Abstract
The utilization of anion exchange membranes (AEMs) has revolutionized the field of electrochemical applications, particularly in water electrolysis and fuel cells. This review paper provides a comprehensive analysis of recent studies conducted on various commercial AEMs, including FAA3-50, Sustainion, Aemion™, XION Composite, and PiperION™ membranes, with a focus on their performance and durability in AEM water electrolysis (AEMWE) and AEM fuel cells (AEMFCs). The discussed studies highlight the exceptional potential of these membranes in achieving high current densities, stable operation, and extended durability. Furthermore, the integration of innovative catalysts, such as nitrogen-doped graphene and Raney nickel, has demonstrated significant improvements in performance. Additionally, the exploration of PGM-free catalysts, such as Ag/C, for AEMFC cathodes has unveiled promising prospects for cost-effective and sustainable fuel cell systems. Future research directions are identified, encompassing the optimization of membrane properties, investigation of alternative catalyst materials, and assessment of performance under diverse operating conditions. The findings underscore the versatility and suitability of these commercial AEMs in water electrolysis and fuel cell applications, paving the way for the advancement of efficient and environmentally benign energy technologies. This review paper serves as a valuable resource for researchers, engineers, and industry professionals seeking to enhance the performance and durability of AEMs in various electrochemical applications.
1. Introduction
Hydrogen has emerged as a highly promising alternative energy carrier for various applications in the domestic, industrial, and automotive sectors [1,2]. Its potential to revolutionize the world’s energy sector is comparable to the transformative impact of computers and the internet on modern information technology [3,4]. Hydrogen stands out due to its chemically lightweight nature and remarkable energy density, surpassing that of all commercially available energy sources, including fossil fuels, with a maximum value of 142 MJ/kg [5].
By harnessing the power of hydrogen, it becomes possible to mitigate the environmental challenges associated with the use of fossil fuels, notably CO2 emissions and other greenhouse gases. The transition to a hydrogen-based economy is supported by the Zero Emissions Scenario of the International Energy Agency (IEA), which forecasts 530 Mt of hydrogen demand in the transport, industry and energy sectors by 2050 [6]. The use of hydrogen in the energy sector is expected to witness significant growth, reaching 102 Mt by 2050, as it offers a suitable solution to balance intermittent generation from solar and wind sources while storing seasonal energy for future use [7]. Green hydrogen production and utilization play a vital role in the decarbonization efforts of key sectors, contributing to the collective goal of achieving net-zero CO2 emissions by 2050.
Alkaline water electrolysis (AWE), proton exchange membrane water electrolysis (PEMWE), and anion exchange membrane water electrolysis (AEMWE) are among the newest and most exciting hydrogen production and utilization technologies suggested by the International Renewable Energy Agency (IRENA) [8]. Each type of hydrogen technology has its distinct characteristic advantages and disadvantages. AEMWE is an emerging technology that offers significant advantages over other hydrogen technologies. While AWE has a long history and benefits from inexpensive electrode materials, it is limited by a corrosive electrolyte and operational capacity constraints [9,10,11]. PEMWE addresses some of these challenges, but it introduces limitations such as a harsh acidic environment and high material costs due to the use of noble-metal catalysts such as IrO2 and Pt [12,13,14,15,16]. On the other hand, AEMWE combines the advantages of AWE and PEM by utilizing a dense polymeric anion exchange membrane while maintaining an alkaline environment [17,18,19]. This enables the use of low-cost and abundant materials, moderate temperatures, and membrane separation. AEM shows promise in overcoming the drawbacks associated with other hydrogen production technologies, making it a compelling solution for efficient and sustainable hydrogen production [20]. The emergence of AEM as a hydrogen technology offers significant advantages over other traditional methods, such as AWE and PEM, making it a promising solution for efficient and sustainable hydrogen technology.
The development of commercial anion exchange membranes (AEMs) has made significant progress in recent years, particularly in the applications of alkaline fuel cells with exchange membranes (AEMFC) and AEMWE. These advancements have greatly influenced environmentally benign options for hydrogen production and OH− transport [21,22]. Both fuel cells (FCs) and water electrolyzers (WEs) rely on the efficient conversion and storage of hydrogen [23]. The transport of OH− ions plays a crucial role in facilitating the electrochemical reactions required for the operation of both FCs and WEs. This similarity in criterion highlights the close relationship between FCs and WEs, emphasizing the need to consider similar criteria for both systems. Therefore, it is essential to gain a comprehensive understanding of the criteria for FCs and WEs, with a specific focus on hydrogen production, power generation, and OH− transport.
Despite extensive research on PEMFC, their need for rare metal catalysts and acid-resistant components has motivated researchers to develop simpler and more accessible alternatives, such as AEMFC [24,25]. The anionic environment of fuel cells has made it feasible to utilize catalysts bereft of precious metals, decreasing expenses. Moreover, these alkaline exchange films have augmented the pliability of the fuel for the cells, empowering them to function on hydrogen, alcohol, or even solid fuels [26]. Furthermore, they have considerably decreased gas seepage velocities, culminating in superior competence and security.
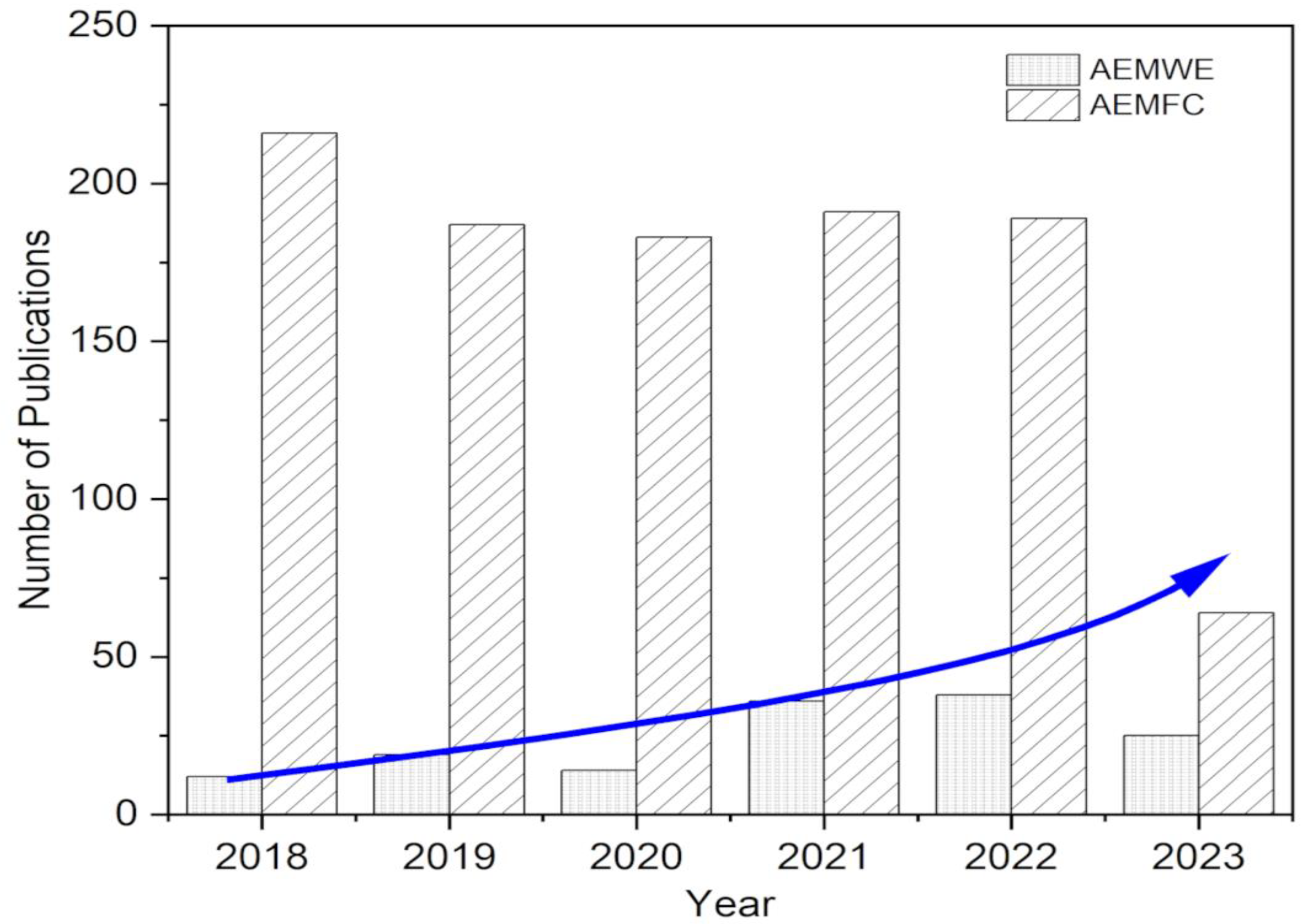
New means of progress in AEMWE science have also changed the way water is split into hydrogen and oxygen [27,28]. Marketable positively charged membrane layers in alkaline electrolyzers have enabled working in basic surroundings, thus unlocking the potential for less expensive, commonly found materials to be utilized as accelerants. This has rendered mass hydrogen generation more practical and affordable, assisting the shift to an energy system powered by hydrogen. The number of articles published has increased steadily, notably in this last half decade, in line with the growth in research focused on AEMFC and AEMWE, as shown in Figure 1 (AEMFC: keywords: anion exchange membrane, fuel cell, polymer; AEMWE: keywords: anion exchange membrane, water electrolysis, polymer). These trends were identified using the Web of Science database, accessed on 11 May 2023.
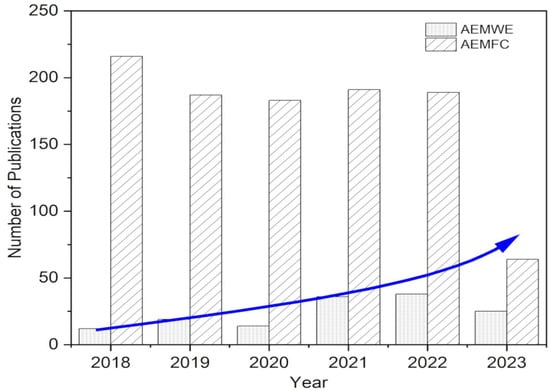
Figure 1.
Various research publications from 2017 to 2023 toward AEM fuel cells and electrolyzers.
It appears that while AEMFC has stagnated in recent years, there has been tremendous research effort and advancement with reference to the development of AEMWE. This shift in emphasis can be attributed to the recognition of the significant potential of AEMWE in advancing sustainable hydrogen production.
It becomes evident that there is tremendous potential to achieve large-scale hydrogen production by utilizing cost-effective catalyst materials and AEMs in alkaline environments. The AEM electrolyzer, with its unique features and capabilities, offers a promising solution for industrial-level hydrogen generation. By harnessing the advantages of low-cost catalytic materials and the utilization of AEMs, the AEM electrolyzer holds great promise in driving the realization of efficient and sustainable hydrogen production. Overall, the advancements in AEMWE, accompanied by the increasing focus on this technology in research, highlight the significant potential for achieving cost-effective and scalable hydrogen production. This progress not only paves the way for a shift towards a hydrogen-based energy system but also underscores the importance of leveraging AEMs and affordable catalyst materials in realizing this vision.
However, despite the existing literature on the properties of commercial anion exchange membranes (AEMs), there is a lack of comprehensive analysis of their performance and durability when coupled with different anode and cathode catalysts in the last half decade [29,30,31]. This review seeks to fill this research gap by conducting an in-depth review and comparison of numerous commercial AEMs in conjunction with various catalysts. The review will specifically highlight the potential of these membranes for applications in AEMWE and AEMFC.
The synergy between the membrane and electrocatalyst in terms of performance and durability is crucial for achieving optimal results in fuel cell (FC) and water electrolyzer (WE) applications [32,33]. While previous studies have primarily focused on the properties of commercial AEMs themselves, this review emphasizes the significance of considering the interaction between the membrane and electrocatalyst and its impact on the overall performance of AEM-based systems. Through a comprehensive analysis, this review paper aims to address this research gap and provide an in-depth discussion about commercial AEMs, their potential when combined with various catalysts, and key factors affecting the performance and durability of AEM, contributing to a better understanding based on the FC and WE system.
