Integration and Operational Application of Advanced Membrane Technologies in Military Water Purification Systems
Abstract
1. Introduction
2. Emerging Technologies Based on Membrane Processes for Water Purification
Basic Principles of the Most Industrially Significant Membrane Processes and Separation Mechanisms
3. Application of Membrane Technologies in Military Wastewater Treatment
3.1. Water Recycling and Reuse in Stationary Military Bases and Mobile Units
3.2. Water Recycling and Reuse in Military Naval Vessels and Submarines
3.3. Treatment of Chemically, Biologically and Radiologically (CBR) Contaminated Water
4. Innovations and Future Trends in Membrane Technologies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBR | Chemical, biological, radiological |
| CMBR | Ceramic membrane bioreactor |
| CEC | Contaminants of emerging concern |
| CNTs | Carbon nanotubes |
| MFI | Mobile Five (zeolite framework type) |
| MOF | Metal organic framework |
| DETS | Decontamination effluent treatment system |
| LWP | Lightweight water purifier |
| ROWPU | Reverse osmosis water purification unit |
References
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Singh, A. A Review of Wastewater Irrigation: Environmental Implications. Resour. Conserv. Recycl. 2021, 168, 105454. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Bera, S.P.; Godhaniya, M.; Kothari, C. Emerging and Advanced Membrane Technology for Wastewater Treatment: A Review. J. Basic Microbiol. 2022, 62, 245–259. [Google Scholar] [CrossRef]
- Peters, T. Membrane Technology for Water Treatment. Chem. Eng. Technol. 2010, 33, 1233–1240. [Google Scholar] [CrossRef]
- Bagwell, T.H.; Shalewitz, B.; Coleman, A. The Army Water Supply Program: An Overview. Desalination 1994, 99, 423–445. [Google Scholar] [CrossRef]
- Atkinson, S. Membranes Help US Military Produce Clean Drinking Water. Membr. Technol. 2006, 2006, 7–8. [Google Scholar] [CrossRef]
- Saritas, O.; Burmaoglu, S. Future of Sustainable Military Operations under Emerging Energy and Security Considerations. Technol. Forecast. Soc. Change 2016, 102, 331–343. [Google Scholar] [CrossRef]
- Othman, N.H.; Alias, N.H.; Fuzil, N.S.; Marpani, F.; Shahruddin, M.Z.; Chew, C.M.; David Ng, K.M.; Lau, W.J.; Ismail, A.F. A Review on the Use of Membrane Technology Systems in Developing Countries. Membranes 2021, 12, 30. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane Distillation: Recent Developments and Perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Turchanin, A.; Gölzhäuser, A. Carbon Nanomembranes. Adv. Mater. 2016, 28, 6075–6103. [Google Scholar] [CrossRef] [PubMed]
- Suwaileh, W.; Pathak, N.; Shon, H.; Hilal, N. Forward Osmosis Membranes and Processes: A Comprehensive Review of Research Trends and Future Outlook. Desalination 2020, 485, 114455. [Google Scholar] [CrossRef]
- Touš, M.; Máša, V.; Vondra, M. Energy and Water Savings in Military Base Camps. Energy Syst. 2021, 12, 545–562. [Google Scholar] [CrossRef]
- Desario, A.P. Technology Assessment of Water Treatment Devices for Small-Scale Production. US Nav. Res. Lab. 2023, 67, 8–9. [Google Scholar]
- Lim, Y.J.; Goh, K.; Kurihara, M.; Wang, R. Seawater Desalination by Reverse Osmosis: Current Development and Future Challenges in Membrane Fabrication–A Review. J. Membr. Sci. 2021, 629, 119292. [Google Scholar] [CrossRef]
- Pasika, S.; Chomko, D.; Opanasenko, O.; Khomiakov, D.; Skyba, O. Legal Regulations of Groundwater Extraction Processes to Support the Needs of Military Units. In Proceedings of the Monitoring 2019, Kyiv, Ukraine, 12–15 November 2019; pp. 1–5. [Google Scholar]
- Li, L.; Shi, W.; Yu, S. Research on Forward Osmosis Membrane Technology Still Needs Improvement in Water Recovery and Wastewater Treatment. Water 2019, 12, 107. [Google Scholar] [CrossRef]
- Loeb, S.; Sourirajan, S. Sea Water Demineralization by Means of an Osmotic Membrane; Advances in Chemistry; ACS, Saline Water Conversion—II; ACS Publications: Washington, DC, USA, 1963; Volume 38, ISBN 978-0-8412-0039-5. [Google Scholar]
- Cadotte, J.E.; Petersen, R.J.; Larson, R.E.; Erickson, E.E. A New Thin-Film Composite Seawater Reverse Osmosis Membrane. Desalination 1980, 32, 25–31. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Fane, A.G.; Wang, R.; Jia, Y. Membrane Technology: Past, Present and Future. In Membrane and Desalination Technologies; Wang, L.K., Chen, J.P., Hung, Y.-T., Shammas, N.K., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 1–45. ISBN 978-1-58829-940-6. [Google Scholar]
- Shenvi, S.S.; Isloor, A.M.; Ismail, A.F. A Review on RO Membrane Technology: Developments and Challenges. Desalination 2015, 368, 10–26. [Google Scholar] [CrossRef]
- Le, N.L.; Nunes, S.P. Materials and Membrane Technologies for Water and Energy Sustainability. Sustain. Mater. Technol. 2016, 7, 1–28. [Google Scholar] [CrossRef]
