Abstract
Fabric phase sorptive extraction (FPSE) is a novel and green sample preparation technique introduced in 2014. FPSE utilizes a natural or synthetic permeable and flexible fabric substrate chemically coated with a sol-gel organic-inorganic hybrid sorbent in the form of ultra-thin coating, which leads to a fast and sensitive micro-extraction device. The flexible FPSE requires no modification of samples and allows direct extraction of analytes. Sol-gel sorbent-coated FPSE media possesses high chemical, solvent, and thermal stability due to the strong covalent bonding between the substrate and the sol-gel sorbent. Therefore, any elution solvent can be used in a small volume, which achieves a high pre-concentration factor without requiring any solvent evaporation and sample reconstitution step. Taking into consideration the complexity of the samples and the need of further minimization and automation, some new, alternative modes of the FPSE have also been developed. Therefore, FPSE has attracted the interest of the scientific community that deals with sample pre-treatment and has been successfully applied for the extraction and determination of many analytes in environmental samples as well as in food and biological samples. The objective of the current review is to present and classify the applications of FPSE according to different sample categories and to briefly show the progress, advantages, and the main principles of the proposed technique.
1. Introduction
It is well known that sample preparation is a very important and inevitable step in the chemical analysis workflow. Most of the real-life analytical samples cannot be directly analyzed with an injection into the analytical instrument. Furthermore, they have to be treated in a way that makes them compatible with the analytical instrument. Additionally, their collection and preparation require more than 80% of the analytical process time. As such, sample preparation is considered to be the most time-consuming but critical part of the whole analysis, which affects its effectiveness and performance. Therefore, the main purposes are the reduction of the matrix complexity and the separation and pre-concentration of the target analytes from the sample matrices in order to be ready for their introduction into the analytical instrument for identification and quantification [1,2].
As time passed, low-cost, fast, and environmentally-friendly procedures become necessary in order to improve quality of life and protect the environment. Scientists try to develop new analytical methodologies compliant with the principles of green analytical chemistry (GAC) [3]. Therefore, the classic sample preparation technique of liquid-liquid extraction (LLE) is replaced by solid-phase extraction (SPE), which has been widely accepted because of the simplicity of the device, the efficiency, the rapid separation process, the ability to extract polar compounds, no requirement of phase separation, and the lower organic solvent consumption [4]. To further minimize the amount of sample and solvent waste during the sample preparation step, to overcome some drawbacks of SPE is a multi-step procedure such as the requirement of solvent evaporation and sample reconstitution. Novel sorbent based micro-extraction techniques have been developed such as solid-phase micro-extraction (SPME) by Pawliszyn and co-workers in 1990, stir-bar sorptive extraction (SBSE) in 1999, magnetic solid-phase extraction technique (MSPE) in 1999, and micro-extraction in a packed syringe (MEPS) in 2004 [4,5].
It is estimated that the major drawbacks of most of the micro-extraction techniques are due to the primary contact surface area (PCSA) of the device and the coating technology that is applied to immobilize the sorbent on the substrate surface [2]. The PCSA is part of the surface area of the extraction media, which can be available for direct interaction with the analytes during the process. The augmentation in PCSA offers higher sorbent loading without any change in the coating technology. Therefore, more target analytes are adsorbed by the sorbent and reduction of the extraction equilibrium time is achieved. Additionally, the sorbent coating technology is very important. From all the alternative ones that have been developed, the sol-gel coating technology, proposed by Malik and co-workers, is the most flexible and convenient. There is a strong chemical bond between the sol-gel coated sorbent and the substrate, which leads to high solvent and chemical stability. Due to its inherent porosity, reduction of the extraction equilibrium time, and higher or equivalent sensitivity is achieved when compared to commercial SPME fibers. In summary, both the coating technology and the PCSA have to be increased, which results in a sensitive and fast sample preparation technique [6,7].
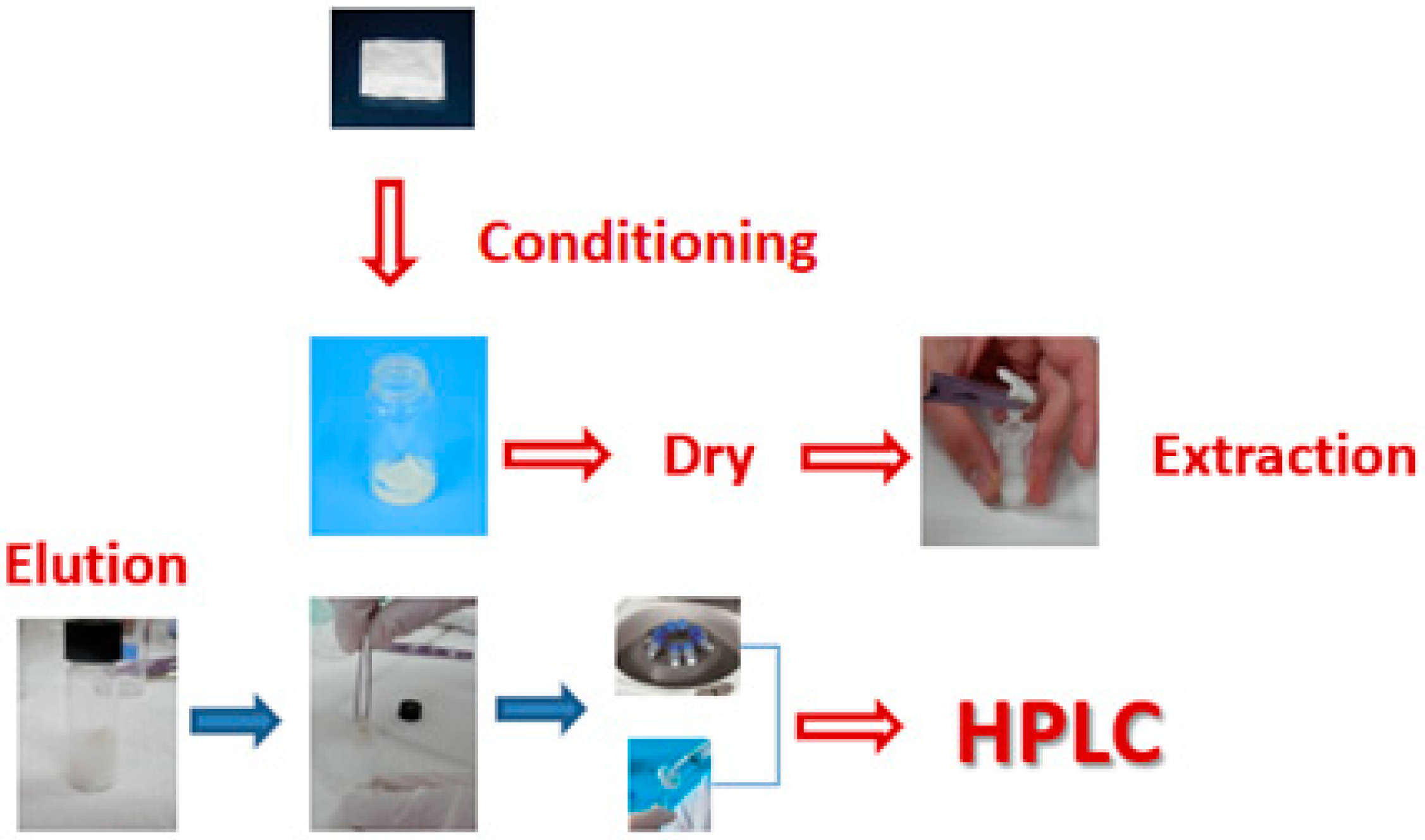
Based on these observations and developments, Kabir and Furton proposed a new, green sample preparation technique in 2014 called fabric phase sorptive extraction (FPSE). FPSE utilizes a natural or synthetic fabric substrate, which is chemically coated in the form of ultra-thin coating with sol-gel organic-inorganic hybrid sorbent as the extraction medium. Generally, the FPSE procedure consists of the following steps: first, the sol-gel sorbent coated FPSE media is submerged into a mixture of appropriate solvents to clean any undesirable impurities from the material and then it is rinsed with deionized water to remove the residues of organic solvents. Afterward, an amount of sample solution is transferred into a glass tube vial that contains a clean Teflon-coated magnetic stir bar inside and the FPSE media is introduced into the vial. The sample is magnetically stirred for an optimum extraction time where the sorption of the target analytes by the sorbent takes place. Subsequently, the FPSE device is removed from the vial and is brought in contact with the eluting solvent into another vial where desorption occurs and the retained analytes are back-extracted to this eluting system. Afterwards, the extract is centrifuged and filtered before being injected into the analytical instrument. The FPSE process is described schematically in Figure 1 [6,8,9]. The FPSE media can be reused for further extraction procedures by washing with a suitable solvent system and drying on a watch glass so that it can be ready for storage in an air-tight glass container for future use.

Figure 1.
General presentation of fabric phase sorptive extraction.
