Use of Mixed Micelles in Micellar Electrokinetic Chromatography Method for Determination of Dexamethasone, Prednisolone and Triamcinolone in Pharmaceutical Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. The Reagents and Solutions
2.2. MEKC Conditions
2.3. Optimization of the BGE Composition
2.4. Sample Preparation
2.5. Method Validation
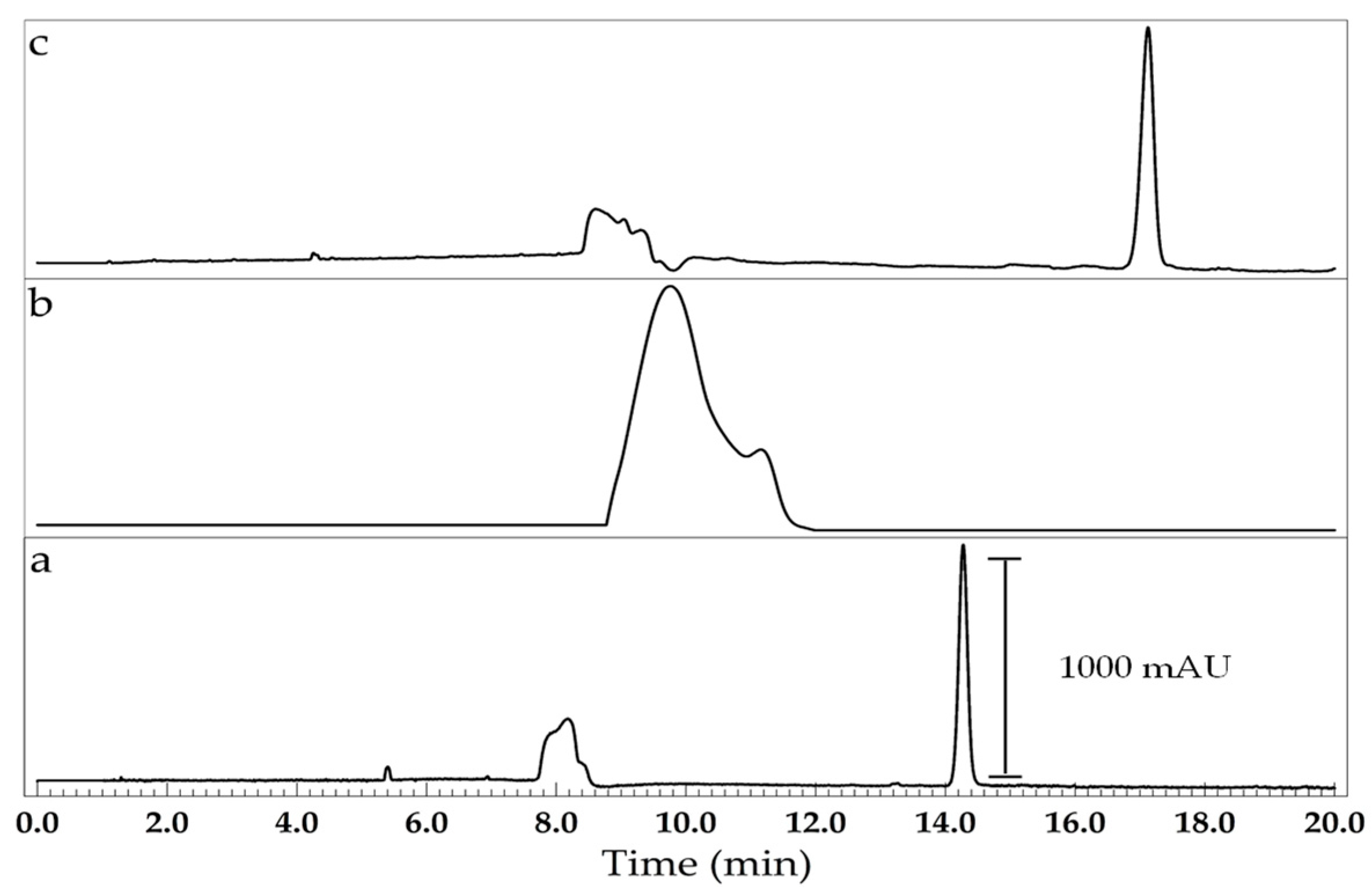
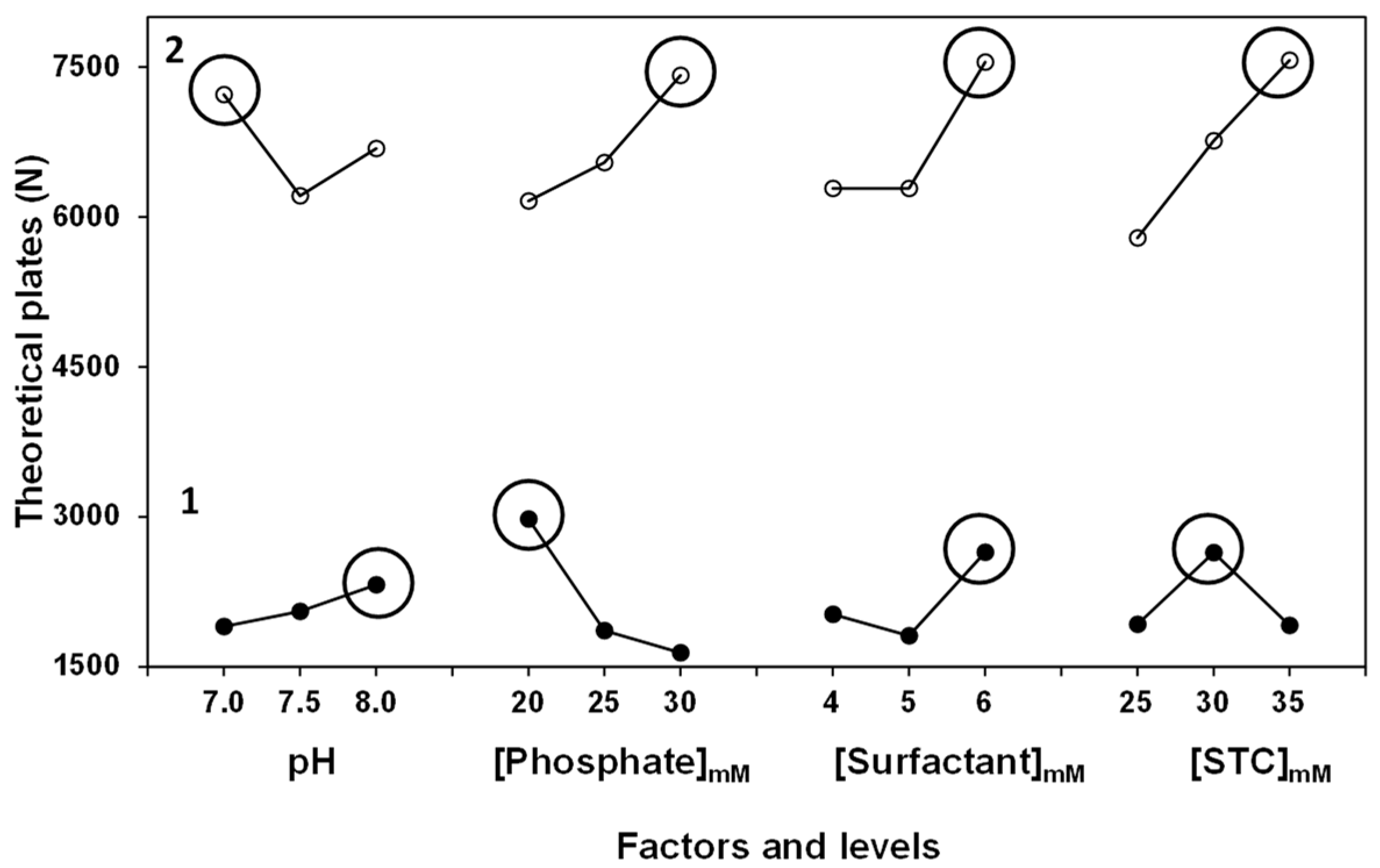
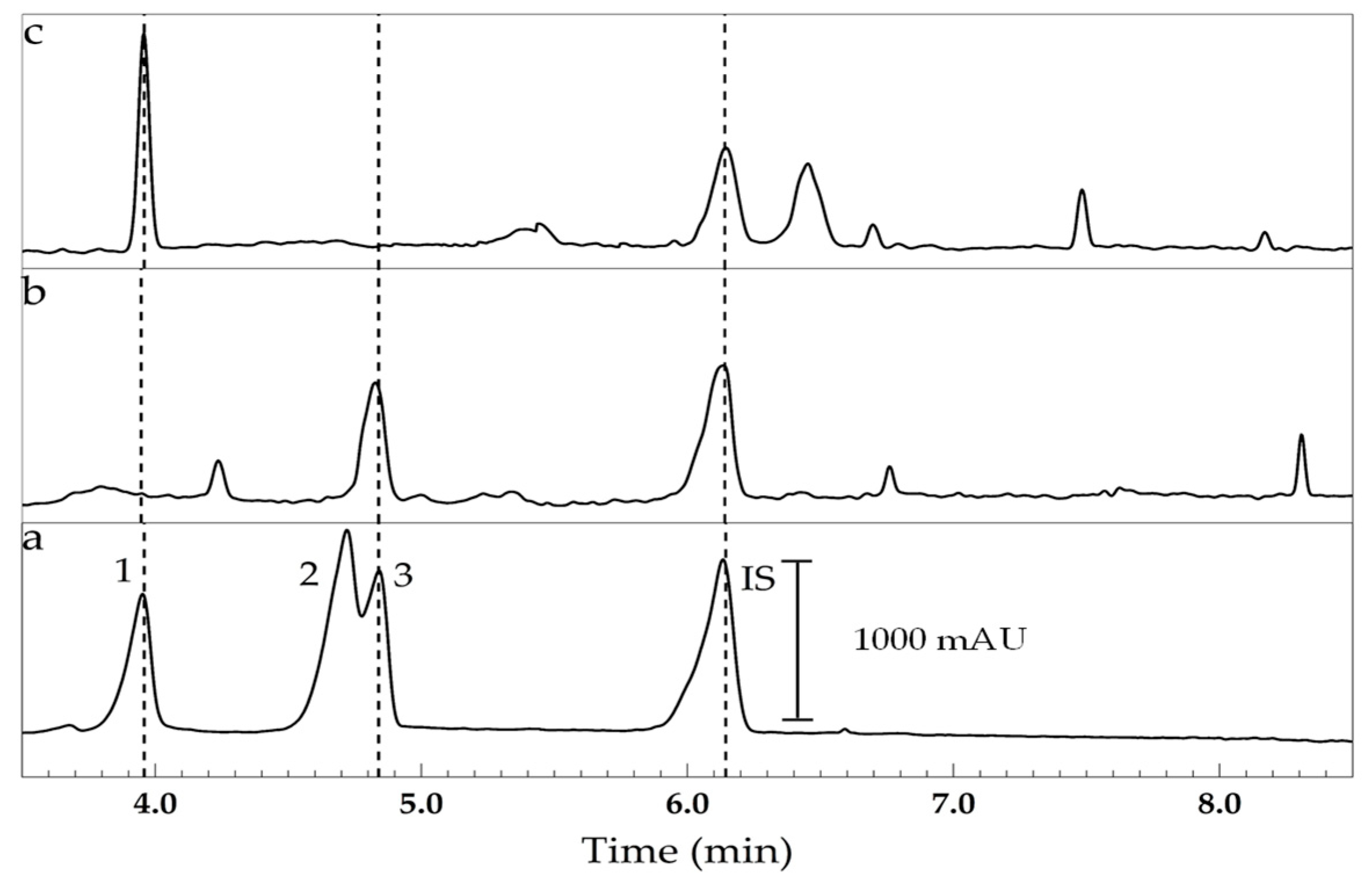
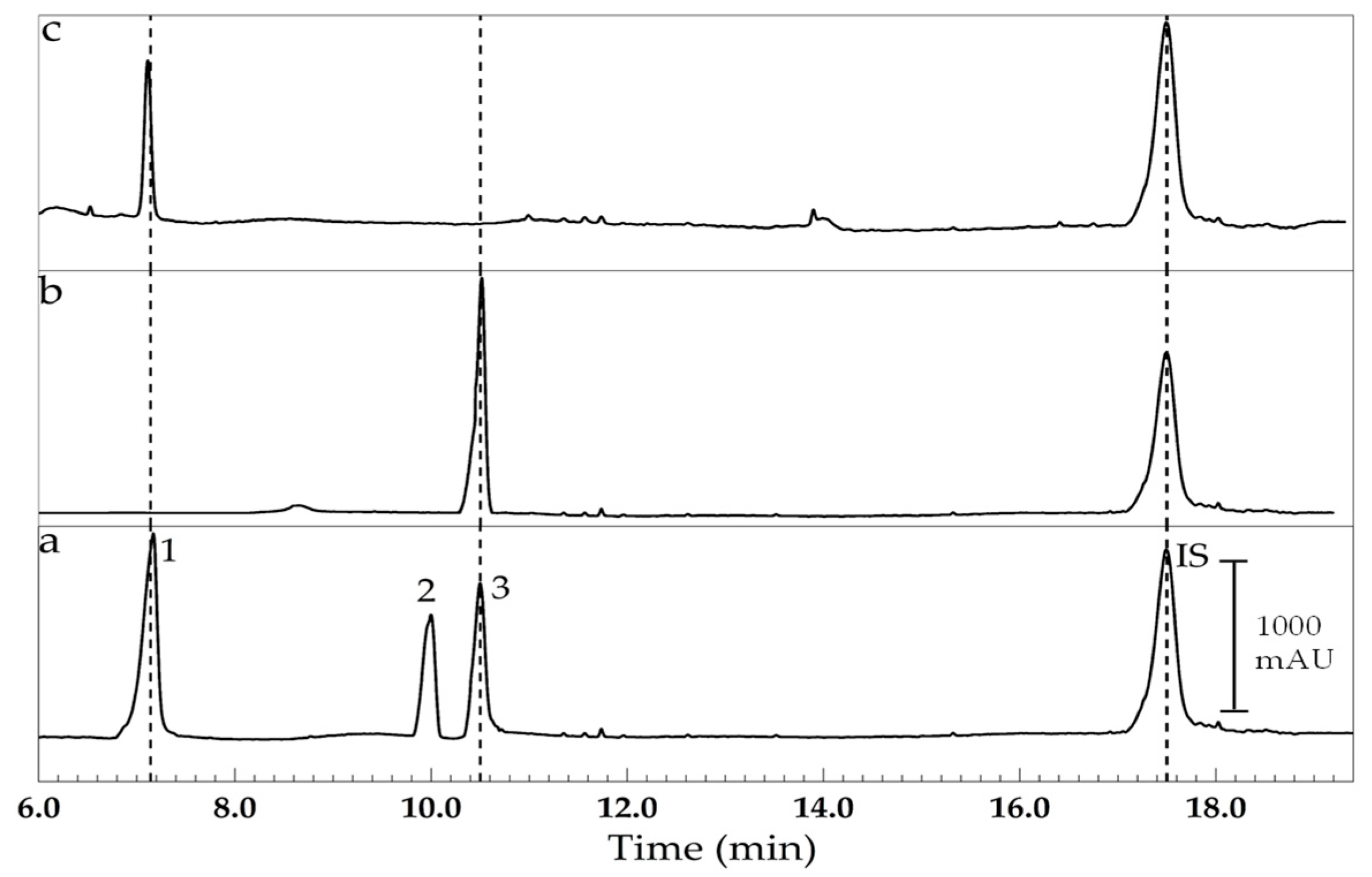
3. Results and Discussion
Optimization of the Background Electrolyte Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BGE | Background electrolyte |
| CE | Capillary electrophoresis |
| DEX | Dexamethasone |
| GC | Gas chromatography |
| HPLC | High-performance liquid chromatography |
| ICH | International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MEKC | Micellar electrokinetic chromatography |
| MEP | Methylprednisolone |
| N | Number of theoretical plates |
| PRE | Prednisolone |
| RSD | Relative standard deviation |
| SDS | Sodium dodecyl sulfate |
| SLES | Sodium lauryl ether sulfate |
| STC | Sodium taurocholate |
| TRI | Triamcinolone |
References
- Kapugi, M.; Cunningham, K. Corticosteroids. Orthop. Nurs. 2019, 38, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.; Gans, E.H. Topical corticosteroids, structure-activity and the glucocorticoid receptor: Discovery and development-A process of “planned serendipity”. J. Pharm. Sci. 2008, 97, 2936–2947. [Google Scholar] [CrossRef]
- Fiori, J.; Andrisano, V. LC-MS method for the simultaneous determination of six glucocorticoids in pharmaceutical formulations and counterfeit cosmetic products. J. Pharmaceut. Biomed. 2014, 91, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M. Clinical pharmacology of corticosteroids. Respir. Care 2018, 63, 655–670. [Google Scholar] [CrossRef]
- Yadav, J.P.; Lodhi, L.; Fatma, T.; Dey, K.K.; Ghosh, M. Investigation of the influence of various functional groups on the dynamics of glucocorticoids. ACS Omega 2022, 7, 43190–43209. [Google Scholar] [CrossRef] [PubMed]
- Gaballa, S.A.; Kompella, U.B.; Elgarhy, O.; Alqahtani, A.M.; Pierscionek, B.; Alany, R.G.; Abdelkader, H. Corticosteroids in ophthalmology: Drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv. Transl. Res. 2021, 11, 866–893. [Google Scholar] [CrossRef]
