Abstract
Gac (Momordica cochinchinensis Spreng.) seeds contain bioactive compounds with medicinal properties. This study aimed to determine a suitable solvent and extraction technique for recovery of important compounds, namely, trypsin inhibitors, saponins, and phenolics. The antioxidant capacity and total solids of derived extracts were also measured. Water with conventional extraction method gave the highest value of trypsin inhibitor activity (118.45 ± 4.90 mg trypsin g−1) while water-saturated n-butanol and methanol extracts were characterized by their highest content of saponins (40.75 ± 0.31 and 38.80 ± 2.82 mg AE g−1, respectively). Aqueous extract with microwave assistance achieved the highest phenolics (3.18 ± 0.04 mg GAE g−1). As a measure of antioxidant capacity, the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) assay gave highest value to the aqueous microwave extract (23.56 ± 0.82 μmol TE g−1) while the ferric reducing antioxidant power (FRAP) assay gave highest values to water-saturated n-butanol and 70% ethanol extracts (5.25 ± 0.04 and 4.71 ± 0.39 μmol TE g−1, respectively). The total solids value was highest using water with microwave assistance (141.5 g kg−1) while ultrasound treatment did not improve any extractions. Therefore, trypsin inhibitors are suitably recovered using water while water-saturated n-butanol or methanol is for saponins, both using a conventional method. Microwave extraction is suitable for phenolics recovery. These conditions are recommended for an efficient recovery of bioactive compounds from defatted Gac seeds.
1. Introduction
Gac (Momordica cochinchinensis Spreng.) is a plant species of the family Cucurbitaceae, whose fruit is also known as red melon, baby jackfruit, spiny bitter gourd, sweet gourd and cochinchin gourd. It is native to the Southeast Asian region and is commonly grown as a crop in countries like Vietnam, Thailand, Laos, Myanmar, and Cambodia [1,2]. The most important part of the mature fruit is the red flesh surrounding the seeds, called the aril, which is used as a colourant in rice or as a material for further processing into functional food ingredients [3].
The seeds are not eaten; they are removed from the aril and are mostly considered waste [3]. However, in traditional medicine, Gac seeds are purported to have a wide array of therapeutic effects on a wide variety of conditions, such as fluxes, liver and spleen disorders, haemorrhoids, wounds, bruises, swelling, and pus [2,4]. Several constituents have been identified, which could be involved in the medicinal effects of Gac seeds. These include trypsin inhibitors, such as MCoTI-I, MCoTI-II, and MCoTI-III [5,6,7]; saponins, such as Momordica Saponin I (Gypsoside), Momordica Saponin II (Quillaic acid) [8,9]; and phenolic compounds such as gallic acid and p-hydroxybenzoic acids [10]. However, studies on how to efficiently extract these various components from Gac seeds are scarce and they are vital for facilitating future applications for these bioactives.
For any given plant bioactive, extractive yield depends on the extraction solvent, the chemical nature of the targeted component, and the characteristics of the extraction procedure. When other factors are kept constant, the extraction solvent plays a key role in obtaining the target constituents in terms of desired quality and quantity [11,12]. The choice of the solvent is mainly done based on the chemical properties, that is, polarity or hydrophobicity, of the target compounds.
Organic solvents with medium or high polarity have been most commonly used in laboratories dealing with natural products and there is evidence that some extracts from these solvents have better activity compared with aqueous extracts [11]. However, organic solvents present safety and environmental issues. Due to the hydrophylic nature of the trypsin inhibitors and phenolics, and the amphiphilic properties of saponins in Gac seeds, aqueous solvents and the alcohols are the safest and the most environmentally-friendly solvents for the extraction of these compounds [13,14].
The conventional aqueous solvent extraction technique, in which the solid material is suspended in water with no assistance for breaking the cell structure of the solid material, is often associated with a long heating time, which risks the degradation of bioactive compounds. This has led to the proposed use of advanced techniques such as microwave-assisted extraction (MAE) and ultrasonic-assisted extraction (UAE) that are efficient in terms of extraction time and water consumption. In MAE, microwave heating is able to disrupt the plant cell structure via an increase in the internal pressure of the cell, thereby releasing the bioactive compounds [15]. Similarly, ultrasonic cavitation during UAE produces shockwaves that are also capable of disrupting the plant cell structure and releasing the plant bioactives [16]. These two advanced extraction methods were reported to be more efficient than the conventional method for recovering carotenoids in Gac peel [17]. They have been widely used for the recovery of bioactive compounds from plant materials and are considered the dominant trends in “green chemistry” extractions [18].
In the present study, it was hypothesised that MAE and UAE would be better than the conventional aqueous extraction technique for the recovery of the important bioactive compounds from Gac seeds. Therefore, the extraction of trypsin inhibitors, saponins, and phenolic compounds, as well as the antioxidant activity of extracts from Gac seeds, using MAE and UAE was compared to conventional extraction with water. Furthermore, the efficiency of extraction for the MAE and UAE techniques was also compared to other alcohol solvents, namely methanol, 50% methanol, ethanol, 70% ethanol, and water-saturated n-butanol.
To date, no study has focused on assisted systems of aqueous extraction for the recovery of bioactive compounds from Gac seeds. The findings will be useful in the selection of best extraction methods specifically for the recovery of trypsin inhibitors, saponins, and phenolic compounds.
2. Materials and Methods
2.1. Materials
2.1.1. Solvents, Reagents, and Chemicals
Solvents, including ethanol, methanol, and n-butanol, and chemicals, including vanillin, sulphuric acid, and potassium persulfate were purchased from Merck (Darmstadt, Germany). Folin-ciocalteu’s phenol reagent, anhydrous sodium carbonate, sodium nitrite, ferric chloride, gallic acid, 2,4,6-Tris(2-pyridyl)-s-triazine; (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2carboxylic acid (Trolox), aecsin, 2,2′-azino-bis(3ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), trypsin (type I) from bovine pancreas, benzyl-dl-arginine-para-nitroanilide (BAPNA), tris, and dimethylsulfoxide (DMSO) were from Sigma-Aldrich Co. (Castle Hill, NSW, Australia). Sodium acetate trihydrate was purchased from Government Stores Department (Kingswood, NSW, Australia). Acetic acid was obtained from BDH Laboratory Supplies (Poole, UK). Sodium hydroxide was from Ajax FineChem (Taren Point, NSW, Australia) and hydrochloride acid was obtained from Lab-scan Ltd. (Bangkok, Thailand).
