Current Perspectives on the Extraction, Isolation, and Identification of Fats and Fatty Acids Using Conventional and Green Methods
Abstract
1. Introduction
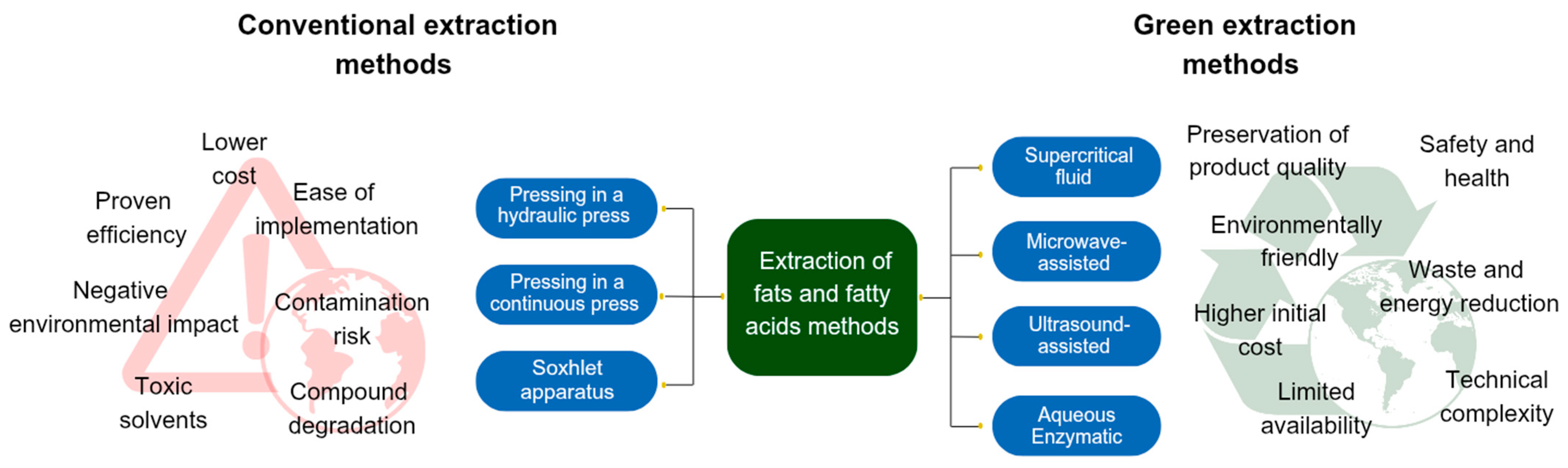
2. Fat Extraction Methods
2.1. Conventional Extraction Methods
2.1.1. Mechanical Extraction Using a Hydraulic and Mechanical Press
2.1.2. Conventional Solvent or Solid–Liquid Extraction
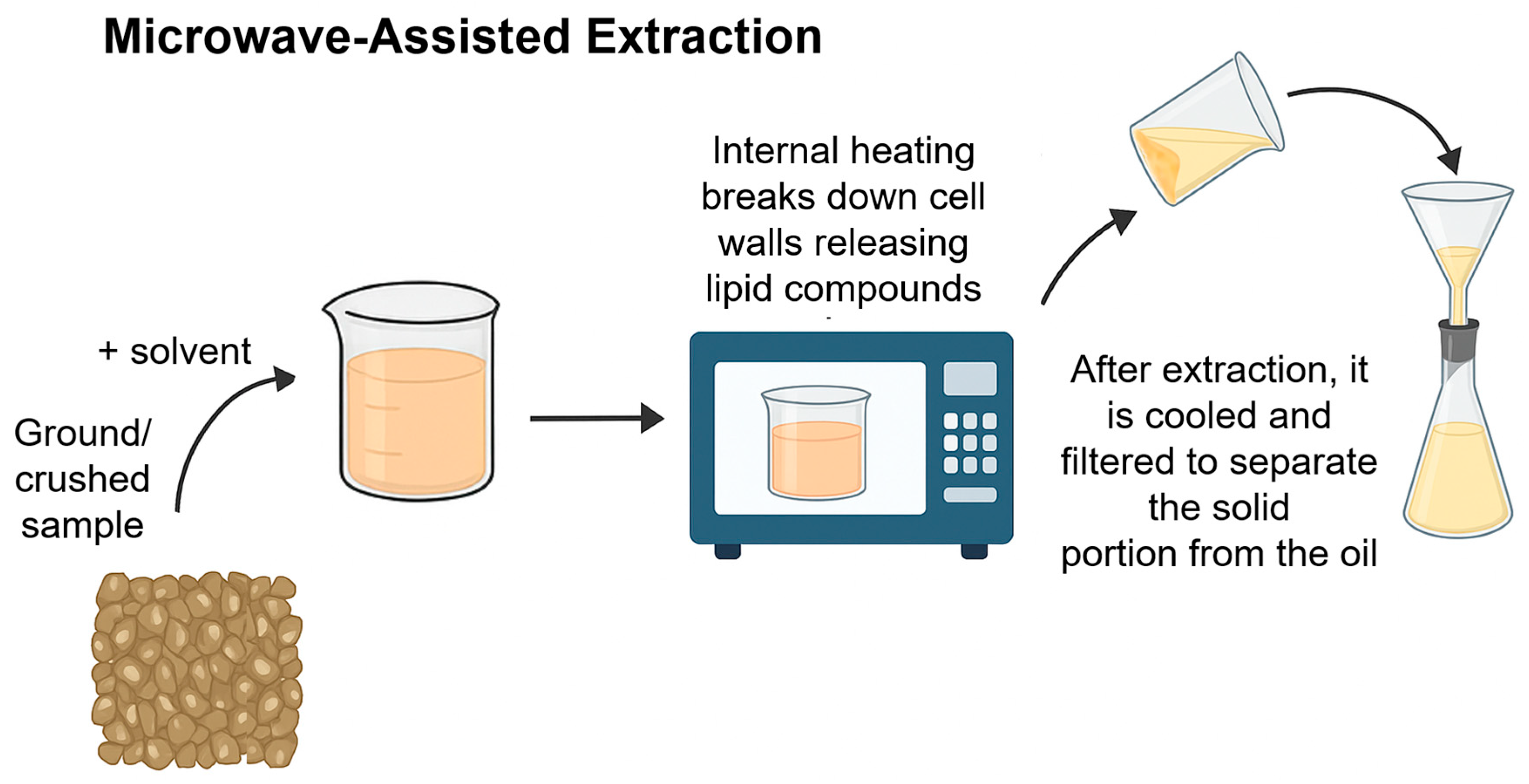
2.2. Green Extraction Methods
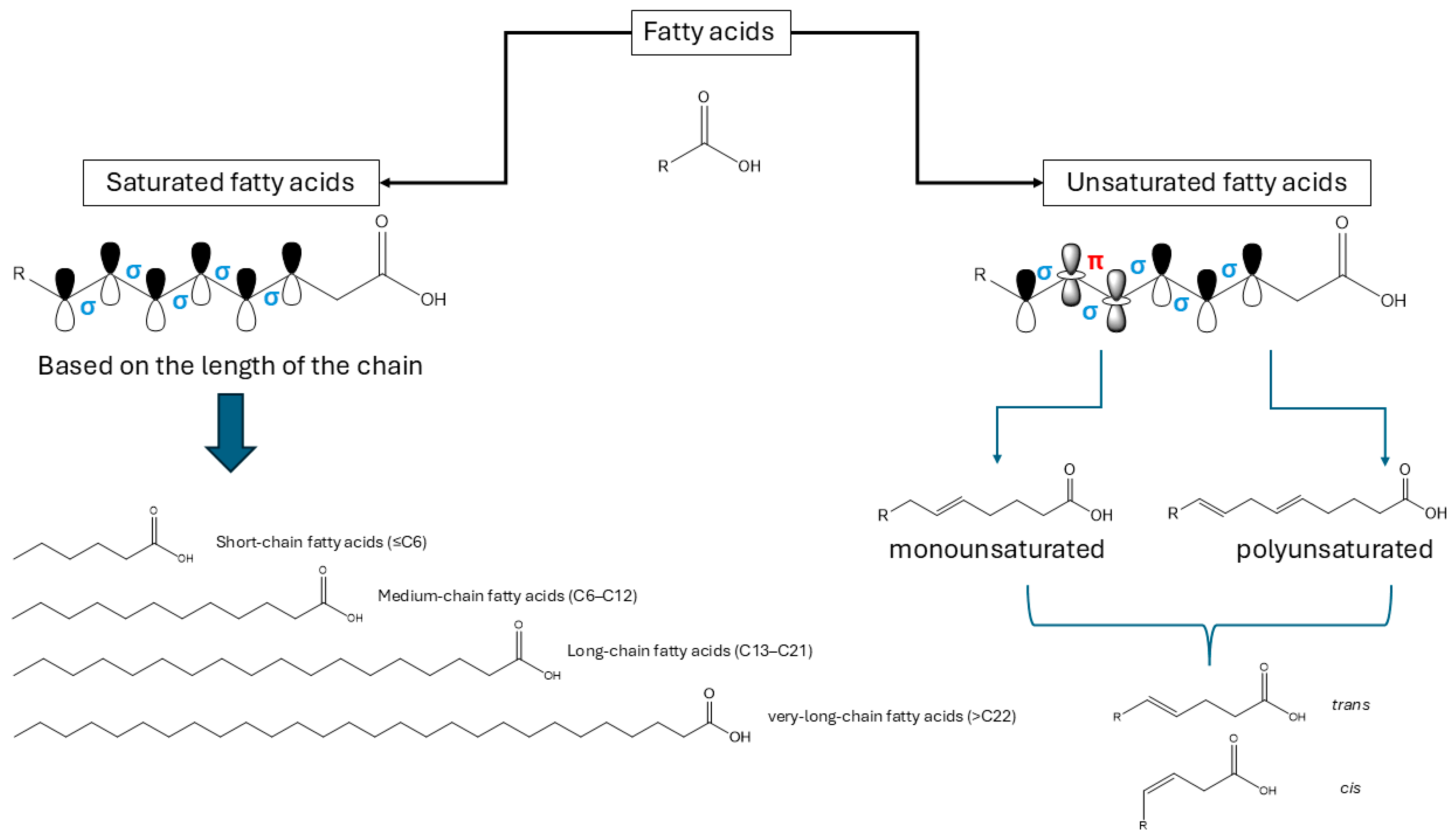
3. Identification of Fatty Acids
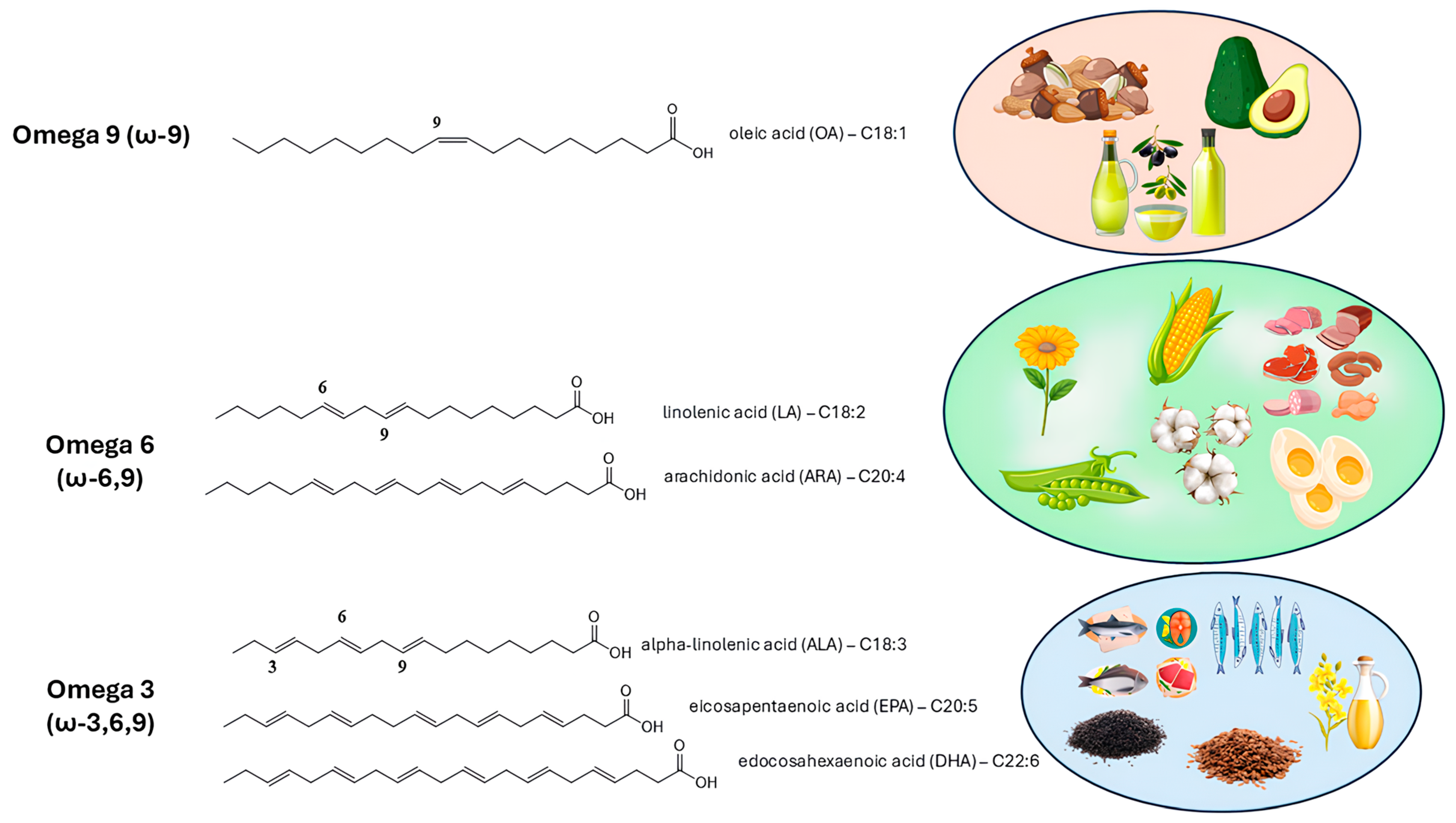
3.1. Chemical Structure
3.2. Identification Techniques
3.2.1. Gas Chromatographic (GC) Analysis
3.2.2. Isolation of Fatty Acids for Structural Analysis by HPLC
3.2.3. Silver-Ion Chromatography
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HPLC | High-performance liquid chromatography; |
| AD | Anno Domini from Gregorian calendar; |
| BTXs | Benzene, toluene, and xylenes; |
| SFE | Supercritical fluid extraction; |
| SFE-CO2 | Carbon dioxide supercritical fluid extraction; |
| MAE | Microwave-assisted extraction; |
| UAE | Ultrasound-assisted extraction; |
| PLE | Pressurized liquid extraction; |
| PEF | Pulsed electric fields; |
| EAE | Enzyme-assisted extraction; |
| SWE | Subcritical water extraction; |
| EACP | Enzyme-assisted cold pressing; |
| EAAE | Enzyme-assisted aqueous extraction; |
| FAs | Fatty acids; |
| MUFAs | Monounsaturated fatty acids; |
| PUFAs | Polyunsaturated fatty acids; |
| SFAs | Saturated fatty acids; |
| ALA | Alpha-linolenic acid; |
| DHA | Docosahexaenoic acid; |
| EPA | Eicosapentaenoic acid; |
| LA | Linoleic acid; |
| ARA | Arachidonic acid; |
| AI | Atherogenicity index; |
| TI | Thrombogenic index; |
| h/H | Hypocholesterolemic/hypercholesterolemic ratio; |
| HPI | Health-promoting index; |
| COX | Calculated oxidizability value; |
| GC | Gas chromatography; |
| GC-MS | Gas chromatography coupled with mass spectrometry detector; |
| GC-FID | Gas chromatography coupled with flame ionization detector; |
| GC-FTIR | Gas chromatography coupled with Fourier transform infrared spectroscopy; |
| Ag-HPLC | Silver-ion high-performance liquid chromatography; |
| NMR | Nuclear magnetic resonance; |
| HP-88 | High-polarity column (88%-cyanopropyl aryl-polysiloxane); |
| DB-FFAP | Nitroterephthalic acid-modified polyethylene glycol column; |
| IL | Ionic liquid; |
| FFAs | Free fat acids; |
| FAMEs | Fatty acid methyl esters; |
| NIST | National Institute of Standards and Technology; |
| TLC | Thin-layer chromatography. |
References
- Cravotto, C.; Claux, O.; Bartier, M.; Fabiano-Tixier, A.-S.; Tabasso, S. Leading Edge Technologies and Perspectives in Industrial Oilseed Extraction. Molecules 2023, 28, 5973. [Google Scholar] [CrossRef]
- Nde, D.; Foncha, A. Optimization Methods for the Extraction of Vegetable Oils: A Review. Processes 2020, 8, 209. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, S.K.; Mondal, A.K. Production and Trade of Major World Oil Crops. In Technological Innovations in Major World Oil Crops; Springer: New York, NY, USA, 2012; Volume 1, pp. 1–15. [Google Scholar] [CrossRef]
- Ramalho, H.F.; Suarez, P.A.Z. The Chemistry of Oils and Fats and Their Extraction and Refining Processes. Rev. Virtual Química 2013, 5, 2–15. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Singh, V.K.; Garg, M.K. Novel Oil Extraction Technologies: Process Conditions, Quality Parameters, and Optimization. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3–20. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Olfert, M.; Knappe, C.; Sievers-Engler, A.; Masberg, B.; Lämmerhofer, M. Determination of Double Bond Positions in Unsaturated Fatty Acids by Pre-Column Derivatization with Dimethyl and Dipyridyl Disulfide Followed by LC-SWATH-MS Analysis. Anal. Bioanal. Chem. 2025, 417, 2753–2766. [Google Scholar] [CrossRef] [PubMed]
- Bizzo, H.R.; Brilhante, N.S.; Nolvachai, Y.; Marriott, P.J. Use and Abuse of Retention Indices in Gas Chromatography. J. Chromatogr. A 2023, 1708, 464376. [Google Scholar] [CrossRef] [PubMed]
- Babushok, V.I. Chromatographic Retention Indices in Identification of Chemical Compounds. TrAC Trends Anal. Chem. 2015, 69, 98–104. [Google Scholar] [CrossRef]
- Lieng, B.Y.; Quaile, A.T.; Domingo-Almenara, X.; Röst, H.L.; Montenegro-Burke, J.R. Computational Expansion of High-Resolution-MSn Spectral Libraries. Anal. Chem. 2023, 95, 17284–17291. [Google Scholar] [CrossRef]
- Pati, S.; Nie, B.; Arnold, R.D.; Cummings, B.S. Extraction, Chromatographic and Mass Spectrometric Methods for Lipid Analysis. Biomed. Chromatogr. 2016, 30, 695–709. [Google Scholar] [CrossRef]
- Rani, H.; Sharma, S.; Bala, M. Technologies for Extraction of Oil from Oilseeds and Other Plant Sources in Retrospect and Prospects: A Review. J. Food Process. Eng. 2021, 44, e13851. [Google Scholar] [CrossRef]
