Abstract
This study focuses on optimizing two tandem columns to separate ethylbenzene and styrene. A steady-state model is developed to minimize total energy consumption (TEC) and total annualized cost (TAC) by optimizing the reflux flow rates. An integrated dynamic model is created using the Multi-Objective Particle Swarm Optimization (MOPSO) algorithm. This model is designed to account for transitions in operating conditions and to identify optimal dynamic strategies for adjusting operations to maintain optimal performance. The optimization considers factors such as fluctuation amplitude, the number of fluctuations, and fluctuation duration. The aim is to reduce fluctuation amplitudes while ensuring higher energy efficiency and stable operation. The results reveal that the optimal reflux flow rates are 41,152.2 kg/h and 1012.7 kg/h, leading to reductions in TEC and TAC by 16.7% and 17.4%, respectively. Compared with the industry standard level, the energy consumption has decreased by 11.25%. Against the backdrop of increasingly strict global carbon emission control, the market competitiveness of ethylbenzene/styrene production has been significantly enhanced. The variable-step adjustment method requires less time to reach a stable state, while the equal-step fluctuation method provides more stability. The Pareto solution set derived from the two optimization techniques can be used to select the most suitable adjustment strategy, ensuring a fast and smooth transition.
1. Introduction
Styrene, a cornerstone monomer in the global polymer industry, is indispensable for the manufacture of polystyrene, acrylonitrile–butadiene–styrene (ABS) resins, and synthetic rubbers. The market for styrene is projected to reach USD 62.81 billion by 2026, with a compound annual growth rate (CAGR) of 4.7% [1]. Catalytic dehydrogenation of ethylbenzene accounts for ~90% of global styrene production [2]. This process involves separating isomers that have a relative volatility (α) close to 1, making it one of the more challenging separations in distillation engineering [3,4]. Industrial processes rely on energy-intensive distillation, necessitating the optimization of parameters such as reflux ratio, feed stage, and interstage flows to mitigate high reboiler duties and associated carbon emissions [5,6,7].
The optimization for ethylbenzene–styrene separation uses a mixed-integer nonlinear programming (MINLP) problem, which includes both discrete design variables (such as the number of trays and the location of the feed stage) and continuous operational parameters (like the reflux ratio) [8,9]. Metaheuristic algorithms have proven effective in solving these types of problems. For instance, Chandra et al. [10] employed multi-objective genetic algorithms (MOGA) to optimize multi-effect distillation configurations, balancing steam consumption and capital costs. Su et al. [11] minimized total annualized cost (TAC) in pressure swing distillation by employing genetic algorithms (GA), which resulted in a 12% cost savings compared to conventional designs. For extraction distillation systems, Wang et al. [12,13] utilized MOGA to optimize the separations of cyclohexane–isopropanol and methanol–water–toluene, respectively, achieving Pareto-optimal solutions for TAC, CO2 emissions, and extraction efficiency. Yin et al. [14] employed particle swarm optimization (PSO) in reactive extraction distillation, achieving an 8.7% reduction in TAC through adaptive tuning of the reflux ratio. Advanced methodologies for simultaneously minimizing TAC and the total energy consumption (TEC) of multi-column systems include the Boltzmann univariate marginal distribution algorithm [15], Mesh adaptive direct search [16], nondominated sorting genetic algorithm II [17], and sequential iterative methods [18].
Despite recent advancements, most studies focus primarily on steady-state optimization while neglecting the dynamic coupling inherent in industrial tandem distillation systems. The transition between operating points, such as adjusting reflux ratios to achieve optimized steady states, generates transient dynamics governed by mass and energy balances, as well as transfer rates and equilibria. If this dynamic process is not managed properly, it can destabilize control loops [19]. Control theory suggests that these transitions require strategic adjustment profiles to reduce fluctuations in critical variables, such as sensitive tray temperatures and intercolumn flow rates, ensuring a safe and efficient convergence to target conditions [20,21,22]. However, the integration of steady-state optimization with dynamic control frameworks, which is essential for industrial implementations, remains underdeveloped, particularly for multi-column systems, where interstage holdups and thermal lags further complicate transient dynamics.
This study addresses this research gap by proposing a holistic optimization framework for a two-column ethylbenzene–styrene separation process. The framework integrates steady-state design optimization with the synthesis of dynamic transition strategies. During the steady-state phase, detailed simulations using Aspen Plus are employed to minimize the total equivalent cost (TEC) and TAC through the optimization of the reflux ratio. The dynamic phase formulates the transition as a state-space control problem, focusing on identifying trajectories that minimize overshoot in controlled variables and reduce settling time. The multi-objective particle swarm optimization (MOPSO) algorithm is adopted for its superior performance in optimizing continuous variables. Unlike other algorithms, such as NSGA-II, which may introduce discretization errors for real-valued parameters like flow rates and temperature setpoints, MOPSO’s velocity–position update mechanism enables the seamless exploration of continuous solution spaces. By incorporating realistic process lags, such as thermocouple response delays and tray-to-tray heat transfer delays, and utilizing Aspen Dynamics for dynamic validation, the proposed framework aims to provide operationally feasible solutions that bridge theoretical optimality with industrial practicability. The optimization accounts for material balance constraints and control system limitations, ensuring robust performance across both transient and steady-state operational regimes.
2. Problem Description
2.1. General Process for Separating the Ethylbenzene–Styrene Mixture
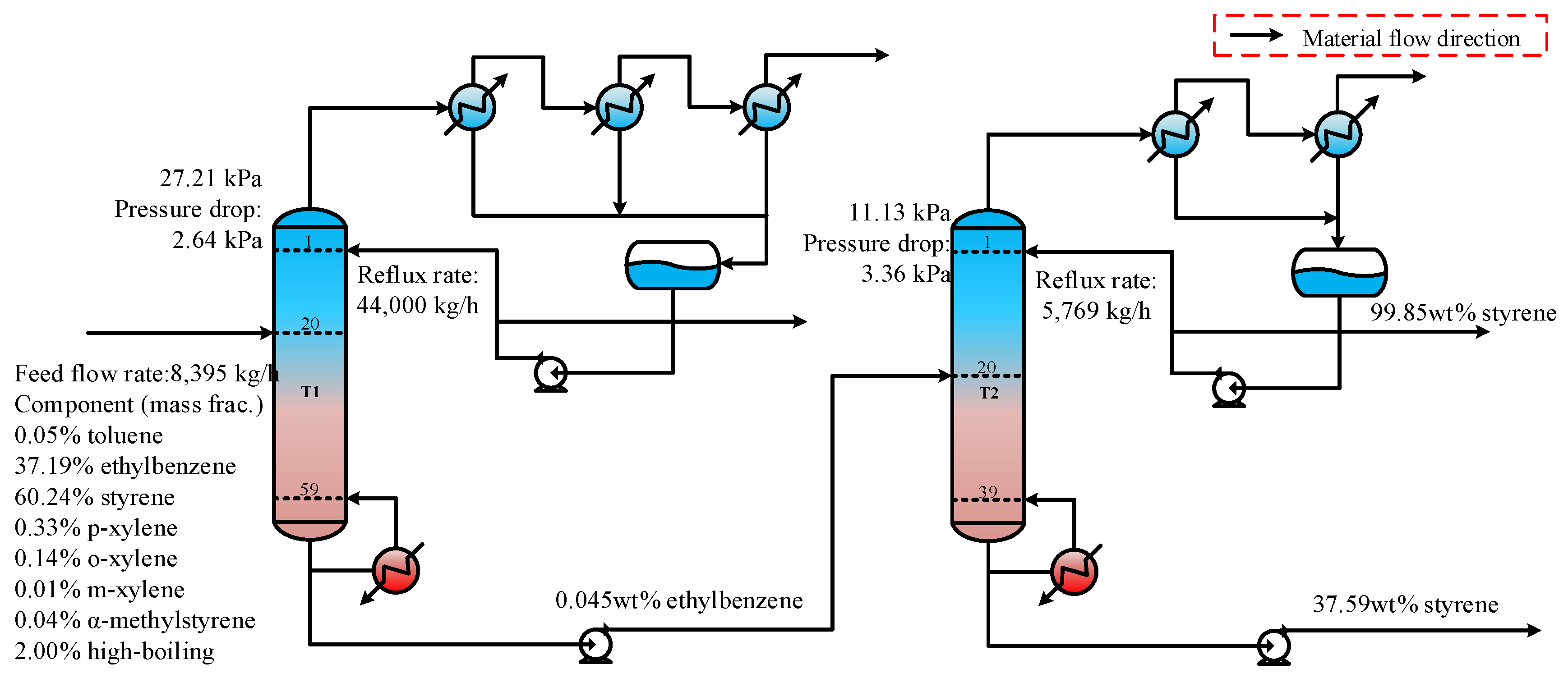
In industrial processes, two tandem columns are commonly used to separate and refine ethylbenzene and styrene, as shown in Figure 1. The feed mixture primarily consists of ethylbenzene, styrene, and trace amounts of other components, such as toluene and xylene. Ethylbenzene is separated from the top of column T1, while styrene is collected at the top of column T2 after removing the high-boiling components. Both columns operate under a high vacuum to prevent styrene polymerization [4]. To ensure that the styrene produced from column T2 meets purity standards, the ethylbenzene content at the bottom of column T1 must be strictly controlled. This requirement creates a significant interdependence between the two tandem columns. Meanwhile, since ethylbenzene and styrene have similar boiling points, the energy consumption for separation is considerable. Therefore, it is essential to optimize the operational parameters to minimize energy usage.
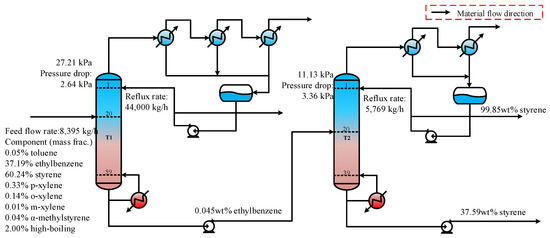
Figure 1.
A flowsheet with two tandem columns for separating the ethylbenzene–styrene mixture.
In practice, the transition from the initial operating conditions to the optimal ones cannot be achieved all at once due to the interconnection between devices and parameters. The ability to adapt to adjustments relies on the rates of mass and energy transfer. Consequently, this transition is a gradual process that occurs in several steps. The adjustment strategy employed will influence the magnitude of fluctuations within the dynamic system as well as the time required to reach the steady state at optimal parameters. Ultimately, this affects both the safety and economic efficiency of the production process. The dynamic variations during the adjustment processes toward the optimal state need further verification.
2.2. Dynamic Optimization Problem
In chemical production, optimizing operating conditions can significantly enhance both production efficiency and economic benefits. While many studies have focused on refining operational parameters and achieved notable results, these optimizations are mainly carried out under steady-state conditions. However, actual chemical production processes are typically dynamic. Unlike steady-state conditions, dynamic chemical processes exhibit significant temporal dependence and complexity. During dynamic processes, system parameters, such as temperature, pressure, and flow rate, change over time, leading to a transitional period often characterized by lag effects and nonlinear behavior. This makes it more challenging to predict the system’s performance. When operating conditions change, the system may enter an unstable transitional stage, resulting in fluctuations in key parameters such as temperature, pressure, and flow rate [23,24,25]. These fluctuations, in turn, can impact production efficiency. If certain safety-related parameters experience severe fluctuations, this may increase the safety risks and potentially lead to accidents. Therefore, it is essential to optimize the process surrounding variations in operating conditions, reduce the duration of the transition period, and enhance production efficiency. Simultaneously, it is important to mitigate drastic fluctuations in control parameters to ensure the safety and stability of the production process.
