Supercritical Fluid Chromatography as a Technique to Fractionate High-Valued Compounds from Lipids
Abstract
1. Introduction
1.1. Natural Products
1.2. SFC Overview
2. Current State
2.1. Lipids
2.1.1. Polyunsaturated Fatty Acids (PUFA)
- DHA and EPA: contribute to normal function of the heart, to the maintenance of normal blood pressure and triglycerides levels.
- DHA: contributes to maintenance of normal brain function, to the normal brain and eyes development of the foetus and breastfed infants
- ALA: contributes to brain development.
2.1.2. Phospholipids
2.2. Carbohydrates
2.3. Other Applications
3. Future
Conflicts of Interest
References
- Gagliardi, N. Consumers Want Healthy Foods—And Will Pay More for Them. Available online: https://www.forbes.com/sites/nancygagliardi/2015/02/18/consumers-want-healthy-foods-and-will-pay-more-for-them/#6571812875c5 (accessed on 27 July 2018).
- Research, A.M. Omega-3 Market to Reach $6,955 Million, Globally. 2022. Available online: https://www.alliedmarketresearch.com/press-release/omega-3-market.html (accessed on 27 July 2018).
- Markets, M.A. Lecithin Market Worth 1.11 Billion Usd by 2020. Available online: https://www.marketsandmarkets.com/PressReleases/lecithin-phospholipids.asp (accessed on 27 July 2018).
- Herrero, M.; Ibañez, E. Green extraction processes, biorefineries and sustainability: Recovery of high added-value products from natural sources. J. Supercrit. Fluids 2017, 134, 252–259. [Google Scholar] [CrossRef]
- Iannuzzi, A. Greener Products: The Making and Marketing of Sustainable Brands; CRC Press: Boca Raton, FL, USA, 2011; p. 222. [Google Scholar]
- Taylor, L.T. Supercritical fluid chromatography. Anal. Chem. 2010, 82, 4925–4935. [Google Scholar] [CrossRef] [PubMed]
- Lesellier, E.; West, C. Description and comparison of chromatographic tests and chemometric methods for packed column classification. J. Chromatogr. A 2007, 1158, 329–360. [Google Scholar] [CrossRef] [PubMed]
- West, C.; Lesellier, E. Characterisation of stationary phases in subcritical fluid chromatography with the solvation parameter model iv. Aromatic stationary phases. J. Chromatogr. A 2006, 1115, 233–245. [Google Scholar] [CrossRef] [PubMed]
- West, C.; Lesellier, E. Characterisation of stationary phases in subcritical fluid chromatography by the solvation parameter model: Ii. Comparison tools. J. Chromatogr. A 2006, 1110, 191–199. [Google Scholar] [CrossRef] [PubMed]