2. Current Achievement of Commercial AEM for AEMWE and AEMFC Applications
2.1. Working Principles of AEMWE and AEMFC
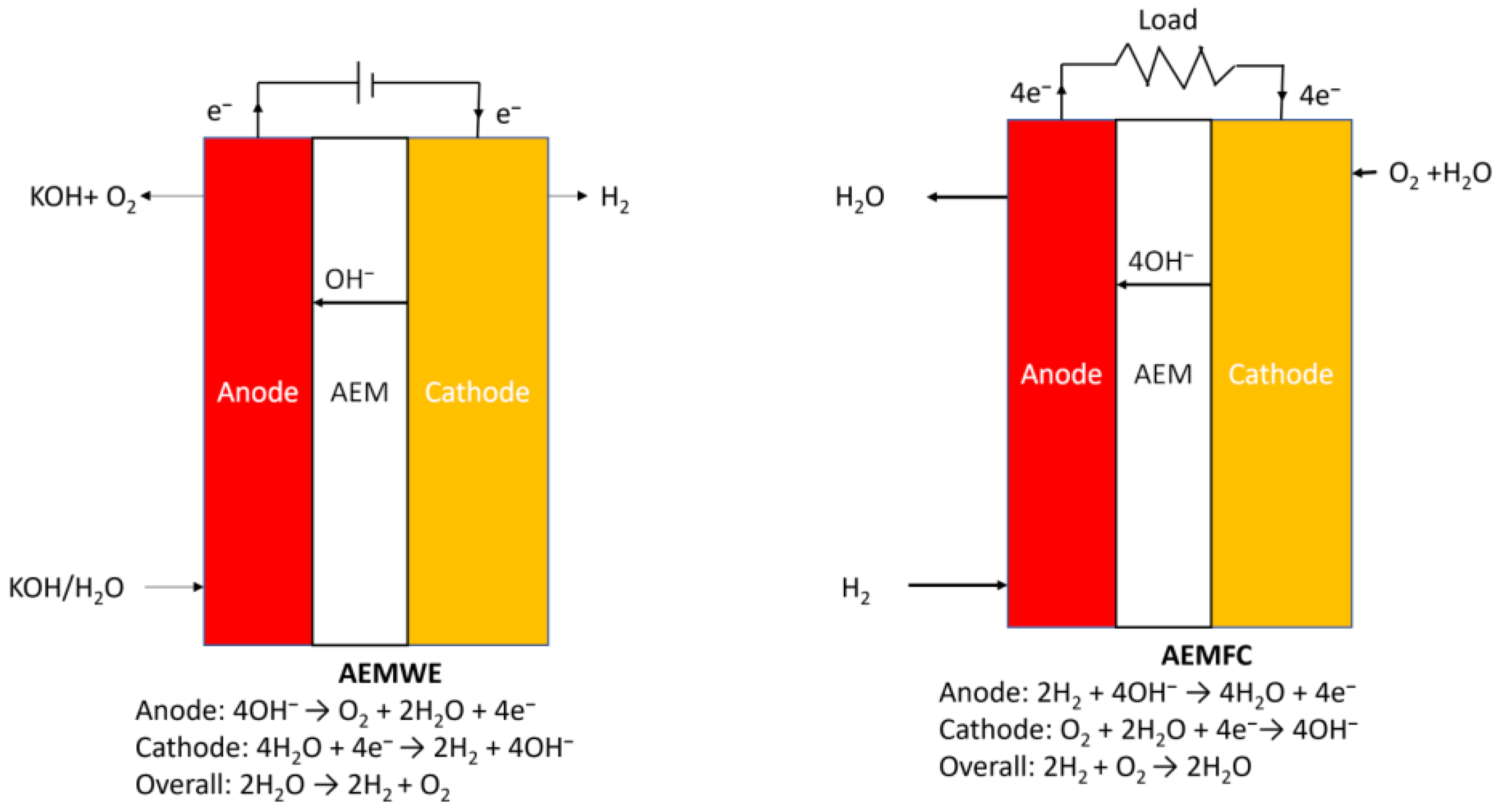
AEMFCs are fuel cells that utilize AEMs to transport hydroxide ions (OH−) from the cathode to the anode. They combine hydrogen and oxygen to produce electricity, with water and heat as products. Equations (1)–(3) below describe the electrochemical reactions that occur on the surface of the catalyst in AEMFC with a direct four-electron path. One of the most important advantages of AEMFCs over PEMFCs is their potential to achieve higher efficiency, mainly due to the enhanced kinetics of the oxygen reduction reaction (ORR) in alkaline conditions typical of AEMFCs [34]. ORR occurs more easily in alkaline than acidic conditions.
Anode: 2H2 + 4OH− → 4H2O + 4e−,
Cathode: O2 + 2H2O + 4e−→ 4OH−,
Overall: 2H2 + O2 → 4H2O.
In the context of producing pure hydrogen and electrical energy through chemical fuel generation such as H2 and O2, the reverse working principle of AEMFC, AEMWE, has emerged as an effective approach [35]. AEMWE involves two electrochemical half-reactions occurring in the electrolyte: the hydrogen evolution reaction (HER) at the cathode, where protons and electrons recombine, and the oxygen evolution reaction (OER) at the anode, which splits water into oxygen gas, protons, and electrons [36,37]. These reactions can be represented by Equations (4)–(6) below. However, AEMWE faces a specific challenge related to high overvoltages. While theoretically, AEMWE only requires a voltage of 1.23 V, the actual operating voltage of the cell ranges from 1.7 to 2.3 V in practice. This discrepancy is primarily attributed to unfavorable characteristics of electrode materials, such as overpotential and poor ion and gas dispersion, as well as various device-related factors, including solution concentration, wire and electrode resistance, electrolyte dispersion blockage, bubble evolution, and heat release [38]. Figure 2 shows the schematic diagram of AEMWE and AEMFC.
Anode: 4OH− → O2 + 2H2O + 4e−,
Cathode: 4H2O + 4e− → 2H2 + 4OH−,
Overall: 2H2O → 2H2 + O2.
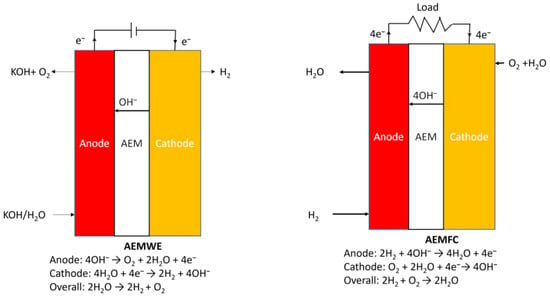
Figure 2.
Schematic diagram of AEMWE and AEMFC.
In addition to these challenges, there are still significant problems to overcome before AEMFCs and AEMWEs can effectively replace PEM technology. Questions remain regarding the stability and endurance of AEMs during extended use, as well as the improvement of ion mobility. Furthermore, the market availability of alkaline exchange membranes has not reached the same level as their proton exchange counterparts. To address these disparities, further scientific research and increased industry participation are crucial. A deeper understanding of AEM degradation and the development of standardized testing procedures are essential to advance the field.
2.2. Progress of Commercial AEMs in AEMWE and AEMFC
The polymer backbone is a crucial element in determining the mechanical strength and stability of AEM membranes. The inclusion of a rigid structure, such as aromatic rings or perfluorinated structures, plays a significant role in ensuring the membrane’s mechanical stability [39]. However, it is essential to address the issue of poor dimensional stability, as it can compromise the overall mechanical strength of the membrane. Various materials have been employed as backbones in anion exchange membranes (AEMs), including oxidation-resistant fluorinated polymers, aromatic polymers derived from hydrocarbons, condensation polymers, and block polymers [40]. The selection of the polymer backbone directly affects multiple membrane properties, such as rigidity, tensile strength, water absorption, OH− conductivity, and chemical stability [41].
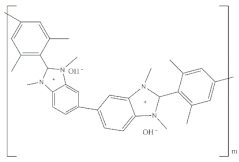
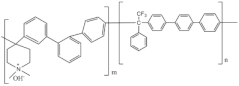
In the past few years, there has been a growing interest in aromatic polymers due to their inherent rigidity and impressive thermal stability [42]. However, it is important to acknowledge that aromatic polymers may be vulnerable to damage from reactive oxygen radicals, especially at the benzylic carbon sites [43,44]. To address this vulnerability, the introduction of a crosslinking structure can offer protection to the polymer chains or head groups, shielding them from radical attacks [45,46]. To provide a comprehensive overview of commercial membranes and their properties, Table 1 presents a summary of different membranes based on their chemical structure, types of backbones, functional groups for OH− transfer, and relevant properties.

Table 1.
Properties of AEMs available commercially.
To date, there is no universally accepted commercial standard within the scientific community for this specific water electrolyzer (WE) and fuel cell (FC) application. However, various commercial membranes are utilized for these purposes. AEM membranes are generally designed for a wide range of applications, including electrolysis, fuel cells, electrodialysis, desalination, and redox flow battery, among others. It is noteworthy that extensive research and advancements in the field of anion exchange resin membranes have been taking place for over seven decades [55]. Because of this, Table 1 only includes a selection of membranes that have been primarily investigated for use in AEMFCs and AEMWEs, respectively, over the most recent half decade, even though there are many other membrane companies and membranes available for other applications, such as those from Asahi Chemical Industry [56], AGC Engineering Co., Ltd. [57], Mega a.s. [58,59], Solvay Specialty Polymers [60], and Tianwei Membrane Technology [61,62,63]. Additionally, not all available membranes or grades are shown in the table; often, additional membranes with different thicknesses, porous supports, or other reinforcements are available upon request.
2.2.1. Fumatech: FAA Series
The FAA series membrane is an anionic ion exchange membrane that can be obtained from independent distributors or directly from Fumatech. It is available in various thicknesses and can be acquired as a non-supported membrane or with PEEK- or PP-reinforcement. This membrane is composed of a polyaromatic polymer that features quaternary ammonium groups covalently bound to the main chain, as well as ether bonds present within the main chain [47]. FAA series membranes have attracted significant attention in research endeavors exploring different catalysts for applications in AEMFC and AEMWE.
These commercially available FAA series membranes have found utility in a range of electrochemical and non-electrochemical applications. Specifically, these membranes have demonstrated their suitability in electrochemical CO2 reduction applications where the electrolyte solutions typically consist of 0.1 to 0.5 M KOH or NaOH, as well as solutions containing 0.1 to 0.5 M water-soluble carbonate or bicarbonate salts. These membranes offer flexibility in terms of their application, allowing researchers to explore various experimental setups and conditions to achieve efficient CO2 reduction [64,65,66]. Moreover, their versatility extends beyond CO2 reduction to encompass other electrochemical processes such as electrodialysis, desalination, reverse electrodialysis, and acid recovery [67,68,69,70]. The FAA series has been readily accessible in the market for a substantial period, offering researchers and industry professionals a dependable and commercially viable solution for these specific applications.
The availability and utilization of the FAA series membranes in both academic and industrial settings have contributed to notable advancements in the field of electrochemical technologies and related disciplines. Researchers have explored the integration of various catalysts with these membranes, paving the way for advancements in anion exchange membrane-based fuel cells and water electrolysis systems. FAA series membranes, with their well-established presence and wide range of applications, continue to play a pivotal role in driving progress in the field of electrochemical science and technology.
2.2.2. Dioxide Materials: Sustainion® X37-50
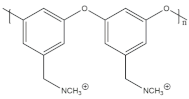
Sustainion membranes, developed by Richard Masel’s research team and produced by Dioxide Materials, are commercially available for direct purchase from Dioxide Materials or their independent distributors [48]. These membranes utilize a unique design based on imidazole-functionalized poly(4-vinylbenzyl chloride-co-styrene). The specific product code, Sustainion 37-50, indicates a copolymer composition with a 37% molar percentage of 4-vinylbenzyl chloride and a thickness of 50 μm. Sustainion membranes were initially developed and patented in 2015 [71]. While originally designed for water electrolyzers, Sustainion X37-50 membranes have been optimized for use in other applications such as CO2 reduction [72] and batteries [73]. This optimization reflects the versatility and adaptability of these membranes for various electrochemical processes. Their unique composition and structure contribute to their performance and suitability in these specific applications. Researchers and industry professionals can benefit from the availability of Sustainion membranes, enabling advancements in CO2 reduction technology and battery systems.
2.2.3. Tokuyama: A201 and A901
Tokuyama’s AEM products were exclusive to the company, and, to acquire them, a non-disclosure agreement had to be signed. Unfortunately, this product line has been discontinued. The Tokuyama A201 membrane, which differs from the preceding Aciplex A201 version, and the structurally identical but thinner (11 µm) A901 membranes are both made of hydrocarbons and have quaternary ammonium groups. However, their exact chemical structures remain undisclosed due to a signed non-disclosure agreement. The A201 and A901 membranes exhibit a compact arrangement of their structure, resulting in a highly interconnected network of ionic pathways. This characteristic facilitates the enhancement of ionic conductivity.
2.2.4. Aemion™ Series
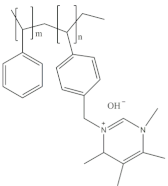
Aemion™ membranes, developed by the Holdcroft group at Simon Fraser University, are based on the chemistry of methylated polybenzimidazoles (PBI). In their early stages, these membranes were initially referred to as “functionalized-PBI,” denoting their origin as polybenzimidazole (PBI) synthesized from a tetraamine and a mesitylene-containing diacid. However, as the development progressed and the unique characteristics of these membranes were recognized, they were eventually named AemionTM membranes [50].
