Nanoplastics: From Separations to Analysis—Challenges and Limitations
Abstract
1. Introduction
2. Materials and Methods
3. Nanoplastics
3.1. Classification and Properties
3.2. Occurrence and Matrices
4. Challenges and Problems Related to NPs Isolation
4.1. Quality Control
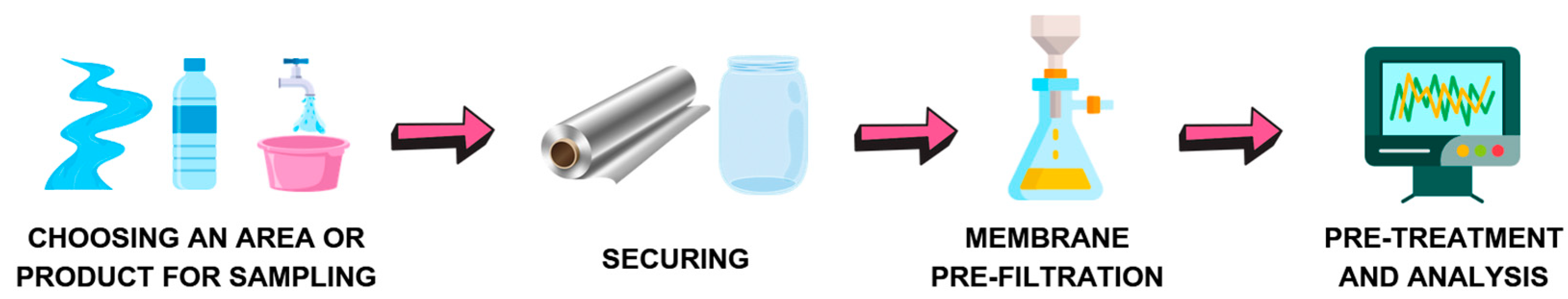
4.2. Collecting Samples
4.3. Separation of NPs from Matrix
4.3.1. Chemical Digestion and Filtration
4.3.2. Magnetic Extraction
4.3.3. Field-Flow Fractionation
4.3.4. Ultracentrifugation
4.3.5. Capillary Electrophoresis
4.3.6. Separation Techniques Coupled with Surface-Enhanced Raman Spectroscopy
5. Analytical Methods
5.1. Methods for the Analysis of NPs
5.1.1. Microscopic Techniques
5.1.2. Spectroscopic Techniques
5.1.3. Thermal Analysis
5.1.4. Other Methods
5.2. Required Properties of Analytical Methods and Challenges in Nanoplastic Analysis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- British Plastics Federation Plastics Applications. Available online: https://www.bpf.co.uk/plastipedia/applications/Default.aspx (accessed on 1 June 2025).
- International Union for Conservation of Nature and Natural Resources. Plastic Pollution; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2024. [Google Scholar]
- Busch, M.; Brouwer, H.; Aalderink, G.; Bredeck, G.; Kämpfer, A.A.M.; Schins, R.P.F.; Bouwmeester, H. Investigating Nanoplastics Toxicity Using Advanced Stem Cell-Based Intestinal and Lung in Vitro Models. Front. Toxicol. 2023, 5, 1112212. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Shelver, W.L. Micro- and Nanoplastic Induced Cellular Toxicity in Mammals: A Review. Sci. Total Environ. 2021, 755, 142518. [Google Scholar] [CrossRef] [PubMed]
- Facciolà, A.; Visalli, G.; Pruiti Ciarello, M.; Di Pietro, A. Newly Emerging Airborne Pollutants: Current Knowledge of Health Impact of Micro and Nanoplastics. Int. J. Environ. Res. Public Health 2021, 18, 2997. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (Contam). Presence of Microplastics and Nanoplastics in Food, with Particular Focus on Seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef] [PubMed]
- Pradel, A.; Catrouillet, C.; Gigault, J. The Environmental Fate of Nanoplastics: What We Know and What We Need to Know about Aggregation. NanoImpact 2023, 29, 100453. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Reverón, R.; Álvarez-Méndez, S.J.; González-Sálamo, J.; Socas-Hernández, C.; Díaz-Peña, F.J.; Hernández-Sánchez, C.; Hernández-Borges, J. Nanoplastics in the Soil Environment: Analytical Methods, Occurrence, Fate and Ecological Implications. Environ. Pollut. 2023, 317, 120788. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pal, D.; Pilechi, A.; Ariya, P.A. Nanoplastics in Water: Artificial Intelligence-Assisted 4D Physicochemical Characterization and Rapid In Situ Detection. Environ. Sci. Technol. 2024, 58, 8919–8931. [Google Scholar] [CrossRef] [PubMed]
- Jing, B.; Wan, W.; Hu, B.; Jin, W.; Zhang, Z.; Peng, C.; Wang, M.; Deng, J.; Dong, X.; Liu, Y.; et al. Plastic Nanoparticles Cause Proteome Stress and Aggregation by Compromising Cellular Protein Homeostasis Ex Vivo and in Vivo. Ecotoxicol. Environ. Saf. 2023, 262, 115347. [Google Scholar] [CrossRef] [PubMed]
- Asmatulu, E.; Andalib, M.N.; Subeshan, B.; Abedin, F. Impact of Nanomaterials on Human Health: A Review. Environ. Chem. Lett. 2022, 20, 2509–2529. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, X.; Zhang, J.; Wu, L.; Wei, W.; Ni, B.-J. Nanoplastics Are Significantly Different from Microplastics in Urban Waters. Water Res. X 2023, 19, 100169. [Google Scholar] [CrossRef] [PubMed]
- Joksimovic, N.; Selakovic, D.; Jovicic, N.; Jankovic, N.; Pradeepkumar, P.; Eftekhari, A.; Rosic, G. Nanoplastics as an Invisible Threat to Humans and the Environment. J. Nanomater. 2022, 2022, 6707819. [Google Scholar] [CrossRef]