- Görög, S. Advances in the analysis of steroid hormone drugs in pharmaceuticals and environmental samples (2004–2010). J. Pharmaceut. Biomed. 2011, 55, 728–743. [Google Scholar] [CrossRef]
- Pujos, E.; Flament-Waton, M.M.; Paisse, O.; Grenier-Loustalot, M.F. Comparison of the analysis of corticosteroids using different techniques. Anal. Bioanal. Chem. 2005, 381, 244–254. [Google Scholar] [CrossRef]
- Amendola, L.; Garribba, F.; Botrè, F. Determination of endogenous and synthetic glucocorticoids in human urine by gas chromatography-mass spectrometry following microwave-assisted derivatization. Anal. Chim. Acta 2003, 489, 233–243. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Li, J.; Wu, Z.; Wang, F.; Liu, L.; Tan, X.; Lei, F. Selective separation and determination of glucocorticoids in cosmetics using dual-template magnetic molecularly imprinted polymers and HPLC. J. Colloid Interf. Sci. 2017, 504, 124–133. [Google Scholar] [CrossRef]
- Arif, S.; Ata, S. Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics. Open Chem. 2020, 18, 962–973. [Google Scholar] [CrossRef]
- Zivanovic, L.; Zecevic, M.; Markovic, S.; Petrovic, S.; Ivanovic, I. Validation of liquid chromatographic method for analysis of lidocaine hydrochloride, dexamethasone acetate, calcium dobesilate, buthylhydroxyanisol and degradation product hydroquinone in suppositories and ointment. J. Chromatogr. A 2005, 1088, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Smits, E.A.; Smits, C.J.; Vromans, H. The development of a method to quantify encapsulated and free prednisolone phosphate in liposomal formulations. J. Pharmaceut. Biomed. 2013, 75, 47–54. [Google Scholar] [CrossRef]
- Araujo, J.; Gonzalez-Mira, E.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int. J. Pharm. 2010, 393, 168–176. [Google Scholar] [CrossRef]
- Razzaq, S.N.; Khan, I.U.; Mariam, I.; Razzaq, S.S. Stability indicating HPLC method for the simultaneous determination of moxifloxacin and prednisolone in pharmaceutical formulations. Chem. Cent. J. 2012, 6, 1–10. [Google Scholar] [CrossRef]
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.C.; Santos, A.L.A.; Bonfilio, R.; de Araújo, M.B. A critical review of analytical methods in pharmaceutical matrices for determination of corticosteroids. Crit. Rev. Anal. Chem. 2020, 50, 111–124. [Google Scholar] [CrossRef]
