Application of the Precolumn Derivatization Reagent CIM-C2-NH2 for Labeling Carboxyl Groups in LC-MS/MS Analysis of Primary Organic Acids in Japanese Sake
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
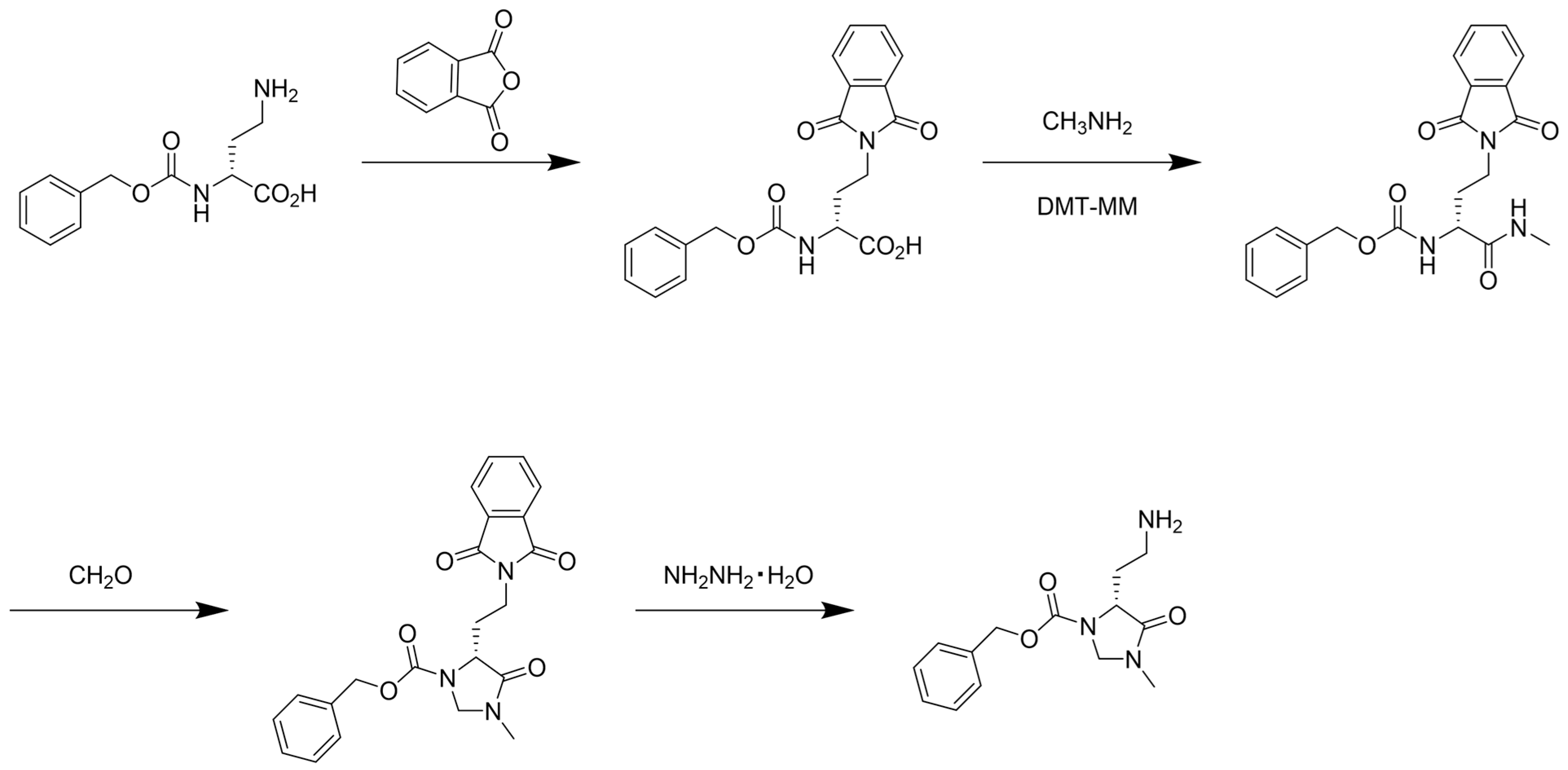
2.3. Synthesis of CIM-C2-NH2
2.3.1. (R)-2-(((benzyloxy)carbonyl)amino)-4-(1,3-dioxoisoindolin-2-yl)butanoic Acid
2.3.2. Benzyl (R)-(4-(1,3-dioxoisoindolin-2-yl)-1-(methylamino)-1-oxobutan-2-yl)carbamate
2.3.3. Benzyl (R)-5-(2-(1,3-dioxoisoindolin-2-yl)ethyl)-3-methyl-4-oxoimidazolidine-1-carboxylate
2.3.4. Benzyl (R)-5-(2-aminoethyl)-3-methyl-4-oxoimidazolidine-1-carboxylate, (R)-CIM-C2-NH2
2.4. Sample Preparation
2.5. LC-MS/MS Parameters
2.6. Linearity, Precision, and Accuracy
2.7. Neutralization Titration
2.7.1. Actual Titration Volume
2.7.2. Predicted Titration Volume
3. Results and Discussion
3.1. Synthesis of CIM-C2-NH2
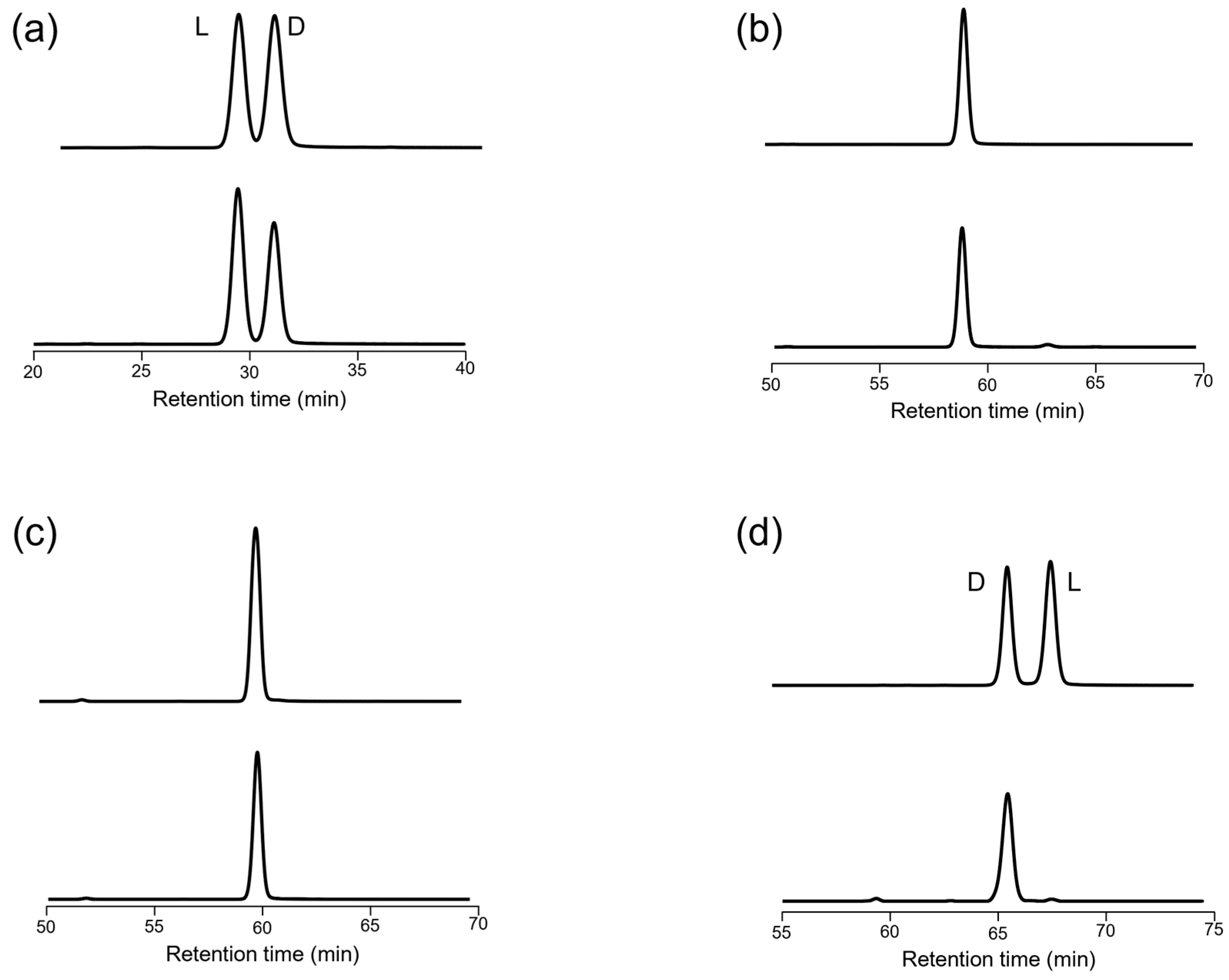
3.2. Chromatographic Detection of Organic Acids in Sake
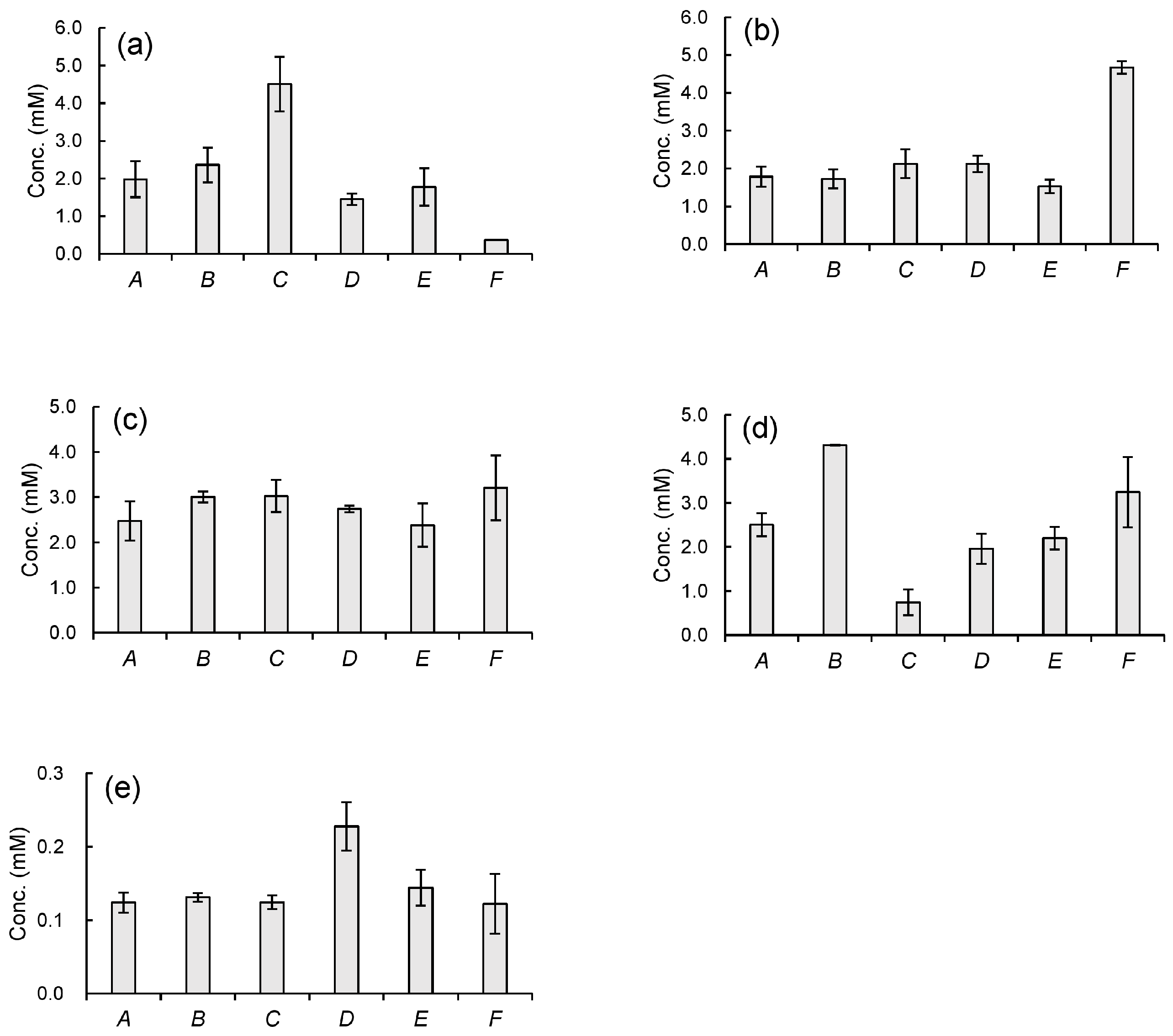
3.3. Organic Acid Concentrations
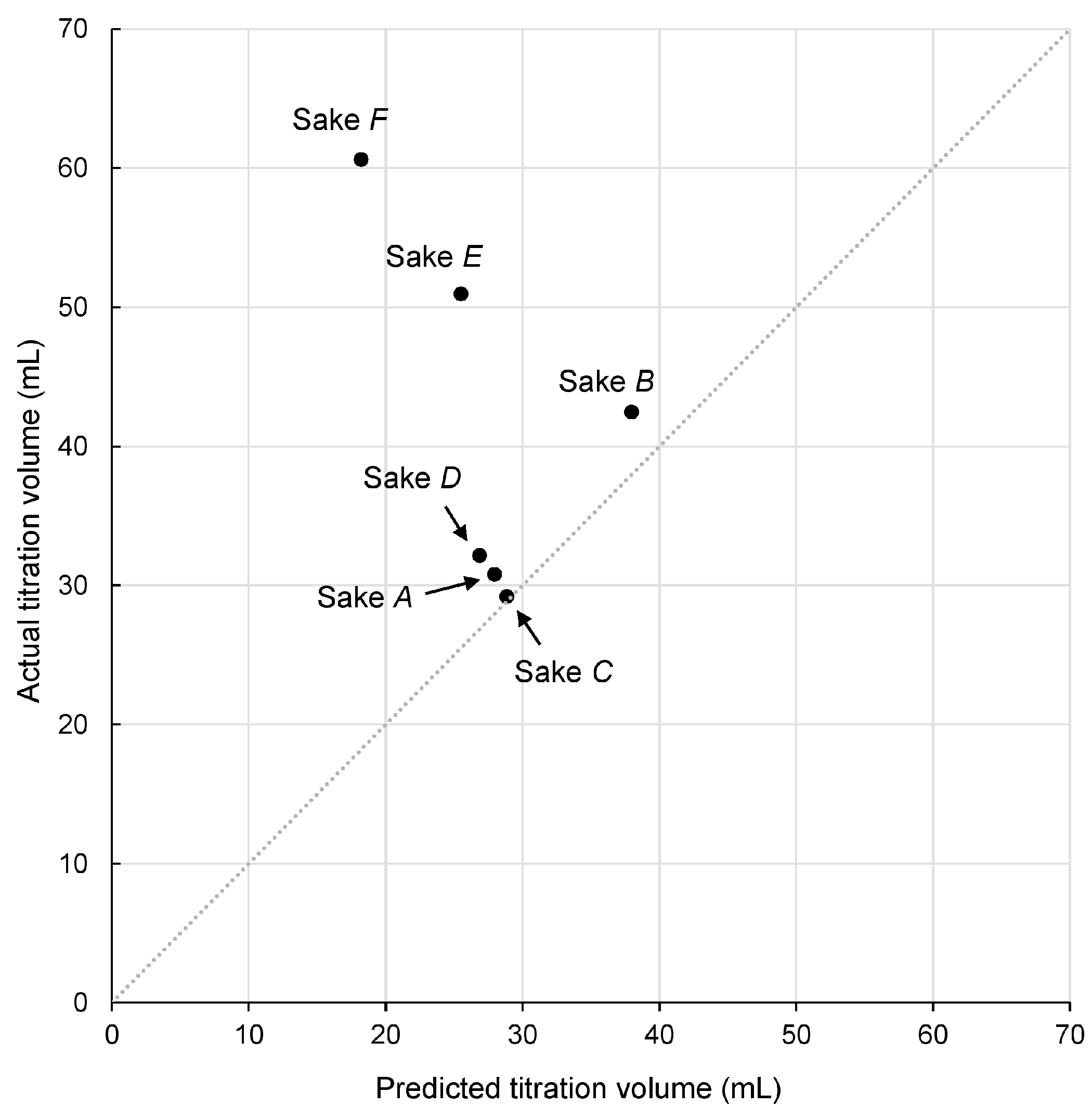
3.4. Acidic Components Determined by Neutralization Titration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LA | l-Lactic acid |
| SA | Succinic acid |
| MA | Malic acid |
| CMA | Citramalic acid |
| D-LA | d-Form of lactic acid |
| L-LA | l-Form of lactic acid |
| D-CMA | d-Form of citramalic acid |
| IS | Internal standard |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| CIM-C2-NH2 | Benzyl 5-(2-aminoethyl)-3-methyl-4-oxoimidazolidine-1-carboxylate |