The FPSE combines the extraction mode of SPME/SPE into a single technology platform. At the beginning of the whole procedure, the FPSE media is in contact with the sample or the aqueous solution along with the analytes of interest whose mass transfers the sorbent until an equilibrium between the sorbent and the sample matrix is established, which mimics the direct-immersion SPME (equilibrium extraction mode). The extraction process can be facilitated with magnetic stirring, sonication, and more. The porous network of sol-gel sorbent coating and the permeability of the substrate lead to the existing flow-through system, which mimics the solid phase extraction (exhaustive extraction mode) [7,10]. The fabric substrates that are used can be hydrophilic (cotton cellulose), hydrophobic (polyester), or both (cotton-polyester). The different nature of the substrates is very important and determines the selectivity and the polarity of the FPSE media. The strong chemical bonding between permeable fabric and porous sol-gel sorbent offers high chemical and solvent stability. Therefore, the FPSE device is reusable and can be exposed to any organic solvent or harsh chemical environment, which keeps its sorption capability unaffected. Additionally, it provides a high primary contact surface area (PCSA) for rapid analyte-sorbent interaction and, as a result, for rapid and efficient analyte extraction (fast extraction equilibrium). The flexible FPSE requires no modified samples but provides extraction of analytes directly from the sample matrices. Therefore, it eliminates any prior and post sample preparation procedure like filtration, centrifugation, solvent evaporation, and sample reconstitution, which avoids the risk of potential analyte loss, experimental errors, and sample preparation costs. A remarkable benefit of this two-step micro-extraction technique is the variety of the available sorbents that allows the application of the FPSE device in different kind of samples. Therefore, this simple, fast, and sensitive sample preparation technique can be successfully applied in environmental samples, biological samples, or food products [2,8,9,10,11,12]. Taking into account all the reported FPSE methods in the literature so far, it is concluded that the majority of them (57.14%) was applied to environmental samples while applications to food samples (25%) and finally to biological samples (17.86%) follow. All FPSE techniques reported in the literature and studied in the present review article are presented in Table 1 and classified according to the three sample categories in which they were applied. Experimental information of the FPSE methods, the target analytes, the samples, and the main analytical parameters obtained were included.

Table 1.
Classification of the applications of the FPSE technique.
The aim of this review article is to briefly present the origin and the main principles of the FPSE technique and also presents the current applications in a wide range of fields in analytical chemistry. To the best of our knowledge, it is the first attempt to classify the different applications according to the various sample types.
2. Environmental Samples
The FPSE technique was applied to determine substituted phenols in water samples followed by HPLC-UV detection [2]. These compounds are a class of significant industrial raw materials known as very toxic environmental pollutants that are found in wastewater and cause serious health problems. With regard to the extraction procedure, cellulose was the preferred substrate for sol-gel PEG sorbent coating. After cleaning the sol-gel PEG coated FPSE device, it was inserted into the sampling vial (10 mL) and magnetically stirred to promote diffusion of the analytes throughout the sample. This was followed by drying. Among MeOH (methanol), ACN (acetonitrile) and ACN:MeOH, a volume of 500 μL of the ACN:MeOH solvent system was used for a five-minute back-extraction of substituted phenols since it provided better reproducibility in analyte desorption. After centrifugation of the extract, it was injected into the HPLC system. The present FPSE media showed stability and a high sample capacity due to its ability to retain an amount of phenols, which was about 10% of the sorbent loading. After one day and between days repeatability, expressed as RSD% (relative standard deviation) values of analytes were in the range of 1.3–5.1 and 0.5–26.0, respectively. After comparing with other methods used for determining substituted phenols, it is evident that the present method presents higher simplicity, sensitivity, and precision.
Rajesh Kumar et al. [13] used the FPSE technique for determining four endocrine disruptor alkylphenol molecules (4-TBP, 4-SBP, 4-TAP, and 4-CP) in aqueous and soil samples followed by HPLC-UV detection. Alkyl phenols are used in manufacturing many industrial, agricultural, and domestic consumables. Therefore, they are found in the environment and foods, which causes a world wild pollution and severe health implications. The sol-gel poly-Tetrahydrofurane (PTHF) coated on cellulose substrate was selected because both analytes and the PTHF polymer were of medium polarity. The sample was magnetically stirred for extracting target analytes and then eluted with 0.5 mL of MeOH for six minutes. The extract was centrifuged, filtered with a syringe filter, and injected into the HPLC system. The separation of target analytes was carried out by RP-HPLC (reversed phase-high performance liquid chromatography) with H2O/ACN (60:40 v/v) as the mobile phase, which was followed by UV detection with λmax at 225 nm. Extraction efficiency values were 74.0%, 75.6%, 78.0%, and 78.3% for 4-TBP, 4-SBP, 4-TAP, and 4-CP, respectively.
Steroid hormones have been widely used in both human and veterinary medicine, but it proved to be the main source of contamination of the aquatic environment. They can be divided into five subclasses: estrogens, androgens, progestogens, glucocorticoids, and mineral-corticoids. In the first FPSE application, Kumar et al. [12] developed a simple, fast, and sensitive FPSE-HPLC-FLD method for the quantification of estrogens (EE2, E2, and BPA) in different environmental water samples. Due to the medium polarity of studied analytes, a medium polar FPSE media prepared by sol-gel PTHF coated on a cellulose substrate was used for the extraction procedure. The sample (10 mL) along with clean-coated FPSE medium were put inside a glass vial and magnetically stirred for efficient adsorption. For back-extraction of the analytes, the FPSE fiber was soaked into 500 μL of MeOH for 8 min. After centrifugation and filtration of the extract, it was injected into the HPLC system. LOD values were lower than LODs obtained from previously reported methods.
Another application for determining natural and synthetic steroid hormones was presented by Rayco Guedes-Alonso et al. [14]. They reported an FPSE-UHPLC-MS/MS method to quantify six androgens and four progestogens in environmental samples. Androgens and progestogens are endocrine disrupting chemicals (EDCs) that cause serious detrimental impact over aquatic biota even in very low amounts of ng/L. As a result, their determination is of crucial importance. The FPSE procedure took place in a glass vial with a Teflon coated magnetic stirrer where thesol-gel PTHF coated FPSE medium was immersed into the sample solution and was stirred. Three-minute back-extraction of the analytes was occurred using 0.75 mL of MeOH as the elution solvent. Lastly, the extract was injected into the UHPLC system coupled with a triple quadrupole detector. The LOQ values were in the range of 5.7–880 ng/L and RSD values were below 10% in tap and osmosis water and below 20% in wastewater. The applicability of the present method was evaluated by analyzing real environmental samples like wastewater handled with different processes and tap water. In the osmosis treatment samples and tap water samples, no hormones were detected in contrast with wastewater samples. In the WWTP secondary effluent samples, testosterone was detected at 28.3 ng/L while the other analytes either were not detected or were detected below the quantification limit. Yet, in the hospital untreated wastewater samples, progesterone was detected at 227.3 ng/L while the other analytes either were not detected or were detected below the quantification limit.
M. Carmen Alcudia-León et al. [15] developed for the first time an innovative, simple, and cheap laboratory-made air sampling unit that integrates sol-gel polymeric coated fabric extracting phase, which works under a sampling and analysis mode and eliminates the sorptive phase preparation steps before the instrumental analysis and the possibility of cross contamination between sampling and analysis. The performance of the present approach was evaluated by determining two components of the sexual pheromone of Tuta absoluta [(3E,8Z,11Z)-tetradecatrien-1-yl acetate and (3E,8Z)-tetradecadien-1-yl acetate] in environmental air collected in tomato crops. Silica fiber glass fabric provided a strong covalent bonding with sol-gel PDMDPS networks, which led to great thermal stability of the media used and decreased phase bleeding during the elution process. Considering that sol-gel PDMDPS showed the best results, the materials mentioned were selected for the fabric phase sorptive extraction media. The unit connected to a sampling pump allowed the sample collection and run through the fabric sorptive phase at a flow-rate of 2 L/min for a certain time. Afterward, the fabric holder was placed into the gas chromatograph autosampler without any instrumental changes. It was moved into the oven by the robotic arm, pressurized with Helium, and heated at 150 °C for 45 min for thermal desorption of the analytes to the headspace, which was magnetically stirred. Lastly, 2 mL of the headspace were injected into the GC-MS for analysis. The unit was used for the analysis of some real environmental air samples, which verified that some of them contained the target analytes at a really low concentration level. Besides its applicability, the present method has some disadvantages, too. From the sampling to the analysis mode, a lot of time was consumed while the sensitivity was decreased due to some drawbacks of headspace mode in comparison with the conventional thermal desorption.