2.1.2. Gac Seeds
Gac seeds were collected from 450 kg of fresh Gac fruit from accession VS7 as classified by Wimalasiri et al. [1]. These fruits were bought at Gac fruit fields in Dong Nai province, Ho Chi Minh city, Vietnam (Latitude: 10.757410; Longitude: 106.673439). After their separation from the fresh fruit, the seeds were vacuum dried at 40 °C for 24 h to reduce moisture and increase the crispness of the shell, which would facilitate shell removal. The dried seeds were de-coated to get the kernels, which were then packaged in vacuum-sealed aluminium bags and stored at −18 °C until used.
Preparation of Defatted Gac Seed Powder
Gac seed kernels were ground in an electric grinder (100 g ST-02A Mulry Disintegrator), to pass through a sieve of 1.4 mm. The powder was then freeze-dried (Dynavac FD3 Freeze Dryer (Sydney, NSW, Australia)) for 48 h at −45 °C under vacuum at a pressure loading of 10−2 mbar (1 Pa), to reduce the moisture content to 12.1 ± 0.2 g kg–1. The powder was then defatted using hexane (1:5 w/v, 30 min, ×3) on a magnetic stirrer at room temperature. The resulting slurry was suction filtered and the residue (defatted powder) was air-dried for 12 h and stored in a desiccator at room temperature until used. The moisture content of the meal was recorded prior to weighing for extraction, using a MOC63u moisture analyzer (Shimazdu, Kyoto, Japan).
2.2. Methods
2.2.1. Extraction Methods
Initially, for the conventional extraction (CE) method, there were six extraction solvents: water (DIW), pure methanol (Methanol), 50% methanol (Methanol50%), pure ethanol (Ethanol), 70% ethanol (Ethanol70%), and water-saturated n-butanol (Butanol), examined for the extraction of bioactive compounds from Gac seeds. For the two assisting methods of UAE and MAE, only water was chosen to investigate because in the CE method it was much better than the other solvents in terms of the extraction yield of trypsin inhibitors and phenolic compounds. As such, the aqueous extract obtained using the CE method was used as a control for comparing among the extracts obtained from the different solvents using the same CE method, and also for comparing the extracts obtained with the UAE- and MAE-assisted aqueous extractions.
Based on preliminary experiments on the suitable ratio of solvent to Gac seed powder (data not shown), the ratio of 20 mL solvent per g of defatted Gac seed powder was chosen for the following extraction procedures. Preliminary experiments were also done for extraction conditions (time, temperature, microwave, and ultrasonic power) to assure these conditions were around the optimal area.
• Conventional extraction (CE)
1.5 g of defatted Gac seed powder was suspended with 30 mL of each solvent in a covered tube. The mixture was kept under constant shaking in a shaking water bath at 40 ± 1 °C for 30 min.
• Ultrasonic-assisted extraction (UAE)
UAE was investigated with only water because it was the best solvent for both trypsin inhibitors and phenolics in the conventional extraction.
1.5 g of defatted Gac seed powder was suspended with 30 mL of deionized water (DIW). UAE was carried out in an ultrasonic bath (Soniclean, 220 V, 50 Hz and 250 W, Soniclean Pty Ltd., Thebarton, SA, Australia) with the working power and temperature were set at 250 W and 40 °C, respectively. The maximum power of 250 W was chosen based on previous studies on ultrasound-assisted extraction of phenolic compounds [19] and saponins [20]. The suspensions were sonicated for 30 min in covered vessels to avoid evaporation. To measure the temperature of the ultrasonic bath, an external digital thermometer was also used. In the case that the ultrasonic bath exceeded the designated temperature, tap water was used to maintain the required temperature.
• Microwave-assisted extraction (MAE)
MAE was investigated with only water because it was the best solvent for both trypsin inhibitors and phenolics in the conventional extraction.
MAE was performed using a R395YS Sharp Carousel microwave oven (1200 W, Sharp Corporation, Bangkok, Thailand) at the set radiation power of 600 W. This level of power was chosen based on previous studies on microwave-assisted extraction of phenolic compounds [21] and saponins [22]. An amount of 1.5 g of defatted Gac seed powder was mixed with 30 mL DIW in a 100 mL conical flask. The flask mouth was tightly wrapped with plastic film and the suspension was left soaking for 45 min at ambient temperature of 22 ± 1 °C before the microwave treatment was applied (four cycles of 10 s power on and 15 s power off per cycle). The temperature of the suspension was recorded as 66 ± 1 °C at the end of the extraction process.
• Filtration
After all extractions, the suspensions were rapidly cooled to ambient temperature in an ice water bath, and filtered through a Whatman No.1 filter paper. The clear extracts were collected and kept at −20 °C for less than a week before analysis. Before analysis, the different filtrates needed to be diluted appropriately with DIW.
2.2.2. Determination of Trypsin Inhibitor Activity (TIA)
The TIA assay was performed as described by Makkar et al. [23] and Stauffer [24]. A synthetic substrate, benzyl-dl-arginine-para-nitroanilide (BAPNA), was subjected to hydrolysis by trypsin to produce the yellow-coloured p-nitroanilide. The degree of inhibition of the development of the yellow colouring by the extracts, a measure of trypsin inhibitor activity, was measured at 385 nm.
Reagent preparation:
- Substrate solution: A substrate solution of 92 mmol L−1 BAPNA was made in 0.05 mol L−1 Tris-buffer (pH 8.2) containing 0.02 mol L−1 CaCl2. The BAPNA was first dissolved in DMSO and then diluted with the buffer solution pre-warmed to 37 °C. This solution was prepared daily and kept at 37 °C while in use.
- Trypsin solution: 20 mg of trypsin (type I) from bovine pancreas was dissolved in 0.001 mol L−1 HCl to make 1 L, stored at 4 °C for use within 2–3 weeks. When subjected to the analytical procedure for the standard, 2 mL of this solution gave an absorbance value in the range of 0.576 ± 0.026 after subtracting the reagent blank at 385 nm.
Determination of TIA
From each appropriately diluted filtrate, 4 test tubes were prepared according to Table 1. All the prepared test tubes were kept in a water bath at 37 °C for 10 min to promote the formation of an enzyme-inhibitor complex; 5.0 mL of BAPNA solution pre-warmed to 37 °C was then added into each tube. The contents of the tubes were well mixed after each addition. The tubes were then incubated in the water bath at 37 °C for another 10 min before 1 mL of 30% acetic acid solution was added to each tube to stop the reaction. Then 2.0 mL of trypsin solution was added into each blank tube (Table 1). After thorough mixing, the absorbance of the reaction mixture (due to the release of p-nitroanilide) was measured at 385 nm using a Cary 50 UV–vis spectrophotometer (Agilent Technologies, Santa Clara, CA, USA).