- Muhamad, M.S.; Salim, M.R.; Lau, W.J.; Yusop, Z. A Review on Bisphenol A Occurrences, Health Effects and Treatment Process via Membrane Technology for Drinking Water. Environ. Sci. Pollut. Res. 2016, 23, 11549–11567. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.M.; Adam, M.R.; Mohamad Kamal, S.N.E.A.; Jaafar, J.; Othman, M.H.D.; Ismail, A.F.; Aziz, F.; Yusof, N.; Bilad, M.R.; Mohamud, R.; et al. A Review of the Potential of Conventional and Advanced Membrane Technology in the Removal of Pathogens from Wastewater. Sep. Purif. Technol. 2022, 286, 120454. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, S.; Delagah, S.; Stone, S.; Mouawad, J.; Sharbatmaleki, M. Assessing Closed-Circuit Reverse Osmosis (CCRO) Efficiency in Removing Contaminants of Emerging Concern (CECs) in a High-Recovery Water Reclamation Pilot Study. Water Pract. Technol. 2025, 20, 314–323. [Google Scholar] [CrossRef]
- Bashir, J.; Ilyas, S.; Asif, A.; de Vos, W.M.; Khan, A.L.; Akhtar, F.H. Polyelectrolyte Multilayer-Based Nanofiltration Membranes with Tunable Performance for Target Pollutants. ACS Appl. Polym. Mater. 2025, 7, 3147–3156. [Google Scholar] [CrossRef]
- Behroozi, A.H.; Meunier, L.; Mirahsani, A.; Champagne, P.; Koupaie, E.H. Graphene-Based Materials and Technologies for the Treatment of PFAS in Water: A Review of Recent Developments. J. Hazard. Mater. Adv. 2025, 17, 100626. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, S.; Sha, Q.; Wang, Y.; Ma, C.; Zhang, X.; Yan, X.; Han, N. Positively Charged Thin-Film Nanocomposite Membrane Doped with Functionalized Covalent Organic Frameworks Nanosphere for Heavy Metal Ion Removal. Sep. Purif. Technol. 2025, 363, 131996. [Google Scholar] [CrossRef]
- Mulder, M. Marcel Mulder Basic Principles of Membrane Technology; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1996; pp. 280–415. [Google Scholar]
- Gavrilova, N.; Gubin, S.; Myachina, M.; Skudin, V. Transport Reagents through the Pore Structure of a Membrane Catalyst under Isothermal and Non-Isothermal Conditions. Membranes 2021, 11, 497. [Google Scholar] [CrossRef]
- Pochivalov, K.V.; Basko, A.V.; Lebedeva, T.N.; Yurov, M.Y.; Yushkin, A.A.; Bronnikov, S.V.; Volkov, A.V. PVDF Membrane Formation via NIPS in Isothermal and Non-Isothermal Conditions: Thermodynamics, Structure, and Properties. Membr. Membr. Technol. 2024, 6, 473–490. [Google Scholar] [CrossRef]
- Malaeb, L.; Ayoub, G.M. Reverse Osmosis Technology for Water Treatment: State of the Art Review. Desalination 2011, 267, 1–8. [Google Scholar] [CrossRef]
- Wang, L.; He, J.; Heiranian, M.; Fan, H.; Song, L.; Li, Y.; Elimelech, M. Water Transport in Reverse Osmosis Membranes Is Governed by Pore Flow, Not a Solution-Diffusion Mechanism. Sci. Adv. 2023, 9, eadf8488. [Google Scholar] [CrossRef]
- Wang, R.; Elimelech, M. Revisiting Solute Transport in Polyamide Membranes: Insights from Neutral Solute Partitioning. J. Membr. Sci. 2025, 728, 124117. [Google Scholar] [CrossRef]
- Fan, H.; Heiranian, M.; Elimelech, M. The Solution-Diffusion Model for Water Transport in Reverse Osmosis: What Went Wrong? Desalination 2024, 580, 117575. [Google Scholar] [CrossRef]
- Zubair, M.M.; Saleem, H.; Zaidi, S.J. Recent Progress in Reverse Osmosis Modeling: An Overview. Desalination 2023, 564, 116705. [Google Scholar] [CrossRef]
- Wenten, I.G. Khoiruddin Reverse Osmosis Applications: Prospect and Challenges. Desalination 2016, 391, 112–125. [Google Scholar] [CrossRef]
- Benítez, F.J.; Acero, J.L.; Leal, A.I.; González, M. The Use of Ultrafiltration and Nanofiltration Membranes for the Purification of Cork Processing Wastewater. J. Hazard. Mater. 2009, 162, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Al Aani, S.; Mustafa, T.N.; Hilal, N. Ultrafiltration Membranes for Wastewater and Water Process Engineering: A Comprehensive Statistical Review over the Past Decade. J. Water Process Eng. 2020, 35, 101241. [Google Scholar] [CrossRef]
- Awad, E.S.; Sabirova, T.M.; Tretyakova, N.A.; Alsalhy, Q.F.; Figoli, A.; Salih, I.K. A Mini-Review of Enhancing Ultrafiltration Membranes (UF) for Wastewater Treatment: Performance and Stability. ChemEngineering 2021, 5, 34. [Google Scholar] [CrossRef]
- Freger, V. Ion Uptake and Pairing in Membranes: The Pore Model. J. Membr. Sci. 2025, 722, 123795. [Google Scholar] [CrossRef]
- Oatley-Radcliffe, D.L.; Walters, M.; Ainscough, T.J.; Williams, P.M.; Mohammad, A.W.; Hilal, N. Nanofiltration Membranes and Processes: A Review of Research Trends over the Past Decade. J. Water Process Eng. 2017, 19, 164–171. [Google Scholar] [CrossRef]
- Maroufi, N.; Hajilary, N. Nanofiltration Membranes Types and Application in Water Treatment: A Review. Sustain. Water Resour. Manag. 2023, 9, 142. [Google Scholar] [CrossRef]
- Khan, N.A.; Singh, S.; López-Maldonado, E.A.; Narasimhappa, P.; Méndez-Herrera, P.F.; López-López, J.R.; Baig, U.; Ramamurthy, P.C.; Mubarak, N.M.; Karri, R.R.; et al. Emerging Membrane Technology and Hybrid Treatment Systems for the Removal of Micropollutants from Wastewater. Desalination 2023, 565, 116873. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mehejabin, F.; Momtahin, A.; Tasannum, N.; Faria, N.T.; Mofijur, M.; Hoang, A.T.; Vo, D.-V.N.; Mahlia, T.M.I. Strategies to Improve Membrane Performance in Wastewater Treatment. Chemosphere 2022, 306, 135527. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Nguyen, N.C.; Chen, S.-S.; Duong, H.C.; Nguyen, M.L.; Tran, C.-S.; Nguyen, P.-D. Exploration of a Cost-Effective Draw Solution Based on Mixing Surfactant and Sodium Chloride for Forward Osmosis Desalination Process. Environ. Technol. Innov. 2023, 30, 103088. [Google Scholar] [CrossRef]
