Adsorption Performance and Mechanism of Gallium from Sulfuric Acid Leach Liquor of High-Alumina Fly Ash
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Static Adsorption
2.3. Characterization of Samples
3. Results and Discussion
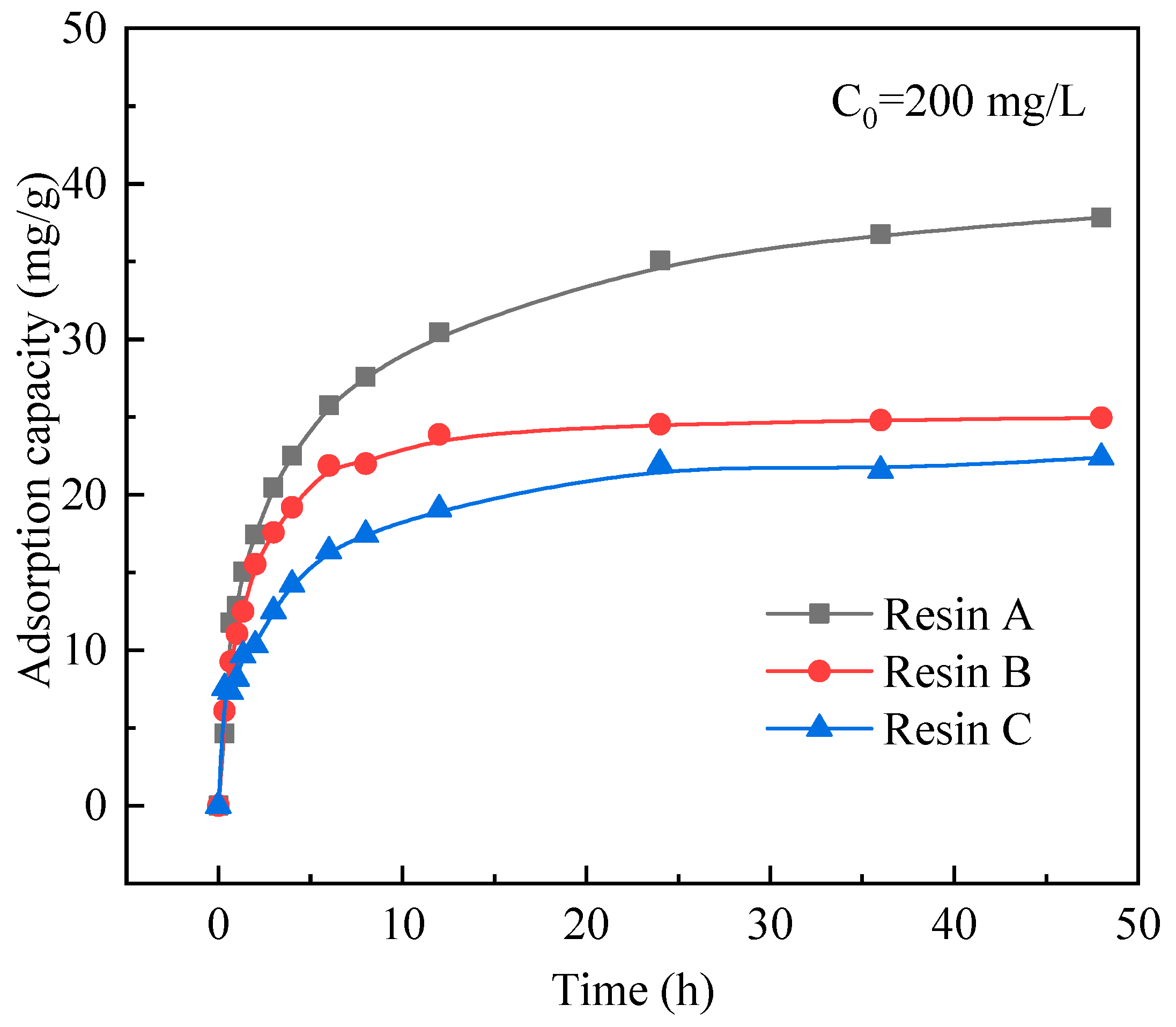
3.1. Screening of Resin
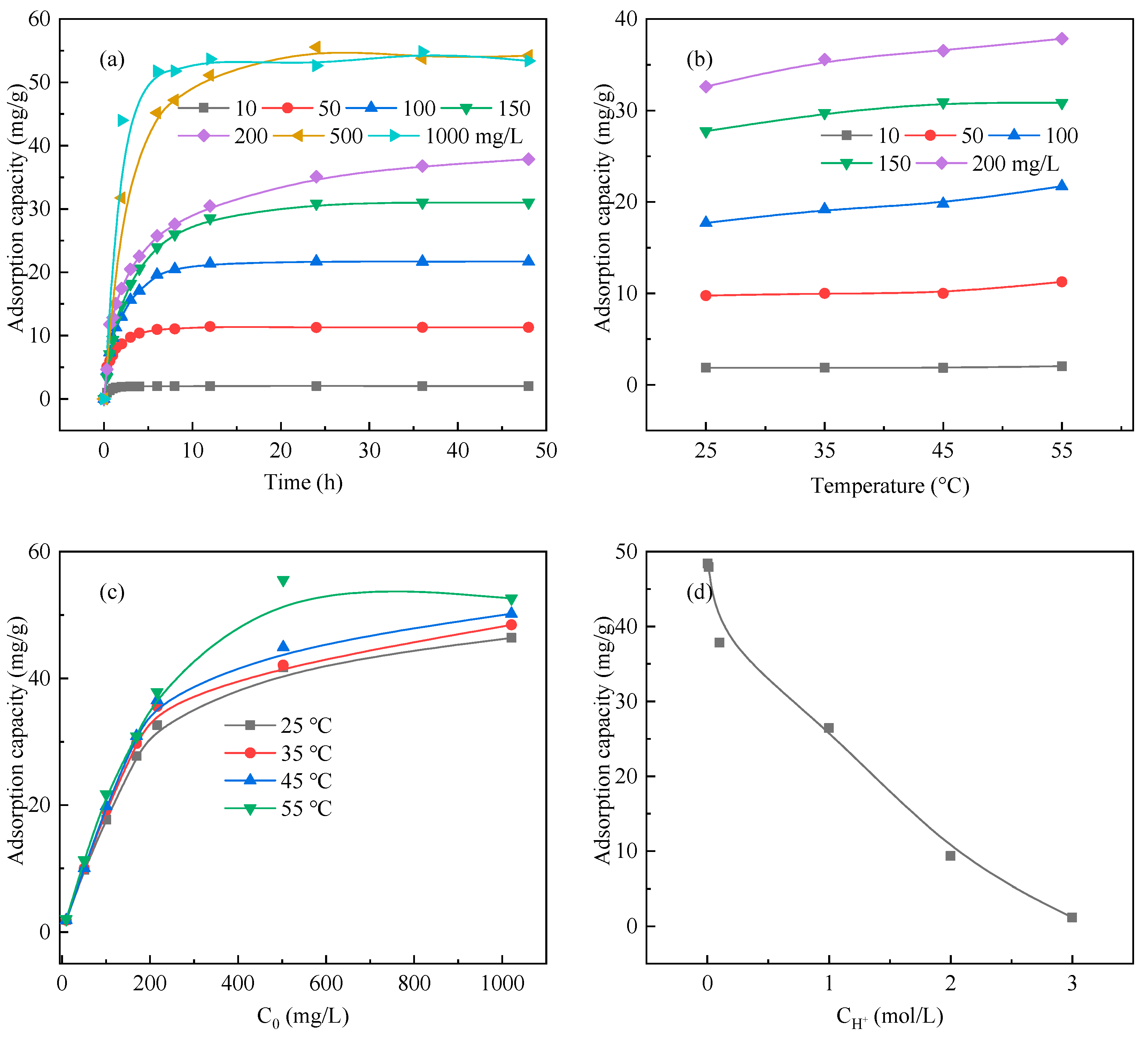
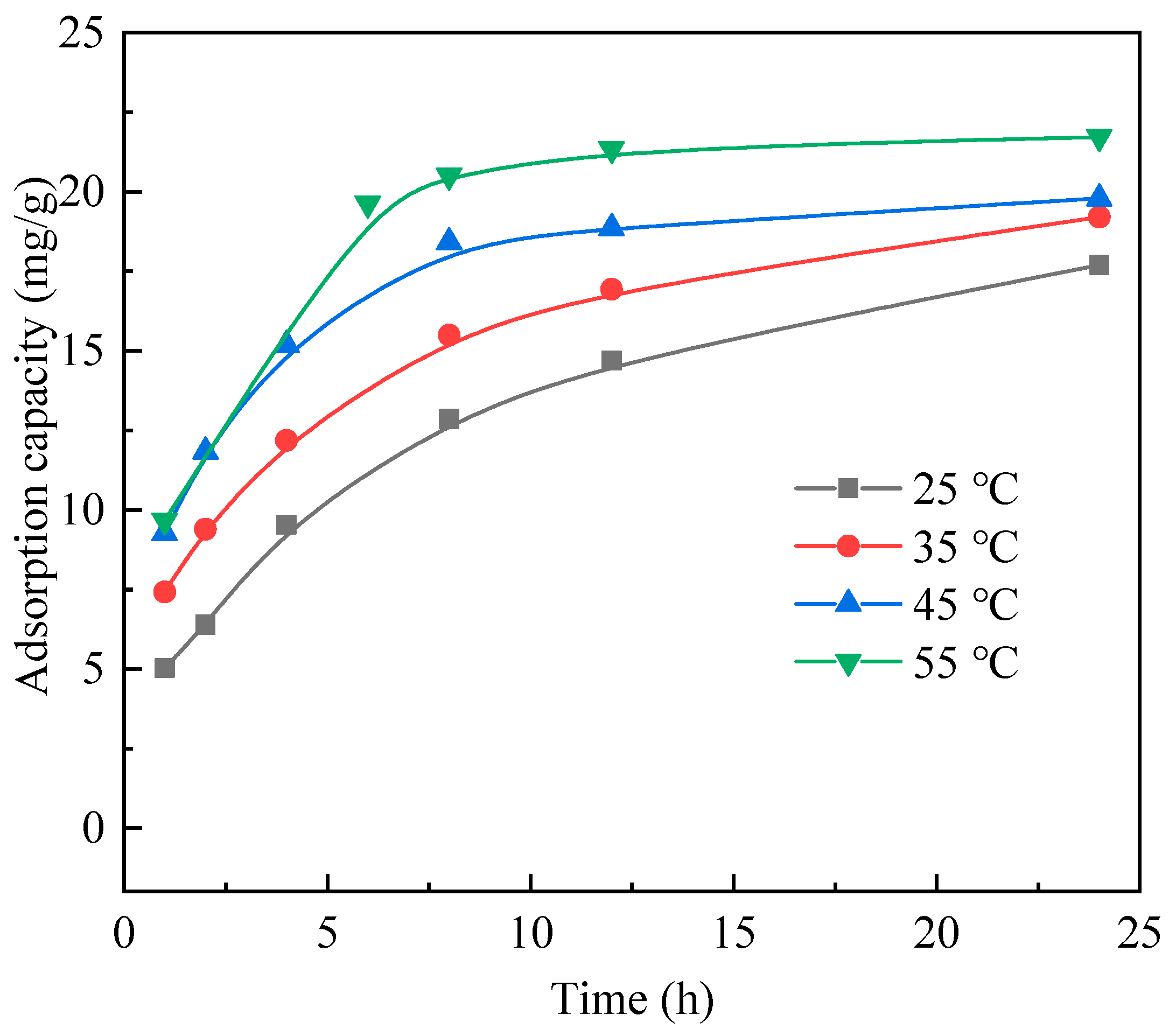
3.2. Adsorption Behaviors of Resin A
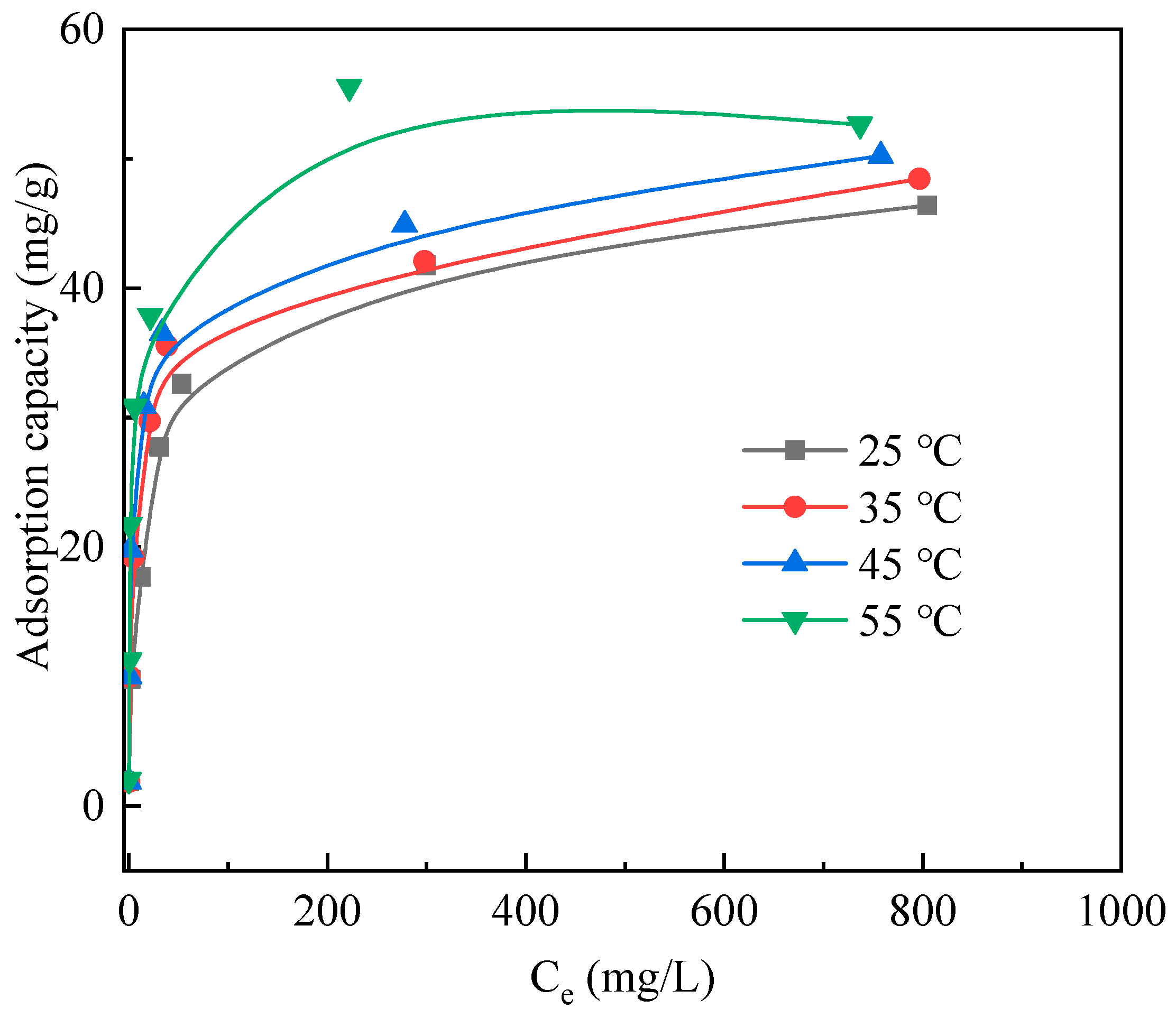
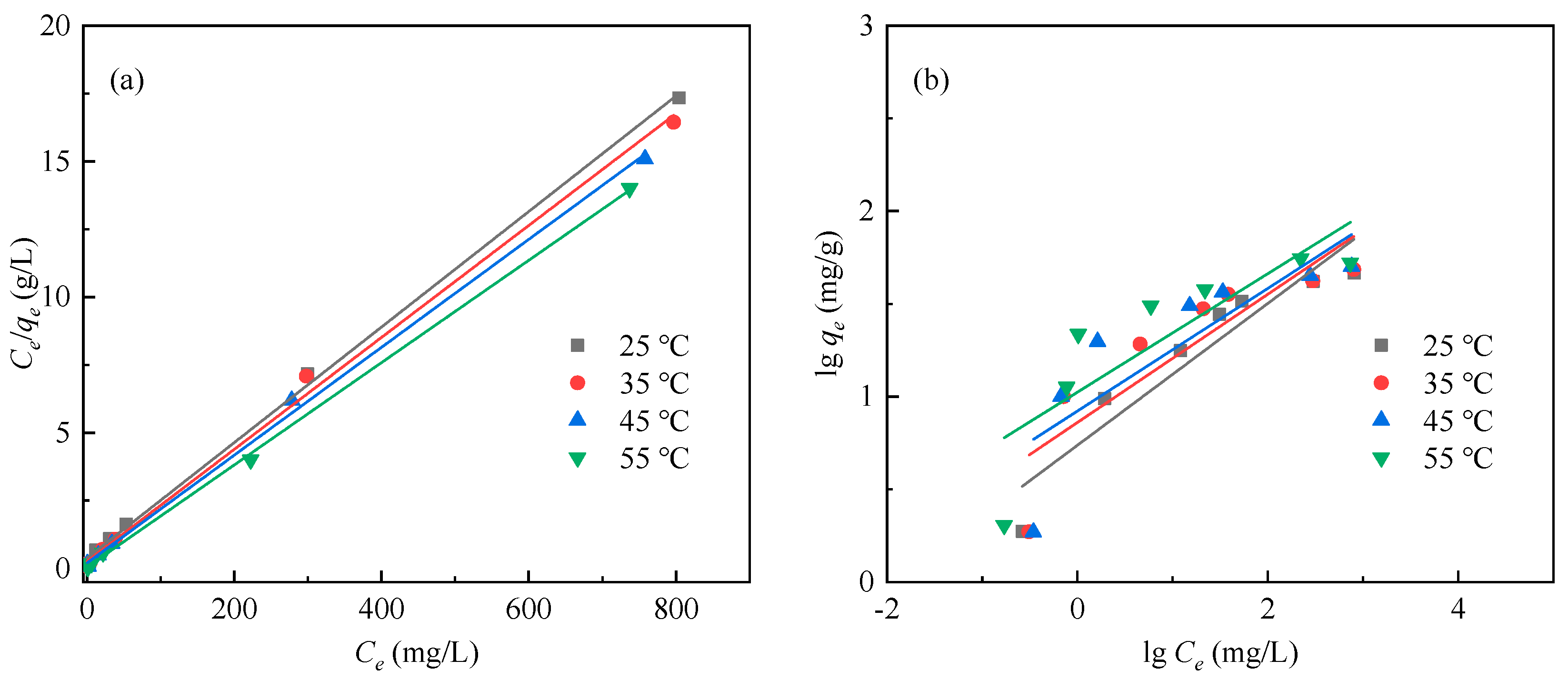
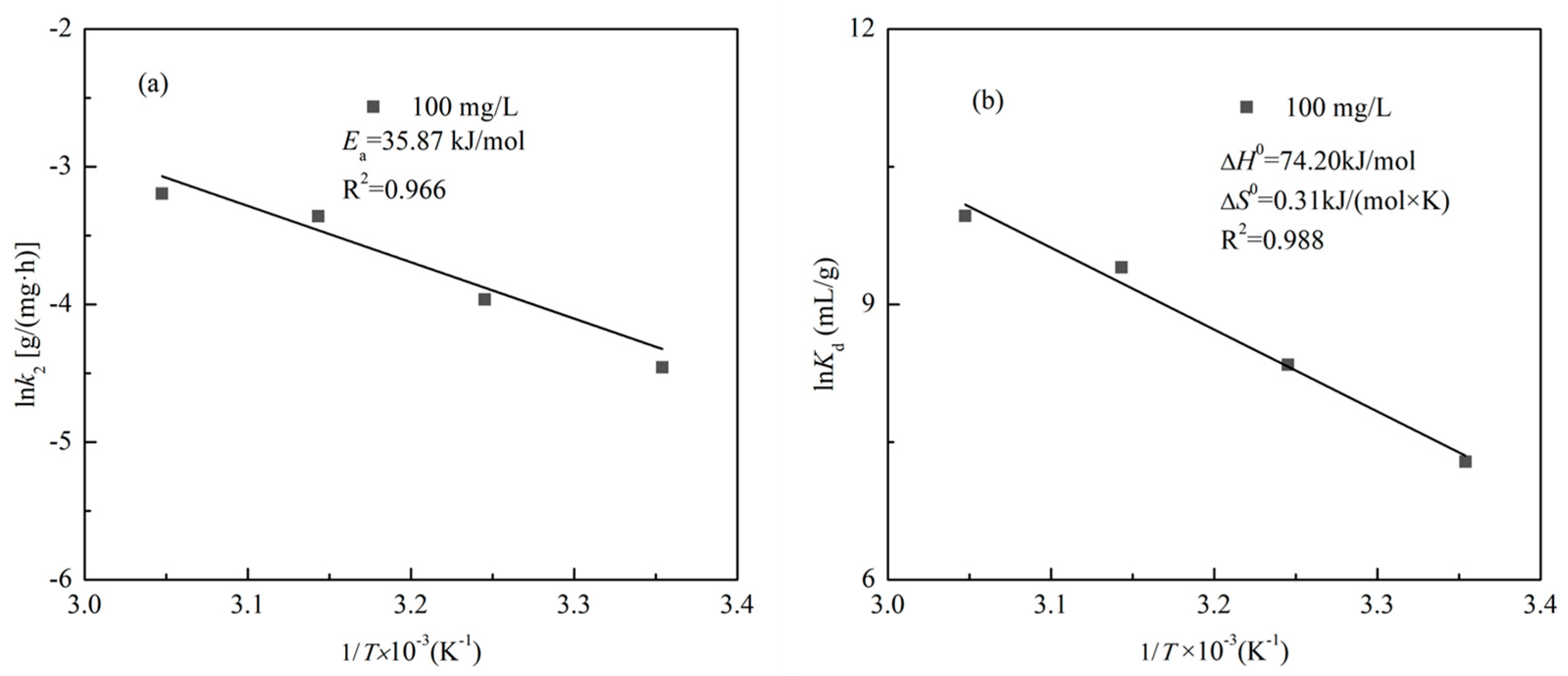
3.3. Adsorption Thermodynamics
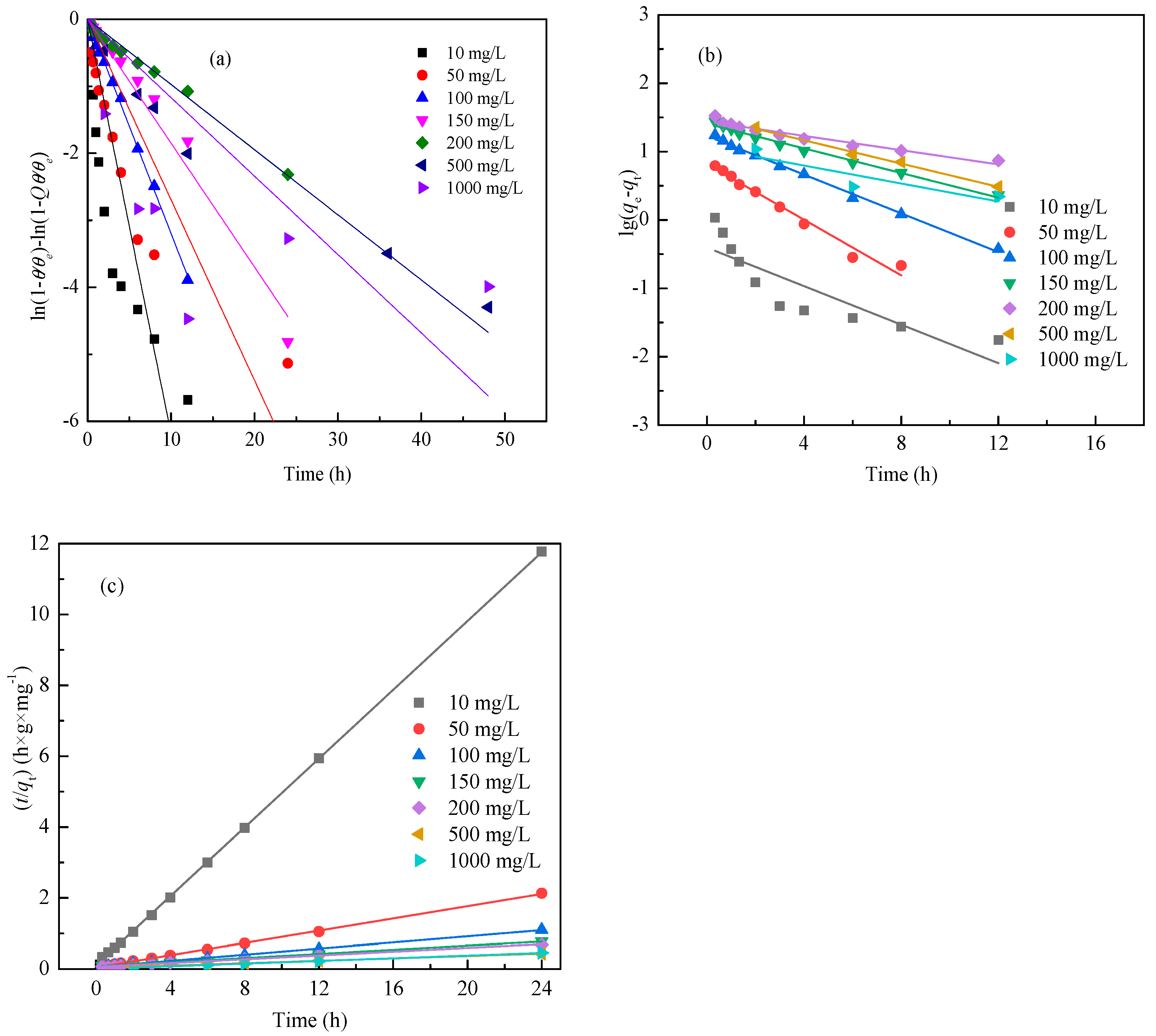
3.4. Adsorption Kinetics
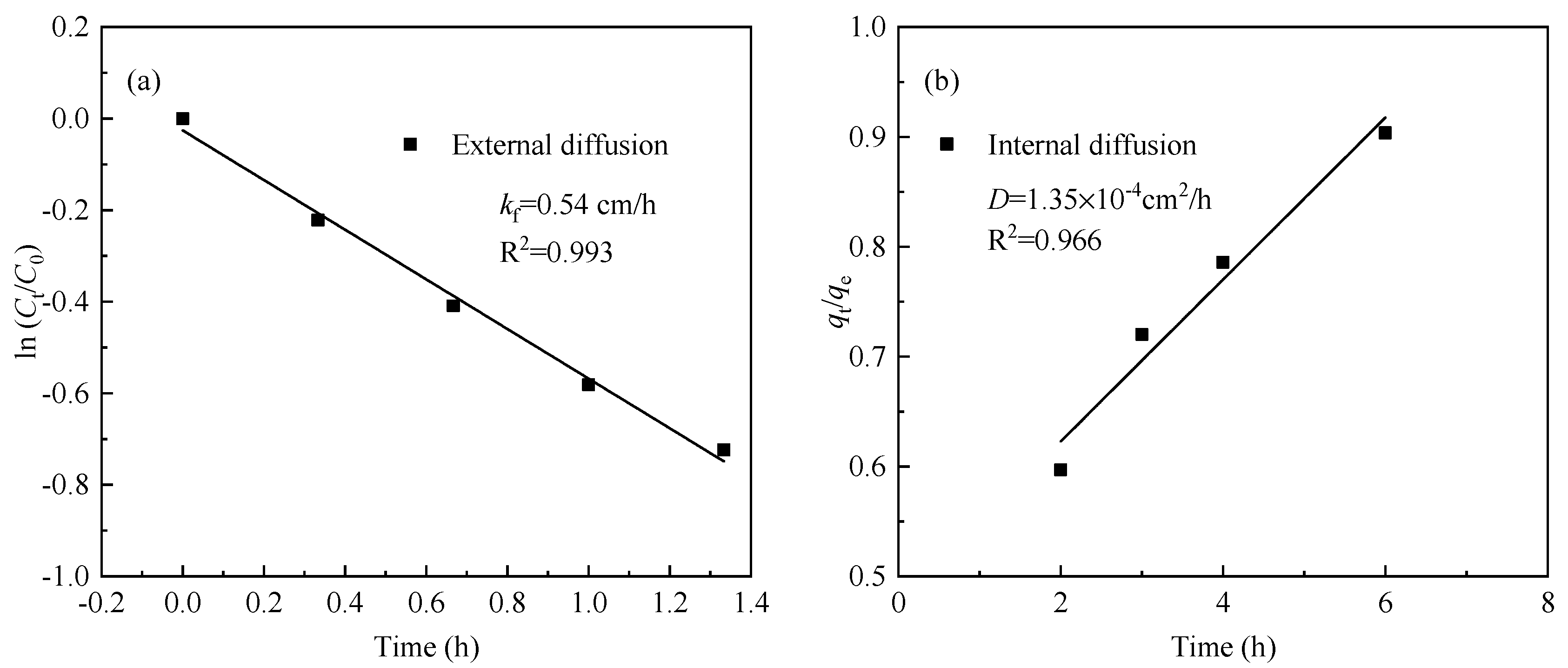
3.5. Adsorption Rate Control Mechanism
3.6. Effect of Ca2+, K+, Al3+, Mg2+ and Fe2+ on Ga3+ Adsorption
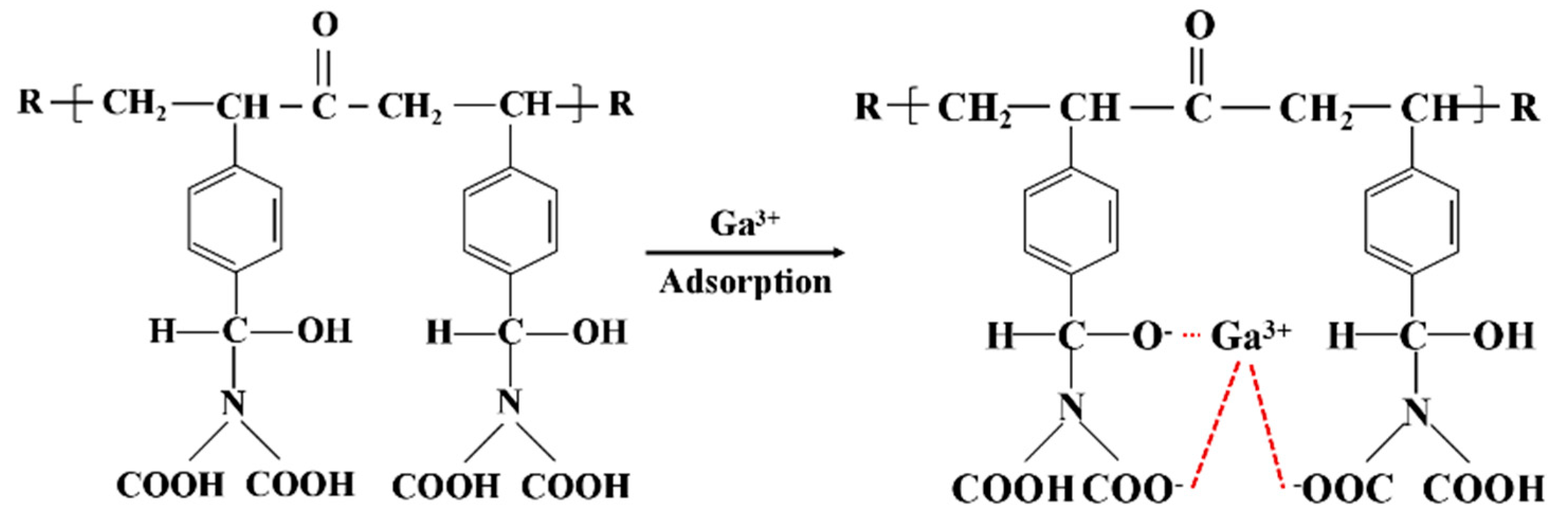
3.7. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, F.; Xiao, T.; Lin, J.; Ning, Z.; Long, Q.; Xiao, L.; Huang, F.; Wang, W.; Xiao, Q.; Lan, X.; et al. Resources and extraction of gallium: A review. Hydrometallurgy 2017, 174, 105–115. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Pomper, M.G. Clinical applications of Gallium-68. Appl. Radiat. Isot. 2013, 76, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Clarkin, O.M.; Wu, B.; Cahill, P.A.; Brougham, D.F.; Banerjee, D.; Brady, S.A.; Fox, E.K.; Lally, C. Novel injectable gallium-based self-setting glass-alginate hydrogel composite for cardiovascular tissue engineering. Carbohydr. Polym. 