The Development of Silica Hydride Stationary Phases for High-Performance Liquid Chromatography from Conception to Commercialization
Abstract
1. Introduction
- MONOMERIC BONDING
- Si–OH + X–Si R’2 R ----> Si–O–Si R’2 R + HX
- X-halide and R-alkyl
- POLYMERIC BONDING
- Si–OH + X3-Si-R ----> Si–O–Si–R + 3HX
- Si–OH + SOCl2 ----> Si–Cl + SO2 + HCl
- Si–Cl + BrMgR ----> Si–R + MgClBr
- Or
- Si–Cl + Li–R ----> Si–R + Li–Cl
2. Evolution of Silica Hydride Stationary Phases

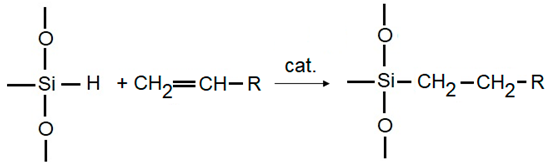
3. Characterization of Silica Hydride Materials
4. Separation Mechanisms on Silica Hydride
5. Applications of Silica Hydride HPLC Columns
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Unger, K.K. Porous Silica; Elsevier Science: Amsterdam, The Netherlands, 1979; ISBN 97800808166. [Google Scholar]
- Pesek, J.J.; Swedberg, S.A. Allyl bonded stationary Phase as a possible intermediate in the synthesis of novel high-performance liquid chromatographic phases. J. Chromatogr. 1986, 361, 83–109. [Google Scholar] [CrossRef]
- Sandoval, J.E.; Pesek, J.J. Synthesis and characterization of a hydride modified porous silica material as an intermediate in the preparation of chemically bonded chromatographic stationary phases. Anal. Chem. 1989, 61, 2067–2075. [Google Scholar] [CrossRef]
- Sandoval, J.E.; Pesek, J.J. Hydrolytically stable bonded chromatographic phases prepared through hydrosilation of olefins on a hydride-modified silica intermediate. Anal. Chem. 1991, 63, 2634–2641. [Google Scholar] [CrossRef]
- Chu, C.H.; Jonsson, E.; Auvinen, M.; Pesek, J.J.; Sandoval, J.E. A new approach for the preparation of a hydride-modified substrate used as an intermediate in the synthesis of surface-bonded materials. Anal. Chem. 1993, 65, 808–816. [Google Scholar] [CrossRef]
- Soukup, J.; Jandera, P. Adsorption of water from aqueous acetonitrile on silica-based stationary phases in aqueous normal-phase liquid chromatography. J. Chromatogr. A 2014, 1374, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Pesek, J.J.; Matyska, M.T.; Oliva, M.; Evanchic, M. Synthesis and characterization of bonded phases made via hydrosilation of alkynes on silica hydride surfaces. J. Chromatogr. A 1998, 818, 145–154. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T.; Soczewinski, E.; Christensen, P. Spectroscopic studies of butylphenyl, mono-ol and perfluorinated bonded phases. Chromatographia 1994, 39, 520–528. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T. Synthesis and spectroscopic characterization of a true diol bonded phase. J. Chromatogr. 1994, 687, 33–44. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T.; Hemphala, H. HPLC evaluation of mono-ol, butylphenyl, and perfluorinated columns prepared via olefin hydrosilation on a silica hydride intermediate. Chromatographia 1996, 43, 10–16. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T.; Williamsen, E.J.; Evanchic, M.; Hazari, V.; Konjuh, K.; Takhar, S.; Tranchina, R. The synthesis and characterization of alkyl bonded phases from a silica hydride intermediate via hydrosilation with free radical initiation. J. Chromatogr. A 1997, 786, 219–228. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T.; Williamsen, E.; Tam, R.; Wang, Z. Synthesis and characterization of liquid crystal type stationary phases on a silica hydride surface. J. Liq. Chromatogr. Rel. Technol. 1998, 21, 2747–2762. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T.; Takhar, S. Synthesis and characterization of long chain alkyl stationary phases on a silica hydride surface. Chromatographia 1998, 48, 631–636. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T.; Muley, S. Synthesis and characterization of a new type of chemically bonded liquid crystal stationary phase for HPLC. Chromatographia 2000, 52, 439–444. [Google Scholar] [CrossRef]
- Pesek, J.; Matyska, M.T.; Fu, P.F. Evaluation of the silanization/hydrosilation process for the synthesis of chiral stationary phases. Chromatographia 2001, 53, 635–640. [Google Scholar] [CrossRef]
- Matyska, M.T.; Pesek, J.J.; Grandhi, V. Charge transfer-like stationary phase for HPLC prepared via hydrosilation on silica hydride. J. Sep. Sci. 2002, 25, 741–748. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T.; James, S. Synthesis and characterization of a C8 stationary phase bonded with 2-acrylamido-2-methyl-1-propanesulfonic acid for HPLC. J. Liq. Chromatogr. Rel. Technol. 