Abstract
A series of homologous saturated fatty acids were introduced and evaluated as collectors for ilmenite flotation using a combination of micro-flotation tests and surface tension measurements. The results showed that ilmenite exhibited good flotation behaviour when decanoic and dodecanoic acids were used as collectors; however, saturated fatty acids with shorter or longer carbon chains were not suitable for ilmenite flotation (caused either by poor collection ability or limited solubility in water). The optimum flotation pH range was also dependent on the carbon chain length of saturated fatty acids, and the solution surface tension did not always match well with the ilmenite flotation behaviour when using a series of saturated fatty acids as the collector. The associated solution chemistry properties under series saturated fatty acid flotation systems were discussed, and the adsorption mechanism of decanoic acid onto the ilmenite surface was also investigated via FTIR, zeta potential, and contact angle measurements.
1. Introduction
Flotation is a widely used technology in mineral processing, especially for processing low-grade, fine-particle, and complex ores which were initially considered uneconomical [1,2,3,4,5]. The use of fatty acids (usually categorized as saturated and unsaturated fatty acids) or their soaps as collectors to modify the physicochemical properties of oxide mineral particles for subsequent flotation separation is a common practice [6,7,8,9]. The chemistry of such solutions becomes complicated when these reagents are added due to changes in the solution pH and reagent concentration. Therefore, methods for determining the relationship between the flotation behaviours of mineral particles and the solution chemistry properties of fatty acid systems, such as experimental tools including micro-flotation tests and surface tension measurements, and computational tools including solution chemistry diagram calculation, have been widely employed [10,11,12,13]. For unsaturated fatty acids, such as sodium oleate, mechanisms including electrostatic interactions of individual ions (physical adsorption), chemisorption, ion-molecule dimeric complex adsorption, and co-adsorption of ions and molecules have been proposed [14,15,16]. For saturated fatty acids, physical adsorption, chemisorption, or co-adsorption, depending on the solution pH and fatty acid type, has been discussed for haematite flotation [11]. However, the influence of saturated fatty acids, which are sometimes considered weak electrolyte surfactants, on the solution chemistry properties and associated flotation behaviours of mineral particles has not yet been sufficiently studied, although some works have been conducted on hematite flotation [11,17,18,19].
Ilmenite is the main titanium-containing mineral and has recently become much more attractive owing to its wide applications in medicine, navigation, aerospace, functional materials, and industrial catalysis [20,21,22]. For the mineral processing of ilmenite-containing ores, magnetic separation and flotation are two typical techniques used to upgrade the TiO2 content in ilmenite concentrate [23,24]. Particularly when ores are of low grade and have complex mineralogy, flotation is an essential option for obtaining ilmenite concentrates. In ilmenite flotation, collectors play a pivotal role, and common collectors include sodium oleate, organic phosphonic salts, and chelating collectors. [20,25,26,27]. The development of novel collectors for efficient ilmenite flotation has always been a research hotspot. Typically, collectors contain a hydrophobic carbon chain and a hydrophilic functional group (such as a carboxyl, sulfonic acid, or phosphonic acid group), both of which collectively affect the effectiveness of the collector in ilmenite flotation [4]. Collectors with various hydrophilic functional groups have been frequently reported in ilmenite flotation [20,25,26,27,28,29]. However, the influence of the carbon chain length of collectors on ilmenite floatability remains unclear, and the use of saturated fatty acids as collectors for ilmenite flotation has not been well studied. To address this, the ilmenite flotation behaviour using a series of saturated fatty acids as collectors was evaluated by micro-flotation tests in this study. The associated solution chemistry properties affected by saturated fatty acids were discussed by referring to surface tension tests and solution chemistry diagram analysis.
2. Materials and Methods
2.1. Materials and Reagents
Pure ilmenite was obtained from Panzhihua in Sichuan Province, China. The mineral was ground in a porcelain mill using an agate ball. The products were then sieved, and the −0.074 + 0.038 mm size fraction was used for the micro-flotation experiments. The X-ray fluorescence (XRF) results indicated that the content of the element titanium was 30.58%, showing that the purity of ilmenite was above 90%. Figure 1 shows the X-ray diffraction (XRD) pattern of the pure ilmenite samples used in this study, which indicates that the main mineral phase was ilmenite. Based on the XRF and XRD results, the purity of the ilmenite was sufficiently high to meet the requirements for micro-flotation.
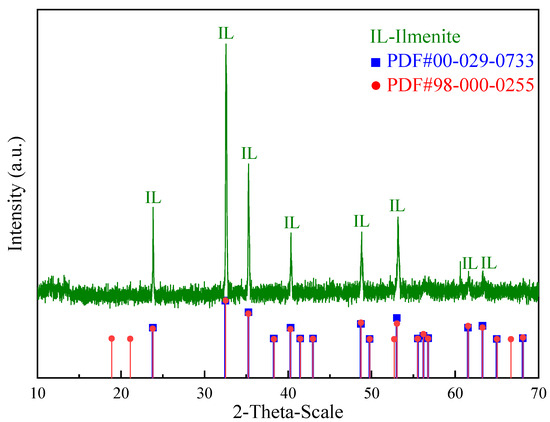
Figure 1.
