Impact of Use of Ultrasound-Assisted Extraction on the Quality of Brazil Nut Oil (Bertholletia excelsa HBK)
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Brazil Nut: General Aspects
3.2. Traditional Extraction Methods Versus Ultrasound-Assisted
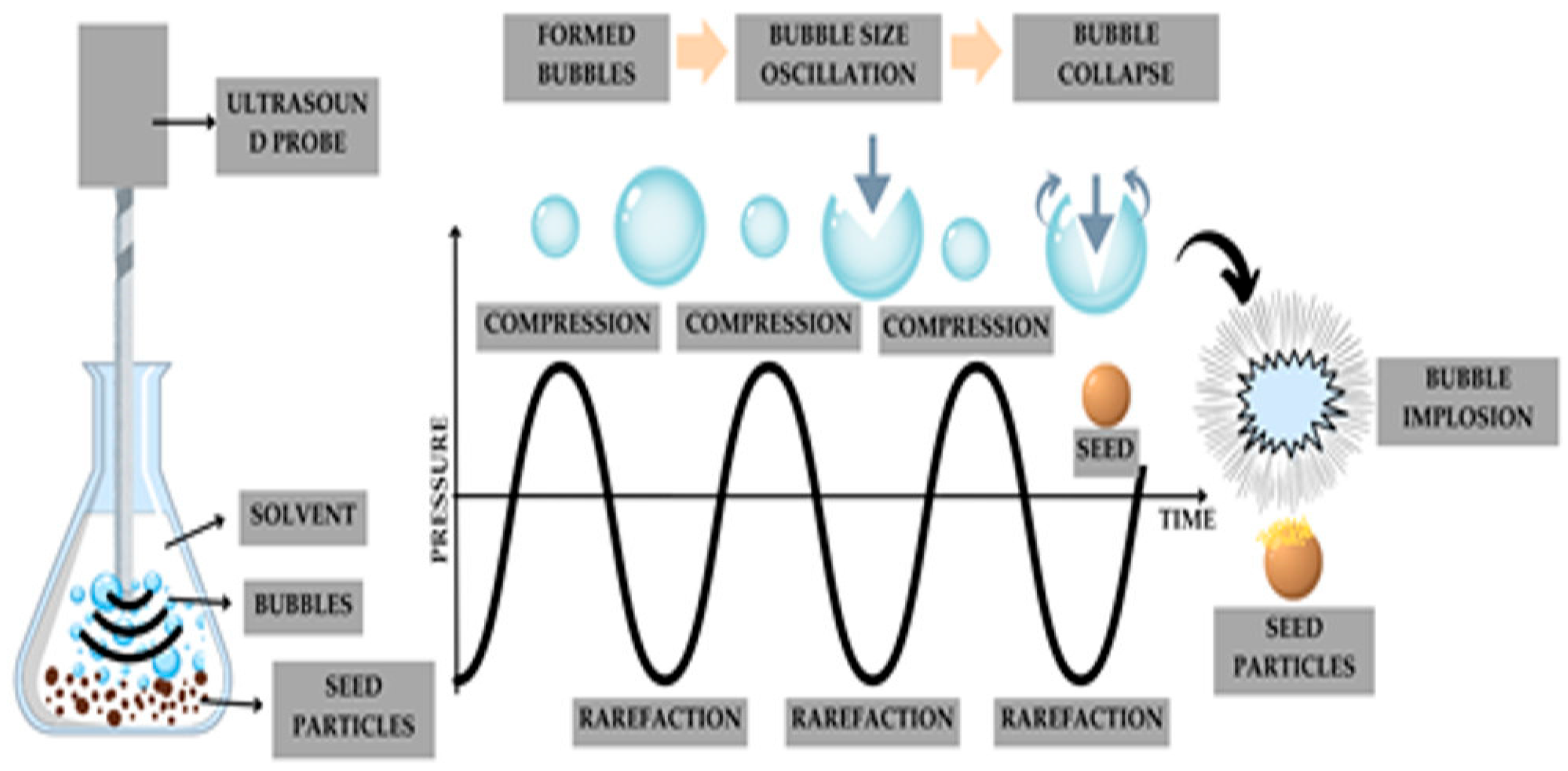
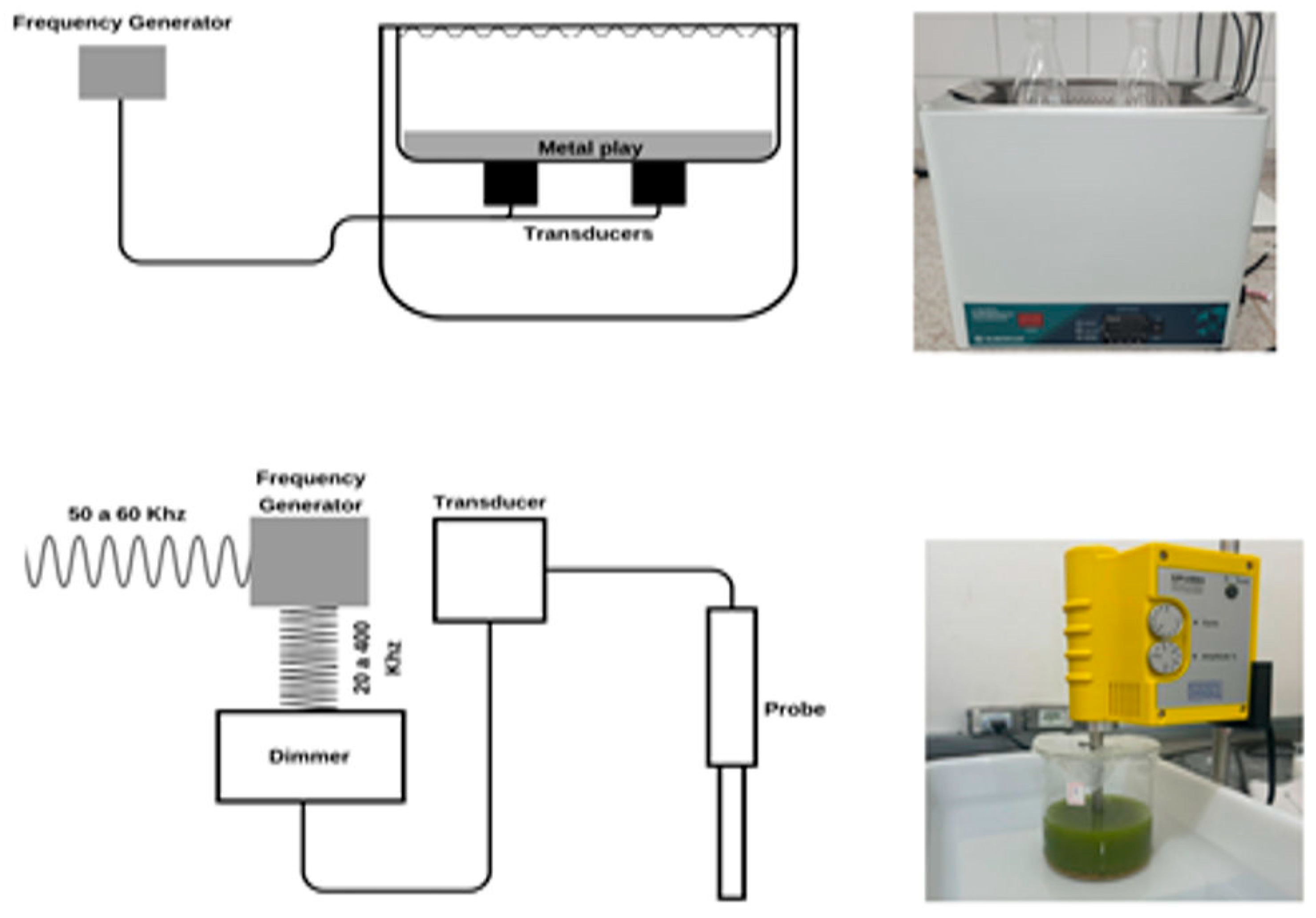
3.3. Ultrasound-Assisted Extraction (UAE)
- High-power (low-frequency) ultrasound, operating in the range of 16–100 kHz with intensities between 10 and 1000 W/cm2, and;
- Low-power (high-frequency) ultrasound, operating between 100 kHz and 1 MHz with intensities below 1 W/cm2 [58].
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Market Reports. Brazil Nuts Oils Market Insights. Study Period: 2021–2031. 220-2024. ID: 762386. Available online: https://www.transparencymarketresearch.com/us-brazil-nut-oil-market.html (accessed on 13 January 2024).
- Santos, O.V.; Azevedo, G.O.; Santos, Â.C.; Lopes, A.S. Development of a Nutraceutical Product Derived from By-Products of the Lipid Extraction of the Brazil Nut (Bertholletia excelsa H.B.K.). Foods 2023, 12, 1446. [Google Scholar] [CrossRef]
- Perez-Nakai, A.; Lerma-Canto, A.; Dominguez-Candela, I.; Ferri, J.M.; Fombuena, V. Novel Epoxidized Brazil Nut Oil as a Promising Plasticizing Agent for PLA. Polymers 2023, 15, 1997. [Google Scholar] [CrossRef]
- Takeda, L.N.; Omine, A.; Laurindo, L.F.; Araújo, A.C.; Machado, N.M.; Dias, J.A.; Kavalakatt, J.; Banerjee, S.; Goulart, R.A.; Atanasov, A.G.; et al. Brazil Nut (Bertholletia excelsa Bonpl.) in Health and Disease: A Narrative Review. Food Chem. 2025, 477, 143425. [Google Scholar] [CrossRef]
- Capaldi, G.; Binello, A.; Aimone, C.; Mantegna, S.; Grillo, G.; Cravotto, G. New Trends in Extraction-Process Intensification: Hybrid and Sequential Green Technologies. Ind. Crops Prod. 2024, 209, 117906. [Google Scholar] [CrossRef]