| DMT-MM | 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride |
| TPP | Triphenylphosphine |
| DPDS | 2,2′-dipyridyl disulfide |
| HPLC | High-performance liquid chromatography |
| HRMS | High-resolution mass spectrometry |
| CID | Collision-induced dissociation |
References
- Suto, M.; Kawashima, H. Discrimination for sake brewing methods by compound specific isotope analysis and formation mechanism of organic acids in sake. Food Chem. 2022, 381, 132295. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Yamada, Y.; Arite, T.; Nakamura, S.; Yano, T. Selection of yeasts for giving a good sour taste to sake and a sake brewing test. J. Brew. Soc. Jpn. 2010, 105, 39–48. [Google Scholar] [CrossRef]
- Takao, Y.; Takahashi, T.; Yamada, T.; Goshima, T.; Isogai, A.; Sueno, K.; Fujii, T.; Akao, T. Characteristic features of the unique house sake yeast strain Saccharomyces cerevisiae Km67 used for industrial sake brewing. J. Biosci. Bioeng. 2018, 126, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Yamamoto, A.; Matsunaga, A.; Matsui, K.; Nakagomi, K.; Hayakawa, K. Behaviors of D- and L-lactic acids during the brewing process of sake (Japanese rice wine). J. Agric. Food Chem. 2002, 50, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Yamamoto, A.; Matsunaga, A.; Soga, T.; Minoura, K. Direct chiral resolution of lactic acid in food products by capillary electrophoresis. J. Chromatogr. A 2000, 875, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Umino, M.; Sakamoto, T.; Onozato, M.; Fukushima, T. Preparation of imidazolidinone compounds as derivatization reagent for diastereomerization and chromatographic separation of chiral organic acids. J. Chromatogr. A 2022, 1675, 463159. [Google Scholar] [CrossRef] [PubMed]
- Umino, M.; Onozato, M.; Sakamoto, T.; Koishi, M.; Fukushima, T. Analyzing citramalic acid enantiomers in apples and commercial fruit juice by liquid chromatography-tandem mass spectrometry with pre-column derivatization. Molecules 2023, 28, 1556. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, H.; Ishii, K.; Hirasa, Y.; Kubo, K.; Fukushima, T. Fluorescence determination of D- and L-tryptophan concentrations in rat plasma following administration of tryptophan enantiomers using HPLC with pre-column derivatization. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 3208–3213. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Shurrush, K.; Kulkarni, S. Asymmetric synthesis of anti-aldol segments via a nonaldol route: Synthetic applications to statines and (−)-tetrahydrolipstatin. J. Org. Chem. 2009, 74, 4508–4518. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Sarkar, A.; Brindisi, M. The Curtius rearrangement: Mechanistic insight and recent applications in natural product syntheses. Org. Biomol. Chem. 2018, 16, 2006–2027. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ang, W.; Long, H.; Chang, Y.; Li, Z.; Zhou, L.; Yang, T.; Deng, Y.; Luo, Y. Scaffold hopping toward agomelatine: Novel 3, 4-dihydroisoquinoline compounds as potential antidepressant agents. Sci. Rep. 2016, 6, 34711. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, M.; Bertini, R.; Cesta, M.C.; Bizzarri, C.; Di Bitondo, R.; Di Cioccio, V.; Galliera, E.; Berdini, V.; Topai, A.; Zampella, G.; et al. 2-arylpropionic CXC chemokine receptor 1 (CXCR1) ligands as novel noncompetitive CXCL8 inhibitors. J. Med. Chem. 2005, 48, 4312–4331. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Adachi, S.; Ichihara, H.; Al-Kindy, S.; Imai, K. Fluorimetric determination of D- and L-lactate derivatized with 4-(N,N-dimethylaminosulfonyl)-7-piperazino-2,1,3-benzoxadiazole (DBD-PZ) by high-performance liquid chromatography. Biomed. Chromatogr. 1999, 13, 418–424. [Google Scholar] [CrossRef]
- Murooka, Y.; Yamshita, M. Traditional healthful fermented products of Japan. J. Ind. Microbiol. Biotechnol. 2008, 35, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Lanznaster, D.; Bruno, C.; Bourgeais, J.; Emond, P.; Zemmoura, I.; Lefevre, A.; Reynier, P.; Eymieux, S.; Blanchard, E.; Vourc’h, P.; et al. Metabolic profile and pathological alterations in the muscle of patients with early-stage amyotrophic lateral sclerosis. Biomedicines 2022, 10, 1307. [Google Scholar] [CrossRef] [PubMed]
- Beksac, K.; Reçber, T.; Çetin, B.; Alp, O.; Kaynaroglu, V.; Kir, S.; Nemutlu, E. GC-MS Based Metabolomics Analysis to Evaluate Short-Term Effect of Tumor Removal on Patients with Early-Stage Breast Cancer. J. Chromatogr. Sci. 2023, 61, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cai, Z.; Ferrari, M.; Liu, Y.; Li, C.; Zhang, T.; Lyu, G. The correlation between gut microbiota and serum metabolomic in elderly patients with chronic heart failure. Mediat. Inflamm. 