2019, 217, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.; Backes, C.; Gholamvand, Z.; Hanlon, D.; McAteer, D.; Nerl, H.C.; McGuire, E.; Seral-Ascaso, A.; Ramasse, Q.M.; McEvoy, N.; et al. Preparation of Gallium Sulfide Nanosheets by Liquid Exfoliation and Their Application As Hydrogen Evolution Catalysts. Chem. Mater. 2015, 27, 3483–3493. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Song, D.; Ma, Q.; Zhang, L.; Zhang, H.; Zhang, L.; Wu, R. Investigation of aluminum–gallium co-doped zinc oxide targets for sputtering thin film and photovoltaic application. J. Alloys Compd. 2013, 575, 174–182. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; Wu, Y. pH controlled synthesis of UV excited host-sensitized luminescence in Dy3+ doped Ga2O3. J. Lumin. 2019, 212, 29–37. [Google Scholar] [CrossRef]
- Lovik, A.N.; Restrepo, E.; Muller, D.B. The global anthropogenic gallium system: Determinants of demand, supply and efficiency improvements. Environ. Sci. Technol. 2015, 49, 5704–5712. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, M.; Ketris, M.P.; Seifert, T.; Gutzmer, J. On the current and future availability of gallium. Resour. Policy 2016, 47, 38–50. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Du, Y.; Sun, J.; Yang, H.; Yan, L. Recovery of Gallium in the Alumina Production Process from High-Alumina Coal Fly Ash. Rare Met. Mater. Eng. 2016, 45, 1893–1897. [Google Scholar]
- Fang, Z.; Gesser, H.D. Recovery of gallium from coal fly ash. Hydrometallurgy 1996, 41, 187–200. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, S.; Zhang, H.; Cheng, F. Novel extraction of valuable metals from circulating fluidized bed-derived high-alumina fly ash by acid–alkali–based alternate method. J. Clean. Prod. 2019, 230, 302–313. [Google Scholar] [CrossRef]