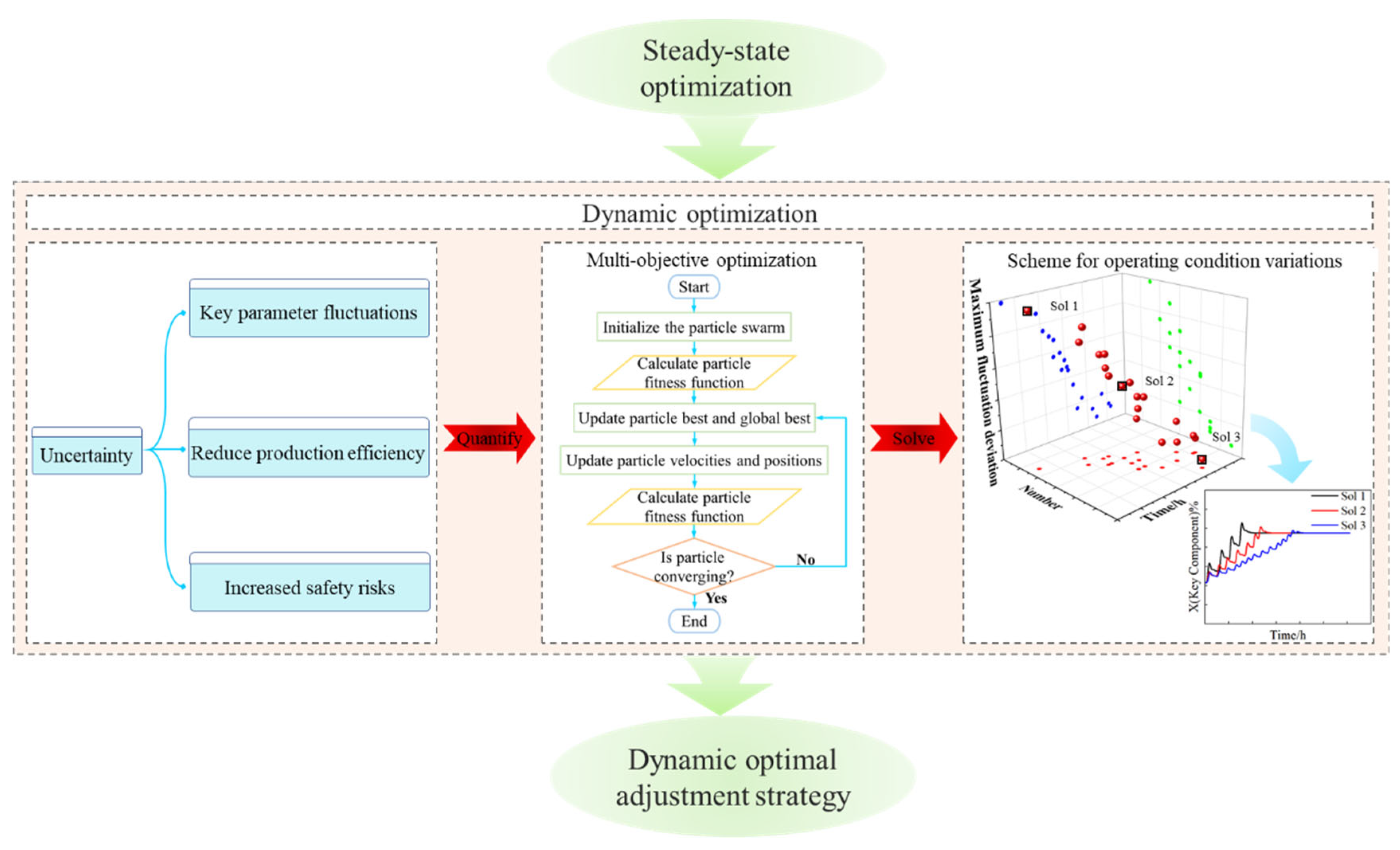
For two tandem columns separating ethylbenzene and styrene, this work will investigate dynamic optimization based on steady-state optimization. The optimal reflux flow rates will be identified through steady-state optimization to minimize the TEC and TAC. The fluctuations caused by variations in reflux flow rates will be quantified, and the optimization can be treated as a multi-objective optimization problem, which is solved using the PSO algorithm. Minimizing the transition time and the amplitude fluctuation of key parameters will be taken as the objective functions. An effective optimization scheme for managing variations in operating conditions will be developed, providing valuable guidance for the actual production process. The framework of dynamic optimization is illustrated in Figure 2.
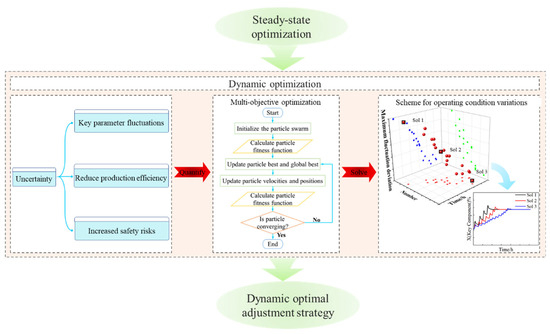
Figure 2.
The framework of dynamic optimization.
3. Optimization Model
3.1. Steady-State Optimization
For the ethylbenzene–styrene separation process illustrated in Figure 1, a steady-state simulation model was developed using Aspen Plus V14 with the NRTL thermodynamic method [4,26]. This model will be utilized to optimize the reflux rates of T1 and T2, thereby minimizing energy consumption and overall annual costs.
The TEC of the system mainly includes reboiler duty and condenser duty, which can be calculated by Equation (1). The TAC is used for the economic evaluation of different processes and includes both the total operating cost (TOC) and the annualized total investment cost (TCI), as shown in Equation (2) [27]. For the optimization of the existing tandem columns separating the ethylbenzene–styrene mixture, only the TOC is considered and can be calculated using Equation (3).
where is the reboiler duty, kW; and represent the duties of condensers cooled by cooling water and chilled water, respectively, kW; and represent the standard coal equivalent coefficients for steam and cooling water, respectively, kgce/t; denotes the standard coal equivalent coefficient for electricity, kgce (coal equivalent)/(kWh); r indicates the latent heat of vaporization of water vapor, kJ/kg; is the specific heat capacity of water, kJ/(kg∙°C); PP is the payback period, year; and represent the differences between the supply and return temperatures of cooling and chilled water, respectively,∙°C; and , , and are the prices of steam, cooling water, and chilled water, and are taken as 91.74 Chinese Yuan (CNY)/t, 0.25 CNY/t, and 5.96 CNY/t, respectively. The annual operating time is 8000 h.
To ensure that the final product’s purity meets the purity requirements, the product purity is used as the constraint, as shown in Equations (4)–(6).
The objective of the steady-state optimization is to minimize both TEC and TAC through optimizing the reflux rates, as shown in Equation (7). The problem is a multi-objective optimization problem.
where L1 and L2 are the reflux rates (kg/h) of T1 and T2, with the value ranges of [40,000, 44,000] and [1000, 5769], respectively.
Both MOPSO and NSGA-II are classic algorithms for solving multi-objective optimization problems. Based on Pareto optimization theory, they generate non-dominated solution sets (Pareto fronts) through iteration, effectively addressing multi-objective conflicts such as energy consumption, economic efficiency, safety, and productivity in chemical distillation processes, and providing trade-off optimization schemes for complex systems [8,28,29]. MOPSO simulates the “social–cognitive” learning process through particle velocity–position update mechanisms, making it naturally suitable for continuous parameter spaces (e.g., distillation column temperature, flow rate, reflux ratio, etc.) and capable of generating mutation-free, continuous, and smooth Pareto solutions to avoid parameter jump errors introduced by discretization operations. Its core advantages include low computational complexity, supporting sub-second iteration to meet the online real-time optimization needs of multi-unit coupled systems; balancing global exploration and local exploitation through the adaptive adjustment of inertia weight to maintain solution diversity; and strong anti-interference ability, suppressing measurement noise and external disturbances through group collaborative learning, making it suitable for robust optimization in dynamic scenarios. NSGA-II, based on genetic algorithm operations such as selection, crossover, and mutation, excels in discrete or mixed-variable optimization (e.g., number of trays, equipment models, etc.) and generates multi-modal Pareto fronts through non-dominated sorting and crowding distance calculation, making it suitable for multi-scheme comparison in offline steady-state design scenarios. Its technical features include an outstanding global search capability that breaks through local optima in discrete spaces, efficiently handling complex constraints by quickly eliminating inferior solutions through non-dominated sorting, while maintaining solution diversity via the use of crowding distance. For this study, the decision variables in the multi-objective optimization problem are continuous (e.g., transition time, control parameter fluctuation range), with constraints primarily on the boundaries of continuous variables. The dynamic model involves multi-variable coupling and time-varying characteristics, requiring continuity in step-size optimization, matching of different vector dimensions, and real-time anti-interference capability in dynamic processes. MOPSO’s continuous dynamic optimization capability, real-time computational efficiency, and noise robustness precisely meet these requirements. Therefore, this study selects MOPSO as the optimization algorithm to achieve steady-state parameter optimization and fast, safe parameter adjustment during dynamic condition transitions.
The MOPSO algorithm, which is adopted to address the optimization challenges in the distillation process, is a variant of the Particle Swarm Optimization (PSO) algorithm [28,29]. Derived from the collaborative foraging behavior of bird flocks, PSO is a heuristic optimization technique originally designed for single-objective optimization [30]. In PSO, each particle represents a candidate solution with its position (encoding decision variables such as reflux ratio or tray temperature in distillation) and flight velocity, which is dynamically adjusted by two critical factors: the particle’s historical best position and the swarm’s global best position. By iteratively updating velocity and position based on these two reference points, particles converge toward the optimal solution through a balance of individual experience and social learning. The updates to the velocity and position of the particles occur according to Equations (8) and (9), respectively.
where v and x denote the velocity and position of the particles, respectively; i and t indicate the number of particles and that of iterations, respectively; w is known as the inertia weight coefficient; c1 and c2 are the learning factors; r1 and r2 are random numbers that range between 0 and 1; refers to the optimal position of the particles; and denotes the optimal position of the entire particle swarm.
MOPSO is built upon the fundamental framework of PSO and incorporates techniques such as non-dominated sorting and crowding distance calculations to address multi-objective optimization problems effectively. By introducing the Pareto optimal boundary, the multi-objective optimization challenge is transformed into an equilibrium problem among multiple objective functions. In the study, the Pareto front is employed to consider the objectives of minimizing TEC and TAC, while also balancing these objectives. The identified Pareto solution set includes all solutions that meet the objective functions and constraints. The final solution can be selected based on the specific requirements of the optimization problem [31].
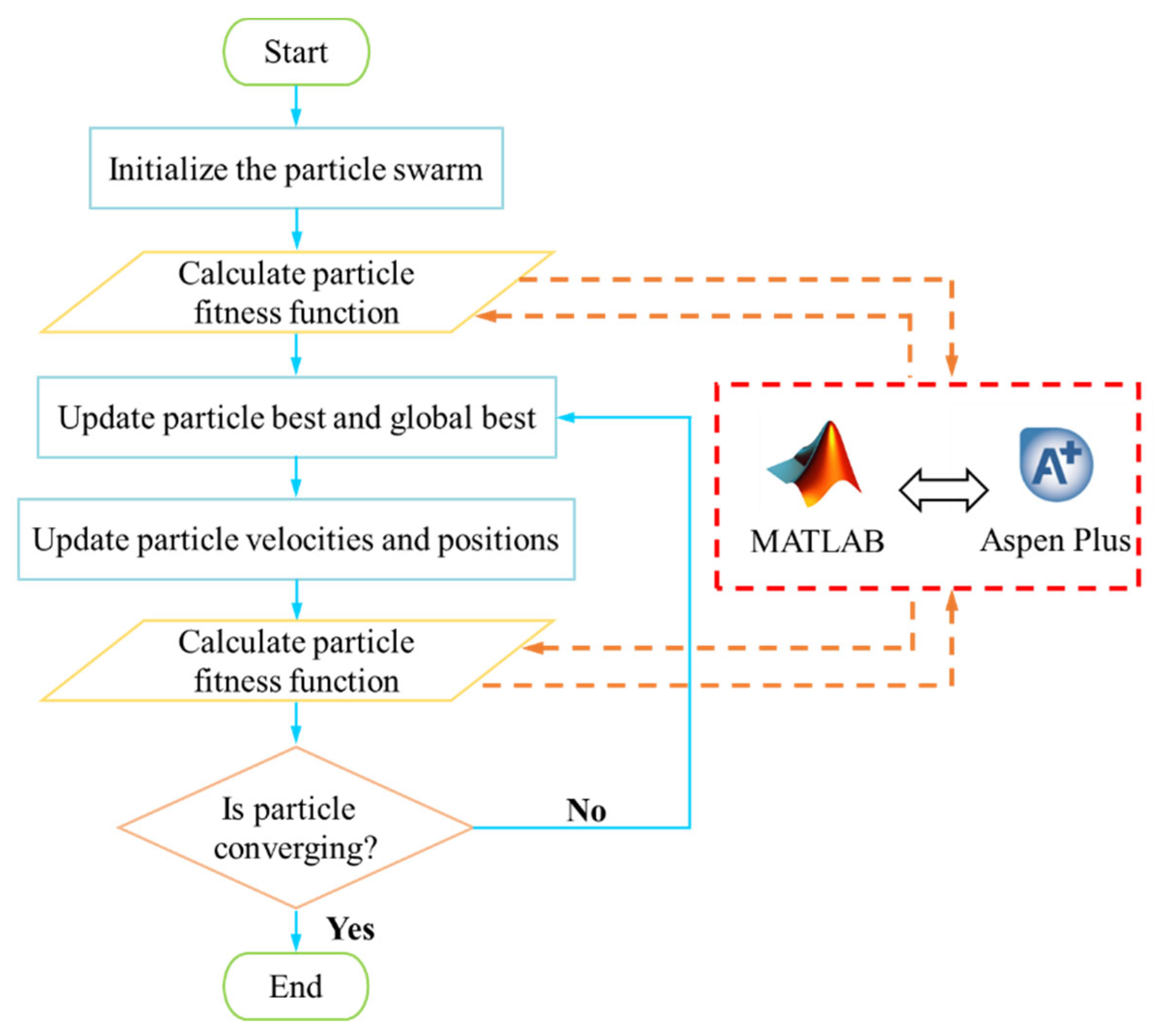
The optimization is carried out by coupling Aspen Plus V14 and MATLAB R2024a. Aspen Plus V14 is utilized to calculate the fitness of the particles, while the optimization algorithm is executed in MATLAB R2024a. The flowchart of the MOPSO is illustrated in Figure 3. Initially, the best individual and the global best individual are updated according to fitness by initializing the particle swarm within a specified range. The velocity and position of the particle swarm are updated, followed by a recalculation of fitness. If the convergence condition is met, the search terminates; otherwise, the algorithm continues iterating. This process ultimately results in a series of mutually non-dominated Pareto solution sets.