- Blandin, G.; Verliefde, A.; Comas, J.; Rodriguez-Roda, I.; Le-Clech, P. Efficiently Combining Water Reuse and Desalination through Forward Osmosis—Reverse Osmosis (FO-RO) Hybrids: A Critical Review. Membranes 2016, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Sayyad, S.U.; Kamthe, N.K.; Sarvade, S.M. Design and Simulation of Reverse Osmosis Process in a Hybrid Forward Osmosis-Reverse Osmosis System. Chem. Eng. Res. Des. 2022, 183, 210–220. [Google Scholar] [CrossRef]
- Patel, D.; Mudgal, A.; Patel, V.; Patel, J. Water Desalination and Wastewater Reuse Using Integrated Reverse Osmosis and Forward Osmosis System. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1146, 012029. [Google Scholar] [CrossRef]
- Sedighi, M.; Behvand Usefi, M.M.; Ismail, A.F.; Ghasemi, M. Environmental Sustainability and Ions Removal through Electrodialysis Desalination: Operating Conditions and Process Parameters. Desalination 2023, 549, 116319. [Google Scholar] [CrossRef]
- Cournoyer, A.; Bazinet, L. Electrodialysis Processes an Answer to Industrial Sustainability: Toward the Concept of Eco-Circular Economy?—A Review. Membranes 2023, 13, 205. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y. Recent Developments and Future Perspectives of Reverse Electrodialysis Technology: A Review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Setodeh, M.; Osfouri, S.; Abbasi, M.; Azin, R. Experimental Analysis of Hybrid Electrodialysis (ED)-Reverse Electrodialysis (RED) Process for the Desalination of Brackish Waters and Generation of Renewable Energy in a Pilot Scale. Desalination Water Treat. 2021, 231, 101–112. [Google Scholar] [CrossRef]
- Shalaby, S.M.; Kabeel, A.E.; Abosheiasha, H.F.; Elfakharany, M.K.; El-Bialy, E.; Shama, A.; Vidic, R.D. Membrane Distillation Driven by Solar Energy: A Review. J. Clean. Prod. 2022, 366, 132949. [Google Scholar] [CrossRef]
- Julian, H.; Nurgirisia, N.; Qiu, G.; Ting, Y.-P.; Wenten, I.G. Membrane Distillation for Wastewater Treatment: Current Trends, Challenges and Prospects of Dense Membrane Distillation. J. Water Process Eng. 2022, 46, 102615. [Google Scholar] [CrossRef]
- Hai, F.; Riley, T.; Shawkat, S.; Magram, S.; Yamamoto, K. Removal of Pathogens by Membrane Bioreactors: A Review of the Mechanisms, Influencing Factors and Reduction in Chemical Disinfectant Dosing. Water 2014, 6, 3603–3630. [Google Scholar] [CrossRef]
- Criscuoli, A.; Bamaga, O.; Wang, Q.; Cui, Z.; Nahri, A.; Albeirutty, M.; Jin, W.; Drioli, E. Integration of Membrane Bioreactors with Reverse Osmosis and Membrane Distillation Units for Wastewater Treatment: Recent Developments and Future Perspectives. Sep. Purif. Rev. 2023, 52, 400–412. [Google Scholar] [CrossRef]
- Rubel, J.; Buysschaert, F.; Vandeginste, V. Review and Selection Methodology for Water Treatment Systems in Mobile Encampments for Military Applications. Clean. Water 2025, 3, 100065. [Google Scholar] [CrossRef]
- Fuller, M.E.; Hedman, P.C.; Chu, K.-H.; Webster, T.S.; Hatzinger, P.B. Evaluation of a Sequential Anaerobic-Aerobic Membrane Bioreactor System for Treatment of Traditional and Insensitive Munitions Constituents. Chemosphere 2023, 340, 139887. [Google Scholar] [CrossRef]
- Manikandan, S.; Subbaiya, R.; Saravanan, M.; Ponraj, M.; Selvam, M.; Pugazhendhi, A. A Critical Review of Advanced Nanotechnology and Hybrid Membrane Based Water Recycling, Reuse, and Wastewater Treatment Processes. Chemosphere 2022, 289, 132867. [Google Scholar] [CrossRef] [PubMed]
- Cevallos-Mendoza, J.; Amorim, C.; Rodríguez-Díaz, J.; Montenegro, M. Removal of Contaminants from Water by Membrane Filtration: A Review. Membranes 2022, 12, 570. [Google Scholar] [CrossRef]
- Abdelrasoul, A. (Ed.) Advances in Membrane Technologies; IntechOpen: London, UK, 2020; ISBN 978-1-78984-806-9. [Google Scholar]
- Gullinkala, T.; Escobar, I.C. Membranes for Water Treatment Applications–An Overview. In ACS Symposium Series; Escobar, I., Van Der Bruggen, B., Eds.; American Chemical Society: Washington, DC, USA, 2011; Volume 1078, pp. 155–170. ISBN 978-0-8412-2618-0. [Google Scholar]
- Abd El-Ghaffar, M.A.; Tieama, H.A. A Review of Membranes Classifications, Configurations, Surface Modifications, Characteristics and Its Applications in Water Purification. Chem. Biomol. Eng. 2017, 2, 57–82. [Google Scholar] [CrossRef]
- Souza, V.C.; Quadri, M.G.N. Organic-Inorganic Hybrid Membranes in Separation Processes: A 10-Year Review. Braz. J. Chem. Eng. 2013, 30, 683–700. [Google Scholar] [CrossRef]
- Kostas-Polston, E.A.; Terehoff, C.B.; Nash, L.N.; Brown, A.M.; Delabastide, Z.A.; Andersen, E.W.; Brown, W.J.; Stucky, C.H.; Norcross, K.R.; Smith, H.N.; et al. Patterns in Urogenital Health in Active Duty Servicewomen: A Prospective Cross-Sectional Survey Evaluating Impacts of Water, Sanitation, and Hygiene Resources Across Three Military Environments. Mil. Med. 2023, 188, e2567–e2575. [Google Scholar] [CrossRef] [PubMed]
- Kupenko, O.; Kostenko, A.; Kalchenko, L.; Pehota, O.; Kubatko, O. Resilience and Vulnerability of a Person in a Community in the Context of Military Events. Probl. Perspect. Manag. 2023, 21, 154–168. [Google Scholar] [CrossRef]
- NATO Standardization Office (NSO). AMedP-4.9 Requirements for Water Quality During Operations; NSO: Brussels, Belgium, 2022.