Table 1.
Reagent composition in extracted filtrate.
Calculation
The change in absorbance (AI) due to the trypsin inhibitor per mL of diluted extract is (Ab − Aa) − (Ad − Ac), where the subscripts refer to tubes (a) to (d) above. Since 1 μg pure trypsin would give an absorbance of 0.0190, the weight of pure trypsin inhibited per mL of diluted extract is AI/0.019 μg. From this value, the trypsin inhibitor activity (TIA) is calculated in terms of milligrams of pure trypsin per gram of defatted Gac seed powder on a dry-weight basis (mg g–1) (Equation (1)).
TIA = mg of pure trypsin inhibited per gram of dried defatted Gac seed powder
where,
- AI: Change in absorbance due to inhibition per 1 mL of diluted extract
- V: Original volume of solvent (mL)
- D: Dilution factor for the filtered extract
- S: Weight of defatted Gac seed powder sample extracted (g)
- 19: Constant figure based on the absorbance given by 1 mg of pure trypsin
- m%: moisture content of defatted Gac seed powder
2.2.3. Determination of Total Saponin Content (TSC)
The TSC was determined according to Tan et al. [25] with some modifications. Briefly, 0.25 mL of diluted extract was mixed with 0.25 mL of 8% vanillin solution in ethanol (w/v) and 2.5 mL 72% sulfuric acid (v/v). The mixture was vortexed and incubated in a water bath at 60 °C for 15 min and then cooled on ice for 10 min. The absorption of the mixture was measured at 560 nm using a Cary 50 UV–vis spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) against a reagent blank. Aecsin was used as a standard and the results were expressed as Aecsin equivalents per gram dry weight of defatted Gac seed (mg AE g−1).
2.2.4. Determination of Total Phenolic Content (TPC)
The total phenolic content of Gac seed extracts was determined according to the method of Tan et al. [26] with some modifications. Briefly, 0.5 mL of diluted extract was mixed with 2.5 mL of 10% (v/v) Folin-Ciocalteu reagent in water and incubated at room temperature for 2 min to equilibrate. Then, 2 mL of 7.5% (w/v) sodium carbonate solution in water was added and the mixture was incubated at room temperature for 1 h. The absorption of the reaction mixture was recorded at 765 nm using a Cary 50 UV–vis spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) against a reagent blank. Gallic acid was used as a standard and results were expressed as Gallic acid equivalents per gram dry weight of defatted Gac seed (mg GAE g−1).
2.2.5. Determination of Antioxidant Capacity
Two methods, namely the 2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and ferric reducing antioxidant power (FRAP) assays, were performed to assess the antioxidant capacity of the Gac seed extracts.
• ABTS
The ABTS assay was as described by Tan et al. [26] with slight modifications. A stock solution of 7.4 mmol L−1 ABTS was freshly prepared and 2.6 mmol L−1 potassium persulfate solution was prepared and kept at 4 °C in dark bottle for use within a month. A fresh working solution was prepared for each assay by mixing the previous stock solutions in equal quantities and incubating them for 15 to 16 h in the dark at room temperature. Then, 1 mL of the working solution was diluted with approximately 30 mL of methanol to obtain an absorbance of 1.1 ± 0.02 units at 734 nm using a UV spectrophotometer (Varian Australia Pty. Ltd., Mulgrave, VIC, Australia). An amount of 0.15 mL of each sample was mixed with 2.85 mL of the working solution and incubated for 2 h in the dark at room temperature. The absorption of the reaction mixture at 734 nm was measured using the spectrophotometer. Trolox was used as a standard and results were expressed as mg Trolox equivalents per gram of dry defatted Gac seed sample (mg TE g−1).
• FRAP
FRAP was estimated following the method of Thaipong et al. [27], based on the increase in absorbance at 593 nm. The fresh FRAP working solution was initially prepared by mixing 300 mmol L−1 acetate buffer (pH 3.6), 10 mmol L−1 iron reagent (TPTZ) in 40 mmol L−1 HCl, and 20 mmol L−1 FeCl3.6H2O in the ratio of 10:1:1. The fresh working solution was warmed at 37 °C before using. An amount of 2.85 mL of the working FRAP solution was added to 0.15 mL of diluted sample and incubated at room temperature in the dark for 30 min before its absorbance was read at 593 nm using a Cary 50 UV–vis spectrophotometer (Varian Australia Pty. Ltd., Mulgrave, VIC, Australia). Trolox was used as a standard and the antioxidant capacity of each sample, based on its ability to reduce ferric ions, was expressed as mg Trolox equivalents per gram of dry defatted Gac seed sample (mg TE g−1).
2.2.6. Determination of Total Solids
To determine the total solids, 10 mL of each filtered extract was transferred to a tared flat-bottomed glass vial and then dried at 95 °C and vacuum pressure of 60 KPa for 24 h in a vacuum oven (Thermoline, Wetherill Park, NSW, Australia) until constant weight was achieved. These vials were cooled in a desiccator for 30 min and weighed. The total solids were calculated in g dried extract per kg of dried defatted Gac seed powder, using Equation (2), where TS is the total solids, DE (g) is the mass of dried extract after the drying, and DW (g) is the mass of dried defatted powder which had been used for the extraction.
2.2.7. Statistical Analyses
Experiments were performed in triplicate, and means ± SD were assessed using one-way ANOVA and Tukey’s Post Hoc Multiple Comparisons test with the IBM SPSS Statistics 24 program (IBM Corp., Armonk, NY, USA). Differences in means were considered statistically significant at p < 0.05. The correlations and significance of correlations were tested using the same software at the level of 0.05.