XRD pattern of pure ilmenite samples used in the study.
Analytical grade saturated fatty acids were used as collectors in the flotation experiments and were supplied by Baisaiqin Chemical Technology Co., Ltd., Shanghai, China. The pH was adjusted using sodium hydroxide and sulphuric acid stock solutions. Deionised water (resistivity = 18.3 MΩ × cm) was used in all experiments.
2.2. Flotation Tests
Ilmenite micro-flotation experiments were conducted in a 40 mL plexiglass cell using an XFG-type self-aerated flotation machine purchased from Prospecting Machinery Factory, Changchun, Jilin, China. The spindle speed (agitation speed) of the flotation machine was controlled at 1700 rpm. For each test, 2 g samples were used and placed into the flotation cell, followed by the addition of 35 mL deionised water to adjust the slurry. After the desired amounts of reagents, such as the pH modifier and collector, were added, the suspension was conditioned for 3 min. After the suspension was completely adjusted, flotation was conducted for 3 min. The concentrate and tailing samples were filtered, dried, and weighed to calculate flotation recovery. In order to assess the accuracy of the flotation tests, at least three tests were performed in each case, and the mean values and standard deviations were calculated and plotted.
A binary mineral mixture was prepared by combining ilmenite and titanaugite in a 1:1 mass ratio. The slurry preparation, pH regulation, and collector addition procedures were conducted following the same protocols as those used in the single-mineral micro-flotation experiments. After flotation, the resulting concentrates and tailings were dried and analysed for TiO2 content to determine the recovery [28].
2.3. Surface Tension Measurements
The surface tensions of the aqueous solutions containing surfactants or pure water were measured using a Kruss K10 automatic tensiometer (Krüss GmbH, Hamburg, Germany) with a platinum plate. The plate was burned in an alcohol flame after washing to completely remove any adsorbed surfactants before each measurement. The temperature was maintained at room temperature (25.0 °C). In all cases, more than three successive measurements were performed, and the average values are reported in this paper.
2.4. Surface Characterisation Techniques
Fourier-transform infrared (FTIR) spectroscopy was performed on three specimens: untreated ilmenite, C10, and ilmenite modified with C10. The spectra were recorded using a Bruker Alpha FTIR spectrophotometer (Bruker Corporation, Billerica, MA, USA) employing the KBr pellet technique, in which the sample-to-KBr mass ratio was maintained at 1:100. The zeta potentials of ilmenite, both untreated and after C10 adsorption, were evaluated as a function of pH using a ZS90 Zeta Meter (Malvern Instruments Ltd., Malvern, UK). To prepare the suspension, 0.05 g of the mineral sample was dispersed in 500 mL of 10 mM KNO3 solution and stirred for 5 min. Subsequently, the fine particle suspension was transferred to a capillary cell for analysis. Each reported zeta potential value was the mean of three independent measurements. For the contact angle measurements, the mineral sample with a particle size below 2 μm was pressed into pellets under 20.0 kPa using a tablet press. The resulting thin-layer specimens were immersed in reagent solutions of specified concentrations and stirred for 10 min. After treatment, the excess surface solution was rinsed off with deionised water, and the samples were dried at low temperature in a vacuum oven. The dried pellets were carefully placed in the rectangular glass chamber of a JC2000C contact angle goniometer for measurement. Each sample was tested at least three times, and the average value was reported as the final result.
3. Results and Discussion
3.1. Micro-Flotation Analysis
Figure 2a,b show the flotation behaviour of ilmenite as a function of pH (collector dosage fixed at 0.5 kg/t) and collector dosage (five levels at 0.1, 0.3, 0.5, 0.7, and 1.0 kg/t), respectively, when using homologous series of saturated fatty acids as collectors.
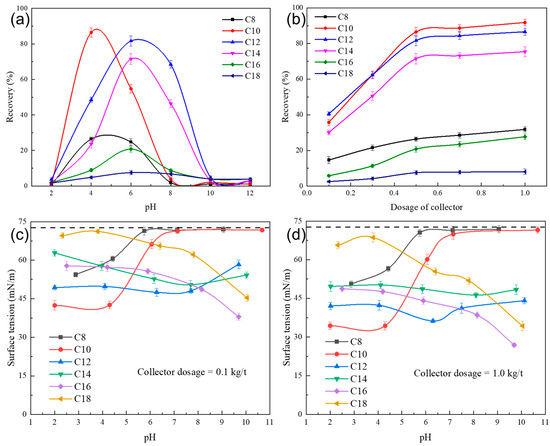
Figure 2.
