Inverse Punicines: Isomers of Punicine and Their Application in LiAlO2, Melilite and CaSiO3 Separation
Abstract
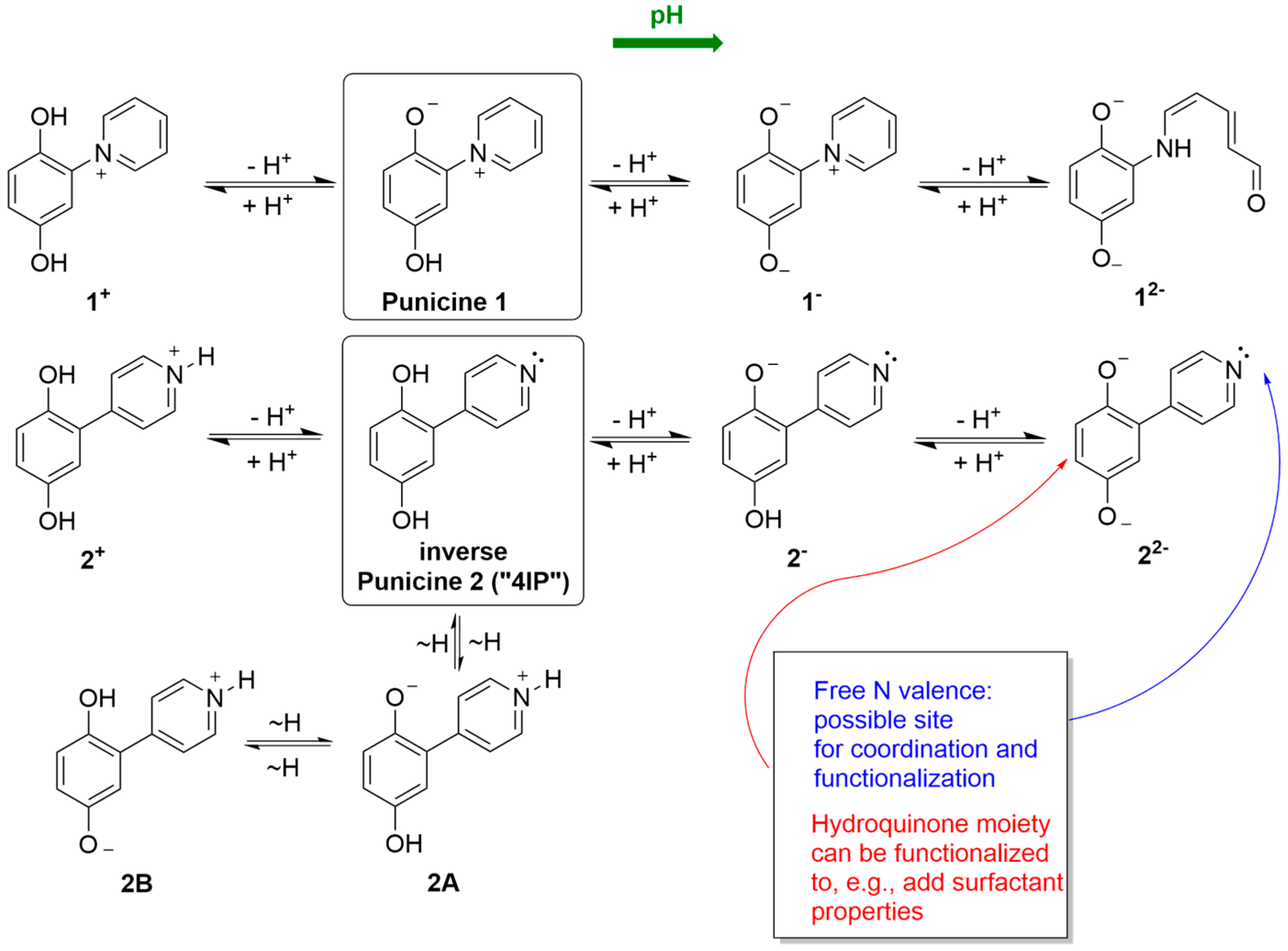
1. Introduction
2. Materials and Methods
2.1. General
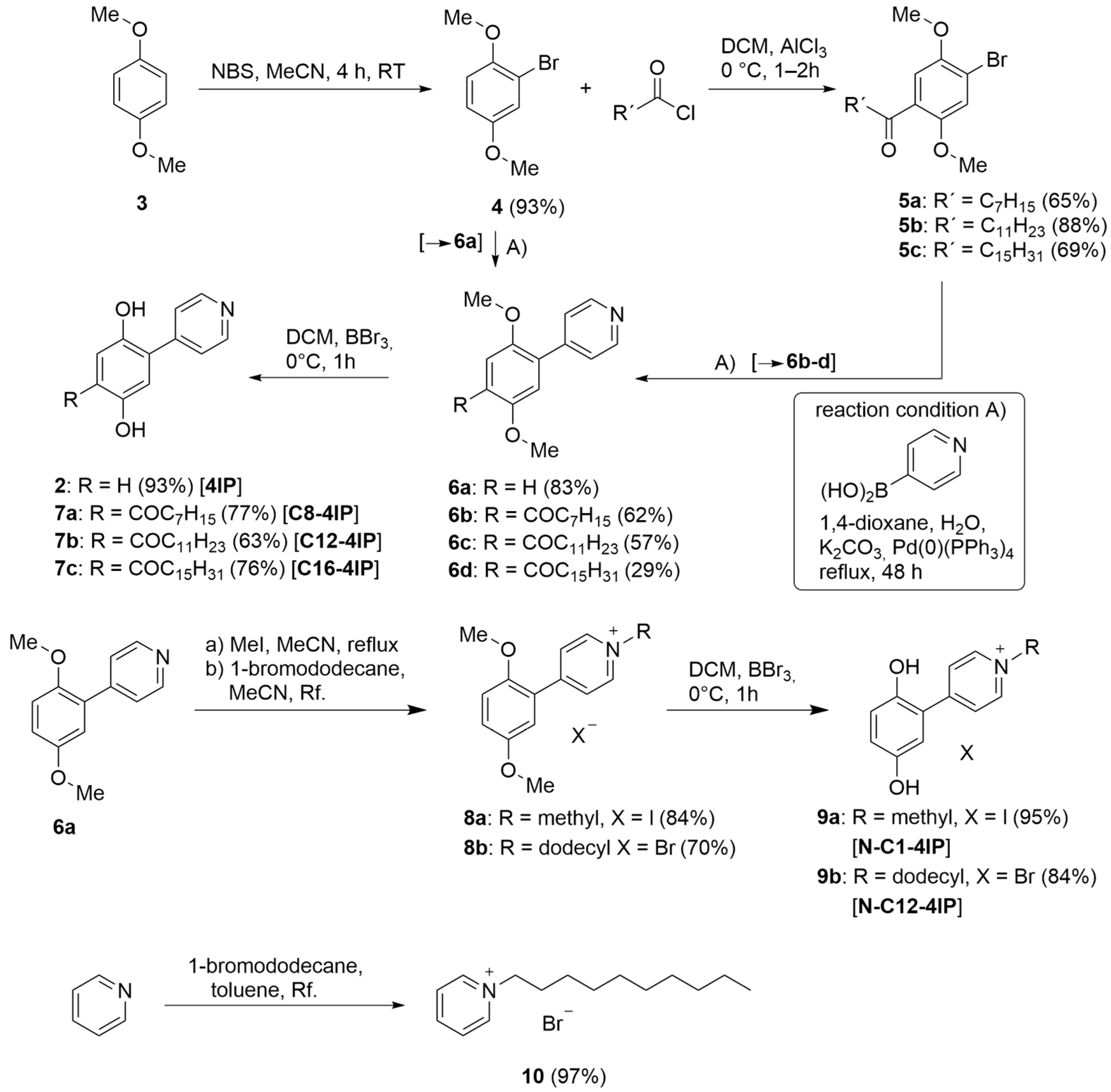
2.2. Syntheses
2.3. Contact Angle Measurements: Procedures
2.4. Froth Flotation
2.5. Particle Size Measurements
2.6. X-Ray Diffraction Analysis (XRD)
2.7. Electron Spin Resonance Spectroscopy (ESR): Procedures
2.8. Zeta Potential Measurements
3. Results
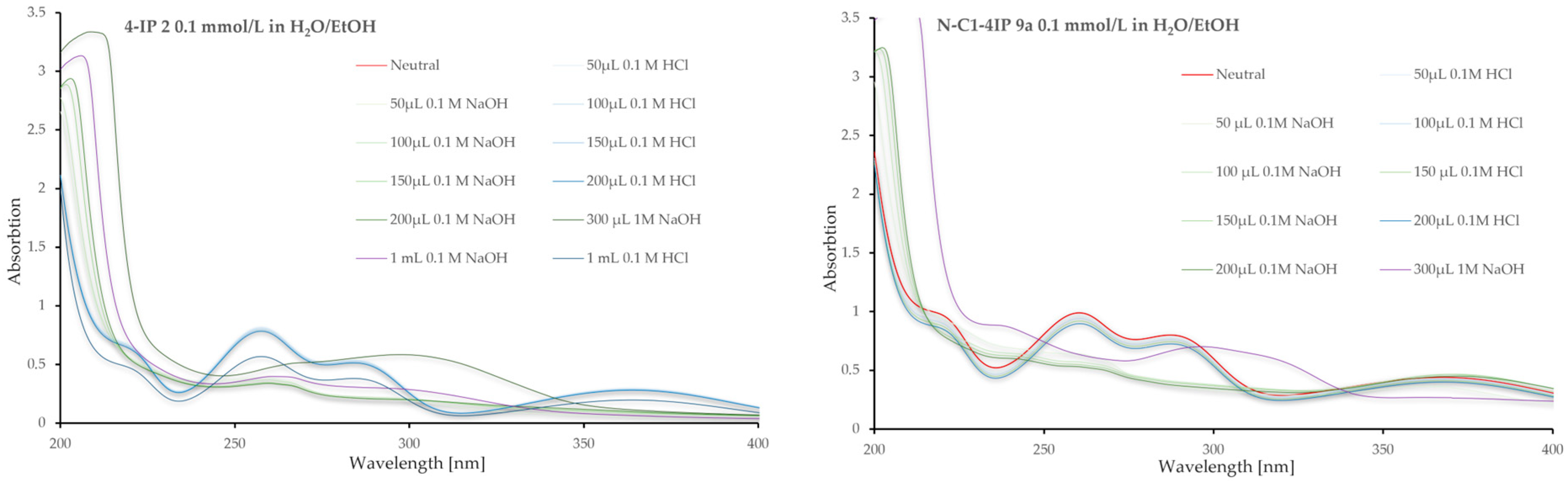
3.1. UV-Vis
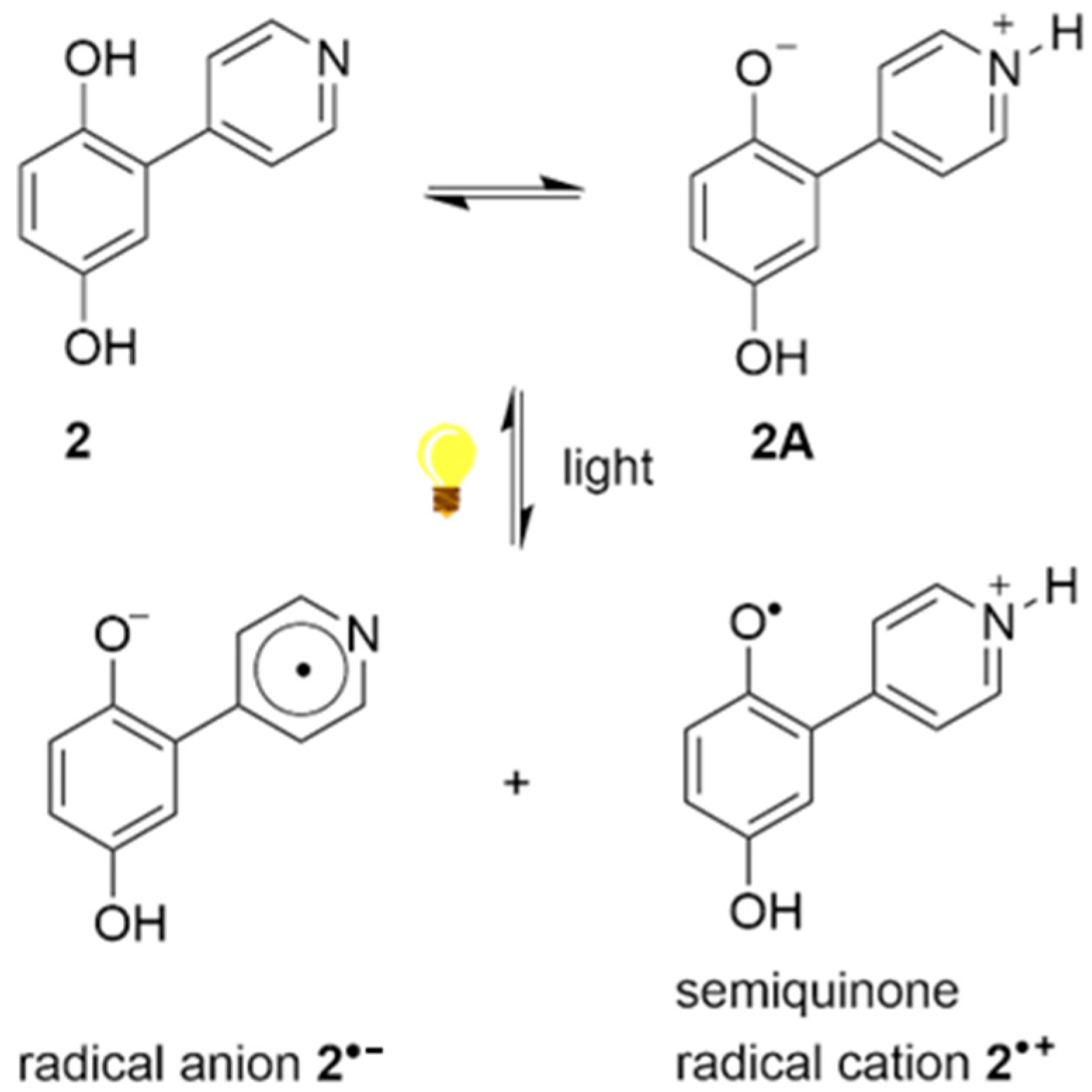
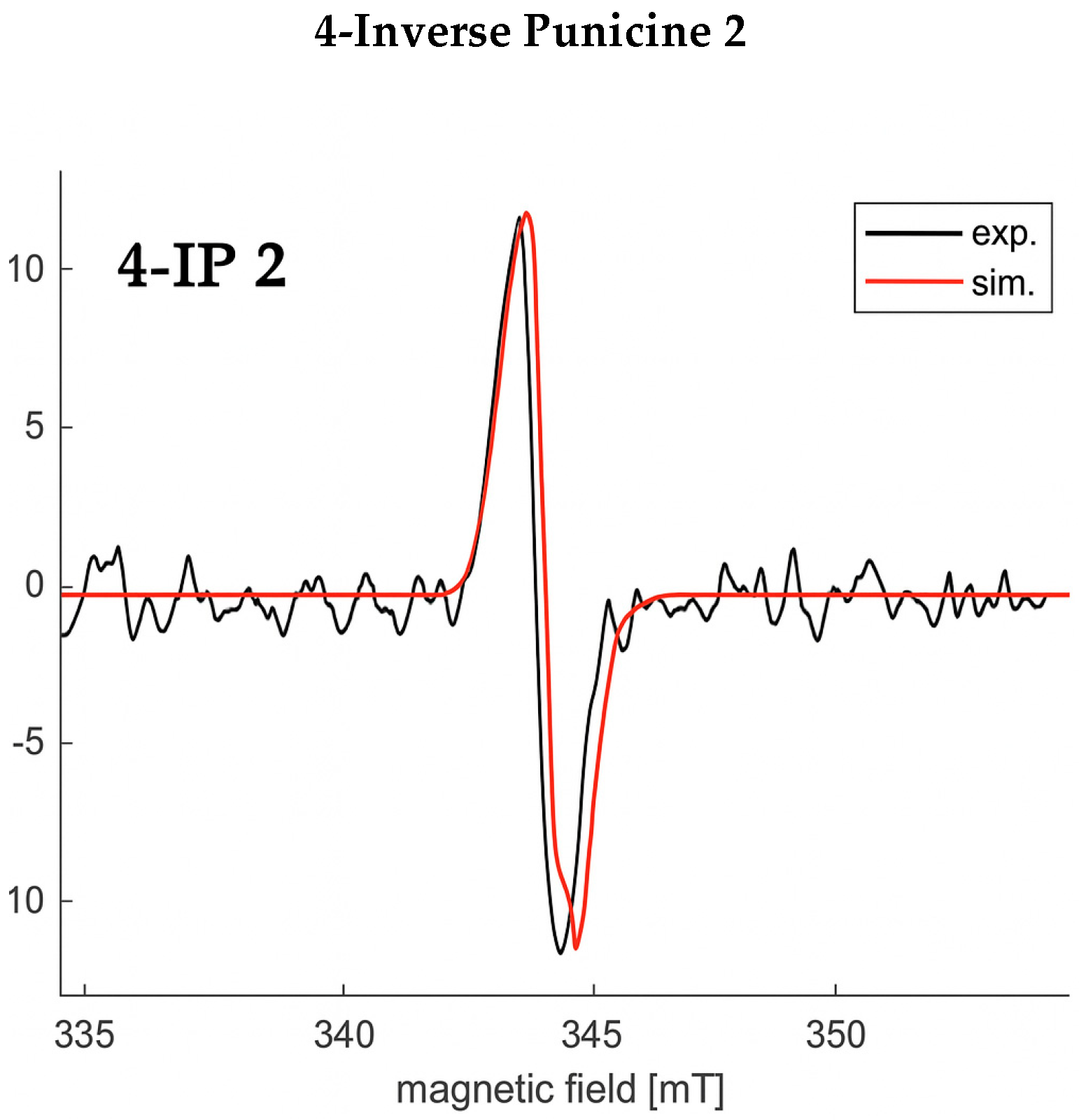
3.2. Electron Spin Resonance Spectroscopy (ESR)
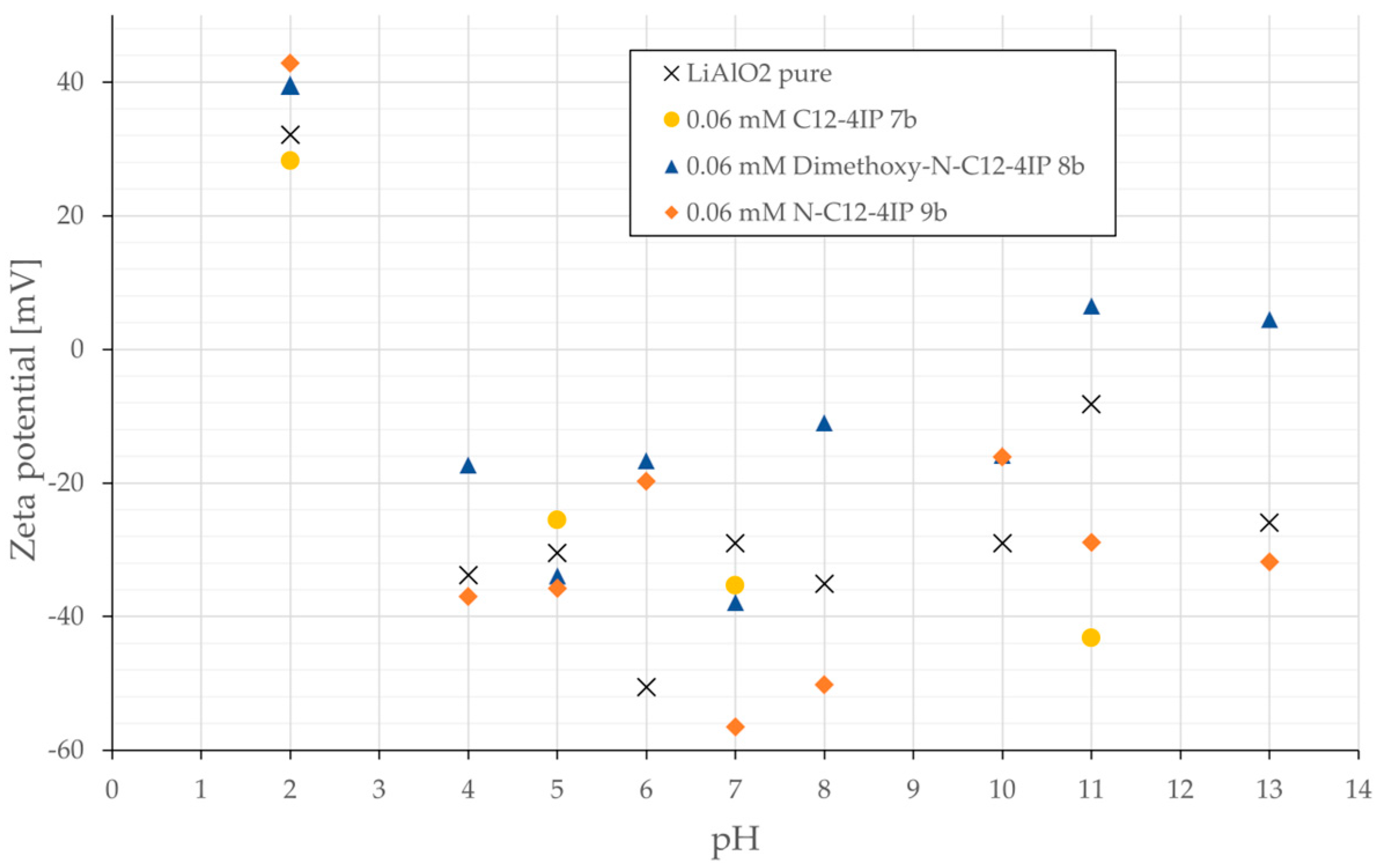
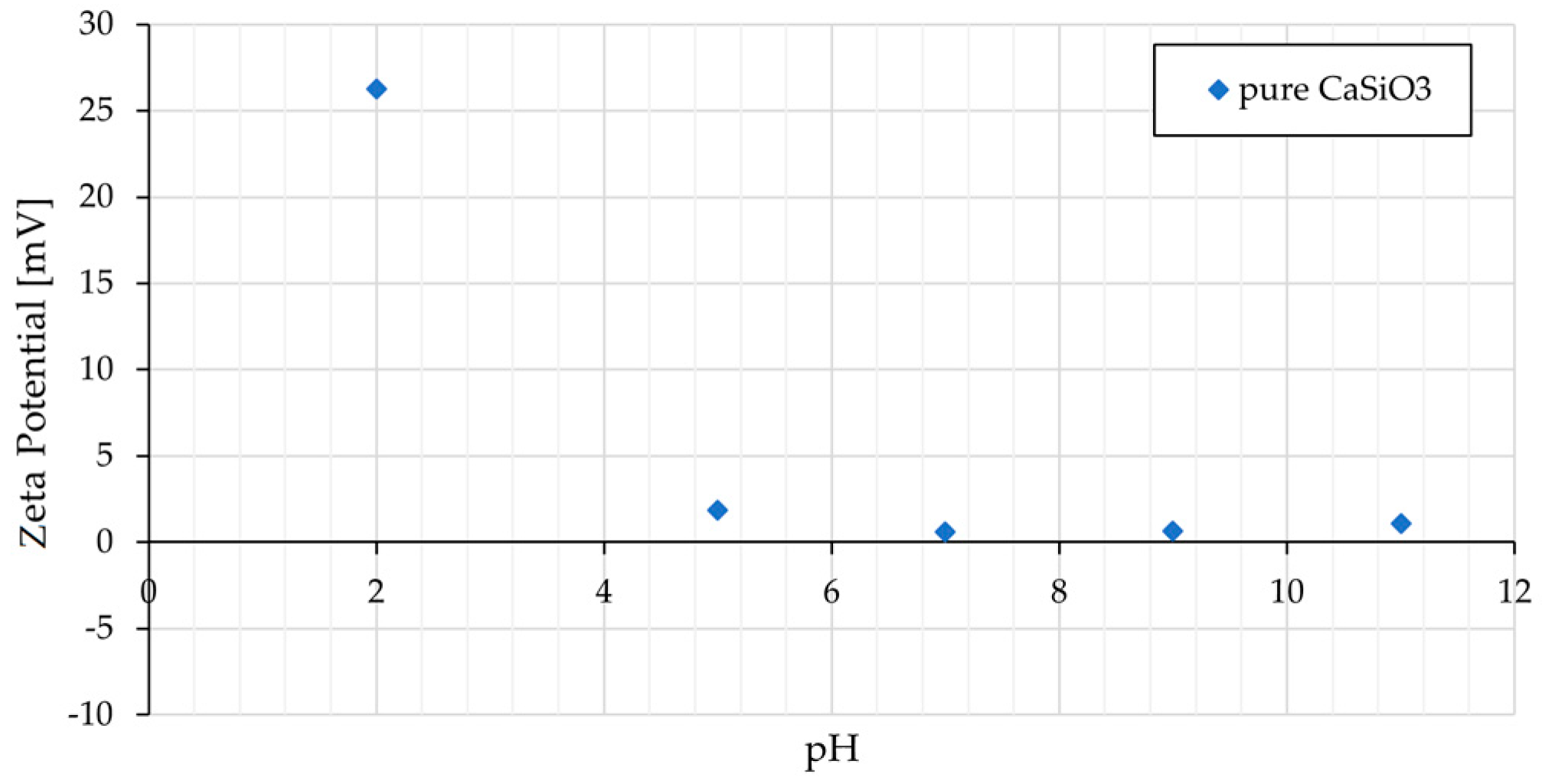
3.3. Zeta Potentials
3.4. Contact Angle Measurements
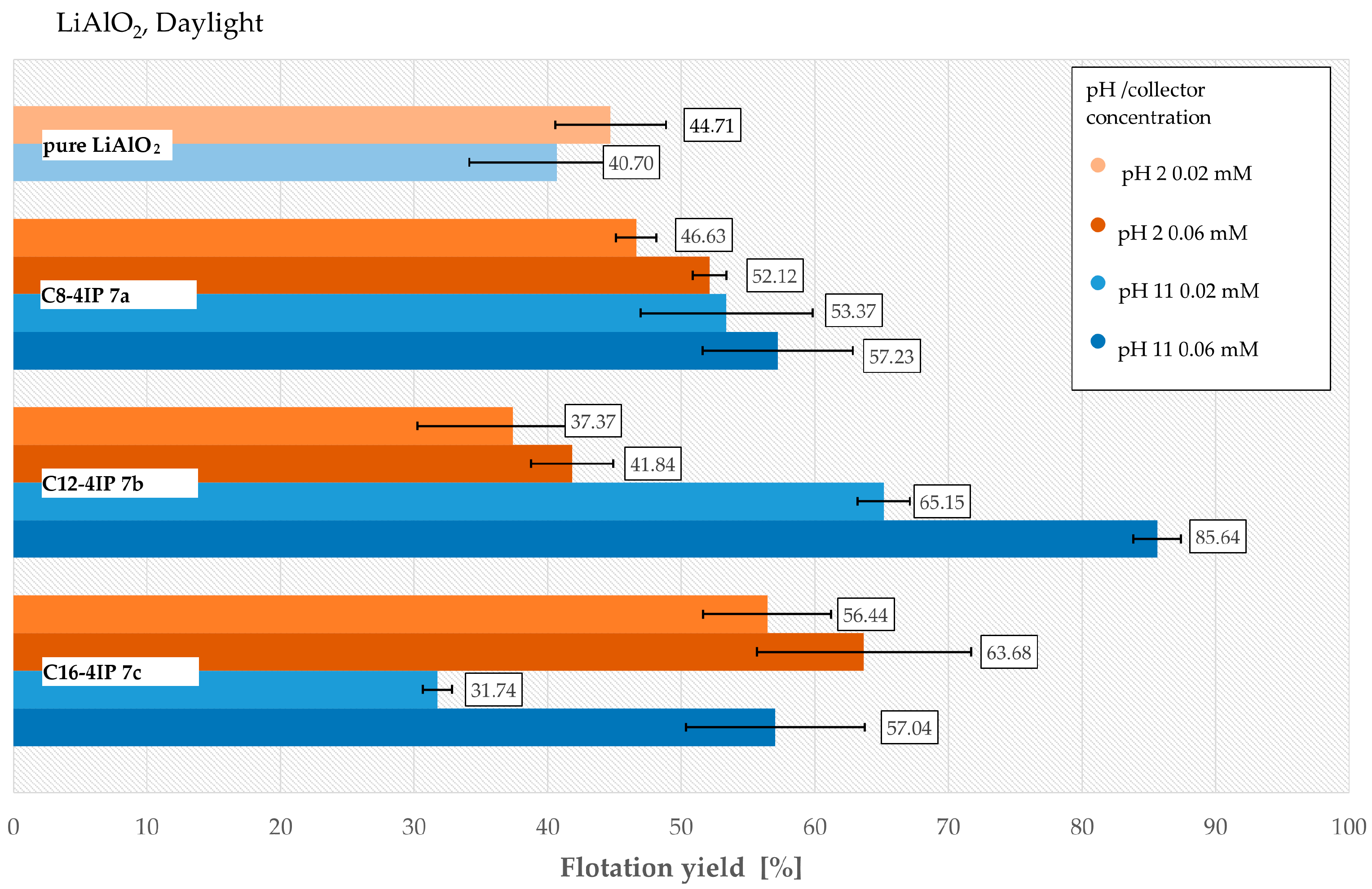
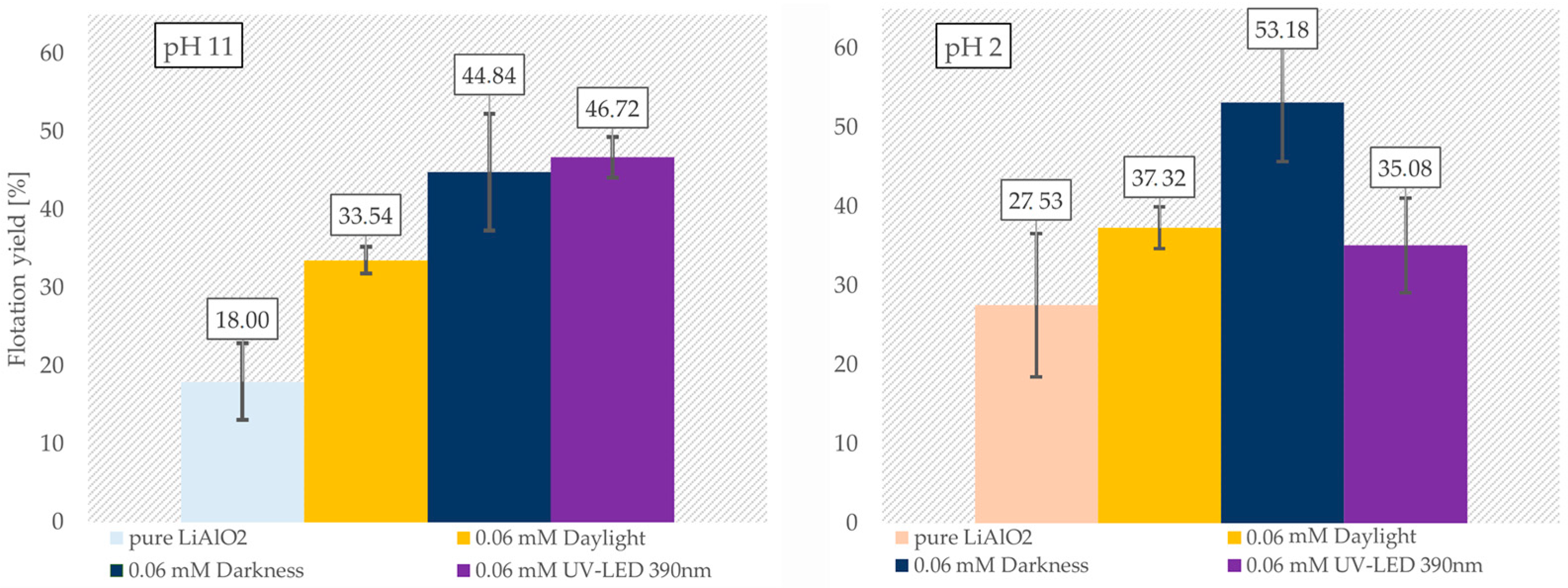
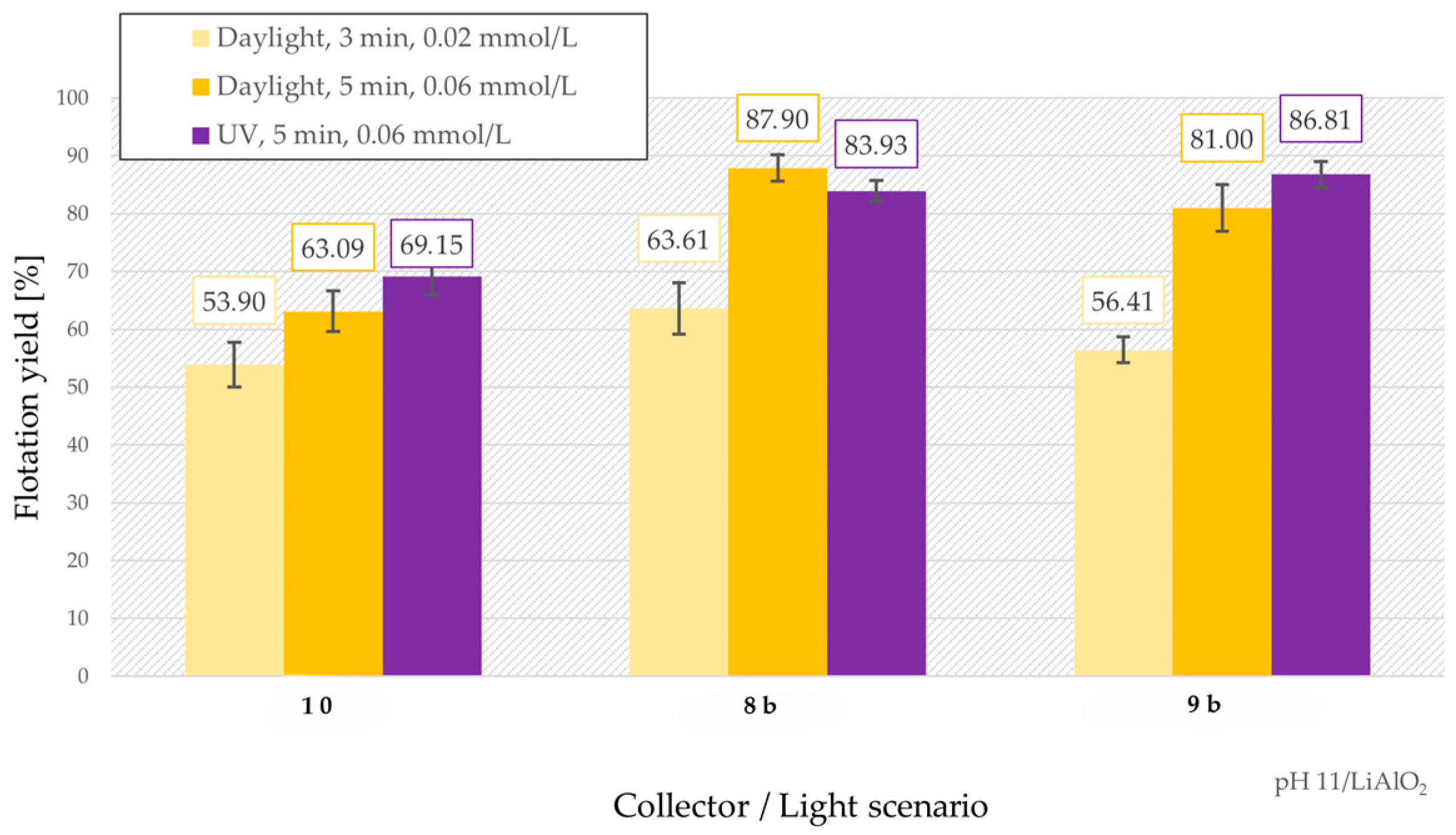
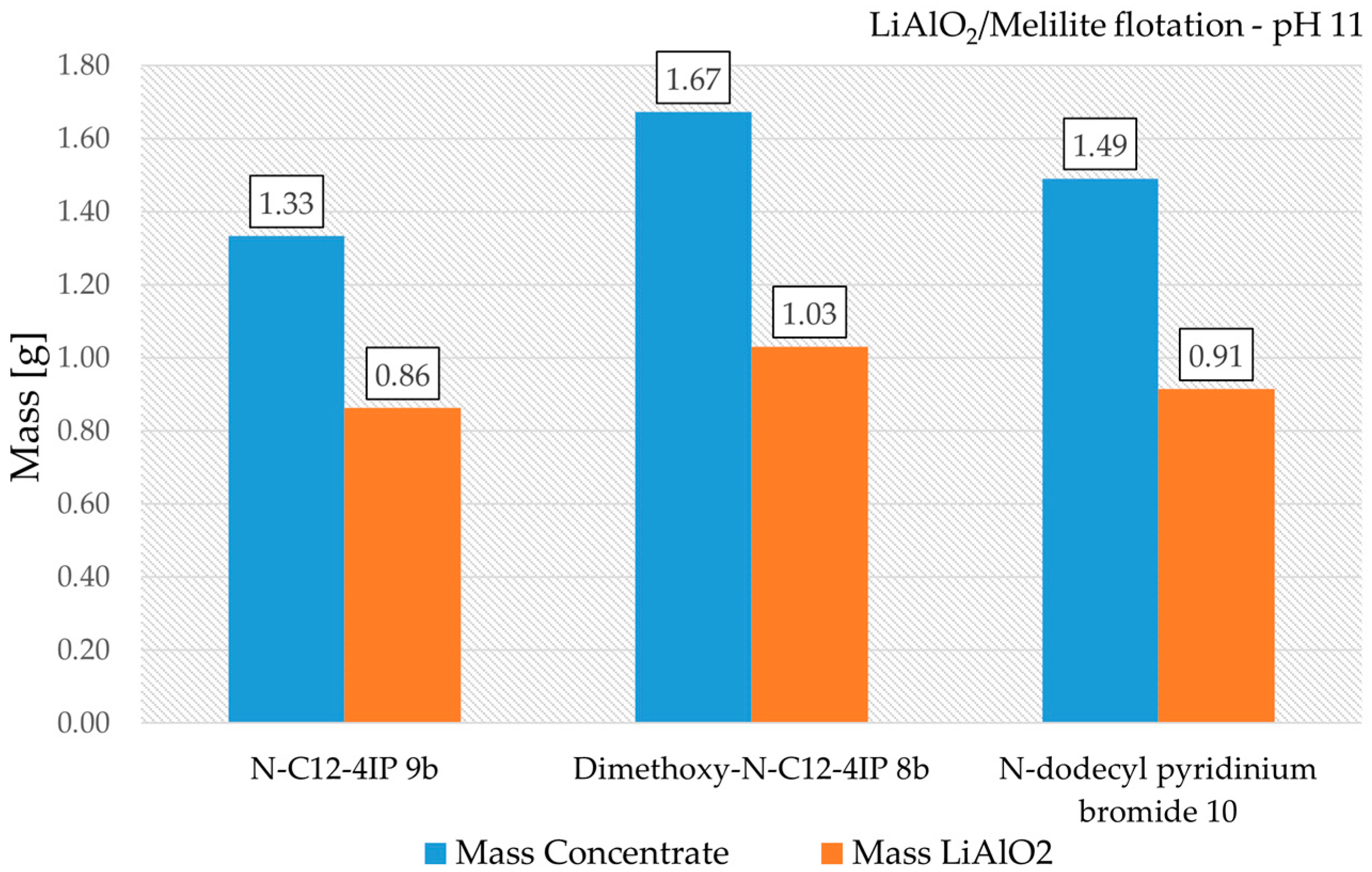
3.5. Froth Flotation of LiAlO2
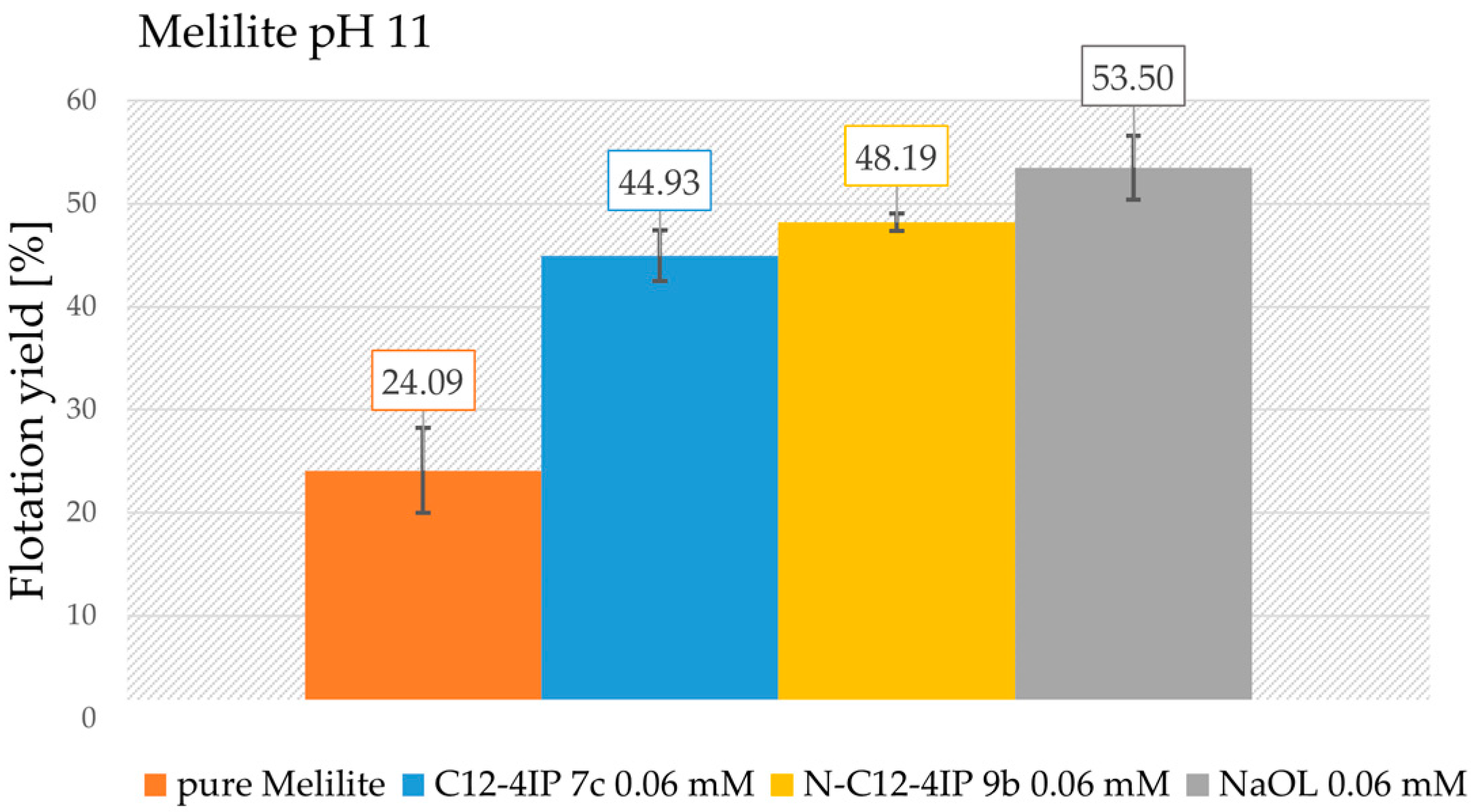
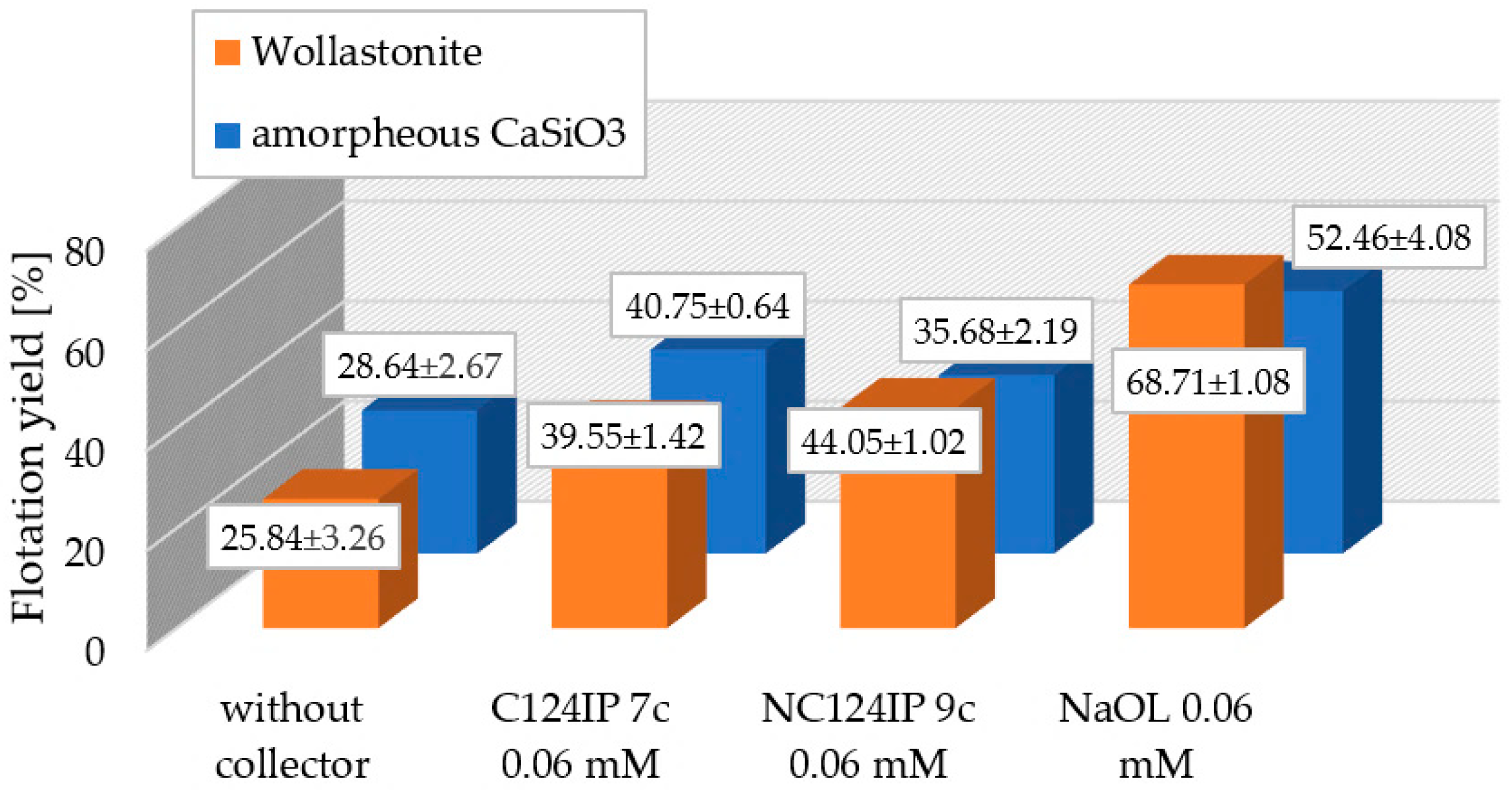
3.6. Froth Flotation of Gangue Material: Melilite and Calcium Silicate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- “COMMUNICATION FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE REGIONS Critical Raw Materials Resilience: Charting a Path towards greater Security and Sustainability. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0474 (accessed on 19 June 2025).