- Ofori-Boateng, C.; Keat Teong, L.; JitKang, L. Comparative Exergy Analyses of Jatropha curcas Oil Extraction Methods: Solvent and Mechanical Extraction Processes. Energy Convers. Manag. 2012, 55, 164–171. [Google Scholar] [CrossRef]
- Subroto, E.; Manurung, R.; Heeres, H.J.; Broekhuis, A.A. Optimization of Mechanical Oil Extraction from Jatropha curcas L. Kernel Using Response Surface Method. Ind. Crops Prod. 2015, 63, 294–302. [Google Scholar] [CrossRef]
- Wilhelm, A.E.; Antoniassi, R.; Faria-Machado, A.F.; Bizzo, H.R.; Reis, S.L.R.; Cenci, S.A. Different Feed Rates of Expeller Press on the Extraction Efficiency and Quality of Passion Fruit Seed Oil. Ciência Rural. 2014, 44, 1312–1318. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Priego-Capote, F. Soxhlet Extraction: Past and Present Panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef]
- Danlami, J.M.; Arsad, A.; Ahmad Zaini, M.A.; Sulaiman, H. A Comparative Study of Various Oil Extraction Techniques from Plants. Rev. Chem. Eng. 2014, 30, 605–626. [Google Scholar] [CrossRef]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green Extraction Techniques of Bioactive Compounds: A State-of-the-Art Review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Usman, M.; Nakagawa, M.; Cheng, S. Emerging Trends in Green Extraction Techniques for Bioactive Natural Products. Processes 2023, 11, 3444. [Google Scholar] [CrossRef]
- Masoodi, L.; Gull, A.; Masoodi, F.A.; Gani, A.; Nissar, J.; Ahad, T.; Nayik, G.A.; Mukarram, S.A.; Kovács, B.; Prokisch, J.; et al. An Overview on Traditional vs. Green Technology of Extraction Methods for Producing High Quality Walnut Oil. Agronomy 2022, 12, 2258. [Google Scholar] [CrossRef]
- Vági, E.; Balázs, M.; Komoczi, A.; Mihalovits, M.; Székely, E. Fractionation of Phytocannabinoids from Industrial Hemp Residues with High-Pressure Technologies. J. Supercrit. Fluids 2020, 164, 104898. [Google Scholar] [CrossRef]
- Rai, A.; Mohanty, B.; Bhargava, R. Supercritical Extraction of Sunflower Oil: A Central Composite Design for Extraction Variables. Food Chem. 2016, 192, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Jokic, S.; Svilovic, S.; Vidovic, S. Modelling the Supercritical CO2 Extraction Kinetics of Soybean Oil. Croat. J. Food Sci. Technol. 2015, 7, 52–57. [Google Scholar] [CrossRef]
- de Oliveira, N.A.; Mazzali, M.R.; Fukumasu, H.; Gonçalves, C.B.; Oliveira, A.L.d. Composition and Physical Properties of Babassu Seed (Orbignya phalerata) Oil Obtained by Supercritical CO2 Extraction. J. Supercrit. Fluids 2019, 150, 21–29. [Google Scholar] [CrossRef]
- Pinto, R.H.H.; Menezes, E.G.O.; Freitas, L.C.; Andrade, E.H.d.A.; Ribeiro-Costa, R.M.; Silva Júnior, J.O.C.; Carvalho Junior, R.N. Supercritical CO2 Extraction of Uxi (Endopleura uchi) Oil: Global Yield Isotherms, Fatty Acid Profile, Functional Quality and Thermal Stability. J. Supercrit. Fluids 2020, 165, 104932. [Google Scholar] [CrossRef]
- Santos, O.V.d.; Carvalho, R.N.; Costa, C.E.F.d.; Lannes, S.C.d.S. Chemical, Chromatographic-Functional, Thermogravimetric-Differential and Spectroscopic Parameters of the Sapucaia Oil Obtained by Different Extraction Methods. Ind. Crops Prod. 2019, 132, 487–496. [Google Scholar] [CrossRef]
- Santos, O.V.; Corrêa, N.C.F.; Carvalho, R.N.; Costa, C.E.F.; Lannes, S.C.S. Yield, Nutritional Quality, and Thermal-Oxidative Stability of Brazil Nut Oil (Bertolletia excelsa H.B.K) Obtained by Supercritical Extraction. J. Food Eng. 2013, 117, 499–504. [Google Scholar] [CrossRef]
- Costa, B.E.T.; Santos, O.V.d.; Corrêa, N.C.F.; França, L.F.d. Comparative Study on the Quality of Oil Extracted from Two Tucumã Varieties Using Supercritical Carbon Dioxide. Food Sci. Technol. 2016, 36, 322–328. [Google Scholar] [CrossRef]
- Santos, O.V.; Lorenzo, N.D.; Souza, A.L.G.; Costa, C.E.F.; Conceição, L.R.V.; Lannes, S.C.d.S.; Teixeira-Costa, B.E. CO2 Supercritical Fluid Extraction of Pulp and Nut Oils from Terminalia catappa Fruits: Thermogravimetric Behavior, Spectroscopic and Fatty Acid Profiles. Food Res. Int. 2021, 139, 109814. [Google Scholar] [CrossRef]
- Scognamiglio, M.; Baldino, L.; Reverchon, E. Fractional Separation and Characterization of Cuticular Waxes Extracted from Vegetable Matter Using Supercritical CO2. Separations 2022, 9, 80. [Google Scholar] [CrossRef]
- Hernández-Santos, B.; Rodríguez-Miranda, J.; Herman-Lara, E.; Torruco-Uco, J.G.; Carmona-García, R.; Juárez-Barrientos, J.M.; Chávez-Zamudio, R.; Martínez-Sánchez, C.E. Effect of Oil Extraction Assisted by Ultrasound on the Physicochemical Properties and Fatty Acid Profile of Pumpkin Seed Oil (Cucurbita pepo). Ultrason. Sonochem. 2016, 31, 429–436. [Google Scholar] [CrossRef]
- de Oliveira, R.C.; de Barros, S.T.D.; Gimenes, M.L. The Extraction of Passion Fruit Oil with Green Solvents. J. Food Eng. 2013, 117, 458–463. [Google Scholar] [CrossRef]
- Destandau, E.; Michel, T.; Elfakir, C. CHAPTER 4. Microwave-assisted Extraction. In RSC Green Chemistry; Mauricio, A.R., Juliana, M.P., Eds.; Royal Society of Chemistry: London, UK, 2013; pp. 113–156. [Google Scholar] [CrossRef]