Flotation recovery of ilmenite (a) as a function of pH using a series of saturated acids as the collector at a fixed dosage of 0.5 kg/t and (b) as a function of the collector dosage at the optimum pH of each (pH = 4 for C8 and C10; pH = 6 for C12, C14, C16, and C18); surface tension as a function of pH (c,d) using saturated acids as the collector (dashed line—surface tension of pure water).
Ilmenite floatability was significantly influenced by pH when saturated fatty acids were used as collectors (Figure 2a). For C8, ilmenite exhibited some floatability in the pH range of 3–6, while the recovery was nearly zero when the pH was over 8. Overall, the optimum pH range for ilmenite flotation using C8 as the collector was in the acidic region. When C10 was used as the collector, the optimum pH range for ilmenite flotation was around 3–7, and ilmenite recovery reached a maximum at pH≈4; this behaviour was similar to that observed in the C8-ilmenite flotation system. Figure 2a demonstrates that C12 was also an effective collector for ilmenite flotation, and its optimum pH range was 5–8. Compared to C8 and C10, the optimum flotation pH range of C12 shifted to a more neutral region. C14 had a lower capability for ilmenite flotation than C10 and C12, and its optimum pH range was about 5–8, which was similar to that of C12. C16 and C18 had limited collecting abilities (Figure 2a), even within their optimum pH range (5–7). Overall, Figure 2a indicates a typical changing pattern of ilmenite flotation recovery using series saturated fatty acids (C8–C18) as affected by pH: both highly acidic and alkaline pH environments could deteriorate ilmenite flotation, and each fatty acid had its own optimum pH range in which ilmenite recovery reached a maximum. Similar trends have been found for ilmenite flotation using oleate as the collector [20,30,31]: (1) in highly acidic pH slurries, fatty acids are mostly present in the form of neutral R–COOH molecules (undissociated form), which have low water solubility and weak adsorption affinity for the mineral surface; (2) while in highly alkaline pH slurries, ilmenite surfaces undergo hydroxylation, forming strong hydrated oxide layers that hinder collector adsorption.
The influence of collector dosage on ilmenite flotation recovery was studied at the respective optimum pH of each fatty acid. As shown in Figure 2b, ilmenite recovery increased with increasing collector dosage, and the increase was particularly evident for C10, C12, and C14. When C10 was used as the collector and its dosage was increased from 0.1 kg/t to 0.5 kg/t, ilmenite flotation recovery increased by about 50% to ~86%. The recovery exceeded 90% at a C10 concentration of 1.0 kg/t. However, increasing the C10 dosage did not significantly increase ilmenite recovery when the C10 dosage was above 0.5 kg/t. Similar trends were also observed when C12 or C14 was used as the collector. For C12, ilmenite recovery increased by ~40% when its dosage was increased from 0.1 kg/t to 0.5 kg/t. At a C12 concentration of 1.0 kg/t, the flotation recovery of ilmenite surpassed 85% within the optimum pH range. Although C14 showed certain ilmenite collecting ability, its ability to effectively float ilmenite was limited (maximum recovery below 80%). For C8, C16, and C18, the collector dosage had a limited ability to increase ilmenite flotation recovery, all remaining at a relatively low level. Therefore, C10 and C12 can be used as effective collectors for ilmenite flotation, with higher recovery than the other tested fatty acids.
3.2. Surface Tension Analysis
To ensure successful flotation, surfactants are utilised to modify the physicochemical properties of mineral particles, causing a difference in wettability between the valuable minerals and gangue and facilitating effective separation by flotation [32,33,34]. The addition of surfactants changes the surface tension of the solution/slurry, which is highly related to the solution pH, surfactant type, and concentration [35,36,37]. In this study, the surface tension changes resulting from the addition of homologous saturated fatty acids at two dosage levels (0.1 and 1.0 kg/t) were studied. The corresponding results are shown in Figure 2c,d.
Figure 2c,d showed that the surface tension was markedly affected by the solution pH, and increasing collector dosage can improve the collector’s ability to reduce the solution surface tension. A continuously decreasing trend in surface tension was observed when the pH was lower than 6 (C8) or 7 (C10); however, the ability of C8 and C10 to reduce the solution surface tension was quite poor when the pH was higher than 7. A different pattern of changes in surface tension affected by pH (surface tension decreased at first and then increased) was found for C12 and C14 compared to C8 and C10. The optimum pH range for C12 in terms of surface tension reduction was 5–8, and for C14, the pH range in which relatively lower surface tension values were observed was around 6–9. Compared with the C8, C10, C12, and C14 systems, the relationship between the surface tension and pH in the C16 and C18 systems was somewhat different; specifically, the solution surface tension decreased gradually with increasing pH values.