One severe problem worldwide is heavy metal pollution due to their bioaccumulation and toxicity in the environment. They come from many sources and activities in everyday life and seem to be a serious threat for aquatic environment (oceans, lakes, rivers) and living organisms (humans, animals, plants). Heena et al. [16] reported a simple FPSE-HPLC-UV method for determining cobalt, nickel, and palladium ions in alloys and aqueous samples. In order to quantify trace metals, a chelating agent is necessary. Therefore, the medium polar morpholino dithiocarbamate (MDTC) was used due to its hydrophilic nature. Co(II), Ni(II), and Pd(II) metal ions react very quickly with MDTC to form colored and stable metal dithiocarbamates, which can be easier detected. The sol-gel poly-THF coated FPSE media was chosen for the extraction of metal ions as Co(MDTC)2, Ni(MDTC)2 and Pd(MDTC)2 complexes. Hydrophilic cellulose was selected as the fabric substrate because of its numerous sol-gel active functional groups in each of its dimer, which resulted in its unique ability to hold a high amount of sol-gel sorbents during the coating process. Taking into account both medium polar polymer (MDTC) and hydrophilic substrate, medium polar poly-THF was selected. A sol-gel poly-THF coated FPSE media was immersed into a 10 mL aqueous sample and magnetically stirred for the sorption of analytes. After that, the FPSE media was removed from the sample and then cleaned and transferred into the vial containing 500 μL ACN for 15 min of desorption. The extract was centrifuged and the solution was injected into the HPLC system. The LOD values were found at much lower concentration levels in comparison with already reported literature with excellent reproducibility. The present method was applied to ground and industrial wastewater and also to the borcher and oakay alloy where satisfactory values of recoveries were obtained. Among the three metal ions studied, Co(II) was not recovered in the oakay alloy while Pd(II) showed this behavior in the borcher alloy.
The same research group applied the FPSE-HPLC-UV method for determining Cr(III) and Cr(IV) ions in aqueous samples [17]. The Cr(III) ion is an important nutrient for human health. On the contrary, the Cr(VI) ion does not exist in a natural way and is toxic, which affects the environmental quality and causes severe problems in humans. Therefore, chromium speciation is necessary and of great interest. A sol-gel poly-THF coated cellulose media was used for extraction of the studied ions in the form of MDTC complexes. The FPSE procedure followed was the same as the previous method including some differences about the extraction time [16]. The separation of complexes took place by RP-HPLC with ACN/H2O (65:35 v/v) as the mobile phase and UV detection at 320 nm. The Cr(III) ion formed a single product as Cr(MDTC)3, which leads to a single peak (λmax = 298 nm) in UV-Vis spectra while the Cr(VI) ion formed two different products as Cr(MDTC)3 and Cr(MDTC)2(OMDTC) where OMDTC stands for oxy-MDTC, which leads to two peaks (λmax = 298 nm, 350 nm). The present method is faster and the LOD values were found lower than the previously published reports.
During recent years, many efforts have been noticed in analytical chemistry in order to support the main principles of GAC and to improve the quality of results. Flow injection (FI) techniques are used for fluidic handling and on-line sample preparation, which offers many benefits in comparison with the batch mode of sample pretreatment such as low amounts of solvents and enhanced repeatability of the extraction process. Recently, Anthemidis et al. [18] proposed for the first time the automation of the FPSE technique. A novel on-line flow injection fabric disk sorptive extraction (FI-FDSE) structure was coupled with flame atomic absorption spectrometry (FAAS) for determining lead and cadmium traces in water samples from the environment. A mini-column was filled in a series with sol-gel coated FPSE media formed in the shape of disks and then it was integrated into an FI system, which was related to the FI-FDSE platform. Among four different FPSE media, sol-gel PDMDPS were coated on a non-polar polyester substrate, sol-gel PTHF, sol-gel PEO-PPO-PEO (sol-gel poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymer), and sol-gel graphene coated on polar cellulose substrate. The first one was chosen as the sorbent fabric media because of its uniform surface coating and the greatest sensitivity and repeatability about the extraction procedure in comparison with the other studied media. The applied mini-column offered easy transportation of the entering flow through the fabric media because of the reduced backpressure. As a result, higher extraction efficiency and shorter analytical cycles were achieved. Furthermore, the mini-column was found to be reliable and unaffected for at least 500 sorption/desorption cycles. The applicability of the FI-FDSE-FAAS method was investigated by analyzing standard reference materials and real spiked environmental water samples such as river water, ditch water, and costal seawater. Additionally, the student t-test showed that no statistically important differences were found for the probability level of 95%, which confirms the accuracy of the developed method for comparable samples.
Sarah Montesdeoca-Esponda et al. [19] proposed an FPSE-UHPLC-MS/MS method for determining seven benzotriazole UV stabilizers (BUVSs) in sewage samples from wastewater treatment plants. Although BUVSs are employed in various personal care products, they are categorized as emerging pollutants and present detrimental effects to an aqueous environmental ecosystem and to the human health. The non-polar analytes were UV P, UV 329, UV 326, UV 328, UV 327, UV 571, and UV 360. The sol-gel PDMDPS coated polyester substrate was used for extracting the target analytes. The polyester was selected due to its hydrophobic nature that would favor the whole FPSE procedure. PDMDPS was chosen because it carries methyl and phenyl functional groups resulting in high selectivity toward the analytes. Sample (10 mL) along with FPSE media were magnetically stirred for a specific time. For desorption of the analytes, the sol-gel sorbent coated fabric was soaked for 5 min into 1 mL of MeOH. The back-extraction solution was filtered and injected into the UHPLC system. Considering these circumstances, intra-day and inter-day RSDs were found lower than 15% and 30% for all compounds, respectively. Applying the proposed method for three real non-spiked sewage water samples, two UV stabilizer compounds were detected in the ranges of 17.0 ng/mL to 60.5 ng/mL (UV 328) and 69.3 ng/mL to 99.2 ng/mL (UV 360).
Romualdo B. García-Guerra et al. [20] developed an FPSE-UHPLC-MS/MS method for determining six benzotriazole UV stabilizers in seawater samples collected from beaches where these compounds come directly from tourist bathing. For the extraction of non-polar analytes (UV P, UV 329, UV 326, UV 328, UV 327, and UV 360), sol-gel PDMDPS coated on polyester substrate was preferred among sol-gel PDMDPS, sol-gel PTHF, and sol-gel PEG coated FPSE media due to its greater extraction capacity in a wide range of concentrations. This method reported LOD values six times lower than the previous method [19] possibly because of the higher complexity of wastewater samples compared with the seawater samples. Absolute recoveries were found between 40.9% and 44.3% for all the target analytes at the lowest spiked concentration except for UV P and UV 329 that presented lower recovery values. The application of this optimum method permitted the detection and quantification of UV 360 in nine different seawater samples in the range of 41.12 ng/L to 544.9 ng/L.
Lakade et al. [21] compared four FPSE media: non-polar sol-gel PDMDPS, medium polar sol-gel PTHF, polar sol-gel PEG-PPG-PEG (sol-gel poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol)), and polar sol-gel PEG for extracting a group of pharmaceuticals and personal care products (PPCPs) with a wide polarity range at trace concentration levels from environmental water samples. The sol-gel PEG coated FPSE media was selected for the extraction procedure due to satisfactory recoveries obtained (24% to 85%) for all the target analytes (MPB, CBZ, PrPB, DHB, BzPB, DHMB, DICLO, BP-3, TCC, and TCS) except for the most polar ones known as PARA (paracetamol), CAFF (caffeine), APy (antipyrine), and PROP (propranolol hydrochloride) that were not included in the current method. The sample (10 mL) adjusted to a pH of 3 along with 10% of NaCl (w/v) (sodium chloride) was magnetically stirred in the presence of FPSE media. Then, after removal and dryness of the FPSE media, elution by 1 mL of MeOH was carried out in an ultrasonic bath for 5 min. The extract was evaporated and dried. The residue was reconstituted in 1 mL of H2O/ACN (80:20 v/v) solution. The determination occurred by the LC-MS/MS method. Generally, LOD values found to be lower than those obtained in a previously mentioned SBSE technique followed by the same LC-MS/MS instrument (5 ng/L to 10 ng/L). Real samples from the Ebre River known as effluent and influent wastewater were analyzed. All analytes were detected in the investigated samples except for TCS in effluent samples and, among them, MPB, CBZ, DICLO, and BP-3 presented higher concentrations in wastewater samples. Comparing the concentrations in influent and effluent samples, analytes were lower in the second one because of the treatment process.
