The Selective Extraction of Natural Sesquiterpenic Acids in Complex Matrices: A Novel Strategy for Isolating Zizanoic Acid in Vetiver Essential Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
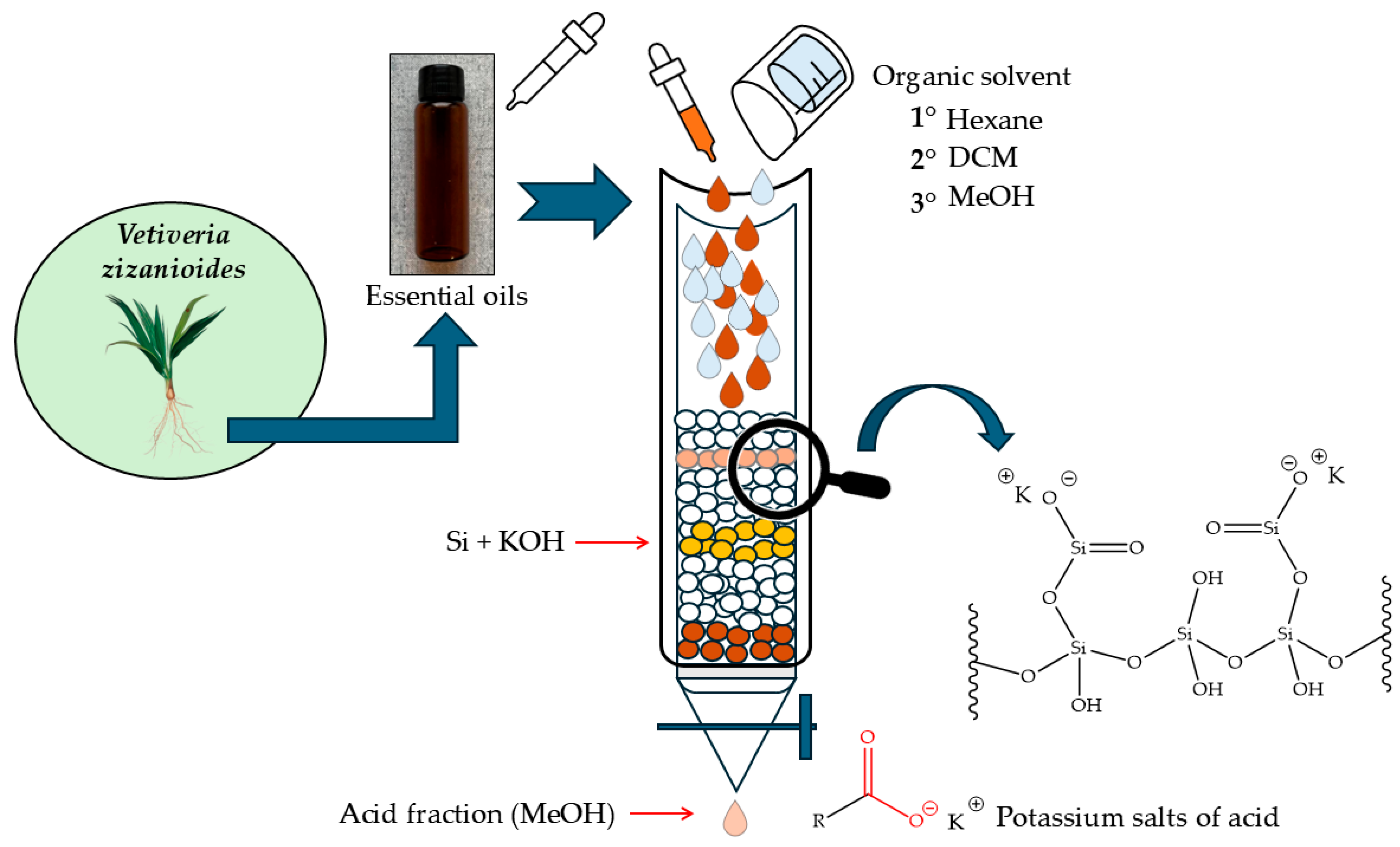
Preparation of the KOH Solution and Impregnation in Silica Gel
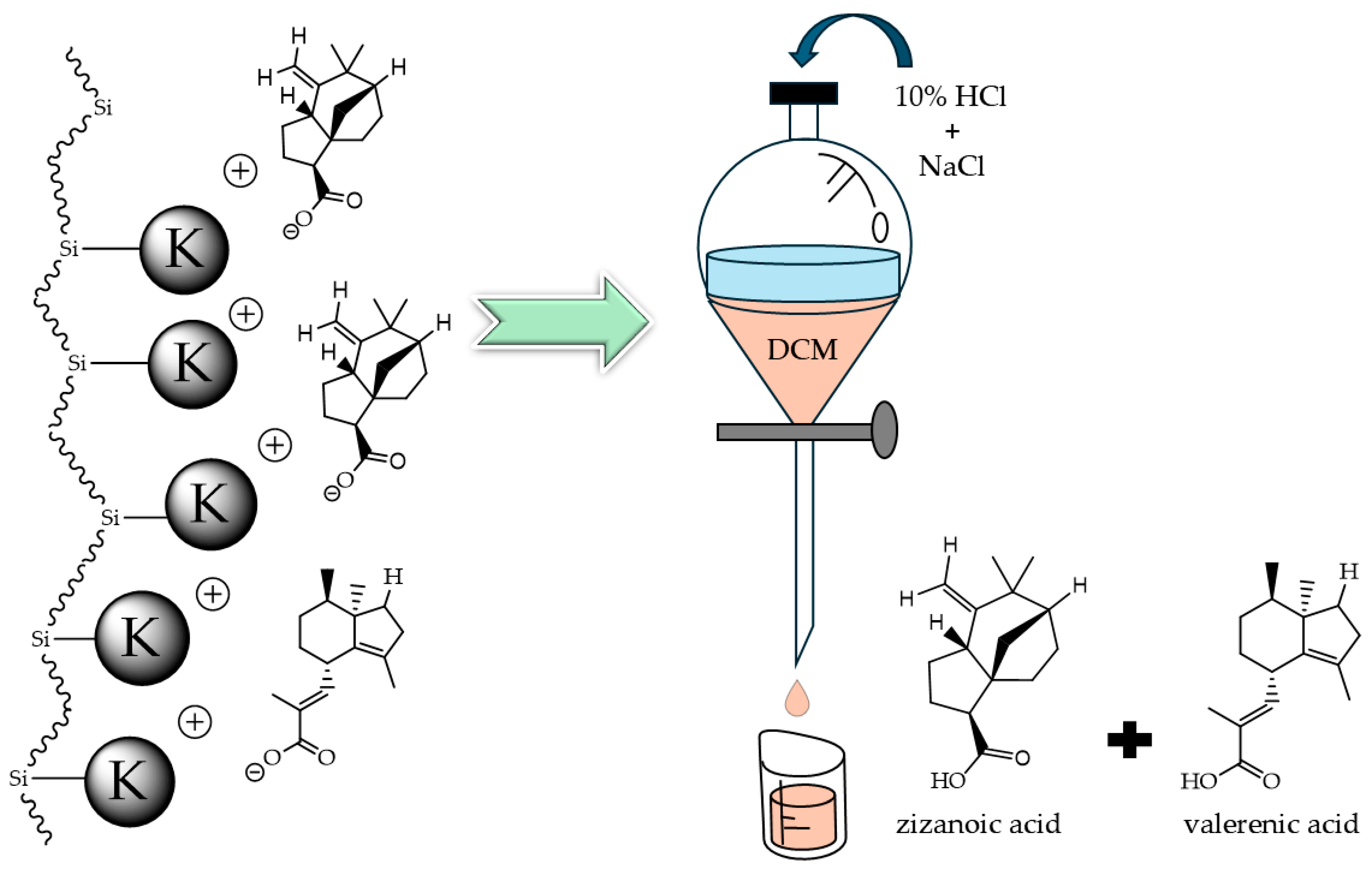
2.2. Filtration Column (Anion Exchange)
2.3. Percentage Yield Calculation
2.4. Instrumental Analysis
2.4.1. UHPLC-HRMS Experiments
2.4.2. GC-MS and GC-MS-MS Experiments
2.4.3. NMR Experiments
3. Results and Discussion
3.1. Ion Exchange Column Yields
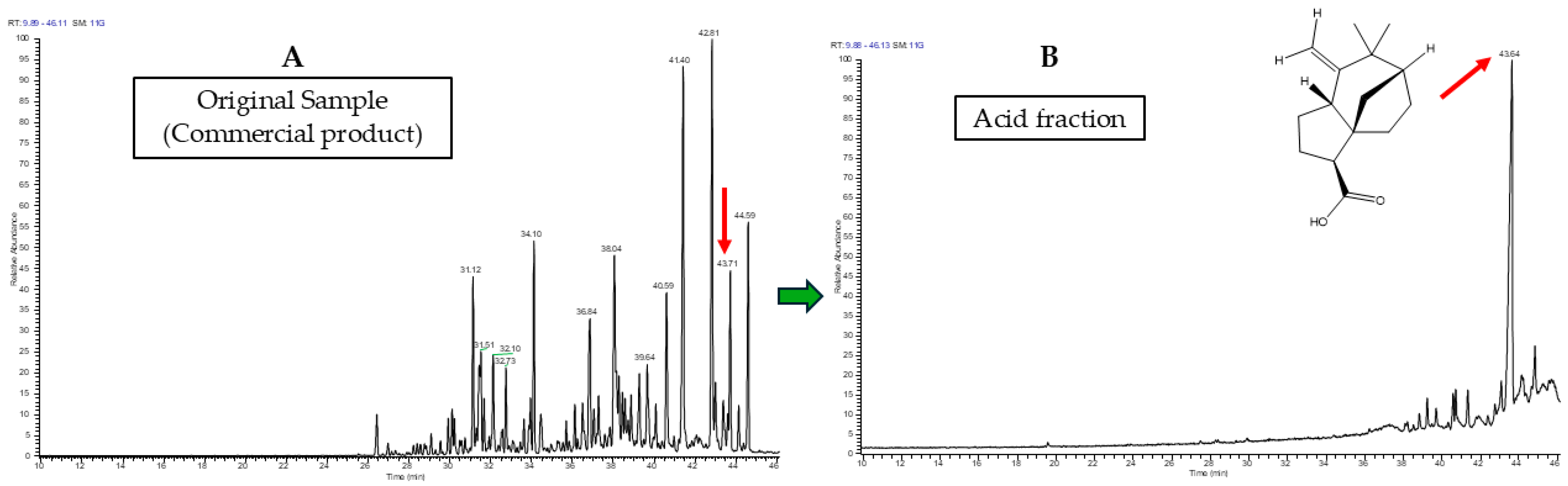
3.2. Identification in GC-MS
3.3. Identification in GC-MS/MS