3. Results and Discussion
3.1. Effect of Extraction Methods on the Trypsin Inhibitor Yield
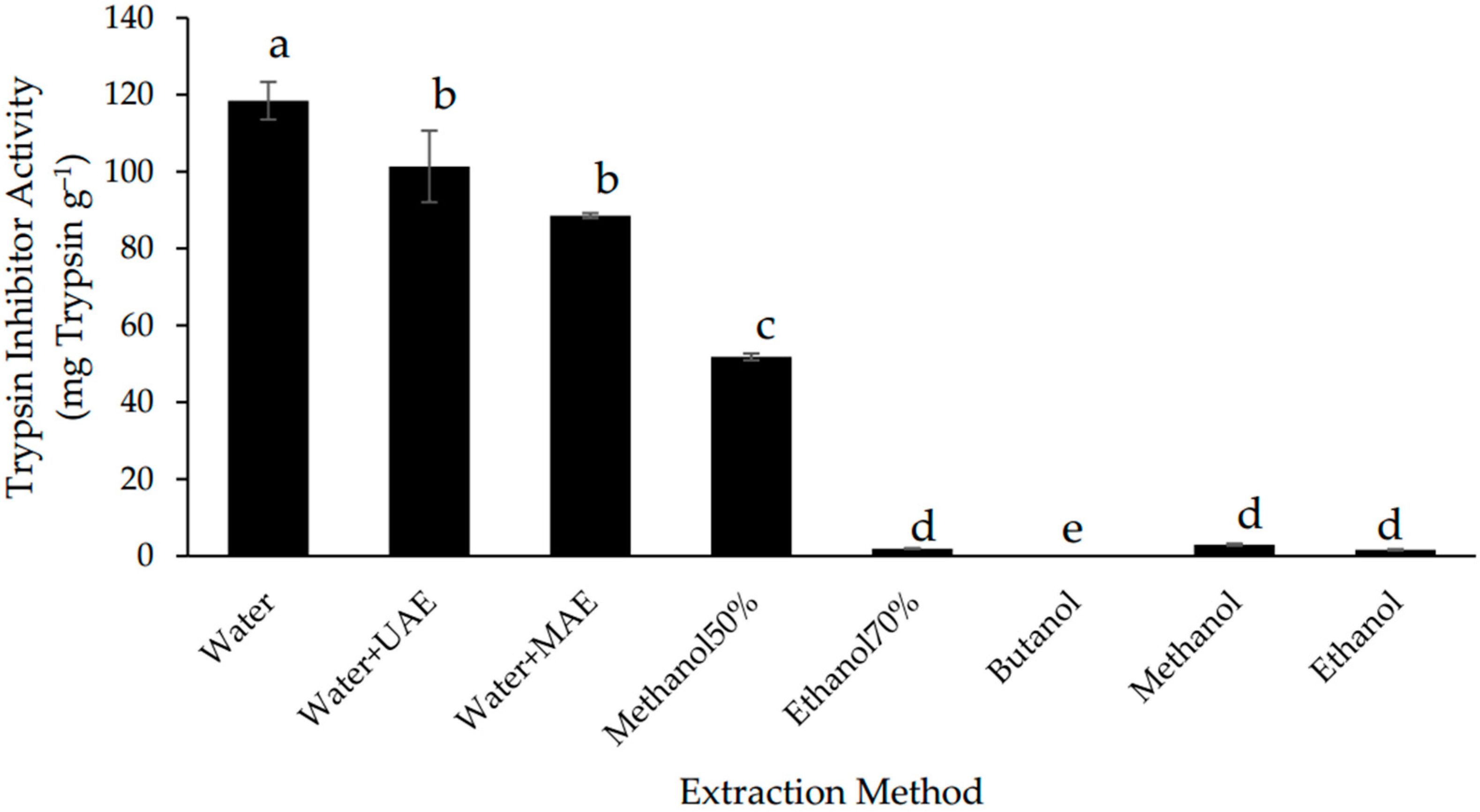
The extraction yield of trypsin inhibitors was measured by the trypsin inhibitor activity (TIA) of the extracts. The highest TIA (118.45 ± 4.90 mg g−1) was found in the extract produced using the conventional aqueous extraction method (Figure 1). This value is higher compared with the ones that have been reported for other seeds, such as 0.34–12.50 mg g−1 for peas [28], 3 mg g−1 for soybean [29], and 18.9 mg g−1 for fresh soya meal [30], revealing that Gac seeds can be an important source for recovery trypsin inhibitors.
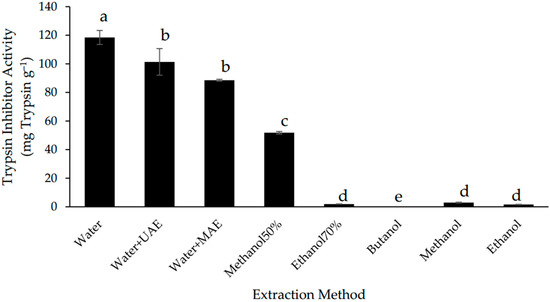
Figure 1.
Trypsin inhibitor activity of defatted Gac seed extracts affected by extraction method. The values are the means of three replicates for each extraction. Columns not sharing the same superscript letter are significantly different at p < 0.05.
Of the aqueous extracts, the extract with the conventional method gave the highest TIA value, followed by UAE and MAE. Although these two assisted extraction techniques have been reported to improve the extraction of bioactives from plant materials [18], it was not the case for the extraction of trypsin inhibitors from the Gac seeds (Figure 1). In fact, it appears that the ultrasound and microwave treatments had a negative impact on the Gac seed trypsin inhibitors as has previously been observed for trypsin inhibitors in soybeans [31,32] and in bean seeds [33].
The temperature achieved during MAE (66 °C) may have caused some denaturation of the trypsin inhibitors and therefore may explain the loss of TIA when using this method; however, temperature was unlikely to be an issue with UAE as the same temperature (40 °C) was used as for the conventional aqueous extraction. Additionally, two of the known trypsin inhibitors in Gac seeds, MCoTI-I and MCoTI-II, are known to be cysteine knot peptides, which can withstand being extracted with boiling water for 1 h [14], which means that the temperature is not a crucial factor.
As shown in Figure 1, the TIA values of all the aqueous extracts were significantly higher than for the organic solvent extracts. This is due to trypsin inhibitors being polypeptides, which are likely to be more soluble in aqueous media than in organic solvents [5,6,7].
Of the alcohol solvents, only 50% methanol was able to extract appreciable TIA but this was less than 50% of the activity observed with the conventional water extraction (Figure 1). The TIA for the extract with saturated n-butanol was undetectable while the values for the extracts of methanol, ethanol, and 70% ethanol were low and insignificantly different from each other (Figure 1).
It is well known that alcohols can denature and precipitate proteins and the Gac seed trypsin inhibitors may be similarly affected. This is consistent with the observations by Nicholls, Sharp, and Honig [34], who found that the conformation of trypsin inhibitors from soybean was changed due to the effect of n-propanol. Others have also reported on the use of organic solvents to deactivate trypsin inhibitors in soybeans [35,36,37]. Furthermore, a more hydrophobic solvent, dichloromethane/methanol (1:1), was not suitable for the extraction of the two known trypsin inhibitors in Gac seeds, MCoTI-I, and MCoTI-II [14].