Unlike conventional PBI membranes, which are prone to degradation due to hydroxide attacks on the imidazolium component’s C2 position, Aemion™ membranes employ a modified structure with at least two phenyl rings to stabilize the positive charges of neighboring imidazolium ions. This design enhancement results in remarkable chemical stability across the entire pH scale (0–14), making Aemion™ membranes highly suitable for the efficient recovery and reuse of strong chemicals. These membranes have demonstrated exceptional performance in various applications, including hot and strong alkaline environments in AEMFC [74] and AEMWE [75]. Their chemical stability is particularly advantageous in the recovery of spent acids in industrial processes such as steel production pickling and mining leaching solutions. Furthermore, Aemion™ membranes enable the selective transport of ions and the removal of unwanted metal salts through processes such as diffusion and electrodialysis. This capability contributes to cost reduction, raw material conservation, and compliance with environmental regulations [76]. Moreover, their high selectivity, proton-blocking characteristics, and tolerance to oxidizing impurities such as chlorine make them valuable for generating high-strength chemicals from waste industrial brine streams. Ongoing testing confirms their stability under high current conditions, providing promising indications for extended lifetime and improved process economics.
2.2.5. Orion TM1
The initial pioneering papers on the membrane now known as Orion TM1 did not originally refer to it by that name. Instead, it was referred to as poly(terphenylene) in the scientific literature [54]. Over time, as research and development progressed, the membrane was further refined and eventually designated as Orion TM1. In response to the growing interest in AEMs, the Orion TM1 membrane is available for purchase from renowned international AEM vendors, including Fuel Cell Store and Dioxide Materials.
Orion Polymer offers a range of Orion TM1 membranes, including both unreinforced membranes with an ion exchange capacity (IEC) of 2.19 meq/g and reinforced membranes with thicknesses varying from 5 to 50 µm. These membranes, developed by Chulsung Bae’s lab at the Rensselaer Polytechnic Institute, have shown promising potential for applications in water electrolyzers [54]. One of the challenges encountered with the TM1 membranes, also known as TPN1-100 in scientific literature, is their mechanical stability when hydrated. While they have demonstrated stable initial performance, issues related to their mechanical integrity have been identified. However, it is important to note that there is currently a lack of comprehensive literature available regarding the Orion TM1 membranes, primarily due to their recent development. In a recent study conducted by Khalid et al. [77], the focus was primarily on investigating the mechanical properties, dimension stability, and hydroxide conductivity of the Orion TM1 membrane. However, comprehensive data on the membrane’s performance in terms of current density at different operating voltages and its long-term durability are still lacking. As a newly developed membrane, further research and investigation are required to address the concerns related to the mechanical stability of the TM1 membranes when hydrated. It is crucial to understand and optimize the membrane’s structural design, fabrication methods, and material composition to enhance its mechanical stability while maintaining its desirable electrochemical properties.
3. Electrochemical Applications of Commercial AEMs
The application of commercial AEMs in electrochemical systems has garnered significant attention due to their potential for various electrochemical applications, including AEMWEs and AEMFCs. This section focuses on exploring the catalysts and performance evaluation of commercial AEMs with A tables and self-plotted figures present detailed performance data. The critical analysis focuses on identifying key factors that significantly impact the performance of AEMWE and AEMFC, facilitating a deeper understanding of the strengths and limitations of commercial AEMs in these specific applications. This section aims to contribute to the existing knowledge of the electrochemical applications of commercial AEMs, particularly in AEMWEs and AEMFCs. The comprehensive analysis and critical discussions serve as valuable resources for researchers, engineers, and industry professionals seeking to optimize the performance and durability of commercial AEMs in AEMWE and AEMFC applications.
3.1. Commercial AEMs in Anion Exchange Membrane Water Electrolysis (AEMWE) Systems
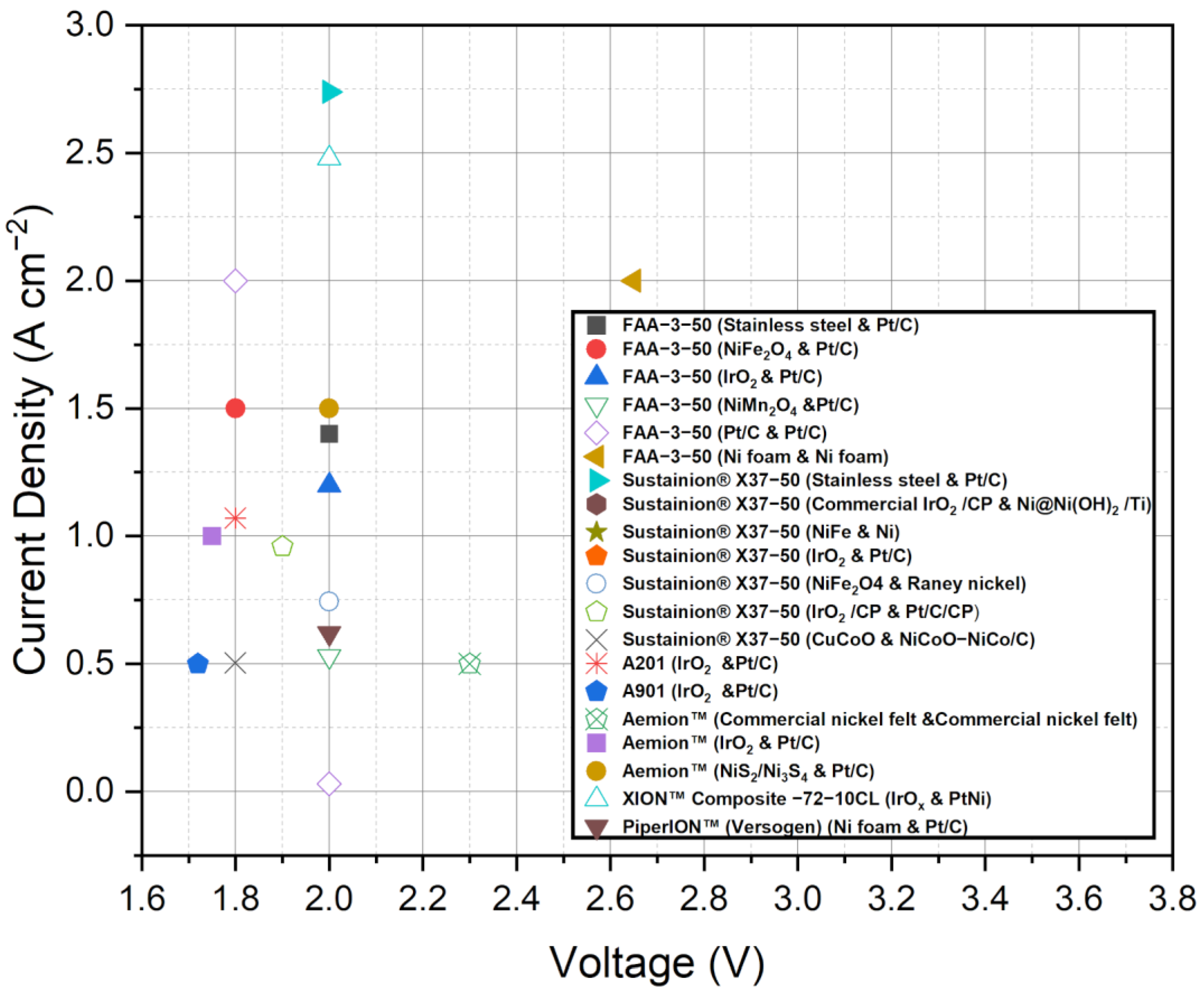
Figure 3, presented in the study, offers a comprehensive compilation of current density and voltage data for the various commercial membranes discussed. This figure provides a visual representation of the performance characteristics of each membrane, allowing for quick comparison and assessment of their relative performance in AEMWE systems. By analyzing the data in Figure 3, trends and differences in current density and voltage among the membranes can be observed, aiding in the evaluation of their suitability for specific applications. Additionally, Table 2 summarizes the performance of AEMWEs in the last half-decade, specifically highlighting the performance of commercial AEMs when coupled with different catalysts. This table provides a concise overview of the current density, voltage, and other relevant performance metrics achieved by these systems. By examining Table 2, it becomes evident how the choice of catalyst can influence the overall performance of AEMWE systems using commercial membranes. Figure 3 and Table 2 can complement the textual information provided and offer readers a more comprehensive understanding of the performance characteristics and trends observed among different commercial membranes and AEMWE systems. This visual representation and summary help support your conclusions and provide valuable insights into the suitability and potential of these membranes in practical applications.
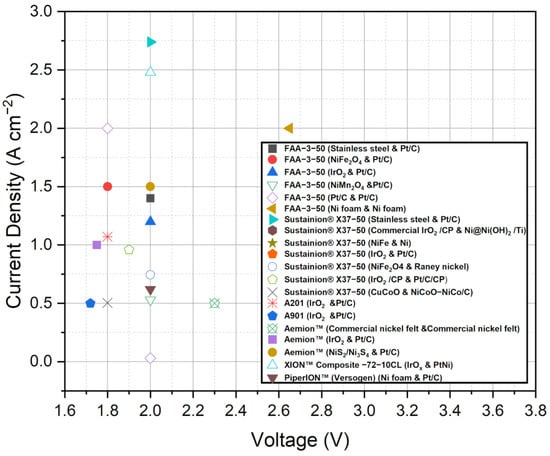
Figure 3.
Comparison of the current density and voltage data for the various AEMWE membranes available commercially. Data adapted from [51,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94].

Table 2.
Performance of AEMWEs in the last half-decade with AEMs available commercially coupled with different catalysts.
The data and references were analyzed comprehensively to examine the performance and durability of various anion exchange membranes (AEMs) and catalyst configurations in electrochemical water splitting. System performance is evaluated by current density at specific voltages, while durability is assessed by system stability during extended operation. Among the membranes tested, Fumasep FAA-3-50 and Sustainion X37-50 were widely investigated. Starting with FUMASEP FAA-3-50, it has been used with various anode catalysts such as stainless steel, NiFe2O4, IrO2, and NiO. The membrane exhibited moderate performance, with current densities ranging from 0.53 A cm−2 to 2 A cm−2 at 1.8 V to 2.2 V. Fumasep FAA-3-50 membrane, in combination with different catalysts, has shown moderate performance but limited durability. For instance, when using the conventional combination of IrO2 as the anode catalyst and platinum as the cathode catalyst, the Fumasep FAA-3-50 membrane achieved a maximum current density of 2 A cm−2 at 2.2 V at 60 °C with a 1 M KOH feed. This performance demonstrates the membrane’s capability to facilitate efficient ion transport and catalytic reactions, resulting in a significant current density. In addition to investigating different catalyst configurations, researchers have also explored the impact of temperature on the performance of anion exchange membranes (AEMs) in electrochemical water splitting. Gatto et al. [80] investigated the influence of temperature on the performance of AEMs in electrochemical water splitting using membrane electrode assemblies (MEAs). The results of their study revealed that varying the temperature had a significant effect on the performance of the MEAs. For instance, by increasing the cell temperature from 60 °C to 80 °C, a notable improvement in the maximum current density was observed when the temperature increased from 60 °C to 70 °C. The current density increased from 1.2 A cm−2 to 1.7 A cm−2 at 2 V, indicating enhanced reaction kinetics and improved ionic conductivity of the AEMs at higher temperatures. Moreover, the analysis of Nyquist plots revealed a decrease in both the series resistance (Rs) and charge transfer resistance (Rct) as the temperature increased. This decrease in resistance suggested an enhancement in the ionic conductivity of the AEMs in the MEAs, leading to improved reaction rates at elevated temperatures [98]. However, it is important to note that beyond a certain temperature, specifically at 80 °C, the current density did not exhibit further increases but rather experienced a decline. This decrease in performance was accompanied by an increase in both Rs and Rct. The degradation of system performance at high temperatures can be attributed to dehydration phenomena, which negatively impact the resistance of the cell and the catalyst/electrolyte interface of MEAs. It is crucial to strike a balance between temperature optimization and the potential drawbacks associated with excessive temperatures.
Lim et al. [97] conducted a study where they utilized the FAA-3-PK-75 membrane, which is an anion exchange membrane (AEM) reinforced with polyketone (PK) and has a thickness of 70–80 μm. Through their research, they were able to achieve a current density of 1 A cm−2 at 1.8 V by employing an optimized manufacturing process and operating the system at a temperature of 90 °C. The membrane’s polyketone reinforcement likely contributed to its mechanical strength and stability, enabling it to withstand operating conditions and maintain its structural integrity during prolonged operation. Polyketone materials, known for their high-temperature resistance and mechanical properties, act as reinforcing components in the membrane. They contribute to improving the membrane’s dimensional stability and resistance to thermal expansion and contraction, reducing the likelihood of deformation or failure under high-temperature conditions. The reinforced membrane’s higher thermal stability enables it to maintain its structural integrity and functional properties at elevated temperatures. This characteristic is particularly crucial for applications such as electrochemical water splitting, where high temperatures are often employed to enhance reaction kinetics and overall system performance.