3.4. Identification in UHPLC-HRMS
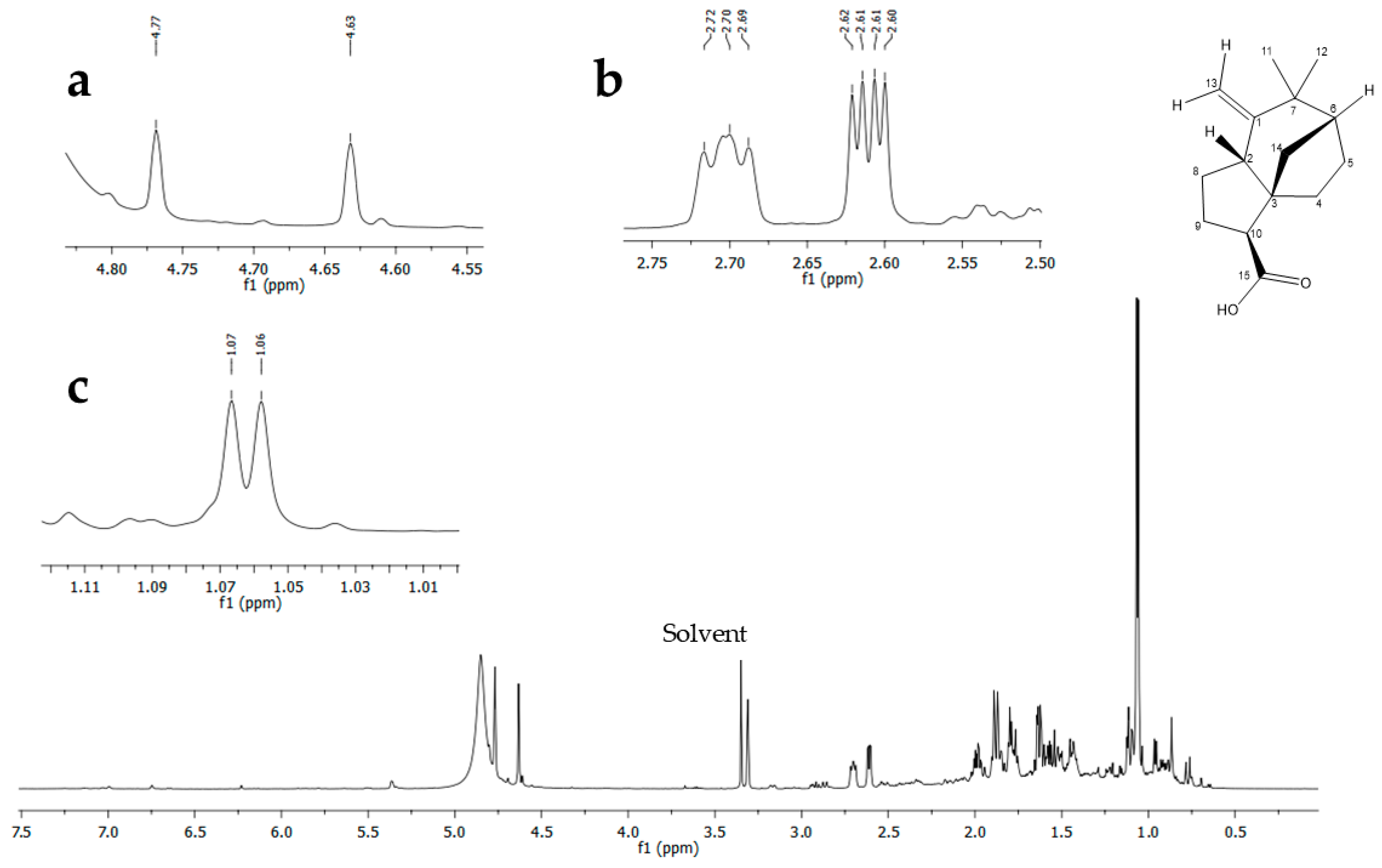
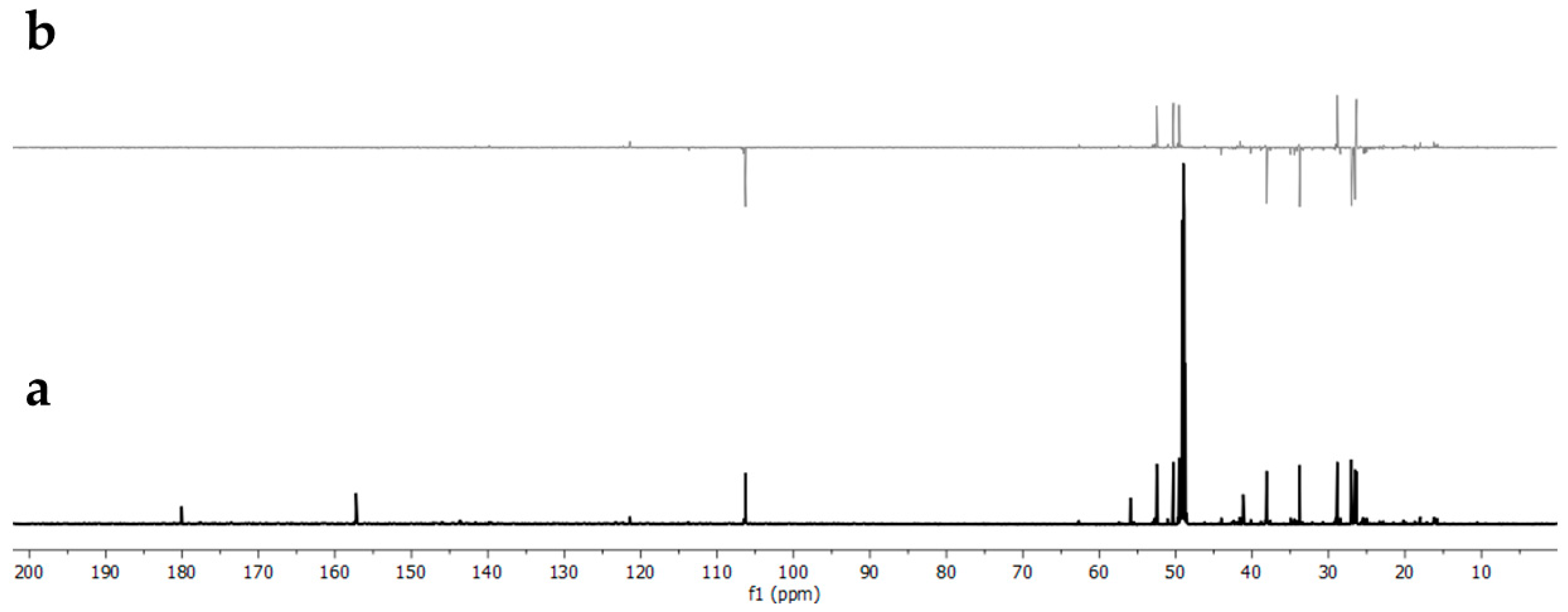
3.5. Identification in NMR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGC | Automatic Gain Control |
| DDA | Data Dependent Analysis |
| DCM | Dichloromethane |
| DEPT | Distortionless Enhancement by Polarization Transfer |
| EI | Electron Impact Ionization |
| ESI | Electrospray Ionization |
| ETOAc | Ethyl Acetate |
| FWHM | Full Widths at Half-Maximum |
| GC | Gas Chromatography |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| HCD | Higher Energy Collisional Dissociation |
| ISQ-LT | Internal Standards Quadrupole—Low Tech |