2002, 25, 2749–2765. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T.; Dawson, G.B.; Wilsdorf, A.; Marc, P.; Padki, M. The cholesterol bonded phase as a separation medium in high performance liquid chromatography. Evaluation of properties and applications. J. Chromatogr. A 2003, 986, 253–262. [Google Scholar] [CrossRef]
- Matyska, M.T.; Pesek, J.J.; Tong, S.; Sandoval, J.E. Adamantyl-modified silica via olefin hydrosilation on a hydride Intermediate. J. Liq. Chromatogr. Rel. Technol. 2003, 26, 1169–1195. [Google Scholar] [CrossRef]
- Matyska, M.T.; Pesek, J.J.; Pan, X. Synthesis and evaluation of a C8 phase on a silica hydride surface by hydrosilation of 1-octyne. J. Chromatogr. A 2003, 992, 57–65. [Google Scholar]
- Matyska, M.T.; Pesek, J.J.; Suryadevara, R. Synthesis and characterization of amino-based columns for HPLC made by silanization/ hydrosilation. J. Liq. Chromatogr. Rel. Technol. 2005, 28, 2111–2139. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T.; Prabhakaran, S. Synthesis and characterization of chemically bonded stationary phases on hydride surfaces by hydrosilation of alkynes and dienes. J. Sep. Sci. 2005, 28, 2437–2443. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T.; Prajapati, K. Synthesis and evaluation of silica hydride-based fluorinated stationary phases. J. Sep. Sci. 2010, 33, 2908–2916. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T.; Lee, P. Synthesis of a preparative C30 stationary phase on a silica hydride surface and its application to carotenoid separation. J. Liq. Chromatogr. Rel. Technol. 2011, 34, 231–240. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T.; Salehi, N. Evaluation of stationary phases made by hydrosilation of alkynes on silica hydride. Curr. Chromatogr. 2015, 2, 41–47. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T. Silica hydride surfaces: Versatile separation media for chromatographic and electrophoretic analyses. J. Liq. Chromatogr. Rel. Technol. 2006, 29, 1105–1124. [Google Scholar] [CrossRef]
- Pesek, J.J.; Williamsen, E. Spectroscopic characterization of chemically modified oxide surfaces. Trends Appl. Spectrosc. 1993, 1, 41–50. [Google Scholar]