- Kumar, S.P.J.; Prasad, S.R.; Banerjee, R.; Agarwal, D.K.; Kulkarni, K.S.; Ramesh, K.V. Green Solvents and Technologies for Oil Extraction from Oilseeds. Chem. Cent. J. 2017, 11, 9. [Google Scholar] [CrossRef]
- Prommaban, A.; Kuanchoom, R.; Seepuan, N.; Chaiyana, W. Evaluation of Fatty Acid Compositions, Antioxidant, and Pharmacological Activities of Pumpkin (Cucurbita moschata) Seed Oil from Aqueous Enzymatic Extraction. Plants 2021, 10, 1582. [Google Scholar] [CrossRef]
- Thilakarathna, R.C.N.; Siow, L.F.; Tang, T.-K.; Chan, E.-S.; Lee, Y.-Y. Physicochemical and Antioxidative Properties of Ultrasound-Assisted Extraction of Mahua (Madhuca longifolia) Seed Oil in Comparison with Conventional Soxhlet and Mechanical Extractions. Ultrason. Sonochem 2023, 92, 106280. [Google Scholar] [CrossRef]
- Thomsen, K.; Raak, N.; Gregersen, S.B.; Månsson, L.; Miquel Becker, E. Enzyme-Assisted Extraction of Rapeseed Oil with Minimum Water Addition: A Proof-of-Concept Study. Int. J. Food Sci. Technol. 2024, 5, 3013–3019. [Google Scholar] [CrossRef]
- Sabarish, C.S.; Sebastian, J.; Muraleedharan, C. Extraction of Oil from Rubber Seed through Hydraulic Press and Kinetic Study of Acid Esterification of Rubber Seed Oil. Procedia Technol. 2016, 25, 1006–1013. [Google Scholar] [CrossRef]
- Polmann, G.; Teixeira, G.L.; Santos, P.H.; Rivera, G.Á.; Ibañez, E.; Cifuentes, A.; Ferreira, S.R.S.; Block, J.M. Chemical Characterization of Gurguéia Nut (Dipteryx lacunifera Ducke) and Press Cake Oil Obtained by Hydraulic Pressing and Supercritical Extraction. Biomass Conv. Bioref. 2024, 14, 19065–19080. [Google Scholar] [CrossRef]
- Dar, I.H.; Junaid, P.M.; Ahmad, S.; Shams, R.; Dash, K.K.; Shaikh, A.M.; Béla, K. Optimization of Ultrasound-Assisted Extraction of Nigella Sativa Seed Oil for Enhancement of Yield and Antioxidant Activity. Discov. Appl. Sci. 2024, 6, 104. [Google Scholar] [CrossRef]
- Senrayan, J.; Venkatachalam, S. Optimization of ultrasound-assisted solvent extraction (UASE) based on oil yield, antioxidant activity and evaluation of fatty acid composition and thermal stability of Coriandrum sativum L. seed oil. Food Sci. Biotechnol. 2019, 28, 377–386. [Google Scholar] [CrossRef]
- da Rosa, A.C.S.; Stevanato, N.; Iwassa, I.; dos Santos Garcia, V.A.; da Silva, C. Obtaining Oil from Macauba Kernels by Ultrasound-Assisted Extraction Using Ethyl Acetate as the Solvent. Braz. J. Food Technol. 2019, 22, e2018195. [Google Scholar] [CrossRef]
- Santos, O.V.; Lemos, Y.S.; da Conceição, L.R.V.; Teixeira-Costa, B.E. Lipids from the Purple and White Açaí (Euterpe Oleracea Mart) Varieties: Nutritional, Functional, and Physicochemical Properties. Front. Nutr. 2024, 11, 1385877. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Palheta, H.C.; Lima dos Santos, M.P.; Vasconcelos dos Santos, O.; Miranda Mendes, P.; Macedo Rogero, M.; Vieira da Conceicão, L.R.; Carrera Silva-Junior, J.O.; Teixeira-Costa, B.E.; De Souza Figueira, M. Functionality of Lipid Extracts Obtained by Green Extraction from Red Pupunha (Bactris Gasipaes Kunt). Sci. Plena 2025, 21. [Google Scholar] [CrossRef]
- Yeasmin, M.S.; Uddin, M.J.; Dey, S.S.; Barmon, J.; Ema, N.T.; Rana, G.M.M.; Rahman, M.M.; Begum, M.; Ferdousi, L.; Ahmed, S.; et al. Optimization of Green Microwave-Assisted Extraction of Essential Oil from Lemon (Citrus limon) Leaves: Bioactive, Antioxidant and Antimicrobial Potential. Curr. Res. Green Sustain. Chem. 2024, 8, 100413. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Fernandes, S.C.M.; Andrés, M.A.; Labidi, J. Microwave-Assisted Extraction of Curcuma longa l. Oil: Optimization, Chemical Structure and Composition, Antioxidant Activity and Comparison with Conventional Soxhlet Extraction. Molecules 2021, 26, 1516. [Google Scholar] [CrossRef] [PubMed]
- Buranachokpaisan, K.; Muangrat, R.; Chalermchat, Y. Supercritical CO2 Extraction of Residual Oil from Pressed Sesame Seed Cake: Optimization and Its Physicochemical Properties. J. Food Process Preserv. 2021, 45, e15722. [Google Scholar] [CrossRef]
- Janßen, H.; Steinbüchel, A. Fatty Acid Synthesis in Escherichia Coli and Its Applications towards the Production of Fatty Acid Based Biofuels. Biotechnol. Biofuels 2014, 7, 7. [Google Scholar] [CrossRef]
- Mu, H. The Digestion of Dietary Triacylglycerols. Prog. Lipid Res. 2004, 43, 105–133. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Dietary Sources, Metabolism, and Significance—A Review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Micha, R.; Mozaffarian, D. Saturated Fat and Cardiometabolic Risk Factors, Coronary Heart Disease, Stroke, and Diabetes: A Fresh Look at the Evidence. Lipids 2010, 45, 893–905. [Google Scholar] [CrossRef]