- Mariano, S.; Tacconi, S.; Fidaleo, M.; Rossi, M.; Dini, L. Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques. Front. Toxicol. 2021, 3, 636640. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Ma, M.; Ge, Q.; Liu, Y.; Zhang, L. Machine Learning Advancements and Strategies in Microplastic and Nanoplastic Detection. Environ. Sci. Technol. 2025, 59, 8885–8899. [Google Scholar] [CrossRef] [PubMed]
- Nene, A.; Sadeghzade, S.; Viaroli, S.; Yang, W.; Uchenna, U.P.; Kandwal, A.; Liu, X.; Somani, P.; Galluzzi, M. Recent Advances and Future Technologies in Nano-Microplastics Detection. Environ. Sci. Eur. 2025, 37, 7. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Wang, Z.; Li, M.; Wang, W.; Lu, R.; Wang, S.; Li, Q.; Hu, Z.; Wu, Y.; et al. Human Exposure to Micro(Nano)Plastics: Health Risks and Analysis Methods. TrAC 2024, 178, 117835. [Google Scholar] [CrossRef]
- Allouzi, M.M.A.; Tang, D.Y.Y.; Chew, K.W.; Rinklebe, J.; Bolan, N.; Allouzi, S.M.A.; Show, P.L. Micro (Nano) Plastic Pollution: The Ecological Influence on Soil-Plant System and Human Health. Sci. Total Environ. 2021, 788, 147815. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and Nano-Plastics in Marine Environment: Source, Distribution and Threats—A Review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Liu, X.; Qu, M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials 2022, 12, 1298. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.-K.; Brehm, J.; Völkl, M.; Jérôme, V.; Laforsch, C.; Freitag, R.; Greiner, A. Disentangling Biological Effects of Primary Nanoplastics from Dispersion Paints’ Additional Compounds. Ecotoxicol. Environ. Saf. 2022, 242, 113877. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are There Nanoplastics in Your Personal Care Products? Environ. Sci. Technol. Lett. 2017, 4, 280–285. [Google Scholar] [CrossRef]
- Mathissen, M.; Scheer, V.; Vogt, R.; Benter, T. Investigation on the Potential Generation of Ultrafine Particles from the Tire–Road Interface. Atmos. Environ. 2011, 45, 6172–6179. [Google Scholar] [CrossRef]
- Sillanpää, M.; Sainio, P. Release of Polyester and Cotton Fibers from Textiles in Machine Washings. Environ. Sci. Pollut. Res. 2017, 24, 19313–19321. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.E.; Sharpe, E.E.; Brander, S.M.; Landis, W.G.; Harper, S.L. Critical Gaps in Nanoplastics Research and Their Connection to Risk Assessment. Front Toxicol. 2023, 5, 1154538. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lin, X.; Lu, J.; Zhao, X.; Wu, D.; Kim, H.; Su, L.; Cai, L. Effect of Shape on the Transport and Retention of Nanoplastics in Saturated Quartz Sand. J. Hazard. Mater. 2024, 479, 135766. [Google Scholar] [CrossRef] [PubMed]
- Goral, K.D.; Guler, H.G.; Larsen, B.E.; Carstensen, S.; Christensen, E.D.; Kerpen, N.B.; Schlurmann, T.; Fuhrman, D.R. Settling Velocity of Microplastic Particles Having Regular and Irregular Shapes. Environ. Res. 2023, 228, 115783. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Ma, X.; Lichtfouse, E.; Robert, D. Nanoplastics Are Potentially More Dangerous than Microplastics. Environ. Chem. Lett. 2023, 21, 1933–1936. [Google Scholar] [CrossRef]
- European Foood Safety Authority. Peter Hollman Microplastics and Nanoplastics in Food–an Emerging Issue 2016; European Foood Safety Authority: Parma, Italy, 2016. [Google Scholar]
- Okoffo, E.D.; Thomas, K.V. Quantitative Analysis of Nanoplastics in Environmental and Potable Waters by Pyrolysis-Gas Chromatography–Mass Spectrometry. J. Hazard. Mater. 2024, 464, 133013. [Google Scholar] [CrossRef] [PubMed]