- Pesek, J.J.; Matyska, M.T. Methods for the modification and characterization of oxide surfaces. J. Interface Sci. 1997, 5, 103–117. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T. Spectroscopic characterization of chemically modified oxide surfaces. In Adsorption and Its Application in Industry and Environmental Protection; Dabrowski, A., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 1, pp. 117–142. [Google Scholar]
- Pesek, J.J.; Matyska, M.T.; Williamsen, E.J.; Tam, R. Variable-temperature solid-state NMR studies of bonded liquid crystal stationary phases for HPLC. Chromatographia 1995, 41, 301–310. [Google Scholar] [CrossRef]
- Freibolin, V.; Bayer, M.P.; Matyska, M.T.; Pesek, J.J.; Albert, K. 1H HR/MAS NMR in the suspended state: Molecular recognition processes in liquid chromatography between steroids and a silica hydride-based cholesterol phase. J. Sep. Sci. 2009, 32, 1722–1728. [Google Scholar] [CrossRef]
- Yeman, H.; Friebolin, V.; Steinhauser, L.; Matyska, M.T.; Pesek, J.J.; Albert, K. Time dependant column performance of cholesterol based stationary phases for HPLC by LC characterization and solid state NMR spectroscopy. J. Sep. Sci. 2012, 35, 1582–1588. [Google Scholar] [CrossRef]
- Berendsen, G.E.; De Galan, L. Preparation and chromatographic properties of some chemically bonded phases for reversed-phase liquid chromatography. J. Liq. Chromatogr. 1978, 1, 561–568. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T. How to retain polar and nonpolar Compounds on the same HPLC Column with an isocratic mobile phase. LC/GC 2006, 24, 296–303. [Google Scholar]
- Zhang, L.; Dai, Q.; Qiao, X.; Yu, C.; Qin, X.; Yan, H. Mixed-mode chromatographic stationary phases: Recent advancements and its applications for high-performance liquid Chromatography. TrAC Trends Anal. Chem. 2016, 82, 143–163. [Google Scholar] [CrossRef]
- Zhang, K.; Xiaodong, L. Mixed-mode chromatography in pharmaceutical and biopharmaceutical applications. J. Pharm. Biomed. Anal. 2016, 128, 73–88. [Google Scholar] [CrossRef]
- Wang, L.; Wei, W.; Zhining, X.; Xu, J.; Zeng, Z.X. Recent advance in materials form stationary phases of mixed-mode high-performance liquid chromatography. TrAC Trends Anal. Chem. 2016, 80, 495–506. [Google Scholar] [CrossRef]