Furthermore, investigations into novel catalyst configurations and modifications could provide insights into enhancing the performance of FAA-3-50 membrane systems. The Fumasep FAA-3-50 membrane has been tested with other anode catalysts, such as stainless steel, NiFe2O4, and NiO with the conventional platinum cathode catalyst, yielding current densities ranging from 1.4 A cm−2 to 2.0 A cm−2 at voltages ranging from 1.8 V to 2.2 V with the temperature of 60 °C and a feed of 1 M KOH. Notably, the combination of NiFe2O4 as the anode catalyst and Pt/C as the cathode catalyst has demonstrated a current density of 1.5 A cm−2 at 1.8 V and 2.0 A cm−2 at 2 V. NiFe2O4 has shown promising performance as an anode catalyst when used in combination with the Fumasep FAA-3-50 membrane in electrochemical water splitting systems. NiFe2O4 has shown promising performance as an anode catalyst when used in conjunction with the Fumasep FAA-3-50 membrane in electrochemical water-splitting systems. It exhibits excellent electrocatalytic activity for the oxygen evolution reaction (OER), which is a crucial step in the water-splitting process. The composition, structure, morphology, and surface properties of NiFe2O4 contribute to its high catalytic activity, enabling the efficient electrochemical oxidation of water and resulting in improved current density performance [99,100]. One advantage of NiFe2O4 is its cost-effectiveness compared to more expensive catalyst materials such as Iridium. Its favorable performance, combined with its relatively lower cost, makes it an attractive option for practical applications, especially when cost considerations are important. It is important to note that the Ni foam membrane, which is not specified in terms of its composition, can only achieve a high current density of 2 A cm−2 at a relatively high cell voltage of 2.65 V with a feed of 2 M KOH [82]. The high molarity of KOH decreased the electrical resistance of the electrolyte. The excessively high cell voltage can lead to low energy efficiency, which is not desirable for actual operation. Notably, the high applied potential with high current density informed that the membrane could withstand such aggressive operating conditions, despite no further durability data obtained [101].
To comprehensively evaluate the membrane’s suitability for FAA-3-50 membrane systems, it is necessary to investigate its long-term stability and degradation behavior. Understanding the degradation mechanisms can help identify potential challenges and guide the development of strategies to enhance its durability. When using the conventional combination of IrO2 as the anode catalyst and platinum as the cathode catalyst, the degradation rate only achieves 3 mV h−1 at 500 mA cm−2 for 8 h [95] and 1 mA cm−2 h−1 at 1.8 V for 120 h [80]. Further investigations are required to assess the membrane’s stability and degradation mechanisms under prolonged operation. The Pt/C membrane, with a loading of 1 mg cm−2 on both the anode and cathode, exhibits a degradation rate of 50 μA cm−2 h−1 at 2 V for 100 h [96]. Notably, while these catalysts are commonly used and exhibit good electrocatalytic activity, they may still contribute to some degree of degradation over time [102,103]. Hence, it cannot be deduced whether the membrane is the sole contributor to the performance degradation. The NiMn2O4-based membrane demonstrated an impressive degradation rate of approximately 120 μV h−1 at 400 mA cm−2 for 1000 h at 80 °C [81]. This indicates a relatively stable performance under the specified conditions, supporting the membrane’s potential durability. The NiMn2O4 robust structure and good adhesion to the electrode support contribute to its long-term durability and resistance to degradation [104]. The porous structure formed by vertically aligned nanosheets increases surface wettability, which enables the rapid dissipation of gas bubbles along with excellent durability. This structural stability, in addition to the thermal stability of Fumasep membranes at a specific operating temperature, plays a crucial role in preventing catalyst detachment and the loss of active surface area during prolonged operation. The thermal stability of the Fumasep membranes at the designated temperature range ensures that the membrane can withstand the elevated temperatures typically encountered during water electrolysis. This attribute is of paramount importance in maintaining the integrity and effectiveness of the catalyst, aligning with the stringent operational requirements for efficient water electrolysis. While specific temperature values may vary depending on the application and specific membrane composition, studies have shown that Fumasep membranes exhibit notable thermal stability at elevated temperatures. For instance, research conducted by Roschger et al. [105] demonstrated the thermal stability of Fumasep membranes at 80 °C during extended electrolyzer operation. Their findings indicated that the Fumasep membrane retained its structural integrity, ensuring the catalyst remained securely attached, and the active surface area was preserved, contributing to stable electrolysis performance. Therefore, the combination of both structural and thermal stability in Fumasep membranes addresses the critical requirements for water electrolysis systems, safeguarding the catalyst’s attachment and preventing the loss of active surface area.
To understand the impact of different membrane materials and designs on overall performance, it is important to compare the performance of different membranes. The Sustainion® X37-50 membrane has demonstrated both good performance and durability in electrochemical water-splitting applications. Its optimized membrane composition and design contribute to higher efficiency, improved ion conductivity, enhanced electrochemical performance, and efficient utilization of electrical energy [106]. When comparing the Sustainion® X37-50 membrane with Fumasep membranes using Pt/C catalysts, the Sustainion membrane consistently exhibited higher current densities (1.8 A cm−2 at 2.0 V) [85] compared to the Fumasep membranes (1.2 A cm−2 at 2.0 V) [80]. This comparison highlights the influence of different ionic transport groups in the membranes on overall performance. Despite concerns about corrosion, the use of stainless steel substrates with the Sustainion membrane demonstrated the exceptional electrocatalytic performance of the oxygen evolution reaction (OER). The formation of a metal oxide layer, comprising mixed oxides of nickel, iron, and chromium, contributes to the high electrocatalytic activity [107]. Moreover, the Sustainion® X37-50 membrane, with its higher OH− conductivity (115 mS cm−1 at 60 °C), enables even higher current densities ranging from 1.4 A cm−2 to 2.74 A cm−2 at 2.0 V [78]. In summary, the comparison between different membranes, such as Sustainion and Fumasep, highlights the impact of membrane material on overall performance. Additionally, the use of stainless-steel substrates with the Sustainion membrane showcases the importance of membrane conductivity in achieving high current densities. The Sustainion® X37-50 membrane, with its superior performance and ability to operate at a lower potential, offers a favorable balance between power output and energy consumption, making it a promising choice for achieving higher efficiency in AEMWE systems over long durations of performance. Researchers have identified the significance of alkaline stability in membrane materials, such as the recent advancements showcased in the development of Sustainion® 37-50 anion exchange. These membranes have demonstrated exceptional chemical stability in KOH compared to Fumasep membranes containing quaternary ammonium compounds [48]. The absence of active hydrogen sites of membranes due to the 1,2,4,5-tetramethylimidazole group in the Sustainion® membrane plays a crucial role in its superior stability, as it prevents hydroxide ions from initiating SN2 reactions with the methyl groups. The hydroxide ion performs an SN2 attack on the methyl groups. Ensuring high alkaline stability is crucial for achieving long-term performance in anion exchange membrane water electrolyzers (AWMWE). Maintaining excellent stability in alkaline environments is essential to mitigate degradation and enhance the durability of these electrolyzers over extended periods. This stability is particularly important in preserving the integrity and functionality of the anion exchange membrane, which serves as a critical component in AWMWE systems. By withstanding high alkaline conditions, the membrane can effectively resist degradation mechanisms, such as hydroxide attack or other chemical reactions, ensuring sustained performance and prolonged operational lifetimes for AWMWE devices.
The durability performance of the Sustainion X37-50 membrane in AEMWE systems is indeed remarkable. The membrane’s ability to sustain its functionality at high current densities is noteworthy. When paired with NiFe2O4 anode and Raney nickel cathode catalysts, the membrane exhibited consistent performance, maintaining a current density of 0.744 A cm−2 at 1.8 V for over 10,000 h [86] Raney nickel, used as the cathode catalyst, is known for its stability and resistance to degradation under harsh operating conditions. Its robustness ensures the long-term stability and performance of the cathode, contributing to the overall durability of the system [108]. The combination of NiFe2O4 anode and Raney nickel cathode catalysts with the Sustainion X37-50 membrane may exhibit synergistic effects, where the individual components complement each other’s performance. This synergy can enhance the overall stability and durability of the system, leading to long-term operation without significant degradation. The membrane exhibited a degradation rate of 0.7 μV h−1 at 1000 mA cm−2 for 10,100 h, indicating its stability over an extended period. Using the same configuration, PTFE reinforced Sustainion® (Grade-T) membranes exhibited a degradation rate of 0.7 μV h−1 at 1000 mA cm−2 for 12,180 h. This suggests the membrane’s potential for long-term durability in AEMWE systems. This extended durability suggests that the Sustainion membrane has the potential to operate for more than 20 years, making it highly attractive for long-term and sustainable AEMWE applications. These exceptional performances suggest that the Sustainion® membrane is well-suited for achieving high current densities with long duration in AEMWE systems compared to the FAA-3-50 membrane. The Sustainion® 37–50 anion exchange membrane with an imidazole group was chemically very stable in KOH compared to the quaternary ammonium group of the FAA-3-50 membrane. The quaternary ammonium group of the FAA-3-50 membrane is susceptible to attack by hydroxide ions (OH−), leading to degradation over time and limiting its long-term durability. This susceptibility arises from the nature of the quaternary ammonium structure, which contains active hydrogen atoms that can undergo reactions with hydroxide ions. During operation, OH− ions can attack the quaternary ammonium groups through processes such as Hofmann elimination and nucleophilic substitution reactions [109,110,111,112,113]. These reactions result in the degradation of the AEM, leading to a decrease in its performance and durability over extended periods of use. In the research conducted by Khalid and his team, it was observed that FAA3-50 lost all its nitrogen due to an attack by hydroxide ions at the benzylic position [77]. This occurred after it was immersed in 1 M of KOH at 60 °C over a period of 28 days. The deterioration of the quaternary ammonium groups was attributed to the assault from hydroxide ions.
Interestingly, the Aemion™ membrane, based on methylated polybenzimidazoles, has demonstrated outstanding performance and durability in AEMWE systems. Xia et al. [93] reported a current density of 1.5 A cm−2 at a stable voltage of 2 V in an AEMWE system utilizing a NiS2/Ni3S4 anode, a Pt/C cathode, and a 1 M KOH feed at 60 °C. This system maintained a current density of 1000 mA cm−2 over a testing period of 500 h with a voltage degradation rate of 0.12 mV h−1. Moreover, in a study conducted by Ruck et al. [92], a significant current density of 1.0 A cm−2 was attained at a voltage of 1.75 V in an AEMWE system. This achievement was accomplished by employing an IrO2 anode, a Pt/C cathode, and a 1 M KOH feed at an elevated temperature of 70 °C. These results demonstrate the capability of the Aemion™ membrane to support high-performance AEMWE systems at elevated temperatures. In contrast to the previous study, Khataee et al. observed a degradation rate of approximately 2 mV h−1 at 200 mA cm−2 for 100 h when using commercial nickel felt as the anode and cathode catalysts [91]. This indicates a relatively higher degradation rate compared to the previous findings. This is most likely due to the formation of nickel hydride and nickel hydroxide layers during electrolysis on the cathode and anode sides, respectively [114]. The presence of these nickel hydride and nickel hydroxide layers significantly influences the degradation mechanism. Khataee et al. [114] hypothesized that the degradation rate is a cumulative effect resulting from both electron and ion transport within the membrane and the electrolyte. In this context, the imidazolium groups present in the membrane structure may facilitate the transport of nickel hydride and nickel hydroxide ions, thus contributing to the higher degradation rate observed. This finding sheds light on the importance of considering the specific catalyst and its interactions with the membrane and electrolyte when evaluating degradation rates in water electrolysis systems. The presence of certain catalysts, such as commercial nickel felt, can lead to the formation of degradation-inducing layers, ultimately affecting the overall performance and stability of the electrolyzer.
Major degradation of Aemion™ occurs via ring-opening through an OH attack at the C2 position of the imidazolium group. The ring-opening degradation of imidazolium leads to the formation of N–H groups [115]. When it comes to durability, the Sustainion® X37-50 membrane surpasses both Fumasep FAA-3 and Aemion™ membranes, highlighting its appropriateness for high-performance AEMWE systems. It is confirmed that the instability of the Aemion™ membrane, which is functionalized with 1-methyl imidazole, results from the active hydrogen present in the ring [74]. On the other hand, the imidazole group of Sustainion® X37-50 exhibits substantial chemical stability in KOH [116]. This further substantiates the notion that the active hydrogen in the ring is responsible for the Aemion™ membrane’s instability. In contrast, the imidazole group in the Sustainion® X37-50 membrane exhibits marked resistance to chemical reactions in KOH. This is because the three active hydrogen atoms in the 1-methyl imidazole ring are prone to be targeted by hydroxyl radicals (·OH) in a 1 M KOH solution [117,118].
Additionally, XION Composite membranes, which are reinforced AEMs, have demonstrated exceptional performance and mechanical properties. These ultra-thin membranes (thickness = 10 µm) comprising a poly(norbornene) backbone with quaternary ammonium functional groups exhibit high stability and durability. Preliminary tests with XION Composite-72-10CL membranes in a water electrolysis setup utilizing IrOx anodes and PtNi cathodes have shown a stable performance of 2.48 A cm−2 at 2.0 V [51]. While further research is necessary to explore the full potential of XION Composite membranes in water electrolysis and fuel cell applications, these initial results indicate their promise in enhancing electrolysis processes.