2021, 2021, 5587428. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, N.; Engelgau, P.; Jones, A.D.; Song, J.; Beaudry, R. Citramalate synthase yields a biosynthetic pathway for isoleucine and straight- and branched-chain ester formation in ripening apple fruit. Proc. Natl. Acad. Sci. USA 2021, 118, e2009988118. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, Y.; Guo, X.; Ren, S.; Staempfli, A.; Chiao, J.; Jiang, W.; Zhao, G. Isoleucine biosynthesis in Leptospira interrogans serotype lai strain 56601 proceeds via a threonine-independent pathway. J. Bacteriol. 2004, 186, 5400–5409. [Google Scholar] [CrossRef] [PubMed]
- Amaha, M.; Sai, T. Some aspects of (-)-citramalic acid accumulation by respiration-deficient mutants of yeasts. Antonie Van Leeuwenhoek 1969, 35, G15–G16. [Google Scholar]
- Yin, Q.C.; Ji, J.B.; Zhang, R.H.; Duan, Z.W.; Xie, H.; Chen, Z.; Hu, F.C.; Deng, H. Identification and verification of key taste components in wampee using widely targeted metabolomics. Food Chem. X 2022, 13, 100261. [Google Scholar] [CrossRef] [PubMed]
| Organic Acid | m/z | Q1 Prebias (V) | Collision Energy (CE) | Q3 Prebias (V) | |
|---|---|---|---|---|---|
| Precursor | Product | ||||
| LA | 349.9 | 91.15 | −14 | −28 | −18 |
| LA-IS | 353.45 | 91.10 | −17 | −39 | −18 |