- Mineral Requirements for Clean Energy Transitions. Available online: https://www.iea.org/reports/the-role-of-critical-minerals-in-clean-energy-transitions/mineral-requirements-for-clean-energy-transitions (accessed on 27 September 2024).
- Bae, H.; Kim, Y. Technologies of lithium recycling from waste lithium ion batteries: A review. Mater. Adv. 2021, 2, 3234–3250. [Google Scholar] [CrossRef]
- Song, J.; Yan, W.; Cao, H.; Song, Q.; Ding, H.; Lv, Z.; Zhang, Y.; Sun, Z. Material flow analysis on critical raw materials of lithium-ion batteries in China. J. Cleaner Prod. 2019, 215, 570–581. [Google Scholar] [CrossRef]
- Schirmer, T.; Qiu, H.; Li, H.; Goldmann, D.; Fischlschweiger, M. Li-Distribution in compounds of the Li2O-MgO-Al2O3-SiO2-CaO System—A first survey. Metals 2020, 10, 1633. [Google Scholar] [CrossRef]
- Schirmer, T.; Qiu, H.; Goldmann, D.; Stallmeister, C.; Friedrich, B. Influence of P and Ti on phase formation at solidification of synthetic slag containing Li, Zr, La, and Ta. Minerals 2022, 12, 310. [Google Scholar] [CrossRef]
- Elwert, T.; Goldmann, D.; Strauss, K.; Schirmer, T. Phase composition of high lithium slags from the recycling of lithium ion batteries. World Metall. ERZMETALL 2012, 65, 163–171. [Google Scholar]