3.2. Effect of Extraction Methods on the Total Saponin Content (TSC)
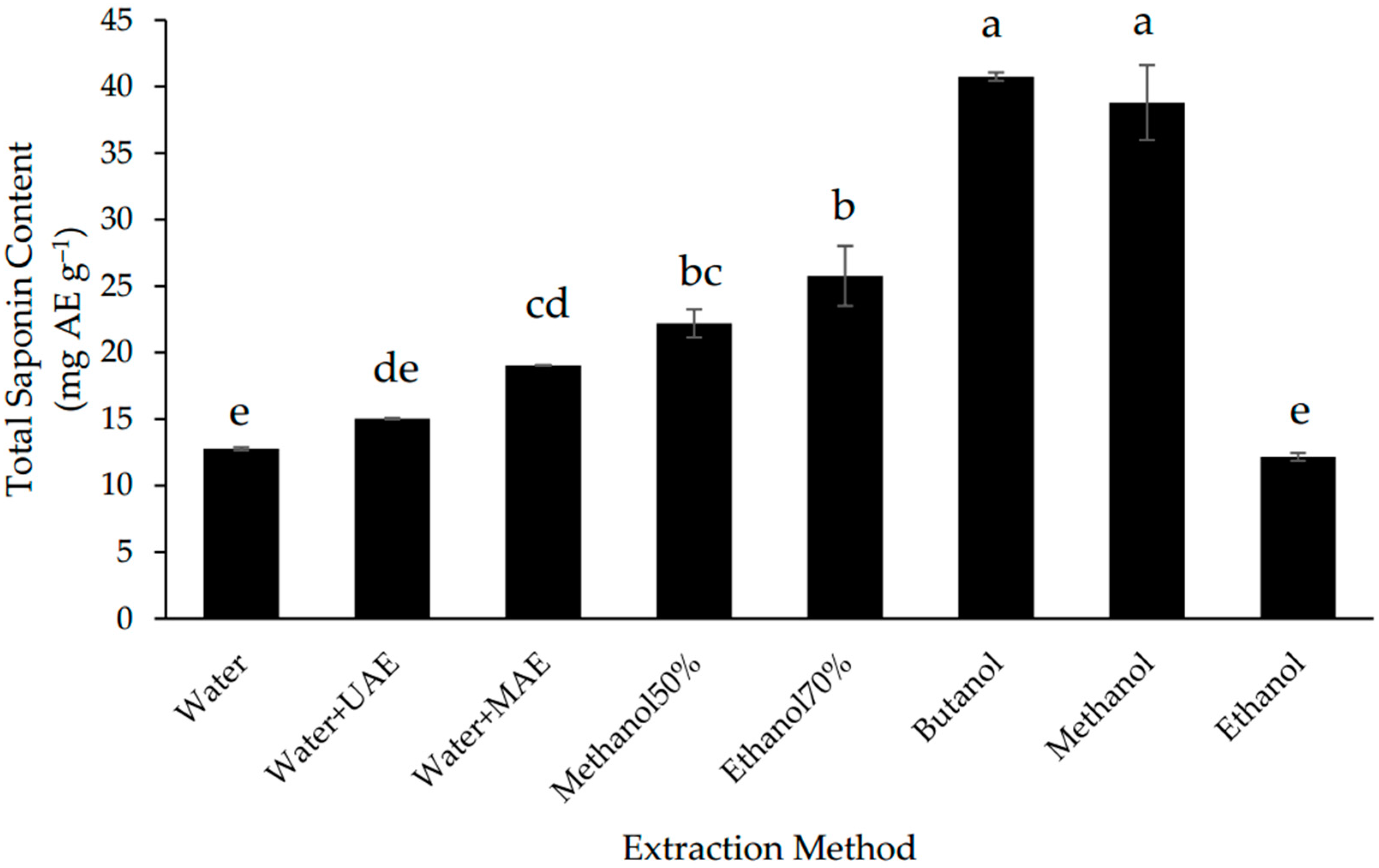
In this study, MAE increased the extraction of saponins from Gac seeds by 40% in comparison to the conventional aqueous extraction method but UAE did not have a significant effect (Figure 2). Despite of MAE improved the recovery of saponins, the extraction was less efficient than for most of the non-water solvents; the highest TSC was found in the butanol and methanol extracts (Figure 2), which were 2-fold higher than the TSC of the MAE extract and not significantly different from each other.
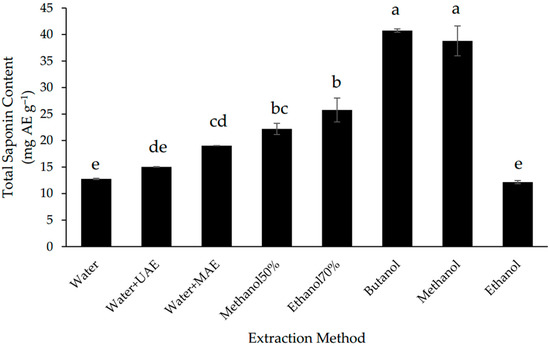
Figure 2.
Total saponin content of defatted Gac seed extracts affected by extraction method. The values are the means of three replicates for each extraction. Columns not sharing the same superscript letter are significantly different at p < 0.05. AE: aescin equivalents.
Due to their amphiphylic nature, saponins are usually extracted with water or alcohols using many different techniques [13,38]. The present results indicate that the Gac seed saponins are more soluble in alcohols than in water (Figure 2), which is consistent with these saponins being triterpenoids [8,9]. The finding that butanol and methanol were the best solvents for the extraction of the Gac seed saponins (Figure 2) is also consistent with previous studies. For example, butanol has been reported to be the solvent of choice for the extraction of saponins from the hull of Chenopodium quinoa seeds [39] while methanol has been used widely to extract saponins from a wide range of plant matrices [13,38]. Nonetheless, if the safety and economical characteristics of the solvent extraction system are a high priority, then water with MAE could be a reasonable choice for the extraction of Gac seed saponins.
