Titania and Zinc Oxide Nanoparticles: Coating with Polydopamine and Encapsulation within Lecithin Liposomes—Water Treatment Analysis by Gel Filtration Chromatography with Fluorescence Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating of TiO2 or ZnO Nanoparticles with Polydopamine
2.3. Lecithin Liposomes Blank
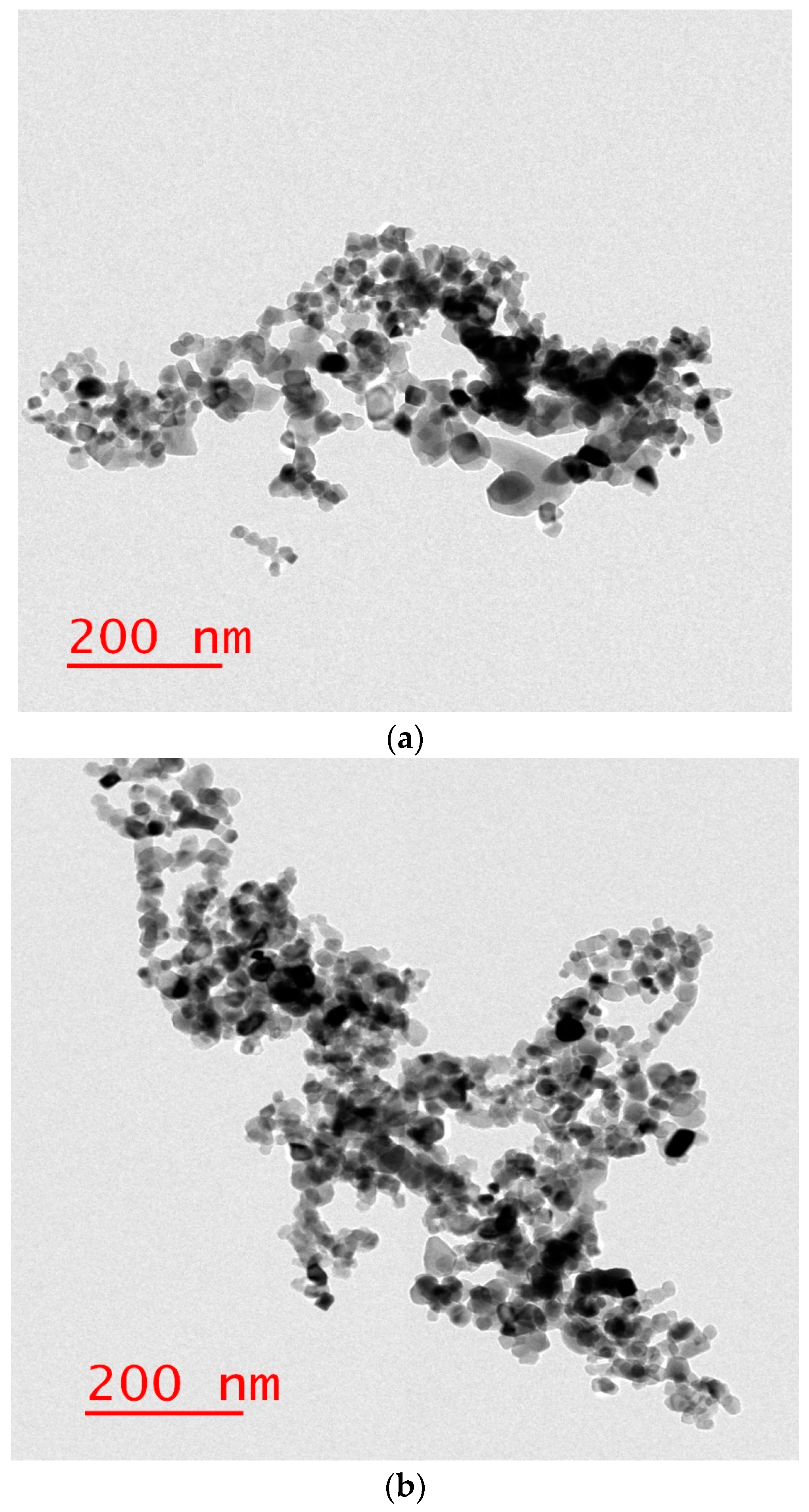