- de Abreu, D.A.; Schnickmann, A.; Chakrabarty, S.; Fischlschweiger, M.J.; Schirmer, T.; Fabrichnaya, O. Stability of crystalline compounds in slag systems mainly composed of Li2O-SiO2-CaO-MnOx. JOM 2024, 76, 6472–6486. [Google Scholar] [CrossRef]
- Fuerstenau, D.W. Pradip A Century of Research Leading to Understanding the Scientific Basis of Selective Mineral Flotation and Design of Flotation Collectors. Min. Metall. Explor. 2019, 36, 3–20. [Google Scholar] [CrossRef]
- Mweene, L.; Prasad Khanal, G. Insights into the separation of chalcopyrite from pyrite in Mg and Ca using gum acacia, xanthomonas campestris and guar gum: An experimental study validated by theoretical investigations. Min. Eng. 2024, 218, 109047. [Google Scholar] [CrossRef]
- Cook, B.K.; Gibson, C.E. A review of fatty acid collectors: Implications for spodumene flotation. Minerals 2023, 13, 212. [Google Scholar] [CrossRef]
- Li, F.; Zhong, H.; Zhao, G.; Wang, S.; Liu, G. Adsorption of a-hydroxyoctyl phosphonic acid to ilmenite/water interface and its application in flotation. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 67–73. [Google Scholar] [CrossRef]
- Liu, G.; Yang, X.; Zhong, H. Molecular design of flotation collectors: A recent progress. Adv. Colloid. Interface Sci. 2017, 246, 181–195. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Z.; Wang, H.; Liu, R.; Cheng, C.; Shuai, S.; Hu, Y.; Guo, Z.; Yu, X.; He, G.; et al. Flotation performance of a novel Gemini collector for kaolinite at low temperature. Int. J. Mining Sci. Technol. 2021, 31, 1145–1152. [Google Scholar] [CrossRef]
