Abstract
Bisphenol-A, a synthetic organic compound with estrogen mimicking properties, may enter bloodstream through either dermal contact or ingestion. Probiotic bacterial uptake of bisphenol can play a major protective role against its adverse health effects. In this paper, a method for the quantification of BPA in bacterial cells of L. lactis and of BPA and its potential metabolites 4-hydroxybenzoic Acid, 4-hydroxyacetophenone and hydroquinone in the culture medium is described. Extraction of BPA from the cells was performed using methanol–H2O/TFA (0.08%) (5:1 v/v) followed by SPE. Culture medium was centrifuged and filtered through a 0.45 μm syringe filter. Analysis was conducted in a Nucleosil column, using a gradient of A (95:5 v/v H2O: ACN) and B (5:95 v/v H2O: ACN, containing TFA, pH 2), with a flow rate of 0.5 mL/min. Calibration curves (0.5–600 μg/mL) were constructed using 4-n-Octylphenol as internal standard (1 > R2 > 0.994). Limit of Detection (LOD) and Limit of Quantification (LOQ) values ranged between 0.23 to 4.99 μg/mL and 0.69 to 15.1 μg/mL respectively. A 24 h administration experiment revealed a decline in BPA concentration in the culture media up to 90.27% while the BPA photodegradation levels were low. Our results demonstrate that uptake and possible metabolism of BPA in L. lactis cells facilitates its removal.
1. Introduction
Bisphenol-A is the organic synthetic compound 4, 4′-Isopropylidenediphenol, which is widely used in thermal paper industry and as a component of synthetic plastic (vinyl-chloride) due to its mechanical and extreme-temperature resistance [1]. BPA, was found to exhibit estrogenic properties, arising from its structural resemblance to the human 17β-estradiol [2], thus it is also referred to as xenoestrogen or endocrine disruptor. The main routes of human exposure to BPA are the consumption of food in BPA containing packages [3] and through dermal contact with BPA rich materials [4]. Following exposure, BPA is mainly metabolized in humans to BPA-glucuronide through the hepatic glucuronide transferase and excreted from the body [5,6]. However, a percentage of BPA, referred to as “free” or “active” BPA, remains in the blood circulation for up to a week and can interfere with endogenous biological processes [4]. Due to its estrogenic and genotoxic effects and its wide occurrence, BPA presents a risk to humans and animals and hence it is the object of great environmental concern [7,8,9].
Although part of bisphenol is degraded via photo-oxidation [10,11,12], monitoring of its levels is deemed necessary in various environmental sources [13]. To this end, biodegradation has been proposed as an advanced technique for BPA elimination [13,14,15], since a variety of microorganisms have been identified to decompose BPA [15,16]. Probiotics are bacteria beneficial to the host, which have been recognized as safe for human consumption and are used in the production of food products [17,18]. Recently they have been reported to be ideal against heavy metal toxicity since they bind and sequester metals [19]. Moreover, the probiotic bacteria Bifidobacterium breve and Lactobacillus casei when administered to rats showed a prophylactic effect against the detrimental effects of BPA, by reducing its intestinal absorption and facilitating its excretion, [18]. Likewise, the ability of Bacillus strains [20,21,22], certain Lactococcus strains [23] and Shingomonas paucimobilis [24] to degrade bisphenol has been documented. Recently a simplified method for the extraction of bisphenol from bacterial culture suspensions has been reported [25]. Moreover, the compounds 4-hydroxy acetophenone (HAP), 4-hydroxy benzoic acid (HBA) and Hydroquinone (HQ) have been proposed as major bacterial metabolites of BPA bacterial degradation pathways [14,26,27]. However, all these studies employed the determination of bisphenol in the cell free culture supernatant and not in the bacterial cells even though they examine the kinetics of bisphenol.
Analytical methods for the determination of bisphenol in various food samples [3], as well as in biological fluids and environmental samples [28], have been extensively reviewed. The determination of BPA has also been investigated in more complex matrices such as rat tissues [29], human breast milk [30] resin-based dental restorative materials [31] and saliva [32]. However, to the best of our knowledge, no method has been reported for the determination of bisphenol in bacterial cells.
In the present study, the development and validation of an analytical method for the quantification of BPA in bacterial cells is reported for the first time using the widespread probiotic bacterium Lactococcus lactis (ATC 11454). The method is also validated in bacterial culture supernatants for the simultaneous determination of the bacterial metabolites reported to be produced during the bacterial major metabolic pathway [14], namely 4-hydroxy-acetophenone (HAP), 4-hydroxy benzoic acid (HBA) and Hydroquinone (HQ). The application of this method demonstrates the uptake of bisphenol by Lactococcus lactis cells and allows its quantification.