- Sykora, E.; Rezanak, P.; Zaruba, K.; Kral, V. Recent advances in mixed-mode chromatographic stationary phases. J. Sep. Sci. 2019, 42, 89–129. [Google Scholar] [CrossRef]
- Kulsing, C.; Yang, Y.; Munera, C.; Tse, C.; Matyska, M.T.; Pesek, J.J.; Boysen, R.I.; Hearn, M.T.W. Correlation between zeta potential and analyte retention using ionic interaction descriptors for silica hydride-based stationary phases. Anal. Chim. Acta 2014, 817, 48–60. [Google Scholar] [CrossRef]
- Kulsing, C.; Nolvachi, Y.; Marriott, P.; Boysen, R.; Matyska, M.; Pesek, J.; Hearn, M. Insights into the origin of the separation selectivity with silica hydride adsorbents. J. Phys. Chem. Part B 2015, 119, 3063–3069. [Google Scholar] [CrossRef]
- Boysen, R.I.; Yang, Y.; Chowdhury, J.; Matyska, M.T.; Pesek, J.J.; Hearn, M.T.W. Simultaneous separation of hydrophobic and hydrophilic peptides with a silica hydride stationary phase using aqueous normal phase conditions. J. Chromatogr. A 2011, 1218, 8021–8026. [Google Scholar] [CrossRef]
- Nguyen, T.; Felker, P.; Hurst, J.W.; Ly, C.; Jarman, S.; Diep, D.; Pham, C.; Pesek, J.J.; Matyska, M.T.; Young, J.E.; et al. LC-MS characterization of phenolics in mesquite flour. LCGC N. Am. 2016, 34, 28–31. [Google Scholar]
- Pesek, J.; Matyska, M.; Hoffman, J.F.; Madruga, N.; Crizel, R.I.; Elias, M.C.; Vanier, N.L.; Chaves, F.C. Analysis of mycotoxins in grains using silica hydride-based stationary phases. J. Sep. Sci. 2017, 40, 1953–1959. [Google Scholar] [CrossRef]
- Young, J.E.; Pan, Z.; Teh, H.E.; Menon, V.; Modegerer, B.; Pesek, J.J.; Matyska, M.T.; Takeoka, G. Phenolic composition of phenolic extracts using an LCMS approach with silica hydride columns. J. Sep. Sci. 2017, 40, 1449–1456. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T.; Sieng, M.; Doan, L. Analysis of capsaicinoids in hot sauces using a silica hydride-based stationary phase. Curr. Chromatogr. 2016, 3, 12–16. [Google Scholar] [CrossRef]
- Young, J.E.; Lim, M.V.; Topete, J.; Hang, H.; Gahol, M.; Pesek, J.J.; Matyska, M.T. Improved sensitivity and specificity of trans-resveratrol in red wine analysis with HPLC-UV and LC-MS. LCGC N. Am. 2016, 34, 205–213. [Google Scholar]