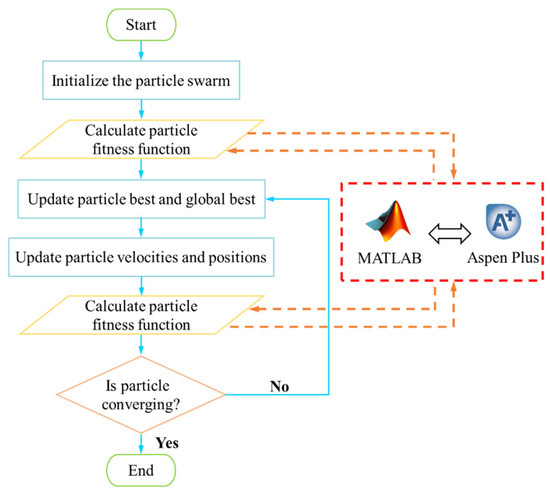
Figure 3.
Flowchart of MOPSO algorithm.
3.2. Dynamic Control and Optimization
3.2.1. Establishment of Dynamic Control Structure
In chemical production, adjusting the distillation process to steady-state optimal operating conditions is a dynamic task. The strategy for adjustment and the associated fluctuation are of great significance for energy conservation and maintaining safety and stability.
To explore the robustness of the control structure, based on the steady-state model, a dynamic model for the ethylbenzene–styrene separation process was constructed using Aspen Plus Dynamics V14. The thermodynamic methods, ideal stages of the two columns, and purity targets of the products are the same as those in the steady-state model. This model ensures stability even in the presence of disturbances [32]. Following the criterion that the bottom of the column and reflux tank should be filled with liquid for ten minutes, the height and diameter of the kettle and reflux tank were determined, and the height-to-diameter ratio of the column was set at 2 [33]. Additionally, valves were incorporated into the steady-state simulation model with an appropriate pressure drop set. Pressure testing was subsequently conducted on the separation process.
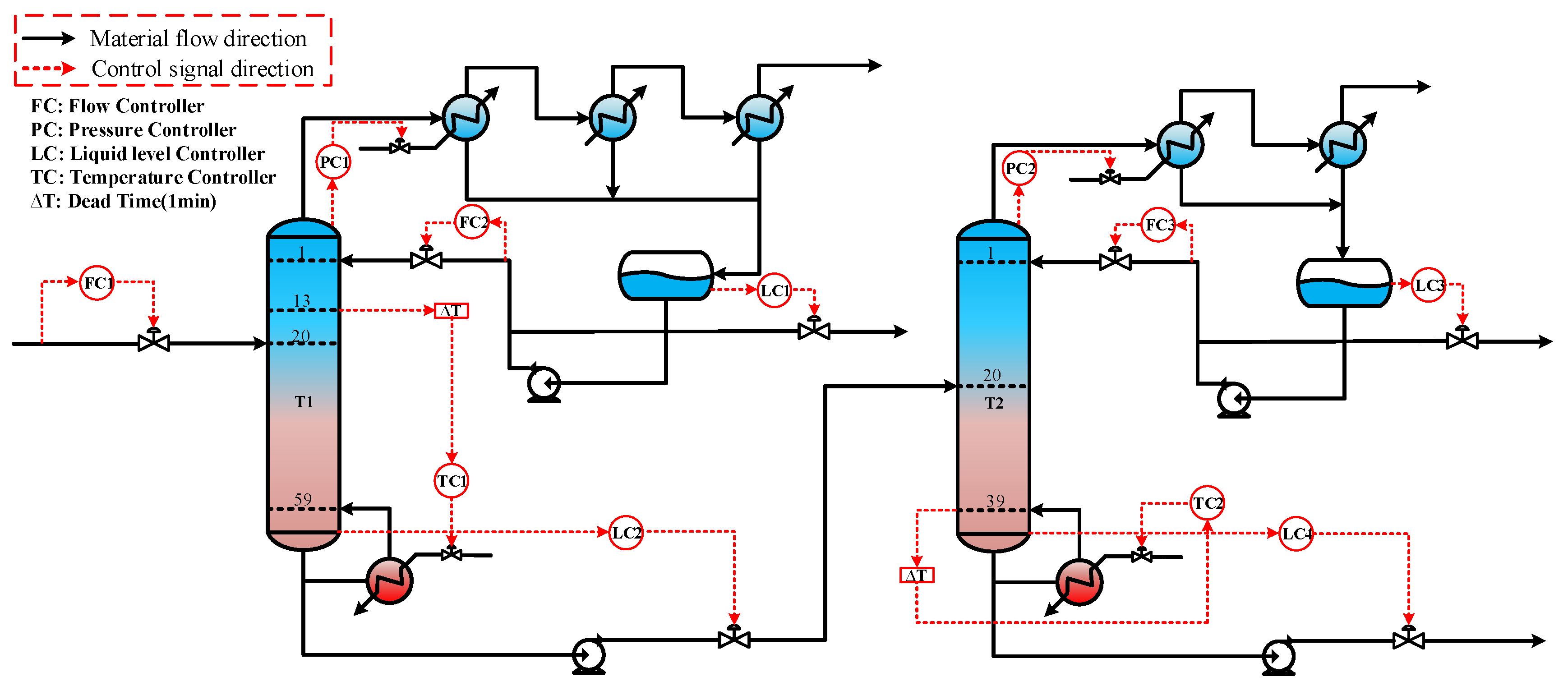
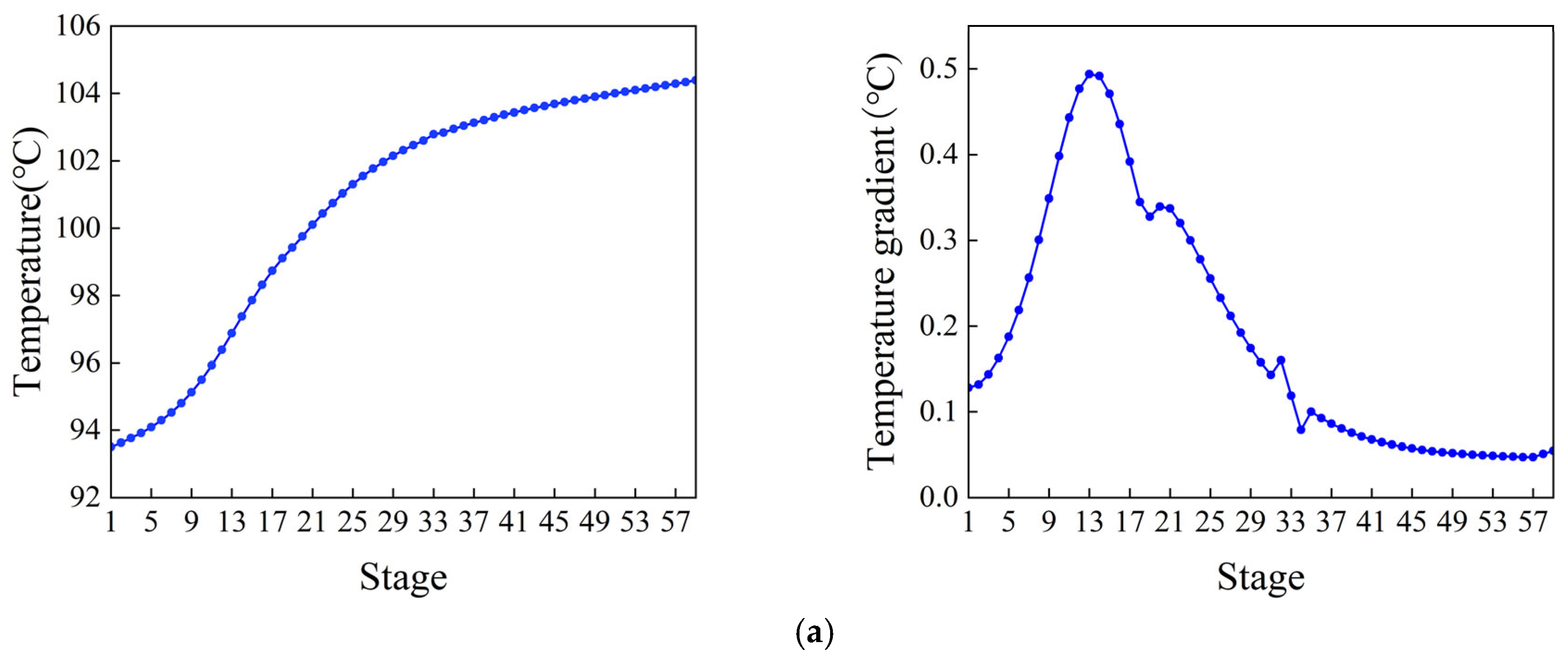
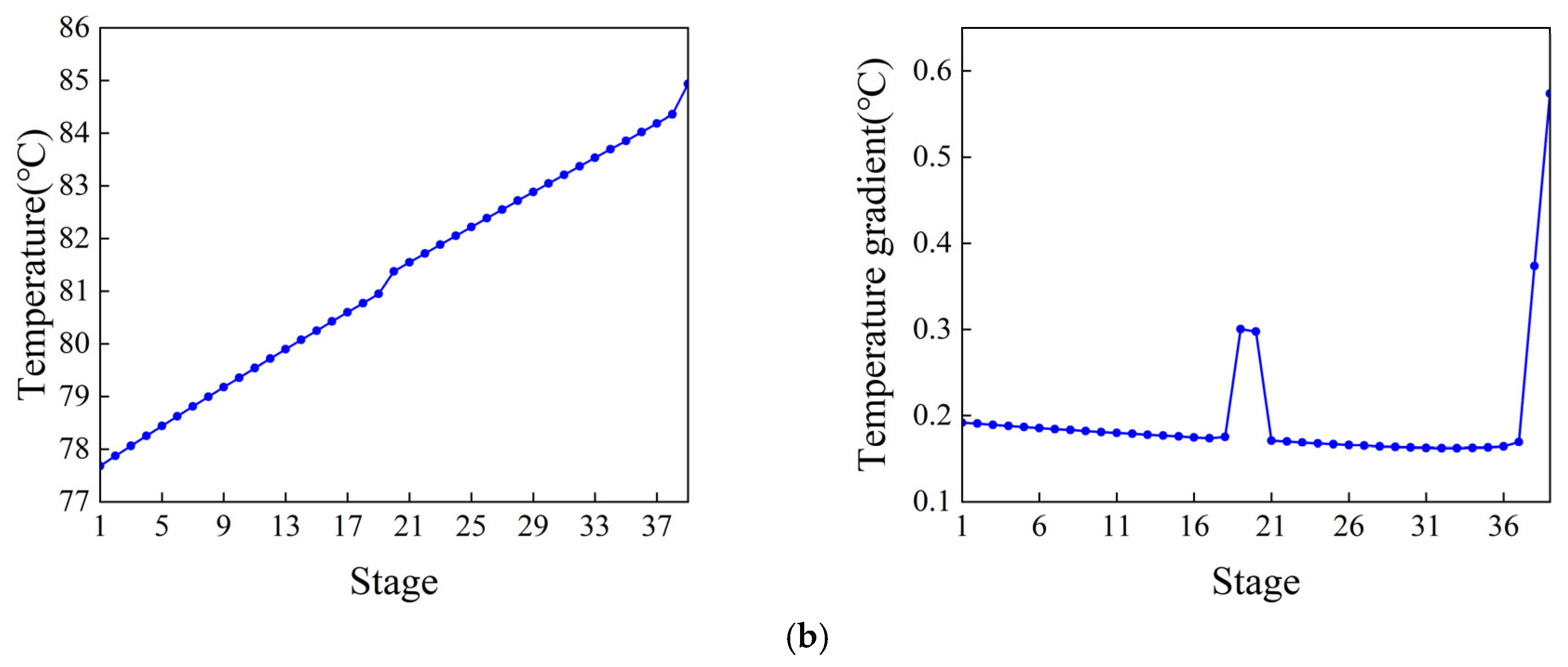
The control scheme for the ethylbenzene–styrene separation process is shown in Figure 4. This scheme encompasses flow control, pressure control, level control, and temperature control, all implemented using Proportional Integral Derivative (PID) controllers [34,35]. The feed flow rate and the reflux flow rate of the two columns are regulated according to specific requirements. The operating pressure is managed by adjusting the cooling medium flow rate in the first-stage condenser. The levels in both the reflux tank and the kettle are maintained by adjusting the product flow rate. Temperatures on the sensitivity plates are controlled by modulating the utility supplied to the reboiler. The slope criterion method is utilized to identify the temperature-sensitive plate [36], where the temperature control of the two columns is set. The tray temperature distribution across the two columns and the slope of each section are depicted in Figure 5. From the data, it is evident that the temperature-sensitive plates in the two columns are the 13th and the 39th plates, respectively. The gain constant (Kc) and integration time (τ1) of the temperature controllers can be calculated using the relay feedback test and the Tyreus–Luyben tuning rule [37]. Additionally, a retardation time of one minute has been incorporated into the temperature control loop to simulate the delays in measurement and execution typically encountered in the actual process [38,39]. The parameters for each controller [40] are presented in Table 1.