By comparing the results in Figure 2, it was found that: (1) for saturated fatty acids with a short carbon chain length (octanoic acid and decanoic acid), their optimum pH range for ilmenite flotation was consistent with their ability to lower the solution surface tension; (2) for saturated fatty acids with a medium chain length (dodecanoic acid and tetradecanoic acid), their optimum pH range for the lowering of solution surface tension roughly agreed with the flotation behaviours (i.e., higher recovery corresponded with lower solution surface tension); and (3) for saturated fatty acids with a longer carbon chain length (hexadecanoic acid and octadecanoic acid), their effects on lowering solution surface tension cannot correspond to or predict ilmenite flotation behaviour well. Similar phenomena have been reported for hematite flotation systems [11]. This indicates that as the carbon chain length of the saturated fatty acids increased, their limited solubility began to influence the results to a large extent.
Therefore, it can be seen that solution surface tension as affected by collector cannot always match the performance of such collector in mineral flotation, although, as a common technique, solution surface tension has proved to be useful in flotation mechanism elaboration [38,39,40,41]. A similar phenomenon has also been observed when using adsorption experiments to elucidate the flotation mechanism of a hematite-ilmenite-oleate system (the measured amount of the collector adsorbed on the hematite/ilmenite surface did not match well with its flotation behaviour) [30,42,43]. Revisiting the relevant literature showed that the measured collector adsorption amount generally decreased as the solution pH increased, but the flotation of hematite/ilmenite reached a maximum at a neutral or weakly alkaline pH. To directly measure the amount of collector adsorbed in the hematite-oleate system, some thermodynamic studies have been conducted, and the results are satisfactory [42,44].
3.3. Solution Chemistry Diagram Analysis
According to previous reports, undissociated acids, ions, dimers, and acid-soap complexes are generally considered to be the active species present in fatty acid systems [10,45,46]. Which of these species acts as the main functional species depends on the pH value and the collector concentration. Studies have shown that, as ion-molecular species, acid-soap complexes (RCOOH·RCOO−) are larger surfactants with high surface activity because of the increase in molecular size and their low intrinsic solubility [10,31,38]. In general, the amount of acid-soap complex reaches a maximum around the pHeq, near which RCOOH(aq) and RCOOH(l) can form a saturated solution (which is closely related to the solubility and pKa values of the fatty acids) [31,46]. However, most previous studies have focused on the solution chemistry analyses of unsaturated fatty acids, and little information is available on the series of saturated fatty acids. To provide a better understanding, the combined solution chemistry diagrams of the series of saturated fatty acids examined in this work were calculated, and the results are shown in Figure 3. The basic data used for solution chemistry analyses, such as pKa and solubility values, were obtained from Quast’s study [11]. Furthermore, only one-stage hydrolysis was considered while creating Figure 3; thus, dimers and acid-soap complexes are not shown. In general, if dimers and acid-soap complexes are present in the solution, they would reach their maximum concentration at pHeq; therefore, the solution chemistry diagrams in Figure 3 could be used for qualitative analyses.
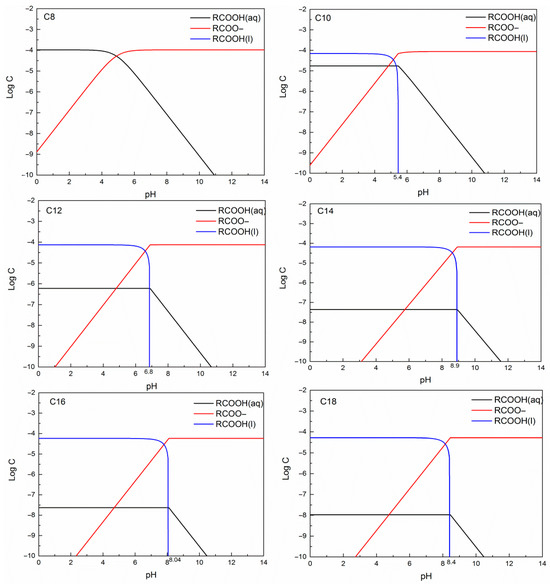
Figure 3.
Solution chemistry diagrams of a series of saturated fatty acids.
As shown in Figure 3, the pHeq values increased with increasing carbon chain length. The abnormal pHeq value of C14 could be due to its relatively higher pKa value (normally 4.7–4.9 for this series of saturated fatty acids, except for that of C14, which was around 5.75 [11]). As the acid-soap complex is the most effective active species, the optimum pH range for better flotation activity shifts from lower to higher pH values as the carbon chain length of the fatty acids increases. To some extent, this could be responsible for the changes in the optimum pH range for ilmenite flotation from the acidic region for short-chain-length fatty acids to the neutral region for longer ones.