The PiperION™ (Versogen) membrane stands out for its unique characteristics, featuring a rigid, hydrophobic, and ether-bond-free aryl backbone with stable piperidinium cations. In a study by Caielli et al. [94], the remarkable performance of the PiperION™ membrane in anionic water electrolyzers was demonstrated. The membrane exhibited an impressive current density of 0.62 A cm−2 at a voltage of 2 V under the specific conditions of 1 M KOH electrolyte at 60 °C. This finding highlights the membrane’s capability to facilitate efficient ion transport and its suitability for applications requiring high performance in electrolysis processes. However, no further durability testing was conducted. Furthermore, the PiperION™ membrane holds great promise for fuel cell applications, given its robust properties and high ionic conductivity. In conclusion, the performance and durability of anion exchange membranes (AEMs) in electrochemical water-splitting systems are influenced by various factors, including the selection of cathode and anode catalysts. This comprehensive analysis has shed light on the performance characteristics of different AEMs, highlighting their strengths and limitations. It is important to note that the contribution of the membrane itself to performance degradation cannot be deduced in these cases, as the catalysts may also play a role. The Sustainion® X37-50 membrane has demonstrated excellent performance and durability, making it a promising choice for achieving high current densities and long-term operation. Further research and analysis, including oxidative stability tests and evaluation of MEAs, are necessary to gain deeper insights into the membrane’s performance and identify strategies for further improvement.
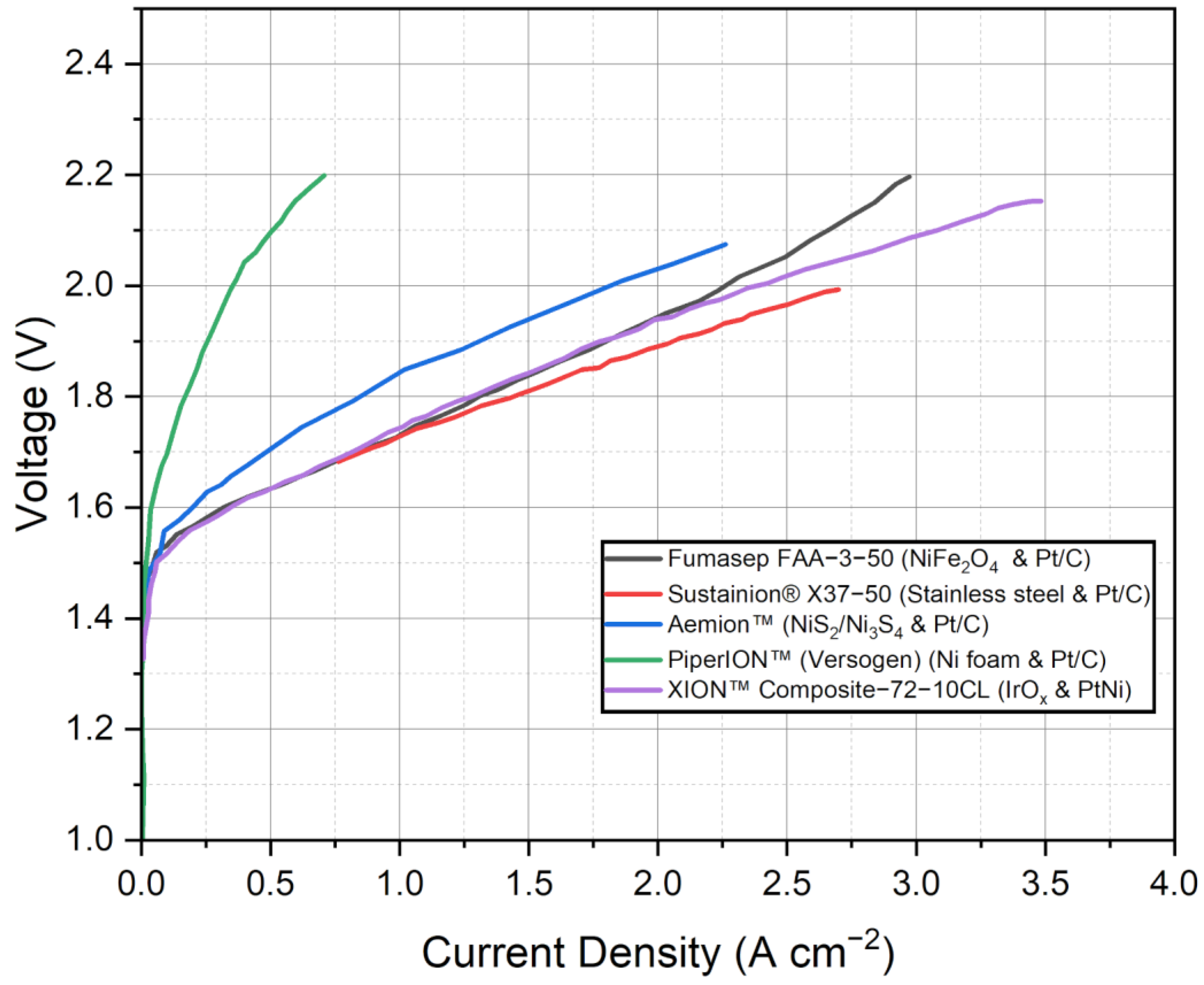
While the findings suggest the potential of these commercial AEMs, it is important to note that the discussion provided thus far is limited by the available information in the referenced studies. More detailed and comprehensive investigations are necessary to evaluate the long-term durability and degradation rates of these membranes. Such studies would provide a deeper understanding of their performance and stability over extended periods. Figure 4 presents the polarization curves of the selected commercial AEMWE membranes with the best-performing MEA configuration, providing a direct comparison of their performance characteristics. By analyzing the polarization curves, which depict the relationship between voltage and current density, we can gain insights into the membrane’s electrochemical behavior and its efficiency in water electrolysis. These curves allow for a visual assessment of the membrane’s performance at different operating conditions, aiding in the evaluation of its suitability for specific applications. In the referenced studies, it was observed that the Fumasep FAA-3-50 membrane exhibited its best performance at low current densities and high voltages. This optimal set of operating parameters resulted in the highest efficiency and favorable electrochemical behavior for the Fumasep membrane in water electrolysis.
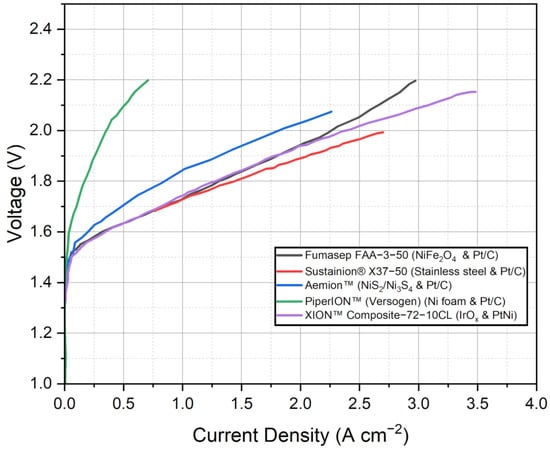
Figure 4.
Polarization curves of the selected commercial AEMWE membranes. Data adapted from [51,78,79,93,94].
The efficiency of electrolyzers is a critical factor in their commercial viability. High-efficiency electrolyzers can reduce the energy required to produce hydrogen, reducing costs and environmental impact. Electrolysis efficiency is the efficiency with which the electrolysis converts electricity into hydrogen. It is equal to the theoretical energy consumption for 1 kg of hydrogen gas divided by energy to produce 1 kg of hydrogen gas. The energy efficiency for the AEM electrolysis unit to produce 1 kg of hydrogen can be determined by Equation (7) [119]. This will enable researchers to identify the most suitable membrane for specific AEMWE system requirements, considering factors such as performance, durability, cost-effectiveness, and scalability. Table 3 comprises energy efficiency when using the conventional combination of IrO2 as the anode catalyst and platinum as the cathode catalyst and the cost of the membrane (at the time of writing). Comparing the conventional combination of IrO2 as the anode catalyst and platinum as the cathode catalyst, as well as the cost of the membrane, provides a baseline reference point for evaluating the performance and economic aspects of the system. This particular combination is widely studied and commonly used in electrochemical water-splitting applications. It allows for meaningful comparisons between different membranes in terms of their performance and cost-effectiveness. By focusing on this specific catalyst combination, researchers and industry professionals can assess the relative performance and cost efficiency of different membranes under standardized conditions. This comparison helps to establish benchmarks and identify potential areas for improvement in terms of performance, durability, and cost reduction. Regarding the cost of the membrane, it can vary depending on the specific type and brand of the membrane. The cost is influenced by factors such as the materials used, manufacturing processes, and market demand. At the time of writing, the cost of the membrane can fluctuate due to various factors such as supply and demand dynamics, technological advancements, and economies of scale.
Energy efficiency (%) = theoretical energy consumption/energy to produce hydrogen gas.

Table 3.
Comparison of energy efficiency and cost of the selected AEMWE membrane.
The theoretical energy consumption is 39.4 kWh/kg H2.
Based on the detailed analysis, the results suggest that the selection of an appropriate membrane for electrochemical water-splitting systems involves a careful consideration of both energy efficiency and cost. The evaluated membranes, namely Fumasep FAA-3-50, Sustainion X37-50, and Aemion™, provide insights into this trade-off. The Fumasep FAA-3-50 membrane demonstrates a commendable energy efficiency of 73.7% when used in combination with the conventional IrO2 anode catalyst and platinum cathode catalyst. Furthermore, it offers a relatively affordable cost of USD 22 for a size of 10 cm × 10 cm. This makes the Fumasep FAA-3-50 membrane an attractive option for short-term tests or applications where cost considerations play a significant role.
On the other hand, the Sustainion X37-50 membrane exhibits a slightly higher energy efficiency of 74.4% under similar conditions. However, it comes with a higher price tag of USD 73 for a size of 10 cm × 10 cm. The Sustainion membrane’s superior energy efficiency and presumably enhanced durability make it a compelling choice for long-term stability tests or applications where long-term performance is a critical factor. The Aemion™ membrane stands out with an impressive energy efficiency of 83.6%. However, due to the non-disclosure agreement, the cost of this membrane remains undisclosed. Consequently, it is challenging to make a direct cost comparison with the other membranes. In your specific scenario, where cost considerations are important, the Aemion™ membrane may not be a practical choice unless the undisclosed cost aligns with your budget constraints.
To summarize, the Fumasep FAA-3-50 membrane offers a good balance between energy efficiency and cost, making it a cost-effective solution for short-term tests. The Sustainion X37-50 membrane provides slightly better energy efficiency and is recommended for long-term stability due to its superior durability. The Aemion™ membrane showcases remarkable energy efficiency, but the cost is much higher compared to Fumasep FAA-3-50 and Sustainion X37-50. Ultimately, the decision on the most suitable membrane should consider your specific requirements, priorities, and budgetary considerations. Further research and evaluation of these membranes, including their long-term stability and performance under various operating conditions, would contribute to a more comprehensive understanding of their suitability for electrochemical water-splitting applications. The evaluated membranes, namely Fumasep FAA-3-50, Sustainion X37-50, and Aemion™, meet the energy efficiency requirements set by the Department of Energy (DOE), which targeted a minimum efficiency of 70% for electrochemical water splitting systems [120].