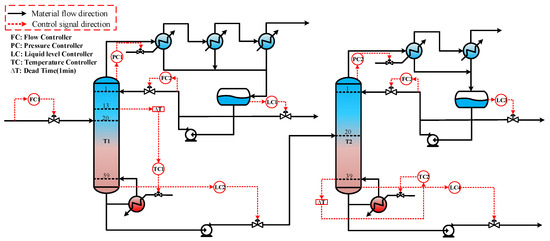
Figure 4.
Dynamic control structure of ethylbenzene–styrene separation process.
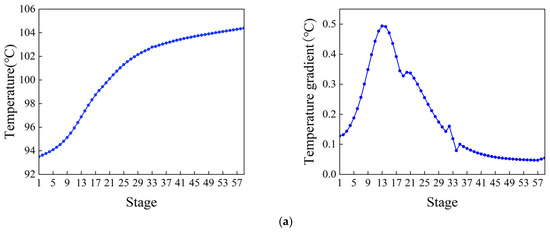
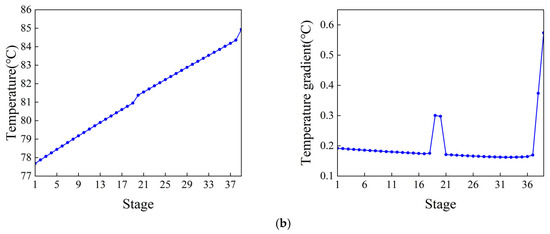
Figure 5.
Temperature distribution and the temperature gradient inside the column. (a) Ethylbenzene–styrene separation column T1. (b) Styrene refining column T2.

Table 1.
Control parameters for columns separating an ethylbenzene–styrene mixture.
3.2.2. Performance of Dynamic Control
The robustness of the dynamic control structure was evaluated by introducing ±10% disturbances in both feed flow and feed composition. Furthermore, ethylbenzene and styrene are the key components of the separation process. The feed composition with ±10% disturbances is detailed in Table 2.

Table 2.
Composition of feed with the addition of ±10% disturbances.
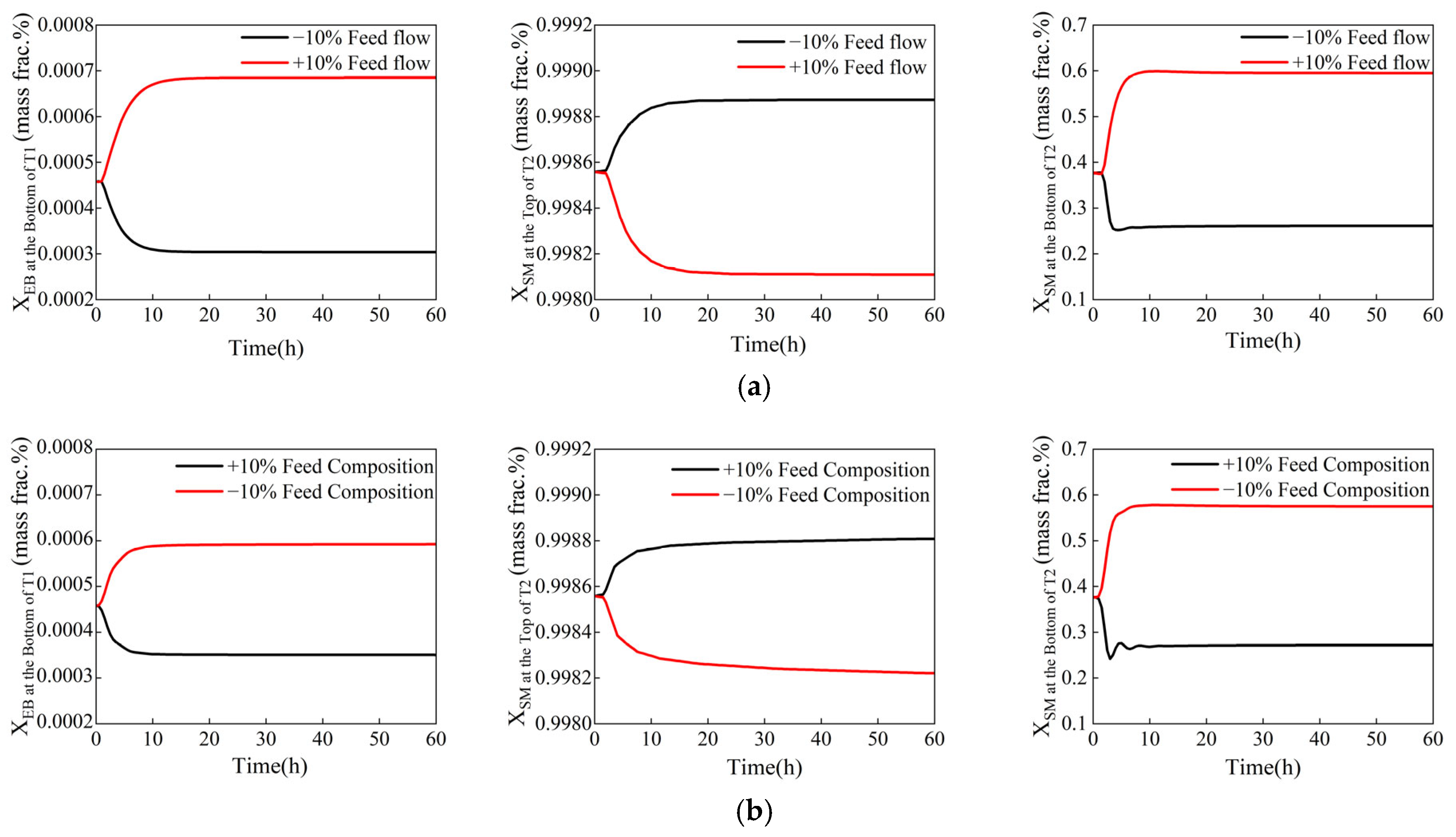
Figure 6 illustrates the system’s dynamic responses to the disturbance in the feed. When disturbances occur, the product compositions stabilize within 10 to 20 h. During this time, the purity of all key components meets the specified product standards. This indicates that the control system demonstrates a strong ability to resist disturbances in the feed.
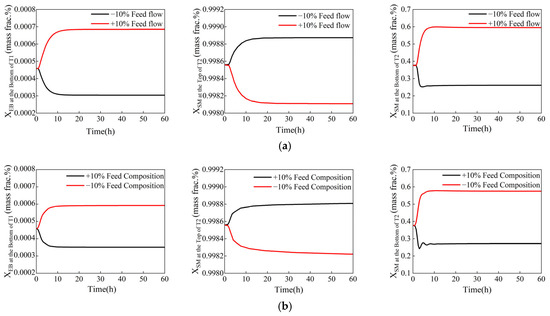
Figure 6.
Dynamic responses of the control components to feed disturbances (xB1, EB ≤ 0.08 wt%, xD2, SM ≥ 99.8 wt%, xB2, SM ≤ 50 wt%). (a) The ±10% disturbance in feed flow rate. (b) The ±10% disturbance in feed composition.
3.2.3. Dynamic Optimization Model
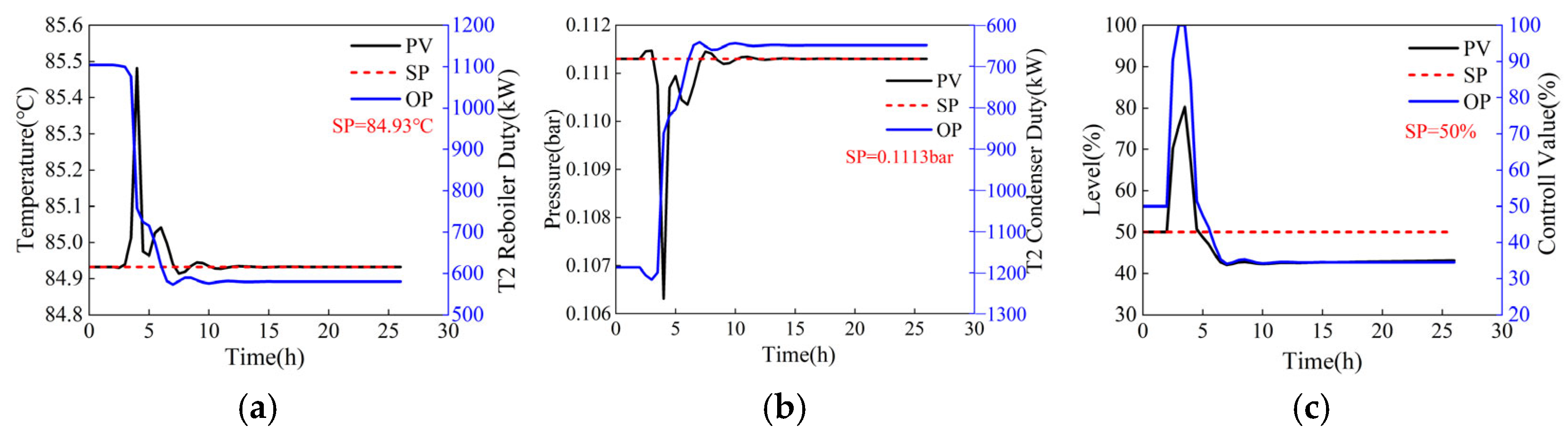
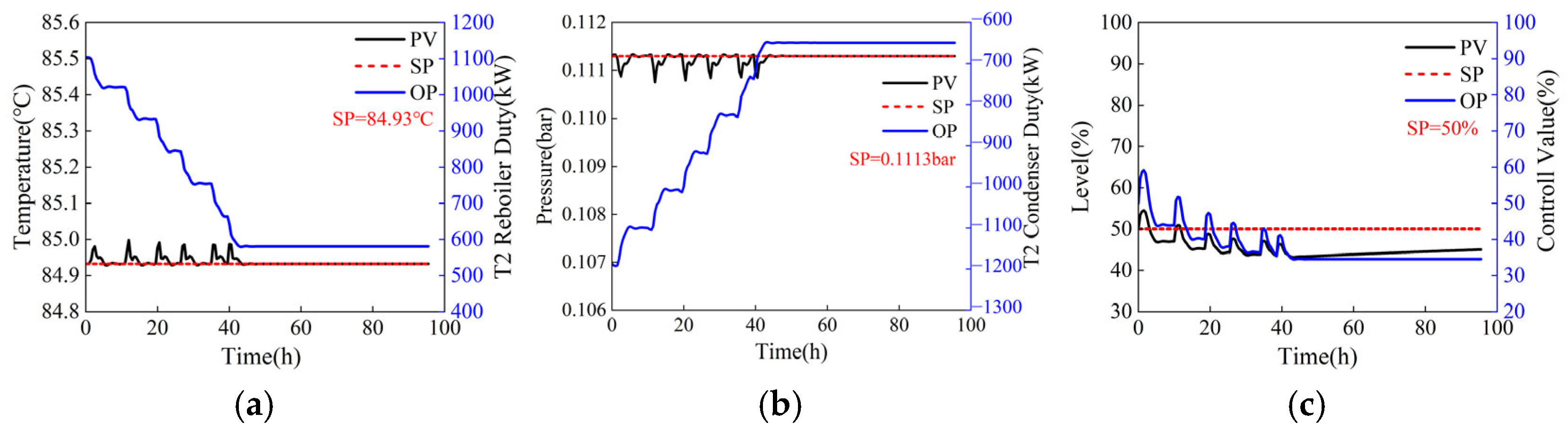
For a process operating under steady-state conditions, adjustments to operating parameters are equivalent to introducing a disturbance that leads to fluctuations in control variables, such as temperature, pressure, and liquid level. When operating parameters are adjusted directly to their target values (the reflux flow rates of the two columns step from 44,000 kg/h and 5769 kg/h to 41,152.2 kg/h and 1012.7 kg/h, with step sizes of 2847.8 kg/h and 4756.3 kg/h, respectively), the dynamic responses of the temperature-sensitive plate, operating pressure, and reflux tank level of T2 are illustrated in Figure 7. Although the Process Variable (PV) and Output (OP) can eventually stabilize near their setpoints (SP), this direct adjustment can cause significant fluctuations, as shown in Figure 7c, where the liquid level PV of the reflux tank steps from 50% to 80%, increasing the system safety risk coefficient. To ensure system safety, operating parameters should not be adjusted directly to the target values. Instead, they should be modified gradually through a series of minor adjustments. Under the step size adjustment mode (the reflux flow rates of the two columns adjusted in steps of 500 kg/h and 1000 kg/h, from 44,000 kg/h and 5769 kg/h to 41,152.2 kg/h and 1012.7 kg/h, respectively), the dynamic responses of the temperature-sensitive plate, operating pressure, and reflux tank level of column T2 are depicted in Figure 8. As shown in Figure 8, the time required for the step adjustment method to reach a stable state is longer. However, the fluctuation amplitude of PV has decreased significantly, thereby enhancing the system’s safety. Then, the adjusted step size can also be optimized to minimize the fluctuation amplitude of the control parameters and the time required to reach a stable state, thereby obtaining a fast and safe two-column reflux adjustment scheme.
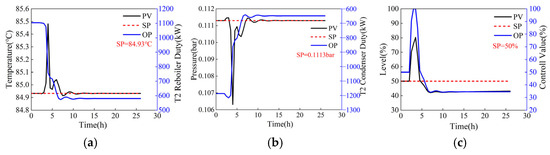
Figure 7.