3.4. Adsorption Mechanism of Decanoic Acid on Ilmenite Surface
In this study, C10 (decanoic acid) was found to be the most effective collector for ilmenite flotation, as demonstrated in micro-flotation tests (Figure 2a,b) among a series of saturated fatty acids, and its optimum flotation pH range was in the acidic region. This finding is of significant importance in the ilmenite cleaning flotation process. In China, the Panzhihua Iron and Steel Limited Corporation, located in the Panzhihua region, is the largest titanium concentrate producer. During industrial production, the cleaning stages are carried out under acidic conditions, which are maintained by adding sulphuric acid [47,48]. Therefore, studies on improving the collecting performance of collectors in the acidic region are urgently needed. After determining that C10 was a much more effective collector than C8, C12, C14, C16, and C18, the adsorption mechanism of C10 on the ilmenite surface was further explored.
Figure 4a shows the FT-IR spectra of C10, ilmenite, and ilmenite treated with C10 at pH = 4. In the C10 spectra, the bands at 2800–3000 cm−1 were attributed to the C-H stretching vibrations of the -CH2–and -CH3 groups, respectively. A series of characteristic absorption bands corresponding to -COO- appeared at around 900–1750 cm−1. A comparison of the spectra of ilmenite and C10-treated ilmenite revealed that no characteristic bands of C10 were observed on the ilmenite surface after the addition of C10 to ilmenite. This result shows that chemisorption did not occur between C10 and ilmenite at pH = 4. Zeta potential measurements (Figure 4b) indicated that ilmenite had an isoelectric point (IEP) of approximately pH 5.3; thus, the ilmenite particles were positively charged at the optimum pH for ilmenite flotation when using C10 as the collector. After C10 adsorption, the zeta potential of ilmenite became more negative, indicating that negatively charged C10 species were adsorbed on the ilmenite surface. It has been previously demonstrated that the IEP value for oleic acid is approximately 2–3, indicating that the undissociated acid molecules/colloidal precipitates are negatively charged when the solution pH is above the IEP [18,19,49]. C10 has similar solution chemistry properties to oleic acid, and thus the colloidal precipitates of C10 at pH = 4 would be negatively charged, which was beneficial for interactions between ilmenite (positively charged) and C10 (negatively charged) via electrostatic forces. After the adsorption of C10 on the ilmenite surface, the ilmenite wettability changed, as demonstrated by the contact angle measurement (Figure 4c). Without C10 treatment, ilmenite had a contact angle of 35.62°, suggesting a hydrophilic surface in its natural form. After C10 adsorption at pH = 4, the contact angle of ilmenite increased to 88.35°, indicating that a more hydrophobic surface was formed. It should be noted that in the C10-ilmenite flotation system, the bubbles were typically negatively charged. This means that after C10 adsorption, negatively charged ilmenite particles and bubbles cannot interact via electrostatic attraction, and in such conditions, hydrophobic interactions play a crucial role based on the Extended DLVO theory [50].
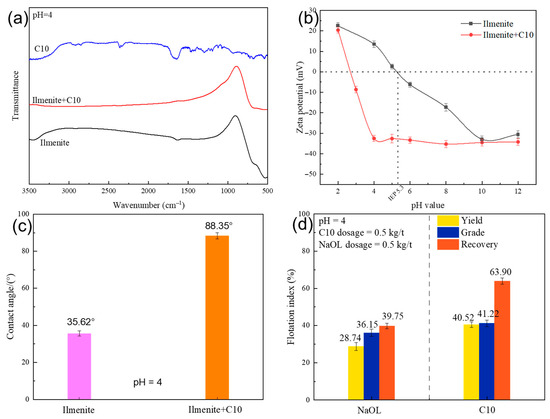
Figure 4.
FT-IR spectra of ilmenite, C10, and ilmenite treated with C10 at pH = 4 (a); zeta potential of ilmenite as a function of solution pH before and after C10 treatment (b); contact angle of ilmenite and ilmenite treated with C10 at pH = 4 (c); artificial binary flotation results of ilmenite/titanaugite (d).
To further explore the effectiveness of C10 in ilmenite flotation, flotation tests of artificial binary minerals of ilmenite and titanaugite at a mass ratio of 1:1 were conducted at pH = 4. For comparison, the most common collector in ilmenite flotation, sodium oleate (NaOL), was also used, and the results are shown in Figure 4d. The results indicated that C10 was much more effective than NaOL in the flotation separation of ilmenite from titanaugite at pH 4. The TiO2 grade and recovery increased by 5.07% and 24.15%, respectively.