- West, C.; Lesellier, E. Characterisation of stationary phases in subcritical fluid chromatography with the solvation parameter model: Iii. Polar stationary phases. J. Chromatogr. A 2006, 1110, 200–213. [Google Scholar] [CrossRef] [PubMed]
- West, C.; Lesellier, E. Characterization of stationary phases in subcritical fluid chromatography by the solvation parameter model: I. Alkylsiloxane-bonded stationary phases. J. Chromatogr. A 2006, 1110, 181–190. [Google Scholar] [CrossRef] [PubMed]
- West, C.; Lesellier, E. Characterisation of stationary phases in supercritical fluid chromatography with the solvation parameter model. V. Elaboration of a reduced set of test solutes for rapid evaluation. J. Chromatogr. A 2007, 1169, 205–219. [Google Scholar] [CrossRef] [PubMed]
- West, C.; Lesellier, E. A unified classification of stationary phases for packed column supercritical fluid chromatography. J. Chromatogr. A 2008, 1191, 21–39. [Google Scholar] [CrossRef] [PubMed]
- West, C.; Lesellier, E. Comments on the paper “characterization of stationary phases by a linear solvation energy relationship utilizing supercritical fluid chromatography” by C.R. Mitchell, N.J. Benz, S. Zhang. J. Sep. Sci. 2011, 34, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Taguchi, K.; Bamba, T. Chapter 14—separation of lipids a2—Poole, colin f. In Supercritical Fluid Chromatography; Elsevier: New York, NY, USA, 2017; pp. 419–438. [Google Scholar]
- Fornari, T.; Stateva, R.P. High Pressure Fluid Technology for Green Food Processing; Springer International Publishing: Basel, Switzerland, 2014. [Google Scholar]
- Montanes, F.; Catchpole, O.; Tallon, S.; Rose, P.; Moreno-Rueda, T. Advances in analytical and preparative supercritical fluid chromatography. Food and nutraceutical applications. In High Pressure Fluid Technology for Green Food Processing; Tiziana, F., Stateva, R.P., Eds.; Springer International Publishing: Basel, Switzerland, 2015. [Google Scholar]
- Poole, C. Supercritical Fluid Chromatography; Elsevier: New York, NY, USA, 2017; pp. i–iii. [Google Scholar]