2. Materials and Methods
2.1. Chemicals and Reagents
Acetonitrile was purchased from VWR Chemicals (Paris, France). Methanol and Absolute ethanol were purchased from Fisher Scientific (Loughborough, UK). Trifluoroacetic acid (TFA), KH2PO4 and FeCl3 were purchased from AppliChem GmbH (Darmstadt, Germany), while NaCl, CaCl2, (NH4)2SO4 and MgSO4 were purchased from Merck KGaA (Darmstadt, Germany). All the chemicals used were HPLC Grade, unless specified otherwise.
Bisphenol-A (BPA) and the internal standard 4-n-Octylphenol (OP) were purchased from Alfa Aesar (Karlsruhe, Germany). 4-Hydroxyacetophenone (HAP), 4-Hydroxybenzoic Acid (HBA) and Hydroquinone (HQ) were purchased from Acros Organics (Ceel, Belgium).
The bacterial strain from which the liquid culture supernatant and the pellet originated and used for the spike-recovery studies, was Lactococcus lactis (ATCC 11454). The liquid culture medium that was used for the growth of L. lactis was Brain Heart Infusion broth (BHI broth) and was purchased from AppliChem GmbH (Darmstadt, Germany). An artificial bacterial culture medium was prepared, namely Basal Salts Medium (BSM), composed of KH2PO4 and (NH4)2SO4 1 g/L each, MgSO4 0.1 g/L, NaCl and CaCl2 0.05 g/L each and FeCl3 0.01 g/L. BPA was used as a sole carbon source in BSM in varying concentrations for the feeding experiments. For the retrieval of the bacterial culture supernatant and pellet 100 μL of an L. lactis glycerol stock solution were inoculated in a 250 mL flash with BHI broth. Following a 24-h incubation at 28 °C under constant stirring, the culture was centrifuged at 5000 rpm and the precipitate was transferred to a 250 mL flask containing BSM. At the end of the experiment, the culture was centrifuged, the precipitate was placed on a filtering paper and its weight was recorded following removal of excess water. The recorded weight is referred as ‘wet cell weight’. The culture supernatant and the wet cell weight were used for the spike-recovery assays.
Solid Phase Extraction columns, frits and C18 sorbent material, 35–75 U were purchased from Grace Davison Discovery Sciences (Bannockburn, IL, USA). Membrane filters with 0.45 μm diameter pores were purchased from Merck KGaA (Darmstadt, Germany).
2.2. Standard Preparation
2.2.1. Stock Solution Preparation
Stock solutions 500 μg/mL of BPA, HAP, HBA and HQ were prepared by dissolving 25 mg of the analyte in 50 mL methanol. The stock solution of BPA (50 mg/mL) was prepared by dissolving 2.5 g of BPA in 50 mL ethanol. The internal standard stock solution of 500 μg/mL was prepared by dissolving 25 mg of OP in 50 mL methanol.
2.2.2. Working Standard Solution Preparation
Seven-point and six-point working standard solutions were prepared for analytes at concentrations of 0.5, 10, 50, 100, 200, 400, and 600 μg/mL for BPA, at 0.5, 2, 4, 6, 8 and 10 μg/mL for HAP and HQ and at 1, 2, 4, 6, 8 and 10 μg/mL for HBA and the internal standard 4-n-octylphenol (75 μg/mL).
2.3. Sample Preparation
For the bacterial pellet method development, 0.1 g of wet cell weight L. lactis was added in a test tube with 8 mL methanol, 2 mL of double distilled water containing 0.08% (v/v) TFA and 150 μL internal standard from the 500 μg/mL stock. The concentration of the internal standard in the final 1 mL volume was 75 μg/mL. The test tube was transferred to an ice-filled container and was placed under a homogenizer for 5 min. The homogenate was separated in ten Eppendorf centrifuge tubes and was centrifuged for 10 min in 8000 rpm. The supernatant was collected and mixed with an equal volume of ddH2O containing 0.08% TFA. BPA extraction was performed using Solid Phase Extraction (SPE). An SPE cartridge was prepared using 0.5 g of C18 sorbent in an SPE column. The SPE cartridge was conditioned with 10 mL methanol, 5 mL ddH2O and 5 mL ddH2O containing 0.08% TFA. The sample was loaded on the cartridge and a manual flow of 1 mL/min was employed. The cartridge was, then, washed with 5 mL ddH2O and elution was performed using 4 mL methanol. The eluate was collected and placed under a rotary evaporator in a 37 °C water bath, to dryness. Finally the sample was reconstituted with 1 mL acetonitrile and stored in an aluminum foil-covered Eppendorf tube at −20 °C. All experiments were conducted in dark or covered tubes to avoid photodegradation of BPA.