4. Conclusions
In this research, the flotation behaviour of ilmenite using a series of homologous saturated fatty acids as collectors was evaluated, and surface tension measurements were performed to explore the potential mechanism. Based on the flotation results, a suitable carbon chain length was found to be essential for the saturated fatty acids to exhibit good collecting ability. Saturated fatty acids with shorter or longer carbon chains were unsuitable for ilmenite flotation. Additionally, the optimum flotation pH range shifted from the acidic to the neutral region as the carbon chain length increased. In this study, decanoic and dodecanoic acids were found to have more effective collecting abilities than the other saturated fatty acids. Surface tension measurements further confirmed that within the optimum flotation pH range, octanoic, decanoic, dodecanoic, and tetradecanoic acids could lower the solution surface tension to a relatively low level. However, it should be noted that the surface tension measurements when using hexadecanoic acid or octadecanoic acid as the collector did not match the flotation effectiveness over the entire pH range. Decanoic acid, the most effective collector for ilmenite flotation among the fatty acids studied, was adsorbed onto the ilmenite surface via a physical process at its optimum pH of 4.
Author Contributions
Conceptualization, J.Z. and P.C.; methodology, J.Z., H.H. and L.S.; software, J.Z. and P.C.; validation, J.Z., X.Y. and H.Z.; formal analysis, J.Z. and H.H.; investigation, J.Z. and H.H.; resources, X.Y. and H.Z.; data curation, J.Z. and H.H.; writing—original draft preparation, J.Z.; writ-ing—review and editing, P.C.; visualization, J.Z.; supervision, P.C.; project administration, P.C.; funding acquisition, J.Z. and P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Program of China [2023YFC2908305], National Natural Science Foundation of China (52474296, 52074357), and Sichuan Natural Science Foundation, China (24NSFSC1217, 24NSFSC1323).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Hao He was employed by the company Sichuan Lianxin Mining Co., Ltd., author Lin Song was employed by the company Aba Mining Co., Ltd., author Xiaohai Yao was employed by the company Huili Caitong Iron and Titanium Co., Ltd., author Hongxian Zhang was employed by the company Huili Xiushuihe Mining Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Somasundaran, P.; Wang, D. Solution Chemistry: Minerals and Reagents; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Jia, K.; Ding, R.; Chen, Y.; Lu, T.; Li, G.; Cao, Y.; Wang, C. Green, multiple-ligand collector sodium myristoyl glutamate for flotation of smithsonite. Appl. Surf. Sci. 2024, 660, 159932. [Google Scholar] [CrossRef]
- Slabov, V.; Jain, G.; Larsen, E.; Kota, H.R.; Chernyshova, I. Eco-friendly collectors for flotation of fine hematite and malachite particles. Min. Metall. Explor. 2023, 40, 475–492. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery; Butterworth-Heinemann: Oxford, UK, 2015. [Google Scholar]
- Zhai, J.; Niu, X.; Chen, P.; Fan, C.; Chen, Z.; Yang, Y. Lithium refinery residue reuse in construction materials production: Status, challenges and further perspectives. J. Environ. Manag. 2025, 379, 124801. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gao, Z.; Sun, W.; Yin, Z.; Wang, J.; Hu, Y. Adsorption of a novel reagent scheme on scheelite and calcite causing an effective flotation separation. J. Colloid Interface Sci. 2018, 512, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Satur, J.V.; Calabia, B.P.; Hoshino, M.; Morita, S.; Seo, Y.; Kon, Y.; Takagi, T.; Watanabe, Y.; Mutele, L.; Foya, S. Flotation of rare earth minerals from silicate–hematite ore using tall oil fatty acid collector. Miner. Eng. 2016, 89, 52–62. [Google Scholar] [CrossRef]
- Cook, B.K.; Gibson, C.E. A review of fatty acid collectors: Implications for spodumene flotation. Minerals 2023, 13, 212. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, P.; Zhang, W.; Dai, H.; Tian, Y.; Tian, M.; Sun, W. Study on the adsorption selectivity of a novel fatty acid collector on apatite, dolomite, and calcite surfaces for improved flotation. Miner. Eng. 2025, 232, 109523. [Google Scholar] [CrossRef]