- Dang, A.; Sieng, M.; Pesek, J.J.; Matyska, M.T. Determination of bisphenol A in receipts and carbon paper by HPLC-UV. J. Liq. Chromatogr. Rel. Technol. 2015, 38, 438–442. [Google Scholar] [CrossRef]
- D′Orazio, G.; Rocco, A.; Fanali, S. Fast liquid chromatography using columns of different internal diameters packed with sub-2 µm silica particles. J. Chromatogr. A 2012, 1228, 213–220. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T.; Fischer, S.M.; Sana, T.R. Analysis of hydrophilic metabolites by high-performance liquid chromatography-mass spectrometry using a silica hydride-based stationary phase. J. Chromatogr. A 2008, 1204, 48–55. [Google Scholar] [CrossRef]
- Chalkraft, K.R.; McCarry, B.E. Tandem LC columns for the simultaneous retention of polar and nonpolar molecules in comprehensive metabolomic analysis. J. Sep. Sci. 2013, 36, 3478–3485. [Google Scholar] [CrossRef]
- Gouzy, A.; Larrouy-Maumus, G. Mycobacterium tuberculosis exploits asparagine to assimilate nitrogen and resist acid stress during infection. PLoS Pathogens 2014, 10, e1003928. [Google Scholar] [CrossRef]
- Jenkins, S.; Fischer, S.M.; Chen, L.; Sana, T.R. Global LC/MS metabolomics profiling of calcium stressed and immunosuppressant treated Saccharomyces cerevisiae. Metabolites 2013, 3, 1102–1117. [Google Scholar] [CrossRef]
- Putluria, N.; Shojaiegn, A.; Vasu, V.T. Metabolic profiling reveals potential markers and bioprocesses altered in bladder cancer progression. Cancer Res. 2011, 15, 7376–7386. [Google Scholar] [CrossRef]
- Hellmuth, C.; Koletzko, B.; Peissner, W. Aqueous normal phase cysteine and methionine by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2011, 879, 83–89. [Google Scholar] [CrossRef]
- Gouzy, A.; Larrouy-Maumus, G. Mycobacterium tuberculosis nitrogen assimilation and host colonization require aspartate. Nat. Chem. Biol. 2013, 9, 674–676. [Google Scholar] [CrossRef]
- Sana, T.R.; Gordon, D.B.; Fischer, S.M.; Tichy, S.E.; Kitagawa, N.; Cai, C.; Gosnell, W.L.; Chang, S.P. Global mass spectrometry based metabolomics profiling of erythrocytes infected with Plasmodium falciparum. PLoS ONE 2013, 8, e60840. [Google Scholar] [CrossRef]