- Flor, S.; Lucangioli, S.; Contin, M.; Tripodi, V. Simultaneous determination of nine endogenous steroids in human urine by polymeric-mixed micelle capillary electrophoresis. Electrophoresis 2010, 31, 3305–3313. [Google Scholar] [CrossRef]
- Sirén, H.; Seppänen-Laakso, T.; Orešič, M. Capillary electrophoresis with UV detection and mass spectrometry in method development for profiling metabolites of steroid hormone metabolism. J. Chromatogr. B 2008, 871, 375–382. [Google Scholar] [CrossRef]
- Kravchenko, A.V.; Kolobova, E.A.; Kartsova, L.A. Usage of 3-methyl-1-β-cyclodextrinimidazole tosylate for electrophoretic separation and preconcentration of corticosteroids by capillary electrophoresis. Monatsh. Chem. 2021, 152, 1067–1074. [Google Scholar] [CrossRef]
- Knox, C.; Wilson, M.; Klinger, C.M.; Franklin, M.; Oler, E.; Wilson, A.; Pon, A.; Cox, J.; Wishart, D.S. DrugBank 6.0: The DrugBank knowledgebase for 2024. Nucleic Acids Res. 2024, 52, D1265–D1275. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.A.; Do, D.P.; Saleh, A.M. Fundamentals of micellar electrokinetic chromatography (MEKC). Eur. J. Chem. 2011, 2, 276–281. [Google Scholar] [CrossRef]
- Xu, X.; Ni, X.; Cao, Y.; Zhuo, X.; Yang, X.; Cao, G. Amphiphilic polymeric micelle as pseudostationary phase in electrokinetic chromatography for analysis of eight corticosteroids in cosmetics. Electrophoresis 2014, 35, 827–835. [Google Scholar] [CrossRef]
- Wu, R.; Tian, M.; Shu, C.; Zhou, C.; Guan, W. Determination of the critical micelle concentration of surfactants using fluorescence strategies. Soft Matter 2022, 18, 8920–8930. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Wu, S.M. Micellar electrokinetic chromatography for simultaneous determination of six corticosteroids in commercial pharmaceuticals. J. Sep. Sci. 2005, 28, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Huang, Z.J.; Huang, X.G. Rapid determination of surfactant critical micelle concentration in aqueous solutions using fiber-optic refractive index sensing. Anal. Biochem. 2010, 401, 144–147. [Google Scholar] [CrossRef]