- Hermansson, M.; Uphoff, A.; Käkelä, R.; Somerharju, P. Automated quantitative analysis of complex lipidomes by liquid chromatography/mass spectrometry. Anal. Chem. 2005, 77, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Doughman, S.D.; Krupanidhi, S.; Sanjeevi, C.B. Omega-3 fatty acids for nutrition and medicine: Considering microalgae oil as a vegetarian source of EPA and DHA. Curr. Diabetes Rev. 2007, 3, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.Y.; Kim, J.B.; Roh, K.H.; Kim, Y.M.; Park, J.S. Biosynthesis of polyunsaturated fatty acids: Metabolic engineering in plants. J. Appl. Biol. Chem. 2009, 52, 93–102. [Google Scholar] [CrossRef]
- Joanneke, S. Algae: Our Original Omega-3 Source. Available online: https://www.wur.nl/upload_mm/d/7/4/b749c4e6-c5bf-47f3-9a5e-3308a136c272_Flyer_Algen%20onze%20oorspronkelijke%20omega-3%20bron%2010%20Mei%202017%20ENG.%20Losse%20pagina%27s%20Definitief%20Press.pdf (accessed on 27 July 2018).
- Catchpole, O.; Moreno, T.; Montañes, F.; Tallon, S. Perspectives on processing of high value lipids using supercritical fluids. J. Supercrit. Fluids 2018, 134, 260–268. [Google Scholar] [CrossRef]
- Higashidate, S.; Yamauchi, Y.; Saito, M. Enrichment of eicosapentaenoic acid and docosahexaenoic acid esters from esterified fish oil by programmed extraction-elution with supercritical carbon dioxide. J. Chromatogr. A 1990, 515, 295–303. [Google Scholar] [CrossRef]
- Perrut, M.; Nicoud, R.M.; Breivik, H. Processes for Chromatographic Fractionation of Fatty Acids and Their Derivatives. U.S. Patent 5719302, 17 February 1998. [Google Scholar]
- Alkio, M.; Gonzalez, C.; Jäntti, M.; Aaltonen, O. Purification of polyunsaturated fatty acid esters from tuna oil with supercritical fluid chromatography. J. Am. Oil Chem. Soc. 2000, 77, 315–321. [Google Scholar] [CrossRef]
- Nilsson, W.B.; Gauglitz, E.J., Jr.; Hudson, J.K.; Stout, V.F.; Spinelli, J. Fractionation of menhaden oil ethyl esters using supercritical fluid CO2. J. Am. Oil Chem. Soc. 1988, 65, 109–117. [Google Scholar] [CrossRef]
- Snoey-Elich, H. Novel Method for Preparing Eicosapentaenoic Acid. Patent ES2159257 A1, 2001. [Google Scholar]
- Pettinello, G.; Bertucco, A.; Pallado, P.; Stassi, A. Production of epa enriched mixtures by supercritical fluid chromatography: From the laboratory scale to the pilot plant. J. Supercrit. Fluids 2000, 19, 51–60. [Google Scholar] [CrossRef]
- Lembke, P. Process-scale sfc: A feasibility study. In Proceedings of the 7th International Symposium on Supercritical Fluid Chromatography and Extraction, Indianapolis, IN, USA, 31 March–4 April 1996. [Google Scholar]