- Hashemi, B.; Shiri, F.; Svec, F.; Novakova, L. Green Solvents and Approaches Recently Applied for Extraction of Natural Bioactive Compounds. TrAC-Trends Anal. Chem. 2022, 157, 116732. [Google Scholar] [CrossRef]
- Freitas, L.C.; Santos, R.W.S.; Reis, F.R.; Haminiuk, C.W.I.; Corazza, M.L. Green extraction technologies: A path to the Amazon bioeconomy development. Trends Food Sci. Technol. 2024, 147, 104462. [Google Scholar] [CrossRef]
- Chutia, H.; Mahanta, C.L. Green Ultrasound and Microwave Extraction of Carotenoids from Passion Fruit Peel Using Vegetable Oils as a Solvent: Optimization, Comparison, Kinetics, and Thermodynamic Studies. Innov. Food Sci. Emerg. Technol. 2021, 67, 102547. [Google Scholar] [CrossRef]
- Khalid, S.; Chaudhary, K.; Amin, S.; Raana, S.; Zahid, M.; Namem, M.; Khaneghah, A.M.; Tadil, R.M. Recent advances in the implementation of ultrasound technology for the extraction of essential oils from terrestrial plant materials: A comprehensive review. Ultrason. Sonochem. 2024, 107, 106914. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Singh, V.K.; Garg, M.K. Novel oil extraction technologies: Process conditions, quality parameters, and optimization. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3–20. [Google Scholar] [CrossRef]
- Vasquez, W.V.; Hernandez, D.M.; Hierro, J.N.; Martin, D.; Cano, M.P.; Fornari, T. Supercritical Carbon Dioxide Extraction of Oil and Minor Lipid Compounds of Cake Byproduct from Brazil Nut (Bertholletia excelsa) Beverage Production. J. Supercrit. Fluids 2021, 171, 105188. [Google Scholar] [CrossRef]
- Chang, S.H. Utilização de Solventes Orgânicos Verdes na Extração por Solvente e Membrana Líquida para Tratamento Sustentável de Águas Residuais e Recuperação de Recursos—Uma Revisão. Environ. Sci. Pollut. Res. 2020, 27, 32371–32388. [Google Scholar] [CrossRef]