- Zgheib, A.; Fischer, M.H.; Namyslo, J.C.; Fittschen, U.E.A.; Wollmann, A.; Weber, A.P.; Schmidt, A. Photo-switchable collectors for the flotation of lithium aluminate for the recycling of the critical raw material lithium. ChemSusChem 2024, 17, e202301900. [Google Scholar] [CrossRef]
- Zgheib, A.; Acker, S.; Fischer, M.H.; Namyslo, J.C.; Strube, F.; Rudolph, M.; Fittschen, U.E.A.; Wollmann, A.; Weber, A.P.; Nieger, M.; et al. Lithium aluminate flotation by pH- and light-switchable collectors based on the natural product punicine. RSC Adv. 2024, 14, 9353–9364. [Google Scholar] [CrossRef]
- Schmidt, A.; Topp, M.; Mordhorst, T.; Schneider, O. Redoxactive Derivative of the betaine-alkaloid Punicine from Punica granatum. Synthesis and Cyclovoltammetry. Tetrahedron 2007, 63, 1842–1848. [Google Scholar] [CrossRef]
- Schmidt, A.; Mordhorst, T.; Nieger, M. Investigation of a betainic alkaloid from Punica granatum. Nat. Prod. Res. 2005, 19, 541–546. [Google Scholar] [CrossRef]
- Ren, D.; Hubbard, A.T. Molecular orientation of hydroquinone adsorbed at a platinum(111) thin-layer electrode. J. Colloid. Interface Sci. 1998, 202, 89–94. [Google Scholar] [CrossRef]