Dynamic response of column T2 when operating parameters are adjusted directly (the reflux flow rates of the two columns step from 44,000 kg/h and 5769 kg/h to 41,152.2 kg/h and 1012.7 kg/h, with step sizes of 2847.8 kg/h and 4756.3 kg/h, respectively). (a) Sensitive plate temperature, (b) operating pressure, (c) liquid level of reflux tank.
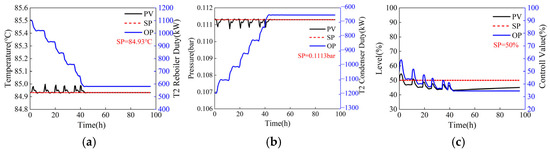
Figure 8.
Dynamic response of column T2 when the operating parameter is adjusted gradually (the reflux flow rates of the two columns adjusted in steps of 500 kg/h and 1000 kg/h, respectively, from 44,000 kg/h and 5769 kg/h to 41,152.2 kg/h and 1012.7 kg/h). (a) Sensitive plate temperature, (b) operating pressure, (c) liquid level of reflux tank.
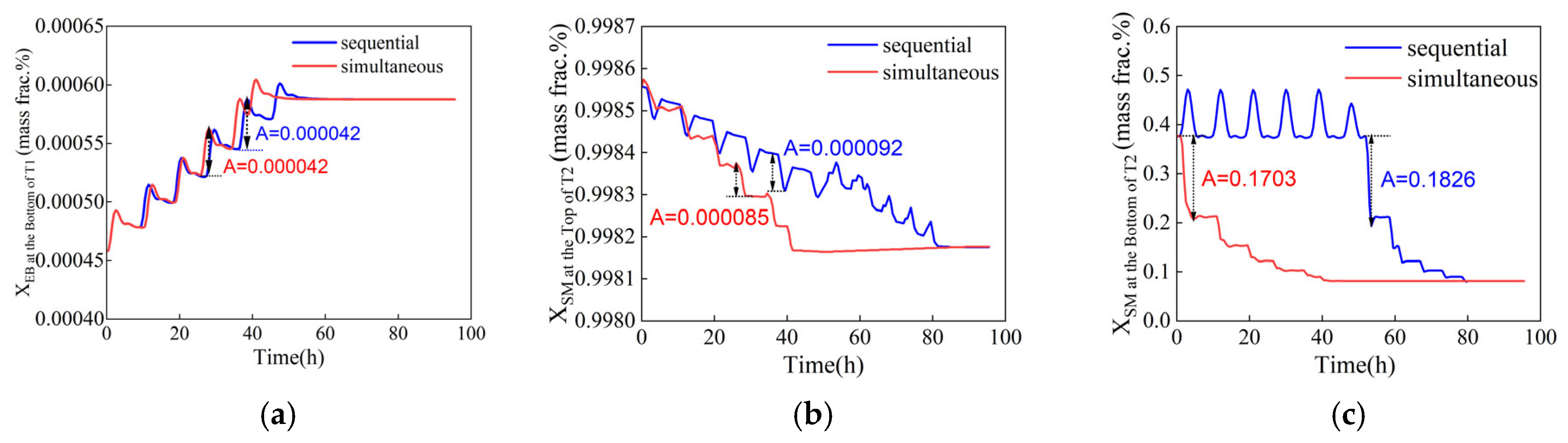
Reducing the duration of fluctuation can improve the stability and safety of chemical production, ultimately increasing economic benefits. The time required for two tandem column reflux flow rates to transition to their optimal values is optimized considering both the sequential and simultaneous adjustment modes, as well as the maximum fluctuation amplitude of control parameters. In both adjustment modes, the same step length is used, and the reflux rates for the two columns are adjusted as LT1 = [44,000, 43,500, 43,000, 42,500, 42,000, 41,500, 41,152.2] and LT2 = [5769, 4969, 4169, 3369, 2569, 1769, 1012.7], respectively. The dynamic response of the two adjustment modes is illustrated in Figure 9. It is evident that under the simultaneous adjustment mode, the system reaches a steady optimal operation status in a shorter time, with a reduced duration of fluctuations. In contrast, the sequential adjustment mode results in more frequent fluctuations in control parameters for column T2. This occurs because the sequential adjustments to the reflux flow rates of columns T1 and T2 introduce additional disturbances to column T2, thereby increasing the number of fluctuations and prolonging the time taken to reach a steady state. Moreover, the maximum fluctuation amplitude is smaller under the simultaneous adjustment mode, indicating its greater effectiveness. When both columns are adjusted simultaneously, the combined positive and negative effects help to stabilize column T2, reducing fluctuation amplitude and allowing the system to achieve a steady state more quickly. The control parameters in Figure 9b clearly illustrate this performance advantage. Therefore, the simultaneous adjustment mode, which requires less time and results in minimal fluctuations, will be adopted for the subsequent optimization.
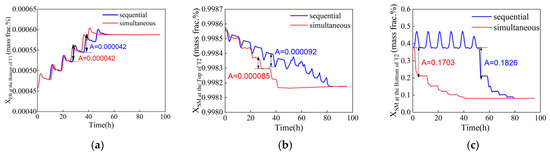
Figure 9.
Dynamic response of control components with sequential and simultaneous adjustments (xB1, EB ≤ 0.08 wt%, xD2, SM ≥ 99.8 wt%, xB2, SM ≤ 50 wt%). (a) Ethylbenzene mass fraction at the bottom of T1, (b) styrene mass fraction at the top of T2, (c) styrene mass fraction at the bottom of T2.
For columns T1 and T2, the differences between the original reflux flow rates and the optimal reflux flow rates are 2847.8 kg/h and 4756.3 kg/h, respectively. During the adjustment process, using a small step can reduce the fluctuation amplitude while increasing the number of adjustment steps and prolonging the time of fluctuation. Conversely, a large step reduces the number of adjustment steps and shortens the fluctuation time while also increasing the system’s fluctuation amplitude, which can challenge the controller. Therefore, the step size should be optimized to find a balance between the system’s fluctuation time, the number of adjustment steps, and the amplitude of fluctuations. The optimization of the operating parameter adjustments will be taken as a multi-objective optimization problem, considering the factors mentioned above. The optimization objectives are presented in Equation (10). The function and the maximum fluctuation amplitude are defined in Equations (11) and (12), respectively.
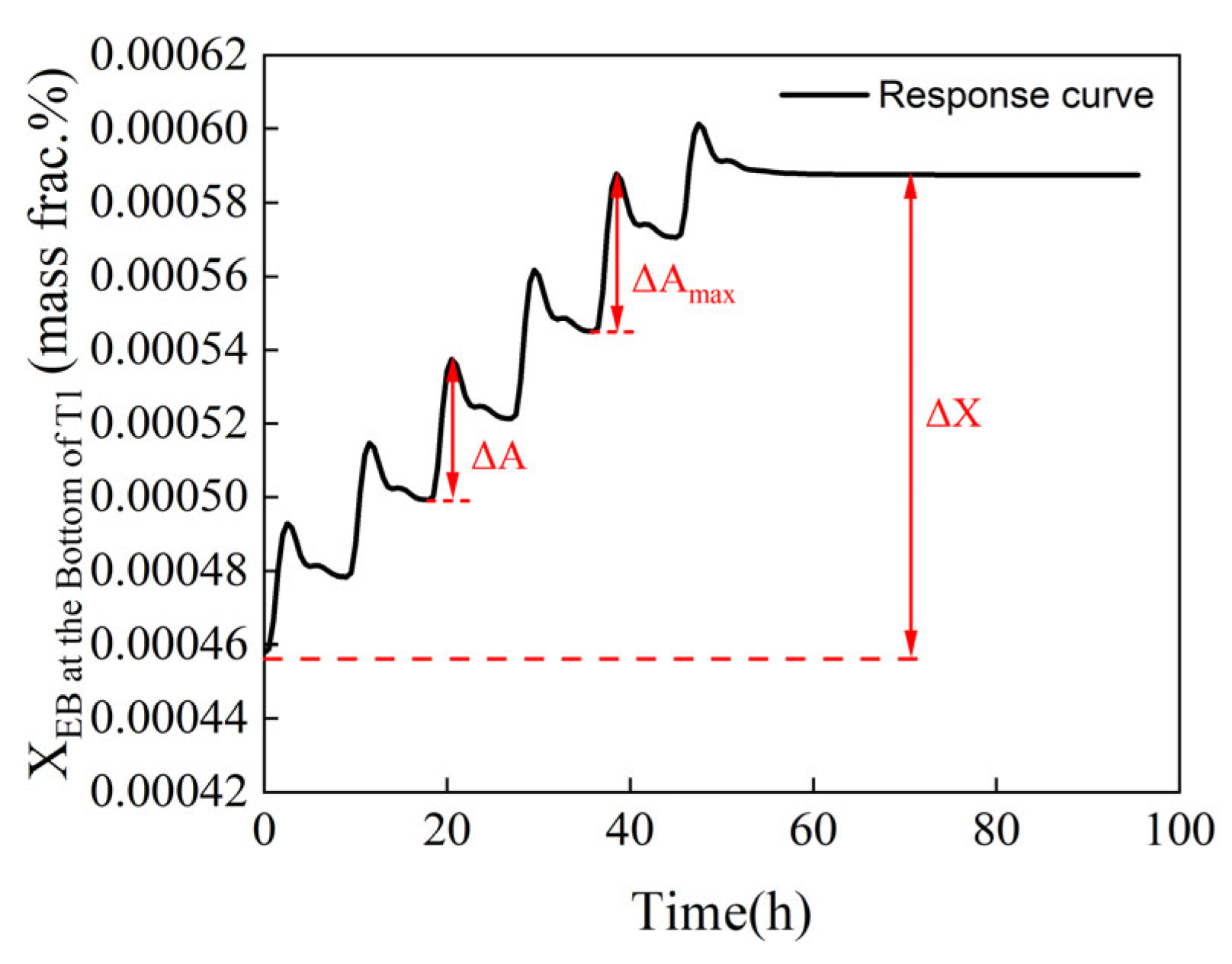
In Equation (10), S1 and S2 represent the step vectors of the reflux flow rates of columns T1 and T2, respectively; A, n, and t denote the maximum fluctuation amplitude, the number of adjustment steps, and the time of system fluctuation, respectively. ΔA indicates the difference between the maximum and minimum values of mass fraction in each adjustment step, reflecting the magnitude of fluctuation caused by each change; ΔX represents the variation in mass fraction when the reflux flowrate is adjusted from the initial value to the optimal value, as shown in Figure 10; and A(1) represents the maximum fluctuation amplitude of the mass fraction of ethylbenzene at the bottom of T1. A denotes the maximum value among the fluctuation amplitudes of the mass fraction of ethylbenzene at the bottom of column T1 and that of styrene at the top of column T2; a smaller value is preferable, indicating a better step size for the entire process.
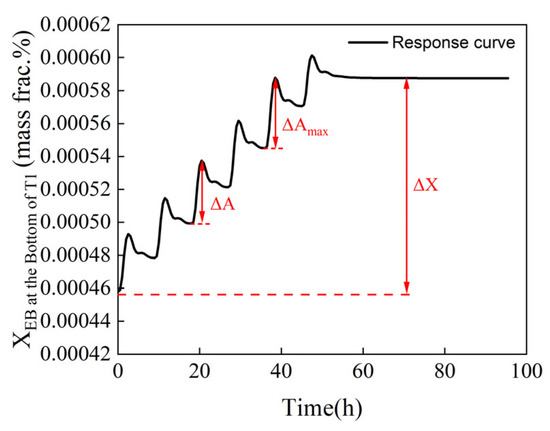
Figure 10.
Variation in mass fraction when the reflux flowrate is adjusted.