- Mokhtar, N.H.; Abdul Rahman, N.S.; Sofian-Seng, S.J.; Lim, W.A.; Wan Mustapha, A.; Abdul Hamid, N.S.; Mohd Razali, N.S.; Mohamed Nazir, M.Y. Comparative Analysis of Process Intensification Technologies (PIT) for Improved Cell Disruption and Lipid Recovery in Aurantiochytrium sp. SW1 Microalgae. Int. J. Food Sci. Technol. 2024, 59, 7827–7836. [Google Scholar] [CrossRef]
- Oliveira, S.H.; Rolim, C.S.S.; Alves, T.C.L.; Santos, C.L.; Lotas, K.M.; Nascimento, I.S.; Rolim, L.N.; Pereira, A.M.; Bonomo, R.C.F. Ultrasound-Assisted Extraction and Characterization of Brazil Nut Oil (Bertholletia excelsa). Sustain. Chem. Environ. 2025, 9, 100218. [Google Scholar] [CrossRef]
- Kluczkovski, A.M.; Oliveira, L.B.; Maciel, B.J.; Kluczkovski-Junior, A. Caracterização e Extração do Óleo de Castanha-do-Brasil: Revisão. Avanços Ciênc. Tecnol. Alimentos 2021, 3, 392–402. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, M.; Yang, C.H. Application of ultrasound technology in processing of ready-to-eat fresh food: A review. Ultrason. Sonochem. 2020, 63, 104953. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Rojo, A.D.; Carrillo-Lopez, L.M.; Reyes-Villagrana, R.; Huerta-Jiménez, M.; Garcia-Galicia, I.A. Ultrasound and meat quality: A review. Ultrason. Sonochem. 2019, 55, 369–382. [Google Scholar] [CrossRef]
- Nicolau-Lapeña, I.; Lafarga, T.; Viñas, I.; Abadias, M.; Bobo, G. Aguiló-Aguayo, I. Ultrasound processing alone or in combination with other chemical or physical treatments as a safety and quality preservation strategy of fresh and processed fruits and vegetables: A review. Food Bioprocess Technol. 2019, 12, 1452–1471. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
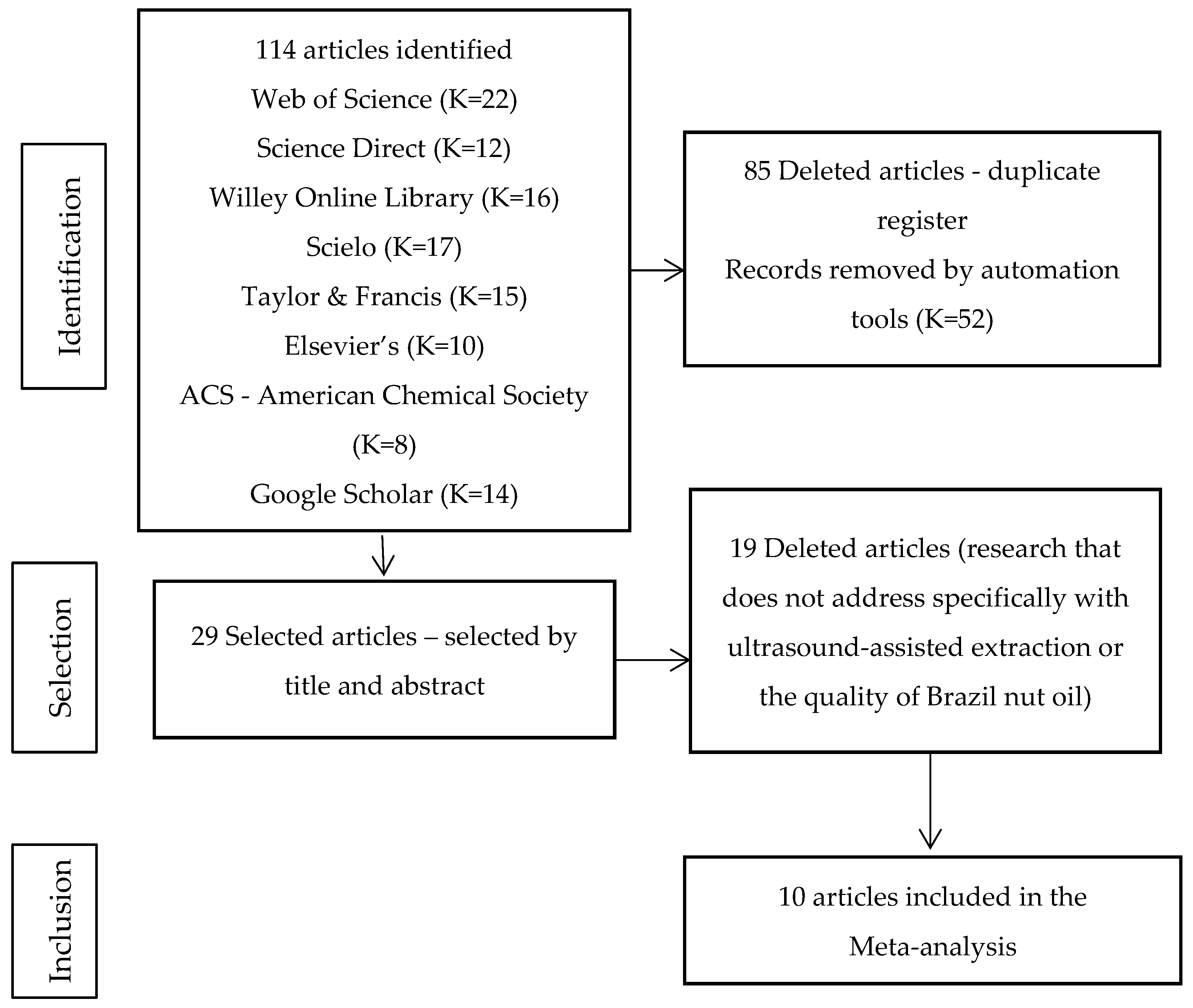
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística. Produção da Extração Vegetal e da Silvicultura: PEVS 2023; IBGE: Rio de Janeiro, Brazil, 2024. Available online: https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/9105-producao-da-extracao-vegetal-e-da-silvicultura.html (accessed on 13 January 2024).