2.4. Lecithin Liposome-Encapsulated TiO2 or ZnO Nanoparticles
2.5. UV-Visible Absorption and Fluorescence Emission Spectroscopy
2.6. Gel Filtration Chromatography
3. Results and Discussion
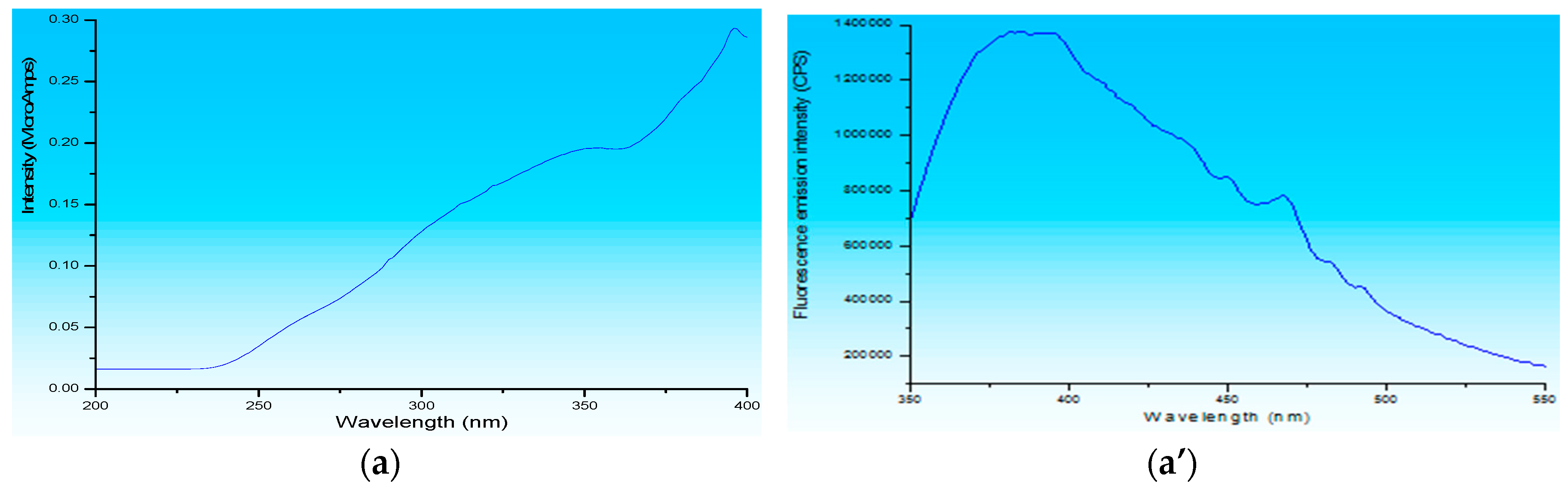
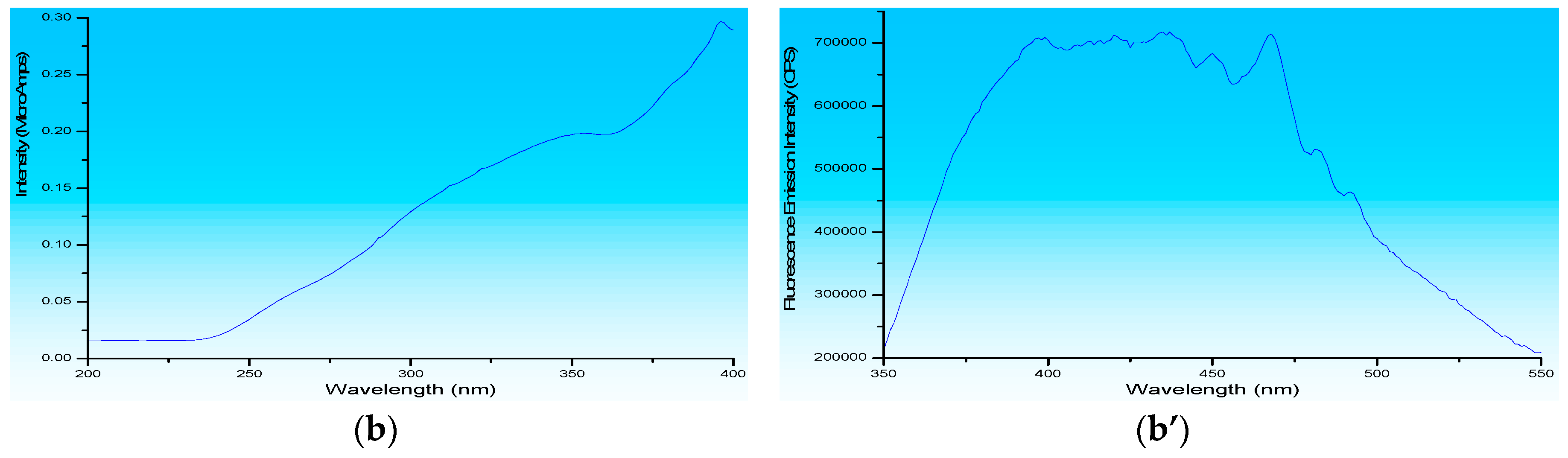
3.1. Fluorescence Spectroscopy
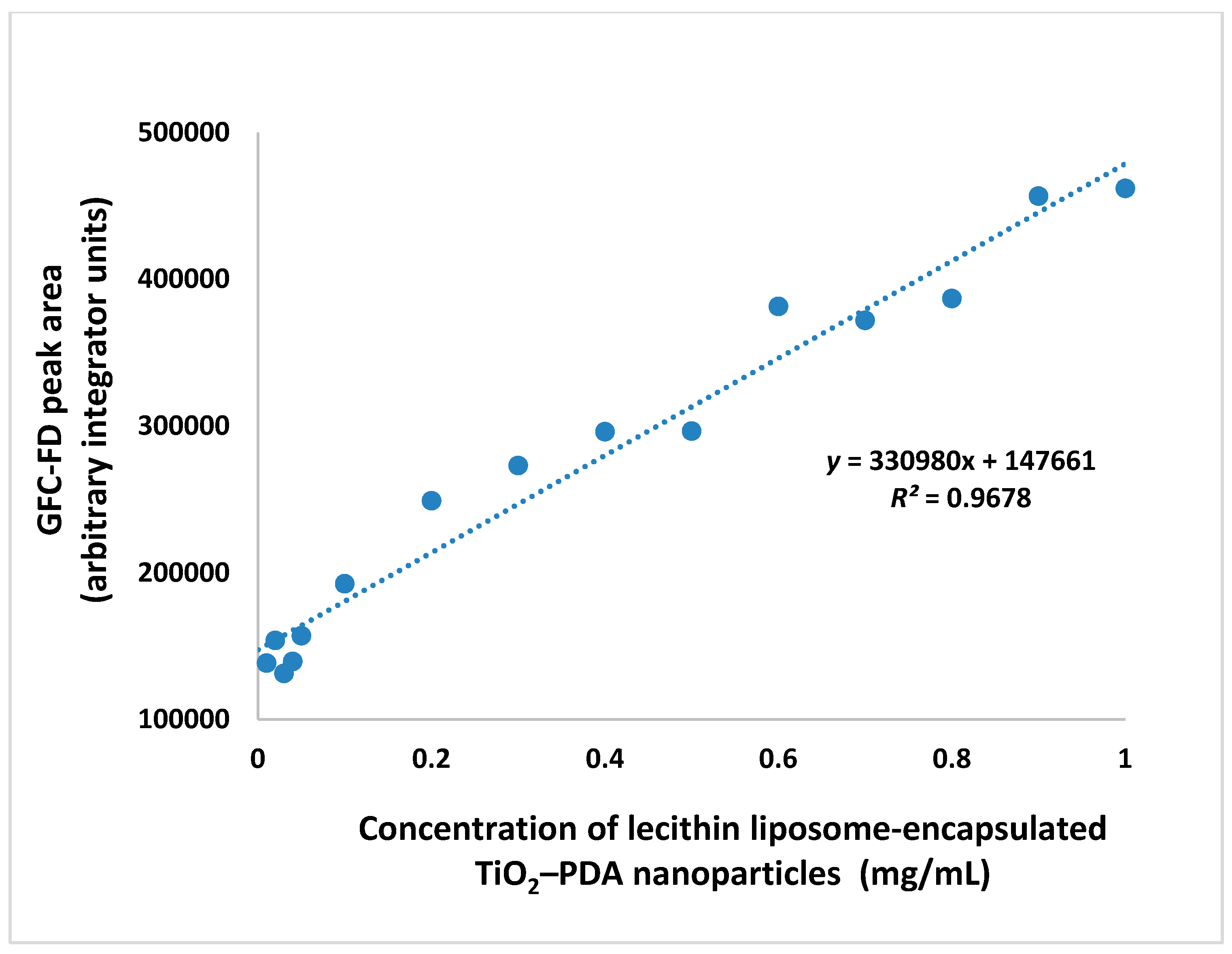
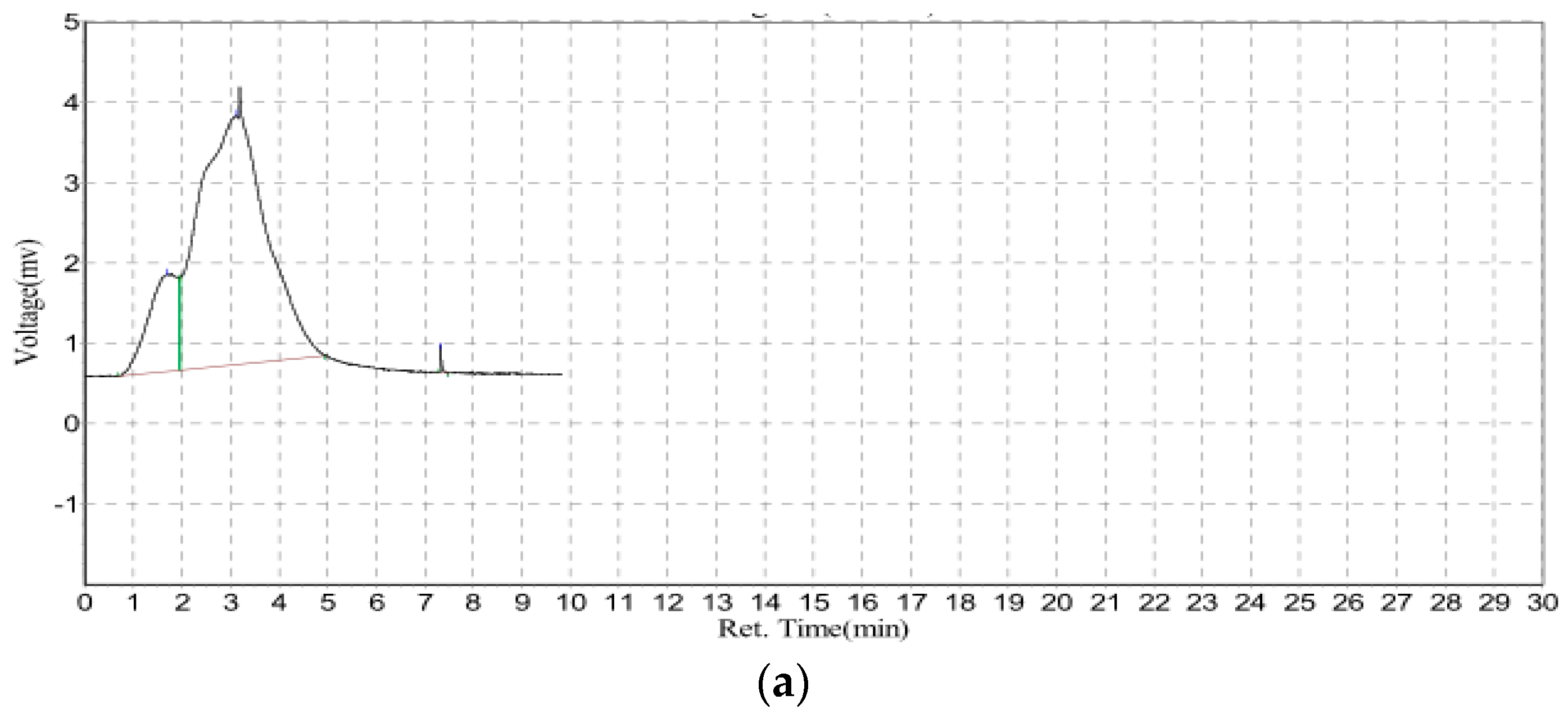