- Chung, F.H. Quantitative interpretation of X-ray diffraction patterns of mixtures. I. Matrix-flushing method for quantitative multicomponent analysis. J. Appl. Crystallogr. 1974, 7, 519–525. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comperhensive software package for spectral simulation and analysis in EPR. J. Mag. Res. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Kosower, E.M. Preparative Organic Chemistry; Springer: Berlin/Heidelberg, Germany, 1983; pp. 117–162. [Google Scholar]
- Albrecht, M.; Yulikov, M.; Kohn, T.; Jeschke, G.; Adams, J.; Schmidt, A. Pyridinium salts and ylides as partial structures of photoresponsive Merrifield resins. J. Mater. Chem. 2010, 20, 3025. [Google Scholar] [CrossRef]
- Schmidt, A.; Mordhorst, T.; Fleischhauer, H.; Jeschke, G. Coupled photocatalytic electron-transfers with derivatives of a betaine alkaloid from Punica granatum. Arkivoc 2005, 2005, 150–164. [Google Scholar] [CrossRef]
- Adachi, K.; Shimizu, M.; Shono, F.; Funatsu, K.; Yamazaki, H. Octanol/water partition coefficients estimated using retention times in reverse-phase liquid chromatography and calculated in silico as one of the determinant factors for pharmacokinetic parameter estimations of general chemical substances. J. Toxicol. Sci. 2024, 49, 127–137. [Google Scholar] [CrossRef]
- Broto, P.; Moreau, G.; Vandycke, C. Molecular structures perception auto correlation descriptor and structure activity relationship studies system of atomic contributions for the calculation of the n-octanol water partition coefficients. Eur. J. Med. Chem. 1984, 19, 71–78, ISSN/ISBN: 0223-5234. [Google Scholar]