- Nematollahi, M.J.; Moore, F.; Keshavarzi, B.; Vogt, R.D.; Nasrollahzadeh Saravi, H.; Busquets, R. Microplastic Particles in Sediments and Waters, South of Caspian Sea: Frequency, Distribution, Characteristics, and Chemical Composition. Ecotoxicol. Environ. Saf. 2020, 206, 111137. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, M.; Palazot, M.; Soccalingame, L.; Falcou-Préfol, M.; Gorsky, G.; Galgani, F.; Bruzaud, S.; Pedrotti, M.L. Chemical Composition of Microplastics Floating on the Surface of the Mediterranean Sea. Mar. Pollut. Bull. 2022, 174, 113284. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, Z.; Wei, W.; Chen, J.; Ni, B.J. Toxicity of Micro/Nanoplastics in the Environment: Roles of Plastisphere and Eco-Corona. Soil Environ. Health 2023, 1, 100002. [Google Scholar] [CrossRef]
- Reynaud, S.; Aynard, A.; Grassl, B.; Gigault, J. Nanoplastics: From Model Materials to Colloidal Fate. Curr. Opin. Colloid. Interface Sci. 2022, 57, 101528. [Google Scholar] [CrossRef]
- Rahman, A.M.N.A.A.; Rusli, A.; Abdullah, M.K.; Shuib, R.K.; Hamid, Z.A.A.; Ku Ishak, K.M.; Mohd Zaini Makhtar, M.; Jaafar, M.; Shafiq, M.D. A Review of Microplastic Surface Interactions in Water and Potential Capturing Methods. Water Sci. Eng. 2024, 17, 361–370. [Google Scholar] [CrossRef]
- Müller, S.; Fiutowski, J.; Rasmussen, M.B.; Balic Zunic, T.; Rubahn, H.-G.; Posth, N.R. Nanoplastic in Aqueous Environments: The Role of Chemo-Electric Properties for Nanoplastic-Mineral Interaction. Sci. Total Environ. 2025, 964, 178529. [Google Scholar] [CrossRef] [PubMed]
- Forster, N.A.; Wilson, S.C.; Tighe, M.K. Microplastic Surface Retention and Mobility on Hiking Trails. Environ. Sci. Pollut. Res. 2023, 30, 46368–46382. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Maity, J.P.; Singha, S.; Mishra, T.; Dey, G.; Chandra Samal, A.; Banerjee, P.; Biswas, C.; Chattopadhyay, S.; Patra, R.R.; et al. Microplastics and Nanoplastics in Environment: Sampling, Characterization and Analytical Methods. Groundw. Sustain. Dev. 2024, 26, 101267. [Google Scholar] [CrossRef]
- Shi, C.; Liu, Z.; Yu, B.; Zhang, Y.; Yang, H.; Han, Y.; Wang, B.; Liu, Z.; Zhang, H. Emergence of Nanoplastics in the Aquatic Environment and Possible Impacts on Aquatic Organisms. Sci. Total Environ. 2024, 906, 167404. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Simnani, F.Z.; Singh, D.; Patel, P.; Sinha, A.; Nandi, A.; Ghosh, A.; Saha, U.; Kumari, K.; Jaganathan, S.K.; et al. Atmospheric Microplastic and Nanoplastic: The Toxicological Paradigm on the Cellular System. Ecotoxicol. Environ. Saf. 2023, 259, 115018. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Li, X.; Lu, C.; Sun, L.; Fan, C.; Fu, M.; Wang, H.; Duan, M.; Xia, S. Micro/Nanoplastics in the Shenyang City Atmosphere: Distribution and Sources. Environ. Pollut. 2025, 372, 126027. [Google Scholar] [CrossRef] [PubMed]
- Kirchsteiger, B.; Materić, D.; Happenhofer, F.; Holzinger, R.; Kasper-Giebl, A. Fine Micro- and Nanoplastics Particles (PM2.5) in Urban Air and Their Relation to Polycyclic Aromatic Hydrocarbons. Atmos. Environ. 2023, 301, 119670. [Google Scholar] [CrossRef]
- Xu, Y.; Ou, Q.; Jiao, M.; Liu, G.; van der Hoek, J.P. Identification and Quantification of Nanoplastics in Surface Water and Groundwater by Pyrolysis Gas Chromatography–Mass Spectrometry. Environ. Sci. Technol. 2022, 56, 4988–4997. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, C.; Tian, Z.; Cai, X.; Guan, B. Identification and Quantification of Nanoplastics (20–1000 Nm) in a Drinking Water Treatment Plant Using AFM-IR and Pyr-GC/MS. J. Hazard. Mater. 2024, 463, 132933. [Google Scholar] [CrossRef] [PubMed]
- Romphophak, P.; Faikhaw, O.; Sairiam, S.; Thuptimdang, P.; Coufort-Saudejaud, C. Removal of Microplastics and Nanoplastics in Water Treatment Processes: A Systematic Literature Review. J. Water Process. Eng. 2024, 64, 105669. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Guan, B. Separation and Identification of Nanoplastics in Tap Water. Environ. Res. 2022, 204, 112134. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Flury, M. Current Understanding of Subsurface Transport of Micro- and Nanoplastics in Soil. Vadose Zone J. 2021, 20, e20108. [Google Scholar] [CrossRef]