- Chelgani, S.C. Investigating the occurrences of valuable trace elements in African coals as potential byproducts of coal and coal combustion products. J. Afr. Earth Sci. 2019, 150, 131–135. [Google Scholar] [CrossRef]
- Ma, Z.; Shan, X.; Cheng, F. Distribution Characteristics of Valuable Elements, Al, Li, and Ga, and Rare Earth Elements in Feed Coal, Fly Ash, and Bottom Ash from a 300 MW Circulating Fluidized Bed Boiler. ACS Omega 2019, 4, 6854–6863. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, Z.; Wang, Y.; Chen, D.; Zhang, Y. Gallium recovery process technology in acid system. Light Met. 2018, 6, 20–24. [Google Scholar] [CrossRef]
- Zheng, F.W.; Sun, C.; Chen, F.J.; Guo-Bin, L.I.; Yi, S.U. A review of extracting alumina from fly ash. Mod. Chem. Ind. 2018, 38, 37–41. [Google Scholar] [CrossRef]
- Gong, B.; Tian, C.; Xiong, Z.; Zhao, Y.; Zhang, J. Mineral changes and trace element releases during extraction of alumina from high aluminum fly ash in Inner Mongolia, China. Int. J. Coal Geol. 2016, 166, 96–107. [Google Scholar] [CrossRef]
- Lu, F.; Xiao, T.; Lin, J.; Li, A.; Long, Q.; Huang, F.; Xiao, L.; Li, X.; Wang, J.; Xiao, Q.; et al. Recovery of gallium from Bayer red mud through acidic-leaching-ion-exchange process under normal atmospheric pressure. Hydrometallurgy 2018, 175, 124–132. [Google Scholar] [CrossRef]
- Wang, Y. Study on influence factors of leaching rate of gallium from fly ash in Jungar area. Glob. Geol. 2014, 33, 730–734. [Google Scholar]