- Huibers, P.D.T.; Lobanov, V.S.; Katritzky, A.R.; Shah, D.O.; Karelson, M. Prediction of Critical Micelle Concentration Using a Quantitative Structure–Property Relationship Approach. J. Colloid. Interface Sci. 1997, 187, 113–120. [Google Scholar] [CrossRef]
- Liu, C.; Han, S.; Zhu, Y.; Xu, W.; Yang, S. Novel collector of a dodecylpyridinium chloride ionic liquid in the reverse flotation separation of muscovite from apatite. Langmuir 2025, 41, 2834–2842. [Google Scholar] [CrossRef]
- Qiu, H.; Kersebaum, J.; Wollmann, A.; Feuge, N.; Haas, A.; Goldmann, D.; Wilhelm, R. Improvement of the froth flotation of LiAlO2 and melilite solid solution via pre-functionalization. Sci. Rep. 2021, 11, 20443. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Hu, T.; Bai, X.; Wang, S.; Xie, W.; Hao, J.; He, Y. Recovery of LiCoO2 and graphite from spent lithium-ion batteries by cryogenic grinding and froth flotation. Min. Eng. 2020, 148, 106223. [Google Scholar] [CrossRef]
- Tanemura, K.; Suzuki, T.; Nishida, Y.; Satsumabayashi, K.; Horaguchi, T. Halogenation of aromatic compounds by N-chloro-, N-bromo-, and N-iodosuccinimide. Chem. Lett. 2003, 32, 932–933. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Y.; Hao, J.; Bao, B.; Bian, X.; Jiang, X.; Pang, J.; Zhang, H.; Jiang, Z.; Jiang, L. A charge-density-tunable three/two-dimensional polymer/graphene oxide heterogeneous nanoporous membrane for ion transport. ACS Nano 2017, 11, 10816–10824. [Google Scholar] [CrossRef]