Time that is required for the extraction process (up to four hours) is one of the main disadvantages of the FPSE technique. To overcome this drawback, Lakade et al. [22] presented a new alteration of the FPSE called dynamic fabric phase sorbent extraction (DFPSE). This approach uses 47 mm circular disks of FPSE media, which are put in a filtration assembly. The target analytes are extracted into the FPSE disks and then they are back-extracted by passing the elution solvent through them. Due to this, the extraction time is decreased. Therefore, a new DFPSE mode followed by LC-MS/MS was developed in order to determine MPB, CBZ, PrPB, DHB, BzPB, DHMB, DICLO, BP-3, TCC, and TCS in river and wastewater samples. At the beginning, the FPSE disks installed into the filtration assembly were conditioned by passing MeOH followed by ultrapure water and they were dried by applying vacuum. With regard to the extraction process, the sample (50 mL) was loaded into the filtration assembly and was left in contact with the disk of the FPSE media for the adsorption of the target analytes. Taking into consideration that most of the analytes are either highly polar (PARA, CAFF, APy, MPB, CBZ) or somewhat polar (PROP, PrPB, DHB, BzPB), cellulose was selected as the substrate because of its hydrophilic nature. In addition, PEG was chosen as the organic polymer since it showed effective results in a previous project [21]. Then, the sample was passed through the FPSE disk by vacuum. Afterwards, the FPSE media was dried by vacuum. The elution of the retained analytes was achieved by passing 10 mL of ethyl acetate since it evaporated faster than the other solvents that were investigated. The analysis occurred by LC-MS/MS. In comparison with static FPSE [21], recovery values were better except for PARA and CAFF whose recoveries were lower than 38%. Additionally, the extraction time was decreased from 240 min to 10 min. After its application in real samples, only MPB and DHMB were found at low ng/L concentrations in river samples while all of the analytes were present in effluent and influent wastewater samples. Most of them were detected at higher concentration levels in influent rather than effluent wastewater due to the wastewater treatment except CBZ and DICLO, which were detected at similar concentrations.
The FPSE technique was also applied for determining four non-steroidal anti-inflammatory drugs (NSAIDs) called IBU, NAP, KET, and DIC in environmental water samples combined with GC-MS [23]. Three different sorbents known as sol-gel PDMDPS (non-polar) coated on a polyester substrate, sol-gel PTHF (somewhat polar), and sol-gel PEG (polar) coated on cellulose substrates, were studied. The sol-gel PEG coated FPSE media was selected because it presented the best extraction efficiency for the target analytes independently of pH and ionic strength. The water sample (30 mL) along with sol-gel PEG coated FPSE media were put into a glass screw-cap vial and were magnetically stirred. The elution of the compounds was performed into a 2 mL screw-cap vial by soaking the FPSE media into 1 mL of ethyl acetate for 15 min. After removal of the FPSE medium from the vial, the extract solution was evaporated to dryness by a nitrogen stream. Afterward, reconstitution of the residue was carried out in a 100 mL insert with ethyl acetate and MTBSTFA (N-Methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide) while the derivatization reaction took place in an oven at 60 °C for 60 min. After room temperature cooling, the extract was inserted into the GC-MS system. In comparison with other techniques, like SPME, SBSE, MEPS, and LOQs obtained with this method were found to be the best while RSDs were between 3.5% and 18%. The method mentioned was successfully applied for the analysis of target analytes in two influent and effluent samples from a wastewater treatment plant and in two river water samples. For influent samples, the concentrations were in the range of 100 ng/L (DIC) up to 10 μg/L (IBU) to 15 μg/L (IBU). For the effluents, the concentrations of IBU were lower (25 ng/L to 100 ng/L) while the concentrations of KET and DIC varied from 90 ng/L to 450 ng/L. Ιt was found that IBU’s concentration ranged from 2 μg/L to 6 μg/L in the period from 2000 to 2002 in the same WWTPs. Therefore, it is observed that the concentration of IBU in the influent has been raised in the last decade. For the Sar river, the concentrations were in the range of 50 ng/L to 150 ng/L while, for the Sarela river, DIC was found to be below the LOQ and the other NSAIDs could be detected in the range of 25 ng/L to 55 ng/L.
Cytostatic drugs treat different types of cancer by inhibiting cell growth. These agents can join the aquatic environment in the range of ng/L through effluents from wastewater treatment plants and hospitals or urinary excretion, which is catastrophic to living organisms. Therefore, Sergio Santana-Viera et al. [24] applied the FPSE micro-extraction technique for the analysis of seven cytostatic drug compounds (MET, GEM, ETO, CP, VINC, VINB, and TAM) in environmental water samples by UHPLC-MS/MS. Six different FPSE media were investigated: sol-gel CN-PEG, sol-gel M-PEG, sol-gel M-Pcaptriol (sol-gel methyl polycaprolactone triol), sol-gel M-UCON (sol-gel methyl UCON), sol-gel M-PEG300, and sol-gel M-Cap-DMS-Cap (sol-gel methyl caprolactone-dimethylsiloxane-caprolactone). Among them, M-PEG and M-UCON showed the best adsorption and desorption efficiencies for all the studied compounds, but M-PEG achieved better absolute recoveries (25% to 90%) than the second one media (20% to 70%), which means it was selected for the current analysis. After cleaning the FPSE media, the extraction of the target analytes from the sample (10 mL) took place. Due to varied physicochemical properties of cytostatic compounds, two pHs were selected to perform extractions: pH 8 for ETO, TAM, and CP and pH 10 for VINB and VINC. GEM and MET presented very low extraction yields, which means they were dismissed. For the elution step, fabric was soaked into 1 mL of MeOH for 5 min. The extracts were injected into the chromatographic system and the fabric media was cleaned and prepared for future use. RSDs were found to be lower than 12%. The developed method was applied to real wastewater samples from an effluent obtained from a hospital and three wastewater treatment plants. No cytostatic compounds were detected in the wastewater from the effluent of the wastewater treatment plants except for 2.6 μg/L of ETO detected in hospital effluents.
Many scientists have tried to increase the contact surface area of micro-extraction devices, which results in increased extraction kinetics. Therefore, Pawliszyn et al. [35] developed a thin film micro-extraction technique. A thin membrane of PDMS (poly(dimethylsiloxane)) was used as the extracting phase and an effective interaction between the sorptive phase and the sample occurred. Taking into account this approach, Mercedes Roldán-Pijuán et al. [25] studied the possible improvement of the conventional FPSE procedure by stirring the entire extraction system. Therefore, a new stir fabric phase sorptive extraction (SFPSE) that incorporates sol-gel hybrid organic-inorganic coated fabric media with a magnetic stirring mechanism was proposed and was followed by UPLC/DAD analysis for determining seven triazine herbicides in water. Cellulose and polyester were investigated as fabric substrates while sol-gel PTHF, sol-gel PEG, and sol-gel PDMDPS were candidates for sorbents. According to the recovery results, better extraction of the target analytes was achieved by sol-gel PEG. Therefore, it was chosen for this study. In order to build the SFPSE unit, a part of the polypropylene SPE cartridge, which is an external piece cut from a pipette tip, an FPSE media for the extraction process and an iron wire to favor the magnetic stirring of the unit were used. After optimization of the extraction conditions, elution time with 1.0 mL methanol was fixed at 5 min. The applicability of the present method was evaluated by determining triazine herbicides in environmental water samples like river water. No traces of the target analytes were found, which means the analysis took place with spiked samples. Due to the stirring system, this approach offers fast analyte diffusion and enlarged contact surface area, which results in a lower extraction time and improved extraction efficiency.
In the context of stirring the fabric substrate, Guiqi Huang et al. [26] designed two extraction devices to apply them toward the determination of three brominated flame retardants (BFRs) (TBBPA, TBBPA-BAE, and TBBPA-BDBPE) in water samples followed by HPLC-DAD analysis. The first stir FPSE device was similar to that described previously [25] where it was used as a magnetic stirring mechanism. The second one is called stir-bar fabric phase sorptive extraction (stir bar-FPSE) and is innovative since it has not been reported yet in the literature. For the construction of the stir bar-FPSE device, the fabric phase media was formed to a house shape and it was pressed and fixed by using a stir bar. Among the investigated FPSE sorbents known as sol-gel PEG, sol-gel PTHF and sol-gel PDMDPS, the sol-gel PTHF was selected to be used on the surface of cellulose substrate because of the medium polarity of the three BFRs. For the two techniques mentioned above, the elution of BFRs took place for 15 min by using 0.3 mL ACN. The developed method was applied in wastewater and reservoir water samples where RSDs were found to be lower than 5.3%. Only TBBPA was detected in wastewater and none of the target analytes were detected in reservoir water. Both analytical procedures presented a fast extraction equilibrium and high extraction efficiency while they could be considered as green ones due to limited solvent utilization.