- Wahl, A.; Le Juge, C.; Davranche, M.; El Hadri, H.; Grassl, B.; Reynaud, S.; Gigault, J. Nanoplastic Occurrence in a Soil Amended with Plastic Debris. Chemosphere 2021, 262, 127784. [Google Scholar] [CrossRef] [PubMed]
- Foetisch, A.; Filella, M.; Watts, B.; Vinot, L.-H.; Bigalke, M. Identification and Characterisation of Individual Nanoplastics by Scanning Transmission X-Ray Microscopy (STXM). J. Hazard. Mater. 2022, 426, 127804. [Google Scholar] [CrossRef] [PubMed]
- Witzig, C.S.; Földi, C.; Wörle, K.; Habermehl, P.; Pittroff, M.; Müller, Y.K.; Lauschke, T.; Fiener, P.; Dierkes, G.; Freier, K.P.; et al. When Good Intentions Go Bad—False Positive Microplastic Detection Caused by Disposable Gloves. Environ. Sci. Technol. 2020, 54, 12164–12172. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Fan, R.; Xie, C.; Liu, Q.; Liu, Q.; Yan, C.; Cui, J.; He, W. Microplastics Are Overestimated Due to Poor Quality Control of Reagents. J. Hazard. Mater. 2023, 459, 132068. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.; Fumagalli, F.; Cella, C.; Gilliland, D.; Tremolada, P.; Valsesia, A. Detection and Formation Mechanisms of Secondary Nanoplastic Released from Drinking Water Bottles. Water Res. 2022, 222, 118848. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Fang, H.; Zhao, X.; Lin, Y.; Su, Z. Identification of Microplastics and Nanoplastics in Environmental Water by AFM-IR. Anal. Chim. Acta 2025, 1354, 343992. [Google Scholar] [CrossRef] [PubMed]
- Primpke, S.; Meyer, B.; Falcou-Préfol, M.; Schütte, W.; Gerdts, G. At Second Glance: The Importance of Strict Quality Control–A Case Study on Microplastic in the Southern Ocean Key Species Antarctic Krill, Euphausia Superba. Sci. Total Environ. 2024, 918, 170618. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.R.; de Jersey, A.M.; Lavers, J.L.; Rodemann, T.; Rivers-Auty, J. Identifying Laboratory Sources of Microplastic and Nanoplastic Contamination from the Air, Water, and Consumables. J. Hazard. Mater. 2024, 465, 133276. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.-Y.; Lee, Y.-M.; Chiu, B.-W.; Hsu, H.-Y.; Chen, C.-W.; Tsai, C.-J. A Comprehensive Study Combing Experiment and CFD Simulation on the Fume Hood Performance for Nanoparticles and Isopropanol Control. Process Saf. Environ. Prot. 2024, 186, 361–375. [Google Scholar] [CrossRef]
- Prata, J.C.; Reis, V.; da Costa, J.P.; Mouneyrac, C.; Duarte, A.C.; Rocha-Santos, T. Contamination Issues as a Challenge in Quality Control and Quality Assurance in Microplastics Analytics. J. Hazard. Mater. 2021, 403, 123660. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, M. Osilla Biological Safety Cabinet vs Laminar Flow Hood. Available online: https://www.ossila.com/pages/biological-safety-cabinets-laminar-flow-hoods (accessed on 1 June 2025).
- Mou, L.; Zhang, Q.; Li, R.; Zhu, Y.; Zhang, Y. A Powerful Method for In Situ and Rapid Detection of Trace Nanoplastics in Water—Mie Scattering. J. Hazard. Mater. 2024, 470, 134186. [Google Scholar] [CrossRef] [PubMed]
- Bogdanowicz, A.; Zubrowska-Sudol, M.; Krasinski, A.; Sudol, M. Cross-Contamination as a Problem in Collection and Analysis of Environmental Samples Containing Microplastics—A Review. Sustainability 2021, 13, 12123. [Google Scholar] [CrossRef]