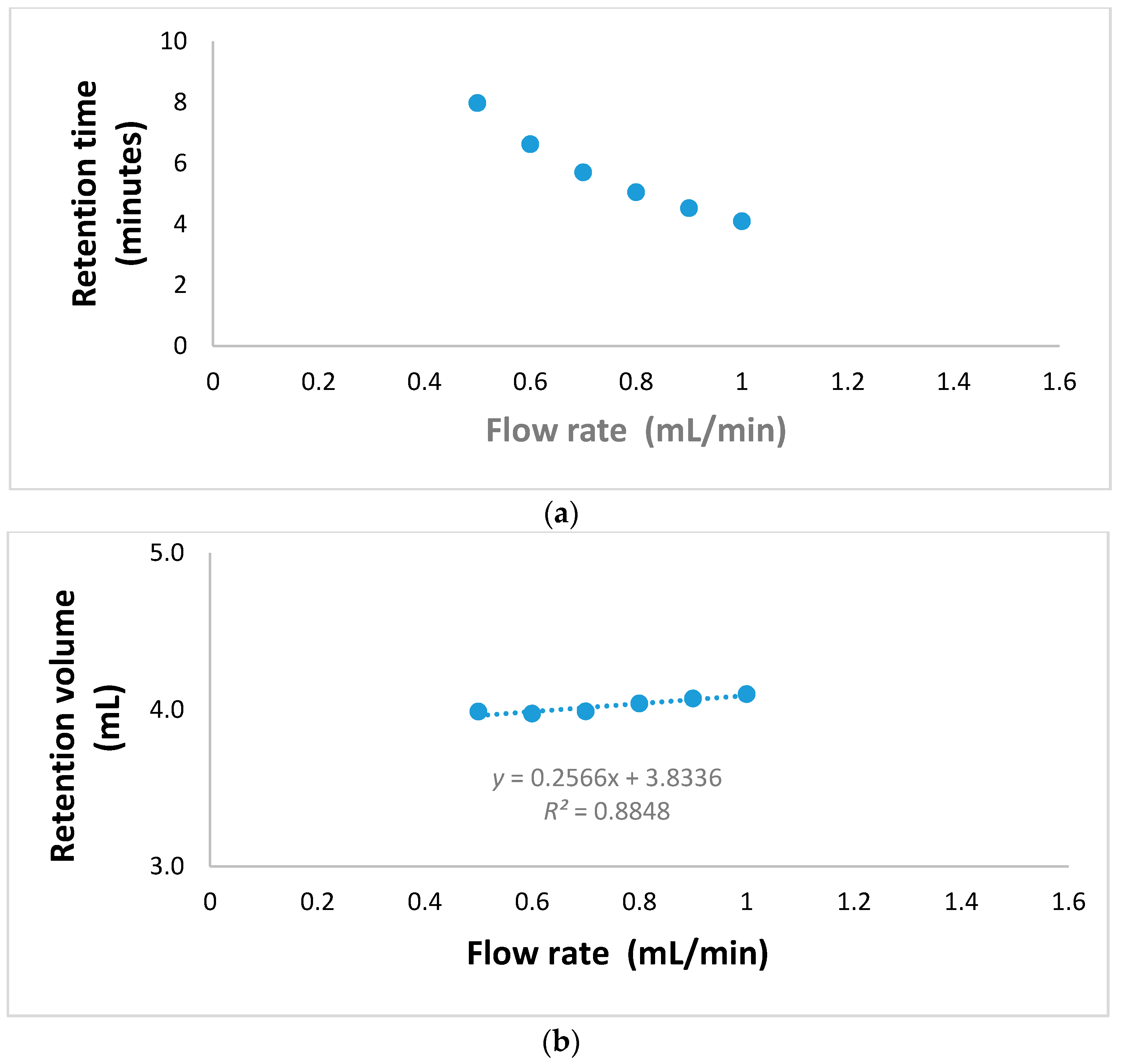
3.2. Rapid GFC-FD Analysis of Lecithin Liposome-Encapsulated TiO2–PDA Nanoparticles
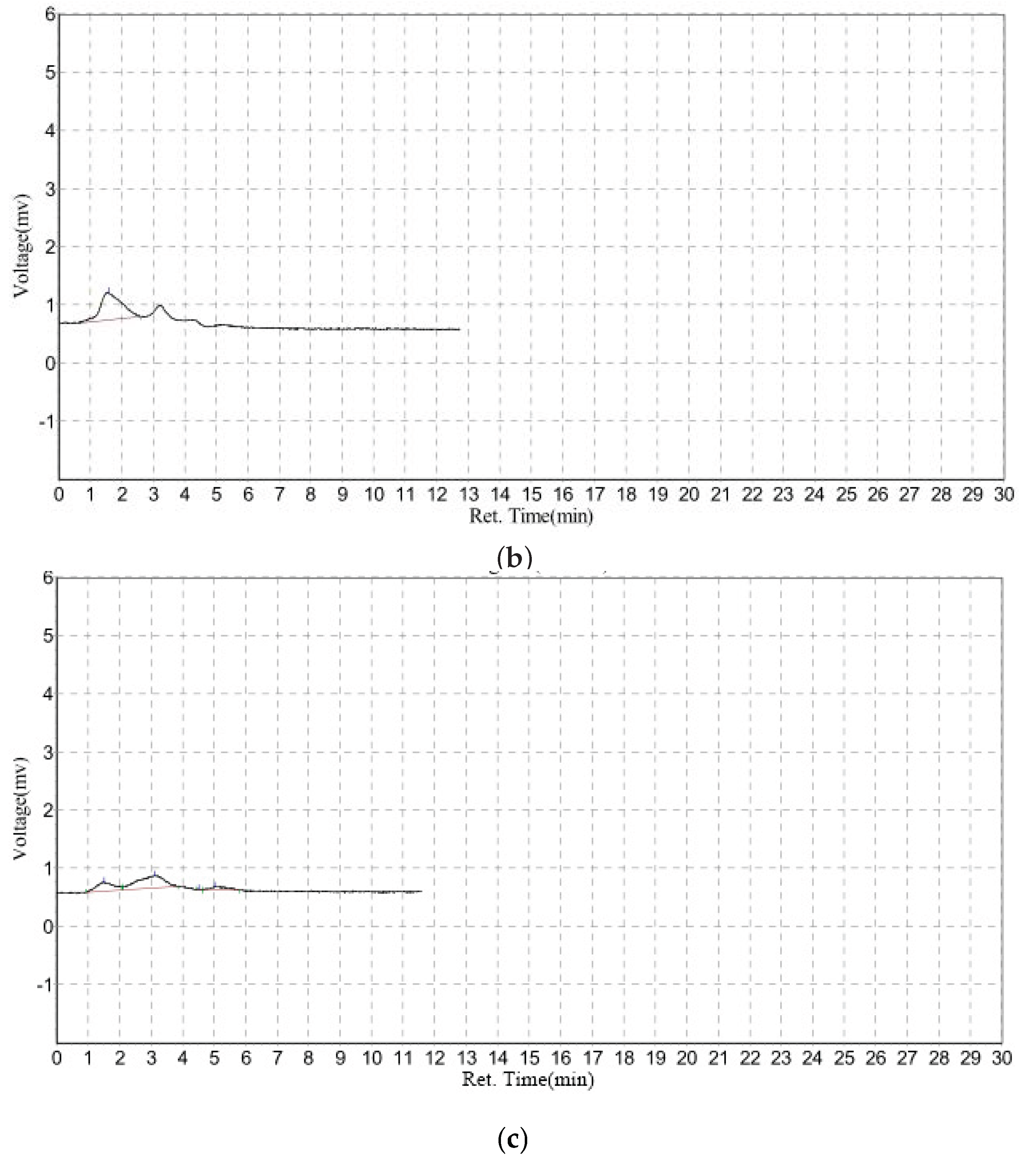
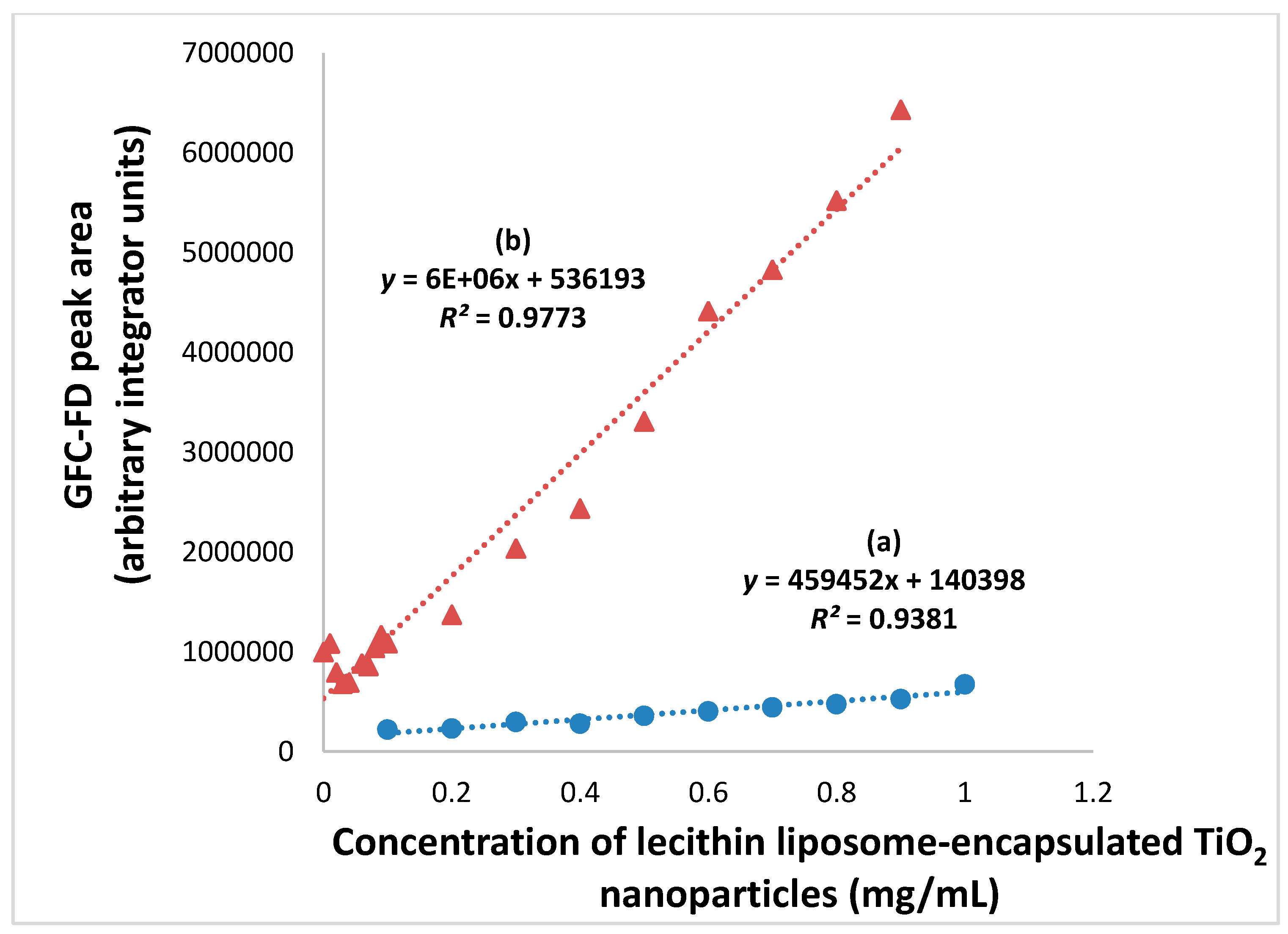
3.3. Rapid GFC-FD Analysis of Lecithin Liposome-Encapsulated TiO2 Nanoparticles
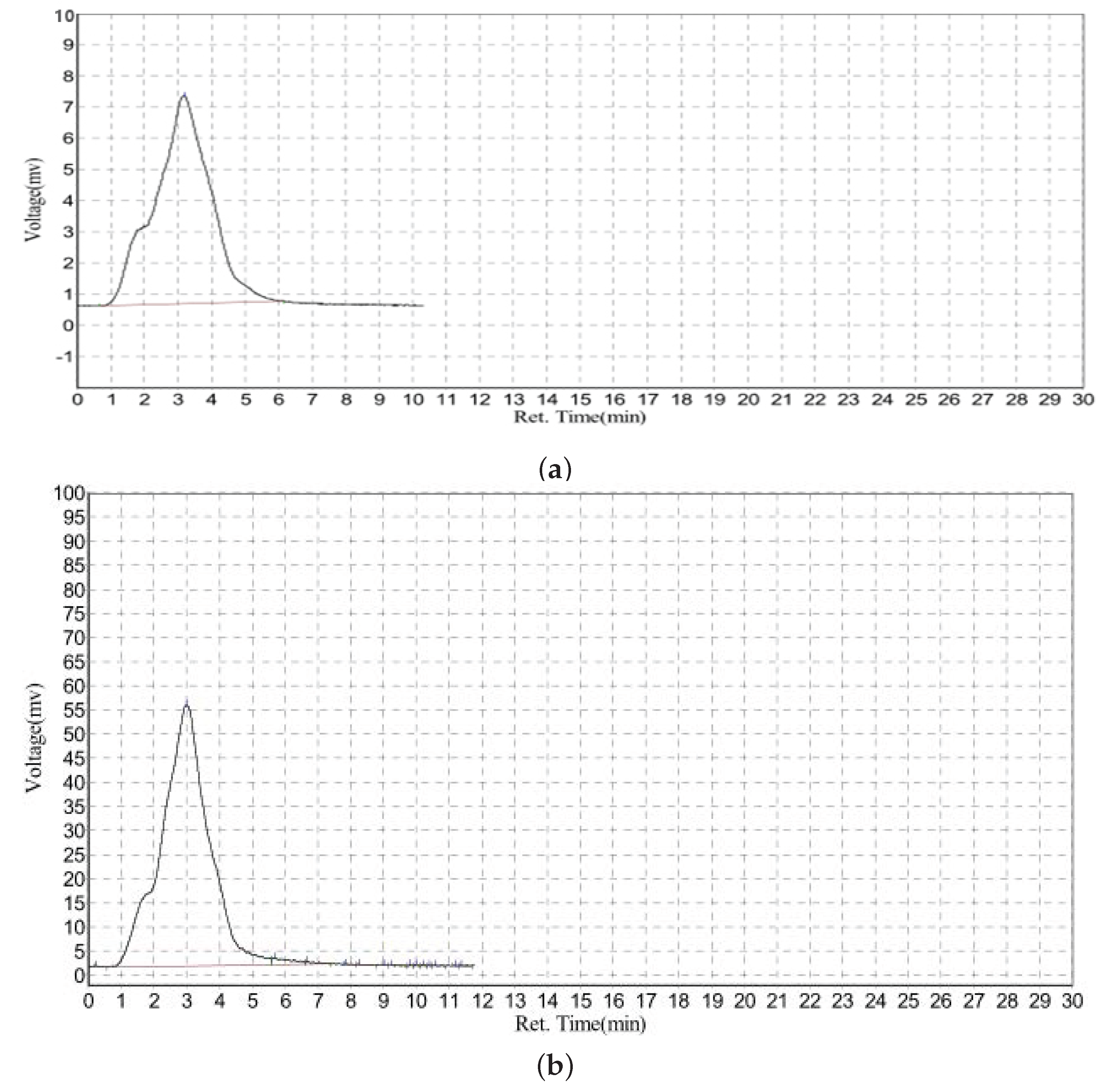
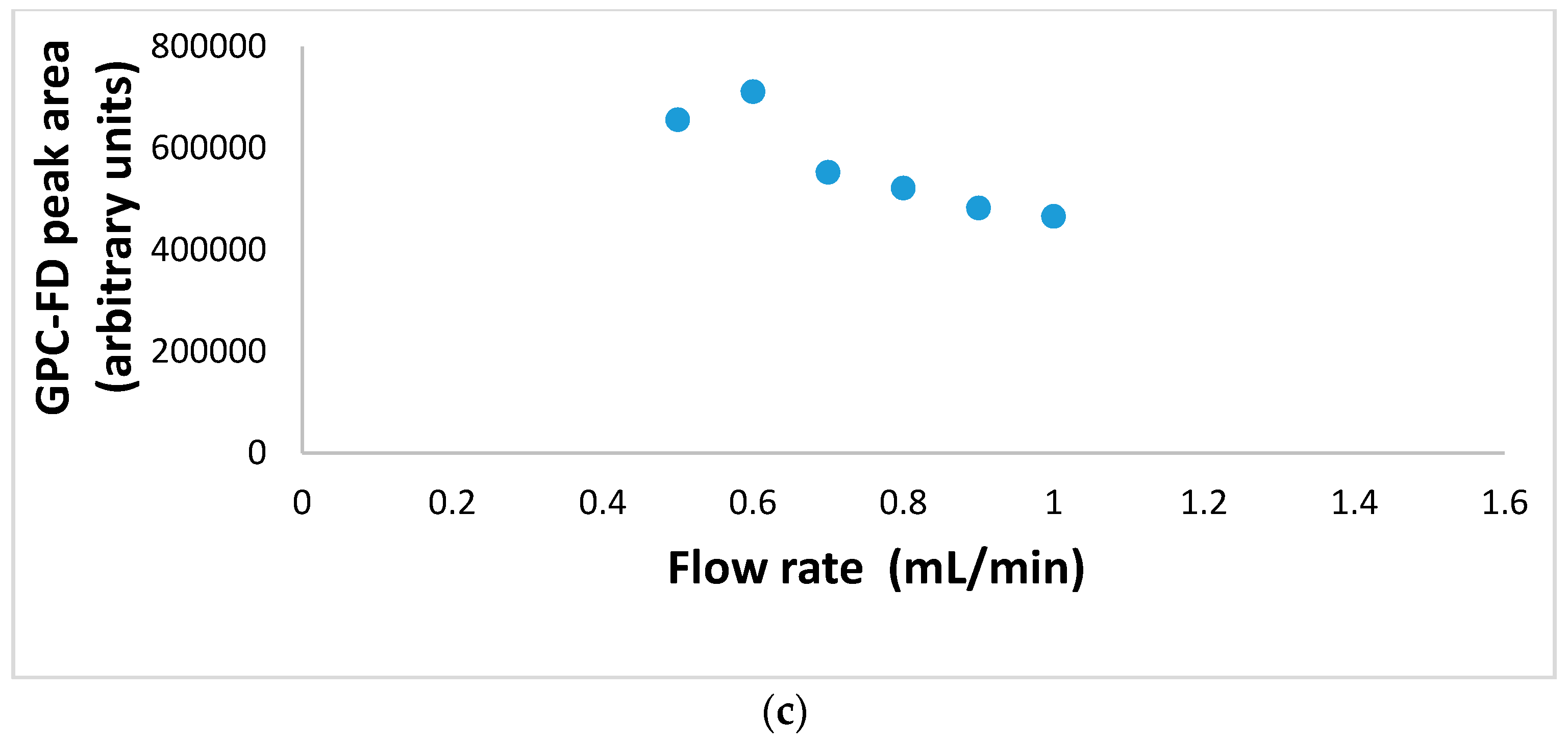
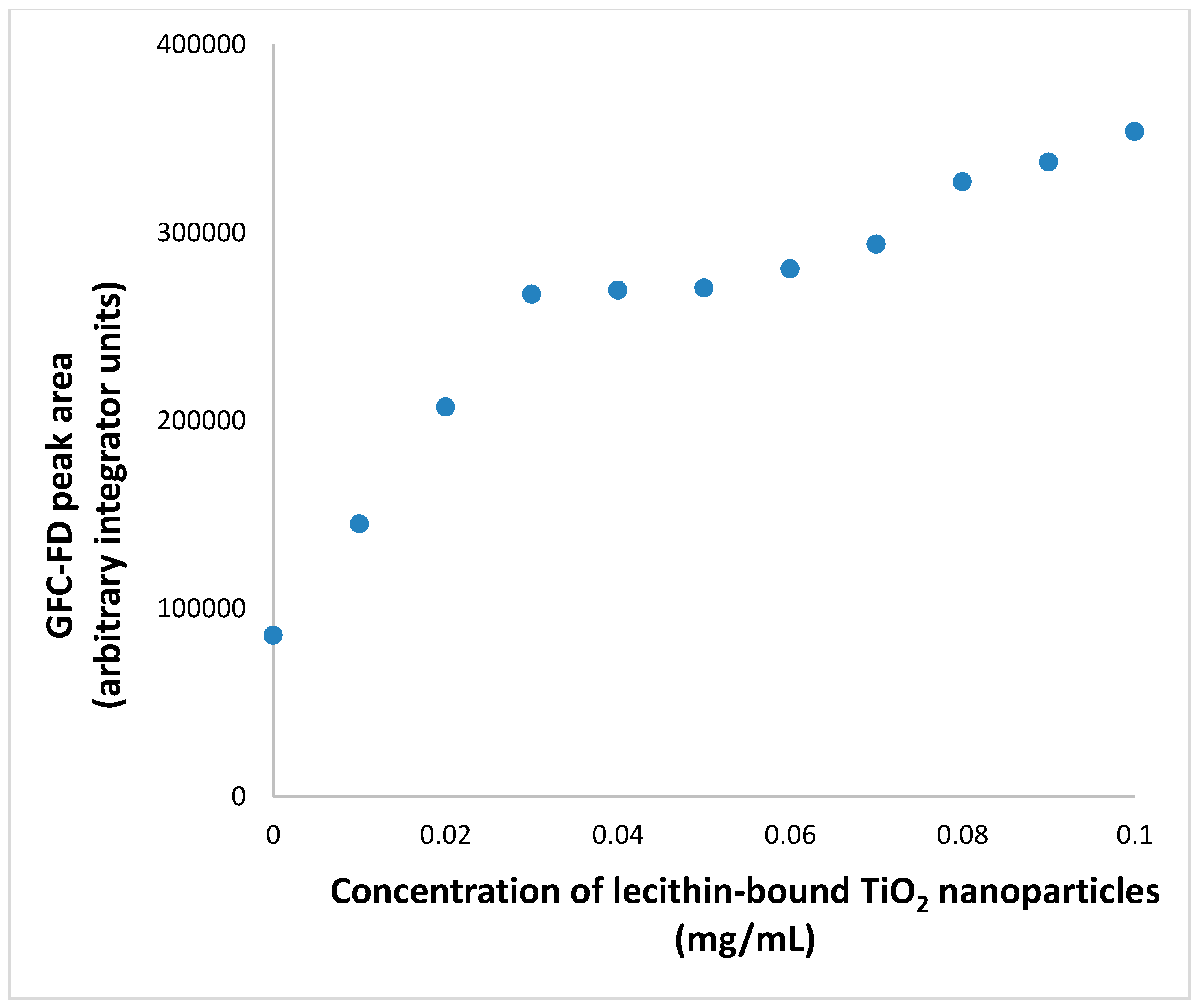