- Semenyuta, I.; Kovalishyn, V.; Hodyna, D.; Startseva, Y.; Rogalsky, S.; Metelytsia, L. New QSTR models to evaluation of imidazolium- and pyridinium-contained ionic liquids toxicity. Comput. Toxicol. 2024, 30, 100309. [Google Scholar] [CrossRef]
- Swainson, I.P.; Dove, M.T.; Schmahl, W.W.; Putnis, A. Neutron powder diffraction study of the akermanite-gehlenite solid solution series. Phys. Chem. Miner. 1992, 19, 185. [Google Scholar] [CrossRef]
- Marezio, M. The crystal structure and anomalous dispersion of γ-LiAlO2. Acta Crystallogr. 1965, 19, 396. [Google Scholar] [CrossRef]
- Thiel, J.P.; Chiang, C.K.; Poeppelmeier, K.R. Structure of LiAl2(OH)7 2H2O. Chem. Mater. 1993, 5, 297. [Google Scholar] [CrossRef]
- Meagher, E.P. The crystal structures of pyrope and grossularite at elevated temperatures. Am. Mineral. 1975, 60, 218. [Google Scholar]
| Collector | Lithium Aluminate | Melilite | Calcium Silicate |
|---|---|---|---|
| pure mineral | 26° | 61° | 65° |
| 7a | 35° | 56° | 69° |
| 7b | 50° | 67° | 68° |
| 7c | >90° | 65° | 72° |
| 8b | 59° | 60° | 74° |
| 9b | >90° | 70° | 78° |
| 10 | 54° | 47° | 75° |
| 7a | 7b | 7c | |
|---|---|---|---|
| Anionic logP | 3.60 | 5.43 | 7.25 |
| Neutral logP | 3.65 | 5.31 | 6.98 |
| LiAlO2 % | Melilite % | LiAl2(OH)7(H2O)2 % | CaCO3 % | Li Grade % | |
|---|---|---|---|---|---|