For the bacterial supernatant, an 8.5 mL sample of the culture was added in a test tube along with 1.5 mL internal standard from the stock solution. The sample was placed in 10 Eppendorf centrifuge tubes (1 mL per tube), and was centrifuged for 10 min in 8000 rpm. The supernatant was collected and filtered through a 0.45 μm diameter pore filter. The filtrate was stored in an aluminum foil-covered Eppendorf tube at −20 °C.
2.4. BPA Administration (Feeding) Assay
For the feeding assays 6 falcon tubes of 50 mL volume were filled with BHI broth and were inoculated with 100 μL of a L. lactis glycerol stock solution. The cultures were incubated for 24 h at 28 °C under constant stirring. The cultures were, then, centrifuged and the medium was discarded. A total of 50 mL of fresh BSM was added to each culture along with varying quantities from the BPA stock solution (50 mg/mL) so that the final BPA concentration in each culture would be 50, 100, 150, 200, 400 και 500 μg/mL respectively. The cultures were incubated for another 24 h at 28 °C under constant stirring. The remaining BPA in the cultures was quantified using the method and the calibration curves that were described.
2.5. Photodegradation Assay
To investigate potential photodegradation of BPA, a falcon tube of 50 mL containing BSM and BPA at a concentration of 50 μg/mL was prepared and was incubated under the same conditions as the feeding assays. BPA was quantified at the beginning and the end of the 24 h incubation period. The presence of photodegradation products was examined in the resulting chromatograms.
2.6. HPLC-DAD Conditions and Methods
The instrument used consisted of an LC20AD pump and an SPD-20A photodiode array detector (DAD) purchased from Shimadzu (Kyoto, Japan). Separation of BPA was achieved on a Nucleosil 100 C18 column (250 × 4.6 mm, 5 μm) purchased by Macherey-Nagel GmbH & Co. (Duren, Germany) with a binary mobile phase system at room temperature. The injection was performed using a 100 μL Hamilton syringe, with an injection volume of 80 μL. Mobile Phase A consisted of a 0.1% TFA in ddH2O solution and Acetonitrile at a ratio of 95:5, v/v. Mobile Phase B consisted of a 0.08% TFA in ddH2O solution and Acetonitrile at a ratio of 5:95, v/v. The gradient started at 100% Mobile Phase A which was brought down to 80% over 1 min, 70% over 4 min, 55% at 15 min, 40% at 17 min, 20% at 20 min and finally to 10% over 2 min and then was kept at this level until the end on the analysis. The flow rate was constant at 0.5 mL/min. BPA was detected at 220 nm with a band width of 2.0 nm. HAP, HBA and HQ were detected at 280 nm with a band width of 2.0 nm. Identification was conducted by matching the peak retention times (Rt) with those of the pure standards. Internal standard calibration was used by plotting the peak area ratio of the analyte to the peak area of the internal standard against the concentration of the analyte.
3. Results and Discussion
3.1. Optimization of Sample Preparation
The homogenization of the sample was performed to lyse the cells and release the adsorbed BPA for the feeding assay. Initially, ethyl acetate was used instead of methanol and a manual homogenization was attempted with a glass homogenizer, but cell lysis was deemed inadequate. Hence methanol was selected as the extraction solvent. The addition of an equal volume ddH2O containing 0.08% (v/v) TFA after centrifugation aimed to increase the solubility of BPA due to further dilution.
An elaborate and efficient vortex assisted liquid-liquid microextraction with octanol was used for the trace analysis of bisphenol in water and wastewater samples [33]. However, an attempt to use liquid-liquid extraction with ethyl acetate in the samples of the present study resulted to the formation of an emulsion zone between the two phases, a fact that led to greatly varying percentages of BPA recovery due to the loss of the analyte during the procedure. Thus, solid phase extraction was implemented, since SPE is a common step in the extraction procedures of bisphenol from complex matrices, such as rat tissues [29] or human serum [5] and its benefits in the biomonitoring of bisphenol have been demonstrated [28,33]. In the present study a flow rate of 1 mL/min was selected throughout the SPE procedure, as effective and timely favorable, while higher flow rates did not provide full retention of BPA by the C18 material, especially at high BPA concentrations employed and resulted in lower recovery values. A 37 °C water bath was selected to facilitate the evaporation of methanol after SPE. The slightly higher evaporation temperatures of 45 °C that have been reported for the analysis of bisphenol and 4-nonylphenol [29] resulted in heat-induced decomposition of the internal standard when used in the present study. Our results for BPA extraction are in accordance with a recently published report on a methanol extraction of bisphenols from bacterial cultures and solid matrices [25] in which, however, SPE was not employed and lower concentrations of BPA were used [25], resulting in recoveries up to 95%. The SPE step used in the present study greatly improved recovery which amounted to 99.38–100.18% in culture supernatants and to 90.8–102.46% in bacterial cells, in the wide range of concentrations employed (Table 1).