- Eoh, H.; Rhee, K.Y. Multifunctional essentiality of succinate metabolism in adaptation to hypoxia in mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2013, 110, 6454–6459. [Google Scholar] [CrossRef]
- Chakraborty, S.; Gruber, T.; Barry, C.E., III; Boshoff, H.I.; Rhee, K.Y. Para-aminosalicylic acid acts as an alternate substrate of folate metabolism in mycobacterium tuberculosis. Science 2013, 339, 88–91. [Google Scholar] [CrossRef]
- Cifkova, E.; Hajek, R.; Lisa, M.; Holcapek, M. Hydrophilic liquid interaction chromatography-mass spectrometry of (lyso)phosphaditic acids, (lyso)phophaditylserines and other lipid classes. J. Chromatogr. A 2016, 1450, 65–73. [Google Scholar] [CrossRef]
- Webster, G.K.; Elliott, A.; Dahan, A.; Miller, J.M. Analysis of PEG 400 in perfusate samples aqueous normal phase (ANP) chromatography with evaporative light scattering detection. Anal. Methods 2011, 1218, 742–744. [Google Scholar] [CrossRef]
- Dang, A.; Matyska, M.T.; Pesek, J.J. The use of aqueous normal phase chromatography as an analytical tool for food analysis. Determination of histamine as a model system. Food Chem. 2013, 141, 4226–4230. [Google Scholar] [CrossRef]
- Young, J.; Matyska, M.T.; Pesek, J.J. Robust HPLC-refractive index analysis of simple sugars in beverages using silica hydride columns. Curr. Nutr. Food Sci. 2016, 12, 125–131. [Google Scholar] [CrossRef]
- Le, R.; Young, J.E.; Pesek, J.J.; Matyska, M.T. Separation of 1,3-Dimethylamine and other polar compounds in dietary supplement formulations using aqueous normal phase chromatography with mass spectrometry. J. Sep. Sci. 2013, 36, 2578–2583. [Google Scholar] [CrossRef]
- Gea, J.; Liu, F.; Holmes, E.H.; Ostrander, G.K.; Li, Q.X. Aqueous normal phase liquid chromatography coupled with tandem time-of-flight quadrupole mass spectrometry for determination of zanamivir in human serum. J. Chromatogr. B 2012, 906, 58–62. [Google Scholar] [CrossRef][Green Version]
- Naffa, R.; Holmes, G.; Meekyung, A.; Harding, D.; Norris, G. Liquid chromatography electrospray ionization mass spectrometry for the simultaneous quantitation of collagen and elastin crosslinks. J. Chromatogr. A 2016, 1478, 60–67. [Google Scholar] [CrossRef]