- Elmansi, H.; El-Awady, M.I.; Barghash, S.; El-Razeq, S.A.; Belal, F. Green versatile micellar electrokinetic chromatographic method for determination of six antimicrobial and anti-inflammatory drugs in combined dosage forms. Acta Chromatogr. 2023, 35, 233–246. [Google Scholar] [CrossRef]
- Essam, H.M.; Saad, M.N.; Elzanfaly, E.S.; Amer, S.M. Stepwise optimization and sensitivity improvement of green micellar electrokinetic chromatography method to simultaneously determine some fluoroquinolones and glucocorticoids present in various binary ophthalmic formulations. Biomed. Chromatogr. 2020, 34, e4941. [Google Scholar] [CrossRef]
- Iadarola, P.; Fumagalli, M.; Viglio, S. Micellar electrokinetic chromatography. In Analytical Separation Science, 1st ed.; Anderson, J.L., Berthod, A., Pino, V., Stalcup, A.M., Eds.; Wiley-VCH: Weinheim, Alemania, 2015; pp. 675–706. [Google Scholar]
- Bessonova, E.A.; Kartsova, L.A.; Gallyamova, V.F. Effect of 3-methyl-1-cetylimidazolium chloride ionic liquid on the electrophoretic preconcentration of steroid hormones. J. Anal. Chem. 2016, 71, 696–702. [Google Scholar] [CrossRef]
- Shakalisava, Y.; Regan, F. Determination of association constants of inclusion complexes of steroid hormones and cyclodextrins from their electrophoretic mobility. Electrophoresis 2006, 27, 3048–3056. [Google Scholar] [CrossRef]
- Marzullo, L.; Gotti, R.; Orlandini, S.; Slavíčková, P.; Jireš, J.; Zapadlo, M.; Dousa, M.; Nekvapilova, P.; Rezanka, P.; Furlanetto, S. Analytical quality by design-compliant development of a cyclodextrin-modified micellar electrokinetic chromatography method for the determination of trimecaine and its impurities. Molecules 2023, 28, 4747. [Google Scholar] [CrossRef] [PubMed]
- Kovač, I.; Jakl, M.; Šolínová, V.; Konášová, R.; Kašička, V.; Dytrtová, J.J. Micellar electrokinetic chromatography in the determination of triazoles in fruit peel. J. Chromatogr. A 2021, 1652, 462385. [Google Scholar] [CrossRef] [PubMed]
- Kartsova, L.A.; Bessonova, E.A. Determination of steroids in biological samples by micellar electrokinetic chromatography. J. Anal. Chem. 2007, 62, 68–75. [Google Scholar] [CrossRef]
- Noe, S.; Böhler, J.; Keller, E.; Frahm, A.W. Evaluation and optimisation of separation buffers for the determination of corticosteroids with micellar electrokinetic capillary chromatography (MECC). J. Pharmaceut. Biomed. 1998, 18, 911–918. [Google Scholar] [CrossRef]