Table 1.
Analyses of the calibration curves for analytes in the cell culture supernatant and the cell culture precipitate.
In an effort to perform the analysis of BPA in the BHI medium, which is recommended for growth of L. lactis, the nutrients in the broth resulted in the presence of peaks in the chromatogram that appeared to interfere with the tested bacterial metabolites peaks. The artificial bacterial medium (BSM) was prepared to tackle the problems that arose in the analysis using the reference BHI medium. Moreover, in this manner BPA was the sole carbon source for the bacterium, a fact that contributes in its biotransformation.
3.2. Method Validation
The results of the linear regression analysis performed using the “Linear Regression” package of Microsoft Excel 2013 software are presented in Table 1, while detection limits and Retention time variance are presented in Table 2. The coefficient of determination values (R2) for the standard curves in BSM supernatant and L. lactis cells were 1 and 0.999 respectively, while for HAP, HBA and HQ in BSM supernatant the values exceeded 0.9994, 0.994 and 0.999 respectively, indicating excellent linearity. The Relative Bias was calculated by computing the percentage of the difference of the mean calculated concentration to the nominal concentration divided by the nominal concentration of the analyte. All results are within the accepted range of ±10%. Intra-day precision was calculated by performing a total of three injections of the nominal concentration on the same day, while inter-day precision was calculated by performing six injections of the nominal concentration over three days. Both were computed as % R.S.D. = (S.D./mean) × 100. None of the values exceeded 6.0% for all analytes, with the exception of the values obtained for the lowest nominal value of 0.5 μg/mL of BPA in bacterial cells and in the medium, which might be due to the fact that this nominal value is below LOD (Table 2). Since an elaborate extraction protocol was required for the bacterial cells, the use of an internal standard was deemed necessary, to compensate for sample losses during sample preparation. The internal standard 4-n-octylphenol was selected, due to the fact that it is well resolved and it has a structure similar to bisphenol. Moreover, it is not expected to be present in samples, since only 4-t-octyphenol has been reported to be found at very low concentrations in environmental samples and human milk [34,35]. Our results, as presented in Table 1, indicate that the method is highly precise and repeatable. Recovery rates were well within range of 10% error, reaching close to 100% recovery, thus further supporting the precision of this method. The recovery results presented in Table 1 are in good correlation with those reported for rat serum (95%) and higher than those reported for rat testis (78.6%) and liver (84.0%) [29].

Table 2.
Analyses LOD, LOQ and Rt values for all analytes.
The nominal concentrations of 0.5 μg/mL BPA in BSM and cell precipitate were below the calculated LOD values (Table 2) and were thus excluded from the estimation of recovery results described above. The values for the Limit of Detection (LOD) and Limit of Quantification (LOQ) were calculated from the calibration curves. The LOD was calculated to 3.3 times the ratio of the Standard Error (intercept) to the Coefficients x, while the LOQ was 10 times this ratio. The retention time means, standard deviations and RSDs were calculated using the data from one chromatogram of each of the nominal concentrations used. The value of an LOD of 4.99 μg/mL estimated in the present study for BPA in bacterial cells at the range of 10–600 μg/mL, is higher than the value of 2.8 ng/mL reported for rat tissues, but over a narrower range of concentrations of 0.15–150 μg BPA/mL [29]. A lower value of LOD was found for BPA in the less complex sample of BSM, amounting to 0.64 μg/mL (Table 2), although the same range of concentrations was employed (Table 1), possibly due to lower matrix complexity. Similar low LOD values were estimated for all possible metabolites tested, as shown in Table 2. Although these compounds have been proposed as possible bisphenol bacterial metabolites, they were identified using MS and NMR analysis of the HPLC isolated fractions [27]. In fact, in the studies on the determination of bisphenol degradation using HPLC-DAD [20,23], no validation was included. The method presented in this work allows the simultaneous quantification of bisphenol A and three of its major bacterial metabolites.