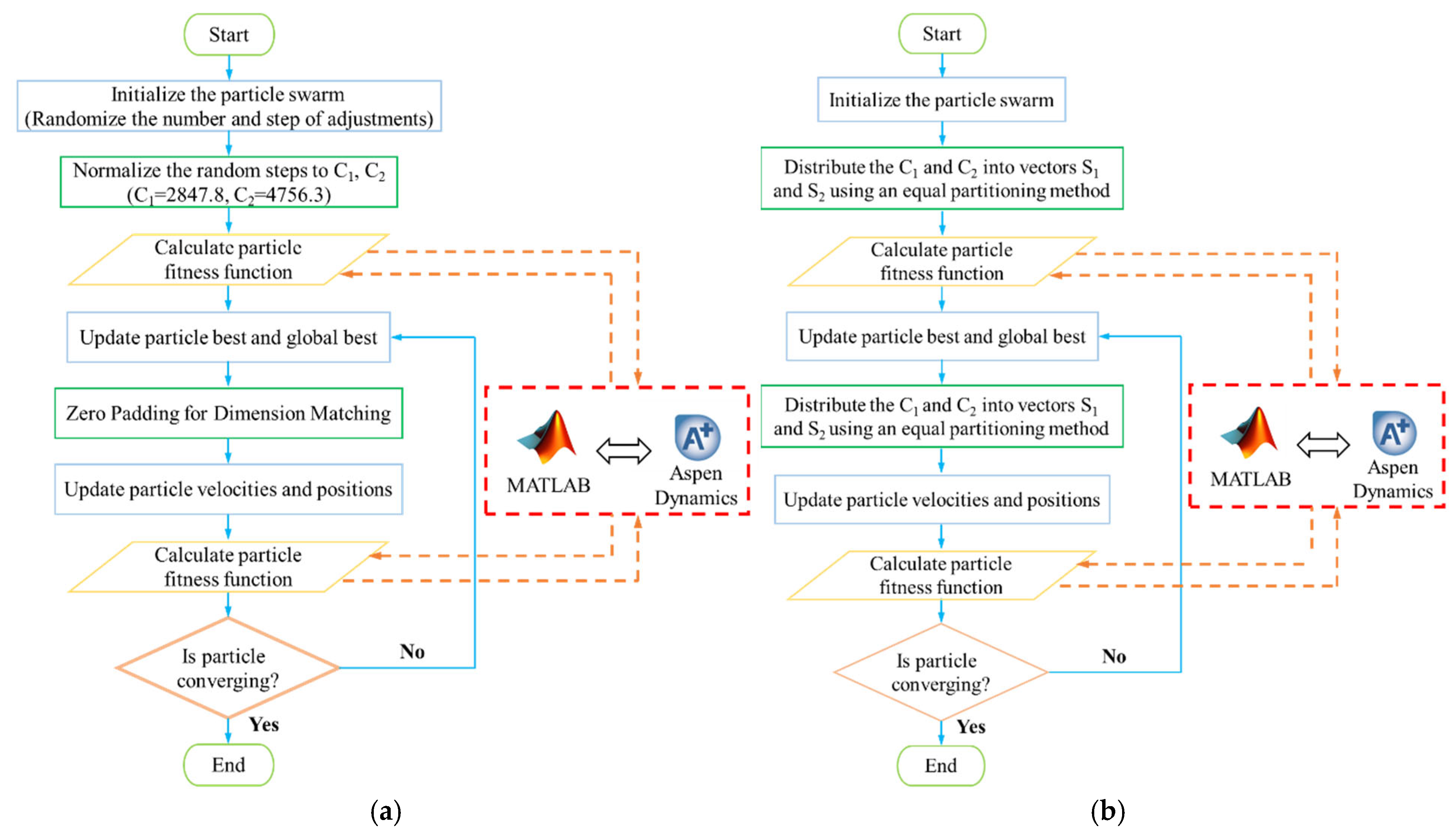
Dynamic optimization is a key aspect of the path planning problem, and the MOPSO algorithm is utilized to address this issue. The flowchart of the MOPSO algorithm is illustrated in Figure 11. The MOPSO algorithm can be implemented using either variable-step or equal-step patterns, with each requiring specific handling based on their characteristics. In variable-step optimization, the particle swarm is initialized by generating a set of random steps within a defined range. These steps are normalized to ensure that the sum of each set of steps corresponds to the variation in the reflux flow rate. Before updating the velocity and position of the particles, it is essential to note that the dimensions of the original velocity and position, as well as the best positions of the particles and the global best position, may differ. To address this, the zero-padding method is used to align the vectors with low dimensions dimensionally [41,42]. In the equal-step optimization, the variations in the reflux flow rates of columns T1 and T2, C1 and C2, need to be divided into vectors S1 and S2, respectively. The division is performed using the equal distribution method before each fitness calculation, ensuring that the sum of each vector equals C1 and C2, respectively. The entire optimization process is executed by coupling MATLAB R2024a with Aspen Plus Dynamics V14, enabling the generation of Pareto solution sets for both approaches.
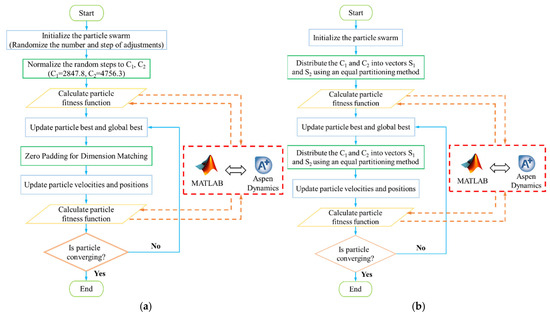
Figure 11.
Flowchart of MOPSO with step variation. (a) Variable-step optimization, (b) equal-step optimization.
4. Results and Discussion
4.1. Steady-State Optimization Results
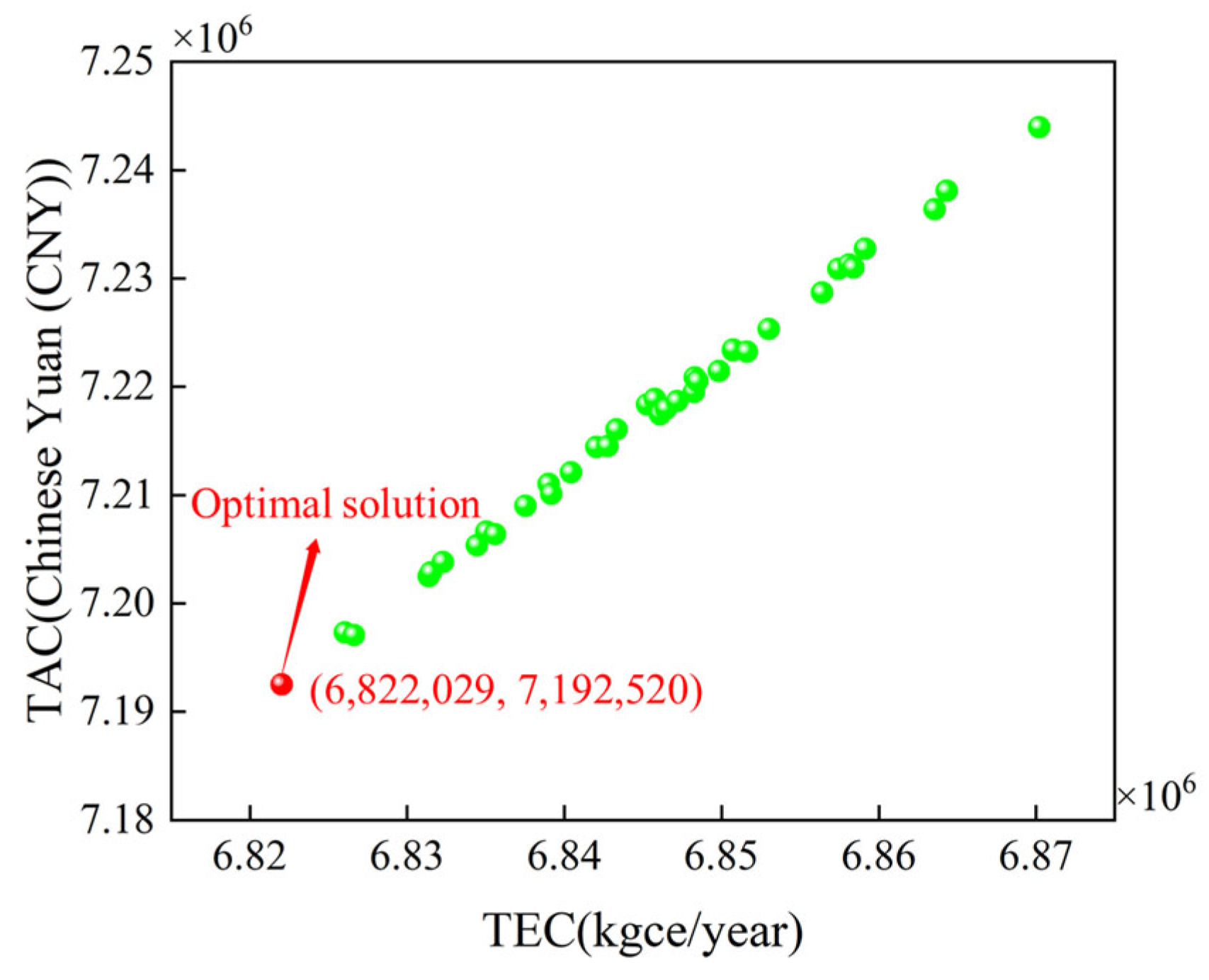
The reflux flow rates of columns T1 and T2 have been optimized using the MOPSO algorithm, with the final solution set illustrated in Figure 12. The steady-state optimization results indicate a trend in which both the TAC and TEC decrease gradually until they reach the optimal solution. This trend can be attributed to the interrelation between the two optimization objectives, as the TAC is calculated based on the TEC, resulting in their consistency. As shown in Figure 12, the TEC of the optimal solution is 6.822 million kgce per year, while the TAC is CNY 7.192 million. Compared to the original process, there is a reduction of 1.36 million kgce/year in TEC and CNY 1.51 million in TAC. Compared to the industry standard, energy consumption has decreased by 11.25%. Against the backdrop of increasingly strict global carbon emission control, the market competitiveness of ethylbenzene/styrene production has been significantly enhanced. The optimal reflux flow rates of columns T1 and T2 are 41,152.2 kg/h and 1012.7 kg/h, respectively, which will be taken as the target reflux flow rates for dynamic optimization.
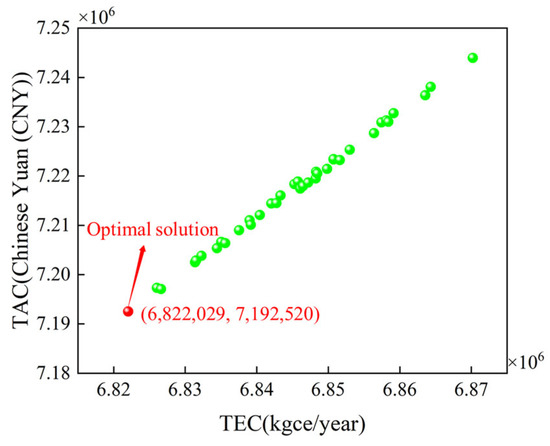
Figure 12.
Steady-state optimization results.
4.2. Dynamic Optimization Results
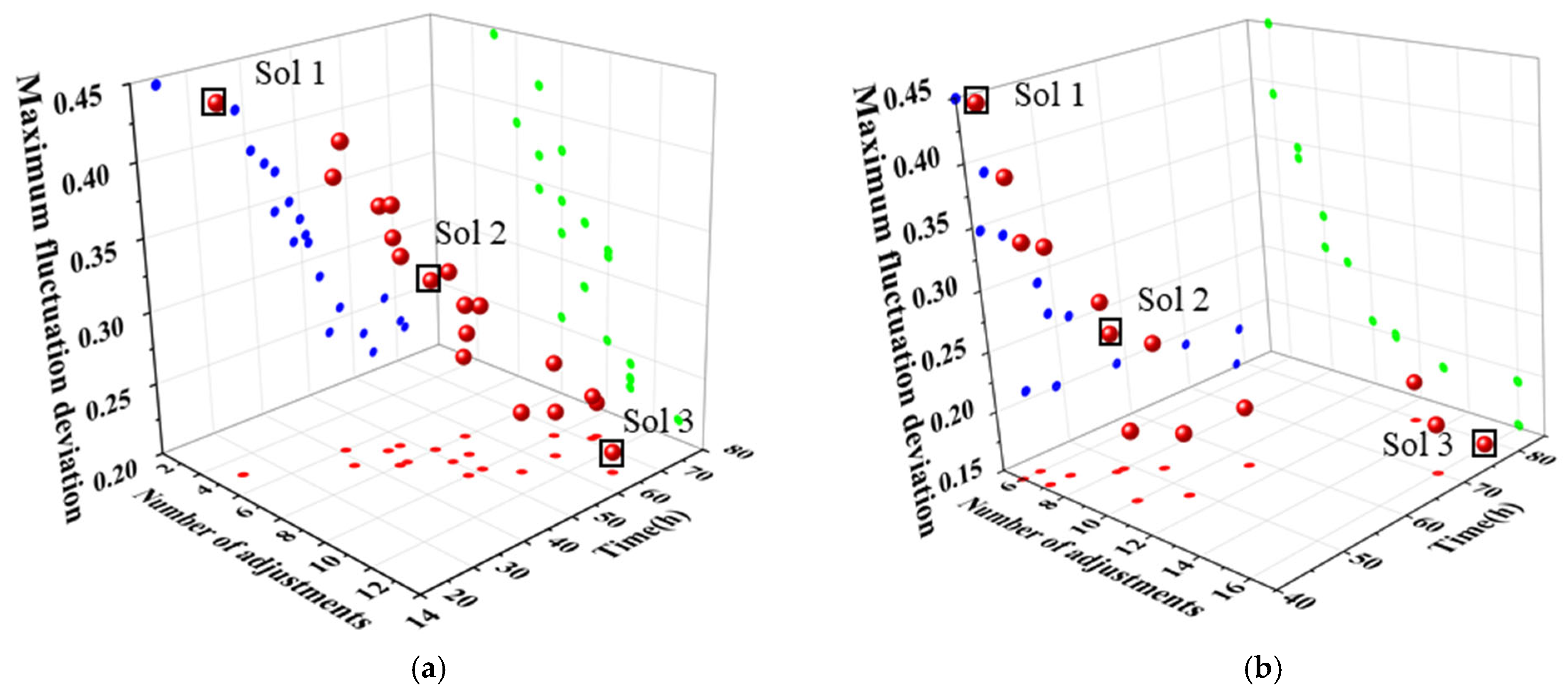
The Pareto solution sets obtained through variable-step and equal-step optimization using the MOPSO algorithm are illustrated in Figure 13a,b, respectively. The maximum fluctuation amplitude ranges from 0.15 to 0.45, indicating that both solution sets contain a broad range of distributions. Since all solutions are mutually independent and it is impossible to minimize all three objective functions simultaneously, a suitable solution can be selected based on specific requirements. In Figure 13a, solution 1 (Sol 1) undergoes 5 adjustment steps and requires 24.5 h to reach a steady state, yet exhibits a maximum fluctuation amplitude of 0.4454. This suggests that a large step length results in more significant fluctuations in the process. If rapid completion of the adjustment of operating parameters is essential, the step length corresponding to solution 1 may be appropriate. For example, when market demand for chemical products surges and a rapid load increase of distillation columns is required within the shortest time, Sol 1 can be selected to complete adjustments via large step sizes, addressing sudden market demand. In contrast, solution 3 (Sol 3) has a maximum fluctuation amplitude of 0.2142, requires 13 adjustment steps, and takes 64.5 h to reach a steady state. This suggests that smaller steps result in reduced fluctuations, although they also prolong the time required to achieve stability. If the goal is to ensure a smoother transition to a steady state, Solution 3 would be the better choice. For example, when high product purity is required, and the distillation process has an extremely low tolerance for parameter fluctuations, Sol 3 is suitable, as it controls fluctuations through small step sizes to meet high-purity production requirements. Solution 2 (Sol 2) represents a compromise, with eight adjustment steps, a maximum fluctuation amplitude of 0.3186, and a time to steady state of 51 h. For routine chemical production processes that require balancing economic efficiency and equipment reliability, the Sol 2 scheme is typically adopted. This solution balances the three objectives: system stability, economic efficiency, and control system capability. Similarly, the optimization results obtained with equal steps, depicted in Figure 13b, show a comparable pattern among the three solutions.