- Alpatova, O.; Maksymenko, I.; Patseva, I.; Khomiak, I.; Gandziura, V. Hydrochemical State of the Post-Military Operations Water Ecosystems of the Moschun, Kyiv Region. In Proceedings of the 16th International Conference Monitoring of Geological Processes and Ecological Condition of the Environment, Kyiv, Ukraine, 15–18 November 2022; pp. 1–5. [Google Scholar]
- Smith, C.B.; Acevedo-Acevedo, D.; Martínez-Guerra, E.; Medina, V.F.; Duczynski, M.P.; Wolters, S.R.; Garfinkle, N.W.; Melendez, L.; Feliciano, L.I. Developing Water Resiliency Solutions at Military Installations. Clim. Risk Manag. 2022, 37, 100451. [Google Scholar] [CrossRef]
- Makropoulos, C.; Koutiva, I.; Kossieris, P.; Rozos, E. Water Management in the Military: The SmartBlue Camp Profiling Tool. Sci. Total Environ. 2019, 651, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Monahan, A.; Dionísio, J.; Delgado, F.; Magro, C. Sustainability Assessment of Wastewater Reuse in a Portuguese Military Airbase. Sci. Total Environ. 2022, 851, 158329. [Google Scholar] [CrossRef]
- Ionita, A. NATO Security Investment Program Consideration. Def. Resour. Manag. 21st Century 2023, 18, 154–164. [Google Scholar]
- Capt. Daniel Mitchell Army Water Purification Systems Can Leave Units Running Dry. Available online: https://www.army.mil/article/218519/army_water_purification_systems_can_leave_units_running_dry (accessed on 4 May 2025).
- Dan Kaszeta Water Purification. Available online: https://euro-sd.com/2019/05/articles/13367/water-purification/ (accessed on 4 May 2025).
- Dong, Z.; Shang, W.; Dong, W.; Zhao, L.; Li, M.; Wang, R.; Sun, F. Suppression of Membrane Fouling in the Ceramic Membrane Bioreactor (CMBR) by Minute Electric Field. Bioresour. Technol. 2018, 270, 113–119. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, W.; Li, K.; Yang, W.; Lee, J.; Xie, B.; Wu, B.; Ren, H.; Hong, S.; Zhan, M. Controlling of Irreversible Fouling and Mechanism in a Hybrid Ceramic Membrane Bioreactor (CMBR)-Reverse Osmosis (RO) Process for Textile Wastewater Reclamation. Desalination 2024, 586, 117914. [Google Scholar] [CrossRef]
- Army Technology LWPS: Lightweight Water Purification System. Available online: https://www.army-technology.com/products/water-purification-system/ (accessed on 4 January 2025).
- Boone, C. Environmental Trade-Offs of Reverse Osmosis Water Purification Units (ROWPUs) and Bottled Water. Master’s Thesis, Air Force Institute of Technology, Wright-Patterson Air Force Base, OH, USA, 2023; pp. 7–10, AFIT-ENV-MS-23-M-170. [Google Scholar]
- Boone, C.; Chini, C.M. Comparative Life Cycle Assessment of Remote Potable Water Supply for the Department of Defense. Sci. Total Environ. 2024, 949, 174732. [Google Scholar] [CrossRef]
- United States Government US Army. Soldier’s Manual and Trainer’s Guide; MOS 92W Water Treatment Specialist Skill Levels 1, 2, 3, and 4; Headquarters, Department of the Army: Washington, DC, USA, 2005.
- Harris, C. Modular Desalting for Specialized Applications. Desalination 2000, 132, 269–274. [Google Scholar] [CrossRef]
- Arnal, J.M.; Sancho, M.; Verdú, G.; Campayo, J.M.; Gozálvez, J.M. Treatment of 137Cs Liquid Wastes by Reverse Osmosis Part II. Real Application. Desalination 2003, 154, 35–42. [Google Scholar] [CrossRef]
- Combernoux, N.; Schrive, L.; Labed, V.; Wyart, Y.; Carretier, E.; Moulin, P. Treatment of Radioactive Liquid Effluents by Reverse Osmosis Membranes: From Lab-Scale to Pilot-Scale. Water Res. 2017, 123, 311–320. [Google Scholar] [CrossRef]
- Ministarstvo obrane Republike Hrvatske Bilateralna Aktivnost Razmjene Iskustva Na Području Pročišćavanja Vode. Available online: https://www.morh.hr/bilateralna-aktivnost-razmjene-iskustva-na-podrucju-prociscavanja-vode/ (accessed on 4 May 2025).
- Hrvatski vojnik Obuka Rukovanjea Sustavom Za Pročišćavanje Voda. Available online: https://hrvatski-vojnik.hr/obuka-rukovanja-sustavom-za-prociscavanje-voda/ (accessed on 4 January 2025).
- Report of the Secretary-General on the United Nations Stabilization Mission in Haiti; United Nations Security Council: New York, NY, USA, 2011; S/2011/183.
- Angelakis, A.N.; Valipour, M.; Choo, K.-H.; Ahmed, A.T.; Baba, A.; Kumar, R.; Toor, G.S.; Wang, Z. Desalination: From Ancient to Present and Future. Water 2021, 13, 2222. [Google Scholar] [CrossRef]
- Toth, A.J. Modelling and Optimisation of Multi-Stage Flash Distillation and Reverse Osmosis for Desalination of Saline Process Wastewater Sources. Membranes 2020, 10, 265. [Google Scholar] [CrossRef]
- Wang, X.J.; Shen, Q.H.; Ji, Y.Z. Research Progress of Ships Waste Treatment Technology. Appl. Mech. Mater. 2014, 587–589, 674–679. [Google Scholar] [CrossRef]
- Moonkhum, M.; Lee, Y.G.; Lee, Y.S.; Kim, J.H. Review of Seawater Natural Organic Matter Fouling and Reverse Osmosis Transport Modeling for Seawater Reverse Osmosis Desalination. Desalination Water Treat. 2010, 15, 92–107. [Google Scholar] [CrossRef]
- Pacenti, P.; De Gerloni, M.; Reali, M.; Chiaramonti, D.; Gärtner, S.O.; Helm, P.; Stöhr, M. Submarine Seawater Reverse Osmosis Desalination System. Desalination 1999, 126, 213–218. [Google Scholar] [CrossRef]
- Al-Kharabsheh, S. An Innovative Reverse Osmosis Desalination System Using Hydrostatic Pressure. Desalination 2006, 196, 210–214. [Google Scholar] [CrossRef]
- U.S. Office of Naval Research Desalination Technology Increases Naval Capabilities. Available online: https://www.onr.navy.mil/media-center/news-releases/desalination-technology-increases-naval-capabilities#:~:text=Armistead%20anticipates%20increased%20capabilities%20from,systems%20will%20likely%20double%20that (accessed on 27 May 2025).