3. Food Samples
M. Aznar et al. [8] applied the FPSE technique followed by UPLC-MS to study the migration of various non-volatile plastic additives from food packaging materials. The 18 plastic additives were 8 plasticizers (DEP, TBC, DBM, TBoAC, TXIB, DBP, 2EHAdip, 2EHSeb), 5 antioxidants (IRGA38, TOPAC, IRGA1076, IRGA168 and IRGA1010), 4 UV absorbers (TINU326, CHIMA81, TINU327, CYA1084), and 1 antistatic agent (HAA C12). Three different sol-gel coated FPSE media were studied including sol-gel PDMS (non-polar), sol-gel PTHF (somewhat polar), and sol-gel PEG (highly polar). All coatings were made using sol-gel coating technology on cotton cellulose fabric as a substrate. In addition, three food simulants with the concentration of the compounds studied known as ethanol 10%, acetic acid 3%, and ethanol 50% were tested. In general, the analytes with the highest polarities (logp < 5), were better extracted using the sol-gel PTHF and sol-gel PEG coated FPSE media, which was dissolved in acetic acid 3% or ethanol 10%. In this case, the percentage of the compound extraction was over 75%. To the contrary, the compounds were hardly extracted in the fabrics using ethanol 50%. The extraction of these analytes with sol-gel PDMS, which is low polar, was lower than the other FPSE media especially for DEP. Regarding the compounds with medium polarities (medium logp values), the best extraction in all FPSE media was achieved only when they were dissolved in acetic acid (3%) and compounds with lower polarities (logp > 10) except for TOPAC were only extracted when ethanol 50% was used as a food simulant. In addition, compounds with low logp values were proven to have higher enrichment factors (EFs) especially with sol-gel PTHF and sol-gel PEG media. For compounds with high logp values, the use of sol-gel PDMS seemed to improve enrichment capacity. Acetonitrile was preferred as the elution solvent because of good recovery results while elution of the studied compounds took place for 10 min. The best extraction recovery values were achieved using the aqueous acetic acid solution 3% for the dissolution of the compounds where 17 out of 18 compounds showed enhancement in their signal intensity after FPSE extraction and 10 presented enrichment factors higher than 3 for all FPSE media. When the extracts were evaporated by nitrogen gas flow, 11 out of 18 compounds presented EFs values above 100.
M. Aznar with the same group [27] presented a simple and sensitive method to detect some possible freshness markers in oranges. Study chemical changes occurred in oranges after storing at 5 °C for two months. Twelve volatile and non-volatile compounds in orange juices were determined by the FPSE technique combined to GC-MS and UPLC-QTOF (quadrupole-time of flight mass spectrometer)-MS. Five FPSE media coated with different sol-gel sorbents were studied including sol-gel long chain PDMS (non-polar), sol-gel short chain PDMS (non-polar), sol-gel short chain PTHF (somewhat polar), sol-gel PEG-PPG-PEG (somewhat polar), and sol-gel PEG (highly polar). The sample (100 mL) was magnetically stirred along with the FPSE medium for the optimum time. The elution of the target analytes assisted by ultrasound was optimized for 10 min. Sol-gel PEG was selected as the most suitable sorbent because of its satisfactory results about enrichment factors for seven out of the ten volatile compounds compared with the other media. Methanol was selected as the elution solvent since, in general, recovery values were higher than 80% except for butyric acid and ethyl butyrate. Between the volatile compounds, monoterpenes and terpenoids showed a final concentration below 10% of their initial concentration after the storage period of two months. As a result, they represent good markers of orange freshness. On the contrary, one amide (subaphyllin) and two flavanoids (tangeretin and nobiletin) that belong to non-volatile compounds presented a remarkable reduction in signal intensity (>70%), which makes them usable as markers for orange quality and freshness.
Samanidou et al. [9] reported a homogenous and ultra-thin sol-gel PEG coated FPSE media in combination with HPLC-DAD detection that was applied for the first time to estimate the concentration levels of highly polar amphenicols residues in raw milk samples. The studied antibiotics were TAP, FFC, and CAP. An aliquot of milk sample (0.5 g) with 500 μL deionized water was magnetically stirred with the FPSE media and the elution of the compounds of interest occurred with 500 μL MeOH for 10 min. The extract was centrifuged and filtered before the HPLC analysis. The FPSE media could be reused by washing with 2 mL ACN:MeOH (50:50 v/v) for 5 min. Afterward, it was dried and stored for future use. Elimination of sample preparation steps was successful because no protein precipitation or solvent evaporation were necessary, which led to a reduction of error sources. The studied method presented good selectivity, sensitivity, and linearity. The decision limits CCa for TAP, FFC, and CAP were 52.49 μg/kg, 55.23 μg/kg, and 53.8 μg/kg, respectively, while the detection capability CCβ were 56.8 μg/kg, 58.99 μg/kg, and 55.9 μg/kg. Additionally, it showed that absolute recovery was 44% for TAP, 66.4% for FFC, and 81.4% for CAP because of the intensive interaction between target antibiotics and sorbent. The evaluated precision within a day and between days varied from 1.0% to 10.7% and 7.6% to 14.0% for the three amphenicols, respectively.
Karageorgou et al. [28] developed for the first time a simple and efficient FPSE-HPLC-UV method for fast simultaneous isolation of three sulfonamides residues, SMTH, SIX, and SDMX from untreated raw milk samples. Sol-gel PEG coated on a cellulose substrate was selected as the extraction medium of the target analytes. The whole FPSE procedure was the same as the previous method [9] including some differences between the elution solvent and elution time. RSDs for within-day repeatability and between-day repeatability were lower than 5.6% and 6.7%, respectively. Additionally, CCa values (decision limit) were 116.5 μg/kg for SMTH, 114.4 μg/kg for SIX, and 94.7 μg/kg for SDMX while CCβ values (detection capability) were 120.4 μg/kg for SMTH, 118.5 μg/kg for SIX, and 104.1 μg/kg for SDMX.
Samanidou et al. [29] developed and validated a new FPSE-HPLC-DAD method for the concurrent extraction of four penicillin antibiotic residues (PENG, CLO, DICLO, and OXA) from milk. Sol-gel PEG FPSE medium was selected among sol-gel C18 and sol-gel PEG-PPG-PEG because of its best absolute recovery values ranging between 22% and 58% while the two other sorbent media provided recovery values lower than 5%. The present micro-extraction method was based to the same FPSE strategy and offered the same noteworthy benefits about simplification of the sample preparation practice as the two previously mentioned methods [9,28]. Penicillins were separated using an RP-HPLC method. The LOQs were under the maximum residue limits (MRLs) set by European legislation (except of PENG). Applying the proposed method to real milk samples, no traces of the target analytes were found or they were detected under the limit of detection.
Samanidou et al. [30] combined the FPSE technique with the impressive characteristics of graphene in order to simplify the extraction procedure of bisphenol A (BPA) and other monomers released from dental restorative materials from milk like TEGDMA, UDMA, and BisGMA. Cotton cellulose has a hydrophilic nature, which leads to better wettability when soaked in water and causes an intensive interaction between the analytes and sorbent. Therefore, it was selected as the used FPSE substrate. Furthermore, among five different FPSE sorbents including sol-gel graphene (for non-polar and medium polar analytes), sol-gel poly-THF (for medium and highly polar analytes), sol-gel PEG (for highly polar analytes), sol-gel C18 (for non-polar and medium polar analytes), and sol-gel PEG-PPG-PEG (for medium and highly polar analytes), sol-gel graphene coated media was applied because it presented the highest extraction sensitivity by considering the concurrent presence of μ-μ interaction, hydrogen bonding, and London dispersion. Sample preparation steps were the same as the previous method [9] developed from the same research team except the de-proteinization step of milk samples by formic acid 10% (v/v) before the extraction procedure and some changes about elution solvents and extraction time. After the extraction process, target analytes were separated by the HPLC-UV validated method. The absolute recovery values from standard solutions were 50% for BPA, 78% for TEGDMA, 110% for UDMA, and 103% for BisGMA and absolute recovery values from milk were 30%, 52%, 104%, and 42%, respectively. After the application of the present method to real samples, no traces of the studied compounds were detected.
In order to increase the contact surface area of the micro-extraction device and improve the extraction efficiency, some impressive and alternative approaches have been proposed. Ionic liquids (ILs) that are liquid salts and consist of organic cations and anions have been applied as green extraction solvents or have been immobilized on the substrate for an extraction procedure, which permits various interactions between the sorbent and the analyte. Additionally, surfactants are amphiphilic organic compounds that are active at the surface or the interface between organic and aqueous phase. Therefore, Miyi Yang et al. [31] used the surfactant-assisted ionic liquid immobilized fabric phase sorptive extraction (SAIL-FPSE) followed by an HPLC-DAD analysis for rapid screening and simultaneous determination of four fungicides (azoxystrobin, chlorothalonil, cyprodinil, and trifloxystrobin) and residues in tea infusions. In the current method, [HMIM]NTf2 was chosen as the coating ionic liquid because of its higher extraction capacity and lower reagent consumption. Furthermore, oil-absorbing cotton showed high hydrophobicity, stability in an organic solution, and a strong ability to recover the target analytes. As a result, it was selected as the fabric substrate. The use of 0.2 mM surfactant Tween 20 increased the extraction efficiency by augmenting the contact of fiber and fungicides. Intra-day RSD% and inter-day RSD% were found to be 1.1% to 4.9% and 4.7% to 7.3% at the concentration of 50 μg/L, respectively.