- Wang, J.; Bao, Y.; Ma, R.; Li, Y.; Gong, L.; Zhang, Y.; Niu, Z.; Xin, B. Gallium recovery from aluminum smelting slag via a novel combined process of bioleaching and chemical methods. Hydrometallurgy 2018, 177, 140–145. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Z.; Li, Y.; Wilson, B.P.; Liu, Z.; Zeng, L.; Lundström, M. Recovery and separation of gallium(III) and germanium(IV) from zinc refinery residues: Part II: Solvent extraction. Hydrometallurgy 2017, 171, 149–156. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Kuang, S.; Li, Y.; Ma, Y.; Li, Y.; Liao, W. Recovery of Ga(III) from chloride solutions by solvent extraction with Cextrant 230. Hydrometallurgy 2019, 185, 76–81. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Y.; Xiao, Y.; Fan, Y. Recovery of gallium from Bayer liquor: A review. Hydrometallurgy 2012, 125–126, 115–124. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Z.; Liu, Y.; Cao, H.; Zhu, W. Recovery of gallium from strong acidic sulphate leach solutions of zinc refinery residues using a novel phosphate ester extractant. Hydrometallurgy 2019, 185, 250–256. [Google Scholar] [CrossRef]
- Li, S.; Wu, W.; Li, H.; Hou, X. The direct adsorption of low concentration gallium from fly ash. Sep. Sci. Technol. 2015, 51, 395–402. [Google Scholar] [CrossRef]
- Lu, S.; Chen, L.; Hamza, M.F.; He, C.; Wang, X.; Wei, Y.; Guibal, E. Amidoxime functionalization of a poly(acrylonitrile)/silica composite for the sorption of Ga(III)—Application to the treatment of Bayer liquor. Chem. Eng. J. 2019, 368, 459–473. [Google Scholar] [CrossRef]