3.3. Feeding Results
A typical chromatogram of HQ, HBA and HAP and BPA is presented in Figure 1. Analyte peaks of HQ, HBA, HAP, BPA and internal standard OP are shown with a retention time (Rt) of 10.671, 12.728, 15.579, 26.241 and 40.771 min respectively. The peak that appears at 25.6 (Figure 1) might be attributed to a BPA photodegradation product, since it was also identified during the photodegradation experiment and is presented in Figure 2. Photodegradation of BPA via photooxidation has been confirmed [10,12], and the products of this process presented higher toxicity than the parent compound [11]. Since the feeding experiments were conducted under normal light, it was deemed appropriate to test if any BPA loss was due to photodegradation. In these experiments the initial BPA concentration was calculated at 48.65 μg/mL and the concentration following the 24 h incubation amounted to 44.18 μg/mL. Hence, the BPA loss due to photodegradation was 9.19%. These values are in accordance with those reported for bisphenol A degradation by UV irradiation in the absence of hydrogen peroxide [36].
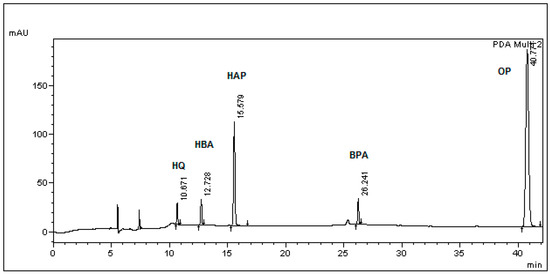
Figure 1.
A chromatogram containing all analytes at a concentration of 10 μg/mL and internal standard at 75 μg/mL in fresh BSM without L. lactis inoculation. The first two peaks with Rt of 5.7 min and 7.5 min are background noise from acetonitrile solvent and TFA.
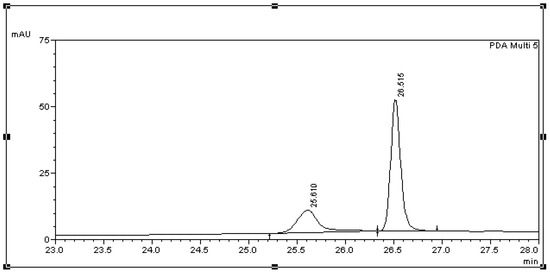
Figure 2.
Chromatogram displaying BPA and its identified photodegradation product with a Rt of 26.515 min and 25.610 min respectively. The compounds were detected at a wavelength of 300 nm.
Typical chromatograms of the feeding assays are presented in Figure 3 (bacterial cells) and Figure 4 (bacterial culture medium). The relative blank chromatograms resulting from bacterial cells and from their culture medium any additions are shown in the Supplemental section. The peaks appearing at 10.2 min in both chromatograms does not represent one of the metabolites described in this paper, as confirmed by comparing the absorbance spectra of the peak to those of the pure metabolites analyzed.
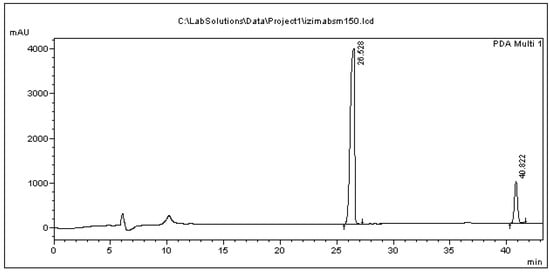
Figure 3.
Chromatogram of cell precipitate originating from the administration of 150 μg/mL BPA in the bacterial cells. The peaks at 26.528 min and 40.822 min correspond to BPA and the internal standard.
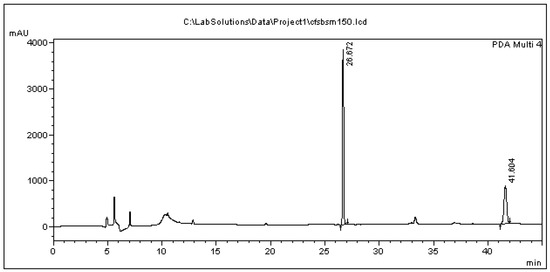
Figure 4.
Chromatogram of culture supernatant originating from the administration of 150 μg/mL BPA in the bacterial cells. The peaks at 26.672 min and 41.604 min correspond to BPA and the internal standard.