4. Biological Samples
Kumar et al. [12] applied the already described FPSE-HPLE-FLD method for determining EE2, E2, and BPA estrogens not only in environmental samples but also in urine samples. The FPSE procedure and the analytical parameters of the method were the same since they were mentioned in environmental applications. The relative recoveries of estrogens from spiked urine samples were good, which proves the successful applicability of the method to biological samples.
Rayco Guedes-Alonso et al. [14] reported an FPSE-UHPLC-MS/MS method for determining four progestogens and six androgens in urine samples. This method has already been described in environmental applications because it was also performed for biological samples. LOQs were from 29.7 ng/L to 440.7 ng/L while RSD values were below 20%. After applying the present method to real urine samples, progesterone, testosterone, and androstenedione were detected at 1100 ng/L, 2300 ng/L, and 3500 ng/L, respectively, which are concentrations that are higher in comparison with those found in environmental samples. The other compounds were either not detected or were detected below the quantification limit.
Azoles drugs are used in pharmaceutical products for fungal infection treatments. Marcello Locatelli et al. [32] reported an FPSE-HPLC-PDA method for the concurrent determination of twelve azole antimicrobial drug residues in human plasma and urine characterized by wide dispersion of logKow values. Therefore, their analysis could be really important for clinical and pharmaceutical applications. Sol-gel PEG coated FPSE media was selected as the optimum sorbent type instead of sol-gel PDMS and sol-gel PCAP-PDMS-PCAP (sol-gel polycaprolactone-polydimethylsiloxane-polycaprolactone block copolymer). After preparing the FPSE media, it was immersed into the sample (500 μL for plasma and 1 mL for urine) for the extraction process. Afterwards, the retained analytes were eluted by using 150 μL of MeOH for both types of samples for 10 min. The extracted azoles were centrifuged and 20 μL of the supernatant were analyzed. The separation took place by using a C18 analytical column and phosphate buffer. ACN as a mobile phase in a gradient elution mode within 36 min while the detector was operated at 210 nm. The intra-day and inter-day RSD% values were lower than 13.1% and 13.9%, respectively, while the intra-day and inter-day bias percentage values ranged from −12.1% to 10.5%. The proposed method was applied to real plasma and urine samples after a single dose oral administration of itraconazole and miconazole to healthy volunteers. The results about the two azoles were similar with pharmacokinetic data mentioned in literature.
Samanidou et al. [33] developed an FPSE-HPLC-DAD method for determining benzodiazepines called APZ, BRZ, DZP, and LRZ in human blood serum. Benzodiazepines are drugs commonly administrated in psychiatric and forensic toxicological incidents so their analysis in biological samples is of great interest. Sol-gel PEG and sol-gel PTHF coated on cellulose substrates for highly and medium polar analytes and sol-gel PDMDPS coated on polyester substrate for less polar analytes were investigated. Among the FPSE sorbents mentioned above, better recovery results were obtained using sol-gel PEG-coated FPSE media so it was selected for the extraction procedure. The FPSE medium was prepared in order to be ready for extraction and was introduced into a glass vial that contained the sample solution and a Teflon-coated magnetic stir. After stirring the sample, the elution of the target analytes took place into another vial by using acetonitrile: methanol (50:50 v/v) as an elution solvent system and lasted for 10 min. Afterwards, direct injection into the HPLC instrument and detection by DAD operated at 240 nm followed. The separation occurred by an RP-analytical column using a solvent system of acetonitrile, methanol, and ammonium acetate as a mobile phase. RSD values were lower than 13.1%. In this work, it did not occur solvent evaporation because of the small amounts of organic solvents that were used or protein precipitation before the FPSE. As a result, the sample preparation time and steps were substantially reduced, which leads to a simple, fast, low cost, and applicable method.
Very recently, Kabir et al. [34] developed a new FPSE-HPLC-PDA method for the simultaneous determination of ciprofloxacin, sulfasalazine, and cortisone in whole blood, plasma, and urine human samples commonly used for inflammatory bowel disease (IBD) treatment. Five FPSE media were investigated including sol-gel PEG, sol-gel PCAP-PDMS-PCAP, sol-gel SCS (sol-gel sucrose), sol-gel PCL (sol-gel poly(caprolactone)), and sol-gel PEG-PPG-PEG. Better enrichment factors were obtained by using the sol-gel PCAP-PDMS-PCAP and sol-gel PEG coated on cellulose substrate. Therefore, they were chosen for further studies. After the sample extraction, back-extraction of target analytes occurred for 10 min by using 500 μL of MeOH. Lastly, the extract was centrifuged and 20 μL of the supernatant were introduced into the analytical instrument. The separation was carried out in 20 min using phosphate buffer: acetonitrile as the mobile phase in gradient elution mode. Both precision (expressed as RSD percentage) and accuracy (expressed as bias percentage) values were lower than 15%. The present method was applied to real samples that were collected from inflammatory bowel disease patients. The data obtained showed that the whole blood could be used as a sample matrix for the direct analysis of the drugs studied. However, further clinical study is required.
5. Fabric Phase Sorptive Extraction: Features of Merit—Comparison with Other Sample Extraction Techniques
Fabric phase sorptive extraction has eloquently integrated both the exhaustive extraction mechanism and the partitioning equilibrium driven extraction into a single technology platform. As such, FPSE possesses the advantages of both sample preparation techniques. Unlike commercially available extraction and micro-extraction techniques, FPSE does not require any special equipment or set-up. The FPSE membrane can be directly introduced into the sampling container for extraction and back-extraction tube for analyte elution. A battery-powered magnetic stirrer can diffuse the solution using a magnetic stir bar. Therefore, the technique is easily field deployable.
Due to the strong chemical bonding between the fabric substrate and the sol-gel sorbents, FPSE membranes can be exposed to any organic or organo-aqueous solvent including a harsh chemical environment (pH 1–13). This offers a unique opportunity for selecting a suitable solvent compatible with both GC and LC and for analyzing simultaneously using both the technique to obtain complementary and holistic chemical information of the sample. On the other hand, the lack of chemical anchorage between the extraction polymer and the substrate limit the solvent selection in most of the classical extraction and micro-extraction techniques.
Although FPSE membranes have been designed as a single use device (like SPE cartridges and disks) to minimize the memory effect from the previous extractions as well as to reduce the risk of cross contamination, it can be reused as many as 50 times without consuming a high volume of organic solvent in the cleaning process.
FPSE eliminates all sample pretreatment steps including filtration, centrifugation, and protein precipitation. It also eliminates solvent evaporation and sample reconstitution from the sample preparation workflow. These steps are often used in SPE.
The extraction time in FPSE varies widely from 20 min to several hours depending on the complexity of the sample matrix and the presence of competing but unwanted compounds in the sample. However, the extraction time can be substantially reduced if FPSE is carried out in a dynamic extraction mode (like an SPE disk). This advantage is only seen in FPSE among all the contemporary sample preparation techniques.
FPSE utilizes sol-gel coating technology for the sorbent coating on the fabric substrates. Unlike the conventional sorbent coating process (physical adhesion of the polymer on the substrate surface followed by free-radical cross-linking reaction), sol-gel coating is a precisely controllable chemical coating process that ensures high batch-to-batch reproducibility, which is a phenomenon of extreme importance in analytical chemistry.
One major advantage of FPSE over other extraction and micro-extraction techniques is its ability to fine tune the overall polarity and selectivity by judiciously selecting the fabric type (hydrophilic or hydrophobic), organic polymer (nonpolar to highly polar), and the inorganic precursor possessing different organic ligands. Conventional extraction and micro-extraction techniques offer limited selectivity towards the target analytes since the organic polymers (in micro-extraction techniques) or the organic ligands (in exhaustive extraction techniques) are the only source of selectivity.
FPSE offers a wide range of sorbents including polar, somewhat polar, nonpolar, cation exchanger, anion exchanger, and mixed mode sorbents. No other extraction and micro-extraction techniques offer such a broad spectrum of sorbent chemistries. In fact, FPSE is the first sample preparation technique that offers traditional SPE sorbents including C8, C18, cyano, diol, phenyl chemistries, and SPME sorbents including poly(dimethylsiloxane) and poly(ethylene glycol) polymeric materials coated on the fabric substrate. As such, FPSE has not only integrated the extraction mechanism of both exhaustive extraction and partitioning equilibrium-based micro-extraction techniques but also makes available all the important sorbent chemistries in both techniques.
In conclusion, FPSE has not only simplified and boosted eco-friendliness in the sample preparation workflow, it has substantially improved the overall sample preparation process.
As an example, Table 2 presents a comprehensive comparison of the performance of the FPSE technique with other sample extraction techniques that have been applied for determining four non-steroidal anti-inflammatory drugs. The selected publications are referred to the analysis using GC-MS so the techniques and numerical data are more comparable. Taking into account the characteristics about the extraction procedure, FPSE can be really practical and easily applicable because it can handle the larger sample volumes and offers the possibility of satisfactory extraction time. Comparing the analytical performance between the techniques, FPSE shows the lowest LOD and LOQ values, which are the most sensitive. In addition, RSDs are below 18% for FPSE, which presents presenting good precision while SPME can be the most repeatable. Lastly, the trueness values, which are expressed as relative recoveries, are quite satisfactory.