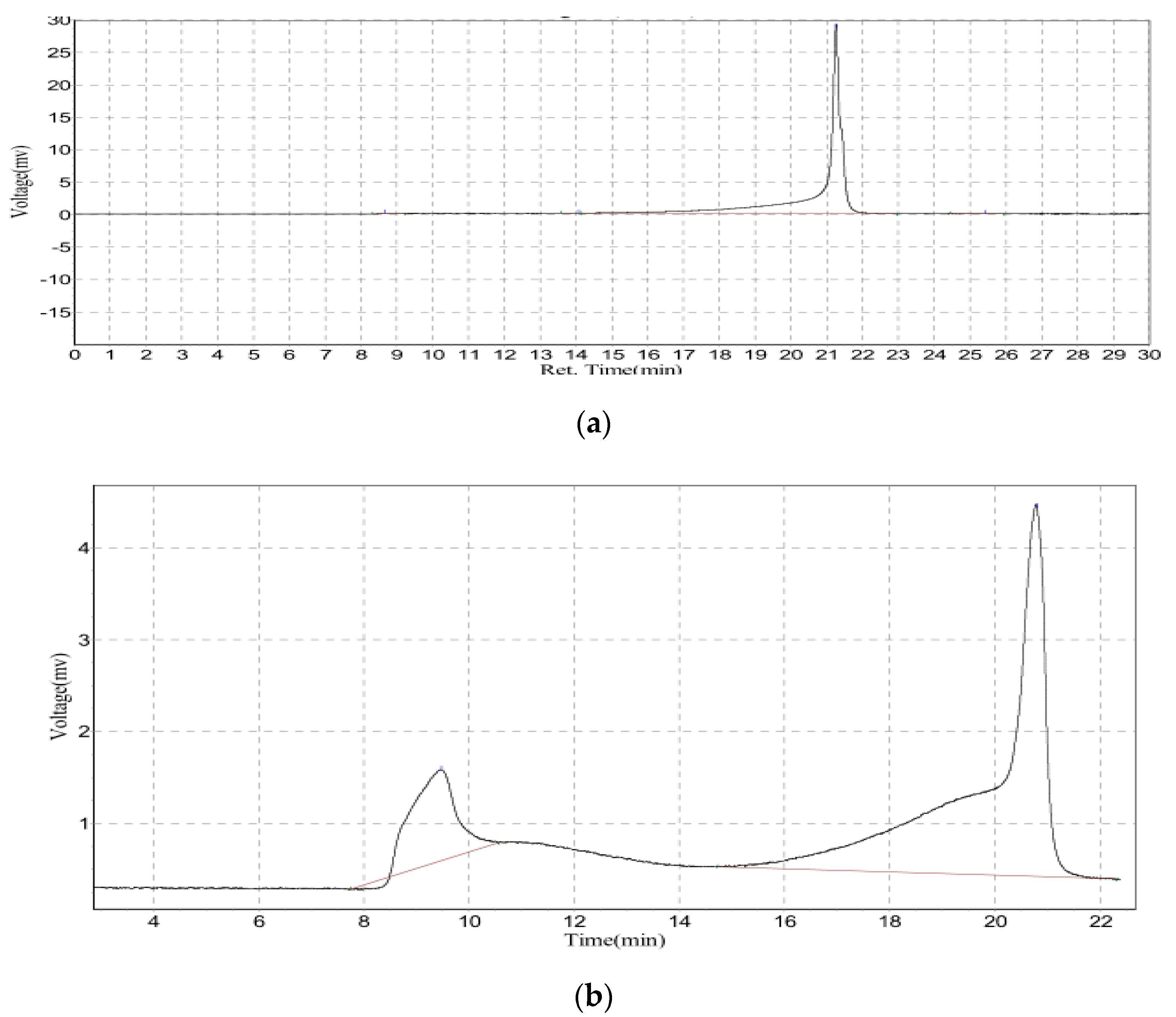
3.4. Extended GFC-FD Analysis of Lecithin Liposome-Encapsulated TiO2 Nanoparticles
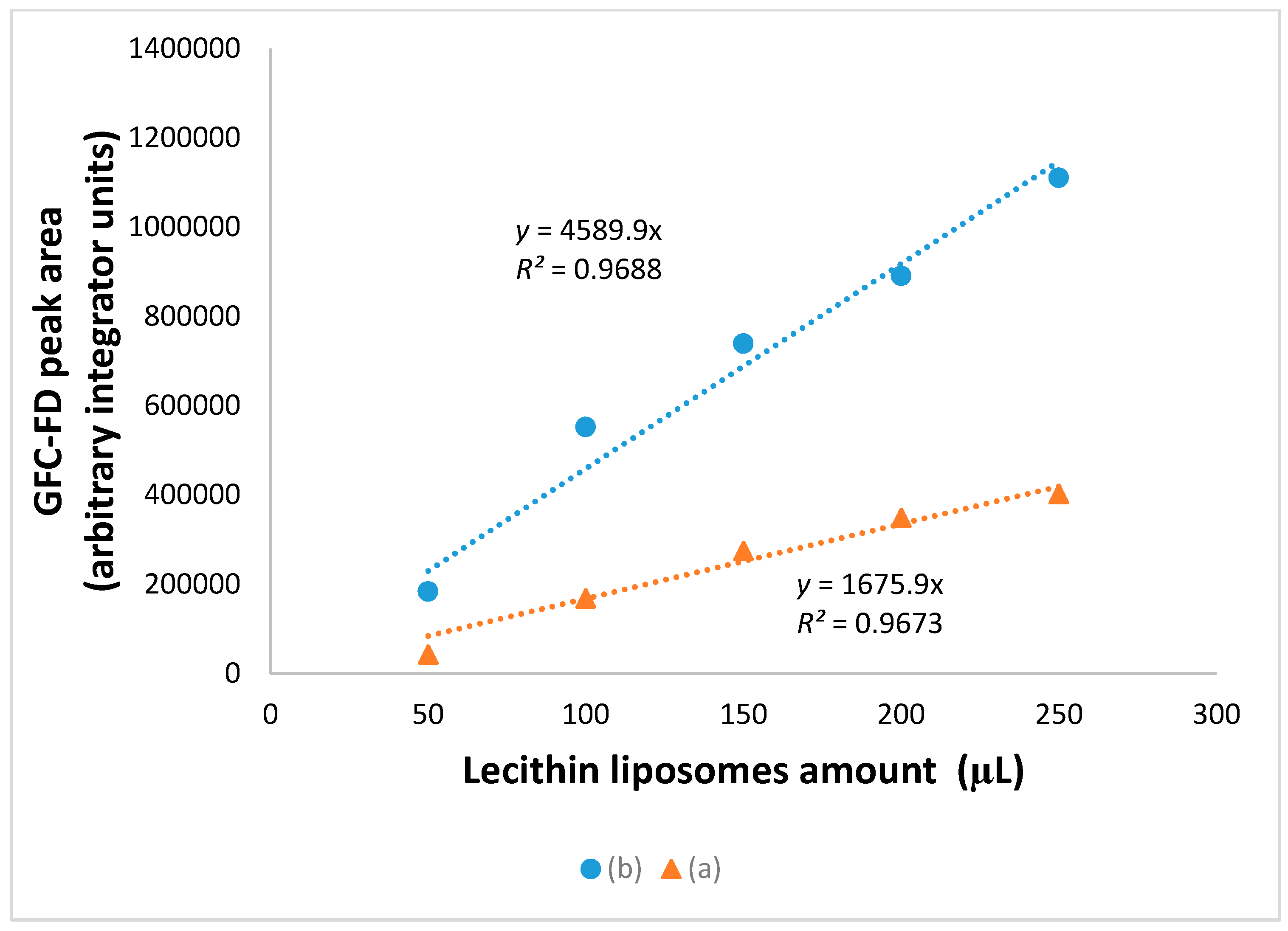
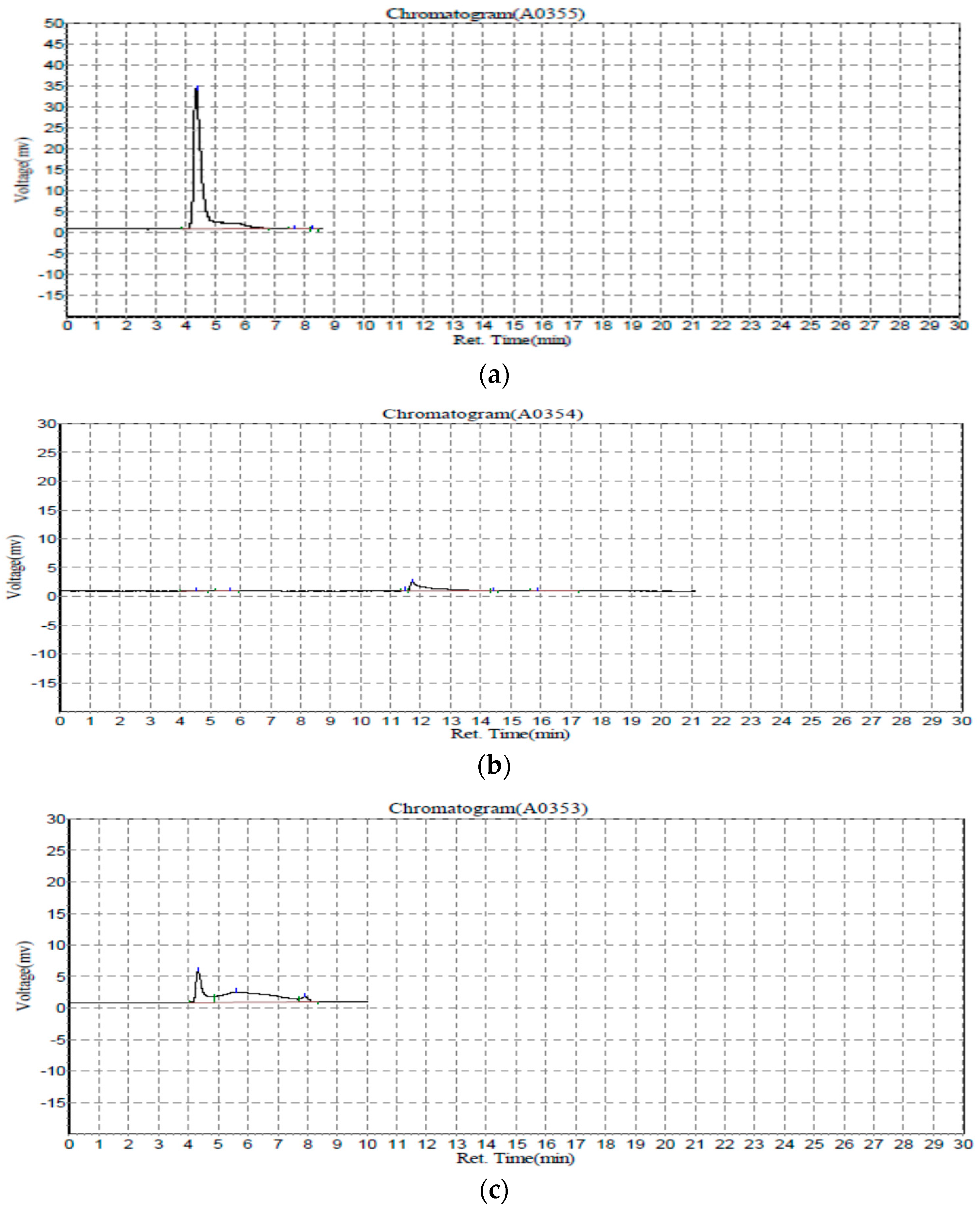
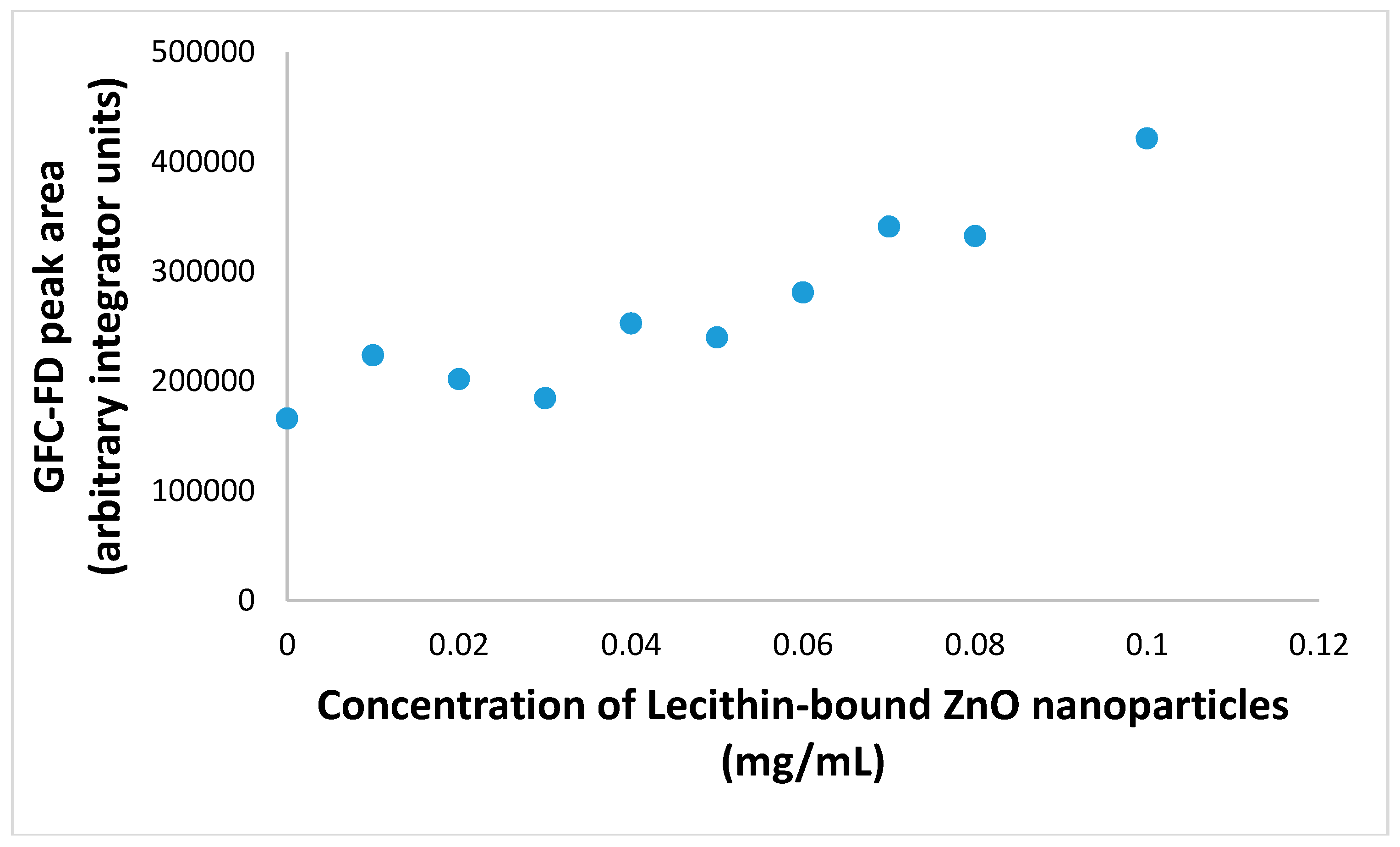
3.5. Extended GFC-FD Analysis of Lecithin Liposome-Encapsulated ZnO Nanoparticles
3.6. Extended GFC-FD Analysis of Lecithin Liposome-Encapsulated Graphene Quantum Dots
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
List of Symbols and Abbreviations
| CeO2 | ceria |
| FD | fluorescence detection |
| GFC | gel filtration chromatography |
| λem,max | maximum emission wavelength |
| λex,max | maximum excitation wavelength |
| PDA | polydopamine |
| TiO2 | titania |
| ZnO | zinc oxide |
References
- Regulatory Cooperation Council. Nanotechnology Initiative. Final Report. Work Element 2, Priority Setting: Development of a Joint Nanomaterials Classification Scheme. Regulatory Cooperation Council. Available online: http://nanoportal.gc.ca/666215CB-929F-4F75-B78C-4D9DD0F1093B/FINAL%20DRAFT%20RCC%20Nanotechnology%20Initiative%20Work%20Element%202.pdf (accessed on 4 February 2018).