- Selvi, P.; Ramasami, M.; Samuel, M.H.P.; Adaikkalam, P.; Srinivasan, G.N. Recovery of Gallium from Bayer Liquor Using Chelating Resins in Fixed-Bed Columns. Ind. Eng. Chem. Res. 2004, 43, 2216–2221. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Chai, Y.; Hua, Z.; Xiao, Y.; Yang, Y. Adsorption Performances and Mechanisms of Amidoxime Resin toward Gallium(III) and Vanadium(V) from Bayer Liquor. ACS Sustain. Chem. Eng. 2015, 4, 53–59. [Google Scholar] [CrossRef]
- Massoud, A.; Mahmoud, H.H. Evaluation of Hybrid Polymeric Resin Containing Nanoparticles of Iron Oxide for Selective Separation of In(III) from Ga(III). J. Inorg. Organomet. Polym. Mater. 2017, 27, 1806–1815. [Google Scholar] [CrossRef]
- Xiong, Y.; Cui, X.; Wang, D.; Wang, Y.; Lou, Z.; Shan, W.; Fan, Y. Diethanolamine functionalized rice husk for highly efficient recovery of gallium(III) from solution and a mechanism study. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.J.; He, C.L.; Li, Y.J.; Zhou, J.; Li, J.; Zheng, C.H.; Zhao, J.; Fujita, T.; Ning, S.Y.; Wei, Y.Z. Enhanced adsorption and separation of gallium using silica-based P507-TBP/SiO2–P adsorbent from sulfuric acid solution. Microp. Mesoporous Mater. 2021, 314, 110859. [Google Scholar] [CrossRef]
- Liu, J.S.; Chen, H.; Chen, X.Y.; Guo, Z.L.; Hu, Y.C.; Liu, C.P.; Sun, Y.Z. Extraction and separation of In(III), Ga(III) and Zn(II) from sulfate solution using extraction resin. Hydrometallurgy 2006, 82, 137–143. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, L.; Wang, Y.; Lou, Z.; Shan, W.; Xiong, Y.; Fan, Y. Preparation of a biomass adsorbent for gallium(III) based on corn stalk modified by iminodiacetic acid. J. Taiwan Inst. Chem. Eng. 2018, 91, 291–298. [Google Scholar] [CrossRef]