- Montañés, F.; Catchpole, O.J.; Tallon, S.; Mitchell, K.; Lagutin, K. Semi-preparative supercritical chromatography scale plant for polyunsaturated fatty acids purification. J. Supercrit. Fluids 2013, 79, 46–54. [Google Scholar] [CrossRef]
- Montañés, F.; Tallon, S.; Catchpole, O. Isolation of non-methylene interrupted or acetylenic fatty acids from seed oils using semi-preparative supercritical chromatography. J. Am. Oil Chem. Soc. 2017, 94, 981–991. [Google Scholar] [CrossRef]
- Chien, T.C.; Lo, S.F.; Ho, C.L. Chemical composition and anti-inflammatory activity of Chamaecyparis obtusa f. Formosana wood essential oil from taiwan. Nat. Prod. Commun. 2014, 9, 723–726. [Google Scholar] [PubMed]
- Morishige, J.I.; Amano, N.; Hirano, K.; Nishio, H.; Tanaka, T.; Satouchi, K. Inhibitory effect of juniperonic acid (δ-5c, 11c, 14c, 17c-20:4, ω-3) on bombesin-induced proliferation of swiss 3t3 cells. Biol. Pharm. Bull. 2008, 31, 1786–1789. [Google Scholar] [CrossRef] [PubMed]
- Asset, G.; Baugé, E.; Wolff, R.L.; Fruchart, J.C.; Dallongeville, J. Pinus pinaster oil affects lipoprotein metabolism in apolipoprotein e-deficient mice. J. Nutr. 1999, 129, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Imasato, Y.; Sasaki, E.; Nakayama, M.; Nagao, H.; Takeo, T.; Yayabe, F.; Sugano, M. Tea catechins decrease micellar solubility and intestinal absorption of cholesterol in rats. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1992, 1127, 141–146. [Google Scholar] [CrossRef]
- Nishiyama, N.; Chu, P.J.; Saito, H. Beneficial effects of biota, a traditional chinese herbal medicine, on learning impairment induced by basal forebrain-lesion in mice. Biol. Pharm. Bull. 1995, 18, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Wang, Y.L.; Saito, H. Beneficial effects of s-113m, a novel herbal prescription, on learning impairment model in mice. Biol. Pharm. Bull. 1995, 18, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Wang, Y.-L.; Saito, H. Effects of biota (bai-zi-ren), a traditional chinese medicine, on learning performances in mice. Jpn. J. Pharmacogn. 1992, 46, 62–70. [Google Scholar]
- Chavali, A.K.; Gianchandani, E.P.; Tung, K.S.; Lawrence, M.B.; Peirce, S.M.; Papin, J.A. Characterizing emergent properties of immunological systems with multi-cellular rule-based computational modeling. Trends Immunol. 2008, 29, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Sugano, M.; Koga, T.; Yamato, T.; Nonaka, M.; Gu, J.Y. Trans fatty acids: Effects on eicosanoid production. World Rev. Nutr. Diet. 1994, 75, 179–182. [Google Scholar] [PubMed]