- De Carvalho, C.; Caramujo, M. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, T.; Brenna, J.T.; Wang, D.H. Fatty Acid Isomerism: Analysis and Selected Biological Functions. Food Funct. 2024, 15, 1071–1088. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Smolińska, K.; Szopa, A.; Sobczyński, J.; Serefko, A.; Dobrowolski, P. Nutritional Quality Implications: Exploring the Impact of a Fatty Acid-Rich Diet on Central Nervous System Development. Nutrients 2024, 16, 1093. [Google Scholar] [CrossRef]
- Botella-Martínez, C.; Pérez-Álvarez, J.Á.; Sayas-Barberá, E.; Navarro Rodríguez de Vera, C.; Fernández-López, J.; Viuda-Martos, M. Healthier Oils: A New Scope in the Development of Functional Meat and Dairy Products: A Review. Biomolecules 2023, 13, 778. [Google Scholar] [CrossRef]
- Tapiero, H.; Nguyen Ba, G.; Couvreur, P.; Tew, K.D. Polyunsaturated Fatty Acids (PUFA) and Eicosanoids in Human Health and Pathologies. Biomed. Pharmacother. 2002, 56, 215–222. [Google Scholar] [CrossRef]
- Santos, O.V.d.; Dias, P.C.S.; Soares, S.D.; da Conceição, L.R.V.; Teixeira-Costa, B.E. Artisanal Oil Obtained from Insects’ Larvae (Speciomerus ruficornis): Fatty Acids Composition, Physicochemical, Nutritional and Antioxidant Properties for Application in Food. Eur. Food Res. Technol. 2021, 247, 1803–1813. [Google Scholar] [CrossRef]
- Dongho Dongmo, F.F.; Fogang Mba, A.R.; Njike Ngamga, F.H.; Djeukeu Asongni, W.; Zokou, R.; Simo Noutsa, B.; Ngo Hagbe, D.; Tchuenbou-Magaia, F.L.; Ebelle Etame, R.M. An Overview of Fatty Acids-Based Nutritional Quality Indices of Fish Oils from Cameroon: Impact of Fish Pre-Treatment and Preservation Methods. J. Food Compos. Anal. 2024, 131, 106250. [Google Scholar] [CrossRef]
- Srivastava, S.; Pandey, V.K.; Singh, K.; Dar, A.H.; Dash, K.K.; Shams, R.; Mukarram Shaikh, A.; Kovács, B. Advances in Detection Technology for Authentication of Vegetable Oils: A Comprehensive Review. Heliyon 2024, 10, e34759. [Google Scholar] [CrossRef]
- Ferreira, R.; Lourenço, S.; Lopes, A.; Andrade, C.; Câmara, J.S.; Castilho, P.; Perestrelo, R. Evaluation of Fatty Acids Profile as a Useful Tool towards Valorization of By-Products of Agri-Food Industry. Foods 2021, 10, 2867. [Google Scholar] [CrossRef]
- Pianosi, F.; Beven, K.; Freer, J.; Hall, J.W.; Rougier, J.; Stephenson, D.B.; Wagener, T. Sensitivity Analysis of Environmental Models: A Systematic Review with Practical Workflow. Environ. Model. Softw. 2016, 79, 214–232. [Google Scholar] [CrossRef]
- Sparkman, O.D.; Penton, Z.E.; Kitson, F.G. Gas Chromatography. In Gas Chromatography and Mass Spectrometry: A Practical Guide; Kitson, F.G., Larsen, B.S., McEwen, C.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 15–83. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Dennis, E.A. High Sensitivity Quantitative Lipidomics Analysis of Fatty Acids in Biological Samples by Gas Chromatography–Mass Spectrometry. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2011, 1811, 648–656. [Google Scholar] [CrossRef]
- Chiu, H.-H.; Kuo, C.-H. Gas Chromatography-Mass Spectrometry-Based Analytical Strategies for Fatty Acid Analysis in Biological Samples. J. Food Drug Anal. 2020, 28, 60–73. [Google Scholar] [CrossRef]
- Salerno, T.M.G.; Donato, P.; Frison, G.; Zamengo, L.; Mondello, L. Gas Chromatography—Fourier Transform Infrared Spectroscopy for Unambiguous Determination of Illicit Drugs: A Proof of Concept. Front. Chem. 2020, 8, 624. [Google Scholar] [CrossRef]
- Rohde, J.K.; Fuh, M.M.; Evangelakos, I.; Pauly, M.J.; Schaltenberg, N.; Siracusa, F.; Gagliani, N.; Tödter, K.; Heeren, J.; Worthmann, A. A Gas Chromatography Mass Spectrometry-Based Method for the Quantification of Short Chain Fatty Acids. Metabolites 2022, 12, 170. [Google Scholar] [CrossRef]
- Holčapek, M.; Lísa, M. Silver-Ion Liquid Chromatography–Mass Spectrometry. In Handbook of Advanced Chromatography/Mass Spectrometry Techniques; Elsevier: Amsterdam, The Netherlands, 2017; pp. 115–140. [Google Scholar] [CrossRef]
- Chardigny, J.; Sébédio, J.; Grandgirard, A.; Martine, L.; Berdeaux, O.; Vatèle, J. Identification of Novel Trans Isomers of 20:5n−3 in Liver Lipids of Rats Fed a Heated Oil. Lipids 1996, 31, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Harvey, D.J. Identification of Long-Chain Fatty Acids and Alcohols from Human Cerumen by the Use of Picolinyl and Nicotinate Esters. Biol. Mass. Spectrom. 1989, 18, 719–723. [Google Scholar] [CrossRef]
- Christie, W.W. Structural Analysis of Fatty Acids. In Advances in Lipid Methodology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 119–169. [Google Scholar] [CrossRef]
- Christie, W.W. Gas Chromatography-mass Spectrometry Methods for Structural Analysis of Fatty Acids. Lipids 1998, 33, 343–353. [Google Scholar] [CrossRef]
- Rohman, A.; Irnawati; Windarsih, A.; Riswanto, F.D.O.; Indrayanto, G.; Fadzillah, N.A.; Riyanto, S.; Bakar, N.K.A. Application of Chromatographic and Spectroscopic-Based Methods for Analysis of Omega-3 (ω-3 FAs) and Omega-6 (ω-6 FAs) Fatty Acids in Marine Natural Products. Molecules 2023, 28, 5524. [Google Scholar] [CrossRef]