- Chatterjee, A.; Schiewer, S. Multi-resistance kinetic models for biosorption of Cd by raw and immobilized citrus peels in batch and packed-bed columns. Chem. Eng. J. 2014, 244, 105–116. [Google Scholar] [CrossRef]
- Ezzati, R.; Ezzati, S.; Azizi, M. Exact solution of the Langmuir rate equation: New Insights into pseudo-first-order and pseudo-second-order kinetics models for adsorption. Vacuum 2024, 220, 112790. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; El-Bindary, A.A.; Kouta, E.Y.; Guibal, E. Functionalization of polyacrylonitrile/Na-Y-zeolite composite with amidoxime groups for the sorption of Cu(II), Cd(II) and Pb(II) metal ions. Chem. Eng. J. 2018, 332, 727–736. [Google Scholar] [CrossRef]
- Guibal, E.; Milot, C.; Tobin, J.M. Metal-anion sorption by chitosan beads: Equilibrium and kinetics studies. Ind. Eng. Chem. Res. 1998, 37, 1454–1463. [Google Scholar] [CrossRef]
- Yu, L.L.; Jiang, L.N.; Wang, S.Y.; Sun, M.M.; Li, D.Q.; Du, G.M. Pectin microgel particles as high adsorption rate material for methylene blue: Performance, equilibrium, kinetic, mechanism and regeneration studies. Int. J. Biol. Macromol. 2018, 112, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Zhao, Z.; Chai, Y.; Li, X.; Hua, Z.; Xiao, Y.; Yang, Y. Binding Mechanism of the Amidoxime Functional Group on Chelating Resins toward Gallium(III) in Bayer Liquor. Ind. Eng. Chem. Res. 2015, 54, 8025–8030. [Google Scholar] [CrossRef]


| Type | Wet Superficial Density (g/mL) | Particle Size (mm) | Maximum Adsorption Capacity (mmol/g) | Functional Structure |