- Carreón, R.V.; Cortázar-Martínez, O.; Rodríguez-Hernández, A.G.; Serrano de la Rosa, L.E.; Gervacio-Arciniega, J.J.; Krishnan, S.K. Ionic Liquid-Assisted Thermal Evaporation of Bimetallic Ag–Au Nanoparticle Films as a Highly Reproducible SERS Substrate for Sensitive Nanoplastic Detection in Complex Environments. Anal. Chem. 2024, 96, 5790–5797. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, S.; Su, J.; Li, S.; Lv, X.; Chen, J.; Lai, Y.; Zhan, J. Identification of Trace Polystyrene Nanoplastics Down to 50 Nm by the Hyphenated Method of Filtration and Surface-Enhanced Raman Spectroscopy Based on Silver Nanowire Membranes. Environ. Sci. Technol. 2022, 56, 10818–10828. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Cai, W.; Li, J.; Wu, D. Visual Monitoring of Polystyrene Nanoplastics < 100 Nm in Drinking Water Based on Functionalized Gold Nanoparticles. Sens. Actuators B Chem. 2023, 392, 134099. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, J.; de Mello, J.C. Detection of Environmental Nanoplastics via Surface-Enhanced Raman Spectroscopy Using High-Density, Ring-Shaped Nanogap Arrays. Front. Bioeng. Biotechnol. 2023, 11, 1242797. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Chen, M.; Du, F.; Matthews, S.; Shi, H. Separation and Enrichment of Nanoplastics in Environmental Water Samples via Ultracentrifugation. Water Res. 2021, 203, 117509. [Google Scholar] [CrossRef] [PubMed]
- Pasanen, F.; Fuller, R.O.; Maya, F. Sequential Extraction, Depolymerization and Quantification of Polyethylene Terephthalate Nanoplastics Using Magnetic ZIF-8 Nanocomposites. Chem. Eng. J. 2024, 490, 151453. [Google Scholar] [CrossRef]
- Surette, M.C.; Mitrano, D.M.; Rogers, K.R. Extraction and Concentration of Nanoplastic Particles from Aqueous Suspensions Using Functionalized Magnetic Nanoparticles and a Magnetic Flow Cell. Microplast. Nanoplast. 2023, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Li, L.; Guo, Z.; Yang, X.; Dong, D. Emerging Isolation and Degradation Technology of Microplastics and Nanoplastics in the Environment. Environ. Res. 2024, 243, 117864. [Google Scholar] [CrossRef] [PubMed]
- Roberto de Bruin, C.; de Rijke, E.; van Wezel, A.P.; Astefanei, A. Methodologies to Characterize, Identify and Quantify Nano- and Sub-Micron Sized Plastics in Relevant Media for Human Exposure: A Critical Review. Env. Sci. Adv. 2022, 1, 238–258. [Google Scholar] [CrossRef]
- Valido, I.H.; Fuentes-Cebrian, V.; Hernández, A.; Valiente, M.; López-Mesas, M. Validated Method for Polystyrene Nanoplastic Separation in Aqueous Matrices by Asymmetric-Flow Field Flow Fraction Coupled to MALS and UV–Vis Detectors. Microchim. Acta 2023, 190, 285. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Huang, Y.; Chen, Y.; He, X.; Chen, Z.; Lu, X.; Wu, M.; Wanger, T.C. Non-Destructive Extraction and Separation of Nano- and Microplastics from Environmental Samples by Density Gradient Ultracentrifugation. Anal. Chem. 2022, 94, 15280–15287. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.P.C.; Onomhante, A.; Tsopmo, A.; Hosseinian, F. Determination of Polystyrene Nanospheres and Other Nanoplastics in Water via Binding with Organic Dyes by Capillary Electrophoresis with Laser-Induced Fluorescence Detection. Talanta 2025, 284, 127265. [Google Scholar] [CrossRef] [PubMed]
- Adelantado, C.; Lapizco-Encinas, B.H.; Jordens, J.; Voorspoels, S.; Velimirovic, M.; Tirez, K. Capillary Electrophoresis as a Complementary Analytical Tool for the Separation and Detection of Nanoplastic Particles. Anal. Chem. 2024, 96, 7706–7713. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Esfahani, E.B.; Chiu, I.; Mohseni, M.; Gao, G.; Yang, T. Quantitative and Rapid Detection of Nanoplastics Labeled by Luminescent Metal Phenolic Networks Using Surface-Enhanced Raman Scattering. J. Hazard. Mater. 2024, 470, 134194. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhu, K.; Yang, K.; Qing, Y.; Zhao, Y.; Wu, L.; Zong, S.; Cui, Y.; Wang, Z. One-Step Detection of Nanoplastics in Aquatic Environments Using a Portable SERS Chessboard Substrate. Talanta 2025, 282, 127076. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-X.; Liu, R.; Hao, L.-T.; Liu, J.-F. Identification of Polystyrene Nanoplastics Using Surface Enhanced Raman Spectroscopy. Talanta 2021, 221, 121552. [Google Scholar] [CrossRef] [PubMed]