- Schlichter, B.; Mavrov, V.; Chmiel, H. Study of a Hybrid Process Combining Ozonation and Membrane Filtration—Filtration of Model Solutions. Desalination 2003, 156, 257–265. [Google Scholar] [CrossRef]
- MECO. (n.d.). Defense Water Purification Systems. Available online: https://www.meco.com/industries/defense/ (accessed on 4 January 2025).
- Raber, E.; Kirvel, R.D. Health Risk Assessment for Radiological, Chemical, and Biological Attacks*. In Wiley Handbook of Science and Technology for Homeland Security; Wiley: Hoboken, NJ, USA, 2008; p. 1. ISBN 978-0-471-76130-3. [Google Scholar]
- Mikelonis, A.M.; Orme, C.J.; Nilkar, A.S.; Szabo, J.G.; Reese, S.J. Rejection of Malathion by Nanofiltration and Reverse Osmosis Membranes Exposed to Foulant and Two Clean-in-Place Procedures. Water Supply 2024, 24, 1196–1206. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, M.; Heo, J.; Jung, S.; Ka, D.; Lee, H.; Kang, S.W.; Jung, H.; Lee, S.; Jin, Y.; et al. Blocking Chemical Warfare Agent Simulants by Graphene Oxide/Polymer Multilayer Membrane Based on Hydrogen Bonding and Size Sieving Effect. J. Hazard. Mater. 2022, 427, 127884. [Google Scholar] [CrossRef] [PubMed]
- Shumeiko, A.E.; Korotkikh, N.I. Chemical Warfare Agents: Structure, Properties, Decontamination (Part 1). J. Org. Pharm. Chem. 2024, 22, 41–52. [Google Scholar] [CrossRef]
- Yousef Motamedhashemi, M.M.; Monji, M.; Egolfopoulos, F.; Tsotsis, T. A Hybrid Catalytic Membrane Reactor for Destruction of a Chemical Warfare Simulant. J. Membr. Sci. 2015, 473, 1–7. [Google Scholar] [CrossRef]
- Talmage, S.; Watson, A.; Hauschild, V.; Munro, N.; King, J. Chemical Warfare Agent Degradation and Decontamination. Curr. Org. Chem. 2007, 11, 285–298. [Google Scholar] [CrossRef]
- Sadare, O.O.; Oke, D.; Olawuni, O.A.; Olayiwola, I.A.; Moothi, K. Modelling and Optimization of Membrane Process for Removal of Biologics (Pathogens) from Water and Wastewater: Current Perspectives and Challenges. Heliyon 2024, 10, e29864. [Google Scholar] [CrossRef]
- Helmi, A.; Gallucci, F. Latest Developments in Membrane (Bio)Reactors. Processes 2020, 8, 1239. [Google Scholar] [CrossRef]
- Obayomi, O.V.; Olawoyin, D.C.; Oguntimehin, O.; Mustapha, L.S.; Kolade, S.O.; Oladoye, P.O.; Oh, S.; Obayomi, K.S. Exploring Emerging Water Treatment Technologies for the Removal of Microbial Pathogens. Curr. Res. Biotechnol. 2024, 8, 100252. [Google Scholar] [CrossRef]
- Montaña, M.; Camacho, A.; Devesa, R.; Vallés, I.; Céspedes, R.; Serrano, I.; Blàzquez, S.; Barjola, V. The Presence of Radionuclides in Wastewater Treatment Plants in Spain and Their Effect on Human Health. J. Clean. Prod. 2013, 60, 77–82. [Google Scholar] [CrossRef]
- Amar, P. Ensuring Safe Water in Post-Chemical, Biological, Radiological and Nuclear Emergencies. J. Pharm. Bioallied Sci. 2010, 2, 253. [Google Scholar] [CrossRef]
- Kumar, V.; Goel, R.; Chawla, R.; Silambarasan, M.; Sharma, R. Chemical, Biological, Radiological, and Nuclear Decontamination: Recent Trends and Future Perspective. J. Pharm. Bioallied Sci. 2010, 2, 220. [Google Scholar] [CrossRef]
- Medina, V.; Waisner, S.; Mattei-Sosa, J.; Martinez-Guerra, E.; Griggs, C.; Lalley, J.; Henderson, D.; Moores, L.; Prager, B. Simulated Field Evaluation of the Decontamination Effluent Treatment System (DETS) for Wash Water from Mass Personnel Decontamination (MPD), Road Test, and Evaluation Treating Perfluorinated Alkyl Substances (PFAS); Engineer Research and Development Center (U.S.): Vicksburg, MS, USA, 2019. [Google Scholar]
- Medina, V. Treating Contaminated Effluent. Mil. Eng. 2018, 712, 37. [Google Scholar]
- Kärcher Futuretech. WTC 8000/15000 RO/UF C–Mobile Reverse Osmosis System. Available online: https://www.karcher-futuretech.com/en/products/mobile-water-supply/mobile-water-treatment-plants/mobile-reverse-osmosis-systems/wtc-8000-15000-ro-uf-c-13431300.html (accessed on 4 January 2025).