- Ichihara, K.; Fukubayashi, Y. Preparation of Fatty Acid Methyl Esters for Gas-Liquid Chromatography. J. Lipid Res. 2010, 51, 635–640. [Google Scholar] [CrossRef]
- Aboelazayem, O.; Gadalla, M.; Saha, B. Derivatisation-Free Characterisation and Supercritical Conversion of Free Fatty Acids into Biodiesel from High Acid Value Waste Cooking Oil. Renew. Energy 2019, 143, 77–90. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Mendonça, M.A.; Pinho, D.M.M.; Resck, I.S.; Suarez, P.A.Z. Chromatographic Analyses of Fatty Acid Methyl Esters by HPLC-UV and GC-FID. J. Braz. Chem. Soc. 2012, 23, 763–769. [Google Scholar] [CrossRef]
- Gerhardtova, I.; Jankech, T.; Majerova, P.; Piestansky, J.; Olesova, D.; Kovac, A.; Jampilek, J. Recent Analytical Methodologies in Lipid Analysis. Int. J. Mol. Sci. 2024, 25, 2249. [Google Scholar] [CrossRef]
- Banni, S.; Carta, G.; Contini, M.S.; Angioni, E.; Deiana, M.; Dessì, M.A.; Melis, M.P.; Corongiu, F.P. Characterization of Conjugated Diene Fatty Acids in Milk, Dairy Products, and Lamb Tissues. J. Nutr. Biochem. 1996, 7, 150–155. [Google Scholar] [CrossRef]
- Guevara-Zambrano, J.M.; Michels, D.; Verkempinck, S.H.E.; Infantes-Garcia, M.R.; Hendrickx, M.E.; Van Loey, A.M.; Grauwet, T. HPLC-CAD Method to Quantify Lipolysis Products from Plant-Based Oils Rich in Unsaturated Fatty Acids. J. Food Compos. Anal. 2023, 121, 105400. [Google Scholar] [CrossRef]
- Teutenberg, T. Potential of High Temperature Liquid Chromatography for the Improvement of Separation Efficiency—A Review. Anal. Chim. Acta 2009, 643, 1–12. [Google Scholar] [CrossRef]
- Poole, C.F.; Lenca, N. Reversed-Phase Liquid Chromatography. In Liquid Chromatography; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 91–123. [Google Scholar] [CrossRef]
- Horká, P.; Vrkoslav, V.; Kindl, J.; Schwarzová-Pecková, K.; Cvačka, J. Structural Characterization of Unusual Fatty Acid Methyl Esters with Double and Triple Bonds Using HPLC/APCI-MS2 with Acetonitrile In-Source Derivatization. Molecules 2021, 26, 6468. [Google Scholar] [CrossRef]
- Dobson, G.; Christie, W.W.; Nikolova-Damyanova, B. Silver Ion Chromatography of Lipids and Fatty Acids. J. Chromatogr. B Biomed. Sci. Appl. 1995, 671, 197–222. [Google Scholar] [CrossRef]
- Tsujikawa, K.; Okada, Y.; Segawa, H.; Yamamuro, T.; Kuwayama, K.; Kanamori, T.; Iwata, Y.T. Thin-Layer Chromatography on Silver Nitrate-Impregnated Silica Gel for Analysis of Homemade Tetrahydrocannabinol Mixtures. Forensic Toxicol. 2022, 40, 125–131. [Google Scholar] [CrossRef]
- Al-Anber, M.A.; Al Ja’afreh, M.; Al-Momani, I.F.; Hijazi, A.K.; Sobola, D.; Sagadevan, S.; Al Bayaydah, S. Loading of Silver (I) Ion in L-Cysteine-Functionalized Silica Gel Material for Aquatic Purification. Gels 2023, 9, 865. [Google Scholar] [CrossRef]
- Czauderna, M.; Kowalczyk, J. Separation of Some Mono-, Di- and Tri-Unsaturated Fatty Acids Containing 18 Carbon Atoms by High-Performance Liquid Chromatography and Photodiode Array Detection. J. Chromatogr. B Biomed. Sci. Appl. 2001, 76, 165–178. [Google Scholar] [CrossRef]
- Liu, P.; Kang, S.; Huang, Y.; Song, T.; Wu, Z.; Lu, Z.; Deng, R. Ultrasonic-Assisted Extraction, Fatty Acids Identification of the Seeds Oil and Isolation of Chemical Constituent from Oil Residue of Belamcanda Chinensis. Ultrason. Sonochem 2022, 90, 106200. [Google Scholar] [CrossRef]
- Liu, P.; Xu, Y.; Gao, X.; Zhu, X.; Du, M.; Wang, Y.; Deng, R.; Gao, J. Optimization of Ultrasonic-Assisted Extraction of Oil from the Seed Kernels and Isolation of Monoterpene Glycosides from the Oil Residue of Paeonia Lactiflora Pall. Ind. Crops Prod. 2017, 107, 260–270. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Monedeiro, F.; Monedeiro-Milanowski, M.; Nowak, Z.; Krakowska-Sieprawska, A.; Pomastowski, P.; Gadzała-Kopciuch, R.; Buszewski, B. Isolation of Omega-3 Polyunsaturated Fatty Acids (Eicosapentaenoic Acid - EPA and Docosahexaenoic Acid - DHA) from Diatom Biomass Using Different Extraction Methods. Algal Res. 2022, 62, 102615. [Google Scholar] [CrossRef]
- Dhayanithy, G.; Mukherjee, S.; Subban, K.; Radhakrishnan, S.; Chelliah, J. Unsaturated Fatty Acid, Nonacosenoic Acid Isolated from an Endophyte Chaetomium Nigricolor Inhabiting the Stem of Catharanthus Roseus and Its Bioactivity. Fungal Biol. 2024, 12, 1876–1884. [Google Scholar] [CrossRef]
- Manjoo, R.; Deepa, S.; Yadav, A.K.; Singh, N.K. Isolation and Characterization of Fusarium Verticillioides NKF1 for Unsaturated Fatty Acid Production. Curr. Microbiol. 2017, 74, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Ntumba, J.K.; Collard, L.; Taba, K.M.; Robiette, R. Isolation of a Series of Fatty Acid Components of Ongokea Gore Seed (Isano) Oil and Their Detailed Structural Analysis. Lipids 2015, 50, 313–322. [Google Scholar] [CrossRef]
- Schlotterbeck, J.; Kolb, A.; Lämmerhofer, M. Free Fatty Acid Profiling in Marine Algae Extract by LC-MS/MS and Isolation as Well as Quantification of the Ω-3 Fatty Acid Hexadeca-4,7,10,13-tetraenoic Acid. J. Sep. Sci. 2018, 41, 4286–4295. [Google Scholar] [CrossRef]