- Silva, R.G.e.; Vilhena, A.E.G.d.; Martelli, M.C. Engenharia de Produtos Naturais: Planejamento, Experimentação, Obtenção de Produtos e Purificação. In Engenharia de Produtos Naturais: Planejamento, Experimentação, Obtenção de Produtos Purificados; Faria, L.J.G., Andrade, E.L., Eds.; Científica Digital: São Paulo, SP, Brasil, 2021; Volume 1, Chapter 28; pp. 183–197. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Cur. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Chalapud, M.C.; Carrín, M.E. Ultrasound-Assisted Extraction of Oilseeds—Sustainability Processes to Obtain Traditional and Non-Traditional Food Ingredients: A Review. Compreh. Rev. Food Sci. Food Saf. 2023, 22, 2161–2196. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, R.C.N.; Siow, L.F.; Tang, T.K.; Lee, Y.Y. A Review on Application of Ultrasound and Ultrasound Assisted Technology for Seed Oil Extraction. J. Food Sci. Technol. 2022, 60, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Farias, G.Y.Y.; Souza, M.M.; Oliveira, J.R.M.; Costa, C.S.B.; Collares, M.P.; Prentice, C. Effect of ultrasound-assisted cold plasma pretreatment to obtain sea asparagus extract and its application in Italian salami. Food Res. Int. 2020, 137, 109435. [Google Scholar] [CrossRef] [PubMed]
- Brandão, R.M.; Ferreira, V.R.F.; Batista, L.R.; Alves, E.; Santiago, W.D.; Barbosa, R.B.; Caetano, A.R.S.; Nelson, D.L.; Cardoso, M.G. Antifungal activity and the effect of the essential oil of Lippia origanoides Kunth on Aspergillus mycotoxins production. Aust. J. Crop Sci. 2021, 15, 1005–1012. [Google Scholar] [CrossRef]
- Carvalho, A.L.S.; Martelli, M.C.; Nascimento, S.C.C.; Brasil, D.d.S.B. Brazil Nut Oil: Extraction Methods and Industrial Applications. Res. Soc. Dev. 2022, 11, e29511427256. [Google Scholar] [CrossRef]
- Bisht, A.; Sahu, S.C.; Barwant, M.M.; Kumar, A.; Maqsood, S.; Barwant, M.M.; Swapnil, G.; Jaiswal, S.G. Recent advances in conventional and innovative extraction techniques for recovery of high-added value compounds for food additives and nutraceuticals. Food Phys. 2025, 2, 100047. [Google Scholar] [CrossRef]
- Senapati, M.R.; Behera, P.C. Novel extraction conditions for phytochemicals. In Recent Frontiers of Phytochemicals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 27–61. [Google Scholar] [CrossRef]
- Lavenburg, V.M.; Rosentrater, K.A.; Jung, S. Extraction Methods of Oils and Phytochemicals from Seeds and Their Environmental and Economic Impacts. Processes 2021, 9, 1839. [Google Scholar] [CrossRef]
- Gaikwad, R.K.; Shaikh, A.M.; Mondal, I.H.; Dash, K.K.; Shaikh, A.M.; Bela, K. Effectiveness of sustainable oil extraction techniques: A comprehensive review. J. Agric. Food Res. 2025, 19, 101546. [Google Scholar] [CrossRef]
- Zhou, J.; Min Wang, M.; Saraiva, J.A.; Martins, A.P.; Pinto, C.A.; Prieto, M.A.; Simal-Gandara, J.; Cao, H.; Xiao, J.; Barba, F.J. Extraction of lipids from microalgae using classical and innovative approaches. Food Chem. 2022, 384, 132236. [Google Scholar] [CrossRef] [PubMed]
- Irianto, I.; Suharmiati, S.; Zaini, A.S.; Zaini, M.A.A.; Airlanngg, B.; Putra, N.R. Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods. Green Process. Synth. 2025, 14, 20240201. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, L.; Liang, Z.; Chen, J.; Zhao, M.; Tang, Q. Comparative analysis of flavonoids extracted from Dendrobium chrysotoxum flowers by supercritical fluid extraction and ultrasonic cold extraction. Sustain. Chem. Pharm. 2023, 36, 101267. [Google Scholar] [CrossRef]
- Ramaswamy, R.; Krishnan, S.B.; Leong, S.S.J. Pulsed electric field technology for recovery of proteins from waste plant resources and deformed mushrooms: A review. Processes 2024, 12, 342. [Google Scholar] [CrossRef]
- Simayi, Z.; Aierken, W.; Rozi, P.; Ababaikeri, G.; Bo, C.; Chenglin, Z.; Xiaojun, Y. Optimization of ultrasound-assisted extraction, structural, functional, and antioxidant properties of Glycyrrhiza uralensis seed protein. Process Biochem. 2023, 124, 1–12. [Google Scholar] [CrossRef]
- Tapia-Quiros, P.; Granados, M.; Sentellas, S.; Saurina, J. Microwave-assisted extraction with natural deep eutectic solvents for polyphenol recovery from agrifood waste: Mature for scaling-up? Sci. Total Environ. 2023, 912, 168716. [Google Scholar] [CrossRef]
- Ferreira, B.; Beik, J.; Alves, S.; Henrique, F.; Sauer, E.; Chornobaid, C.; Bowles, S.; Chaves, E. Extração assistida por ultrassom para determinação de lipídeos em alimentos: Um experimento de laboratório. Quím. Nova 2020, 43, 1320–1325. [Google Scholar] [CrossRef]
- Zhong, J.-L.; Muhammad, N.; Chen, S.-Q.; Guo, L.-W.; Li, J.-S. Pilot-scale supercritical CO2 extraction coupled molecular distillation and hydrodistillation for the separation of essential oils from Artemisia argyi Lévl. et Vant. Sep. Sci. Technol. 2021, 56, 3127–3135. [Google Scholar] [CrossRef]