- Abdelrazeq, H.; Khraisheh, M.; Ashraf, H.M.; Ebrahimi, P.; Kunju, A. Sustainable Innovation in Membrane Technologies for Produced Water Treatment: Challenges and Limitations. Sustainability 2021, 13, 6759. [Google Scholar] [CrossRef]
- Roy, S.; Ragunath, S. Emerging Membrane Technologies for Water and Energy Sustainability: Future Prospects, Constraints and Challenges. Energies 2018, 11, 2997. [Google Scholar] [CrossRef]
- Miller, M.; Kisiel, A.; Cembrowska-Lech, D.; Durlik, I.; Miller, T. IoT in Water Quality Monitoring—Are We Really Here? Sensors 2023, 23, 960. [Google Scholar] [CrossRef]
- Buelke, C.; Alshami, A.; Casler, J.; Lewis, J.; Al-Sayaghi, M.; Hickner, M.A. Graphene Oxide Membranes for Enhancing Water Purification in Terrestrial and Space-Born Applications: State of the Art. Desalination 2018, 448, 113–132. [Google Scholar] [CrossRef]
- Ali, I.; Zenab Hasan, S.; Garcia, H.; Danquah, M.K.; Imanova, G. Recent Advances in Graphene-Based Nano-Membranes for Desalination. Chem. Eng. J. 2024, 483, 149108. [Google Scholar] [CrossRef]
- Tiwary, S.K.; Singh, M.; Chavan, S.V.; Karim, A. Graphene Oxide-Based Membranes for Water Desalination and Purification. Npj 2D Mater. Appl. 2024, 8, 27. [Google Scholar] [CrossRef]
- Raza, A.; Hassan, J.Z.; Mahmood, A.; Nabgan, W.; Ikram, M. Recent Advances in Membrane-Enabled Water Desalination by 2D Frameworks: Graphene and Beyond. Desalination 2022, 531, 115684. [Google Scholar] [CrossRef]
- Omran, B.; Baek, K.-H. Graphene-Derived Antibacterial Nanocomposites for Water Disinfection: Current and Future Perspectives. Environ. Pollut. 2022, 298, 118836. [Google Scholar] [CrossRef]
- Liu, F.; Wang, L.; Sun, D.; Li, B.; Tian, H.; Qi, Z.; Zhou, S. An Efficient Superhydrophilic Photothermal Membrane of Graphene-Decorated TiO2 Nanotube Arrays for Solar Desalination. Desalination 2025, 597, 118398. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A Review of Removal of Pollutants from Water/Wastewater Using Different Types of Nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 825910. [Google Scholar] [CrossRef]
- Thakur, M.; Medintz, I.L.; Walper, S.A. Enzymatic Bioremediation of Organophosphate Compounds—Progress and Remaining Challenges. Front. Bioeng. Biotechnol. 2019, 7, 289. [Google Scholar] [CrossRef]
- Pashirova, T.; Salah-Tazdaït, R.; Tazdaït, D.; Masson, P. Applications of Microbial Organophosphate-Degrading Enzymes to Detoxification of Organophosphorous Compounds for Medical Countermeasures against Poisoning and Environmental Remediation. Int. J. Mol. Sci. 2024, 25, 7822. [Google Scholar] [CrossRef]
- Vitola, G.; Mazzei, R.; Poerio, T.; Porzio, E.; Manco, G.; Perrotta, I.; Militano, F.; Giorno, L. Biocatalytic Membrane Reactor Development for Organophosphates Degradation. J. Hazard. Mater. 2019, 365, 789–795. [Google Scholar] [CrossRef]
- Vitola, G.; Mazzei, R.; Fontananova, E.; Porzio, E.; Manco, G.; Gaeta, S.N.; Giorno, L. Polymeric Biocatalytic Membranes with Immobilized Thermostable Phosphotriesterase. J. Membr. Sci. 2016, 516, 144–151. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, M.; Li, Y.; Chen, Z.; Yang, H.; Wang, X. Advanced Porous Materials and Emerging Technologies for Radionuclides Removal from Fukushima Radioactive Water. Eco-Environ. Health 2023, 2, 252–256. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Hou, L. Reverse Osmosis Membrane with Crown Ethers Decoration for Enhanced Radionuclides Sieving. Surf. Interfaces 2024, 48, 104222. [Google Scholar] [CrossRef]
- Liu, S.; Tang, J.; Ji, F.; Lin, W.; Chen, S. Recent Advances in Zwitterionic Hydrogels: Preparation, Property, and Biomedical Application. Gels 2022, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hong, H.; Cao, J.; Yang, Y. Progress in Marine Antifouling Coatings: Current Status and Prospects. Coatings 2023, 13, 1893. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, R.; Liu, K.; Zhang, Y.; Shi, X.; Sand, W.; Hou, B. Application of Nanomaterials in Antifouling: A Review. Nano Mater. Sci. 2024, 6, 672–700. [Google Scholar] [CrossRef]