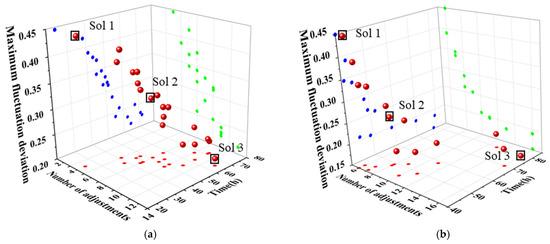
Figure 13.
Pareto solution set obtained by the MOPSO in dynamic variable-step and equal-step optimization. (a) Variable-step optimization, (b) equal-step optimization.
Among the two optimization methods, the dynamic response to the selected solutions is shown in Figure 14 and Figure 15. The corresponding steps for these solutions are detailed in Table 3. The total time to reach a steady state and the number of adjustment steps increase successively from Sol 1 to Sol 3, whereas the maximum fluctuation amplitude decreases in the same order. Table 3 also shows that the step lengths increase sequentially; larger step lengths lead to greater disturbances to the system, resulting in larger variation amplitudes. Consequently, the process associated with Sol 1 experiences a higher fluctuation amplitude. In contrast, when a smaller step length is used, the dynamic response of the system becomes more stable. The total time to reach a steady state is influenced by both the number of adjustment steps and the control system’s ability to resist disturbance. The control system being studied exhibits strong anti-interference capabilities concerning variation in the reflux flow rate. As a result, the time required for each adjustment to reach a steady state remains constant, regardless of whether the step length is large or small. Therefore, the total time taken for the system to reach a steady state is mainly determined by the number of adjustment steps. A small step length combined with a large number of adjustment steps will ultimately lead to a long total time.
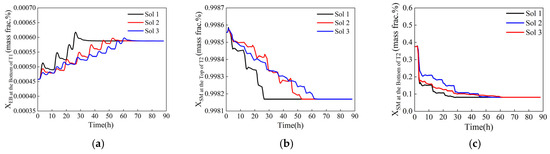
Figure 14.
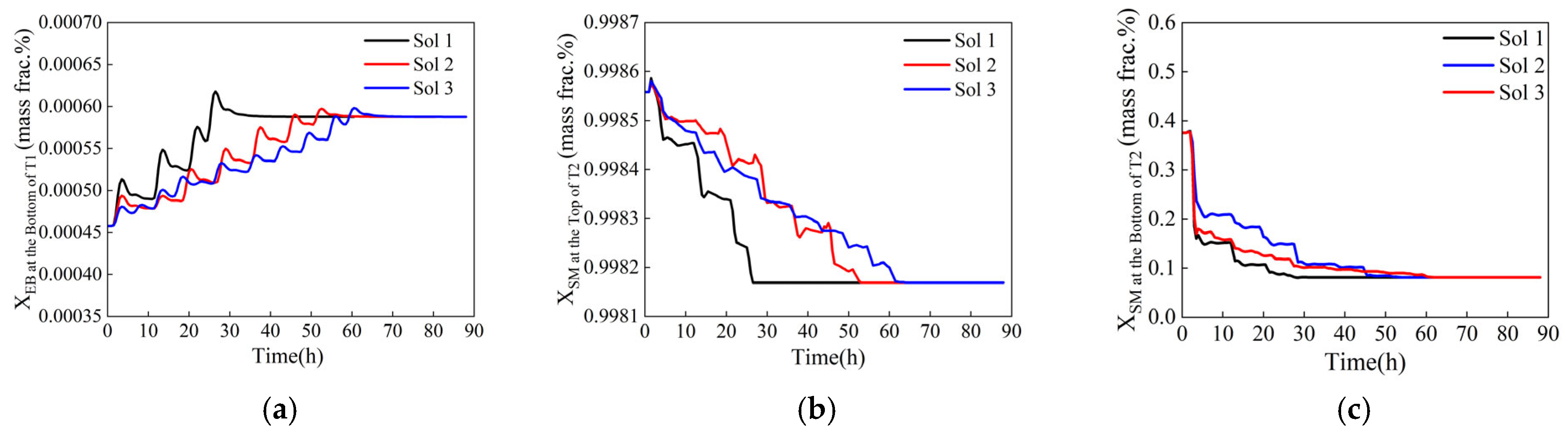
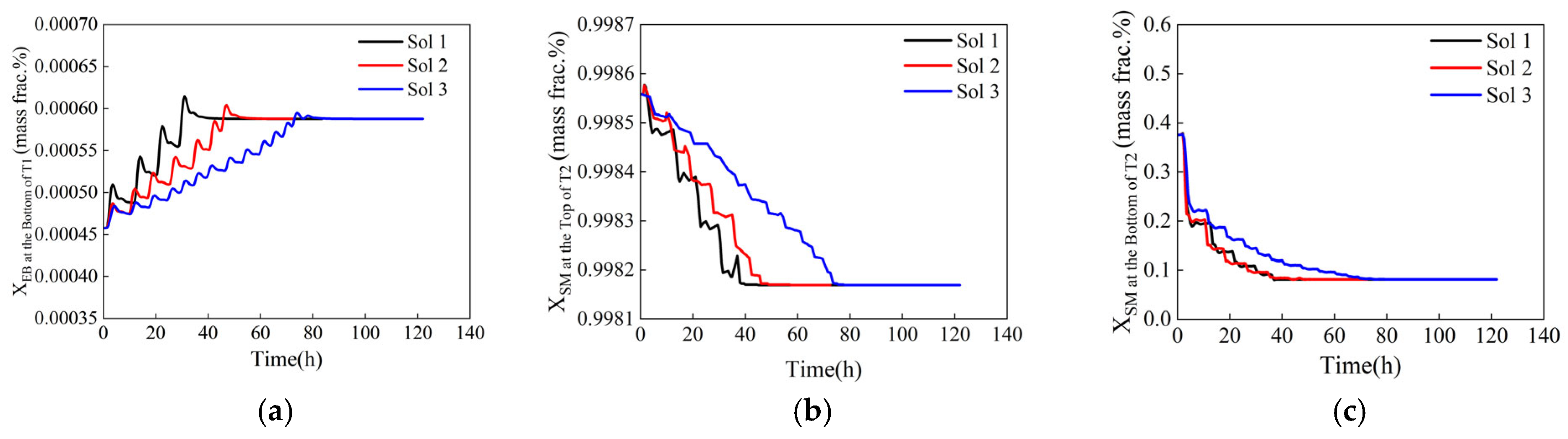
Dynamic response of control components during variable-step optimization. (a) Ethylbenzene mass fraction at the bottom of T1, (b) styrene mass fraction at the top of T2, (c) styrene mass fraction at the bottom of T2.
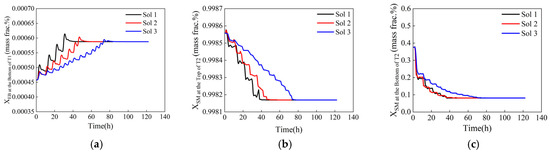
Figure 15.
Dynamic response of control components during equal-step optimization. (a) Ethylbenzene mass fraction at the bottom of T1, (b) styrene mass fraction at the top of T2, (c) styrene mass fraction at the bottom of T2.

Table 3.
The step vectors corresponding to different solutions (Sol1, Sol2, Sol3) during dynamic variable-step and equal-step optimization.
Compared to the equal-step variation, the total time required for the system to reach a steady state is shorter when the reflux flow rates are adjusted using variable-step lengths. This indicates that the variable-step adjustments can facilitate a more efficient transition to optimal operating conditions. However, they tend to exhibit greater fluctuations and relatively poorer stability. In contrast, the equal-step variation results in less fluctuation, a smoother response curve, and better stability. This might be because equal-step adjustments contribute to a more stable process, and the smoother dynamic response helps suppress system fluctuations. In summary, if the process requires efficient changes, variable-step adjustments are advisable, whereas if stability is a priority, equal-step adjustments should be preferred.
5. Conclusions
This study focused on optimizing two tandem columns for the separation of ethylbenzene and styrene, aiming to reduce fluctuation amplitudes while enhancing energy efficiency and ensuring stable operation. A steady-state model developed using Aspen Plus V14 and MATLAB R2024a can minimize the Total Energy Consumption (TEC) and Total Annual Cost (TAC) by optimizing the reflux flow rates. The developed dynamic optimization model, utilizing a MOPSO algorithm, can simulate the transition of operating conditions and identify the optimal strategy for adjusting operations to achieve the best performance, considering fluctuation amplitude, number of fluctuations, and fluctuation duration.
Steady-state optimization of the two-column system yielded optimal reflux rates of 41,152.2 kg/h and 1012.7 kg/h, reducing total energy consumption (TEC) to 6.822 million kgce/year and total annual cost (TAC) to CNY 7.192 million, representing 16.7% (1.36 million kgce/year) and 17.4% (CNY 1.51 million) savings, respectively, compared to the initial process. Energy consumption also decreased by 11.25% compared to industry standards, thereby reducing the process’s carbon emissions and enhancing its market competitiveness. Dynamic optimization via MOPSO revealed that direct transitions between operating conditions induce significant instability. In contrast, stepwise adjustments mitigate fluctuations, thereby reducing control system load and improving safety. Simultaneous tuning of the reflux rate for both columns dampens column downstream dynamic responses, minimizing overshoot and accelerating steady-state convergence. Larger step sizes reduce settling time but increase peak fluctuations, while smaller steps enhance stability. Variable-step strategies optimize transition efficiency, whereas equal-step variations produce smoother response profiles with superior overall stability. This structured approach to reflux adjustment provides a robust framework for balancing efficiency and stability in industrial distillation systems.
In this study, only the adjustment of reflux flow rates is considered; the operating pressure may also be adjusted in conjunction with the reflux. Additionally, other disturbances could arise, leading to deviations in practical applications. Future research should consider these disturbances and explore both variable-step and equal-step optimization methods to develop a more reliable and effective reflux flow step variation strategy.
Author Contributions
Conceptualization, G.J. and Y.S.; methodology, G.J. and Z.S.; software, G.J.; validation, Y.S. and L.Z.; writing—original draft preparation, G.J.; writing—review and editing, L.Z. and Z.S.; funding acquisition, G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (U24B6016) and Higher Education Institution Academic Discipline Innovation and Talent Introduction Plan (“111 Plan”) (No. B23025).
Data Availability Statement
The Data Availability Statement has been supplemented.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature and Abbreviations
| Nomenclature | |
| A | Maximum fluctuation amplitude |
| A(1), A(2) | The maximum fluctuation amplitude of the mass fraction of ethylbenzene at the bottom of T1 and T2 |
| cH2O | Specific heat capacity of water, kJ/(kg·°C) |
| CCW | Prices of cooling water, CNY/t |
| CRW | Prices of chilled water, CNY/t |
| Csteam | Prices of steam, CNY/t |
| gbest | Optimal position of the entire particle swarm |
| Kc | Gain constant |
| L1, L2 | The reflux rates of T1 and T2, kg/h |
| n | The number of adjustment steps |
| pbest | Optimal position of the particles |
| PP | Payback period, year |
| QCW | Duty of condensers cooled by cooling water, kW |
| QR | Reboiler duty, kW |
| QRW | Duty of condensers cooled by chilled water, kW |
| r | The latent heat of vaporization of water vapor, kJ/kg |
| S1, S2 | Step vectors of the reflux flow rates of columns T1 and T2 |
| t | The time of system fluctuation, h |
| αelec | Standard coal equivalent coefficient for electricity, kgce/(kWh) |
| αH2O | Standard coal equivalent coefficients for cooling water, kgce/t |
| αsteam | Standard coal equivalent coefficients for steam, kgce/t |
| ΔA | The difference between the maximum and minimum values of mass fraction in each adjustment step |
| ΔTCW | Difference between the supply and return temperatures of cooling water, °C |
| ΔTRW | Difference between the supply and return temperatures of chilled water, °C |
| ΔX | The variation in mass fraction when the reflux flow rate is adjusted from the initial value to the optimal value |
| τ1 | Integration time, min |
| Abbreviations | |
| ce | Coal equivalent |
| CAGR | Compound annual growth rate |
| CNY | Chinese Yuan |
| GA | Genetic algorithm |
| MINLP | Mixed integer nonlinear programming problem |
| MOPSO | Multi-objective particle swarm optimization |
| OP | Output |
| PID | Proportional integral derivative |
| PSO | Particle swarm optimization |
| PV | Process variable |
| SP | Setpoint |
| TAC | Total annualized cost |
| TCI | Total investment cost |
| TEC | Total energy consumption |
| TOC | Total operating cost |
References
- Research and Markets. Styrene Global Market Report 2023. Available online: https://www.researchandmarkets.com/reports/5733831/styrene-global-market-report (accessed on 14 January 2025).