Two major pathways have been proposed for the metabolism of bisphenol A by bacteria, the first leading to mineralization, with HBA and HAP as intermediates and the second to hydroxylation [6,16]. However, it has been pointed out that the metabolic pathways might be strain specific [14], since certain metabolites produced by one strain are not detected when another bacterial strain is used. In a similar manner, the analytes HQ, HBA and HAP were not detected in the chromatograms of the feeding assays. Absence of bacterial metabolites has also been reported for Bacillus pumilus [20] and certain Lactococcus strains [23]. In all these reports, including the present work, the presence of metabolites was examined at 24 h of culture. However, when various Bacilli and Sphingomonas strains were tested for bisphenol degrading capacity, metabolites were detected for three of them and only from 8–12 h, in a declining manner [37]. These results indicate that the time period of 24 h used in the present paper might be too long, since the metabolites under investigation might have formed and then further transformed. On the other hand, the bacterial metabolites examined in the present paper were reported to be produced by Bacillus sp., yet only after 3 days of incubation in the presence of 20 mM BPA [21]. Moreover, the photodegradation of BPA metabolites has been reported for hydroxybenzoic acid as well as for hydroquinone [38,39]. It is evident that more experiments are needed in the course of bacterial incubation, since metabolism is an ongoing process and each strain presents particular metabolic characteristics.
A depletion of up to 58% in total BPA (μg) was observed in our experiments, in all bacterial cultures as shown in Table 3. Complete degradation of BPA has been reported following a 48 h incubation period [37] or after 9 days of incubation [21]. In our experiments BPA was reduced by 90.27%, 64.16%, 58.60%, 67.74%, 79.83% and 85.05% in the cultures corresponding to initial BPA concentration of 50, 100, 150, 200, 400 and 500 μg/mL. Our results suggest that the initial BPA concentration added has a profound effect on its degradation. Many bacterial strains that can degrade BPA have been reported, and this degrading ability varies among the species [14]. It should also be noted that different initial concentrations of BPA are used in the reports employing bacteria, ranging from 0.1 to 60 mg/L [37,40,22] and for varying incubation periods. Bacteria can clearly employ a variety of metabolic pathways to exploit pollutants as energy sources, under the prerequisite that the pollutants are bioavailable and at levels that could trigger such mechanisms [16].

Table 3.
Comparative data of BPA administered (μg) and found in feeding assays.
4. Conclusions
The developed analytical method provides a reliable, robust and sensitive approach for the simultaneous detection and quantification of bisphenol A and its potential metabolites in the complex matrix sample of bacterial cells. Good chromatography separation was achieved on a Nucleosil 100 C18 column with a longer run time, which however allows the detection and separation of metabolites. LC-DAD combined with solid phase extraction and the use of 4-n-octylphenol as the internal standard, is capable of operating with a detection limit of 4.99 μg/mL for bisphenol and lower limits for metabolites in bacterial cells. The method was also successfully applied in the analysis of bacterial culture medium with a detection limit of 0.64 μg/mL for bisphenol. The feeding experiments revealed that L. lactis is capable to uptake of bisphenol and contribute to its elimination from the culture medium. Further experiments are needed for the detection and quantification of metabolites as a function of culture time and administrated bisphenol concentration.
Supplementary Materials
The following are available online at http://www.mdpi.com/2297-8739/5/1/12/s1, Figure S1: Blank chromatograms of L. lactis cells (A) and cell free bacterial medium (B).
Acknowledgments
No additional funding was received in support of the present research work.