- Toh, K.Y.; Liang, Y.Y.; Lau, W.J.; Fimbres Weihs, G.A. 3D CFD Study on Hydrodynamics and Mass Transfer Phenomena for SWM Feed Spacer with Different Floating Characteristics. Chem. Eng. Res. Des. 2020, 159, 36–46. [Google Scholar] [CrossRef]
- Dieu, B. Application of the SCADA System in Wastewater Treatment Plants. ISA Trans. 2001, 40, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Schlake, J.C. Changes to the Automation Architecture: Impact of Technology on Control Systems Algorithms. In Proceedings of the 2017 22nd IEEE International Conference on Emerging Technologies and Factory Automation (ETFA), Limassol, Cyprus, 12–15 September 2017; pp. 1–8. [Google Scholar]
- Scholze, R.J.; Zaghloul, H.H. Assessment of SCADA Technology Applications to Automate U.S. Army Water and Wastewater Sanitary Systems; ERDC, U.S. Army Corps of Engineers; Defense Technical Information Center: Fort Belvoir, VA, USA, 2001; Report No. ADA398730.
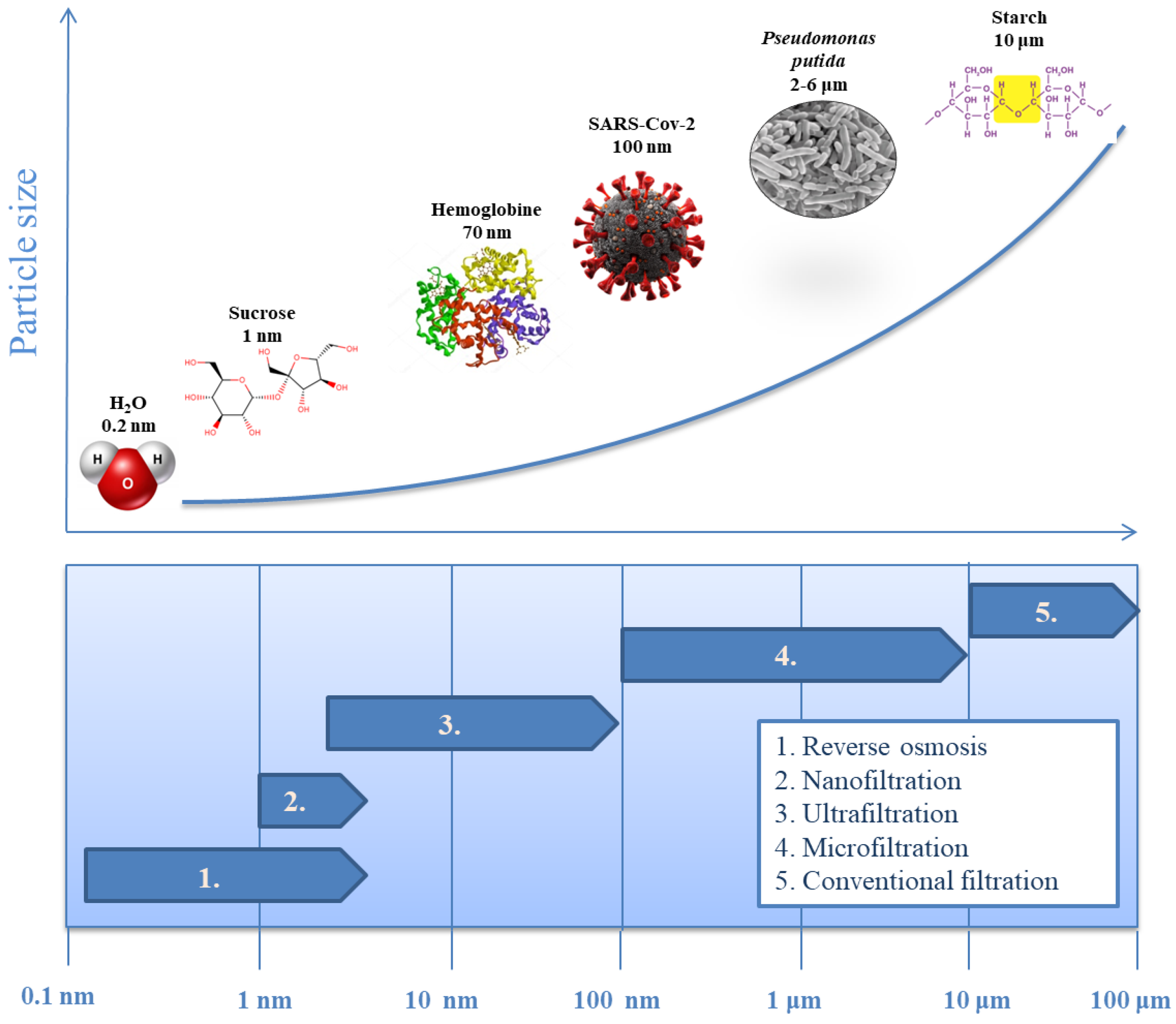


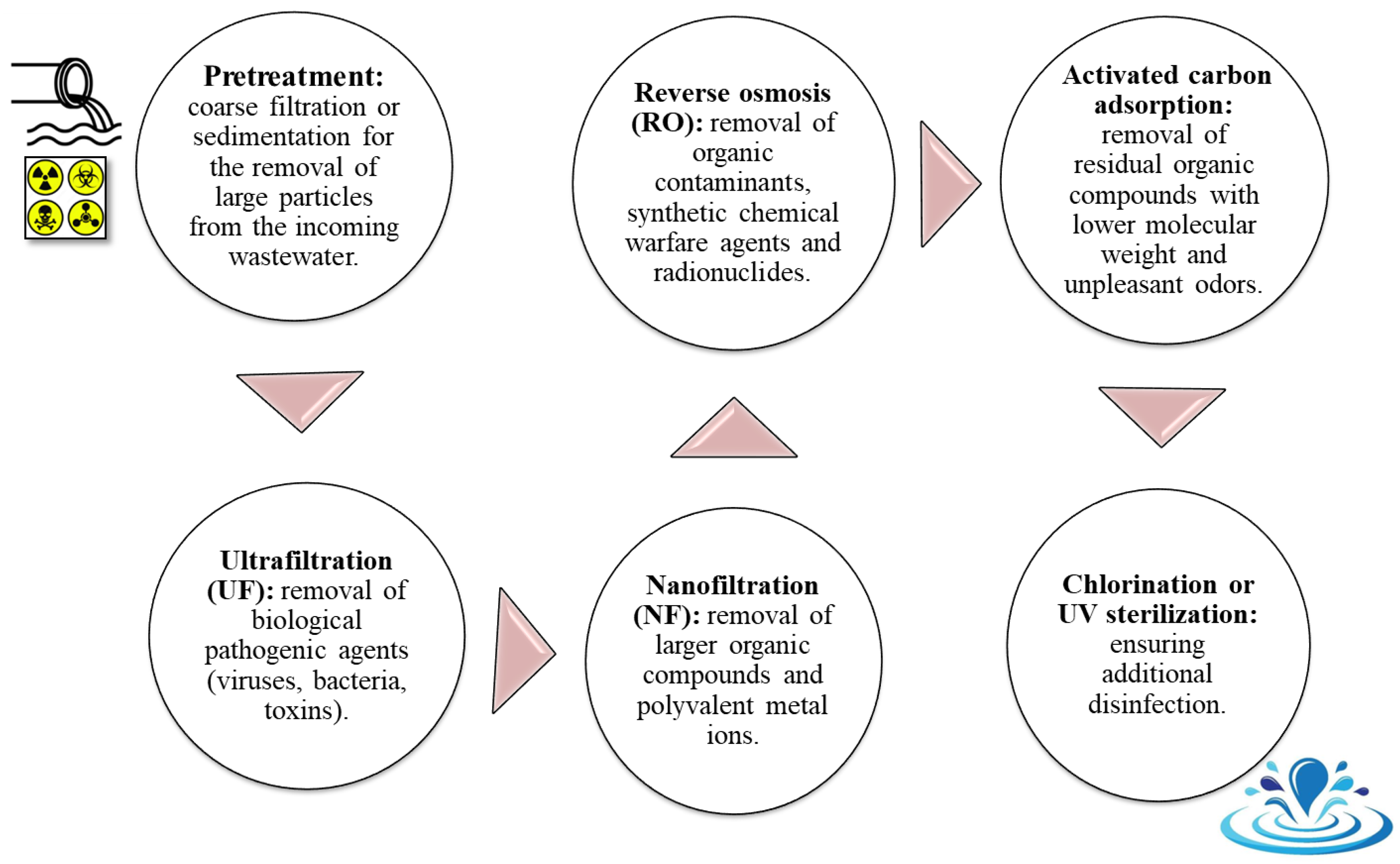
| Pressure Difference | Concentration (Activity) Difference | Temperature Difference | Electrical Potential Difference |
|---|---|---|---|
| Microfiltration | Pervaporation | Thermo-osmosis Membrane distillation | Electrodialysis Electro-osmosis Membrane electrolysis |
| Ultrafiltration | Gas separation | ||
| Nanofiltration | Vapor permeation | ||
| Reverse osmosis | Dialysis | ||
| Forward osmosis | Diffusion dialysis | ||
| Pressure retarded osmosis | Carrier-mediated transport |
| Type of Membranes | Spectrum of Use | Advantages | Disadvantages | |
|---|---|---|---|---|
| Inorganic membranes | Al2O3, TiO2, ZrO2 ceramic membranes, Al-Si oxide membranes, titanium/stainless steel porous metal membranes, carbon based molecular sieves, silicate, borosilicate and porous glass membranes | used in various sectors of the chemical industry for water filtration and gas separation, in analytical sensors, pH electrodes etc. | excellent mechanical, thermal and chemical stability, variable pore size, work under challenging process conditions, separation mechanism: molecular sieving, capillary condensation surface diffusion, Knudsen diffusion | membranes have extremely fragile pores, preparation process is expensive, difficulties in scale-up implementations |
| Organic (polymeric) membranes | polyamide (PA) membranes | used in NF and RO processes, for seawater desalination—thin film composite membrane (TFC), for industrial wastewater treatment—spirally wound membranes (SWM) | low production cost, good mechanical stability, easy for upscaling, realtively easy preparation, separation mechanism: size exclusion, charge exclusion and membrane-solution interactions | plasticization, depending on the nature of the polymer relatively low thermal and chemical stability, not controllable pore size, trade-off between permeability and selectivity, need for regular cleaning procedure (intensive pore fouling) |
| polyvinylidene fluoride (PVDF) membranes | used in UF i MBR processes, used in municipal and industrial plants for wastewater treatment etc. | |||
| polyurethane (PU) membranes | utilised as biosensors for the detection of ions and for the controlled release of drugs in medicine etc. | |||
| membranes based on cellulose derivatives | used in ED i RO, used as membranes for desalination of seawater (1. generation) | |||
| Hybrid and mixed matrix membranes | graphene-modified polymer membranes (graphene-polymethyl methacrylate PMMA nanolaminate) | used in biosensors, electronic and optical devices, for the removal of organic and inorganic pollutants, as well as for water purification and desalination etc. | reduced plasticization, enhanced thermal and mechanical stability, low energy consumption, separation mechanism: combined inorganic and polymeric membrane principle | at high fraction of filler within the polymer matrix, fragility of the system, thermal and chemical stabilities depend on the polymeric matrix |
| zeolite nanoparticles MFI/polycrystalline membrane MOF ZIF-8 | used in biomedicine for drug delivery, catalytic reactions, and in the separation of petroleum fractions etc. | |||
| biomimetric and bioinspired membranes | application in various sectors, for water purification, biosensor development, and industrial processes etc. | |||