- Long, T.C.; Saleh, N.; Tilton, R.D.; Lowry, G.V.; Veronesi, B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): Implications for nanoparticle neurotoxicity. Environ. Sci. Technol. 2006, 40, 4346–4352. [Google Scholar] [CrossRef] [PubMed]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009, 69, 8784–8789. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhang, Y.; Tang, M.; Wang, B. Health effects of exposure to nano-TiO2: A meta-analysis of experimental studies. Nanoscale Res. Lett. 2013. [Google Scholar] [CrossRef] [PubMed]
- Grande, F.; Tucci, P. Titanium dioxide nanoparticles: A risk for human health? Mini Rev. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Disdier, C. Evaluation of the Effects of TiO2 Nanoparticles Exposure on the Adult and Vulnerable Brains—Toxicology and Food Chain. Ph.D. Thesis, University of Paris-Saclay, Paris, France, 2016. Available online: https://tel.archives-ouvertes.fr/tel-01312182/document (accessed on 4 February 2018).
- Shah, S.N.A.; Shah, Z.; Hussain, M.; Khan, M. Hazardous effects of titanium dioxide nanoparticles in ecosystem. Bioinorg. Chem. Appl. 2017. [Google Scholar] [CrossRef] [PubMed]
- Barndõk, H.; Hermosilla, D.; Han, C.; Dionysiou, D.D.; Negro, C.; Blanco, Á. Degradation of 1,4-dioxane from industrial wastewater by solar photocatalysis using immobilized NF-TiO2 composite with monodisperse TiO2 nanoparticles. Appl. Catal. B Environ. 2016, 180, 44–52. [Google Scholar] [CrossRef]
- Wadhwa, U. Removal of Toxic Metal Ions from Industrial Waste Water by Photo Deposition on P-25 TiO2 Nanoparticles. Master’s Thesis, Thapar Univeristy, Patiala, India, 2016. Available online: http://dspace.thapar.edu:8080/jspui/handle/10266/4302 (accessed on 4 February 2018).
- Malakootian, M.; Ranandeh Kalankesh, L.; Loloi, M. Investigation of performance of hybrid nanoparticles TiO2/SiO2 in removing of mercury from industrial waste water. J. Toloo-E-Behdasht 2014, 13, 147–159. [Google Scholar]
- Kohri, M.; Yanagimoto, K.; Kawamura, A.; Hamada, K.; Imai, Y.; Watanabe, T.; Ono, T.; Taniguchi, T.; Kishikawa, K. Polydopamine-based 3D colloidal photonic materials: Structural color balls and fibers from melanin-like particles with polydopamine shell layers. ACS Appl. Mater. Interfaces 2017. [Google Scholar] [CrossRef] [PubMed]
- Nador, F.; Guisasola, E.; Baeza, A.; Villaecija, M.A.M.; Vallet-Regí, M.; Ruiz-Molina, D. Synthesis of polydopamine-like nanocapsules via removal of a sacrificial mesoporous silica template with water. Chem. Eur. J. 2017, 23, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, C.; Zhang, D.; Wang, Y.; Ren, X.; Ai, K.; Chen, X.; Lu, L. Targeted polydopamine nanoparticles enable photoacoustic imaging guided chemo-photothermal synergistic therapy of tumor. Acta Biomater. 2017, 47, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Huang, H.; Yang, W.; Wang, X.; Ren, C.; Hu, Q.; Li, Y.; Ren, B. Ultrathin polydopamine film coated gold nanoparticles: A sensitive, uniform, and stable SHINERS substrate for detection of benzotriazole. Analyst 2017, 142, 3459–3467. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Miao, L.; Yang, H.; Yu, J.; Xie, Y.; Xu, L.; Song, Y. A novel nanoenzyme based on Fe3O4 nanoparticles@thionine-imprinted polydopamine for electrochemical biosensing. Sens. Actuators B Chem. 2017, 253, 108–114. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Li, X.; Pan, S.; Zhang, X.; Sun, X.; Shen, J.; Han, W.; Wang, L. Internal pore decoration with polydopamine nanoparticle on polymeric ultrafiltration membrane for enhanced heavy metal removal. Chem. Eng. J. 2017, 314, 38–49. [Google Scholar] [CrossRef]
- Rao, Z.; Feng, K.; Tang, B.; Wu, P. Surface Decoration of Amino-Functionalized Metal-Organic Framework/Graphene Oxide Composite onto Polydopamine-Coated Membrane Substrate for Highly Efficient Heavy Metal Removal. ACS Appl. Mater. Interfaces 2017, 9, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, L.; Lu, X.; Lee, P.S. Method of Preparing Hollow Metal or Metal Oxide Nano- or Micro-Spheres. U.S. Patents 9,555,557 B2, 31 January 2017. [Google Scholar]
- Wang, K.; Lai, E.P.C. Fluorescence quenching of graphene quantum dots by titania nanoparticles in water analysis after coating with polydopamine. J. Mol. Nanotechnol. Nanomed. 2017, 1, 106–114. [Google Scholar]
- Li, X.; John, V.T.; He, G.; Zhan, J.; Tan, G.; McPherson, G.; Bose, A.; Sarkar, J. Shear induced formation of patterned porous titania with applications to photocatalysis. Langmuir 2009, 25, 7586–7593. [Google Scholar] [CrossRef] [PubMed]
- Shchipunov, Y.; Krekoten, A. Crystalline titania nanoparticles synthesized in nonpolar Lα lecithin liquid-crystalline media in one stage at ambient conditions. Colloids Surf. B Biointerfaces 2011, 87, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Taladrid, D.; Marín, D.; Alemán, A.; Álvarez-Acero, I.; Montero, P.; Gómez-Guillén, M.C. Effect of Chemical Composition and Sonication Procedure on Properties of Food-Grade Soy Lecithin Liposomes with Added Glycerol. Food Res. Int. 2017, 100, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Zabodalova, L.A.; Chernjavskij, V.A.; Ishchenko, T.N.; Skvortcova, N.N. Production of liposomes from soybean lecithin. Sci. J. NRU ITMO 2011, 2, UDC 665.372+544.77. [Google Scholar]
- Mohan, A.; Rajendran, S.R.C.K.; Thibodeau, J.; Bazinet, L.; Udenigwe, C.C. Liposome Encapsulation of Anionic and Cationic Whey Peptides: Influence of Peptide Net Charge on Nanoliposome Formation and Properties. LWT Food Sci. Technol. 2018, 87, 40–46. [Google Scholar] [CrossRef]
- Jin, H.H.; Lu, Q.; Jiang, J.G. Curcumin liposomes prepared with milk fat globule membrane phospholipids and soybean lecithin. J. Dairy Sci. 2016, 99, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent advances on liposomal nanoparticles: Synthesis, characterization and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Guner, S.; Oztop, M.H. Food grade liposome systems: Effect of solvent, homogenization types and storage conditions on oxidative and physical stability. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 468–478. [Google Scholar] [CrossRef]
- Sahu, A.K.; Jain, V. Screening of process variables using Plackett–Burman design in the fabrication of gedunin-loaded liposomes. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Nguyen, T.H.; Nguyen, D.H. Development and in vitro evaluation of liposomes using soy lecithin to encapsulate paclitaxel. Int. J. Biomater. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Nkanga, C.I.; Krause, R.W.; Noundou, X.S.; Walker, R.B. Preparation and characterization of isoniazid-loaded crude soybean lecithin liposomes. Int. J. Pharm. 2017, 526, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Naeem, S.; Kiew, L.V.; Chung, L.Y.; Fui, K.S.; Misran, M.B. A comparative approach for the preparation and physicochemical characterization of lecithin liposomes using chloroform and non-halogenated solvents. J. Surfactants Deterg. 2015, 18, 579–587. [Google Scholar] [CrossRef]
- Roberts, S.A.; Parikh, N.; Blower, R.J.; Agrawal, N. SPIN: Rapid synthesis, purification, and concentration of small drug-loaded liposomes. J. Liposome Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Boxi, A.; Ashe, S.; Thathapudi, N.C.; Nayak, B. Stavudine loaded gelatin liposomes for HIV therapy: Preparation, characterization and in vitro cytotoxic evaluation. Mater. Sci. Eng. C 2017, 73, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi, M.; Negahdari, C.B.; Daraee, H.; Eatemadi, A. Heart targeted nanoliposomal/nanoparticles drug delivery: An updated review. Biomed. Pharmacother. 2017, 86, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, A.L.; Barenholz, Y.; Bercovier, H. Nano-liposomes of crude soy lecithin are effective for cleaning fuel-contaminated sands and soils. Expert Opin. Environ. Biol. 2016, 5. [Google Scholar] [CrossRef]