3.2. Commercial AEMs in Anion Exchange Membrane Fuel Cells (AEMFCs)
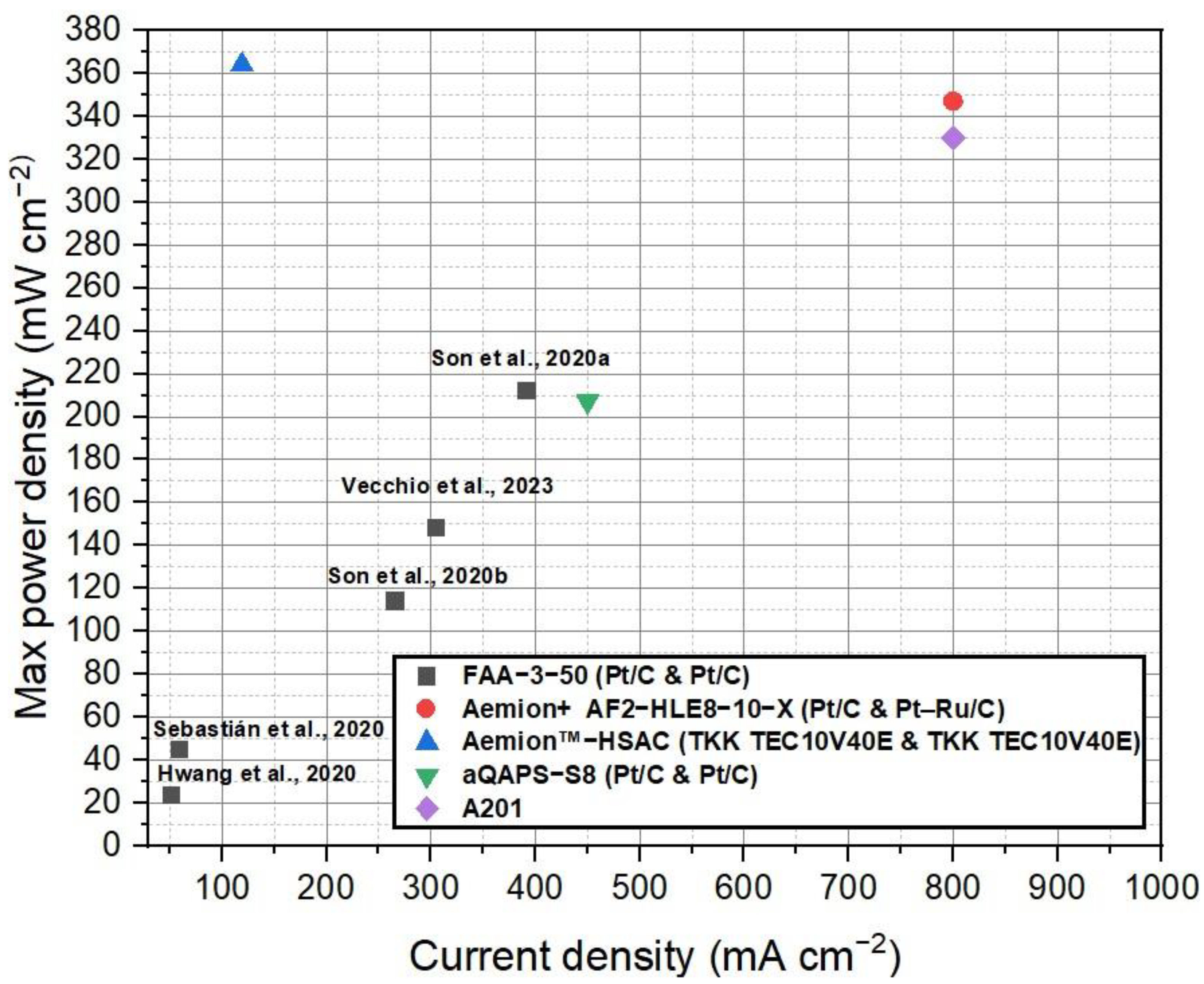
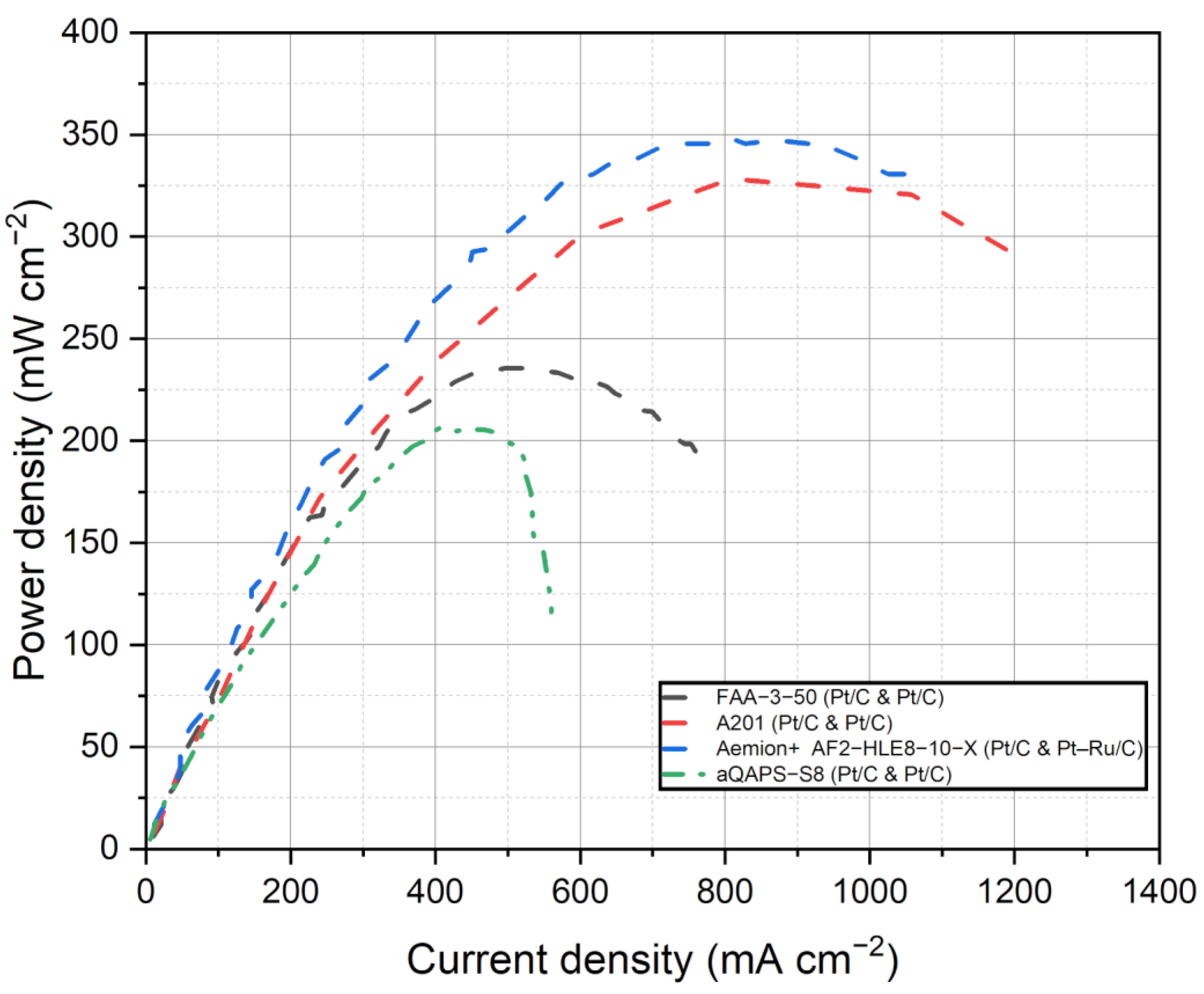
Figure 5 depicts the compilation of current density and voltage data for the various commercial membranes discussed in this study for AEMFCs. This figure provides a visual representation of the performance characteristics of each membrane. Figure 6 shows the power density curves of the selected commercial membranes for AEMFCs. Table 4 summarizes the performance of AEMFCs in the last half-decade with commercial AEMs coupled with different catalysts. The tabulated data and figures provide insights into the performance of different membrane systems in fuel cells, along with the corresponding anode and cathode catalysts, operating conditions, maximum power densities, and durability.
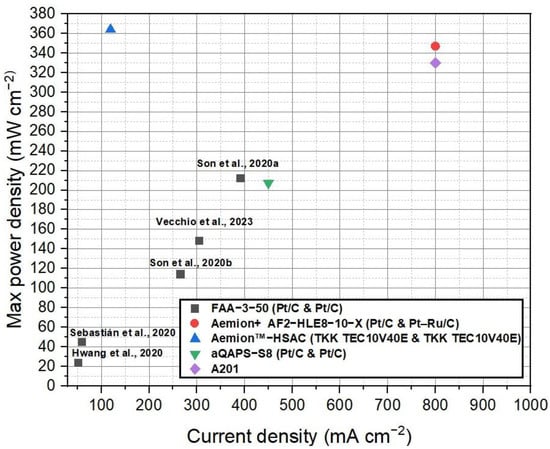
Figure 5.
Comparison of the current density and voltage data for the various commercial AEMFC membranes discussed in this study. Data adapted from [53,121,122,123,124,125,126,127,128].
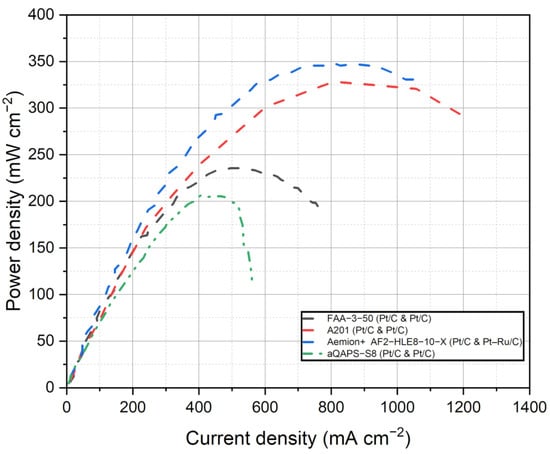
Figure 6.
Power density curves of the selected membranes for AEMFCs. Data adapted from [53,121,125,127].

Table 4.
Performance of AEMFCs in the last half-decade with AEMs coupled with different catalysts.
Upon analysis of the data, it is observed that FAA series membranes have demonstrated promising results in the field of anion exchange membrane fuel cells (AEMFCs). In one study, an AEMFC operating at 60 °C and 100% relative humidity achieved an impressive power density of 212 mW cm−2 with an H2/O2 feed flow rate of 0.3/0.3 cc min−1 and platinum loading of 1.0 mg cm−2 [122]. In another study, the FAA-3-50 membrane, when coupled with Pt/C catalysts (0.4 mg cm−2), exhibited a maximum power density of 23.4 mW cm−2 under the conditions of 1/1 mol/mol H2/O2 at 30 °C, 100% RH, and ambient pressure [121]. A notable observation was made regarding the relationship between platinum (Pt) loading and power density in AEMFCs employing Fumasep membranes. Specifically, an increase in Pt loading led to a substantial improvement in power density. This significant increase in power density can be attributed to several factors. Firstly, the higher Pt loading translates to a greater number of active catalytic sites available for the electrochemical reactions taking place at the anode and cathode interfaces. The increased surface area of the Pt catalyst allows for more efficient fuel oxidation and oxygen reduction reactions, promoting enhanced power generation. Furthermore, higher Pt loading enhances the catalyst’s ability to facilitate rapid charge transfer and efficient electron/ion transport within the fuel cell system. The improved kinetics and enhanced mass transport of reactants contribute to reduced polarization losses and improved overall fuel cell performance. This finding underscores the significant impact of catalyst loading on the performance of the fuel cell system. This indicates that the choice of catalyst and operating parameters significantly influence the performance of the fuel cell system. The presence of quaternary ammonium groups within the FAA membranes also contributes to the performance of the fuel cell to their excellent ion exchange properties and promotes favorable ion transport.
Furthermore, the Tokuyama A201 membrane demonstrated a maximum power density of 330 mW cm−2 in an AEMFC operating at 60 °C and 50% relative humidity, with an H2/O2 flow rate of 250/200 SCCM [128]. On the other hand, the Aemion+ AF2-HLE8-10-X membrane outperformed the Tokuyama A201 membrane, achieving an even higher maximum power density of 347 mW cm−2 when utilizing precious metal catalysts [126]. Aemion™ membranes, based on methylated polybenzimidazoles, hold great potential for AEMFC applications. These membranes demonstrate stable performance and high current densities under various operating conditions, making them well-suited for fuel cell systems. Their stability and impressive performance highlight their potential impact in advancing fuel cell technologies. The successful steric-crowding strategy employed to stabilize the C-2 position of imidazole species has attracted widespread attention in both AEMFC and AEMWE applications. Aemion™ membranes, built on the foundation of methylated polybenzimidazoles, offer promising prospects for AEMFCs and contribute to the advancement of fuel cell technologies. Nonetheless, it is important to mention that the Tokuyama A201 membrane, which previously demonstrated a maximum power density of 330 mW cm−2 in an AEMFC, has been discontinued. This discontinuation indicates the need for alternative solutions in the field of AEMFCs. While the Tokuyama A201 membrane served as a reference point for evaluating AEMFC technology, the superior performance of the Aemion+ AF2-HLE8-10-X membrane underscores the potential of Aemion™ membranes in fuel cell applications. The stability and high current densities exhibited by Aemion™ membranes further highlight their significant impact on advancing fuel cell technologies. The precise nature of the functional groups within the Aemion membrane, such as the steric crowding nature of the imidazole group, may contribute to its enhanced catalytic activity and efficiency. It is important to note that the fuel cell study utilized the in-house TKK TEC10V40E catalyst, featuring a Pt loading of 0.17 mg cm−2. The intriguing observation of an interesting synergy effect between the imidazolium functional group and the PtC catalyst suggests the presence of a complex interplay between these components. Further research is needed to thoroughly elucidate the specific mechanisms and properties responsible for the exceptional performance observed with the Aemion membrane. By unraveling these intricate details, researchers can gain deeper insights into the synergistic interactions between the imidazolium functional group and the PtC catalyst, ultimately paving the way for advancements in AEMFC technology.
Comparing the performance of Pt/C catalysts across FAA, A201, and aQAPS-S8 membranes reveals interesting insights. The A201 membrane, while exhibiting the lowest Pt/C loading, has been discontinued. On the other hand, both FAA and aQAPS-S8 membranes show potential for enhancing AEMFC performance. In a comparative study conducted by Truong et al. [53], an AEMFC using aQAPS-S8 membranes demonstrated varying maximum power densities at different voltages. Specifically, at 0.4 V, the AEMFC achieved a maximum power density of 200 mW cm−2. At 0.3 V and 0.5 V, the maximum power densities recorded were 105 mW cm−2 and 207 mW cm−2, respectively. These studies reported that the aQAPS-S8 membranes were coupled with PtC catalysts at a loading of 0.8 mg cm−2. The incorporation of phenyl groups in the polysulfone backbone of aQAPS-S8 membranes introduces aromatic moieties, which can influence membrane properties such as hydrophilicity, ion conductivity, and mechanical strength. These modifications enhance the overall performance of the membrane in AEMFC applications. The presence of phenyl groups in the polymer backbone contributes to improved ion transport and ion exchange properties. The aromatic structure facilitates better interaction with hydroxide ions, enabling more efficient ion conduction through the membrane. This enhanced ion conductivity allows for lower resistance in the fuel cell, resulting in higher fuel cell intensity and improved power density. The unique characteristics imparted by the polysulfone backbone with phenyl groups enable aQAPS-S8 membranes to exhibit high fuel cell intensity and power density even with lower Pt loading. The improved ion conductivity and stability of the membrane reduce the reliance on higher Pt loading to achieve desired performance levels. This not only enhances the overall efficiency of the AEMFC system but also offers potential cost savings by reducing the amount of expensive Pt catalyst required.