- Souza, R.R.; Gasparoti, P.S.; Paula, J.A.M. Obtenção de extratos de plantas medicinais: Uma Revisão de Escopo dos Métodos Extrativos Modernos em Comparação ao Método Clássico por Soxhlet. Movimenta 2022, 15, e20220013. [Google Scholar] [CrossRef]
- Favaro, S.P.; Miranda, C.H.B.; Lima, K.Q.; Shinzato, N.S.U.; Leal, I.F.C.S.; Gambetta, R.; Rodrigues, D.S. Princípios da Extração sem Solvente e Tecnologias Potenciais Para Obtenção de Óleos Vegetais, 1st ed.; Embrapa Agroenergia: Brasília, Brazil, 2022; Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/1142245/1/DOC-43-SEG-.pdf (accessed on 13 January 2024).
- Rashid, S.N.A.A.; Leong, H.Y.; Cheng, K.K.; Yaakob, H.; Latiff, N.A. Squalene rich virgin palm oil by microwave-assisted enzyme aqueous extraction from palm mesocarp. Biocatal. Agric. Biotechnol. 2023, 47, 102568. [Google Scholar] [CrossRef]
- Nde, D.; Foncha, A. Optimization Methods for the Extraction of Vegetable Oils: A review. Processes 2020, 8, 209. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, J.; Dai, X.; Li, X. Extraction and Analysis of Chemical Compositions of Natural Products and Plants. Separations 2023, 10, 598. [Google Scholar] [CrossRef]
- Souza, T.S.P.; Dias, F.F.G.; Koblitz, M.G.B.; Bell, J.M.L.N.M. Effects of enzymatic extraction of oil and protein from almond cake on the physicochemical and functional properties of protein extracts. Food Bioprod. Process. 2020, 122, 280–290. [Google Scholar] [CrossRef]
- Muñoz, A.M.; Casimiro-Gonzales, S.; Gómez-Coca, R.B.; Moreda, W.; Best, I.; Cajo-Pinche, M.I.; Loja, J.F.; Ibañez, E.; Cifuentes, A.; Ramos-Escudero, F. Comparison of Four Oil Extraction Methods for Sinami Fruit (Oenocarpus mapora H. Karst): Evaluating quality, polyphenol content and antioxidant activity. Foods 2022, 11, 1518. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Li, Y.; Song, J.; Li, H.; Zhou, Q.; Li, C.; Zhang, Z.; Liu, Y.; Liu, A.; Zhang, Q.; et al. Extração de óleo de chufa (Cyperus esculentus L.) usando a combinação de método enzimático aquoso assistido por micro-ondas e ultrassom—Projeto, otimização e avaliação de qualidade. J. Chromatogr. A 2020, 19, 100868. [Google Scholar] [CrossRef]
- Chen, G.; Sun, F.; Wang, S.; Wang, W.; Dong, J.; Gao, F. Enhanced extraction of essential oil from Cinnamomum cassia bark by ultrasound assisted hydrodistillation. Chin. J. Chem. Eng. 2021, 36, 38–46. [Google Scholar] [CrossRef]
- Santos, O.V.; Medeiros, P.G.; Lima, S.M.P.; Carvalho, R.R.; Galvão, M.M.; Das Chagas, A.N.F.; Dias, S.S.; Vieira, C.L.R. Potencial Nutricional e Funcional do Óleo da Pupunha Variedade Amarela (Bactris gasipaes Kunth). Sci. Plena 2022, 18, 061501. [Google Scholar] [CrossRef]
- Rodríguez-Mena, A.; Ochoa-Martínez, L.A.; González-Herrera, S.M.; Rutiaga-Quiñones, O.M.; González-Laredo, R.F.; Olmedilla-Alonso, B.; Vega-Maturino, S. Coloring potential of anthocyanins from purple sweet potato paste: Ultrasound-assisted extraction, enzymatic activity, color and its application in ice pops. Food Chem. Adv. 2023, 3, 100358. [Google Scholar] [CrossRef]
- Mushtaq, A.; Roobab, U.; Denoya, G.I.; Inam-Ur-Raheem, M.; Gullón, B.; Lorenzo, J.M.; Barba, F.J.; Zeng, X.-A.; Wali, A.; Aadil, R.M. Advances in Green Processing of Seed Oils Using Ultrasound-Assisted Extraction: A Review. J. Food Process. Preserv. 2020, 44, e14740. [Google Scholar] [CrossRef]
- Gerhardtova, I.; Jankech, T.; Majerova, P.; Piestansky, J.; Olesova, D.; Kovac, A.; Jampilek, J. Recent Analytical Methodologies in Lipid Analysis. Int. J. Mol. Sci. 2024, 25, 2249. [Google Scholar] [CrossRef]
- Chaves, J.O.; Souza, M.C.; Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F. Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 2020, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ou, H.; Xiang, Z.; Gregersen, H. Pré-tratamento ultrassônico combinado com extração com CO2 supercrítico do óleo de semente de Iberis amara. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100265. [Google Scholar] [CrossRef]
- Marhamati, M.; Kakhaki, Z.K.; Rezaei, M. Advance in Ultrasound Assisted Extraction of Edible Oils: A review. J. Nutr. Fast. Health 2020, 8, 220–230. [Google Scholar] [CrossRef]
- Soares, T.F.; Alves, R.C.; Beatriz, M. From Olive Oil Production to By-Products: Emergent Technologies to Extract Bioactive Compounds. Food Rev. Int. 2024, 40, 3342–3369. [Google Scholar] [CrossRef]
- Abrantes, K.K.B.; Colombo Pimentel, T.; da Silva, C.; Santos Junior, O.d.O.; Barão, C.E.; Cardozo-Filho, L. Brazil Nut SemiDefatted Flour Oil: Impact of Extraction Using Pressurized Solvents on Lipid Profile, Bioactive Compounds Composition, and Oxidative Stability. Plants 2024, 13, 2678. [Google Scholar] [CrossRef]
- Schoss, K.; Glavač, N.K. Supercritical CO2 Extraction vs. Hexane Extraction and Cold Pressing: Comparative analysis of seed oils from six plant species. Plants 2024, 13, 3409. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.M.; Marino, C.J.M.; Cotrim, A.C.M.; Nogueira, R.; Valladão, D.M.S.; Torres, M.P.R.; Ribeiro, E.B. Development and sun protection factor of emusionated formulation containing Brazil nut oil. Sci. Elec. Arch. 2020, 13, 130–141. [Google Scholar] [CrossRef]