- Taylor, S.L.; King, J.W. Preparative-scale supercritical fluid extraction/supercritical fluid chromatography of corn bran. J. Am. Oil Chem. Soc. 2002, 79, 1133–1136. [Google Scholar] [CrossRef]
- Osseo, L.S.; Caputo, G.; Gracia, I.; Reverchon, E. Continuous fractionation of used frying oil by supercritical CO2. J. Am. Oil Chem. Soc. 2004, 81, 879–885. [Google Scholar] [CrossRef]
- Choo, Y.M.; Ma, A.N.; Yahaya, H.; Yamauchi, Y.; Bounoshita, M.; Saito, M. Separation of crude palm oil components by semipreparative supercritical fluid chromatography. J. Am. Oil Chem. Soc. 1996, 73, 523–525. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Puoci, F.; Cirillo, G.; Vinci, G.; Picci, N. Determination of phospholipids in food samples. Food Rev. Int. 2012, 28, 1–46. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhao, S.Q.; Wang, R.A. Analysis of soybean lecithin by supercritical fluid chromatography. Se pu = Chin. J. Chromatogr. 2001, 19, 344–346. [Google Scholar]
- Yamada, T.; Bamba, T. Lipid profiling by supercritical fluid chromatography/mass spectrometry. In Neuromethods; Springer: Berlin, Germany, 2017; Volume 125, pp. 109–131. [Google Scholar]
- Lee, J.W.; Nishiumi, S.; Yoshida, M.; Fukusaki, E.; Bamba, T. Simultaneous profiling of polar lipids by supercritical fluid chromatography/tandem mass spectrometry with methylation. J. Chromatogr. A 2013, 1279, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; King, J.W.; Montanari, L.; Fantozzi, P.; Blanco, M.A. Enrichment and fractionation of phospholipid concentrates by supercritical fluid extraction and chromatography. Ital. J. Food Sci. 2000, 12, 65–76. [Google Scholar]
- Uchikata, T.; Matsubara, A.; Fukusaki, E.; Bamba, T. High-throughput phospholipid profiling system based on supercritical fluid extraction-supercritical fluid chromatography/mass spectrometry for dried plasma spot analysis. J. Chromatogr. A 2012, 1250, 69–75. [Google Scholar] [CrossRef] [PubMed]
- King, J.W.; University of Arkansas, Fayetteville, NC, USA. Theory and Applications of Critical Fluids in Engineering, Unpublished work. 2010. [Google Scholar]