- Kulkarni, R.; Somasundaran, P. Flotation chemistry of hematite/oleate system. Colloids Surf. 1980, 1, 387–405. [Google Scholar] [CrossRef]
- Quast, K. Flotation of hematite using C6–C18 saturated fatty acids. Miner. Eng. 2006, 19, 582–597. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Tian, J.; Wu, H.; Wang, L.; Yang, Y.; Wang, Z. Synergistic effect of mixed cationic/anionic collectors on flotation and adsorption of muscovite. Colloids Surf. A Physicochem. Eng. Asp. 2016, 492, 181–189. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, M.; Han, R.; Li, Z.; Zhou, A.; Wang, H. Enhanced flotation separation of coal gasification fine slag by composite collectors containing polar fatty acids. J. Environ. Chem. Eng. 2024, 12, 114890. [Google Scholar] [CrossRef]
- Rao, K.H.; Cases, J.; De Donato, P.; Forssberg, K. Mechanism of oleate interaction on salt-type minerals: IV. Adsorption, electrokinetic, and diffuse reflectance FT-IR studies of natural fluorite in the presence of sodium oleate. J. Colloid Interface Sci. 1991, 145, 314–329. [Google Scholar] [CrossRef]
- Somasundaran, P.; Ananthapadmanabhan, K.; Ivanov, I. Dimerization of oleate in aqueous solutions. J. Colloid Interface Sci. 1984, 99, 128–135. [Google Scholar] [CrossRef]
- Fu, X.; Gao, Y.; Peng, C.; Han, H.; Sun, W.; Yue, T. Adsorption mechanism of sodium oleate at hematite/quartz–water interfaces: A quantitative molecular insight. Miner. Eng. 2024, 216, 108904. [Google Scholar] [CrossRef]
- Quast, K. Flotation of hematite using 18-carbon fatty acids. Miner. Eng. 2021, 160, 106647. [Google Scholar] [CrossRef]
- Laskowski, J. Electrokinetic measurements in aqueous solutions of weak electrolyte type surfactants. J. Colloid Interface Sci. 1993, 159, 349–353. [Google Scholar] [CrossRef]
- Laskowski, J.; Yordan, J.; Yoon, R. Electrokinetic potential of microbubbles generated in aqueous solutions of weak electrolyte type surfactants. Langmuir 1989, 5, 373–376. [Google Scholar] [CrossRef]
- Chen, P.; Zhai, J.; Sun, W.; Hu, Y.; Yin, Z.; Lai, X. Adsorption mechanism of lead ions at ilmenite/water interface and its influence on ilmenite flotability. J. Ind. Eng. Chem. 2017, 53, 285–293. [Google Scholar] [CrossRef]
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.; Lichtfouse, E. Synthesis and application of titanium dioxide photocatalysis for energy, decontamination and viral disinfection: A review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef] [PubMed]
- García-Muñoz, P.; López-Maxías, C.; Guerra-Rodríguez, S.; Carbajo, J.; Casas, J.A.; Rodríguez-Chueca, J. Photocatalytic activation of peroxymonosulfate using ilmenite (FeTiO3) for Enterococcus faecalis inactivation. J. Environ. Chem. Eng. 2022, 10, 108231. [Google Scholar] [CrossRef]
- Zhai, J.; Chen, P.; Sun, W.; Chen, W.; Wan, S. A review of mineral processing of ilmenite by flotation. Miner. Eng. 2020, 157, 106558. [Google Scholar] [CrossRef]
- Zheng, X.; Du, L.; Li, S.; Jing, Z.; Lu, D.; Jia, K.; Cadiere, K.; Peng, B.; Wang, Y. A novel method for efficient recovery of ilmenite by high gradient magnetic separation coupling with magnetic fluid. Miner. Eng. 2023, 202, 108279. [Google Scholar] [CrossRef]
- Song, Q.; Tsai, S.C. Flotation of ilmenite using benzyl arsonic acid and acidified sodium silicate. Int. J. Miner. Process. 1989, 26, 111–121. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, H.; Tang, Q.; Wang, S.; Zhao, G.; Liu, G. A novel collector 2-ethyl-2-hexenoic hydroxamic acid: Flotation performance and adsorption mechanism to ilmenite. Appl. Surf. Sci. 2015, 353, 882–889. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, G.; Feng, Q.; Yan, D.; Wang, W. Effect of surface dissolution on flotation separation of fine ilmenite from titanaugite. Trans. Nonferrous Met. Soc. China 2011, 21, 1149–1154. [Google Scholar] [CrossRef]
- Zhai, J.; Chen, P.; Wang, H.; Yang, Y.; Guan, C. Eco-friendly sophorolipid enabling the selective and efficient flotation separation of ilmenite from titanaugite. Miner. Eng. 2025, 232, 109567. [Google Scholar] [CrossRef]
- Pan, C.; Liu, J.-y.; Li, W.-s.; Zhai, J.-h.; Yang, Y.-h. Flotation behavior of ilmenite using 1, 10-phenanthroline as novel collector. Trans. Nonferrous Met. Soc. China 2024, 34, 995–1002. [Google Scholar]