- Brasil. Resolução RDC/ANVISA/MS nº 481, de 15 de Março de 2021. Regulamento Técnico Para Óleos Vegetais, Gorduras Vegetais e Creme Vegetal. D.O.U. 2021. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2020/rdc0481_15_03_2021.pdf (accessed on 13 January 2024).
- Silva, B.S.F.; Ferreira, N.R.; Alamar, P.D.; Melo e Silva, T.; Pinheiro, W.B.S.; Santos, L.N.; Alves, C.N. FT-MIR-ATR Associated with Chemometrics Methods: A Preliminary Analysis of Deterioration State of Brazil Nut Oil. Molecules 2023, 28, 6878. [Google Scholar] [CrossRef]
- Chamchomg, M.; Waeruwanaruk, D.; Guntornkun, C.; Alam, T. Effect of storage conditions on rancidity and antioxidant activity of gac oil compared with healthy oils. Agric. Nat. Resour. 2021, 55, 201–208. [Google Scholar] [CrossRef]
- Lemos, L.M.R.; Feltes, M.M.C. Exploring the nutritional and functional properties of Brazil nut (Bertholletia excelsa): A scientific prospection of nut-derived food products. J. Food Compos. Anal. 2025, 141, 107363. [Google Scholar] [CrossRef]

| Traditional Extraction Methodologies | Advantages | Disadvantages | Fonte |
|---|---|---|---|
| Maceration | Simple extraction technique; extraction of thermolabile chemicals, low-temperature extraction | Long period of time Lower yield, not economical, the solvent of choice generates waste Waste solvent can be an environmental pollutant | Bisht et al. (2025) [31] Senapati and Behera (2023) [32] |
| Mechanical pressing | Simple extraction technique, low cost, does not need organic solvents, the extraction temperature can be 50 °C to perform cold pressing, preserving nutritional compounds of the oil, reduces environmental impact, making it ecofriendly extraction technique that supports sustainable food systems | Lower oil recovery; limitation in equipment construction Low extraction capacity exposure of the sample to oxidative agents | Lavenburg et al. (2021) [33] Gaikwad et al. (2025) [34] |
| Soxhlet | Low cost; simple operation; high extraction rate | Long extraction time, large reagent and energy consumption (high extraction temperatures) low efficiency and generation of large amounts of environmentally polluting waste | Zhou et al. (2022) [35] Irianto et al. (2025) [36] Bisht et al. (2025) [31] |
| Folch | Fast, easy to handle large number of samples, the complete process is gentle | Toxic reagents are used, which are harmful to human health and the environment, generation of large amounts of environmentally polluting waste | Zhou et al. (2022) [35] Irianto et al. (2025) [36]. |
| Bligh–Dyer | Lipid extraction and separation can be achieved at the same time, simple extraction technique; extraction of thermolabile chemicals, low-temperature extraction | Extractive reagents are toxic and have few substitutes. The cost is high, generation of large amounts of environmentally polluting waste | Zhou et al. (2022) [35] Irianto et al. (2025) [36] |
| Super-/subcritical fluids/pressurized lipids extraction | High extraction efficiency, less use of toxic reagents and easy separation of lipids; protect bioactive compounds, reduce energy consumption and pollution, functions quicker than conventional techniques, increasing efficiency, and operates at lower temperatures, avoiding thermal degradation of heat-sensitive chemicals | It has selectivity to lipids of different polarity and the equipment is more expensive, not economically viable, requires considered technical knowledge high maintenance cost | Zhou et al. (2022) [35] Irianto et al. (2025) [36] Hu et al. (2023) [37] |
| Pulsed electric fields | The operation is simple and pollution-free, processing large number of samples, eliminating harsh chemicals, heavy solvents, and extreme mechanical effects on cells. By using plain water as a solvent, PEF reduces environmental impact, making it an ecofriendly extraction technique that supports sustainable food systems | It is necessary to control the proper electric field strength. Electric fields are too high and may adversely affect the extraction. Requires considered technical knowledge, high maintenance cost | Ramaswamy et al. (2024) [38] Bisht et al. (2025) [31] |
| Ultrasound-assisted extraction | Simple extraction technique: extraction of thermolabile chemicals, low-temperature extraction, by using plain water as a solvent reduces environmental impact, making it an ecofriendly extraction technique that supports sustainable food systems | Ultrasonic equipment is relatively expensive and sensitive, at least in larger operations, building large-scale equipment for industries that evenly distribute waves is a challenge. Powerful ultrasound may also break fragile compounds, so significant control over parameters is necessary. It is also noisy | Zhou et al. (2022) [35] Irianto et al. (2025) [36] Bisht et al. (2025) [31] Simayi et al. (2023) [39] |