- Sanz, M.L.; Martínez-Castro, I. Recent developments in sample preparation for chromatographic analysis of carbohydrates. J. Chromatogr. A 2007, 1153, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, E.J.; Schwartz, H.E. Integral pressure restrictor for capillary sfc. J. Chromatogr. Sci. 1986, 24, 236–241. [Google Scholar] [CrossRef]
- Lafosse, M.; Herbreteau, B.; Morin-Allory, L. Supercritical fluid chromatography of carbohydrates. J. Chromatogr. A 1996, 720, 61–73. [Google Scholar] [CrossRef]
- Montañés, F.; Corzo, N.; Olano, A.; Reglero, G.; Ibáñez, E.; Fornari, T. Selective fractionation of carbohydrate complex mixtures by supercritical extraction with CO2 and different co-solvents. J. Supercrit. Fluids 2008, 45, 189–194. [Google Scholar] [CrossRef]
- Montañés, F.; Fornari, T.; Martín-Álvarez, P.J.; Corzo, N.; Olano, A.; Ibáñez, E. Selective recovery of tagatose from mixtures with galactose by direct extraction with supercritical CO2 and different cosolvents. J. Agric. Food Chem. 2006, 54, 8340–8345. [Google Scholar] [CrossRef] [PubMed]
- Montañés, F.; Fornari, T.; Martín-Álvarez, P.J.; Montilla, A.; Corzo, N.; Olano, A.; Ibáñez, E. Selective fractionation of disaccharide mixtures by supercritical CO2 with ethanol as co-solvent. J. Supercrit. Fluids 2007, 41, 61–67. [Google Scholar] [CrossRef]
- Montañés, F.; Fornari, T.; Olano, A.; Ibáñez, E. Supercritical fluid purification of complex carbohydrate mixtures produced by enzimatic transglycosilation and isomerized with complexating reagents. J. Supercrit. Fluids 2010, 53, 25–33. [Google Scholar] [CrossRef]
- Montañés, F.; Fornari, T.; Stateva, R.P.; Olano, A.; Ibáñez, E. Solubility of carbohydrates in supercritical carbon dioxide with (ethanol + water) cosolvent. J. Supercrit. Fluids 2009, 49, 16–22. [Google Scholar] [CrossRef]
- Salvador, A.; Herbreteau, B.; Lafosse, M.; Dreux, M. Subcritical fluid chromatography of monosaccharides and polyols using silica and trimethylsilyl columns. J. Chromatogr. A 1997, 785, 195–204. [Google Scholar] [CrossRef]
- Lefler, J.L.; Chen, R. A feasibility study of using supercritical fluid chromatography (sfc)-uv-elsd for food and beverage analyses. LC GC N. Am. 2008, 26, 42–43. [Google Scholar]