| MA | 653.1 | 91.05 | −32 | −54 | −17 |
| SA | 637.1 | 91.15 | −24 | −45 | −18 |
| CMA | 667.1 | 91.05 | −24 | −48 | −18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onozato, M.; Uchida, H.; Ono, M.; Koishi, M.; Oi, M.; Umino, M.; Sakamoto, T.; Fukushima, T. Application of the Precolumn Derivatization Reagent CIM-C2-NH2 for Labeling Carboxyl Groups in LC-MS/MS Analysis of Primary Organic Acids in Japanese Sake. Separations 2025, 12, 186. https://doi.org/10.3390/separations12070186
Onozato M, Uchida H, Ono M, Koishi M, Oi M, Umino M, Sakamoto T, Fukushima T. Application of the Precolumn Derivatization Reagent CIM-C2-NH2 for Labeling Carboxyl Groups in LC-MS/MS Analysis of Primary Organic Acids in Japanese Sake. Separations. 2025; 12(7):186. https://doi.org/10.3390/separations12070186
Chicago/Turabian StyleOnozato, Mayu, Haruna Uchida, Misaki Ono, Mikoto Koishi, Maya Oi, Maho Umino, Tatsuya Sakamoto, and Takeshi Fukushima. 2025. "Application of the Precolumn Derivatization Reagent CIM-C2-NH2 for Labeling Carboxyl Groups in LC-MS/MS Analysis of Primary Organic Acids in Japanese Sake" Separations 12, no. 7: 186. https://doi.org/10.3390/separations12070186
APA StyleOnozato, M., Uchida, H., Ono, M., Koishi, M., Oi, M., Umino, M., Sakamoto, T., & Fukushima, T. (2025). Application of the Precolumn Derivatization Reagent CIM-C2-NH2 for Labeling Carboxyl Groups in LC-MS/MS Analysis of Primary Organic Acids in Japanese Sake. Separations, 12(7), 186. https://doi.org/10.3390/separations12070186