- Singla, R.K.; Ali, M.; Kamal, M.A.; Dubey, A.K. Isolation and Characterization of Nuciferoic Acid, a Novel Keto Fatty Acid with Hyaluronidase Inhibitory Activity from Cocos Nucifera Linn. Endocarp. Curr. Top. Med. Chem. 2019, 18, 2367–2378. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Masaki, T.; Itoh, F.; Kondo, K.; Sudo, K. Isolation of Fatty Acid Amide as an Angiogenic Principle from Bovine Mesentery. Biochem. Biophys. Res. Commun. 1990, 168, 423–429. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Case, B.P.; Mena, E.E.; Kniffin, T.M.; Duke, S.O.; Wedge, D.E. Isolation and Identification of Antifungal Fatty Acids from the Basidiomycete Gomphus Floccosus. J. Agric. Food Chem. 2008, 56, 5062–5068. [Google Scholar] [CrossRef]
- Khan, M.N.A.; Cho, J.Y.; Lee, M.C.; Kang, J.Y.; Nam, G.P.; Fujii, H.; Hong, Y.K. Isolation of Two Anti-Inflammatory and One pro-Inflammatory Polyunsaturated Fatty Acids from the Brown Seaweed Undaria Pinnatifida. J. Agric. Food Chem. 2007, 55, 6984–6988. [Google Scholar] [CrossRef] [PubMed]
- James, D.W.; Dooner, H.K. Isolation of EMS-Induced Mutants in Arabidopsis Altered in Seed Fatty Acid Composition. Theor. Appl. Genet. 1990, 80, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Ito, Y.; Suzuki, A.; Onoue, S.; Noguchi, H.; Yamada, S. Isolation and Pharmacological Characterization of Fatty Acids from Saw Palmetto Extract. Anal. Sci. 2009, 25, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Yang, Q.; Pan, L.; Chen, M.; He, Y.; Yang, Z.; Yu, S. Isolation and Characterization of Fatty Acid Desaturase Genes from Peanut (Arachis hypogaea L.). Plant Cell Rep. 2011, 30, 1393–1404. [Google Scholar] [CrossRef]
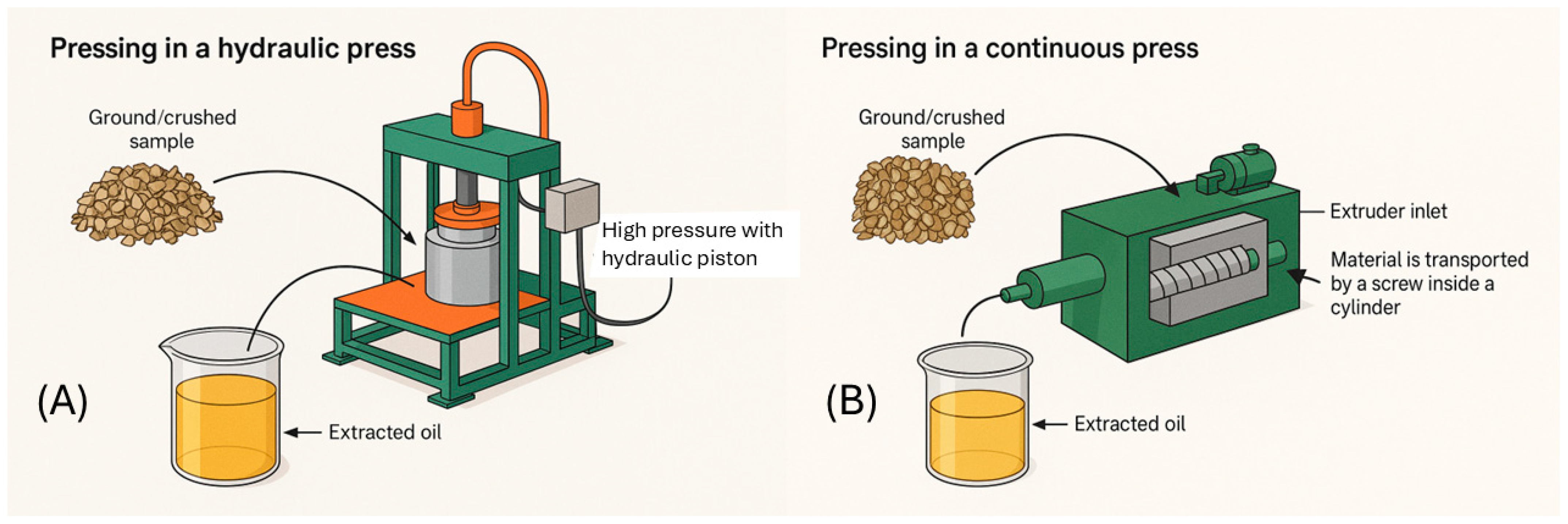
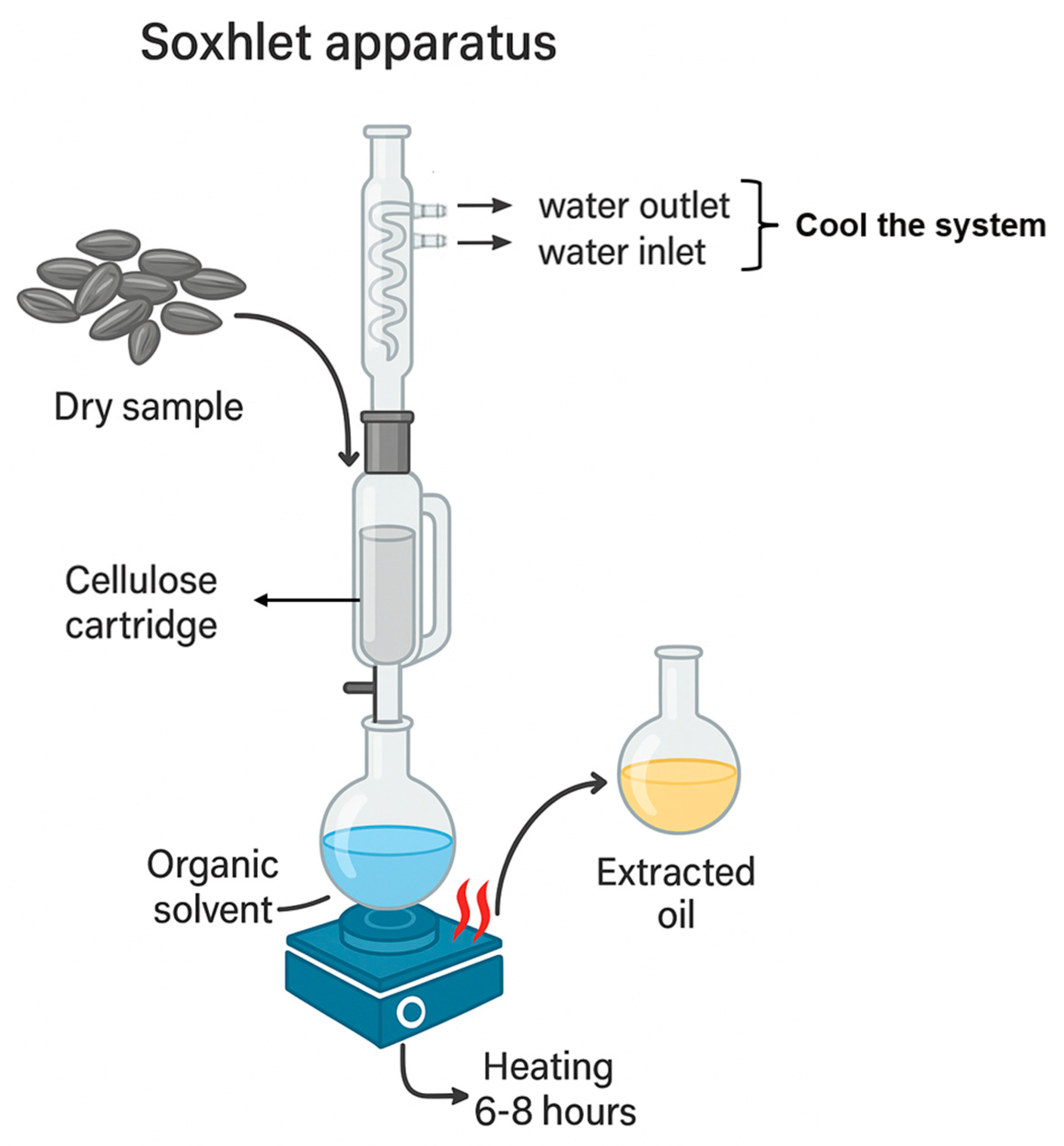
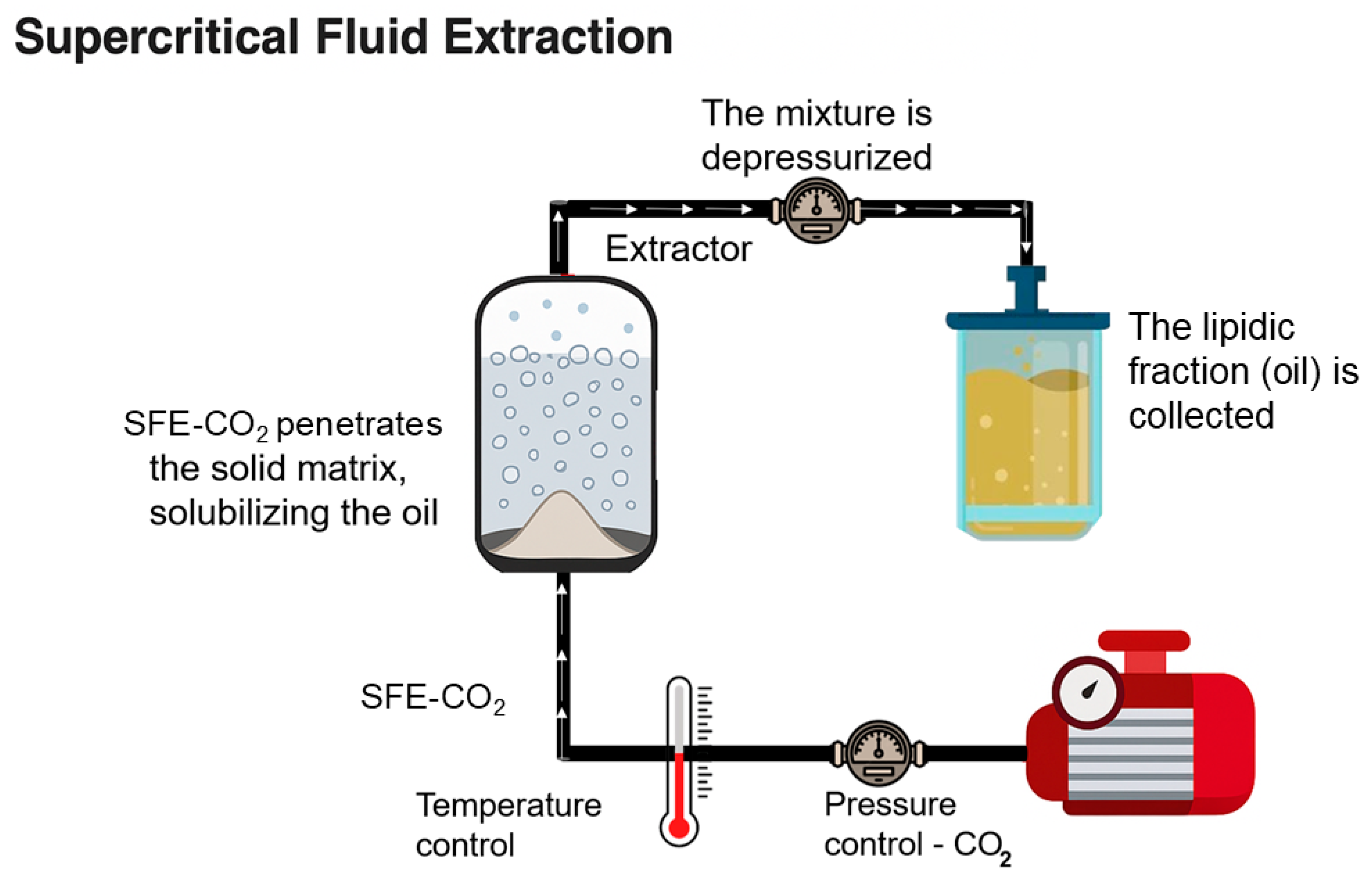
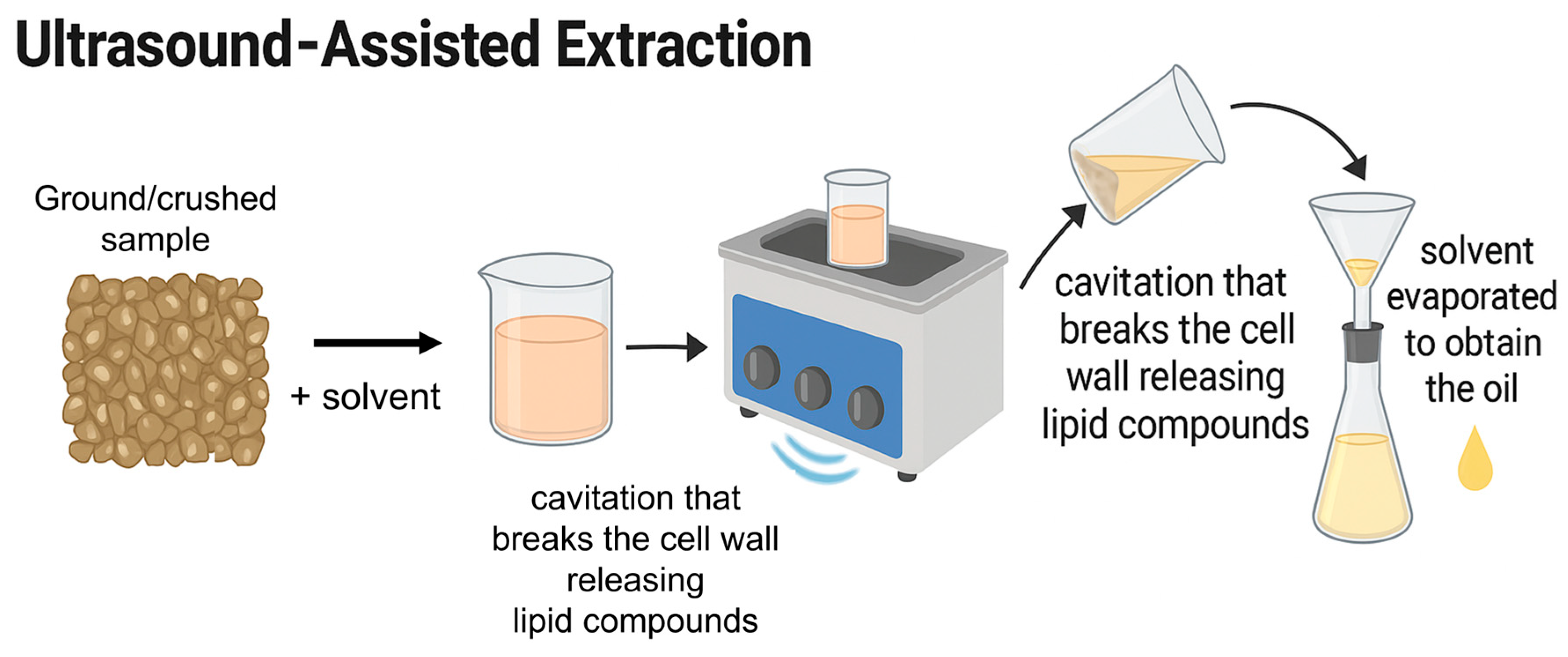

| Extraction Method | Raw Material | Pre-treatment | Conditions | Yield (%) | Reference |
|---|---|---|---|---|---|
| Mechanical extraction | Madhuca longifolia (Mahua) | Air drying, peeling, grinding ≤1.4 mm | 10 g of seeds pressed in a domestic press, heated for 5 min > 90 °C | ≈42.7% | [36] |
| Rapeseed Flakes | Industrial flaking, refrigeration (4 °C), application of enzymes with little water; incubation; thermal conditioning. | Commercial enzymes: Viscozyme® L (pectinase) + Celluclast® 1.5 L (cellulase); 1 mL enzyme solution 100 g−1 seeds, incubation: 50 °C, 2 h; pressing: screw press, 75 °C, 50 rpm, 2 min | 65.0 ± 1.1% | [37] | |
| Hevea brasiliensis (rubber seed) | Peeling, sun drying, muffle drying, fine grinding (300 g sample) | Pressure up to 35 kg cm−2; pressing up to 25 min; drying at 55–65 °C for 1–3 h; manual press with filter and perforated metal cage | ≈16.7% | [38] | |
| Dipteryx lacunifera (press cake) | Drying (48 h at 40 °C), husk removal, grinding | Hydraulic press at 40 °C for 10 min; centrifugation at 1968 g for 10 min | 32.5 ± 0.2 g 100 g−1 | [39] | |
| Conventional solvent or solid-liquid extraction (Soxhlet) | Nigella sativa | Cleaning, sorting by size, fine grinding in blender, sieving to obtain fine powder | 5 g + 70 mL of hexane, 4 h at 80 °C | ≈34.4 ± 0.3% | [40] |
| Madhuca longifolia (mahua) | Air drying, manual husk removal, grinding ≤ 1.4 mm | 10 g + 200 mL of hexane, 8 h at >80 °C | ≈57.2% | [41] | |
| Acrocomia aculeata (macaúba) | Pulp removed; kernels dried at 60 °C for 6 h; ground (avg. 0.841 mm), sieved by granulometry | 5 g + 150 mL of hexane, 8 h at 69.1 °C | ≈51.2 ± 1.2% | [42] | |
| Euterpe oleracea fruit, purple and white varieties | The açaí samples were freeze-dried at −40 °C for 48 h, then the pulp powder was used for hexane extraction | An exaustive extraction | ≈52.2–60.7% | [43] | |
| Dipteryx lacunifera (press cake) | Drying (48 h at 40 °C), husk removal, grinding | 5 g + 150 mL de n-hexano, 6 h, evaporação em rotaevaporador | 18.5 ± 0.05 g 100 g−1 | [39] | |
| Ultrasound-assisted extraction (UAE) | Nigella sativa | Seeds were pre-selected for grinding | 5 g sample with solvent at 1:6 ratio; ultrasonicated in bath at 80 °C for 90 min, 40 kHz, 100 W | ≈34.7% | [40] |
| Acrocomia aculeata (Macaúba) | Fruits depulped, kernels dried at 60 °C for 6 h, ground in blender, sieved by granulometry. | 3 g sample + ethyl acetate (12 mL g−1), ultrasound bath with condenser connected to thermostatic bath at 10 °C | ≈40.6% | [42] | |
| Bactris gasipaes Kunt fruits | The fruits were pressure boiled for 15 min, then freeze-dried | Ethanolic ultrasound- assisted extraction with ratio of 1:5 (w/v) for 30 min with a frequency of 20 kHz and a temperature of 50 ± 2 °C. | ≈9.81% (dry basis) | [44] | |
| Coriandrum sativum L. seed | Air drying, grounded, sieved ≤0.05 mm | Sample-solvent ratio (1:5–1:15), amplitude (70–90%), temperature (40–50 °C), time (5–15 min), 24 kHz, 400 W | ≈8.15–29.98% | [41] | |