- Available online: http://kb.mtc-usa.com/article/AA-02558/0/ (accessed on 5 May 2019).
- Available online: http://kb.mtc-usa.com/article/AA-02185/0/ (accessed on 5 May 2019).
- Pesek, J.J.; Matyska, M.T.; Watanabe, S.; Makhanov, M.; Lopez, A.; Alejo, K.; Orozco, D.; Doan, L. Evaluation of silica hydride materials for the LC-MS analysis of cathinones and benzylpiperazines. Forensic Chem. 2018, 8, 90–94. [Google Scholar] [CrossRef]
- Available online: http://kb.mtc-usa.com/article/AA-01493/0/ (accessed on 5 May 2019).
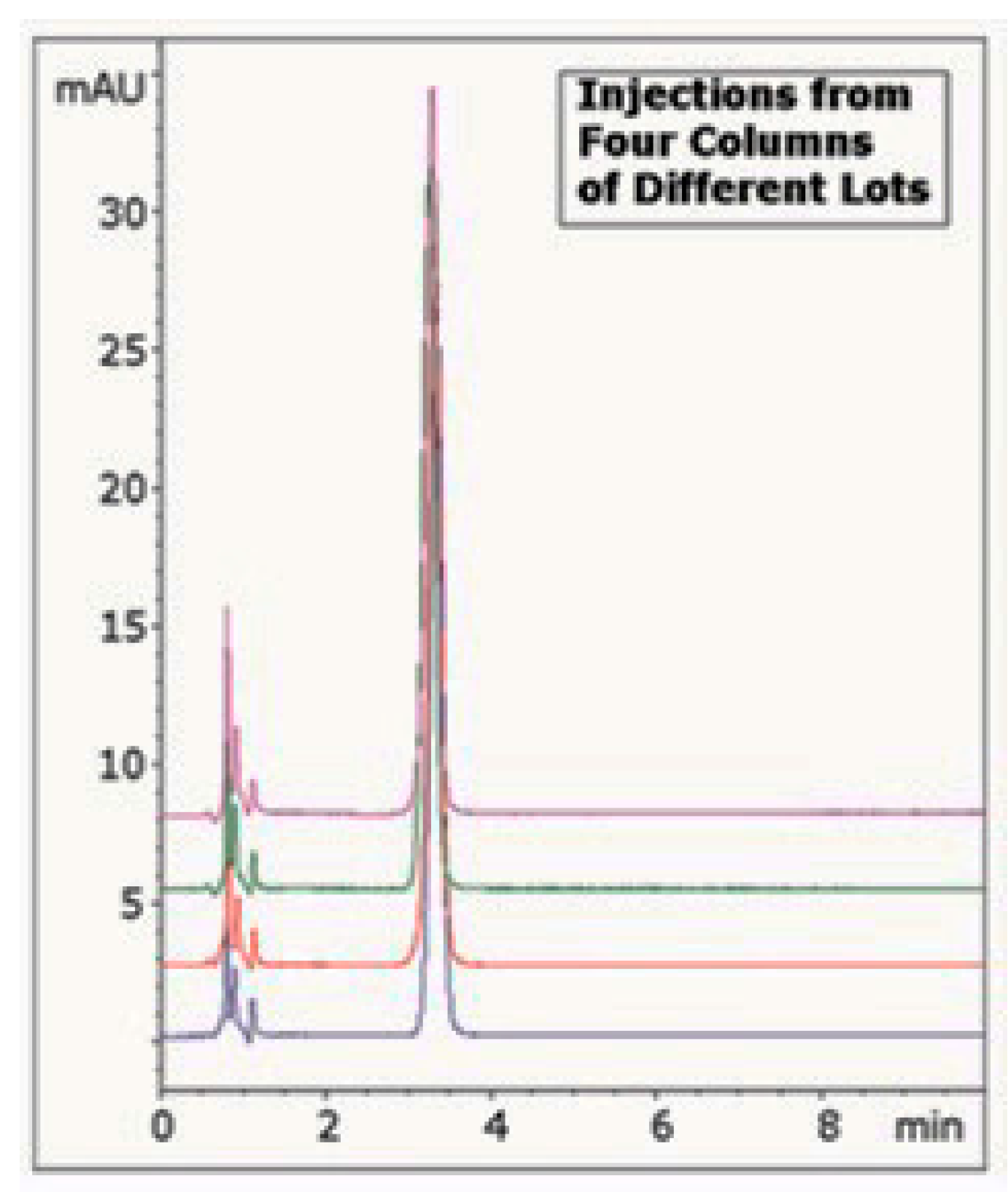
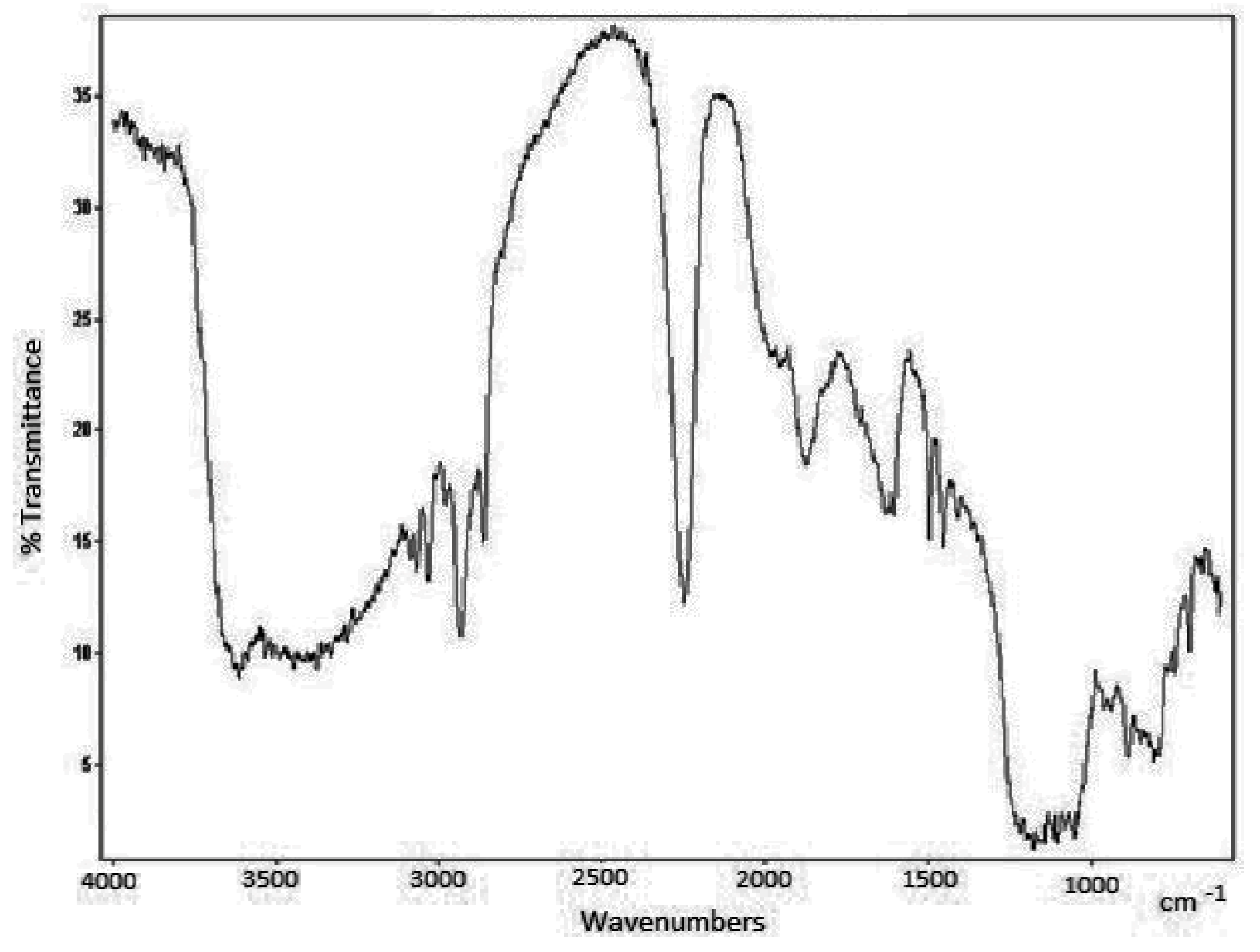
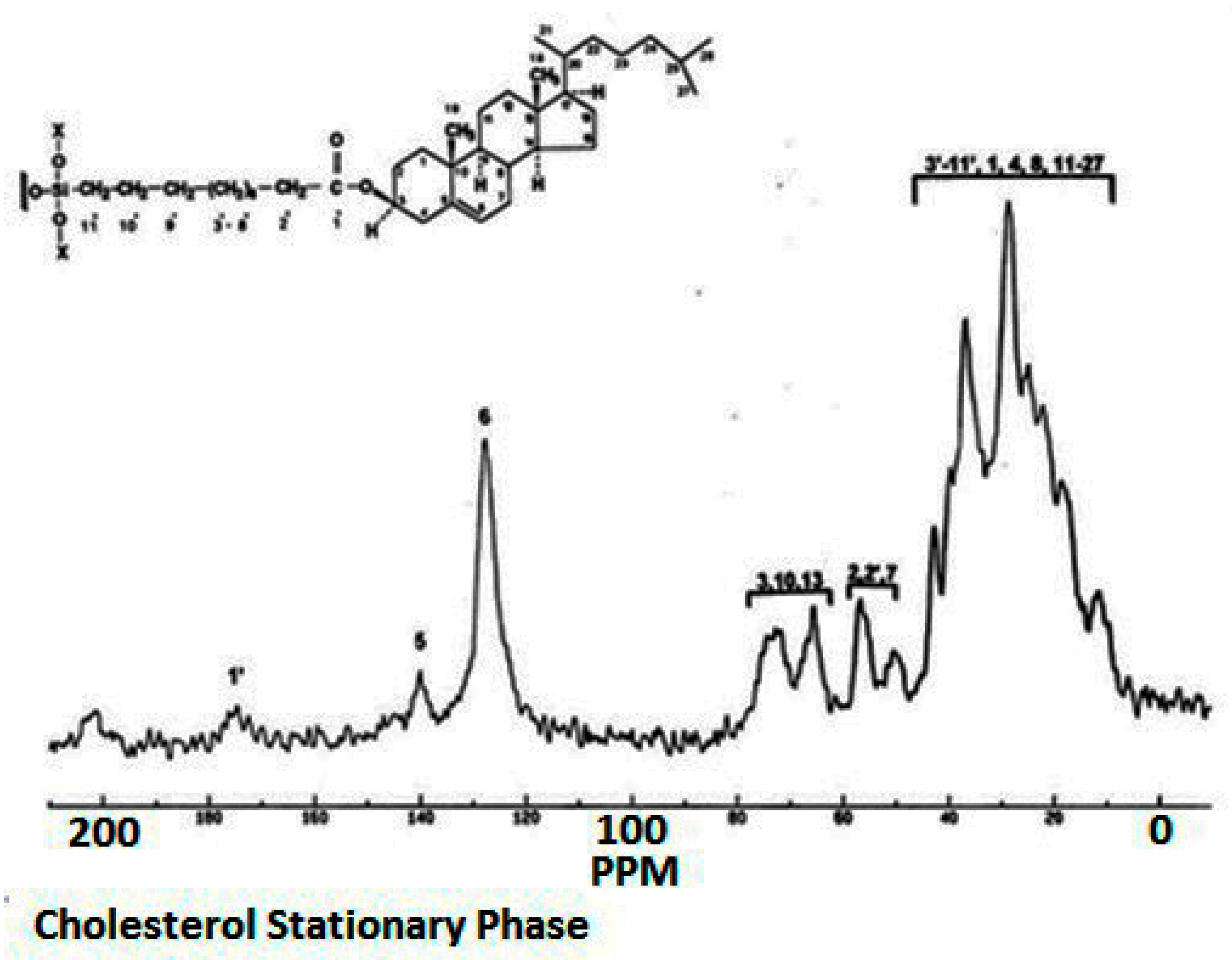

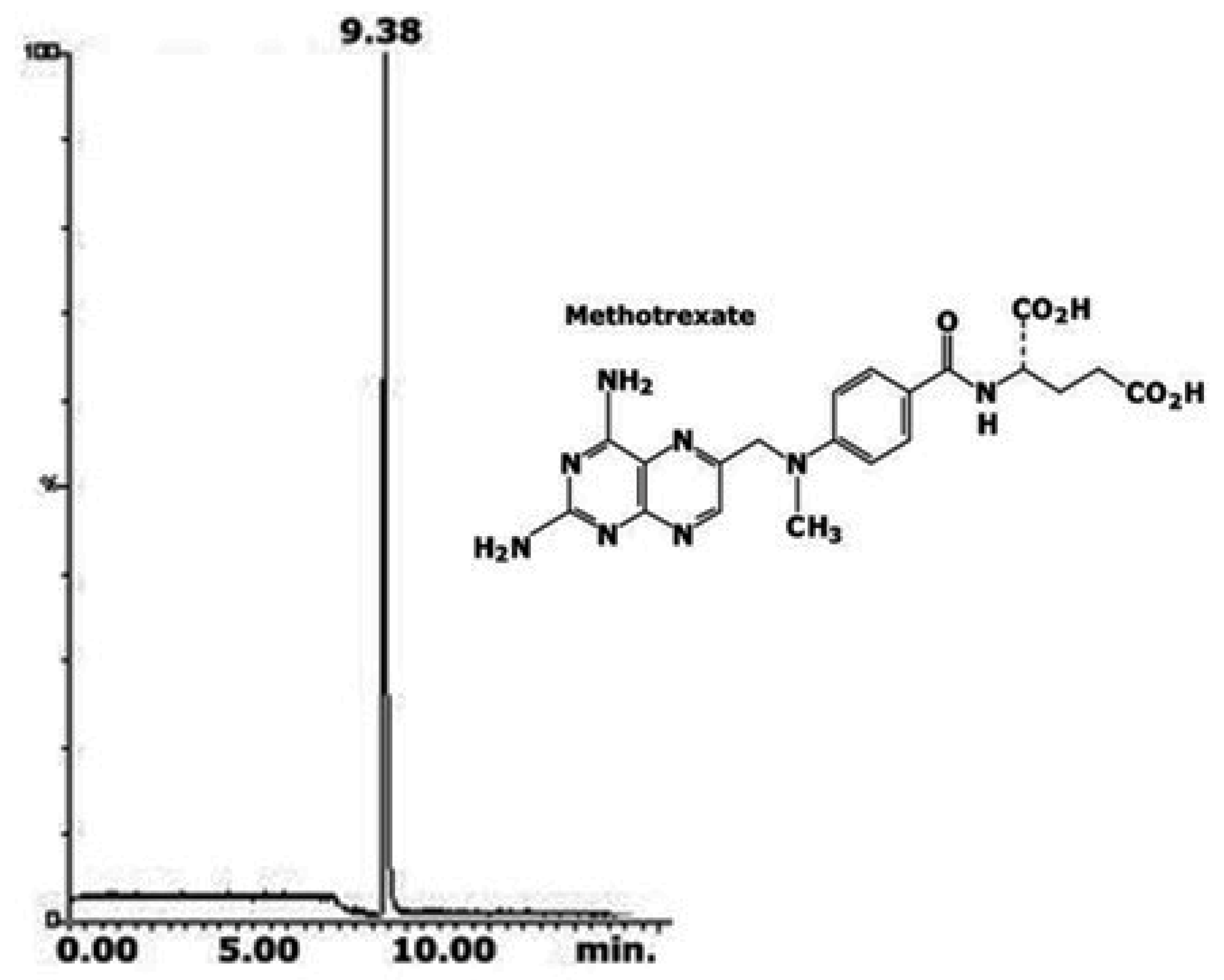
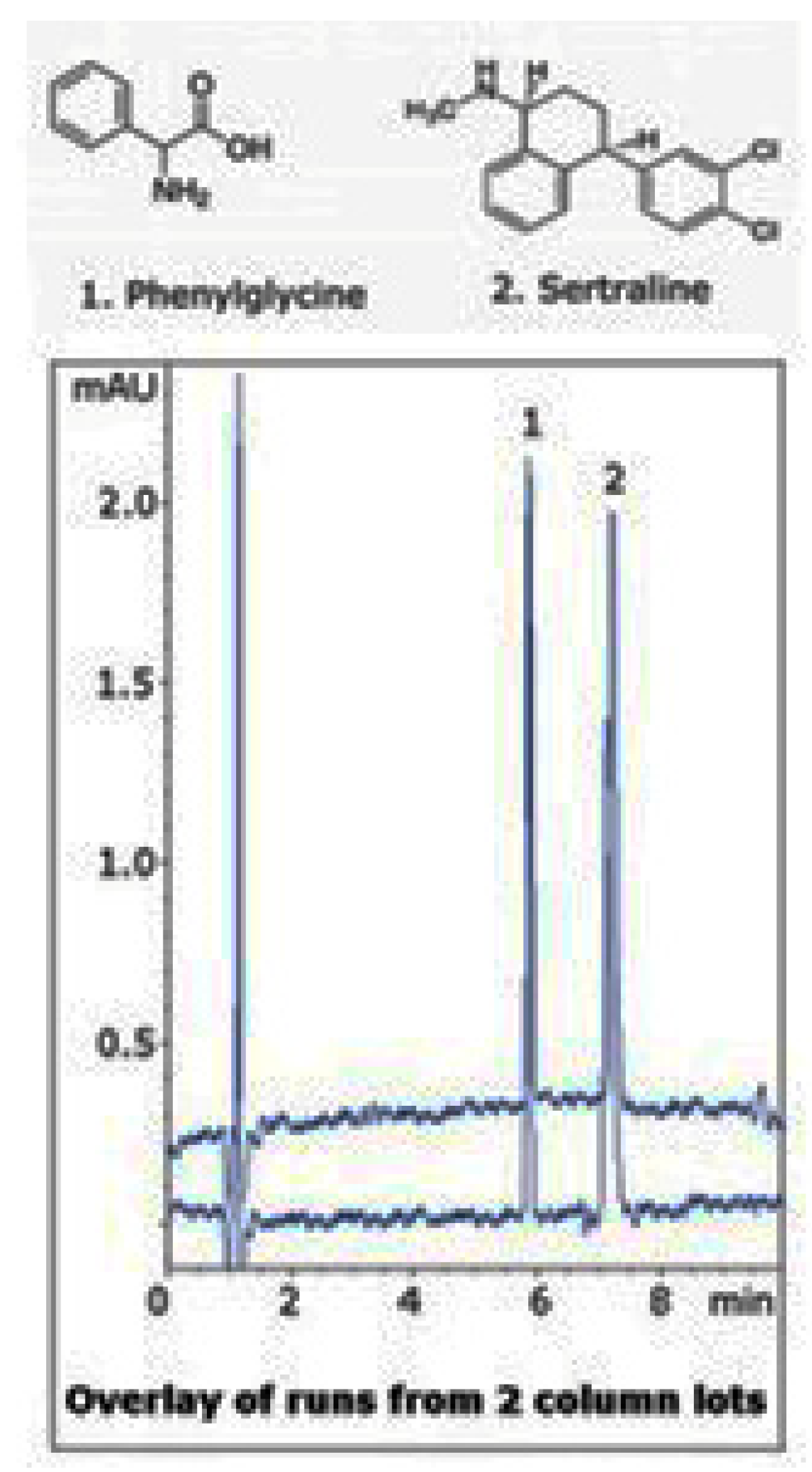
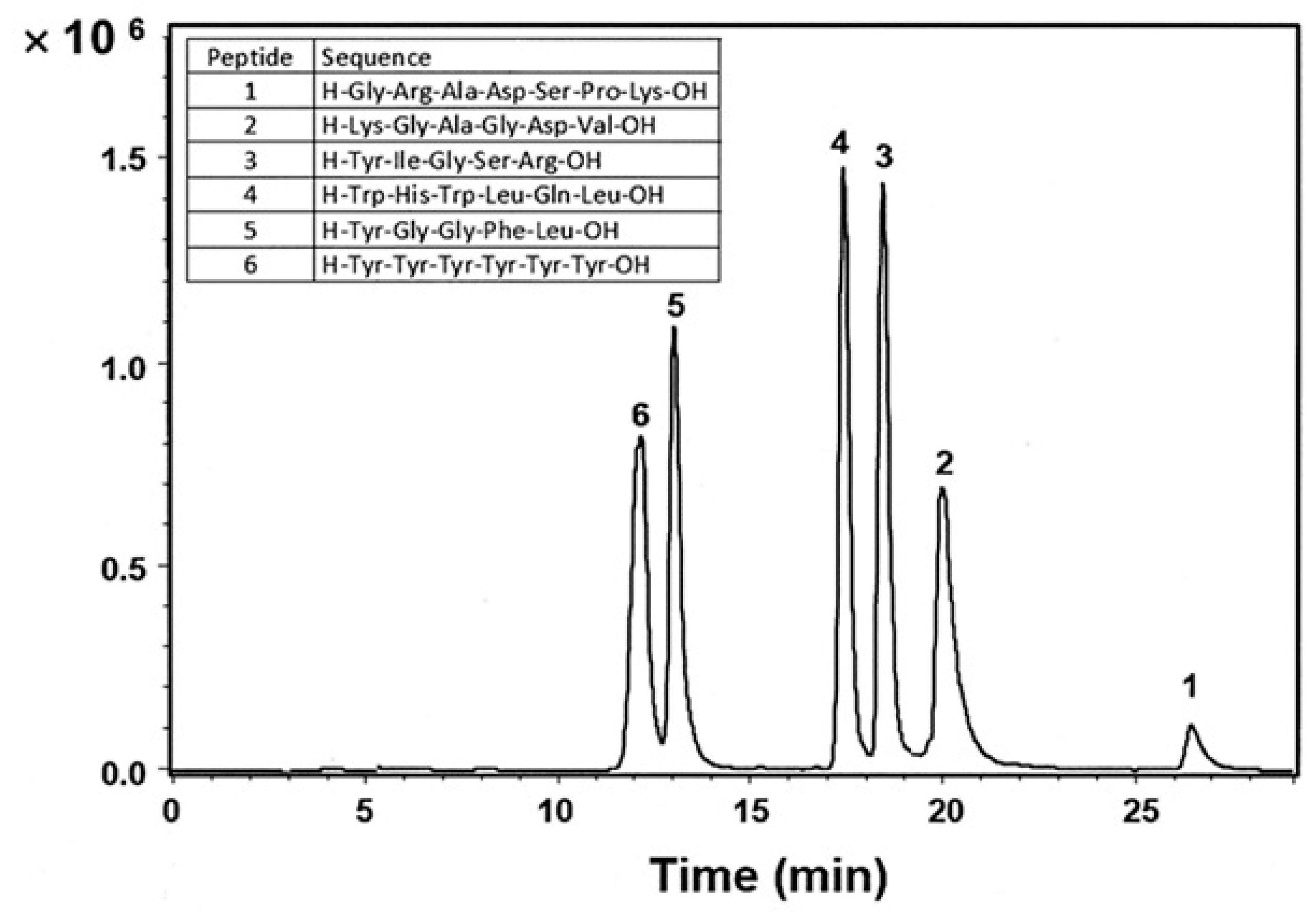
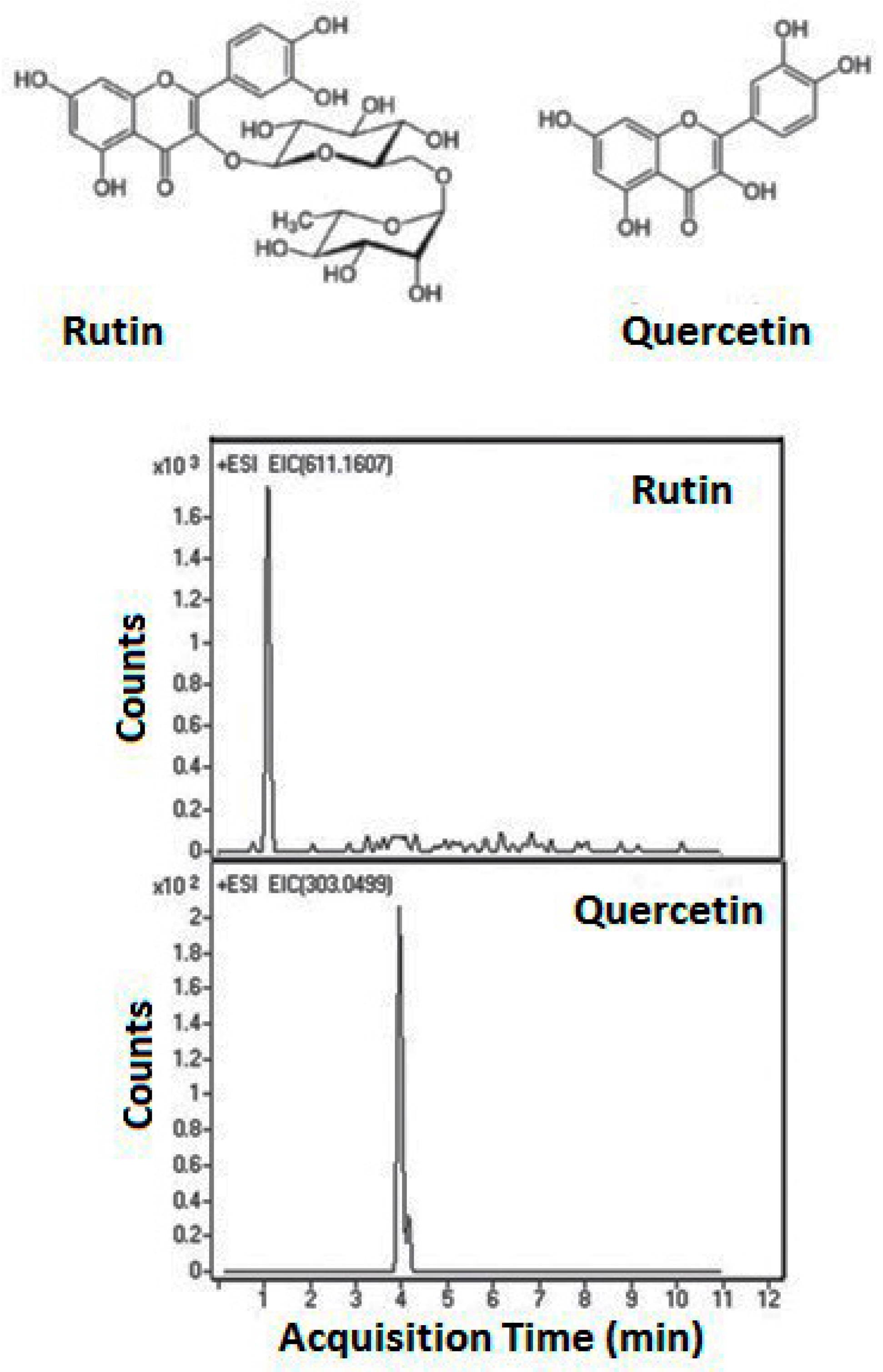
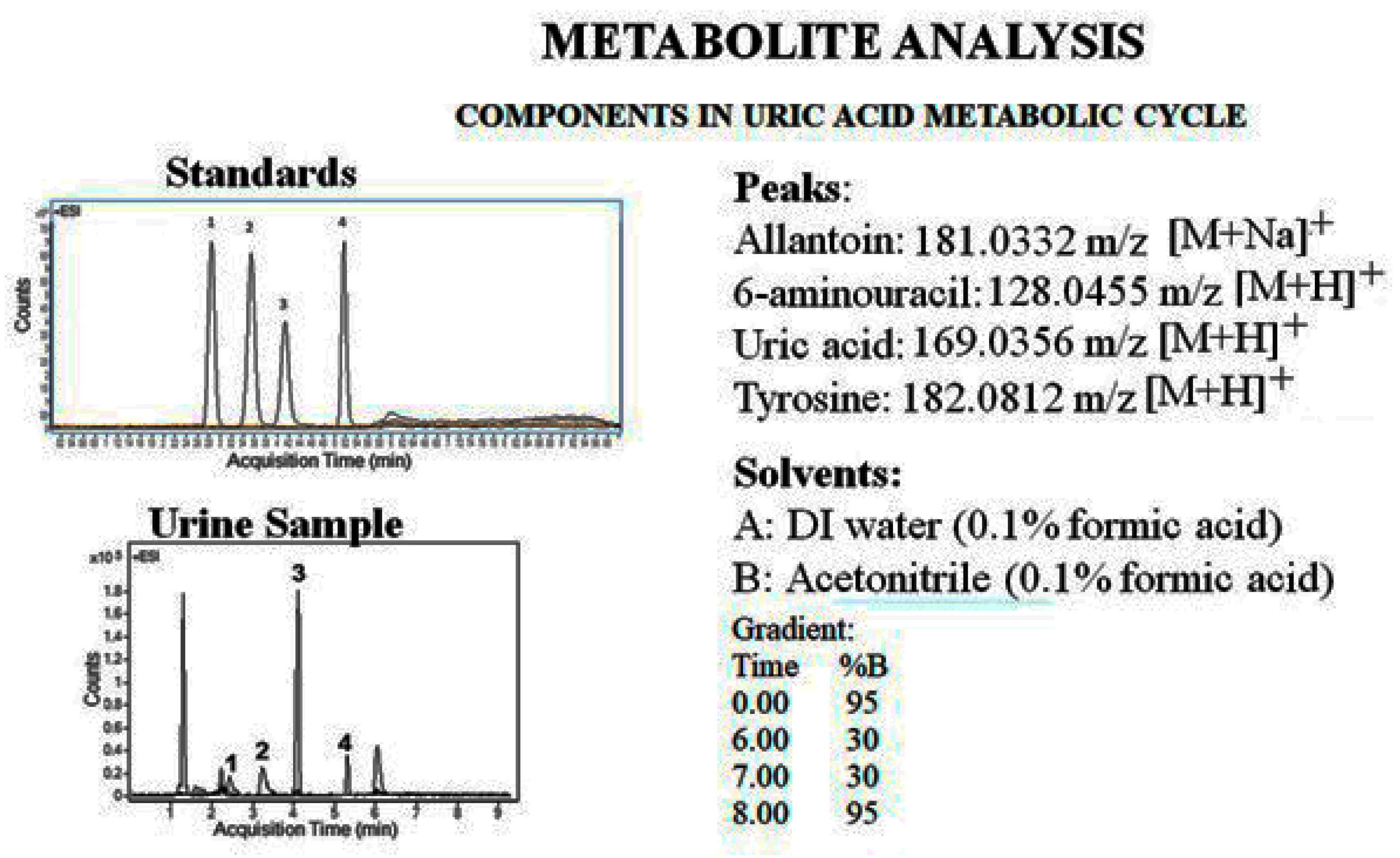
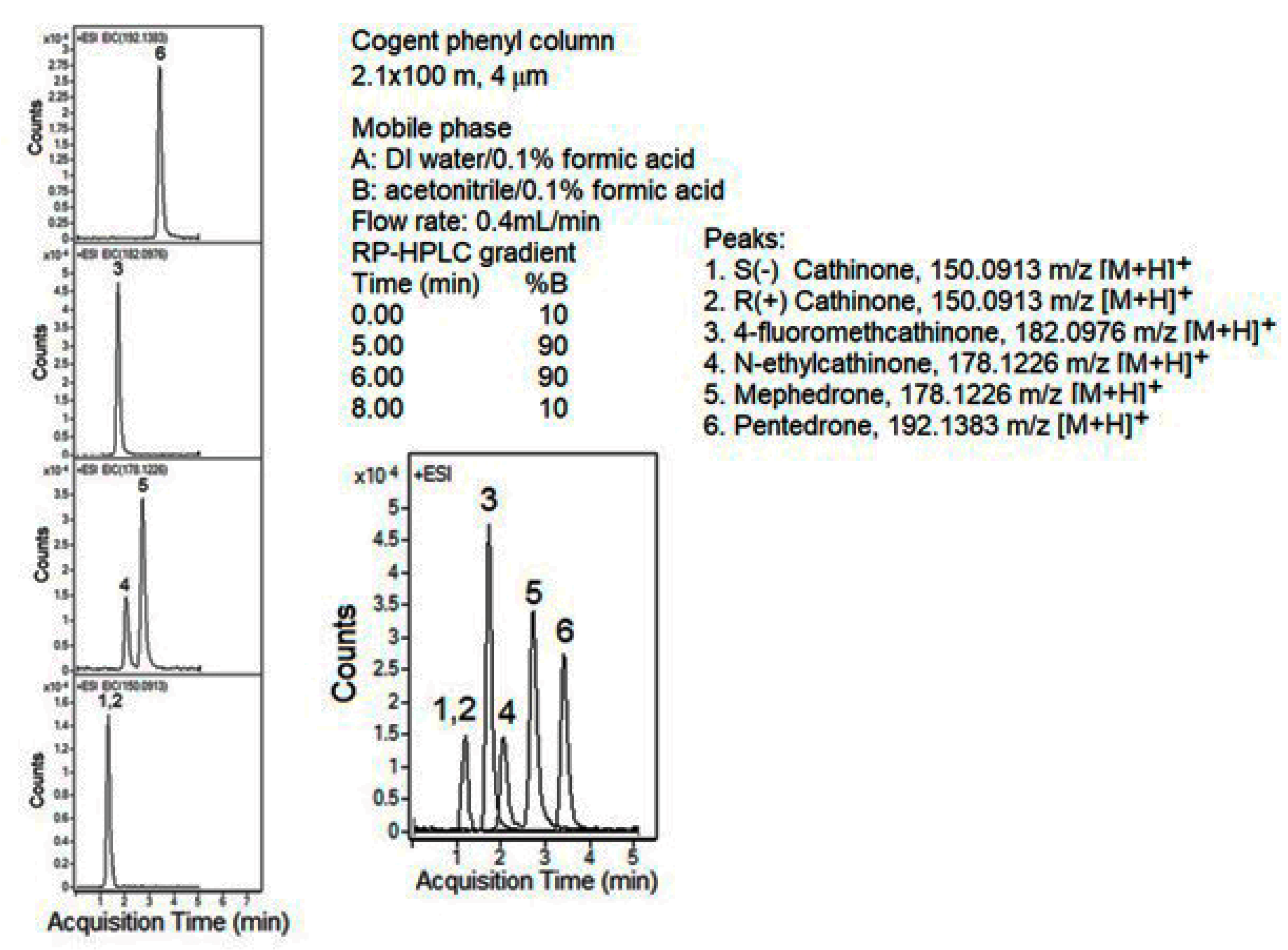
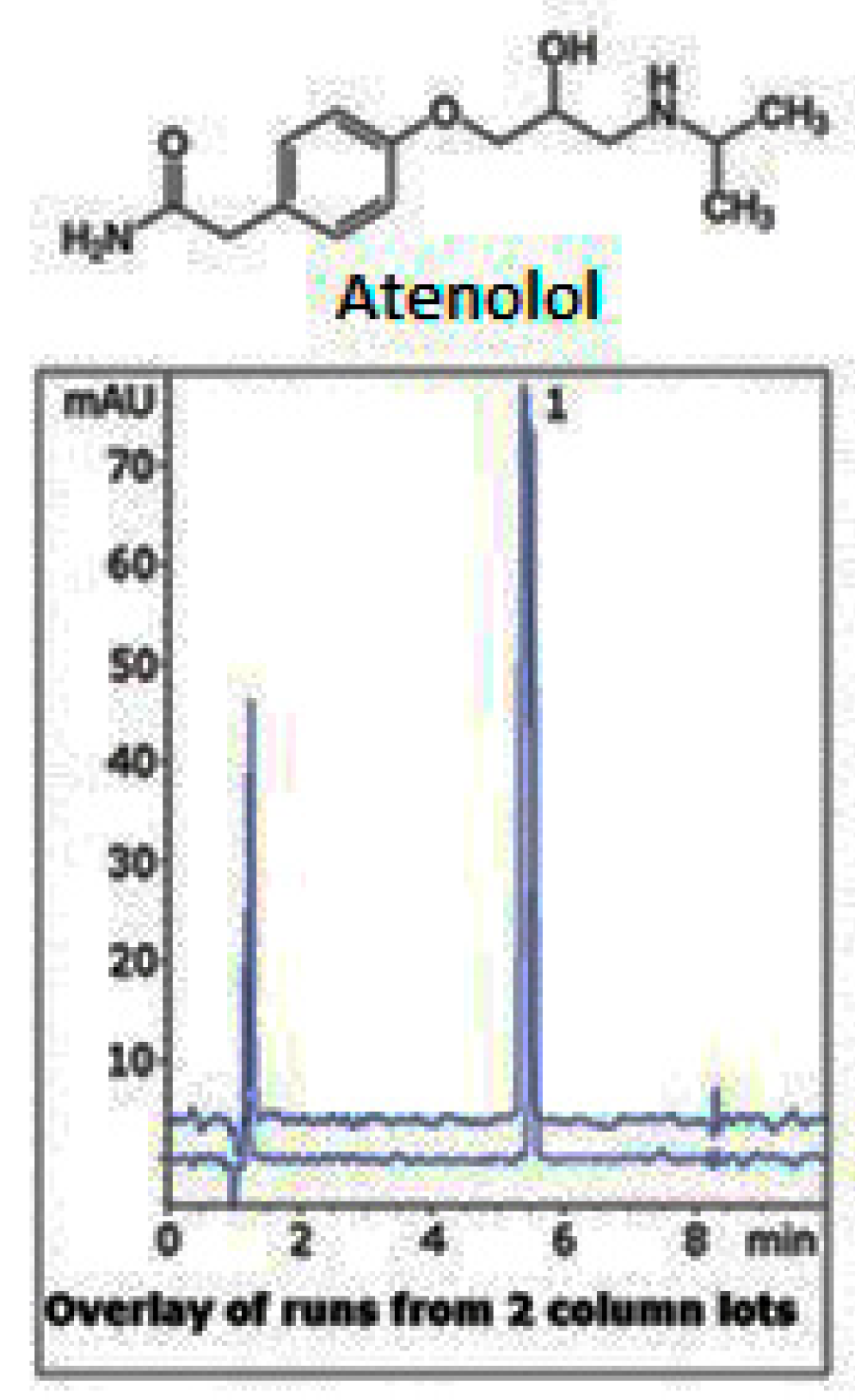
| Column | Nw |
|---|---|
| ZIC HILIC | 6.11 |
| ZIC cHILIC | 9.48 |
| Luna HILIC | 4.72 |
| Triart DIOL | 3.06 |
| Cogent Silica C | 0.45 |
| Cogent Diamond hydride | 0.43 |
| Cogent UDC Cholesterol | 0.32 |
| Cogent Bidentate C18 | 0.28 |
| Cogent Phenyl hydride | 0.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matyska, M.; Pesek, J. The Development of Silica Hydride Stationary Phases for High-Performance Liquid Chromatography from Conception to Commercialization. Separations 2019, 6, 27. https://doi.org/10.3390/separations6020027
Matyska M, Pesek J. The Development of Silica Hydride Stationary Phases for High-Performance Liquid Chromatography from Conception to Commercialization. Separations. 2019; 6(2):27. https://doi.org/10.3390/separations6020027
Chicago/Turabian StyleMatyska, Maria, and Joseph Pesek. 2019. "The Development of Silica Hydride Stationary Phases for High-Performance Liquid Chromatography from Conception to Commercialization" Separations 6, no. 2: 27. https://doi.org/10.3390/separations6020027
APA StyleMatyska, M., & Pesek, J. (2019). The Development of Silica Hydride Stationary Phases for High-Performance Liquid Chromatography from Conception to Commercialization. Separations, 6(2), 27. https://doi.org/10.3390/separations6020027