3.3. Effect of Extraction Methods on Total Phenolic Content (TPC)
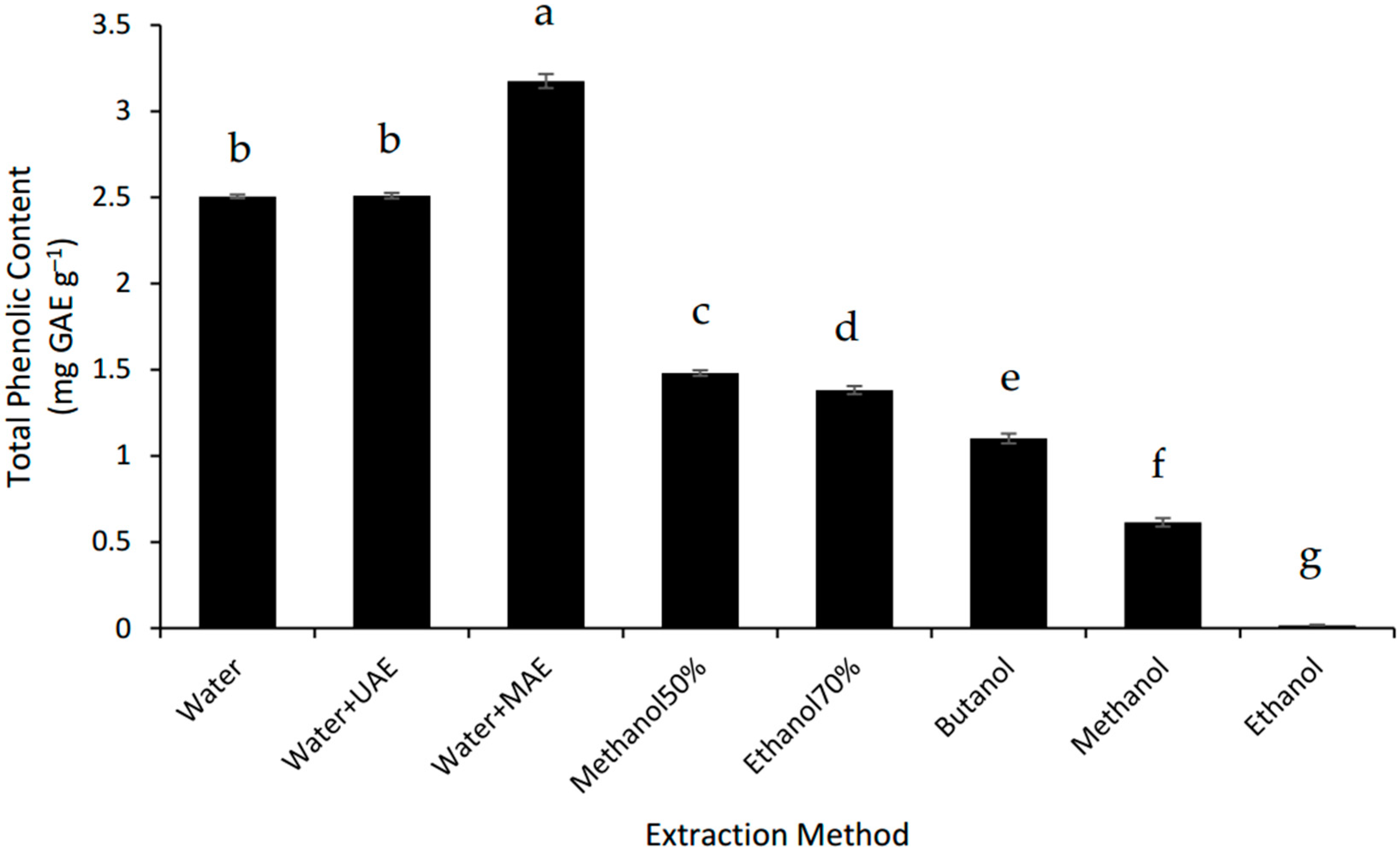
The MAE system was the best way to extract the phenolics from the defatted Gac seed powder. MAE increased the TPC of the Gac seed extract by 25% compared to the conventional water extraction but UAE had no effect (Figure 3). However, the three water-based extractions were better than all the organic solvent extractions tested and the TPC for the MAE was 2.2 times higher than for the best of the alcoholic extractions tested, 70% methanol (Figure 3). These findings suggest that the extractable phenolic compounds from Gac seeds are very hydrophilic, which is consistent with gallic acid and p-hydroxybenzoic acids being previously identified in Gac seeds [10].
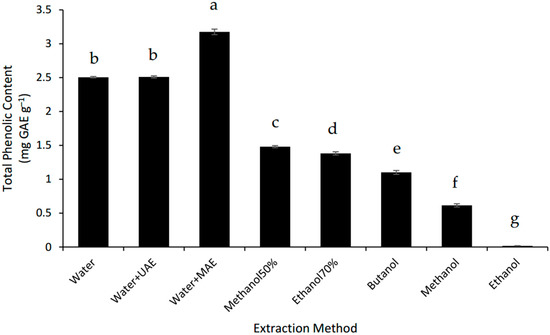
Figure 3.
Total phenolic content of defatted Gac seed extracts affected by extraction method. The values are the mean of three replicates for each extraction. Columns not sharing the same superscript letter are significantly different at p < 0.05. GAE: gallic acid equivalents.
3.4. Effect of Extraction Methods on Total Solids and Antioxidant Capacity
Among the three extraction techniques using water as extracting solvent (CE, MAE, and UAE), MAE gave the highest TS value, followed by the conventional method, and then UAE (Table 2). The three aqueous extraction methods also gave higher TS values than all the alcohol extraction methods. These results suggest that the extractable material in the defatted Gac seed powder is highly polar, which is consistent with the finding that the aqueous extractions are enriched in trypsin inhibitors [6,40] (Figure 1) and phenolics [10] (Figure 3).

Table 2.
Effect of extraction method on total solids and antioxidant capacity of defatted Gac seed powder.
The antioxidant capacity of the extracts was measured using two assays—ABTS and FRAP. The extracts from the MAE and conventional aqueous method gave the highest value for ABTS (Table 2). For the FRAP assay, MAE and UAE did not improve the activity compared to the conventional extraction. Furthermore, the water-saturated butanol and the 70% ethanol extracts gave the highest FRAP values. Therefore, it is likely that the ABTS assay measured the antioxidant activity due to hydrophilic compounds, such as the Gac seed TPC (Figure 3), while the FRAP assay measured antioxidant activity due to more hydrophobic compounds, such as the Gac seed saponins (Figure 2). However, it may also be due to the FRAP assay measuring ferric reducing power of the extract rather than the free radical scavenging activity measured by the ABTS assay [27].