| Microwave-assisted extraction (MAE) | Citrus limon leaves | Fresh lemon leaves washed with clean water, cut and ground in a blender (Mixture Grinder, India) into coarse powder | 50 min at 110 °C, microwave power of 300 watts | ≈2.5% | [45] |
| Curcuma longa root | Root washed and dried at 50 °C; then ground using cutting mill (Retsch SM 2000, Retsch, Haan, Germany), sieved (0.5 × 0.5 mm mesh), stored in the dark | 1:20 (w/v) ratio with ethanol, 30 min, 160 W | ≈10.3% | [46] | |
| Supercritical fluid extraction (SFE) | Nigella sativa seeds | Seeds pre-selected and ground | 50 g placed in extraction chamber; supercritical CO2 injected at 350 bar, 45 °C for 60 min, flow rate 100 mL min−1 | ≈29.9% | [40] |
| Black sesame seed press cake | Cake dried at 65 °C for 16 h and stored at 4 °C | 2 kg of dried cake in 5 L supercritical CO2 extractor at 220 bar, 50 °C for 5 h; oil collected in nitrogen-flushed amber bottle | ≈29.8% | [47] | |
| Babassu (Orbignya phalerata) seed oil | Dried and crushed babassu seeds | 10 g of dried and crushed babassu seeds were used for the experimental design conditions (25 and 35 MPa) and five temperatures (40, 50, 60, 70 and 80 °C) | ≈50.3–59.9% | [24] |
| Material | Sample preparation | Isolation | Identification | Derivatization | Chromatographic Conditions | Reference |
|---|---|---|---|---|---|---|
| Rumen fluid, milk, and duodenal digesta samples from sheep | Lyophilized samples hydrolyzed with 2 M NaOH at 85–95 °C for 35–40 min in sealed tubes. | Preparative HPLC | HLPC-UV | A 0.5 mL in bromacetophenone + 0.5 mL in triethylamine (10 g L−1 in acetone) | Gradient mode with flow rate of 3 mL min−1: solvent A was acetonitrile, while solvent B was acetonitrile-water (85:15, v/v). Column temperature of 38 °C. | [86] |
| Oil residue of Belamcanda chinensis | Prior a methanolic extraction in a Soxhlet apparatus for 4 h. Then filtrated to obtain the sample solution. | HPLC | NMR, UV and MS | A 0.5 mol L−1 KOH-CH3OH solution was added to 400 mg of the oil. | Column at 25 °C, UV detector at 254 nm, mobile phase of methanol (A) + 0.1% phosphoric acid (B) with gradient elution | [87] |
| Oil of Paeonia lactiflora | The oil residue was dried and extracted with methanol for 4 h under reflux. The solution was concentrated. | Silica gel column chromatography and eluted with a mixture CH3Cl-MeOH-H2O gradient system | GC–MS | A 0.5 mol L−1 KOH-CH3OH solution was added to 400 mg of the oil. | Acetonitrile (A) and 0.1% potassium dihydrogen phosphate solution in distilled water (B) as the mobile phase by gradient elution. Flow rate at 1.0 mL min−1. Wavelength detection at 260 nm and 232 nm. | [88] |
| Diatom biomass as Pseudostaurosira trainorii | The biomass was separated by decantation, washed with distilled water, centrifuged and dried at 40 °C in an oven. Then, the dried biomass was ground to form a fine powder. | Soxhlet extraction + Solvent extraction with acetone + Supercritical fluid extraction (SFE | HPLC-DAD | 50 µL of 2,4-dibromoacetophenone solution (10 mg mL 1 in acetone) + 50 µL of triethylamine solution (10 mg mL 1 in acetone) were added to the contents of the sample vial. | The mobile phase flow rate was set to 0.6 mL min−1 and the injection volume was 10 μL. Gradient elution programs were Acetonitrile (A) + Water (B) | [89] |
| Fungus Chaetomium nigricolor culture | The culture filtrate was extracted with hexane (v/v), and the organic portion was concentrated. The condensed crude extract was stored at 4 °C for later analysis. | Thin layer chromatography (TLC) separation using petroleum ether and diethyl ether in a ratio of 20:1 and visualized using UV at 254 nm | UV, HPLC, MS and IR analysis | Not applied | Petroleum ether + diethyl ether in a ratio of 20:1 and visualized in UV 254 nm | [90] |
| Roots of mangrove plants (Fusarium verticillioides fungal culture) | Dried fungal biomass using the Blight and Dyer’s protocol | Not apply | GC-FID | 2 mL of BF3-MeOH were added and incubated in a water bath at 55 °C for 1.5 h, under vigorous shaking for 20 min. | Not applied | [91] |
| Ongokea gore seed oil | The pulverized material was dissolved in methanol for 24 h and then filtered. The cake was then macerated at room temperature with cyclohexane for 48 h and then filtered. | Flash column chromatography using a cyclohexane/ethyl acetate gradient (98:2–0:100) as mobile phase and silica gel as stationary phase + HPLC separation with an isocratic reversed phase. | HPLC + GC-FID | Transesterification under methanol reflux (65 °C) to obtain the corresponding methyl esters. | The mobile phase consisted of a gradient of H2O/CH3CN/TFA/EtOH (20:35:10:35–0:50:0:50), at a flow rate of 1 mL min−1. | [92] |
| Marine algae wakame | Liquid extract in CHCl3 | Purification of the enriched organic phase containing free fatty acids was performed using normal phase SPE. | UHPLC-ESI-QTOF-MS | Not applied | Eluent A consisted of aqueous ammonium acetate buffer and eluent B was composed of 55% v/v ACN, 40% v/v IPA, and 5% v/v aqueous ammonium acetate buffer. | [93] |