Author Contributions
M.T. conceived the experiments; M.T. and V.F.S. designed the experiments; A.T.R. performed the experiments; A.T.R. analyzed the data, A.T.R., M.T. and V.F.S. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- López-Cervantes, J.; Sánchez-Machado, D.; Paseiro-Losada, P.; Simal-Lozano, J. Effects Of Compression, Stacking, Vacuum Packing and Temperature on the Migration of Bisphenol A from Polyvinyl Chloride Packaging Sheeting into Food Simulants. Chromatographia 2003, 58, 327–330. [Google Scholar]
- Kang, J.-H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Toxicology 2006, 226, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Gómez, A.; Rubio, S.; Pérez-Bendito, D. Analytical methods for the determination of bisphenol A in food. J. Chromatogr. A 2009, 16, 449–469. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Martin, J.W. Prolonged exposure to bisphenol A from single dermal contact events. Environ. Sci. Technol. 2017, 51, 9940–9949. [Google Scholar] [CrossRef] [PubMed]
- Völkel, W.; Colnot, T.; Csanady, G.; Filser, J.G.; Dekant, W. Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem. Res. Toxicol. 2002, 15, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Yadav, S.; Prakash, K.; Swati, J.; Singh, S.P. Singh Effect of bisphenol A on human health and its degradation by microorganisms: A review. Ann. Microbiol. 2014, 64, 13–21. [Google Scholar] [CrossRef]
- Kang, J.H.; Kondo, F. Bisphenol A Degradation by Bacteria Isolated from River Water. Arch. Environ. Contam. Toxicol. 2002, 43, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T. Human Health Risk on Environmental Exposure to Bisphenol-A: A Review. J. Environ. Sci. Health 2006, 24, 225–255. [Google Scholar] [CrossRef] [PubMed]
- Oehlmann, J.; Oetken, M.; Schulte-Oehlmann, U. A critical evaluation of the environmental risk assessment for plasticizers in the freshwater environment in Europe, with special emphasis on bisphenol A and endocrine disruption. Environ. Res. 2008, 108, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.A.; Dorn, P.B.; Klecka, G.M.; O’Block, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol-A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Da Silva, J.C.C.; Reis Teodoro, J.A.; Afonso, R.J.; Aquino, S.F.; Augusti, R. Photodegradation of bisphenol A in aqueous medium: Monitoring and identification of by-products by liquid chromatography coupled to high-resolution mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Diepens, M.; Gijsman, P. Photodegradation of bisphenol A polycarbonate. Polym. Degrad. Stab. 2007, 92, 397–406. [Google Scholar] [CrossRef]
- Suzuki, T.; Nakagawa, Y.; Takano, I.; Yaguchi, K.; Yasuda, K. Environmental Fate of Bisphenol A and Its Biological Metabolites in River Water and Their Xeno-estrogenic Activity. Environ. Sci. Technol. 2004, 38, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yin, K.; Chen, L. Bacteria-mediated bisphenol A degradation. Appl. Microbiol. Biotechnol. 2013, 97, 5681–5689. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Katayama, Y.; Kondo, F. Biodegradation or metabolism of bisphenol A: From microorganisms to mammals. Toxicology 2006, 217, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kolvenbach, B.A.; Helbling, D.E.; Kohler, H.P.E.; Corvini, P.F.X. Emerging chemicals and the evolution of biodegradation capacities and pathways in bacteria. Curr. Opin. Biotechnol. 2014, 27, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Giarma, E.; Amanetidou, E.; Toufexi, A.; Touraki, M. Defense systems in developing Artemia franciscana nauplii and their modulation by probiotic bacteria offer protection against a Vibrio anguillarum challenge. Fish Shellfish Immunol. 2017, 66, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Sato, T.; Yokoi, W.; Yoshida, Y.; Ito, M.; Sawada, H. Effect of probiotics, Bifidobacterium breve and Lactobacillus casei, on bisphenol A exposure in rats. Biosci. Biotechnol. Biochem. 2008, 72, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Monachese, M.; Burton, J.P.; Reid, G. Bioremediation and tolerance of humans to heavy metals through microbial processes: A potential role for probiotics? Appl. Environ. Microbiol. 2012, 78, 6397–6404. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, H.; Moriyoshi, K.; Ohmoto, T.; Ohe, T.; Sakai, K. Degradation of bisphenol A by Bacillus pumilus isolated from kimchi, a traditionally fermented food. Appl. Biochem. Biotechnol. 2007, 136, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, G.; Ramadas, V.; Nellaiah, H. Isolation, indentification and degradation of Biphenol A by Bacillus sp. from effluents of thermal paper industry. Int. J. Sci. Eng. Res. 2013, 4, 366–374. [Google Scholar]