|---|---|---|---|---|
| Resin A | 0.65–0.70 | 0.80–1.00 | 5.5 | 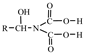 |
| Resin B | 0.70–0.80 | 0.40–1.00 | 5.5 | 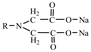 |
| Resin C | 1.04–1.07 | 0.55–1.13 | 4.3 | 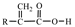 |
| Initial Concentration of Ga (mg/L) | 10 | 50 | 100 | 150 | 200 |
|---|---|---|---|---|---|
| Adsorption capacity of resin A (mg/g) | 2 | 11 | 22 | 31 | 35 |
| Adsorption capacity of resin B (mg/g) | 2 | 11 | 21 | 28 | 24 |
| Adsorption capacity of resin C (mg/g) | 2 | 11 | 19 | 23 | 22 |
| Temperature (°C) | qm,exp (mg/g) | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|---|
| kl (L/mg) | qm (mg/g) | R2 | kF (mg(1−1/n)·L1/n/g) | n | R2 | ||
| 25 | 46.39 | 0.06 | 46.99 | 0.998 | 5.47 | 2.62 | 0.868 |
| 35 | 48.45 | 0.08 | 48.50 | 0.997 | 7.26 | 2.89 | 0.734 |
| 45 | 50.21 | 0.10 | 50.33 | 0.998 | 8.36 | 3.03 | 0.652 |
| 55 | 55.54 | 0.39 | 53.05 | 0.999 | 10.57 | 3.13 | 0.660 |
| C0 (mg/L) | qe,exp. (mg/g) | Exact form of Langmuir | Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|---|---|---|
| ka [L/(mg·h)] | R2 | k1 (h−1) | qe (mg/g) | R2 | k2 [g/(mg·h)] | qe (mg/g) | R2 | ||
| 10 | 2.04 | 2.37 × 10−3 | 0.858 | 0.32 | 0.39 | 0.717 | 2.22 | 2.06 | 0.999 |
| 50 | 11.30 | 1.28 × 10−3 | 0.813 | 0.47 | 6.55 | 0.975 | 0.16 | 11.61 | 0.999 |
| 100 | 21.72 | 1.99 × 10−3 | 0.998 | 0.33 | 16.96 | 0.995 | 0.03 | 23.21 | 0.999 |
| 150 | 31.02 | 1.54 × 10−3 | 0.984 | 0.21 | 25.52 | 0.989 | 0.01 | 34.39 | 0.999 |
| 200 | 37.84 | 8.79 × 10−4 | 0.990 | 0.12 | 27.48 | 0.940 | 0.01 | 37.61 | 0.995 |