| IT | Injection Time |
| LC | Liquid Chromatography |
| MHz | Megahertz |
| MS | Mass Spectrometry |
| NIST17 | National Institute of Standards and Technology Library, 2017 |
| NMR | Nuclear Magnetic Resonance |
| ppm | Parts Per Million |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
| UHPLC-HRMS | Ultra High-Performance Liquid Chromatography-High Resolution Mass Spectrometry |
| VEO | Vetiver Essential Oil |
References
- Jean-Jacques, F.; Emilie, B.; Nicolas, B.; Hugues, B.; Uwe, J.M. Qualitative and quantitative analysis of vetiver essential oils by comprehensive two-dimensional gas chromatography and comprehensive two-dimensional gas chromatography/mass spectrometry. J. Chromat. A 2013, 1288, 127–148. [Google Scholar]
- Ranjana, M.; Pallavi, Y.; Ram, K.; Pallavi, K.; Shubhra, R.; Soumyajit, M.; Shubham, S.; Mohammad, Q.A.; Abhishek, K.S.; Harmesh, S.C.; et al. Transcriptome and metabolome analysis of sesquiterpene diversity in Indian vetiver (Chrysopogon zizanioides L. Roberty). Ind. Crops Prod. 2023, 200, 116798. [Google Scholar]
- Ian, G.C.B.-S.; Filipe, K.F.S.; Harsha, K.; Subhash, C.; Amit, C.K.-W.; Rajendra, A.; Neerupma, D.; Bhupesh, S.; Kulkarni, G.T.; Harold, L.; et al. Vetiver, Vetiveria zizanioides (L.) Nash: Biotechnology, bi-orefineries and the production of volatile phytochemicals. Plants 2025, 14, 1435. [Google Scholar]
- Andreea, D.; Anca, F.; Sonia, A.S. An overview of the chemical composition and bioactivities of Vetiveria zizanioides (L.) Nash essential oil. Trends Food Sci. Technol. 2023, 140, 104153. [Google Scholar]
- Research and Markets. Vetiver Oils Strategic Business Report 2025: Ethically Sourced Vetiver Oil Gains Traction in Organic and Clean Beauty Markets; Discover How Holistic Wellness Trends are Fueling Demand for Vetiver Oil in Aromatherapy. Available online: https://www.globenewswire.com/news-release/2025/01/28/3016641/28124/en/Vetiver-Oils-Strategic-Business-Report-2025-Ethically-Sourced-Vetiver-Oil-Gains-Traction-in-Organic-and-Clean-Beauty-Markets.html (accessed on 24 April 2025).