- Montañés, F.; Rose, P.; Tallon, S.; Shirazi, R. Separation of derivatized glucoside anomers using supercritical fluid chromatography. J. Chromatogr. A 2015, 1418, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Yamauchi, Y. Isolation of tocopherols from wheat germ oil by recycle semi-preparative supercritical fluid chromatography. J. Chromatogr. A 1990, 505, 257–271. [Google Scholar] [CrossRef]
- Johannsen, M.; Brunner, G. Supercritical fluid chromatography as successful separation tool in chemical and pharmaceutical industry. In Proceedings of the 9th Meeting on Supercritical Fluids, Trieste, Italy, 13–16 June 2004. [Google Scholar]
- Peper, S.; Crammerer, S.; Johannsen, M.; Brunner, G. Supercritical fluid chromatography: Process optimization of the separation of tocopherol homologues. In Proceedings of the International Symposium on Supercritical Fluids, Versailles, France, 28–30 April 2003. [Google Scholar]
- Ramírez, P.; García-Risco, M.R.; Santoyo, S.; Señoráns, F.J.; Ibáñez, E.; Reglero, G. Isolation of functional ingredients from rosemary by preparative-supercritical fluid chromatography (prep-sfc). J. Pharm. Biomed. Anal. 2006, 41, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, P.; Santoyo, S.; García-Risco, M.R.; Señoráns, F.J.; Ibáñez, E.; Reglero, G. Use of specially designed columns for antioxidants and antimicrobials enrichment by preparative supercritical fluid chromatography. J. Chromatogr. A 2007, 1143, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Coleman, W.M.; Dube, M.F.; Ashraf-Khorassani, M.; Taylor, L.T. Isomeric enhancement of davanone from natural davana oil aided by supercritical carbon dioxide. J. Agric. Food Chem. 2007, 55, 3037–3043. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lambros, T.; Wang, Z.; Goodnow, R.; Ho, C.T. Efficient and scalable method in isolation of polymethoxyflavones from orange peel extract by supercritical fluid chromatography. J. Chromatogr. B 2007, 846, 291–297. [Google Scholar] [CrossRef] [PubMed]
- García-Risco, M.R.; Vicente, G.; Reglero, G.; Fornari, T. Fractionation of thyme (Thymus vulgaris L.) by supercritical fluid extraction and chromatography. J. Supercrit. Fluids 2011, 55, 949–954. [Google Scholar] [CrossRef]
- Perrotin-Brunel, H.; Van Roosmalen, M.J.E.; Kroon, M.C.; Van Spronsen, J.; Witkamp, G.J.; Peters, C.J. Solubility of cannabinol in supercritical carbon dioxide. J. Chem. Eng. Data 2010, 55, 3704–3707. [Google Scholar] [CrossRef]
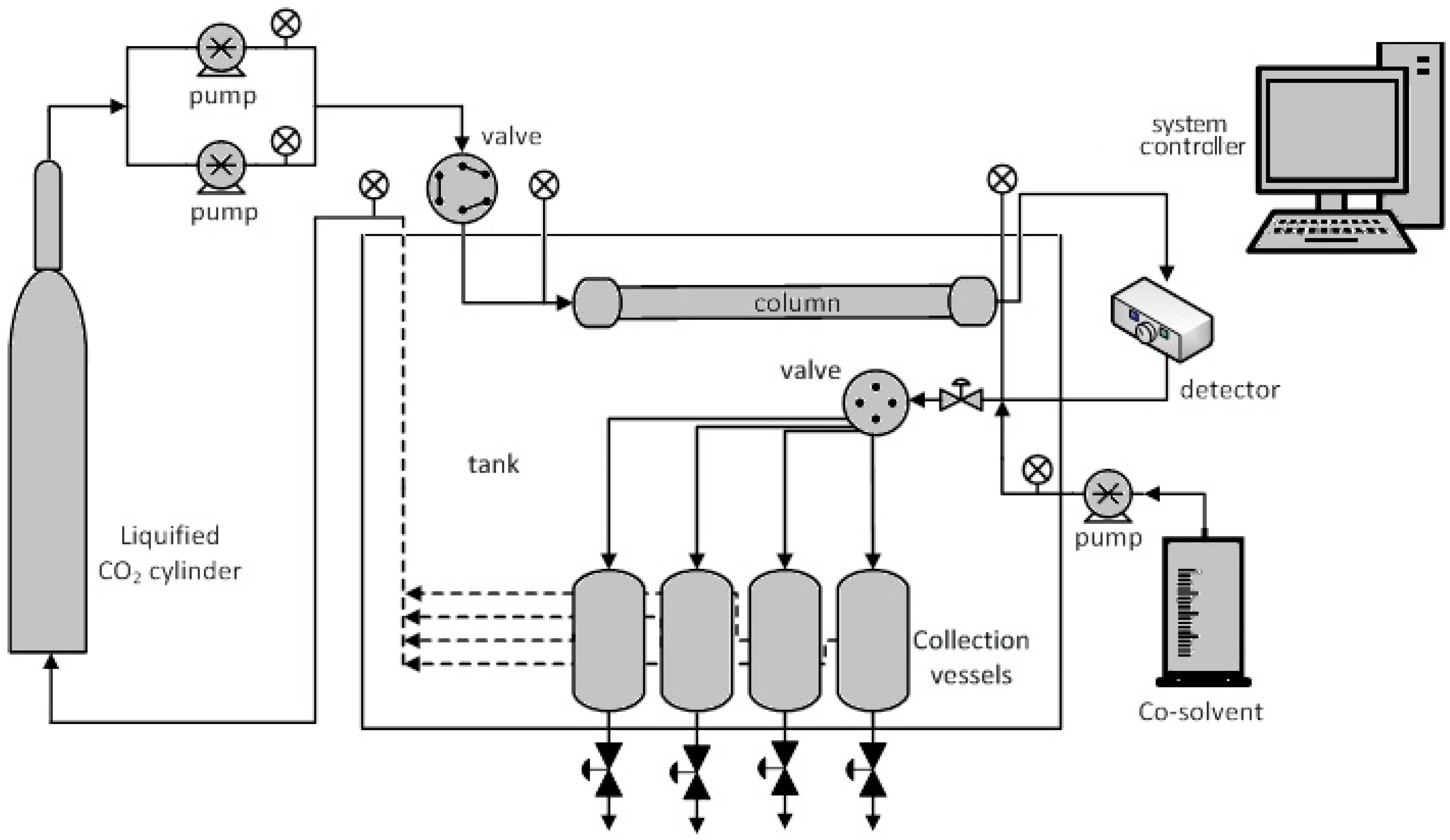
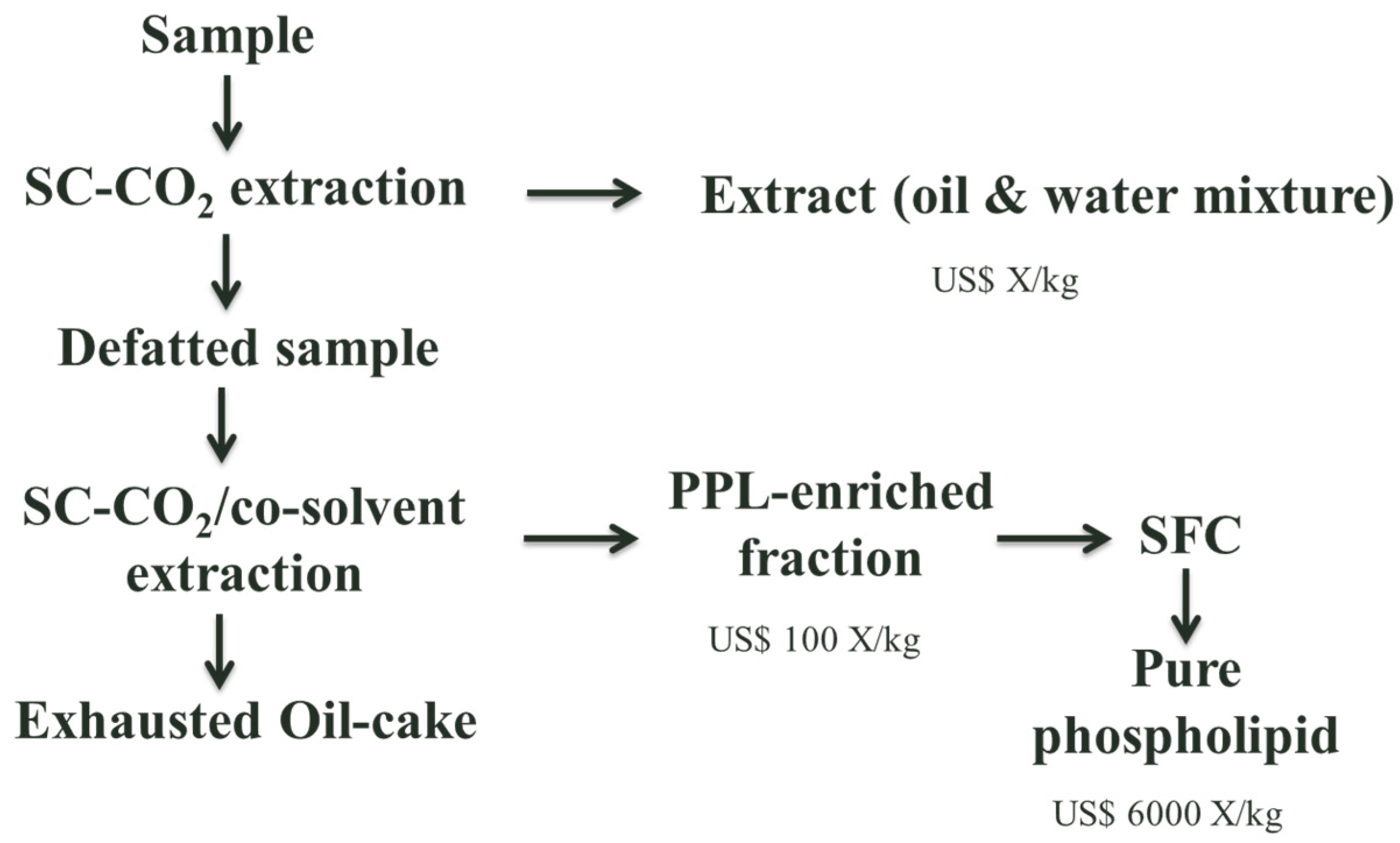