3.5. Correlations between Bioactive Compounds and Total Solids and Antioxidant Activity in the Extracts
As seen in Table 3, TIA was highly correlated with the amount of total solids across all the extracts. This could suggest that the trypsin inhibitors contributed to the total solids in the aqueous extracts (Figure 1). However, it is more likely that different proteins, not just the trypsin inhibitors, contributed to the total solids in the aqueous extracts [41]. In contrast, there were no correlations between TIA and the ABTS or FRAP values across the extracts (Table 3), which suggest that Gac seed trypsin inhibitors are unlikely to have antioxidant activity. However, in another study [42], trypsin inhibitors from Cajanus cajan and Phaseolus limensis were observed to possess FRAP antioxidant activities comparable to that of ascorbic acid. The difference in molecule structure of these trypsin inhibitors and the purity of the extracts possibly explained their different contributions to antioxidant activity [43].

Table 3.
Correlations between bioactive compounds with total solids and antioxidant activity of defatted Gac seed extracts.
There was also a very strong correlation between TPC and TS (Table 3), confirming the highly polar nature of the phenolic compounds in Gac seeds [10]. Phenolic compounds in plant materials are found to be well correlated with antioxidant potential [44,45]. The results in Table 3 also show that the TPC values correlated strongly with the ABTS antioxidant activity but not with the FRAP values. This suggests that the phenolic compounds present in Gac seed extracts acted as antioxidants through the mechanism of free radical scavenging (of ABTS) rather than the mechanism of reducing of the oxidised intermediate in the FRAP chain reaction or through chelation [27]. Polyphenols that can neutralise free radicals by donating an electron or hydrogen atom [46] result in their strong antioxidant capacity possibly explained the correlation between TPC and ABTS in Gac seed extracts.
There were no significant correlations between the TSC and the TS, ABTS, and FRAP values across the extracts (Table 3), revealing that the saponins did not relate to the amount of material extracted from the defatted Gac seed powder and that they were not the main drivers of the ABTS or the FRAP antioxidant activity. However, a report of Tan et al. [26] showed that saponins from bitter melon correlated as strongly as phenolics with antioxidant capacity (R2 ranged from 0.87 to 0.93). The structural diversity of different saponins from different materials possibly explains their variations in antioxidant activity.
4. Conclusions
MAE proved to be the best method for extracting phenolic compounds and the best aqueous extraction method for recovering saponins but it did not improve the extraction of trypsin inhibitors, for which the conventional water extraction was the best method. For saponins, water-saturated n-butanol and methanol were more efficient than MAE. On the other hand, UAE did not show any improvement in comparison to the tested conventional extraction methods.
Therefore, this study demonstrated that the choice of solvent and extraction method plays an important role in the recovery of bioactive compounds from defatted Gac seeds. The MAE method is recommended for the extraction of phenolic compounds and saponins, if an aqueous extraction is preferred due to the safety and environmental concerns when organic solvents are used. However, the conventional water extraction method is the best option for the extraction of trypsin inhibitors.
Author Contributions
Conceptualization, A.V.L., M.H.N. and P.D.R.; Methodology, A.V.L.; Validation, A.V.L; Formal Analysis, A.V.L.; Investigation, A.V.L.; Data Curation, A.V.L. and P.D.R.; Writing-Original Draft Preparation, A.V.L.; Writing-Review & Editing, P.D.R., M.H.N. and S.E.P.; Supervision, P.D.R., M.H.N. and S.E.P.
Funding
This research received no external funding.
Acknowledgments
AVL acknowledges the University of Newcastle and VIED for their financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wimalasiri, D.; Piva, T.; Urban, S.; Huynh, T. Morphological and genetic diversity of Momordica cochinchinenesis (Cucurbitaceae) in Vietnam and Thailand. Genet. Resour. Crop Evol. 2016, 63, 19–33. [Google Scholar] [CrossRef]
- Behera, T.; John, K.J.; Bharathi, L.; Karuppaiyan, R.; Momordica. Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Heidelberg, NY, USA, 2011; pp. 217–246. ISBN 978-3-642-20449-4. [Google Scholar]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Gac fruit (Momordica cochinchinensis Spreng.): A rich source of bioactive compounds and its potential health benefits. Int. J. Food Sci. Technol. 2015, 50, 567–577. [Google Scholar] [CrossRef]
- Masayo, I.; Hikaru, O.; Tatsuo, Y.; Masako, T.; Yoshie, R.; Shuji, H.; Kunihide, M.; Ryuichi, H. Studies on the constituents of Momordica cochinchinensis Spreng. I. Isolation and characterization of the seed saponins, Momordica saponins I and II. Chem. Pharm. Bull. 1985, 33, 464–478. [Google Scholar]
- Chan, L.Y.; Wang, C.K.L.; Major, J.M.; Greenwood, K.P.; Lewis, R.J.; Craik, D.J.; Daly, N.L. Isolation and characterization of peptides from Momordica cochinchinensis seeds. J. Nat. Prod. 2009, 72, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.C.; Fong, W.; Ng, T. Multiple trypsin inhibitors from Momordica cochinchinensis seeds, the Chinese drug mubiezhi. Peptides 2004, 25, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.Y.; He, W.; Tan, N.; Zeng, G.; Craik, D.J.; Daly, N.L. A new family of cystine knot peptides from the seeds of Momordica cochinchinensis. Peptides 2013, 39, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.M.; Mu, B.Z. (Eds.) Semen momordicae. In Chinese Materia Medicia; Traditional Chinese Materia Medica Press: Beijing, China, 2005; pp. 601–602. [Google Scholar]
- Lin, Z.Y.; Liu, X.; Yang, F.; Yu, Y.Q. Structural characterization and identification of five triterpenoid saponins isolated from Momordica cochinchinensis extracts by liquid chromatography/tandem mass spectrometry. Int. J. Mass Spectrom. 2012, 328, 43–66. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phytochemicals and antioxidant activity of different fruit fractions (peel, pulp, aril and seed) of Thai gac (Momordica cochinchinensis Spreng). Food Chem. 2011, 127, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, G.; Kyerematen, G.; Farah, M.H. Preliminary chemical characterization of pharmacologically active compounds in aqueous plant extracts. J. Ethnopharmacol. 1985, 14, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Mahatmanto, T.; Poth, A.G.; Mylne, J.S.; Craik, D.J. A comparative study of extraction methods reveals preferred solvents for cystine knot peptide isolation from Momordica cochinchinensis seeds. Fitoterapia 2014, 95, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Tatke, P.; Jaiswal, Y. An overview of microwave assisted extraction and its applications in herbal drug research. Res. J. Med. Plant 2011, 5, 21–31. [Google Scholar] [CrossRef]