- Holm, R.; Müllertz, A.; Mu, H. Bile salts and their importance for drug absorption. Int. J. Pharm. 2013, 453, 44–55. [Google Scholar] [CrossRef]
- Britz-McKibbin, P.; Ichihashi, T.; Tsubota, K.; Chen, D.D.; Terabe, S. Complementary on-line preconcentration strategies for steroids by capillary electrophoresis. J. Chromatogr A 2003, 1013, 65–76. [Google Scholar] [CrossRef]
- Farmacopea de los Estados Unidos Mexicanos; Undécima Edición; Secretaria de Salud, Comisión Permanente de la Farmacopea de los Estados Unidos Mexicanos: Ciudad de México, Mexico, 2014.
- ICH HARMONISED GUIDELINE. Validation of Analytical Procedures Q2 (R1). ICH: (2022). Geneva, Switzerland. Available online: https://database.ich.org/sites/default/files/ICH_Q2-R2_Document_Step2_Guideline_2022_0324.pdf (accessed on 17 February 2025).
- Jumppanen, J.H.; Wiedmer, S.K.; Sirén, H.; Riekkola, M.L.; Haario, H. Optimized separation of seven corticosteroids by micellar electrokinetic chromatography. Electrophoresis 1994, 15, 1267–1272. [Google Scholar] [CrossRef]
- Rupp, C.; Steckel, H.; Müller, B.W. Mixed micelle formation with phosphatidylcholines: The influence of surfactants with different molecule structures. Int. J. Pharmaceut. 2010, 387, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, H.; Sarkar, M. Optical spectroscopic and TEM studies of catanionic micelles of CTAB/SDS and their interaction with a NSAID. Langmuir 2004, 20, 3551–3558. [Google Scholar] [CrossRef]
- Olvera-Ureña, E.; Lopez-Tellez, J.; Vizueto, M.M.; Hidalgo-Ledezma, J.G.; Martinez-Quiroz, B.; Rodriguez, J.A. Lipase-assisted synthesis of alkyl stearates: Optimization by Taguchi design of experiments and application as defoamers. Molecules 2023, 29, 195. [Google Scholar] [CrossRef]
- Hillaert, S.; De Beer, T.R.M.; De Beer, J.O.; Van den Bossche, W. Optimization and validation of a micellar electrokinetic chromatographic method for the analysis of several angiotensin-II-receptor antagonists. J. Chromatogr. A 2003, 984, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Jimidar, M.I.; Van Ael, W.; Van Nyen, P.; Peeters, M.; Redlich, D.; De Smet, M. A screening strategy for the development of enantiomeric separation methods in capillary electrophoresis. Electrophoresis 2004, 25, 2772–2785. [Google Scholar] [CrossRef] [PubMed]
