- Kalaycioğlu, Z.; Hashemi, P.; Günaydin, K.; Erim, F.B. The Sensitive Capillary Electrophoretic-LIF Method for Simultaneous Determination of Curcuminoids in Turmeric by Enhancing Fluorescence Intensities of Molecules upon Inclusion into (2-Hydroxypropyl)-β-Cyclodextrin. Electrophoresis 2015, 36, 2516–2521. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, R.; Yang, F.; Xiao, W.; Chen, C.; Xia, Z. Determination of Three Curcuminoids in Curcuma Longa by Microemulsion Electrokinetic Chromatography with Protective Effects on the Analytes. Anal. Methods 2014, 6, 2566–2571. [Google Scholar] [CrossRef]
- Unsal, Y.E.; Tuzen, M.; Soylak, M. Ultrasound-Assisted Ionic Liquid-Dispersive Liquid–Liquid of Curcumin in Food Samples Microextraction and Its Spectrophotometric Determination. J. AOAC Int. 2019, 102, 217–221. [Google Scholar] [CrossRef]
- Ali, Z.; Saleem, M.; Atta, B.M.; Khan, S.S.; Hammad, G. Determination of Curcuminoid Content in Turmeric Using Fluorescence Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 192–198. [Google Scholar] [CrossRef]
- Lestari, H.P.; Martono, S.; Wulandari, R.; Rohman, A. Simultaneous Analysis of Curcumin and Demethoxycurcumin in Curcuma Xanthorriza Using FTIR Spectroscopy and Chemometrics. Int. Food Res. J. 2017, 24, 2097–2101. [Google Scholar]
- Zokhtareh, R.; Rahimnejad, M. An Investigation of New Electrochemical Sensors for Curcumin Detection: A Mini Review. Anal. Methods 2019, 11, 4401–4409. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Yang, L.; Wang, L.; Ye, B. The Electrochemical Characterization of Curcumin and Its Selective Detection in Curcuma Using a Graphene-Modified Electrode. Anal. Methods 2014, 6, 7801–7808. [Google Scholar] [CrossRef]
- Chaisiwamongkhol, K.; Ngamchuea, K.; Batchelor-Mcauley, C.; Compton, R.G. Multiwalled Carbon Nanotube Modified Electrodes for the Adsorptive Stripping Voltammetric Determination and Quantification of Curcumin in Turmeric. Electroanalysis 2017, 29, 1049–1055. [Google Scholar] [CrossRef]
- Lucci, P.; Saurina, J.; Núñez, O. Trends in LC-MS and LC-HRMS Analysis and Characterization of Polyphenols in Food. TrAC Trends Anal. Chem. 2017, 88, 1–24. [Google Scholar] [CrossRef]
- Saurina, J. Characterization of Wines Using Compositional Profiles and Chemometrics. TrAC Trends Anal. Chem. 2010, 29, 234–245. [Google Scholar] [CrossRef]
- Sepahpour, S.; Selamat, J.; Manap, M.Y.A.; Khatib, A.; Razis, A.F.A. Comparative Analysis of Chemical Composition, Antioxidant Activity and Quantitative Characterization of Some Phenolic Compounds in Selected Herbs and Spices in Different Solvent Extraction Systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Regueiro, J.; Alvarenga, J.F.R.; Martinez-Huelamo, M.; Leal, L.N.; Lamuela-Raventos, R.M. Characterization of the Phenolic and Antioxidant Profiles of Selected Culinary Herbs and Spices: Caraway, Turmeric, Dill, Marjoram and Nutmeg. Food Sci. Technol. 2015, 35, 189–195. [Google Scholar] [CrossRef]
- Vidal, O.; Castilla, X.; Aliaga-Alcalde, N.; López-Periago, A.; Domingo, C.; Sentellas, S.; Saurina, J. Determination of Curcuminoids by Liquid Chromatography with Diode Array Detection. Application to the Characterization of Turmeric and Curry Samples. Curr. Anal. Chem. 2020, 16, 95–105. [Google Scholar] [CrossRef]
- Ni, Y.; Mei, M.; Kokot, S. Resolution of High Performance Liquid Chromatographic Fingerprints of Rhizoma Curcumae by Application of Chemometrics. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1952–1964. [Google Scholar] [CrossRef]
- Kulyal, P.; Kuchibhatla, L.N.; Uma Maheshwari, K.; Nirmal Babu, K.; Tetali, S.D.; Raghavendra, A.S. Highly Sensitive HPLC Method for Estimation of Total or Individual Curcuminoids in Curcuma Cultivars and Commercial Turmeric Powders. Curr. Sci. 2016, 111, 1816–1824. [Google Scholar] [CrossRef]