Significantly, the AEMFC employing Ag/C and commercial Ag catalysts demonstrated a decrease in performance of 3.5% and 49.3%, respectively, in comparison to the AEMFC using Pt/C as the cathode catalyst [53]. While this difference in performance might initially appear discouraging, it actually presents a promising opportunity for the utilization of PGM-free cathode catalysts, such as Ag/C, in AEMFC applications. The lower performance observed with Ag/C and commercial Ag catalysts indicates the need for further research and development to enhance their activity and optimize their performance. With continued exploration and improvements in catalyst design, Ag/C and other PGM-free catalysts hold the potential to become viable alternatives to Pt/C, offering cost-effective and sustainable options for AEMFC technology.
To further advance the understanding and application of aQAPS-S8 membranes in AEMFCs, additional research is needed. Future investigations should focus on optimizing membrane properties, exploring different catalyst materials, and evaluating performance under various operating conditions. Additionally, studies on durability, long-term stability, and compatibility with other system components are crucial for a comprehensive understanding and effective utilization of aQAPS-S8 membranes in AEMFC technologies. The FAA membrane exhibited improved performance with increased Pt/C loading at higher temperatures, attributed to enhanced ion conductivity and optimized functional group interactions. The Aemion membrane demonstrated exceptional performance with a lower Pt/C loading, suggesting the influence of the steric crowding nature of imidazole species. It is important to note that while the increase in Pt loading resulted in a substantial improvement in power density, it also entails higher material costs. Therefore, careful consideration must be given to striking an optimal balance between performance gains and economic feasibility when determining the Pt loading for AEMFC applications. Further research is necessary to unravel the precise mechanisms behind the exceptional performance of the Aemion membrane. These advancements signal progress in membrane technology and catalyst utilization, driving continued improvements in AEMFCs and fuel cell applications. The choice of catalyst materials, such as Pt/C, Pt-Ru/C, and TKK TEC10V40E, has a significant impact on the power density achieved. It is evident that Pt-based catalysts generally exhibit higher power densities compared to other catalysts. In summary, recent studies on FAA series membranes and aQAPS-S8 membranes have demonstrated their potential for enhancing the performance of AEMFC systems. The findings highlight the suitability of these membranes for fuel cell applications and the viability of PGM-free catalysts in AEMFC cathodes. The choice of catalyst materials, operating conditions, and membrane characteristics significantly influence the power density achieved. Further research and exploration are necessary to fully exploit the capabilities of these membranes and drive advancements in AEMFC technologies.
4. Future Challenges and Perspectives
In order for AEM technology to reach market maturity and compete with PEM technology, it is crucial to achieve cost parity or superiority while ensuring sufficient stability. Although some AEM systems currently outperform PEM systems, further optimization and assessment of durability are necessary. A recent study by Faqeeh and Symes (2023) reported the impressive performance of the AEM water electrolyzer using Fumasep FAA-3-50 with Stainless Steel and Pt/C catalysts, reaching 2.74 A cm−2 at 2.0 V. However, this system requires optimization to enhance its performance, and the durability of such systems needs to be thoroughly evaluated.
The most significant challenge facing AEMFC and AEMWE is durability. The reported lifetime of AEMFC and AEMWE systems is considerably shorter compared to PEM technology. While a few reports have demonstrated longer lifetimes (500–1000 h) under steady-state operating conditions for AEMWE, the longevity of AEM technology is still at least an order of magnitude lower than that of PEM technology. The integration of innovative catalysts has shown promise in enhancing the performance of AEMWE systems. Additionally, the incorporation of Raney nickel on the cathode of the NiFe2O4-based membrane enabled stable performance over 10,100 h of testing, demonstrating the effectiveness of innovative catalysts in enhancing durability [86]. Researchers have proposed a multitude of strategies to improve the performance of AEWEs and AEMFCs. These include the development of highly stable cationic groups [129], such as cycloaliphatic quaternary ammonium groups [130] and the use of aryl ether-free polymer backbones [131,132,133]. Other strategies involve the introduction of crosslinking strategies [46,134,135,136] and the use of filler [137,138,139]. Recent years have witnessed unprecedented progress in AEMFC technology, largely due to the development of high-performance AEMs and ionomers. Specifically, significant advancements have been made in terms of power densities, with some systems achieving over 3 W cm−2. Furthermore, long-term stability has also improved, with some systems demonstrating operational stability for over 500 h under a current density of 0.6 A cm−2. These advancements underscore the potential of AEM technology and the importance of ongoing research in this field.
It is evident from the literature that AEMWE systems achieving superior performance compared to PEM systems often utilize a 1 M KOH feed. This serves as evidence of the potential of AEM technology. However, it is crucial to consider the ultimate goal of using pure water. This goal is twofold: firstly, to facilitate the integration of AEM technology into existing PEM electrolysis systems, and secondly, to address feed concentration rebalancing issues coupled with different catalysts. By focusing on these aspects, further advancements can be made in the field of AEMWE systems. However, it is important to note that the majority of AEM membranes currently exhibit a lifetime of less than 1000 h, which presents a significant challenge for practical implementation. Therefore, extensive research efforts are still required to overcome these challenges and advance the development of platinum group metal- and precious metal-free AEM technology, focusing on improving both performance and durability. This research should include the investigation of alkaline stability and membrane performance over extended periods, such as 1000 h under 1 M KOH at different temperatures, to address the limitations and enhance the potential of AEM membranes in water electrolysis applications. Therefore, extensive research is still required to overcome these challenges, particularly in demonstrating platinum group metal- and precious metal-free AEM technology and improving both performance and durability.
Advancements in commercial AEM have significantly contributed to making technologies that use AEM more accessible and effective. Still, further improvements are essential to fully understand the potential of these renewable energy transition and storage technologies. The area is ready for investigation, giving the promise of a future with sustainable, reasonable, and efficient energy solutions. In AEMFC and AEMWE systems, the catalysts are typically made of nickel or nickel-based catalysts, which may also contain two or three non-precious elements such as cobalt (Co), manganese (Mn), or nickel-molybdenum-iron (NiMoFe). The findings emphasize the suitability and versatility of commercial AEMs, such as FAA3-50 and Sustainion membranes, for diverse water electrolysis applications. These membranes consistently deliver high current densities and demonstrate robust performance, whether utilizing non-precious or precious metal catalysts. Moreover, the integration of novel catalysts such as nitrogen-doped graphene and Raney nickel has shown promising results in enhancing the overall performance of AEMWE systems. It is also possible for them to contain noble metal compounds such as platinum (Pt), palladium (Pd), iridium dioxide (IrO2), or ruthenium dioxide (RuO2). Ni-based electrocatalysts, for example, have benefits over platinum group metals (PGM) in that they are more affordable and exhibit significant durability and performance in an alkaline environment [131,140,141]. The selection of catalyst materials, such as Pt/C, Pt-Ru/C, and TKK TEC10V40E, plays a crucial role in the power density of fuel cells, with Pt-based catalysts generally delivering higher power densities compared to other catalyst types. This emphasizes the critical nature of catalyst choice when it comes to boosting the performance of fuel cells. In conclusion, recent research on FAA series membranes and aQAPS-S8 membranes has revealed their potential to improve the efficiency of AEMFC systems. These studies underscore the aptness of these membranes for fuel cell uses and the potential of PGM-free catalysts in AEMFC cathodes. Factors such as catalyst materials, operating conditions, and membrane properties notably affect the power density obtained. More investigation and experimentation are needed to fully utilize these membranes’ potential and to push forward the development of AEMFC technologies.
One advantage that PEM development has enjoyed is the availability of mass-produced benchmark materials that are easily obtained. To accelerate the progress of AEM technology and evaluate newly developed ionomers more efficiently, it is crucial to establish a stable, repeatable, high-quality, and standardized testing protocol for AEMs as soon as possible. The absence of such a testing protocol could impede the development of AEMs in comparison to PEMs. However, the positive aspect is that the wide range of seemingly viable AEMs currently being produced at the laboratory scale offers numerous potential paths to overcome the limitations of PEM technology. Ultimately, the decision on the most suitable membrane should consider your specific requirements, priorities, and budgetary considerations. Further research and evaluation of these membranes, including their long-term stability and performance under various operating conditions, would contribute to a more comprehensive understanding of their suitability for electrochemical water-splitting applications. Overall, further advancements, extensive research, and the establishment of standardized testing protocols are essential to overcome the challenges related to performance, durability, and cost reduction in AEM technology, ultimately enabling its successful implementation in various applications.
Author Contributions
Conceptualization, W.Y.W. and W.K.N.; formal analysis, W.Y.W. and W.K.N.; resources, W.Y.W. and K.S.L.; data curation, W.K.N. and N.A.H.R.; writing—original draft preparation, W.K.N.; writing—review and editing, W.Y.W. and N.A.H.R.; visualization, W.K.N.; supervision, W.Y.W.; project administration, W.Y.W.; funding acquisition, K.S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Higher Education Malaysia, grant number TRGS/1/2022/UKM/01/8/2.
Data Availability Statement
No new data were created.