- Terabe, S. Selectivity manipulation in micellar electrokinetic chromatography. J. Pharmaceut. Biomed. 1992, 10, 705–715. [Google Scholar] [CrossRef]
- Maher, H.M.; Alzoman, N.Z.; Alshehri, M.M.; Aljohar, H.I.; Sultan, M.A. Microemulsion electrokinetic chromatography with polarity switching stacking mode for the determination of dexamethasone and dexamethasone sodium phosphate: Application to pharmacokinetic studies in rabbit plasma. Anal. Methods 2015, 7, 3260–3267. [Google Scholar] [CrossRef]
- Ćirin, D.M.; Poša, M.M.; Krstonošić, V.S. Interactions between selected bile salts and Triton X-100 or sodium lauryl ether sulfate. Chem. Cent. J. 2011, 5, 1–8. [Google Scholar] [CrossRef]
- Escamilla-Lara, K.A.; Ibarra, I.S.; Paez-Hernandez, M.E.; Gutierrez, E.; Rodriguez, J.A. Determination of prohibited corticosteroids, dexamethasone, prednisolone and triamcinolone in cosmetic creams by combination of dispersive solid phase extraction and high-performance liquid chromatography with diode array detection (HPLC-DAD). Anal. Lett. 2025, 1–16. [Google Scholar] [CrossRef]
- Hoeman, K.W.; Culbertson, C.T. A novel, environmentally friendly sodium lauryl ether sulfate-, cocamidopropyl betaine-, cocamide monoethanolamine-containing buffer for MEKC on microfluidic devices. Electrophoresis 2008, 29, 4900–4905. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- González, A.G.; Herrador, M.Á. A practical guide to analytical method validation, including measurement uncertainty and accuracy profiles. TrAC Trends Anal. Chem. 2007, 26, 227–238. [Google Scholar] [CrossRef]
- Gallego, J.L.; Arroyo, J.P. Determination of prednisolone acetate, sulfacetamide and phenylefrine in local pharmaceutical preparations by micellar electrokinetic chromatography. J. Pharm. Biomed. Anal. 2003, 31, 873–884. [Google Scholar] [CrossRef]
- Lemus Gallego, J.M.; Pérez Arroyo, J. Micellar electrokinetic capillary chromatography as an alternative method for determination of hydrocortisone and its most important associated compounds in local pharmaceutical preparations. Chromatographia 2002, 56, 455–462. [Google Scholar] [CrossRef]
| Factor | Level 1 | Level 2 | Level 3 |
|---|---|---|---|
| pH | 7.0 | 7.5 | 8.0 |
| [Phosphate] mM | 20.0 | 25.0 | 30.0 |
| [Surfactant] mM | 4.0 | 5.0 | 6.0 |
| [STC] mM | 25.0 | 30.0 | 35.0 |