| Microwave-assisted extraction | The temperature in the process is low, and the energy required is less. High extraction rate can be achieved in a short time, faster extraction times, less solvent consumption, greater extraction rates, and cheaper costs | This extraction process is affected by temperature, time, ethanol concentration, and solvent-to-sample ratio. Penetration depth, uneven heating in complex matrices, and the risk of overheating thermolabile compounds can affect extraction efficiency. The cost of specialized equipment is higher than traditional methods, and the need for electromagnetic shielding presents safety concerns | Zhou et al. (2022) [35] Irianto et al. (2025) [36] Bisht et al. (2025) [31] Tapia-Quiros et al. (2023) [40] |
| Enzyme-assisted extraction | Selective to substrate, pretreatment can be completed at room temperature and pressure to reduce energy consumption, sustainable and environmentally friendly extraction method with commercial potential, integrated with green technologies, as it requires less energy than conventional extraction techniques | The price of enzyme preparation is high, it is necessary to optimize the conditions to obtain the highest extraction rate, incomplete hydrolysis of plant cell walls, and scalability issues for industrial applications | Zhou et al. (2022) [35] Irianto et al. (2025) [36] Bisht et al. (2025) [31] |
| Author | Title | Results | Conclusion |
|---|---|---|---|
| Abrantes et al. (2024) [60] | Brazil Nut Semi-Defatted Flour Oil: Impact of Extraction Using Pressurized Solvents on Lipid Profile, Bioactive Compounds Composition, and Oxidative Stability | Pressurized n-propane extraction yielded 13.7–13.8% oil, while the CO2/n-propane mix yielded 2.2%. Squalene reached up to 1007 mg/100 g (4.5× higher than Brazil Nut Kernel Oil—BNKO), β-sitosterol ranged from 40–41 mg/100 g, and linoleic acid from 42.0–42.3%. Cold-pressed oil (BNKO) had higher phenolics (8.23 mg GAE/100 g) and antioxidant activity (DPPH: 366 µmol/100 g) compared to pressurized oils (~5.2–5.8 mg and ~198 µmol, respectively). Oxidative stability was highest (12 h) in Oil Extracted with Pressurized Fluid—OPF[p1], OPF[p2], and OPF[m], and lowest (6.5 h) in OPF[p3]. | Compared oils extracted from Brazil nut flour defatted by cold pressing and by pressurized solvents. The pressurized method outperformed traditional pressing in recovering bioactive compounds and improving lipid profile. |
| Thilakarathna et al. (2022) [27] | A Review on Application of Ultrasound and Ultrasound-Assisted Technology for Seed Oil Extraction | UAE achieved oil yields ranging from 8% to 83%. Kapok seed oil showed 92.29% recovery in 10 min (vs. 5.7 h for SE, 8 h for SXE). Ultrasound-Assisted Enzymatic Extraction (UAEE) increased pomegranate seed oil yield by 18.4% and reduced time by 91.7%. UASE increased passion fruit seed oil yield from 12.3% to 20.6%. Ultrasound Assisted Microwave Extraction (UAME) extracted 85.23% tiger nut oil, and Allanblackia seed oil reached 64.15% yield with 92.16% efficiency. | Comparative review of traditional and innovative extraction methods (ultrasound, enzymatic, supercritical, microwave), evaluating yield, cost–benefit, lipid profile, and oil stability. |
| Chalapud & Carrín (2023) [26] | Ultrasound-Assisted Extraction of Oilseeds—Sustainability Processes to Obtain Traditional and Non-Traditional Food Ingredients: A Review | The article highlights that ultrasound-assisted extraction (UAE) improves oil and bioactive compound yields from oilseeds, using less time, energy, and solvent compared to traditional methods. UAE also enhances the quality of extracts and facilitates the use of green solvents. It supports sustainable food ingredient production but still faces challenges in scalability, economic evaluation, and process modeling. | Use of UAE with milder solvents in food production supports environmentally friendly practices and the development of healthier and safer products. |
| Vasquez-Rojas et al. (2023) [11] | Extraction and Analytical Characterization of Phenolic Compounds from Brazil Nut (Bertholletia excelsa) | Adding 7.5 phr of Epoxidized Brazil Nut Oil (EBNO) to Poly(lactic acid) (PLA) increased elongation at break by 70.9% and crystallinity by over 400%, while tensile strength and Young’s modulus dropped by 40.9% and 11%, respectively. The glass transition temperature (Tg) decreased by up to 3.7 °C. Thermal degradation onset (T5%) was reduced by 14 °C. Biodegradability remained unaffected, with 90% disintegration in 27 days. | Compared different extraction methods and solvents with UAE; evaluated yield, quality, and antioxidant activity indices. |