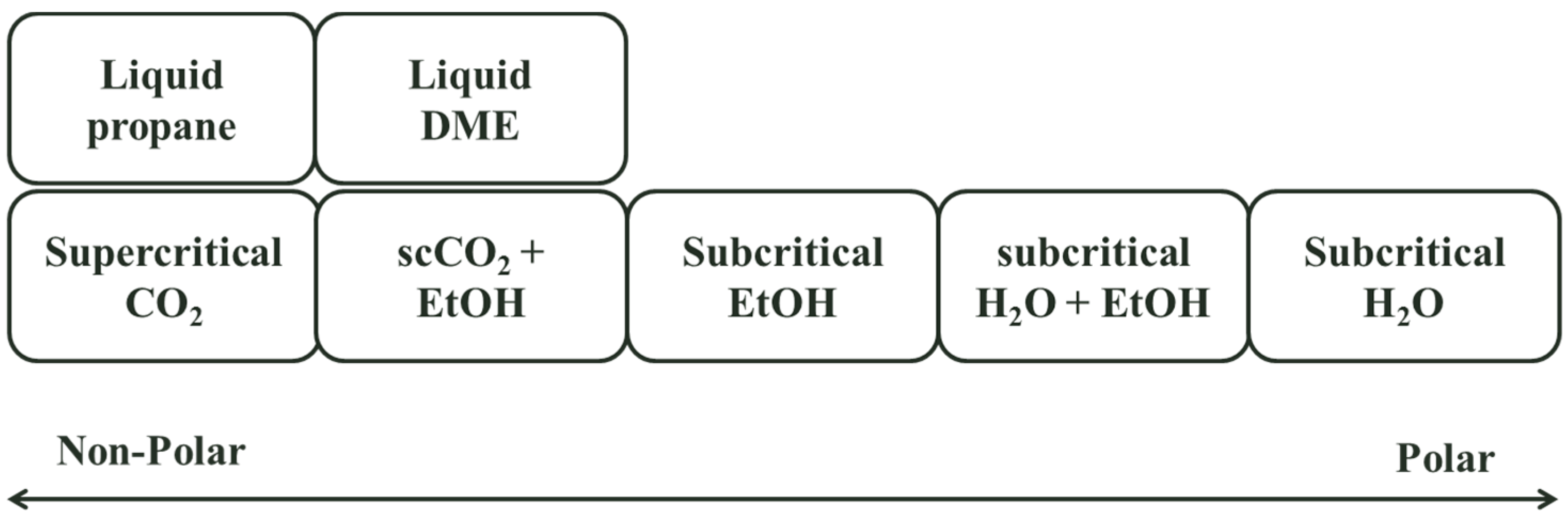
| Reference | Feed Material | Ester Form | Stationary Phase | Mobile Phase | Experimental Conditions (P/T) | Feed Concentration (%) | Final Concentration (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| EPA | DHA | EPA | DHA | ||||||
| [24] | Sardine oil | Methylated | Silica gel coated with silver nitrate | CO2 | 80–200 bar 313 K | 12 | 13 | 93 | 92 |
| [25] * | Marine oil | Ethylated | Reverse phase octadecyl silica gel | Acetonitrile/water or methanol/water | 200 bar 323 K | 50 | 30 | 95 | 90 |
| [26] | Tuna oil | Ethylated | Octadecyl silane | CO2 | 145 bar 338 K | NA | NA | 50 | 90 |
| [27] | Menhaden oil | Ethylated | 0.375″ ball bearings 304 SS or 0.16″ Propak 316 SS | CO2 | 150–170 bar 313–373 K | NA | NA | NA | NA |
| [28] | Fish oil | Ethylated | 220 kg porous silicon dioxide impregnated with (3-aminopropyl)-triethoxysilane | CO2 | 100–150 bar 313–333 K | 50 | --- | 95 | --- |
| [29] | Fish oil | Ethylated | Silica gel | CO2 | 150–220 bar 343–353 K | 68 | --- | 93 | --- |
| [30] ** | Fish oil | Ethylated | Amino-bonded silica | CO2 | 170 bar 310 K | NA | NA | NA | NA |
| [31] | Algae oil | Ethylated | Silica-PFP *** | CO2 | 170 bar 333 K | 16 | 0.7 | 86 | 72 |
| [31] | Fish oil | Ethylated | Silica-PFP *** | CO2 | 170 bar 333 K | 18 | 9.1 | 94 | 83 |
| PPL | % |
|---|---|
| PC | 84.3 |
| 1 LPC | 0.2 |
| 2 LPC | 1.2 |
| PE | 10.5 |
| SM | 2.3 |
| DHSM | 1.1 |
| 2 LPE | 0.3 |
| Total PPLs in sample | 78.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montañés, F.; Tallon, S. Supercritical Fluid Chromatography as a Technique to Fractionate High-Valued Compounds from Lipids. Separations 2018, 5, 38. https://doi.org/10.3390/separations5030038
Montañés F, Tallon S. Supercritical Fluid Chromatography as a Technique to Fractionate High-Valued Compounds from Lipids. Separations. 2018; 5(3):38. https://doi.org/10.3390/separations5030038
Chicago/Turabian StyleMontañés, Fernando, and Stephen Tallon. 2018. "Supercritical Fluid Chromatography as a Technique to Fractionate High-Valued Compounds from Lipids" Separations 5, no. 3: 38. https://doi.org/10.3390/separations5030038
APA StyleMontañés, F., & Tallon, S. (2018). Supercritical Fluid Chromatography as a Technique to Fractionate High-Valued Compounds from Lipids. Separations, 5(3), 38. https://doi.org/10.3390/separations5030038