| Sample | Description |
|---|---|
| 1 | Dexamethasone sodium phosphate (4000 mg L−1), injectable solution. |
| 2 | Prednisolone acetate (10,000 mg L−1), ophthalmic solution. |
| 3 | Dexamethasone sodium phosphate (500 mg kg−1) neomycin and phenylephrine, ophthalmic solution. |
| 4 | Triamcinolone acetonide (1000 mg kg−1) and nystatin, ointment. |
| 5 | Dexamethasone (1000 mg kg−1) and tobramycin sulfate, ointment. |
| Experiment | pH | [Phosphate]mM | [Surfactant]mM | [STC]mM | N 1 | N 2 |
|---|---|---|---|---|---|---|
| 1 | 7 | 20 | 4 | 25 | 2346 | 5337 |
| 2 | 7 | 25 | 5 | 30 | 1736 | 6697 |
| 3 | 7 | 30 | 6 | 35 | 1622 | 9638 |
| 4 | 7.5 | 20 | 5 | 35 | 3780 | 6105 |
| 5 | 7.5 | 25 | 6 | 25 | 1314 | 5977 |
| 6 | 7.5 | 30 | 4 | 30 | 591 | 6555 |
| 7 | 8 | 20 | 6 | 30 | 4160 | 7032 |
| 8 | 8 | 25 | 4 | 35 | 2531 | 6961 |
| 9 | 8 | 30 | 5 | 25 | 2405 | 6055 |
| Parameter | SDS-STC | SLES-STC | ||||
|---|---|---|---|---|---|---|
| TRI | PRE | DEX | TRI | PRE | DEX | |
| Coefficient of determination | 0.9912 | 0.9938 | 0.9934 | 0.9991 | 0.9995 | 0.9994 |
| Analytical sensitivity | 0.81 ± 0.14 | 1.01 ± 0.15 | 0.86 ± 0.13 | 0.96 ± 0.11 | 1.25 ± 0.16 | 0.97 ± 0.13 |
| Intercept | 0.07 ± 0.09 | 0.10 ± 0.13 | 0.12 ± 0.15 | 0.12 ± 0.15 | 0.10 ± 0.12 | 0.10 ± 0.11 |
| Limit of detection (LOD, mg L−1) | 1.13 | 0.95 | 0.98 | 0.35 | 0.26 | 0.28 |
| Limit of quantification (LOQ, mg L−1) | 3.43 | 2.89 | 2.98 | 1.05 | 0.78 | 0.87 |
| Linearity interval (mg L−1) | 3.43–10.00 | 2.89–10.00 | 2.98–10.00 | 1.05–10.00 | 0.78–10.00 | 0.87–10.00 |
| Parameter | SDS-STC | SLES-STC | ||||
|---|---|---|---|---|---|---|
| TRI | PRE | DEX | TRI | PRE | DEX | |
| Repeatability (%RSD, n = 3) | ||||||
| 5 (mg L−1) | 4.5 | 4.3 | 4.4 | 3.1 | 3.8 | 3.2 |
| 7 (mg L−1) | 3.1 | 3.1 | 3.0 | 3.0 | 3.3 | 3.1 |
| 9 (mg L−1) | 3.6 | 3.5 | 4.2 | 3.6 | 3.7 | 3.2 |
| Intermediate precision %RSD, n = 9) | ||||||
| 5 (mg L−1) | 4.9 | 4.4 | 4.9 | 4.1 | 4.4 | 4.2 |
| 7 (mg L−1) | 4.3 | 3.6 | 4.2 | 3.5 | 3.7 | 3.6 |
| 9 (mg L−1) | 4.6 | 3.8 | 4.5 | 3.7 | 3.8 | 3.7 |
| Sample | MEKC (SDS) | MEKC (SLES) | HPLC |
|---|---|---|---|
| 1 a | 3672.1 (3.7) | 3872.1 (2.3) | 3786.8 |
| 2 a | 8891.9 (3.8) | 9007.9 (1.5) | 9174.0 |
| 3 a | 482.5 (3.9) | 452.5 (2.1) | 448.0 |
| 4 b | 901.5 (3.1) | 907.5 (1.8) | 918.9 |