- Windarsih, A.; Rohman, A.; Swasono, R.T. Application of 1H-NMR Based Metabolite Fingerprinting and Chemometrics for Authentication of Curcuma Longa Adulterated with C. Heyneana. J. Appl. Res. Med. Aromat. Plants 2019, 13, 100203. [Google Scholar] [CrossRef]
- Gad, H.A.; Bouzabata, A. Application of Chemometrics in Quality Control of Turmeric (Curcuma Longa) Based on Ultra-Violet, Fourier Transform-Infrared and 1H NMR Spectroscopy. Food Chem. 2017, 237, 857–864. [Google Scholar] [CrossRef]
- Wise, B.; Gallager, N.B. PLS_Toolbox for Use with MATLAB, Version 2.0; Eigenvector Research Inc.: Mason, WA, USA, 1992. [Google Scholar]
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Izquierdo-Llopart, A.; Saurina, J. Characterization of Sparkling Wines According to Polyphenolic Profiles Obtained by HPLC-UV/Vis and Principal Component Analysis. Foods 2019, 8, 22. [Google Scholar] [CrossRef]
- Campmajo, G.; Navarro, G.J.; Nunez, N.; Puignou, L.; Saurina, J.; Nunez, O. Non-Targeted HPLC-UV Fingerprinting as Chemical Descriptors for the Classification and Authentication of Nuts by Multivariate Chemometric Methods. Sensors 2019, 19, 1388. [Google Scholar] [CrossRef] [PubMed]


| Sample Type | Brand | Number of Samples * | Composition |
|---|---|---|---|
| Turmeric | Hacendado | 5 | curcuma longa (Erode) |
| MG | 1 | curcuma longa (Alleppey) | |
| Burriac | 1 | curcuma longa | |
| Carmencita | 1 | curcuma longa (Erode) | |
| Ducros | 1 | curcuma longa | |
| Artemis Bio | 1 | curcuma longa | |
| Natco | 1 | curcuma longa | |
| Pelotari | 1 | Unknown | |
| Dani | 2 | curcuma zedoaria | |
| Especies | 1 | curcuma longa (Alleppey) | |
| Onena | 1 | curcuma longa (Madras) | |
| Tata Sampann | 1 | Unknown | |
| Herbalist | 1 | curcuma longa (Madras) | |
| Street market | 1 | curcuma longa (Madras) | |
| Biospirit | 1 | curcuma longa | |
| NAAI | 1 | Unknown | |
| Curry | Hacendado | 2 | white pepper, coriander, ginger, cardamom, clove, cinnamon, anise, mustard |
| Carrefour | 1 | pepper, coriander, ginger, cumin, fenugreek, laurel, fennel, mustard | |
| Species Kania | 1 | pepper, coriander, cumin, fenugreek, parsley, chili, garlic, fennel | |
| Condis | 1 | pepper, coriander, fennel, cumin, cayenne, garlic, anise | |
| Burriac | 1 | white pepper, coriander, ginger, cardamom, clove, cinnamon, anise, mace | |
| Eroski | 1 | coriander, cardamom, ginger, fenugreek, anise, garlic, clove, mustard | |
| Ducros | 1 | pepper, coriander, cumin, ginger, laurel, anise, garlic, clove, cinnamon, mace | |
| Street market | 1 | Unknown |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal-Casanella, O.; Nuñez, N.; Sentellas, S.; Núñez, O.; Saurina, J. Characterization of Turmeric and Curry Samples by Liquid Chromatography with Spectroscopic Detection Based on Polyphenolic and Curcuminoid Contents. Separations 2020, 7, 23. https://doi.org/10.3390/separations7020023
Vidal-Casanella O, Nuñez N, Sentellas S, Núñez O, Saurina J. Characterization of Turmeric and Curry Samples by Liquid Chromatography with Spectroscopic Detection Based on Polyphenolic and Curcuminoid Contents. Separations. 2020; 7(2):23. https://doi.org/10.3390/separations7020023
Chicago/Turabian StyleVidal-Casanella, Oscar, Nerea Nuñez, Sonia Sentellas, Oscar Núñez, and Javier Saurina. 2020. "Characterization of Turmeric and Curry Samples by Liquid Chromatography with Spectroscopic Detection Based on Polyphenolic and Curcuminoid Contents" Separations 7, no. 2: 23. https://doi.org/10.3390/separations7020023
APA StyleVidal-Casanella, O., Nuñez, N., Sentellas, S., Núñez, O., & Saurina, J. (2020). Characterization of Turmeric and Curry Samples by Liquid Chromatography with Spectroscopic Detection Based on Polyphenolic and Curcuminoid Contents. Separations, 7(2), 23. https://doi.org/10.3390/separations7020023