- Emilie, B.; Jean-Jacques, F.; Hugues, B.; Daniel, J.; Nicolas, B. Volatile constituents of vetiver: A review. Flavour. Fragr. J. 2015, 30, 26–82. [Google Scholar]
- Luu, T.D.; Paul, T.; Raffaela, M.; Tam, T.; Neil, F. Vetiver grass, vetiveria zizanioides: A choice plant for phytoremediation of heavy metals and organic wastes. Int. J. Phytoremed. 2009, 11, 664–691. [Google Scholar]
- Luu, T.D.; Paul, T.; Raffaela, M.; Tam, T.; Neil, F. Extraction of vetiver essential oil by ethanol-modified supercritical carbon dioxide. Chem. Eng. J. 2010, 165, 26–34. [Google Scholar]
- Emeline, T.; Mara, E.M.B.; Paulo, T.V.R.; Delphine, P.-J.; Maria, A.A.M. Supercritical fluid extraction of vetiver roots: A study of SFE kinetics. J. Supercrit. Fluids 2008, 47, 200. [Google Scholar]
- Alifano, P.; Del Giudice, L.; Talà, A.; De Stefano, M.; Maffei, M. Microbes at work in perfumery: The microbial community of vetiver root and its involvement in essential oil biogenesis. Flavour. Fragr. J. 2010, 25, 121. [Google Scholar] [CrossRef]
- Kim, H.-J.; Chen, F.; Wang, X.; Chung, H.; Jin, Z. Evaluation of antioxidant activity of vetiver (Vetiveria zizanioides L.) oil and identification of its antioxidant constituents. J. Agric. Food Chem. 2005, 53, 7691. [Google Scholar] [CrossRef]
- Ruzicka, L.; Capato, E.; Huyser, H. Ernest Guenther—Essential Oils Vol II. Rec. Trav. Chim. 1928, h7, 370. [Google Scholar] [CrossRef]
- Fusao, K.; Hisashl, U.; Akira, Y. The structure of zizanoic acid, a novel sesquiterpene in vetiver oil. Tet. Lett. 1967, 29, 2815–2920. [Google Scholar]
- Hanayamaj, N.; Kmo, F.; Tanaka, R.; Hisashl, U.; Akira, Y. Sesquiterpenoids of vetiver oil-i the structures of zizanoic acid and related constituents1. Tetrahedron 1973, 29, 945–954. [Google Scholar] [CrossRef]
- Gaurav, R.D.; Shikha, G.; Sudeep, R.; Komal, K.; Anirban, P.; Jay, P.T.; Dharmendra, S.; Ashok, S.; Nandan, S.D.; Mahendra, P.D.; et al. Tricyclic Sesquiterpenes from Vetiveria zizanoides (L.) Nash as Antimycobacterial Agents. Chem. Biol. Drug Des. 2013, 82, 587–594. [Google Scholar]
- Gabrielle, M.L.; Lucindo, J.Q.-J.; Sara, M.T.; Emyle, M.S.A.A.; Mônica, S.M.; Mairim, R.S.; Sócrates, C.H.C.; Daniel, P.G.; João, P.A.S.; Arie, F.B.; et al. Phytochemical screening, antinociceptive and anti-inflammatory activities of Chrysopogon zizanioides essential oil. Braz. J. Pharmacog. 2012, 22, 443–450. [Google Scholar]
- Andreea, D.; Fang, W.; Xiaoming, S.; Hongna, L.; Jieru, L.; Peilei, L.; Gang, D. Chemical Composition, Antioxidant, and Antimi-crobial Activities of Vetiveria zizanioides (L.) Nash Essential Oil Extracted by Carbon Dioxide Expanded Ethanol. Molecules 2019, 24, 1897. [Google Scholar]
- Peter, W.; Helga, M.; Ute, S.; Dietmar, W.; Horst, S. Constituents of Haitian vetiver oil. Flavour. Fragr. J. 2000, 15, 395–412. [Google Scholar]
- Sarita, K.; Amit, L.; Anurag, K. Chapter 3.1.7: Valerenic and acetoxyvalerenic acid. In Book: Naturally Occurring Chemicals Against Alzheimer’s Disease; Springer: Cham, Switzerland, 2021; pp. 117–125. [Google Scholar]
- Ekrem-Murat, G.; Omer, B.; Funda-Nuray, Y.; Lutfiye-Omur, D. The roles of valerenic acid on BDNF expression in the SH-SY5Y cell. Saudi Pharm. J. 2018, 26, 960–964. [Google Scholar]
- Ivani, C.T.; José, R.D.; Cleber, R.A.; Renato, C.-B.; Felipe, X.C.; José Ribeiro, L., Jr. Níveis Séricos do BDNF na Proteção Cardi-ovascular e em Resposta ao Exercício. Arq. Bras. Cardiol. 2020, 115, 263–269. [Google Scholar]