| 500 | 54.19 | 4.66 × 10−4 | 0.954 | 0.20 | 32.06 | 0.991 | 0.01 | 59.84 | 0.999 |
| 1000 | 54.83 | 1.63 × 10−4 | 0.607 | 0.21 | 14.79 | 0.627 | 0.08 | 53.45 | 0.999 |
| Ion | C0 (mg/L) | Ce (mg/L) | Qe (mg/g) | Kd (mL/g) | |
|---|---|---|---|---|---|
| Ga3+ | 97.60 | 5.12 | 18.49 | 3611.36 | 1.00 |
| Al3+ | 99.80 | 94.92 | 0.97 | 10.28 | 351.21 |
| Ca2+ | 93.50 | 90.32 | 0.63 | 7.04 | 512.67 |
| K+ | 89.00 | 85.00 | 0.80 | 9.41 | 383.71 |
| Mg2+ | 101.40 | 99.61 | 0.35 | 3.59 | 1006.58 |
| Fe2+ | 102.3 | 95.34 | 1.39 | 14.60 | 247.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Wen, C.; Li, S.; Sun, Z.; Hou, X.; Li, H.; Ma, Z. Adsorption Performance and Mechanism of Gallium from Sulfuric Acid Leach Liquor of High-Alumina Fly Ash. Separations 2025, 12, 190. https://doi.org/10.3390/separations12080190
Wu W, Wen C, Li S, Sun Z, Hou X, Li H, Ma Z. Adsorption Performance and Mechanism of Gallium from Sulfuric Acid Leach Liquor of High-Alumina Fly Ash. Separations. 2025; 12(8):190. https://doi.org/10.3390/separations12080190
Chicago/Turabian StyleWu, Wenfen, Chaolu Wen, Shaopeng Li, Zhenhua Sun, Xinjuan Hou, Huiquan Li, and Zhibin Ma. 2025. "Adsorption Performance and Mechanism of Gallium from Sulfuric Acid Leach Liquor of High-Alumina Fly Ash" Separations 12, no. 8: 190. https://doi.org/10.3390/separations12080190
APA StyleWu, W., Wen, C., Li, S., Sun, Z., Hou, X., Li, H., & Ma, Z. (2025). Adsorption Performance and Mechanism of Gallium from Sulfuric Acid Leach Liquor of High-Alumina Fly Ash. Separations, 12(8), 190. https://doi.org/10.3390/separations12080190