- Zühlke, M.K.; Schlüter, R.; Henning, A.K.; Lipka, M.; Mikolasch, A.; Schumann, P.; Giersberg, M.; Kunze, G.; Schauer, F. A novel mechanism of conjugate formation of bisphenol A and its analogues by Bacillus amyloliquefaciens: Detoxification and reduction of estrogenicity of bisphenols. Int. Biodeterior. Biodegrad. 2016, 109, 165–173. [Google Scholar] [CrossRef]
- Endo, Y.; Kimura, N.; Ikeda, I.; Fujimoto, K.; Kimoto, H. Adsorption of bisphenol A by lactic acid bacteria, Lactococcus, strains. Appl. Microbiol. Biotechnol. 2007, 74, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Soda, S.; Fujita, M.; Ike, M. Kinetics of bisphenol A degradation by Sphingomonas paucimobilis FJ-4. J. Biosci. Bioeng. 2016, 122, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Yip, D.; Lee, J.; Löffler, F.E. Simplified extraction of bisphenols from bacterial culture suspensions and solid matrices. J. Microbiol. Methods 2016, 126, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Spivack, J.; Leib, T.K.; Lobos, J.H. Novel Pathway for Bacterial Metabolism of Bisphenol A. J. Biol. Chem. 1994, 269, 7323–7329. [Google Scholar] [PubMed]
- Lobos, J.H.; Leib, T.K.; Su, T.M. Biodegradation of bisphenol A and other bisphenols by a gram-negative aerobic bacterium. Appl. Environ. Microbiol. 1992, 58, 1823–1831. [Google Scholar] [PubMed]
- Rykowska, I.; Wasiak, W. Properties, threats, and methods of analysis of bisphenol A and its derivatives. Acta Chromatogr. 2006, 16, 7–27. [Google Scholar]
- Xiao, Q.; Li, Y.; Ouyang, H.; Xu, P.; Wu, D. High-performance liquid chromatographic analysis of bisphenol A and 4-nonylphenol in serum, liver and testis tissues after oral administration to rats and its application to toxicokinetic study. J. Chromatogr. B 2006, 830, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.F.; Frysali, M.A.; Papadoyannis, I.N. Matrix solid phase dispersion for the extraction of bisphenol A from human breast milk prior to HPLC analysis. J. Liquid Chromatogr. Relat. Technol. 2014, 37, 247–258. [Google Scholar] [CrossRef]
- Samanidou, V.F.; Kerezoudi, C.; Tolika, E.; Palaghias, G. A Simple Isocratic HPLC Method for the Simultaneous Determination of the Five Most Common Residual Monomers Released from Resin-Based Dental Restorative Materials. J. Liquid Chromatogr. Relat. Technol. 2015, 38, 740–749. [Google Scholar] [CrossRef]
- Samanidou, V.; Hadjicharalampous, M.; Palaghias, G.; Papadoyannis, I. Development and validation of an isocratic HPLC method for the simultaneous determination of residual monomers released from dental polymeric materials in artificial saliva. J. Liquid Chromatogr. Relat. Technol. 2012, 35, 511–523. [Google Scholar] [CrossRef]
- Asimakopoulos, A.G.; Thomaidis, N.S.; Koupparis, M.A. Recent trends in biomonitoring of bisphenol A, 4-t-octylphenol, and 4-nonylphenol. Toxicol. Lett. 2012, 210, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.W.; Ding, W.H.; Ku, H.Y.; Chao, H.R.; Chen, H.Y.; Huang, M.C.; Wang, S.L. Alkylphenols in human milk and their relations to dietary habits in central Taiwan. Food Chem. Toxicol. 2010, 48, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Yang, Y.; Wang, Q.; Li, H.P.; Luo, Z.F.; Qu, Z.P.; Yang, Z.G. Determination of 4-n-octylphenol, 4-n-nonylphenol and bisphenol A in fish samples from lake and rivers within Hunan Province China. Microchem. J. 2017, 132, 100–106. [Google Scholar] [CrossRef]
- Neamtu, M.; Frimmel, F.H. Degradation of endocrine disrupting bisphenol A by 254 nm irradiation in different water matrices and effect on yeast cells. Water Res. 2006, 40, 3745–3750. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Hosokawa, C.; Sasaki-Mori, M.; Akahira, A.; Fukunaga, K.; Ikeuchi, T.; Oshiman, K.; Tsuchido, T. Isolation and characterization of novel bisphenol-A-degrading bacteria from soils. Biocontrol Sci. 2009, 14, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Heredia, J.; Torregrosa, J.; Dominguez, J.R.; Peres, J.A. Comparison of the degradation of p-hydroxybenzoic acid in aqueous solution by several oxidation processes. Chemosphere 2001, 42, 351–359. [Google Scholar] [CrossRef]
- Abbas, S.S.; Elghobashy, M.R.; Bebawy, L.I.; Shokry, R.F. Stability-indicating chromatographic determination of hydroquinone in combination with tretinoin and fluocinolone acetonide in pharmaceutical formulations with a photodegradation kinetic study. RSC Adv. 2015, 5, 43178–43194. [Google Scholar] [CrossRef]
- Eio, E.J.; Kawai, M.; Tsuchiya, K.; Yamamoto, S.; Toda, T. Biodegradation of bisphenol A by bacterial consortia. Int. Biodeterior. Biodegrad. 2014, 96, 166–173. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).