- Angelo, C.P.; Waldenir, F.B.; Claudia, M.R.; Francisco, M.S.G.; Valdir, F.V.-J.; Lothar, B.; Maria, L.P.; Octávio, A.C.A. Sepa-ration of Acid Diterpenes of Copaifera cearensis Huber ex Ducke by Flash Chromatography Using Potassium Hydroxide Im-pregnated Silica Gel. J. Braz. Chem. Soc. 2000, 11, 355–360. [Google Scholar]
- Pierce, V.K.; Ruth, H.D.; Ciaran, A.M.; Rabab, M.; Fiona, K.; Catherine, S.; John, J.W. Detection and Quantification of Valerenic Acid in Commercially Available Valerian Products. J. Chem. Educ. 2007, 84, 829. [Google Scholar]
- Vijaya, S.; Pratibha, S.; Deepak, K.; Rituraj, K.; Bikarma, S.; Brijesh, K. Phytochemical analysis of high value medicinal plant Valeriana jatamansi using LC-MS and it’s in-vitro anti-proliferative screening. Phytomed. Plus. 2021, 1, 10025. [Google Scholar]
- Jan, S.; Sônia, M.S.; Denise, D. Differential NMR and chromatography for the detection and analysis of adulteration of vetiver essential oils. Talanta 2022, 237, 122928. [Google Scholar]

| Vetiveria zizanioides Root Oil | F. Hexane (g) | F. Dichloromethane (g) | F. Methanol (g) |
|---|---|---|---|
| V1 | 3.83 | 0.24 | 0.22 |
| V2 | 2.99 | 0.65 | 0.12 |
| Zizanoic Acid | Valerenic Acid | |||||
|---|---|---|---|---|---|---|
| Sample | Rt (min.) | Area | % | Rt (min.) | Area | % |
| V1 | 43.92 | 580,366,466 | 84.18 | 46.42 | 57,105,897 | 8.28 |
| V2 | 43.64 | 203,248,113 | 87.07 | 46.36 | 15,083,369 | 6.46 |
| Shukla et. al. [24] | V1 Sample | V2 Sample | ||
|---|---|---|---|---|
| Fragments Ions (m/z) | Fragments Ions (m/z) | Error (Δppm) | Fragments Ions (m/z) | Error (Δppm) |
| 235.1693 | 235.1697 | 1.62 | 235.1696 | 1.15 |
| 217.1595 | 217.1591 | 1.70 | 217.1590 | 2.30 |
| 189.1637 | 189.1642 | 2.80 | 189.1642 | 2.54 |
| 123.1174 | 123.1173 | 0.81 | 123.1172 | 1.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcellos-Silva, I.G.C.; da Silva Antonio, A.; da Silva, M.C.C.; de Melo Regazio Cariello, F.; Hallwass, F.; Padilha, M.C.; Veiga-Junior, V.F. The Selective Extraction of Natural Sesquiterpenic Acids in Complex Matrices: A Novel Strategy for Isolating Zizanoic Acid in Vetiver Essential Oil. Separations 2025, 12, 163. https://doi.org/10.3390/separations12060163
Barcellos-Silva IGC, da Silva Antonio A, da Silva MCC, de Melo Regazio Cariello F, Hallwass F, Padilha MC, Veiga-Junior VF. The Selective Extraction of Natural Sesquiterpenic Acids in Complex Matrices: A Novel Strategy for Isolating Zizanoic Acid in Vetiver Essential Oil. Separations. 2025; 12(6):163. https://doi.org/10.3390/separations12060163
Chicago/Turabian StyleBarcellos-Silva, Ian Gardel Carvalho, Ananda da Silva Antonio, Mateus Curty Cariello da Silva, Fernanda de Melo Regazio Cariello, Fernando Hallwass, Monica Costa Padilha, and Valdir Florencio Veiga-Junior. 2025. "The Selective Extraction of Natural Sesquiterpenic Acids in Complex Matrices: A Novel Strategy for Isolating Zizanoic Acid in Vetiver Essential Oil" Separations 12, no. 6: 163. https://doi.org/10.3390/separations12060163
APA StyleBarcellos-Silva, I. G. C., da Silva Antonio, A., da Silva, M. C. C., de Melo Regazio Cariello, F., Hallwass, F., Padilha, M. C., & Veiga-Junior, V. F. (2025). The Selective Extraction of Natural Sesquiterpenic Acids in Complex Matrices: A Novel Strategy for Isolating Zizanoic Acid in Vetiver Essential Oil. Separations, 12(6), 163. https://doi.org/10.3390/separations12060163