Table 2.
Comparison of the FPSE with other extraction techniques regarding the determination of four non-steroidal anti-inflammatory drugs known as ibuprofen, naproxen, ketoprofen, and diclofenac.
6. Conclusions and Future Perspectives
Fabric phase sorptive extraction has been proven to be a green and highly promising sample preparation technique and a useful tool for the extraction and pre-concentration of both volatile and non-volatile analytes from different matrices. The inherently porous structure of the sol-gel sorbent networks along with the great permeability of the host fabric substrate synergistically contribute to the efficient extraction procedure and reduce equilibrium extraction time. The strong chemical bonding between the sol-gel extraction sorbents and the substrates provides excellent chemical and solvent stability to the FPSE media and permits the use of any elution solvent for analyte back extraction/desorption. Immersing the FPSE media directly into the sample solution and eliminating a number of error prone sample preparation steps such as protein precipitation, solvent evaporation followed by sample reconstitution and consumption of small amounts of organic solvent for elution of the target analytes lead to a simple, faster, eco-friendly, and low-cost sample preparation technique that conforms to the principles of GAC. In addition, the potential analyte loss and other sources of errors are greatly minimized.
As presented, FPSE can be successfully used with any chromatographic techniques such as liquid chromatography and gas chromatography coupled with various detectors depending on the needs of the analysis. Μost of the applications of the unique, new generation technique are focused on the isolation and determination of drugs, pharmaceuticals, and other compounds mainly in environmental samples as well as in food samples and biological fluids.
Although FPSE is a novel sorptive extraction technique used in a sample preparation process, many research papers have already been published proposing its application in various scientific fields. It is an easily handled and simple technique. The fact that FPSE medium can be immersed directly into the sample solution requiring no sample modification may offer the possibility of on-site analysis especially for environmental samples. Furthermore, new and innovative chemically-modified sol-gel coatings on the substrates may result in new fabric sorbents that could be used for the sorption of more categories of analytes. In addition to this, enzymatic modification of the FPSE media or the use of biocompatible materials may lead to novel fabrics suitable for clinical and bio-applications. Therefore, the application of FPSE on analytical problems with biological interest, which is limited so far, may be increased. It contributes to the development of minimal invasive and “real time” diagnostics. Additionally, noteworthy efforts have been made in order to improve the FPSE performance as much as possible such as FDSE, DFPSE, SFPSE, and more. The ability to automate the FPSE would be of crucial importance since it could be applied for routine analysis when the FPSE media becomes commercially available. FPSE is a new technique, which can be modified easily in many different ways by offering unique variations. Therefore, due to its adaptability, the future of the FPSE technique is limited only by the imagination of the researchers.
Author Contributions
All authors have equally contributed.
Funding
This work received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ΗPLC, High performance liquid chromatography; UV, UltraViolet; PEG, Poly(ethyleneglycol); MeOH, Methanol; ACN, Acetonitrile; 4-CP, 4-Cumylphenol; 3,5-DMP 3,5-dimethylphenol; 2,6-DCP, 2,6-dichlorophenol; 2,4,6-TCP, 2,4,6-trichlorophenol; 2,4-DIPP, 2,4-diisopropylphenol; PTHF, Poly(tetrahydrofuran); 4-TBP, 4-tert-butylphenol; 4-SBP, 4-s-butylphenol; 4-TAP, 4-tert-amylphenol; FLD, Fluorescence detection; WWTP, Waste water treatment plant; BPA, Bisphenol A; E2, 17β-Estradiol; EE2, 17α-Ethynylestradiol; UHPLC, Ultra high pressure liquid chromatography; MS/MS, Tandem mass spectrometry; NORET, Norethisterone; NOR, Norgestrel; MGA, Megestrol acetate; PRO, Progesterone; BOL, Boldenone; NAN, Nandrolone; TES, Testosterone; DHEA, Dehydroepiandrosterone; AND, Androsterone; ADTD, Androstenedione; HS, headspace; GC, Gas chromatography; MS, Mass spectrometry; PDMDPS, Poly(dimethyldiphenylsiloxane); He, Helium gas; FDSE, Fabric disk sorptive extraction; FI, Flow injection; FAAS, Flame atomic absorption spectrometry; MIBK, Methyl isobutyl ketone; UV P, 2-(benzotriazol-2-yl)-4-methylphenol; UV 329, 2-(benzotriazol-2-yl)-4-(2,4,4-trimethylpentan-2-yl) phenol; UV 326, 2-tert-butyl-6-(5-chlorobenzotriazol-2-yl)-4-methylphenol;UV 328, 2-(benzotriazol-2-yl)-4,6-bis (2-methybutan-2-yl) phenol; UV 327, 2,4-ditert-butyl-6-(5-chlorobenzotriazol-2-yl) phenol; UV 571, 2-(benzotriazol-2-yl)-6-dodecyl-4-methylphenol; UV 360, 2-(benzotriazol-2-yl)-6-[[3-(benzotriazol-2-yl)-2-hydroxy-5-(2,4,4-trimethylpentan-2-yl)phenyl]methyl]-4-(2,4,4-trimethylpentan-2-yl)phenol; LC, Liquid chromatography; MPB, Methylparaben; CBZ, Carbamazepine; PrPB, Propylparaben; DHB, 2,4-di-hydroxy- benzophenone; BzPB, benzylparaben; DHMB, 2,2-dihydroxy-4-4methoxybenzophenone; DICLO, Diclofenac; BP-3, 3-benzophenone; TCC, Triclocarban; TCS, Triclosan; DFPSE, Dynamic fabric phase sorptive extraction; EtOAc, Ethyl Acetate; IBU, Ibuprofen; NAP, Naproxen; KET, Ketoprofen; DIC, Diclofenac; M-PEG, Methyl poly(ethyleneglycol); ETO, Etoposide; CP, Cyclophosphamide; VINC, Vincristine; VINB, Vinblastine; TAM, Tamoxifen; UPLC, Ultra performance liquid chromatography; DAD, Diode array detector; TBBPA, Tetrabromobisphenol A; TBBPA-BAE, Tetrabromobisphenol A bisallylether; TBBPA-BDBPE, Tetrabromobisphenol A bis(2,3-dibromopropyl)ether; DEP, Diethyl phthalate; TBC, Tributyl citrate; DBM, Dibutyl maleate; TBoAC, Tributyl-o-acetyl citrate; TXIB, 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate; DBP, Dibutyl phthalate; 2EHAdip, Bis(2-ethylhexyl) adipate; 2EHSeb, Bis(2-ethylhexyl) sebacate; IRGA38, Irgafos38; TOPAC, Topanol CA; IRGA1076, Irganox 1076; IRGA168, Irgafos 168; IRGA1010, Irganox 1010; TINU326, Tinuvin 326; CHIMA81, Chimassorb 81; TINU327, Tinuvin 327; CYA1084, Cyassorb 1084; HAAC12, N-Lauryldiethano-lamine; TAP, Thiamphenicol; FFC, Florfenicol; CAP, Chloramphenicol; SMTH, Sulfamethazine; SIX, Sulfisoxazole; SDMX, Sulfadimethoxine; PENG, benzylpenicillin; CLO, Cloxacillin; DICLO, Dicloxacillin; OXA, Oxacillin; TEGDMA, Triethylene glycol dimethacrylate; UDMA, Urethane dimethacrylate; BisGMA, Bisphenol A glycerolatedimethacrylate; SAIL, Surfactant-assisted ionic liquid-immobilized; [HMIM]NTf2, 1-hexyl-3-methylimidazolium bis(trifluoromethanesulfonimide); APZ, Alprazolam; BRZ, Bromazepam; DPZ, Diazepam; LRZ, Lorazepam; PCAP-PDMS-PCAP, Polycaprolactone-polydimethylsiloxane-polycaprolactone.