| N-C12-4IP 9b | |||||
| 64.7 | 29.7 | 4.3 | 1.7 | 6.95 | |
| Dimethoxy-N-C12-4IP 8b | |||||
| 61.7 | 33.0 | 4.0 | 1.0 | 6.63 | |
| N-dodecyl pyridinium bromide 10 | |||||
| 61.3 | 34.3 | 3.3 | 1.0 | 6.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, M.H.; Zgheib, A.; El Hraoui, I.; Schnickmann, A.; Schirmer, T.; Jeschke, G.; Schmidt, A. Inverse Punicines: Isomers of Punicine and Their Application in LiAlO2, Melilite and CaSiO3 Separation. Separations 2025, 12, 202. https://doi.org/10.3390/separations12080202
Fischer MH, Zgheib A, El Hraoui I, Schnickmann A, Schirmer T, Jeschke G, Schmidt A. Inverse Punicines: Isomers of Punicine and Their Application in LiAlO2, Melilite and CaSiO3 Separation. Separations. 2025; 12(8):202. https://doi.org/10.3390/separations12080202
Chicago/Turabian StyleFischer, Maximilian H., Ali Zgheib, Iliass El Hraoui, Alena Schnickmann, Thomas Schirmer, Gunnar Jeschke, and Andreas Schmidt. 2025. "Inverse Punicines: Isomers of Punicine and Their Application in LiAlO2, Melilite and CaSiO3 Separation" Separations 12, no. 8: 202. https://doi.org/10.3390/separations12080202
APA StyleFischer, M. H., Zgheib, A., El Hraoui, I., Schnickmann, A., Schirmer, T., Jeschke, G., & Schmidt, A. (2025). Inverse Punicines: Isomers of Punicine and Their Application in LiAlO2, Melilite and CaSiO3 Separation. Separations, 12(8), 202. https://doi.org/10.3390/separations12080202