- Pingret, D.; Fabiano-Tixier, A.S.; Chemat, F. Ultrasound-assisted extraction. In Natural Product Extraction: Principles and Applications; Mauricio, R., Juliana, P., Eds.; RSC Pub: Cambridge, UK, 2013; pp. 89–111, ISBN 1849736065, 9781849736060. [Google Scholar]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-assisted extraction and ultrasound-assisted extraction for recovering carotenoids from Gac peel and their effects on antioxidant capacity of the extracts. Food Sci. Nutr. 2018, 6, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Vuong, Q.V.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Development of the ultrasonic conditions as an advanced technique for extraction of phenolic compounds from Eucalyptus robusta. Sep. Sci. Technol. 2017, 52, 100–112. [Google Scholar] [CrossRef]
- Wu, J.; Lin, L.; Chau, F.T. Ultrasound-assisted extraction of ginseng saponins from ginseng roots and cultured ginseng cells. Ultrason. Sonochem. 2001, 8, 347–352. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Vuong, Q.V.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Microwave-assisted extraction of Eucalyptus robusta leaf for the optimal yield of total phenolic compounds. Ind. Crops Prod. 2015, 69, 290–299. [Google Scholar] [CrossRef]
- Anh, V.L.; Sophie, E.P.; Minh, H.N.; Paul, D.R. Optimisation of the microwave-assisted ethanol extraction of saponins from Gac (Momordica cochinchinensis Spreng.) seeds. Medicines 2018, 5, 70. [Google Scholar] [CrossRef]
- Makkar, H.P.; Siddhuraju, P.; Becker, K. Trypsin Inhibitor. In Plant Secondary Metabolites; Humana Press: New York, NY, USA, 2007; pp. 1–6. ISBN 1588299937. [Google Scholar]
- Stauffer, C.E. Measuring trypsin inhibitor in soy meal: Suggested improvements in the standard method. Cereal Chem. 1990, 67, 296–302. [Google Scholar]
- Tan, S.P.; Kha, T.C.; Parks, S.E.; Stathopoulos, C.E.; Roach, P.D. Effects of the spray-drying temperatures on the physiochemical properties of an encapsulated bitter melon aqueous extract powder. Powder Technol. 2015, 281, 65–75. [Google Scholar] [CrossRef]
- Tan, S.P.; Vuong, Q.V.; Stathopoulos, C.E.; Parks, S.E.; Roach, P.D. Optimized aqueous extraction of saponins from bitter melon for production of a saponin-enriched bitter melon powder. J. Food Sci. 2014, 79, E1372–E1381. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compost. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Boye, J.I.; Ma, Z. Impact of Processing on Bioactive Compounds of Field Peas. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Elsevier: Boston, MA, USA, 2015; pp. 63–70. ISBN 978-0-12-404699-3. [Google Scholar]
- Serrano, M.; Rebollar, P.; Sueiro, S.; Hermida, M.; Mateos, G. Influence of duration of storage on protein quality traits of soybean meals. J. Appl. Poult. Res. 2013, 22, 423–429. [Google Scholar] [CrossRef]
- Smith, C.; van Megen, W.; Twaalfhoven, L.; Hitchcock, C. The determination of trypsin inhibitor levels in foodstuffs. J. Sci. Food Agric. 1980, 31, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Kwok, K.C.; Liang, H.H. Inhibitory activity and conformation changes of soybean trypsin inhibitors induced by ultrasound. Ultrason. Sonochem. 2008, 15, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Vagadia, B.H.; Vanga, S.K.; Raghavan, V. Inactivation methods of soybean trypsin inhibitor—A review. Trends Food Sci. Technol. 2017, 64, 115–125. [Google Scholar] [CrossRef]
- Pysz, M.; Polaszczyk, S.; Leszczyńska, T.; Piątkowska, E. Effect of microwave field on trypsin inhibitors activity and protein quality of broad bean seeds (Vicia faba var. major). Acta. Sci. Pol. Technol. Aliment. 2012, 11, 193–198. [Google Scholar] [PubMed]
- Nicholls, A.; Sharp, K.A.; Honig, B. Protein folding and association: Insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins Struct. Funct. Bioinform. 1991, 11, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, W.; Stark, C.; Ferket, P.; Brake, J. Effects of trypsin inhibitor and particle size of expeller-extracted soybean meal on broiler live performance and weight of gizzard and pancreas. Poult. Sci. 2014, 93, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Jirgensons, B. Effects of n-propyl alcohol and detergents on the optical rotatory dispersion of α-chymotrypsinogen, β-casein, histone fraction F1, and soybean trypsin inhibitor. J. Biol. Chem. 1967, 242, 912–918. [Google Scholar] [PubMed]
- Pesoti, A.R.; Oliveira, B.M.; Oliveira, A.C.; Pompeu, D.G.; Gonçalves, D.B.; Marangoni, S.; Silva, J.A.; Granjeiro, P.A. Extraction, purification and characterization of inhibitor of trypsin from Chenopodium quinoa seeds. Food Sci. Technol. 2015, 35, 588–597. [Google Scholar] [CrossRef]
- Güçlü-Üstündağ, Ö.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Madl, T.; Sterk, H.; Mittelbach, M.; Rechberger, G.N. Tandem mass spectrometric analysis of a complex triterpene saponin mixture of Chenopodium quinoa. J. Am. Soc. Mass Spectrom. 2006, 17, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.F.; Gagnon, J.; Chiche, L.; Nguyen, T.M.; Andrieu, J.P.; Heitz, A.; Trinh, H.T.; Pham, T.T.; Le, N.D. Squash trypsin inhibitors from Momordica cochinchinensis exhibit an atypical macrocyclic structure. Biochemistry 2000, 39, 5722–5730. [Google Scholar] [CrossRef] [PubMed]
- Sastry, M.; Murray, D.R. The contribution of trypsin inhibitors to the nutritional value of chick pea seed protein. J. Sci. Food Agric. 1987, 40, 253–261. [Google Scholar] [CrossRef]
- Shamsi, T.N.; Parveen, R.; Afreen, S.; Azam, M.; Sen, P.; Sharma, Y.; Fatima, S. Trypsin inhibitors from Cajanus cajan and Phaseolus limensis possess antioxidant, anti-inflammatory, and antibacterial activity. J. Diet. Suppl. 2018, 15, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.N.T.; Nguyen, V.T.; Vuong, V.Q.; Bowyer, M.C.; Scarlett, C.J. Bioactive compound yield and antioxidant capacity of Helicteres hirsuta Lour. stem as affected by various solvents and drying methods. J. Food Process. Preserv. 2017, 41, e12879. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).