| 5 b | 931.9 (4.1) | 943.9 (0.9) | 4952.0 |
| Corticosteroid Analyzed | Sample | Electrolyte Composition | pH | Separation Voltage (kV) | Time of Analysis (min) | LOD (mg L−1) | LOQ (mg L−1) | REF |
|---|---|---|---|---|---|---|---|---|
| Prednisolone acetate | Ocular drops and ointments | 5.0 mM phosphate, 5.0 mM borate, 40.0 mM SDS | 8.2 | 30.0 | 10.0 | 0.34 | 1.21 | [53] |
| Hydrocortisone, hydrocortisone hemisuccinate and hydrocortisone acetate | Ocular solution, aerosol and tablet | 15.0 mM phosphate, 15.0 mM borate, 60.0 mM SDS and 10:1 (%, v/v) methanol/water | 8.2 | 25.0 | 25.0 | 0.12–0.50 | 0.41–1.60 | [54] |
| Dexamethasone or prednisolone | Ophthalmic solution | 20.0 mM borate and 50.0 mM SDS | 10.0 | 20.0 | 10.0 | 0.65–0.89 | 1.99–2.70 | [28] |
| Dexamethasone, prednisolone and triamcinolone | Injectable solution, drops solution, ointment | 30.0 mM phosphate, 30.0 mM SLES and 35.0 mM STC | 7.0 | 14.0 | 20.0 | 0.28–0.35 | 0.78–1.05 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escamilla-Lara, K.A.; Ibarra, I.S.; Lopez-Tellez, J.; Rodriguez, J.A. Use of Mixed Micelles in Micellar Electrokinetic Chromatography Method for Determination of Dexamethasone, Prednisolone and Triamcinolone in Pharmaceutical Formulations. Separations 2025, 12, 154. https://doi.org/10.3390/separations12060154
Escamilla-Lara KA, Ibarra IS, Lopez-Tellez J, Rodriguez JA. Use of Mixed Micelles in Micellar Electrokinetic Chromatography Method for Determination of Dexamethasone, Prednisolone and Triamcinolone in Pharmaceutical Formulations. Separations. 2025; 12(6):154. https://doi.org/10.3390/separations12060154
Chicago/Turabian StyleEscamilla-Lara, Karen A., Israel S. Ibarra, Jorge Lopez-Tellez, and Jose A. Rodriguez. 2025. "Use of Mixed Micelles in Micellar Electrokinetic Chromatography Method for Determination of Dexamethasone, Prednisolone and Triamcinolone in Pharmaceutical Formulations" Separations 12, no. 6: 154. https://doi.org/10.3390/separations12060154
APA StyleEscamilla-Lara, K. A., Ibarra, I. S., Lopez-Tellez, J., & Rodriguez, J. A. (2025). Use of Mixed Micelles in Micellar Electrokinetic Chromatography Method for Determination of Dexamethasone, Prednisolone and Triamcinolone in Pharmaceutical Formulations. Separations, 12(6), 154. https://doi.org/10.3390/separations12060154