- Kalaronis, D.; Ainali, N.M.; Evgenidou, E.; Kyzas, G.Z.; Yang, X.; Bikiaris, D.N.; Lambropoulou, D.A. Microscopic Techniques as Means for the Determination of Microplastics and Nanoplastics in the Aquatic Environment: A Concise Review. Green. Anal. Chem. 2022, 3, 100036. [Google Scholar] [CrossRef]
- Yang, T.; Luo, J.; Nowack, B. Characterization of Nanoplastics, Fibrils, and Microplastics Released during Washing and Abrasion of Polyester Textiles. Environ. Sci. Technol. 2021, 55, 15873–15881. [Google Scholar] [CrossRef] [PubMed]
- Davranche, M.; Lory, C.; Le Juge, C.; Blancho, F.; Dia, A.; Grassl, B.; El Hadri, H.; Pascal, P.-Y.; Gigault, J. Nanoplastics on the Coast Exposed to the North Atlantic Gyre: Evidence and Traceability. NanoImpact 2020, 20, 100262. [Google Scholar] [CrossRef]
- Man Thaiba, B.; Sedai, T.; Bastakoti, S.; Karki, A.; Anuradha, K.C.; Khadka, G.; Acharya, S.; Kandel, B.; Giri, B.; Bhakta Neupane, B. A Review on Analytical Performance of Micro- and Nanoplastics Analysis Methods. Arab. J. Chem. 2023, 16, 104686. [Google Scholar] [CrossRef]
- Galluzzi, M.; Lancia, M.; Zheng, C.; Re, V.; Castelvetro, V.; Guo, S.; Viaroli, S. Atomic Force Microscopy (AFM) Nanomechanical Characterization of Micro- and Nanoplastics to Support Environmental Investigations in Groundwater. Emerg. Contam. 2025, 11, 100478. [Google Scholar] [CrossRef]
- Mandemaker, L.D.B.; Meirer, F. Spectro-Microscopic Techniques for Studying Nanoplastics in the Environment and in Organisms. Angew. Chem. Int. Ed. Engl. 2023, 62, e202210494. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Krolis, E.; Molenaar, R.; Claessens, M.M.A.E.; Blum, C. Nile Red Staining for Nanoplastic Quantification: Overcoming the Challenge of False Positive Counts Due to Fluorescent Aggregates. Environ. Chall. 2023, 13, 100744. [Google Scholar] [CrossRef]
- Kousheh, S.; Lin, M. Recent Advancements in SERS-Based Detection of Micro- and Nanoplastics in Food and Beverages: Techniques, Instruments, and Machine Learning Integration. Trends Food Sci. Technol. 2025, 159, 104940. [Google Scholar] [CrossRef]
- Han, K.; Yan, Z.; Ding, Z.; Zhou, P.; Ye, C.; Qin, L.; Bao, Z.; Zhang, M.; Zhang, W. High-Sensitivity SERS Sensor Leveraging Three-Dimensional Ti3C2Tx/TiO2/W18O49 Semiconductor Heterostructures for Reliable Detection of Trace Micro/Nanoplastics in Environmental Matrices. Talanta 2025, 286, 127474. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.N.; Lipp, P.; Zucker, I.; Schäfer, A.I. Quantification of Nanoplastics and Inorganic Nanoparticles via Laser-Induced Breakdown Detection (LIBD). Small Methods 2025, e2402060. [Google Scholar] [CrossRef] [PubMed]
- Foreman-Ortiz, I.U.; Ma, T.F.; Hoover, B.M.; Wu, M.; Murphy, C.J.; Murphy, R.M.; Pedersen, J.A. Nanoparticle Tracking Analysis and Statistical Mixture Distribution Analysis to Quantify Nanoparticle–Vesicle Binding. J. Colloid. Interface Sci. 2022, 615, 50–58. [Google Scholar] [CrossRef] [PubMed]
- James, A.E.; Driskell, J.D. Monitoring Gold Nanoparticle Conjugation and Analysis of Biomolecular Binding with Nanoparticle Tracking Analysis (NTA) and Dynamic Light Scattering (DLS). Analyst 2013, 138, 1212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, M.; Lian, E.; Xia, L.; Asimakopoulos, A.G.; Luo, S.; Wang, L. Identification of Poly(Ethylene Terephthalate) Nanoplastics in Commercially Bottled Drinking Water Using Surface-Enhanced Raman Spectroscopy. Environ. Sci. Technol. 2023, 57, 8365–8372. [Google Scholar] [CrossRef] [PubMed]
- Okoffo, E.D.; Thomas, K.V. Mass Quantification of Nanoplastics at Wastewater Treatment Plants by Pyrolysis–Gas Chromatography–Mass Spectrometry. Water Res. 2024, 254, 121397. [Google Scholar] [CrossRef] [PubMed]