References
- Samanidou, V.F. Trends in microextraction techniques for sample preparation. Separations 2018, 5, 1. [Google Scholar] [CrossRef]
- Kabir, A.; Mesa, R.; Jurmain, J.; Furton, K. Fabric phase sorptive extraction explained. Separations 2017, 4, 21. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. The role of green extraction techniques in Green Analytical Chemistry. TrAC-Trends Anal. Chem. 2015, 71, 2–8. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Miniaturized solid-phase extraction techniques. TrAC-Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Spietelun, A.; Marcinkowski, Ł.; de la Guardia, M.; Namieśnik, J. Recent developments and future trends in solid phase microextraction techniques towards green analytical chemistry. J. Chromatogr. A 2013, 1321, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kazantzi, V.; Anthemidis, A. Fabric sol-gel phase sorptive extraction technique: A review. Separations 2017, 4, 20. [Google Scholar] [CrossRef]
- Silva, F.; Universidade, S.; Fluminense, F.; Semaan, F.S. Sample preparation in food analysis: Practices, problems and future outlook. In Thermal Techniques and Their Applications; Locatelli, M., Celia, C., Eds.; Nova Science Publishers: New York, NY, USA, 2017; pp. 23–53. ISBN 978-1-53612-282-4. [Google Scholar]
- Aznar, M.; Alfaro, P.; Nerin, C.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction: An innovative sample preparation approach applied to the analysis of specific migration from food packaging. Anal. Chim. Acta 2016, 936, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.; Galanopoulos, L.D.; Kabir, A.; Furton, K.G. Fast extraction of amphenicols residues from raw milk using novel fabric phase sorptive extraction followed by high-performance liquid chromatography-diode array detection. Anal. Chim. Acta 2015, 855, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.F. Fabric phase sorptive extraction in pharmaceutical analysis. Pharm. Anal. Acta 2015, 6, 6–8. [Google Scholar] [CrossRef]
- Kabir, A.; Locatelli, M.; Ulusoy, H. Recent trends in microextraction techniques employed in analytical and bioanalytical sample preparation. Separations 2017, 4, 36. [Google Scholar] [CrossRef]
- Kumar, R.; Gaurav; Heena; Malik, A.K.; Kabir, A.; Furton, K.G. Efficient analysis of selected estrogens using fabric phase sorptive extraction and high performance liquid chromatography-fluorescence detection. J. Chromatogr. A 2014, 1359, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gaurav; Kabir, A.; Furton, K.G.; Malik, A.K. Development of a fabric phase sorptive extraction with high-performance liquid chromatography and ultraviolet detection method for the analysis of alkyl phenols in environmental samples. J. Sep. Sci. 2015, 38, 3228–3238. [Google Scholar] [CrossRef] [PubMed]
- Guedes-Alonso, R.; Ciofi, L.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Del Bubba, M.; Kabir, A.; Furton, K.G. Determination of androgens and progestogens in environmental and biological samples using fabric phase sorptive extraction coupled to ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1437, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M.; Kabir, A.; Furton, K.G. Integrated sampling and analysis unit for the determination of sexual pheromones in environmental air using fabric phase sorptive extraction and headspace-gas chromatography-mass spectrometry. J. Chromatogr. A 2017, 1488, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Heena Kaur, R.; Rani, S.; Malik, A.K.; Kabir, A.; Furton, K.G. Determination of cobalt (II), nickel (II) and palladium (II) Ions via fabric phase sorptive extraction in combination with high-performance liquid chromatography-UV detection. Sep. Sci. Technol. 2017, 52, 81–90. [Google Scholar] [CrossRef]
- Furton, K.G.; Rani, S.; Malik, A.K.; Kabir, A.; Furton, K.G. Speciation of Cr (III) and Cr (VI) ions via fabric phase sorptive extraction for their quantification via HPLC with UV detection speciation of Cr (III) and Cr (VI) ions via fabric phase sorptive extraction for their quantification via HPLC with UV. J. Chromatogr. Sep. Tech. 2016. [Google Scholar] [CrossRef]
- Anthemidis, A.; Kazantzi, V.; Samanidou, V.; Kabir, A.; Furton, K.G. An automated flow injection system for metal determination by flame atomic absorption spectrometry involving on-line fabric disk sorptive extraction technique. Talanta 2016, 156–157, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Kabir, A.; Furton, K.G.; Santana-Rodríguez, J.J. Fabric phase sorptive extraction followed by UHPLC-MS/MS for the analysis of benzotriazole UV stabilizers in sewage samples. Anal. Bioanal. Chem. 2015, 407, 8137–8150. [Google Scholar] [CrossRef] [PubMed]
- García-Guerra, R.B.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Kabir, A.; Furton, K.G.; Santana-Rodríguez, J.J. Rapid monitoring of residual UV-stabilizers in seawater samples from beaches using fabric phase sorptive extraction and UHPLC-MS/MS. Chemosphere 2016, 164, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Lakade, S.S.; Borrull, F.; Furton, K.G.; Kabir, A.; Fontanals, N.; Marcé, R.M. Comparative study of different fabric phase sorptive extraction sorbents to determine emerging contaminants from environmental water using liquid chromatography-tandem mass spectrometry. Talanta 2015, 144, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Lakade, S.S.; Borrull, F.; Furton, K.G.; Kabir, A.; Marcé, R.M.; Fontanals, N. Dynamic fabric phase sorptive extraction for a group of pharmaceuticals and personal care products from environmental waters. J. Chromatogr. A 2016, 1456, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Racamonde, I.; Rodil, R.; Quintana, J.B.; Sieira, B.J.; Kabir, A.; Furton, K.G.; Cela, R. Fabric phase sorptive extraction: A new sorptive microextraction technique for the determination of non-steroidal anti-inflammatory drugs from environmental water samples. Anal. Chim. Acta 2015, 865, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Santana-Viera, S.; Guedes-Alonso, R.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Kabir, A.; Furton, K.G. Optimization and application of fabric phase sorptive extraction coupled to ultra-high performance liquid chromatography tandem mass spectrometry for the determination of cytostatic drug residues in environmental waters. J. Chromatogr. A 2017, 1529, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Pijuán, M.; Lucena, R.; Cárdenas, S.; Valcárcel, M.; Kabir, A.; Furton, K.G. Stir fabric phase sorptive extraction for the determination of triazine herbicides in environmental waters by liquid chromatography. J. Chromatogr. A 2015, 1376, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Dong, S.; Zhang, M.; Zhang, H.; Huang, T. Fabric phase sorptive extraction: Two practical sample pretreatment techniques for brominated flame retardants in water. Water Res. 2016, 101, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Aznar, M.; Úbeda, S.; Nerin, C.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction as a reliable tool for rapid screening and detection of freshness markers in oranges. J. Chromatogr. A 2017, 1500, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, E.; Manousi, N.; Samanidou, V.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction for the fast isolation of sulfonamides residues from raw milk followed by high performance liquid chromatography with ultraviolet detection. Food Chem. 2016, 196, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.; Michaelidou, K.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction of selected penicillin antibiotic residues from intact milk followed by high performance liquid chromatography with diode array detection. Food Chem. 2017, 224, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.; Filippou, O.; Marinou, E.; Kabir, A.; Furton, K.G. Sol-gel-graphene-based fabric-phase sorptive extraction for cow and human breast milk sample cleanup for screening bisphenol A and residual dental restorative material before analysis by HPLC with diode array detection. J. Sep. Sci. 2017, 40, 2612–2619. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Gu, Y.; Wu, X.; Xi, X.; Yang, X.; Zhou, W.; Zeng, H.; Zhang, S.; Lu, R.; Gao, H.; et al. Rapid analysis of fungicides in tea infusions using ionic liquid immobilized fabric phase sorptive extraction with the assistance of surfactant fungicides analysis using IL-FPSE assisted with surfactant. Food Chem. 2018, 239, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Kabir, A.; Innosa, D.; Lopatriello, T.; Furton, K.G. A fabric phase sorptive extraction-High performance liquid chromatography-Photo diode array detection method for the determination of twelve azole antimicrobial drug residues in human plasma and urine. J. Chromatogr. B 2017, 1040, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.; Kaltzi, I.; Kabir, A.; Furton, K.G. Simplifying sample preparation using fabric phase sorptive extraction technique for the determination of benzodiazepines in blood serum by high-performance liquid chromatography. Biomed. Chromatogr. 2016, 30, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Furton, K.G.; Tinari, N.; Grossi, L.; Innosa, D.; Macerola, D.; Tartaglia, A.; Di Donato, V.; D’Ovidio, C.; Locatelli, M. Fabric phase sorptive extraction-high performance liquid chromatography-photo diode array detection method for simultaneous monitoring of three inflammatory bowel disease treatment drugs in whole blood, plasma and urine. J. Chromatogr. B 2018, 1084, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Bruheim, I.; Liu, X.; Pawliszyn, J. Thin-Film Microextraction. Anal. Chem. 2003, 75, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.B.; Rodil, R.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Multiresidue analysis of acidic and polar organic contaminants in water samples by stir-bar sorptive extraction-liquid desorption-gas chromatography-mass spectrometry. J. Chromatogr. A 2007, 1174, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.; Carpinteiro, J.; Quintana, J.B.; Carro, A.M.; Lorenzo, R.A.; Cela, R. Solid-phase microextraction with on-fiber derivatization for the analysis of anti-inflammatory drugs in water samples. J. Chromatogr. A 2004, 1024, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Noche, G.G.; Laespada, M.E.F.; Pavón, J.L.P.; Cordero, B.M.; Lorenzo, S.M. Microextraction by packed sorbent for the analysis of pharmaceutical residues in environmental water samples by in situ derivatization-programmed temperature vaporizer-gas chromatography-mass spectrometry. J. Chromatogr. A 2011, 1218, 9390–9396. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).