- Zhu, X.; Gao, Y.; Wang, X.; Haribal, V.; Liu, J.; Neal, L.M.; Bao, Z.; Wu, Z.; Wang, H.; Li, F. A Tailored Multi-Functional Catalyst for Ultra-Efficient Styrene Production under a Cyclic Redox Scheme. Nat. Commun. 2021, 12, 1329. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, C.; Li, H.; Gao, X. Process Synthesis and Simulation-Based Optimization of Ethylbenzene/Styrene Separation Using Double-Effect Heat Integration and Self-Heat Recuperation Technology: A Techno-Economic Analysis. Sep. Purif. Technol. 2019, 228, 115760. [Google Scholar] [CrossRef]
- Jongmans, M.T.G.; Hermens, E.; Raijmakers, M.; Maassen, J.I.W.; Schuur, B.; De Haan, A.B. Conceptual Process Design of Extractive Distillation Processes for Ethylbenzene/Styrene Separation. Chem. Eng. Res. Des. 2012, 90, 2086–2100. [Google Scholar] [CrossRef]
- Muhammed, T. Optimized Energy Efficient Reactive Distillation for Octane Upgrading with Economic and Environmental Considerations. Fuel 2024, 378, 132994. [Google Scholar] [CrossRef]
- Amooey, A.A. Optimization of Operating Parameters for Furfuryl Alcohol Production in a Reactive Distillation Column Using Response Surface Methodology. Results Chem. 2024, 12, 101873. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, B.J.; He, C.; Chen, Q.L. Simultaneous Optimization of Solvent Composition and Operation Parameters for Sulfolane Aromatic Extractive Distillation Processes. In Proceedings of the 13th International Symposium on Process Systems Engineering—PSE 2018, San Diego, CA, USA, 1–5 July 2018; Eden, M.R., Ierapetritou, M., Towler, G.P., Eds.; Elsevier: San Diego, CA, USA, 2018; Volume 44, pp. 1111–1116. [Google Scholar]
- Yang, A.; Ernawati, L.; Wang, M.; Kong, Z.Y.; Sunarso, J.; Sun, S.; Shen, W. Multi-Objective Optimization of the Intensified Extractive Distillation with Side-Reboiler for the Recovery of Ethyl Acetate and Methanol from Wastewater. Sep. Purif. Technol. 2023, 310, 123131. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Shan, B.; Ma, Y.; Xu, Q.; Wang, Y.; Cui, P.; Zhang, F. Sustainable Process Design and Multi-Objective Optimization of Efficient and Energy-Saving Separation of Xylene Isomers via Extractive Distillation Based on Double Extractants. Sep. Purif. Technol. 2025, 354, 128899. [Google Scholar] [CrossRef]
- Chandra, P.; Mudgal, A.; Patel, J.; Patel, V.K. Thermo-Economical Modeling and Multi-Objective Optimization of Thermal Energy Driven Multiple Effect Distillation System for Water Treatment Using NSGA-II Algorithm. Desalination Water Treat. 2024, 320, 100646. [Google Scholar] [CrossRef]
- Su, X.-R.; Kusuma, A.A.N.A.N.; Gunawan, J.E.; Haidar, R.; Adhi, T.P.; Adi, V.S.K. Flexible Design and Optimization of Electronic-Grade Propylene Glycol Monomethyl Ether Acetate Production via Integrated Reactive and Pressure-Swing Distillation. Sep. Purif. Technol. 2025, 358, 130440. [Google Scholar] [CrossRef]
- Wang, K.; Xin, L.; Zhang, Y.; Qi, J.; Zhu, Z.; Wang, Y.; Zhong, L.; Cui, P. Sustainable and Efficient Process Design for Wastewater Recovery of Cyclohexane/Isopropyl Alcohol Azeotrope by Extractive Distillation Based on Multi-Objective Genetic Algorithm Optimization. Chem. Eng. Res. Des. 2024, 201, 593–602. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Sun, K.; Xu, Q.; Wang, Y.; Zhang, F.; Shan, B. Sustainable and Efficient-Saving Process Synthesis Design for Separating Methanol-Water-Toluene via Extractive Distillation Based on Multi-Objective Optimization. Process Saf. Environ. Prot. 2024, 190, 262–276. [Google Scholar] [CrossRef]
- Yin, T.; Zhang, Q.; Chen, Y.; Liu, C.; Xiang, W. Process Design and Optimization of the Reactive-Extractive Distillation Process Assisted with Reaction Heat Recovery via Side Vapor Recompression for the Separation of Water-Containing Ternary Azeotropic Mixture. Process Saf. Environ. Prot. 2024, 184, 1041–1056. [Google Scholar] [CrossRef]
- Murrieta-Dueñas, R.; Cortez-González, J.; Segovia-Hernández, J.G.; Hernández-Aguirre, A.; Gutiérrez-Guerra, R.; Hernández, S. A Comparative Analysis of Differential Evolution and Boltzmann-Based Distribution Algorithms with Constraint Handling Techniques for Distillation Process Optimization. Chem. Eng. Res. Des. 2025, 214, 39–53. [Google Scholar] [CrossRef]
- Leng, J.; Fan, S.; Dong, L.; Feng, Z. Design and Optimization of Energy-Saving Heterogeneous Azeotropic Distillation Processes for the Separation of Ternary Mixture of Ethyl Acetate/n-Propanol/Water. Sep. Purif. Technol. 2025, 359, 130537. [Google Scholar] [CrossRef]
- Li, M.; Peng, J.; Cheng, Y.; Zhang, Z.; Ma, Y.; Gao, J. Dual-Objective Optimization and Energy Efficiency Enhancement of Natural Decantation Assisted Extractive Distillation Process for n-Butanol/Isobutanol/Water Separation. Sep. Purif. Technol. 2024, 336, 126336. [Google Scholar] [CrossRef]
- Tian, X.; Wang, R.; Wang, H.; Li, C.; Liu, J. Energy-Saving Extractive Distillation Processes Design and Optimization for the Separation of Ethyl Acetate and n-Heptane Azeotrope. Fuel 2025, 379, 132974. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Y.; Li, S.; Huang, M. Mechanism-Embedded Neural Network Modeling and Operation Optimization of a Distillation Unit with Varying Production Performance. Chem. Eng. Res. Des. 2022, 183, 221–234. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Y.; Zou, Y.; Li, S. Enhancing Interactive Optimization with Operating Condition Supervision for Distillation Units. Control Eng. Pract. 2024, 148, 105942. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Liu, D. Dynamic Optimization for SP of Control Loops Using Adaptive APC Techniques. J. Taiwan Inst. Chem. Eng. 2025, 167, 105858. [Google Scholar] [CrossRef]
- Shin, Y.; Smith, R.; Hwang, S. Development of Model Predictive Control System Using an Artificial Neural Network: A Case Study with a Distillation Column. J. Clean. Prod. 2020, 277, 124124. [Google Scholar] [CrossRef]
- Huang, D.; Luo, X.-L. Process Transition Based on Dynamic Optimization with the Case of a Throughput-Fluctuating Ethylene Column. Ind. Eng. Chem. Res. 2018, 57, 6292–6302. [Google Scholar] [CrossRef]
- Zhang, Y.; Mo, Y. Dynamic Optimization of Chemical Processes Based on Modified Sailfish Optimizer Combined with an Equal Division Method. Processes 2021, 9, 1806. [Google Scholar] [CrossRef]
- Haider, P.; Freko, P.; Lochner, S.; Reiter, T.; Rehfeldt, S.; Klein, H. Design of a Test Rig for the Simulation of Startup Procedures in Main Heat Exchangers of Air Separation Plants. Chem. Eng. Res. Des. 2019, 147, 90–97. [Google Scholar] [CrossRef]
- Aucejo, A.; Loras, S.; Martínez-Soria, V.; Becht, N.; Del Río, G. Isobaric Vapor−Liquid Equilibria for the Binary Mixtures of Styrene with Ethylbenzene, o-Xylene, m-Xylene, and p-Xylene. J. Chem. Eng. Data 2006, 51, 1051–1055. [Google Scholar] [CrossRef]
- Song, Z.; Cui, W.; Wu, Y.; Wu, B.; Chen, K.; Ji, L. Energy, Exergy, Economic, and Environmental Analysis of a Novel Liquid-Only Transfer Dividing Wall Column with Vapor Recompression. Sep. Purif. Technol. 2024, 329, 125122. [Google Scholar] [CrossRef]
- Parhi, S.S.; Rangaiah, G.P.; Jana, A.K. Multi-Objective Optimization of Vapor Recompressed Distillation Column in Batch Processing: Improving Energy and Cost Savings. Appl. Therm. Eng. 2019, 150, 1273–1296. [Google Scholar] [CrossRef]
- Tang, Y.; Long, W.; Wang, Y.; Xiao, G.; Wang, Y.; Lu, M. Multi-Objective Optimization of Methanol Reforming Reactor Performance Based on Response Surface Methodology and Multi-Objective Particle Swarm Optimization Coupling Algorithm for on-Line Hydrogen Production. Energy Convers. Manag. 2024, 307, 118377. [Google Scholar] [CrossRef]
- Wang, D.; Tan, D.; Liu, L. Particle Swarm Optimization Algorithm: An Overview. Soft Comput. 2018, 22, 387–408. [Google Scholar] [CrossRef]
- Cui, Y.; Geng, Z.; Zhu, Q.; Han, Y. Review: Multi-Objective Optimization Methods and Application in Energy Saving. Energy 2017, 125, 681–704. [Google Scholar] [CrossRef]
- Geng, X.; Xu, D.; Hou, Z.; Li, H.; Gao, X. Novel Dynamic Control Structure of Reactive Distillation Process for Isopropanol Production via Transesterification. Chem. Eng. Res. Des. 2024, 205, 131–147. [Google Scholar] [CrossRef]
- Luyben, W.L. Principles and Case Studies of Simultaneous Design; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; ISBN 978-0-470-92708-3. [Google Scholar]
- Shan, B.; Niu, C.; Meng, D.; Zhao, Q.; Ma, Y.; Wang, Y.; Zhang, F.; Zhu, Z. Control of the Azeotropic Distillation Process for Separation of Acetonitrile and Water with and without Heat Integration. Chem. Eng. Process.—Process Intensif. 2021, 165, 108451. [Google Scholar] [CrossRef]
- Luyben, W.L. Control of a Three-Column Distillation Process for Separating Acetonitrile, Chloroform and Ethanol. Sep. Purif. Technol. 2025, 360, 131081. [Google Scholar] [CrossRef]
- Luyben, W.L. Distillation Design and Control Using AspenTM Simulation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; ISBN 978-1-118-41143-8. [Google Scholar]
- Luyben, W.L. Tuning Proportional−Integral−Derivative Controllers for Integrator/Deadtime Processes. Ind. Eng. Chem. Res. 1996, 35, 3480–3483. [Google Scholar] [CrossRef]
- Shan, B.; Sun, D.; Zheng, Q.; Zhang, F.; Wang, Y.; Zhu, Z. Dynamic Control of the Pressure-Swing Distillation Process for THF/Ethanol/Water Separation with and without Thermal Integration. Sep. Purif. Technol. 2021, 268, 118686. [Google Scholar] [CrossRef]
- Luyben, W.L. Design and Control of a Pressure-Swing Distillation Process with Vapor Recompression. Chem. Eng. Process.—Process Intensif. 2018, 123, 174–184. [Google Scholar] [CrossRef]
- Dai, Y.; Xu, Y.; Wang, S.; Li, S.; Wang, Y.; Gao, J. Dynamics of Hybrid Processes with Mixed Solvent for Recovering Propylene Glycol Methyl Ether from Wastewater with Different Control Structures. Sep. Purif. Technol. 2019, 229, 115815. [Google Scholar] [CrossRef]
- Bravo Sanchez, F.J.; English, N.B.; Hossain, M.R.; Moore, S.T. Improved Analysis of Deep Bioacoustic Embeddings through Dimensionality Reduction and Interactive Visualisation. Ecol. Inform. 2024, 81, 102593. [Google Scholar] [CrossRef]
- Xiong, H.; Song, J.; Liu, J.; Han, Y. Deep Transfer Learning-Based SSVEP Frequency Domain Decoding Method. Biomed. Signal Process. Control 2024, 89, 105931. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).