| Constituents | Unit | Min. Emergency Standards | Potential Health Effect |
|---|---|---|---|
| Phisical | |||
| colour | CU/cobalt-platinum method | 50 | Risk of dehydration due to reduced water consumption caused by decreased palatability; symptoms of dehydration include weariness apathy, impaired co-ordination, delirium, heat stroke. |
| turbidity | NTU | 1 | Risk of dehydration due to reduced water consumption caused by decreased palatability. Mostly gastro-intestinal effects due to presence of pathogenic microorganisms, caused by decreased disinfection efficiency. |
| conductivity | µS cm−1 | 1500 | Risk of dehydration due to reduced water consumption caused by decreased palatability. |
| pH | - | 5–9.5 | More corrosive activity on lower pH and decreased disinfection efficiency at higher pH. |
| Microbiological | |||
| Escherichia coli | CFU 100 mL−1 | 0 | Mostly gastro-intestinal effects due to presence of pathogenic microorganisms. Symptoms: dehydration, abdominal cramps, diarrhea, vomiting, bloating, high fever, HUS syndrome, etc. |
| coliform bacteria | CFU 100 mL−1 | 0 | Gastrointestinal infections, diarrhea, vomiting, abdominal pain, urinary tract infections, hemolytic syndrome caused by Shiga toxin, etc. |
| Chemical | |||
| cyanide | mg L−1 | 6 | Headache, breathlessness, weakness, palpitation, nausea, vomiting, giddiness, tremor, rapid heartbeat, dizziness, confusion, anxiety, agitation, cardiac arrhythmias, seizures, stupor, coma. |
| arsenic | mg L−1 | 0.3 | Facial swelling, vomiting, loss of appetite, abdominal pain, diarrhoea, shock, muscle cramps, headache, chill, cardiac abnormalities, anaemia, decreased white blood cell count, enlargement of liver, delayed effects including sensory and motor peripheral polyneuropathies. |
| sulphate | mg L−1 | 300 | Laxative effect that can lead to symptoms of dehydration including weariness apathy, impaired co-ordination, delirium, heat stroke. |
| inorganic mercuric compounds | mg L−1 | 0.003 | Mercury compounds mainly have health effects on the kidney and the central nervous system. |
| lewisite (arsenic fraction) | mg L−1 | 0.080 | Nausea, vomiting, diarrhoea, abdominal pain, intense thirst, weakness, hypotension, hypothermia. |
| sulphur mustard | mg L−1 | 0.140 | Nausea, vomiting of blood, diarrhoea, abdominal pain, fever, headache, cardiac arrhythmias, dizziness, malaise, loss of appetite, lethargy, convulsion, leukopenia, anemia, immumosuppression. |
| nerve agents | mg L−1 | 0.012 | Nausea, vomiting, diarrhea, abdominal cramps, headache, giddiness, dizziness, excessive salivation, tearing, miosis, blurred or dim vision, difficult breathing, cardiac arrhythmias, loss of muscle coordination, muscle twitching, random jerking movements, convulsions, coma. |
| T-2 toxins | mg L−1 | 0.026 | Nausea, vomiting, diarrhea, generalised, burning erythema, mental confusion. |
| Radiological | |||
| alpha, Pu239 | activity limit, Bq L−1 | 28,500 | Nausea, vomiting, diarrhea The standard of each type of radiation correspondents with an exposure of 250 mSv. |
| beta, St90 | 255,000 | ||
| gamma, I131 | 300,000 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volf, M.; Morović, S.; Košutić, K. Integration and Operational Application of Advanced Membrane Technologies in Military Water Purification Systems. Separations 2025, 12, 162. https://doi.org/10.3390/separations12060162
Volf M, Morović S, Košutić K. Integration and Operational Application of Advanced Membrane Technologies in Military Water Purification Systems. Separations. 2025; 12(6):162. https://doi.org/10.3390/separations12060162
Chicago/Turabian StyleVolf, Mirela, Silvia Morović, and Krešimir Košutić. 2025. "Integration and Operational Application of Advanced Membrane Technologies in Military Water Purification Systems" Separations 12, no. 6: 162. https://doi.org/10.3390/separations12060162
APA StyleVolf, M., Morović, S., & Košutić, K. (2025). Integration and Operational Application of Advanced Membrane Technologies in Military Water Purification Systems. Separations, 12(6), 162. https://doi.org/10.3390/separations12060162