| Perez-Nakai et al. (2023) [3] | Novel Epoxidized Brazil Nut Oil as a Promising Plasticizing Agent for PLA | Epoxidized Brazil nut oil (EBNO) increased the elongation at break of PLA by 70.9% with 7.5 phr added, while reducing tensile strength and Young’s modulus by 40.9% and 11%, respectively. EBNO also increased PLA crystallinity from 5.3% to 27.1%, lowered the glass transition temperature by up to 3.7 °C, and did not impair biodegradability—achieving 90% disintegration within 27 days under composting conditions. | Demonstrated the potential of epoxidized Brazil nut oil, extracted via UAE, as a bioplasticizer for PLA, increasing resistance and durability. |
| Carvalho et al. (2022) [30] | Brazil Nut Oil: Extraction Methods and Industrial Applications | The review study compared the efficiency of extracting oil from Brazil nuts using different solvents, with hexane having the highest yield with 69% oil, followed by petroleum ether with 66%, ethyl alcohol with 31.7% and isopropyl alcohol with 54.6%. Brazil nut oil using UAE indicates parameters compatible with current legislation in Brazil. | Found isopropyl alcohol to deliver better extraction yield than ethanol (54.6% vs. 31.7%) in Brazil nut oil extraction. |
| Oliveira et al. (2025) [14] | Ultrasound-Assisted Extraction and Characterization of Brazil Nut Oil (Bertholletia excelsa) | The acidity index found for Brazil nut oil was 0.45 ± 0.09, while the saponification index was 522.89 ± 9.00, and the refractive index was 1.7107 ± 0.001. The fatty acid composition consisted of 36–45% oleic acid and 33–38% linoleic acid. | UAE proved to be a practical, fast, and cost-effective method, maintaining oil quality and suitability for both food and cosmetics applications. |
| Freitas et al. (2024) [7] | Green Extraction Technologies: A Path to the Amazon Bioeconomy Development | Highlights EAU with promising results in yield, extract quality, and environmental aspects. Cites a study on the optimization of the extraction of antioxidant phenolic compounds from Brazil nut cake, obtained under the following conditions: ethanol–water (40:60; v/v); 2.5 min of homogenization; and 1 h of extraction at 60 °C. Also discusses a study by authors who identified high yields of oil extraction from the Brazil nut beverage by-product, by supercritical fluid with carbon dioxide (SC–CO2) under conditions of 400 bar and 60 °C. | Optimization of UAE parameters (frequency, power, time) enables extraction of key compounds with yields comparable to classical methods, but in less time. |
| Khalid et al. (2023) [9] | Recent Advances in the Implementation of Ultrasound Technology for the Extraction of Essential Oils from Terrestrial Plant Materials: A Comprehensive Review | Studies with UAE demonstrate a yield of up to 71% for essential oil extraction, higher than that found with the conventional Soxhlet method, which obtained 54%. Another study conducted with carrot seed essential oil obtained a 33% increase in oil yield using the UAE technique. UAE demonstrates greater efficiency, high selectivity, durability, scalability, and cost-effectiveness. | UAE was shown to be efficient, selective, durable, scalable, and cost-effective; compatible with integration into hybrid techniques. |
| Gomes et al. (2020) [62] | Development and Sun Protection Factor of Emulsified Formulation Containing Brazil Nut Oil | Emulsion formulations using Brazil nut oil were incorporated into the Octyl-methoxycinnamate UV filter at a concentration of 1%. The potential antioxidant activity test showed EC50 values of 7.41 mgmL−1 and 5.73 mgmL−1 for the chosen emulsions. The base formulation developed with Brazil nut oil showed adequate characteristics for incorporation of a sunscreen. | Developed and tested emulsified formulations containing Brazil nut oil extracted via ultrasound-assisted hexane method for sun protection factor (SPF) efficacy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, O.V.d.; Freires, S.C.V.; Palheta, H.C.d.O.; Ferreira, P.H.d.M. Impact of Use of Ultrasound-Assisted Extraction on the Quality of Brazil Nut Oil (Bertholletia excelsa HBK). Separations 2025, 12, 182. https://doi.org/10.3390/separations12070182
Santos OVd, Freires SCV, Palheta HCdO, Ferreira PHdM. Impact of Use of Ultrasound-Assisted Extraction on the Quality of Brazil Nut Oil (Bertholletia excelsa HBK). Separations. 2025; 12(7):182. https://doi.org/10.3390/separations12070182
Chicago/Turabian StyleSantos, Orquidea Vasconcelos dos, Sara Camila Vidal Freires, Helen Cristina de Oliveira Palheta, and Paulo Henrique de Melo Ferreira. 2025. "Impact of Use of Ultrasound-Assisted Extraction on the Quality of Brazil Nut Oil (Bertholletia excelsa HBK)" Separations 12, no. 7: 182. https://doi.org/10.3390/separations12070182
APA StyleSantos, O. V. d., Freires, S. C. V., Palheta, H. C. d. O., & Ferreira, P. H. d. M. (2025). Impact of Use of Ultrasound-Assisted Extraction on the Quality of Brazil Nut Oil (Bertholletia excelsa HBK). Separations, 12(7), 182. https://doi.org/10.3390/separations12070182