- Seggio, M.; Arcadio, F.; Cennamo, N.; Zeni, L.; Bossi, A.M. A Plasmonic Gold Nano-Surface Functionalized with the Estrogen Receptor for Fast and Highly Sensitive Detection of Nanoplastics. Talanta 2024, 267, 125211. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wei, W.; Liu, X.; Ni, B.J. Emerging Electrochemical Techniques for Identifying and Removing Micro/Nanoplastics in Urban Waters. Water Res. 2022, 221, 118846. [Google Scholar] [CrossRef] [PubMed]
- Donisi, I.; Colloca, A.; Anastasio, C.; Balestrieri, M.L.; D’Onofrio, N. Micro(Nano)Plastics: An Emerging Burden for Human Health. Int. J. Biol. Sci. 2024, 20, 5779–5792. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, A.; Huopalainen, J.; Mäkilä, E.; Häkkilä, S.; Damlin, P.; Hänninen, J. Expanding Sample Volume for Microscopical Detection of Nanoplastics. Mar. Environ. Res. 2024, 202, 106806. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Jiang, S.; Yan, Y.; Zhao, B.; Grant, E.R.; Kitts, D.D.; Yada, R.Y.; Pratap-Singh, A.; Baldelli, A.; Yang, T. Integrating Metal–Phenolic Networks-Mediated Separation and Machine Learning-Aided Surface-Enhanced Raman Spectroscopy for Accurate Nanoplastics Quantification and Classification. ACS Nano 2024, 18, 26281–26296. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lee, L.M.; Yu, D.; Chan, S.H.; Li, A. An Optimized Multi-Technique Based Analytical Platform for Identification, Characterization and Quantification of Nanoplastics in Water. Talanta 2024, 272, 125800. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Han, K.; Zhang, A.; Wang, T.; Yan, Z.; Ding, Z.; Shen, Y.; Zhang, M.; Zhang, W. Honeycomb-like AgNPs@TiO2 Array SERS Sensor for the Quantification of Micro/Nanoplastics in the Environmental Water Samples. Talanta 2024, 266, 125070. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, Y.; Wu, Q.; Yan, B.; Zhou, X. Quantifying the Occurrence of Polystyrene Nanoplastics in Environmental Solid Matrices via Pyrolysis-Gas Chromatography/Mass Spectrometry. J. Hazard. Mater. 2022, 440, 129855. [Google Scholar] [CrossRef]
- Ranasinghe, M.; Breadmore, M.C.; Maya, F. Preconcentration of Nanoplastics Using Micro-Electromembrane Extraction across Free Liquid Membranes. Adv. Sample Prep. 2024, 11, 100125. [Google Scholar] [CrossRef]
- Battistini, B.; Petrucci, F.; Bocca, B. In-House Validation of AF4-MALS-UV for Polystyrene Nanoplastic Analysis. Anal. Bioanal. Chem. 2021, 413, 3027–3039. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Skoczynska, E.; Siddhanti, D.; van Putten, R.-J.; Leslie, H.A.; Gruter, G.-J.M. Quantification of Polyethylene Terephthalate Microplastics and Nanoplastics in Sands, Indoor Dust and Sludge Using a Simplified in-Matrix Depolymerization Method. Mar. Pollut. Bull. 2022, 175, 113403. [Google Scholar] [CrossRef] [PubMed]
- Carreón, R.V.; Rodríguez-Hernández, A.G.; Serrano de la Rosa, L.E.; Gervacio-Arciniega, J.J.; Krishnan, S.K. Mechanically Flexible, Large-Area Fabrication of Three-Dimensional Dendritic Au Films for Reproducible Surface-Enhanced Raman Scattering Detection of Nanoplastics. ACS Sens. 2025, 10, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
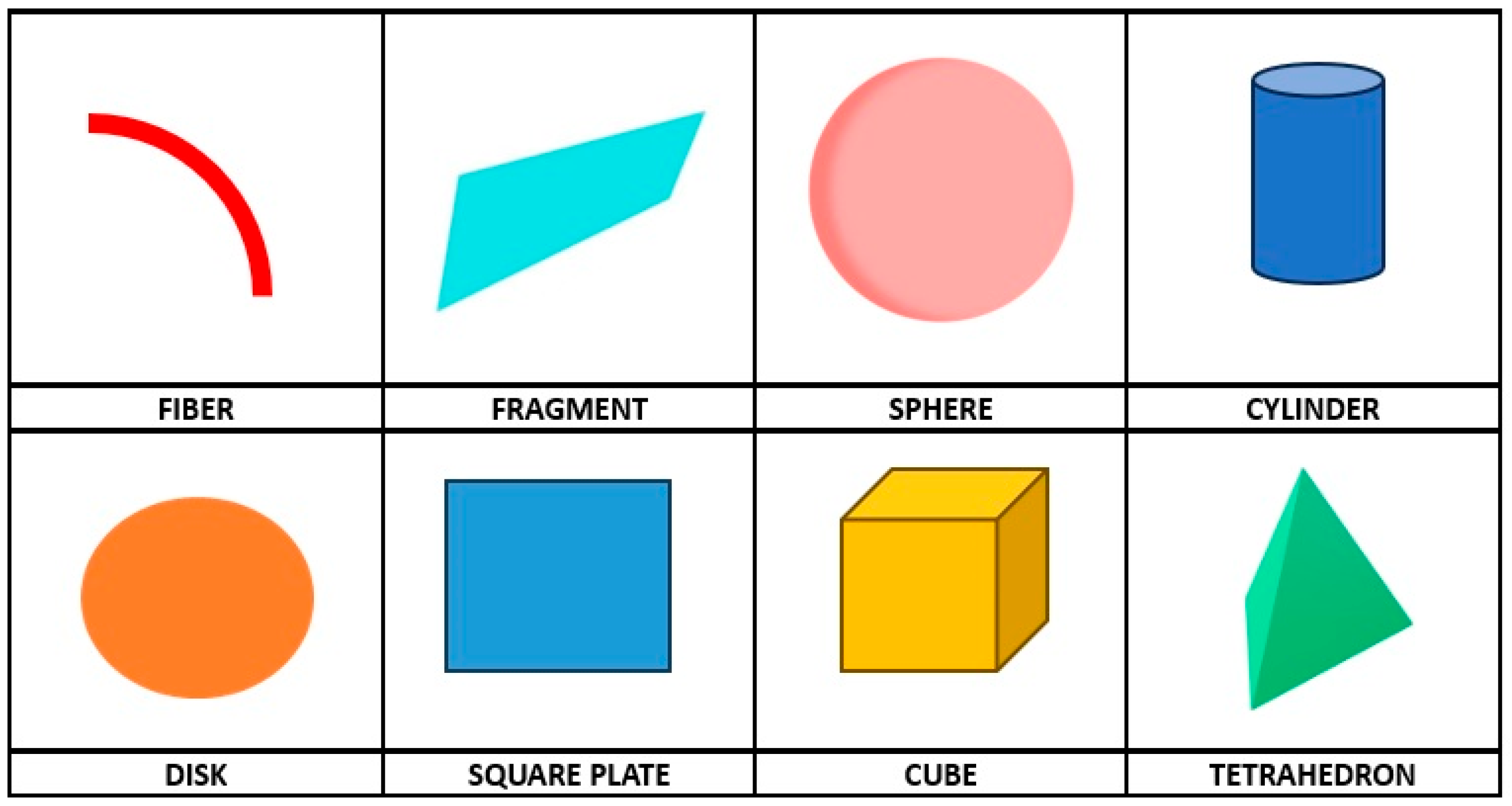
| Properties | MPs | NPs | Reference |
|---|---|---|---|
| Size | 100 nm–5 mm | 1 nm–100 nm | [29] |
| Identify polymer type | Polyethylene (PE), polypropylene (PP), polystyrene (PS), poly (ethylene terephthalate) (PET) | [30,31,32] | |
| Nylon (NYL) | - | ||
| Environmental risk | Plastisphere | Eco-corona | [33] |
| Colloidal behavior | Creates a colloid | Creates a colloid | [34,35] |
| Mobility and Dispersion | Low mobility | High mobility | [36,37] |
| ResearcherProtection | Workplace | ToolMaterial | Reagent Purity | Sample Protection | Blank Sample | Type of Sample | Reference |
|---|---|---|---|---|---|---|---|