- Simões, M.G.; Alves, P.; Carvalheiro, M.; Simões, P.N. Stability effect of cholesterol-poly(acrylic acid) in a stimuli-responsive polymer-liposome complex obtained from soybean lecithin for controlled drug delivery. Colloids Surf. B Biointerfaces 2017, 152, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Guo, B.; Yu, S.; Zhang, W.; Zhang, D.; Wang, J.; Wang, Y. Encapsulation of the flavonoid quercetin with chitosan-coated nano-liposomes. LWT Food Sci. Technol. 2017, 85A, 37–44. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J.; Shan, G.; Yan, D.; Chen, Y.; Liu, Y. SERS-active liposome@Ag/Au nanocomposite for NIR light-driven drug release. Colloids Surf. B Biointerfaces 2017, 154, 150–159. [Google Scholar] [CrossRef] [PubMed]
- O’Fagain, C.; Cummins, P.M.; O’Connor, B.F. Protein Chromatography; Methods in Molecular Biology Book Series; Humana Press: New York, NY, USA, 2017; Volume 681, pp. 25–33. [Google Scholar]
- Hagel, L. Gel-Filtration Chromatography. Curr. Protoc. Mol. Biol. 2001. [Google Scholar] [CrossRef]
- Fedotov, P.S.; Vanifatova, N.G.; Shkinev, V.M.; Spivakov, B.Y. Fractionation and characterization of nano- and micro-particles in liquid media. Anal. Bioanal. Chem. 2011, 400, 1787–1804. [Google Scholar] [CrossRef] [PubMed]
- Malvern Instruments Limited. Size Exclusion Chromatography/Gel Permeation Chromatography: An Introduction in 30 Minutes; Malvern Instruments Limited: Malvern, UK, 2015; Available online: https://cdn.technologynetworks.com/TN/Resources/PDF/WP150625IntroToSECGPC.pdf (accessed on 4 February 2018).
- Preecha, P.; Jettanasen, J. Investigation of functionalized silicon nanoparticles by size exclusion chromatography. Mater. Res. Express 2017, 4, 045007. [Google Scholar] [CrossRef]
- Pitkänen, L.; Bustos, A.R.M.; Murphy, K.E.; Winchester, M.R.; Striegel, A.M. Quantitative characterization of gold nanoparticles by size-exclusion and hydrodynamic chromatography, coupled to inductively coupled plasma mass spectrometry and quasi-elastic light scattering. J. Chromatogr. A 2017, 59–67. [Google Scholar] [CrossRef] [PubMed]
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P.C. Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ. Sci. Technol. 2009, 43, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Ikeguchi, Y.; Nakamura, H. Selective enrichment of phospholipids by Titania. Anal. Sci. 2000, 16, 541–543. [Google Scholar] [CrossRef]
- Chibowski, E.; Holysz, L.; Terpilowski, K. Influence of ionic surfactants and lecithin on stability of titanium dioxide in aqueous electrolyte solution. Croat. Chem. Acta 2007, 80, 395–403. [Google Scholar]
- Byung, G.; Jong, H.; Jin, H.; Kyoung, C. Inorganic-organic nano-hybrid: Preparation of nano-sized TiO2 paste trapped OMC nano-emulsion and its application for cosmetics. J. Soc. Cosmet. Sci. Korea 2004, 30, 181–187. [Google Scholar]
- Logeta, G.; Yoo, J.E.; Mazare, A.; Wang, L.; Schmuki, P. Highly controlled coating of biomimetic polydopamine in TiO2 nanotubes. Electrochem. Commun. 2015, 52, 41–44. [Google Scholar] [CrossRef]
- Mao, W.X.; Lin, X.J.; Zhang, W.; Chi, Z.X.; Lyu, R.W.; Cao, A.M.; Wan, L.J. Core–shell structured TiO2@polydopamine for highly active visible-light photocatalysis. Chem. Commun. 2016, 52, 7122–7125. [Google Scholar] [CrossRef] [PubMed]
- Krüger, S.; Bürmann, L.; Morlock, G.E. Comparison and characterization of soybean and sunflower lecithins used for chocolate production by high-performance thin-layer chromatography with fluorescence detection and electrospray mass spectrometry. J. Agric. Food Chem. 2015. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, S.; Rhodes, C.T. Separation of liposomes by a gel filtration chromatographic technique: A preliminary evaluation. Pharm. Acta Helv. 1994, 69, 107–113. [Google Scholar] [CrossRef]
- Hara, I.; Kaneko, H.; Kato, A. The critical micelle concentration of phospholipids—Lecithin and lysolecithin. J. Jpn. Oil Chem. Soc. 1960, 9, 407–409. [Google Scholar] [CrossRef]
- Parham, H.; Zargar, B.; Rezazadeh, M. Removal, preconcentration and spectrophotometric determination of picric acid in water samples using modified magnetic iron oxide nanoparticles as an efficient adsorbent. Mater. Sci. Eng. C 2012, 32, 2109–2114. [Google Scholar] [CrossRef]
- Li, L.; Braiteh, F.S.; Kurzrock, R. Liposome-encapsulated curcumin: In vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer 2005, 104, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Xu, C.; Fletcher, P.D.I.; Paunov, V.N. A novel technique for preparation of monodisperse giant liposomes. Chem. Commun. 2003, 14, 1732–1733. [Google Scholar] [CrossRef]
- Duke, M.; Zhao, D.; Semiat, R. (Eds.) Functional Nanostructured Materials and Membranes for Water Treatment; Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Saleh, T.A.; Gupta, V.K. (Eds.) Nanomaterial and Polymer Membranes: Synthesis, Characterization, and Applications; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Grumezescu, A.M. (Ed.) Water Purification; Academic Press: London, UK, 2017. [Google Scholar]
- Zhou, S.; Xu, H.; Gan, W.; Yuan, Q. Graphene quantum dots: Recent progress in preparation and fluorescence sensing applications. RSC Adv. 2016, 6, 110775–110788. [Google Scholar] [CrossRef]
- Lai, E.P.C. Carbon, graphene and graphene oxide quantum dots for analytical biochemistry research. Biochem. Anal. Biochem. 2016, 5. [Google Scholar] [CrossRef]
- Lai, E.P.C. Graphene quantum dots for bioanalytical sensors and biochemical imaging applications. J. Res. Anal. 2017, 3, 84–90. [Google Scholar]
- Chen, C.S.; Yao, J.; Durst, R.A. Liposome encapsulation of fluorescent nanoparticles: Quantum dots and silica nanoparticles. J. Nanopart. Res. 2006, 8, 1033–1038. [Google Scholar] [CrossRef]
- Bennett, A. Membrane Technology: Developments in Ultrafiltration Technologies. Filtr. Sep. 2012, 49, 28–33. [Google Scholar] [CrossRef]
- Lohwacharin, J.; Takizawa, S. Effects of nanoparticles on the ultrafiltration of surface water. J. Membr. Sci. 2009, 326, 354–362. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Lai, E.P.C. Titania and Zinc Oxide Nanoparticles: Coating with Polydopamine and Encapsulation within Lecithin Liposomes—Water Treatment Analysis by Gel Filtration Chromatography with Fluorescence Detection. Separations 2018, 5, 13. https://doi.org/10.3390/separations5010013
Zhao X, Lai EPC. Titania and Zinc Oxide Nanoparticles: Coating with Polydopamine and Encapsulation within Lecithin Liposomes—Water Treatment Analysis by Gel Filtration Chromatography with Fluorescence Detection. Separations. 2018; 5(1):13. https://doi.org/10.3390/separations5010013
Chicago/Turabian StyleZhao, Xuhao, and Edward P. C. Lai. 2018. "Titania and Zinc Oxide Nanoparticles: Coating with Polydopamine and Encapsulation within Lecithin Liposomes—Water Treatment Analysis by Gel Filtration Chromatography with Fluorescence Detection" Separations 5, no. 1: 13. https://doi.org/10.3390/separations5010013
APA StyleZhao, X., & Lai, E. P. C. (2018). Titania and Zinc Oxide Nanoparticles: Coating with Polydopamine and Encapsulation within Lecithin Liposomes—Water Treatment Analysis by Gel Filtration Chromatography with Fluorescence Detection. Separations, 5(1), 13. https://doi.org/10.3390/separations5010013