| Cocos nucifera Linn. endocarp | Ethanolic extract of endocarp | Silica gel chromatography of the ethanol extract using pre-coated TLC plates. Visualization under short UV (254 nm) and long UV (366 nm). | LC-MS (QTOF) + 1H-NMR + 13C-NMR + HMBC and HSQC | Not applied | Isocratic mode of gradients ranging from nonpolar to polar solvents (hexane, chloroform, ethyl acetate, methanol and ultrapure water). | [94] |
| Bovine mesentery | The tissue was thawed and cut in a blender with nonpolar organic solvent. The white fat mass was recovered and filtered. | Silicic acid column chromatography | silicic acid HPLC + EI-MS + FT-RT + 1H-NMR | Not applied | Gradient elution with solvent mixtures n-hexanediisopropylether/ethanol/water (from 5:20:4:10 plus 1.5% ethanol to 5:20:5:10 plus 2% ethanol), in 30 min. Flow rate of 1 mL min−1. | [95] |
| The basidiomycete Gomphus floccosus | Crude ethyl acetate extract was dissolved in CH3OH/H2O (90/10) and placed in a separatory funnel. The phase was extracted by means of hexane portions, dried and evaporated to dryness. The dried sample was dissolved in MeOH. | Reversed-phase C-18 HPLC column | GC-MS + High-Resolution LC-MS + 1H and 13C NMR | Not applied | A linear gradient from 40/60 (H2O with 0.1% trifluoroacetic acid/acetonitrile) to 0/100 (H2O with 0.1% trifluoroacetic acid/acetonitrile). | [96] |
| Brown seaweed Undaria pinnatifida | The seaweed powder was extracted with acetonitrile, and the crude extract was evaporated under vacuum to give a dark brown residue. | silica gel column + RP-HPLC | GC-MS + GC-FID + 1-D NMR (1H, 13C, and DEPT)+ 2-D NMR (HMQC, HMBC, and COSY) | Not applied | The analysis was performed on a gradient liquid chromatograph monitored at 213 nm. The mobile phase consisted of two solvent systems: acetonitrile with 0.1% TFA and distilled water with 0.1% TFA. Elution was performed with a linear gradient from 0 to 100% v/v acetonitrile over 33 min and with 100% v/v acetonitrile over 40 min for the compounds at a flow rate of 2 mL/min. | [97] |
| Seeds Arabidopsis thaliana (L.) Heynh. | Fatty acids were cleaved from triacylglycerol and methylated by treatment with 1.0 mL of 1 N HCl in 100% methanol for 1 h. After incubation, 0.9% (w/v) NaCl and hexane were added to the tubes and centrifuged. | GC-FID | GC-FID | Not applied | Samples were injected via autosampler. The chromatograph was programmed as follows: 160 °C for 2.0 rain, ramp to 220 °C at 30 °C rain, hold at 2213 °C for 12.0 rain. The injector and FID detector were held at 250 °C and 300 °C r. | [98] |
| Saw Palmetto | Saw palmetto in hexane and diethyl ether extract | Silica-gel column + preparative HPLC. | LC/ESI-MS + NMR | Not applied | The samples were separated using a gradient mobile phase composed of distilled water (A) and acetonitrile (B), with a detection wavelength of 220 nm. The mobile phase gradient conditions were 0–15 min, 70–95% B; 15–20 min, 95% B, and the flow rate was set at 0.5 mL/min. The column oven temperature was set at 30 °C. | [99] |
| Peanut (Arachis hypogaea L.) | Fatty acid methyl esters (FAMEs) were recovered with hexane. | GC–MS | GC–MS | Lipids were extracted with dichloromethane/methanol (2:1) from dried cells, solidified under nitrogen gas ventilation, and transmethylated with methanol containing 0.5 M KOH-methanol/H2O (95:5) at 100 °C for 2 h. | Not informed | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima-Pereira, Y.; de Souza, E.M.O.; dos Reis, D.S.; Barcellos-Silva, I.G.C.; Miki, K.S.L.; Veiga-Júnior, V.F.; Teixeira-Costa, B.E. Current Perspectives on the Extraction, Isolation, and Identification of Fats and Fatty Acids Using Conventional and Green Methods. Separations 2025, 12, 160. https://doi.org/10.3390/separations12060160
Lima-Pereira Y, de Souza EMO, dos Reis DS, Barcellos-Silva IGC, Miki KSL, Veiga-Júnior VF, Teixeira-Costa BE. Current Perspectives on the Extraction, Isolation, and Identification of Fats and Fatty Acids Using Conventional and Green Methods. Separations. 2025; 12(6):160. https://doi.org/10.3390/separations12060160
Chicago/Turabian StyleLima-Pereira, Ytaiara, Esther Maria Oliveira de Souza, David Silva dos Reis, Ian Gardel Carvalho Barcellos-Silva, Karine Sayuri Lima Miki, Valdir F. Veiga-Júnior, and Barbara Elisabeth Teixeira-Costa. 2025. "Current Perspectives on the Extraction, Isolation, and Identification of Fats and Fatty Acids Using Conventional and Green Methods" Separations 12, no. 6: 160. https://doi.org/10.3390/separations12060160
APA StyleLima-Pereira, Y., de Souza, E. M. O., dos Reis, D. S., Barcellos-Silva, I. G. C., Miki, K. S. L., Veiga-Júnior, V. F., & Teixeira-Costa, B. E. (2025). Current Perspectives on the Extraction, Isolation, and Identification of Fats and Fatty Acids Using Conventional and Green Methods. Separations, 12(6), 160. https://doi.org/10.3390/separations12060160