| Nitrile gloves and 100% cotton laboratory coats | Fume hood | Glass and metal | Analytical grade without additional purification Milli-Q ultrapure water | Dichloromethane pre-cleaned aluminum foils | Yes | Environmental and potable water | [30] |
| Nitrile butadiene gloves and cotton laboratory coats | Laminar flow Cabinets | Glass or stainless steel | Milli-Q ultrapure water | No information | Yes | Water from drinking water plant | [44] |
| Cotton laboratory coats | Laminar air flow fume hood | Glass or stainless steel | Analytical grade Ultrapure water | Aluminum foil | Yes | Tap water | [46] |
| No information | No information | Glass | Ultrapure water | No information | Yes | Environmental water | [53] |
| Type of Method | Advantages | Limitations |
|---|---|---|
| Microscopic | ||
| Transmission electron microscopy (TEM) | Observation of agglomerated and single-molecule NPs | Lack of selectivity for NPs |
| Atomic force microscopy (AFM) | Observation of NPs aggregates and aging processes | May damage or contaminate the sample |
| Fluorescence microscopy | Rapid Cost-effective Does not necessitate sample pretreatment | Suitable only for spiked samples, not real samples Non-fluorescent polymers cannot be detected |
| Spectroscopic | ||
| SERS | Rapid Non-destructive Can be coupled with separation techniques | NPs dimensions cannot be distinguished |
| Mie scattering | Rapid Suitable for mixture of particles of varying sizes | Suitable only for pure or simple water matrices |
| Laser-Induced Breakdown Detection (LIBD) | Can be coupled with separation techniques | Suitable only for pure or simple water matrices |
| Nanoparticle Tracking Analysis (NTA) | Sensitive | NPs dimensions cannot be distinguished High limit of detection (LOD) |
| Thermal | ||
| Pyr-GC/MS | Sensitive Identification and quantification of NPs | Time-consuming Destructive |
| Others | ||
| Estrogen Receptor–Gold Nanograting (ER-GNG) | No pretreatment of sample needed | Two weeks lifetime of the sensor |
| Electrochemical methods | Identification of the size and material of plastic particles Identification of contaminants carried by NPs | The minimum particle size is 1 µm |
| Method | Matrix | Polymer Types | NPs Size [nm] | Linearity Range | LOD | LOQ | Reference |
|---|---|---|---|---|---|---|---|
| SERS | Water | PS | 50/100 | 50–1000/200–1000 μg/mL | 12.5/6.25 μg/mL | - | [62] |
| LIBD | Water | PS | 20–400 | - | 104−105 particles/mL | - | [86] |
| ER-GNG biosensor | Seawater | PMMA | 100 | 1–100 ng/mL | 0.39 ng/mL | - | [91] |
| AF4-MALS + Pyr-GC/MS | Bottled water | PMMA, PS | 50–350 | 0.02–10 µg; R2 = 0.9993–0.9996 | 0.01 µg | - | [96] |
| AF4-MALS + UV-DAD | Water | PS | 20–200 | 50–1000 μg/mL; R2 = 0.998–0.999 | 15–33 μg/mL | - | [100] |
| LC-UV | Indoor dust | PET | 100 | - | 0.3 mg/g | 1 mg/g | [101] |
| SERS | Tap and pond water, diluted milk, wine | PS | 100 | 10−5–10−1 g/mL | 8.2 μg/mL | - | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ośko, J.; Kadac-Czapska, K.; Jażdżewska, K.; Nowak, N.; Kowalczyk, P.; Grembecka, M. Nanoplastics: From Separations to Analysis—Challenges and Limitations. Separations 2025, 12, 185. https://doi.org/10.3390/separations12070185
Ośko J, Kadac-Czapska K, Jażdżewska K, Nowak N, Kowalczyk P, Grembecka M. Nanoplastics: From Separations to Analysis—Challenges and Limitations. Separations. 2025; 12(7):185. https://doi.org/10.3390/separations12070185
Chicago/Turabian StyleOśko, Justyna, Kornelia Kadac-Czapska, Katarzyna Jażdżewska, Natalia Nowak, Piotr Kowalczyk, and Małgorzata Grembecka. 2025. "Nanoplastics: From Separations to Analysis—Challenges and Limitations" Separations 12, no. 7: 185. https://doi.org/10.3390/separations12070185
APA StyleOśko, J., Kadac-Czapska, K., Jażdżewska, K., Nowak, N., Kowalczyk, P., & Grembecka, M. (2025). Nanoplastics: From Separations to Analysis—Challenges and Limitations. Separations, 12(7), 185. https://doi.org/10.3390/separations12070185