- Chen, P.; Zhai, J.; Sun, W.; Hu, Y.; Yin, Z. The activation mechanism of lead ions in the flotation of ilmenite using sodium oleate as a collector. Miner. Eng. 2017, 111, 100–107. [Google Scholar] [CrossRef]
- Mehdilo, A.; Irannajad, M.; Rezai, B. Effect of crystal chemistry and surface properties on ilmenite flotation behavior. Int. J. Miner. Process. 2015, 137, 71–81. [Google Scholar] [CrossRef]
- Gao, Z.; Li, C.; Sun, W.; Hu, Y. Anisotropic surface properties of calcite: A consideration of surface broken bonds. Colloids Surf. A: Physicochem. Eng. Asp. 2017, 520, 53–61. [Google Scholar] [CrossRef]
- Jiang, W.; Gao, Z.; Khoso, S.A.; Gao, J.; Sun, W.; Pu, W.; Hu, Y. Selective adsorption of benzhydroxamic acid on fluorite rendering selective separation of fluorite/calcite. Appl. Surf. Sci. 2018, 435, 752–758. [Google Scholar] [CrossRef]
- Von Rybinski, W.; Schwuger, M. Adsorption of surfactant mixtures in froth flotation. Langmuir 1986, 2, 639–643. [Google Scholar] [CrossRef]
- de Castro, F.H.B.; Borrego, A.G. Modification of surface tension in aqueous solutions of sodium oleate according to temperature and pH in the flotation bath. J. Colloid Interface Sci. 1995, 173, 8–15. [Google Scholar] [CrossRef]
- Pugh, R.; Weissenborn, P.; Paulson, O. Flotation in inorganic electrolytes; the relationship between recover of hydrophobic particles, surface tension, bubble coalescence and gas solubility. Int. J. Miner. Process. 1997, 51, 125–138. [Google Scholar] [CrossRef]
- Yarar, B.; Kaoma, J. Estimation of the critical surface tension of wetting of hydrophobic solids by flotation. Colloids Surf. 1984, 11, 429–436. [Google Scholar] [CrossRef]
- Pugh, R.; Stenius, P. Solution chemistry studies and flotation behaviour of apatite, calcite and fluorite minerals with sodium oleate collector. Int. J. Miner. Process. 1985, 15, 193–218. [Google Scholar] [CrossRef]
- Man, X.; Wang, C.; Yu, S.; Yang, X.; Liu, J.; Fu, Y.; Dong, Z.; Zhi, H.; Ou, L. Low-Temperature Flotation Separation of Diaspore from Kaolinite by Using a Mixed Collector. Minerals 2022, 12, 891. [Google Scholar] [CrossRef]
- Sun, W.; Lan, L.; Zeng, H.; Zhou, J.; Khoso, S.A.; Wang, L. Study on the flotation separation mechanism of diaspore from kaolinite using mixed NaOL/BHA collector. Miner. Eng. 2022, 186, 107719. [Google Scholar] [CrossRef]
- Jia, W.; Jiao, F.; Zhu, H.; Xu, L.; Qin, W. Mitigating the negative effects of feldspar slime on spodumene flotation using mixed anionic/cationic collector. Miner. Eng. 2021, 168, 106813. [Google Scholar] [CrossRef]
- Morgan, L.; Somasundaran, P.; Partyka, S. Adsorption of hydrolyzable surfactants: Effect of precipitation on adsorption of oleate on hematite. Colloids Surf. 1987, 27, 15–27. [Google Scholar] [CrossRef]
- Morgan, L.J.; Ananthapadmanabhan, K.; Somasundaran, P. Oleate adsorption on hematite: Problems and methods. Int. J. Miner. Process. 1986, 18, 139–152. [Google Scholar] [CrossRef]
- Quast, K. Direct measurement of oleate adsorption on hematite and its consequences for flotation. Miner. Eng. 2018, 118, 122–132. [Google Scholar] [CrossRef]
- Antti, B.-M.; Forssberg, E. Pulp chemistry in industrial mineral flotation. Studies of surface complex on calcite and apatite surfaces using FTIR spectroscopy. Miner. Eng. 1989, 2, 217–227. [Google Scholar] [CrossRef]
- Jung, R.F.; James, R.O.; Healy, T.W. Adsorption, precipitation, and electrokinetic processes in the iron oxide (Goethite)—Oleic acid—Oleate system. J. Colloid Interface Sci. 1987, 118, 463–472. [Google Scholar] [CrossRef]
- Guan, C.; Yin, Z.; Zhai, J.; Hu, Y.; Chen, P.; Sun, W. Surface modification of ilmenite by a novel surfactant dodecyliminodimethylenediphosphoinc acid and its sequent influence on ilmenite floatability. Sep. Sci. Technol. 2019, 55, 358–368. [Google Scholar] [CrossRef]
- Zhai, J.; Chen, P.; Wang, H.; Hu, Y.; Sun, W. Flotability improvement of ilmenite using attrition-scrubbing as a pretreatment method. Minerals 2017, 7, 13. [Google Scholar] [CrossRef]
- Vurdela, R.; Laskowski, J. Positively charged colloidal species in aqueous anionic surfactant solutions. Colloids Surf. 1987, 22, 77–80. [Google Scholar] [CrossRef]
- Yoon, R.-h.; Mao, L. Application of Extended DLVO Theory, IV: Derivation of Flotation Rate Equation from First Principles. J. Colloid Interface Sci. 1996, 181, 613–626. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