Acknowledgments
The authors acknowledge the support of the Universiti Kebangsaan Malaysia by providing resources in conducting this study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Szulejko, J.E.; Kwon, E.E.; Kim, K.-H. Is mass-scale electrocatalysis of aqueous methanol an energetically and economically viable option for hydrogen production? J. Ind. Eng. Chem. 2022, 105, 58–62. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Arham Khan, M.; Ibrahim, H.; Ekeoma, B.C.; Kamyab, H.; Rahman, M.M.; Nadda, A.K.; Chelliapan, S. A State-of-The-Art Review on the Latest trends in Hydrogen production, storage, and transportation techniques. Fuel 2023, 340, 127574. [Google Scholar] [CrossRef]
- Tang, D.; Tan, G.-L.; Li, G.-W.; Liang, J.-G.; Ahmad, S.M.; Bahadur, A.; Humayun, M.; Ullah, H.; Khan, A.; Bououdina, M. State-of-the-art hydrogen generation techniques and storage methods: A critical review. J. Energy Storage 2023, 64, 107196. [Google Scholar] [CrossRef]
- Neumann, J.; da Rocha, R.C.; Debiagi, P.; Scholtissek, A.; Dammel, F.; Stephan, P.; Hasse, C. Techno-economic assessment of long-distance supply chains of energy carriers: Comparing hydrogen and iron for carbon-free electricity generation. Appl. Energy Combust. Sci. 2023, 14, 100128. [Google Scholar] [CrossRef]
- Rasul, M.G.; Hazrat, M.A.; Sattar, M.A.; Jahirul, M.I.; Shearer, M.J. The future of hydrogen: Challenges on production, storage and applications. Energy Convers. Manag. 2022, 272, 116326. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Oko, E.; Kuang, B.; Bello, A.; Onwualu, A.P.; Oyagha, S.; Whidborne, J. The prospects of hydrogen in achieving net zero emissions by 2050: A critical review. Sustain. Chem. Clim. Action 2023, 2, 100024. [Google Scholar] [CrossRef]
- İnci, M. Future vision of hydrogen fuel cells: A statistical review and research on applications, socio-economic impacts and forecasting prospects. Sustain. Energy Technol. Assess. 2022, 53, 102739. [Google Scholar] [CrossRef]
- Yang, D.; Lee, J.; Song, N.C.; Lee, S.; Kim, S.; Lee, S.; Choi, S. Patent analysis on green hydrogen technology for future promising technologies. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Mohamad Nor, N.A.; Mohamed, M.A.; Jaafar, J. Modified sulfonated polyphenylsulfone proton exchange membrane with enhanced fuel cell performance: A review. J. Ind. Eng. Chem. 2022, 116, 32–59. [Google Scholar] [CrossRef]
- Ul Haq, T.; Haik, Y. A roadmap towards sustainable anode design for alkaline water electrolysis. Appl. Catal. B Environ. 2023, 334, 122853. [Google Scholar] [CrossRef]
- Wang, S.; Geng, Z.; Bi, S.; Wang, Y.; Gao, Z.; Jin, L.; Zhang, C. Recent advances and future prospects on Ni3S2-Based electrocatalysts for efficient alkaline water electrolysis. Green Energy Environ. 2023. [Google Scholar] [CrossRef]
- Seo, K.; Nam, K.-H.; Han, H. Chain end-termination of p-polybenzimidazole by bulk segment for efficient electrochemical power generation and hydrogen separation. J. Ind. Eng. Chem. 2020, 91, 85–92. [Google Scholar] [CrossRef]
- Ayers, K. High efficiency PEM water electrolysis: Enabled by advanced catalysts, membranes, and processes. Curr. Opin. Chem. Eng. 2021, 33, 100719. [Google Scholar] [CrossRef]
- Samad, S.; Loh, K.S.; Wong, W.Y.; Lee, T.K.; Sunarso, J.; Chong, S.T.; Wan Daud, W.R. Carbon and non-carbon support materials for platinum-based catalysts in fuel cells. Int. J. Hydrogen Energy 2018, 43, 7823–7854. [Google Scholar] [CrossRef]
- Sinniah, J.D.; Wong, W.Y.; Loh, K.S.; Yunus, R.M.; Timmiati, S.N. Perspectives on carbon-alternative materials as Pt catalyst supports for a durable oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2022, 534, 231422. [Google Scholar] [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Ramya, K.; Khalid, M.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Walvekar, R.; Kadhum, A.A.H. Additives in proton exchange membranes for low- and high-temperature fuel cell applications: A review. Int. J. Hydrogen Energy 2019, 44, 6116–6135. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.A.H.; Rooney, D.W. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Zhang, M.; Ma, Y.; Hu, J.; Qu, Y. Sustainable production of hydrogen with high purity from methanol and water at low temperatures. Nat. Commun. 2022, 13, 5527. [Google Scholar] [CrossRef]
- Lim, K.L.; Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Selambakkannu, S.; Othman, N.A.; Yang, H. Radiation-Grafted Anion-Exchange Membrane for Fuel Cell and Electrolyzer Applications: A Mini Review. Membranes 2021, 11, 397. [Google Scholar] [CrossRef]
- Li, C.; Baek, J.-B. The promise of hydrogen production from alkaline anion exchange membrane electrolyzers. Nano Energy 2021, 87, 106162. [Google Scholar] [CrossRef]
- Raja Sulaiman, R.R.; Wong, W.Y.; Loh, K.S. Recent developments on transition metal–based electrocatalysts for application in anion exchange membrane water electrolysis. Int. J. Energy Res. 2022, 46, 2241–2276. [Google Scholar] [CrossRef]
- Rahim Malik, F.; Yuan, H.-B.; Moran, J.C.; Tippayawong, N. Overview of hydrogen production technologies for fuel cell utilization. Eng. Sci. Technol. Int. J. 2023, 43, 101452. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Nam, S.Y. Recent advancements in applications of alkaline anion exchange membranes for polymer electrolyte fuel cells. J. Ind. Eng. Chem. 2019, 70, 70–86. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Chen, Y.-Y.; Zhuang, Q.; Li, G. Recent advances in MOFs-based proton exchange membranes. Coord. Chem. Rev. 2022, 471, 214740. [Google Scholar] [CrossRef]
- Peera, S.G.; Liu, C. Unconventional and scalable synthesis of non-precious metal electrocatalysts for practical proton exchange membrane and alkaline fuel cells: A solid-state co-ordination synthesis approach. Coord. Chem. Rev. 2022, 463, 214554. [Google Scholar] [CrossRef]
- Feng, Z.; Gupta, G.; Mamlouk, M. A review of anion exchange membranes prepared via Friedel-Crafts reaction for fuel cell and water electrolysis. Int. J. Hydrogen Energy 2023, 48, 25830–25858. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Park, S.-J.; Thorat, H.N.; Bodkhe, G.A.; Ramanavicius, A.; Ramanavicius, S.; Shirsat, M.D.; Ha, T.-J. Advanced energy materials: Current trends and challenges in electro- and photo-catalysts for H2O splitting. J. Ind. Eng. Chem. 2023, 119, 90–111. [Google Scholar] [CrossRef]
- Palmas, S.; Rodriguez, J.; Mais, L.; Mascia, M.; Herrando, M.C.; Vacca, A. Anion exchange membrane: A valuable perspective in emerging technologies of low temperature water electrolysis. Curr. Opin. Electrochem. 2023, 37, 101178. [Google Scholar] [CrossRef]
- Henkensmeier, D.; Najibah, M.; Harms, C.; Žitka, J.; Hnát, J.; Bouzek, K. Overview: State-of-the Art Commercial Membranes for Anion Exchange Membrane Water Electrolysis. J. Electrochem. Energy Convers. Storage 2020, 18, 024001. [Google Scholar] [CrossRef]
- Thompson, S.T.; Peterson, D.; Ho, D.; Papageorgopoulos, D. Perspective—The Next Decade of AEMFCs: Near-Term Targets to Accelerate Applied R&D. J. Electrochem. Soc. 2020, 167, 084514. [Google Scholar] [CrossRef]
- Mustain, W.E.; Chatenet, M.; Page, M.; Kim, Y.S. Durability challenges of anion exchange membrane fuel cells. Energy Environ. Sci. 2020, 13, 2805–2838. [Google Scholar] [CrossRef]
- Liu, J.; Choi, H.J.; Meng, L.-Y. A review of approaches for the design of high-performance metal/graphene electrocatalysts for fuel cell applications. J. Ind. Eng. Chem. 2018, 64, 1–15. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, L.; Yuan, Z.-Y. Recent advances in transition metal layered double hydroxide based materials as efficient electrocatalysts. J. Ind. Eng. Chem. 2023, 120, 27–46. [Google Scholar] [CrossRef]
- Fashedemi, O.O.; Bello, A.; Adebusuyi, T.; Bindir, S. Recent trends in carbon support for improved performance of alkaline fuel cells. Curr. Opin. Electrochem. 2022, 36, 101132. [Google Scholar] [CrossRef]
- Du, N.; Roy, C.; Peach, R.; Turnbull, M.; Thiele, S.; Bock, C. Anion-Exchange Membrane Water Electrolyzers. Chem. Rev. 2022, 122, 11830–11895. [Google Scholar] [CrossRef]
- David, M.; Ocampo-Martínez, C.; Sánchez-Peña, R. Advances in alkaline water electrolyzers: A review. J. Energy Storage 2019, 23, 392–403. [Google Scholar] [CrossRef]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weißgärber, T.; Röntzsch, L.; Meier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: A review of recent developments in critical materials and operating conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Zhang, J.; Wang, J.; Hu, Y.; Jiang, H.; Li, C. Anion Exchange Membrane Water Electrolyzer: Electrode Design, Lab-Scaled Testing System and Performance Evaluation. EnergyChem 2022, 4, 100087. [Google Scholar] [CrossRef]
- Gohil, J.M.; Dutta, K. Structures and properties of polymers in ion exchange membranes for hydrogen generation by water electrolysis. Polym. Adv. Technol. 2021, 32, 4598–4615. [Google Scholar] [CrossRef]
- Salvatore, D.A.; Gabardo, C.M.; Reyes, A.; O’Brien, C.P.; Holdcroft, S.; Pintauro, P.; Bahar, B.; Hickner, M.; Bae, C.; Sinton, D.; et al. Designing anion exchange membranes for CO2 electrolysers. Nat. Energy 2021, 6, 339–348. [Google Scholar] [CrossRef]
- Han, J.; Song, W.; Cheng, X.; Cheng, Q.; Zhang, Y.; Liu, C.; Zhou, X.; Ren, Z.; Hu, M.; Ning, T.; et al. Conductivity and Stability Properties of Anion Exchange Membranes: Cation Effect and Backbone Effect. ChemSusChem 2021, 14, 5021–5031. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Noonan, K.J.T.; Coates, G.W. Alkaline-stable anion exchange membranes: A review of synthetic approaches. Prog. Polym. Sci. 2020, 100, 101177. [Google Scholar] [CrossRef]
- Wei, C.; Yu, W.; Zhang, Y.; Zhang, F.; Li, M.; Shen, X.; Zhang, K.; Ge, X.; Wu, L.; Xu, T. Alkaline anion exchange membrane containing pyrene-based π-π stacking interactions. J. Power Sources 2023, 553, 232247. [Google Scholar] [CrossRef]
- Raja Rafidah, R.S.; Rashmi, W.; Khalid, M.; Wong, W.Y.; Priyanka, J. Recent Progress in the Development of Aromatic Polymer-Based Proton Exchange Membranes for Fuel Cell Applications. Polymers 2020, 12, 1061. [Google Scholar] [CrossRef]
- Hossain, M.M.; Wu, L.; Liang, X.; Yang, Z.; Hou, J.; Xu, T. Anion exchange membrane crosslinked in the easiest way stands out for fuel cells. J. Power Sources 2018, 390, 234–241. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Wang, J. Crosslinked anion exchange membrane with improved membrane stability and conductivity for alkaline fuel cells. J. Appl. Polym. Sci. 2019, 136, 48169. [Google Scholar] [CrossRef]
- Konovalova, A.; Kim, H.; Kim, S.; Lim, A.; Park, H.S.; Kraglund, M.R.; Aili, D.; Jang, J.H.; Kim, H.-J.; Henkensmeier, D. Blend membranes of polybenzimidazole and an anion exchange ionomer (FAA3) for alkaline water electrolysis: Improved alkaline stability and conductivity. J. Membr. Sci. 2018, 564, 653–662. [Google Scholar] [CrossRef]
- Kaczur, J.J.; Yang, H.; Liu, Z.; Sajjad, S.D.; Masel, R.I. Carbon Dioxide and Water Electrolysis Using New Alkaline Stable Anion Membranes. Front. Chem. 2018, 6, 263. [Google Scholar] [CrossRef]
- Marinkas, A.; Struźyńska-Piron, I.; Lee, Y.; Lim, A.; Park, H.S.; Jang, J.H.; Kim, H.-J.; Kim, J.; Maljusch, A.; Conradi, O.; et al. Anion-conductive membranes based on 2-mesityl-benzimidazolium functionalised poly(2,6-dimethyl-1,4-phenylene oxide) and their use in alkaline water electrolysis. Polymer 2018, 145, 242–251. [Google Scholar] [CrossRef]
- Thomas, O.D.; Soo, K.J.W.Y.; Peckham, T.J.; Kulkarni, M.P.; Holdcroft, S. A Stable Hydroxide-Conducting Polymer. J. Am. Chem. Soc. 2012, 134, 10753–10756. [Google Scholar] [CrossRef]
- Hassan, N.U.; Motyka, E.; Kweder, J.; Ganesan, P.; Brechin, B.; Zulevi, B.; Colón-Mercado, H.R.; Kohl, P.A.; Mustain, W.E. Effect of porous transport layer properties on the anode electrode in anion exchange membrane electrolyzers. J. Power Sources 2023, 555, 232371. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Setzler, B.P.; Rojas-Carbonell, S.; Ben Yehuda, C.; Amel, A.; Page, M.; Wang, L.; Hu, K.; Shi, L.; et al. Poly(aryl piperidinium) membranes and ionomers for hydroxide exchange membrane fuel cells. Nat. Energy 2019, 4, 392–398. [Google Scholar] [CrossRef]
- Truong, V.M.; Yang, M.-K.; Yang, H. Functionalized Carbon Black Supported Silver (Ag/C) Catalysts in Cathode Electrode for Alkaline Anion Exchange Membrane Fuel Cells. Int. J. Precis. Eng. Manuf.-Green Technol. 2019, 6, 711–721. [Google Scholar] [CrossRef]
- Lee, W.-H.; Park, E.J.; Han, J.; Shin, D.W.; Kim, Y.S.; Bae, C. Poly(terphenylene) Anion Exchange Membranes: The Effect of Backbone Structure on Morphology and Membrane Property. ACS Macro Lett. 2017, 6, 566–570. [Google Scholar] [CrossRef]
- Kressman, T.R.E. Ion Exchange Resin Membranes and Resin-Impregnated Filter Paper. Nature 1950, 165, 568. [Google Scholar] [CrossRef]
- Bayer, T.; Cunning, B.V.; Selyanchyn, R.; Daio, T.; Nishihara, M.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. Alkaline anion exchange membranes based on KOH-treated multilayer graphene oxide. J. Membr. Sci. 2016, 508, 51–61. [Google Scholar] [CrossRef]
- Moruno, F.L.; Rubio, J.E.; Santoro, C.; Atanassov, P.; Cerrato, J.M.; Arges, C.G. Investigation of patterned and non-patterned poly(2,6-dimethyl 1,4-phenylene) oxide based anion exchange membranes for enhanced desalination and power generation in a microbial desalination cell. Solid State Ion. 2018, 314, 141–148. [Google Scholar] [CrossRef]
- Sarapulova, V.; Pismenskaya, N.; Titorova, V.; Sharafan, M.; Wang, Y.; Xu, T.; Zhang, Y.; Nikonenko, V. Transport Characteristics of CJMAED™ Homogeneous Anion Exchange Membranes in Sodium Chloride and Sodium Sulfate Solutions. Int. J. Mol. Sci. 2021, 22, 31415. [Google Scholar] [CrossRef]
- Čížek, J.; Cvejn, P.; Marek, J.; Tvrzník, D. Desalination Performance Assessment of Scalable, Multi-Stack Ready Shock Electrodialysis Unit Utilizing Anion-Exchange Membranes. Membranes 2020, 10, 347. [Google Scholar] [CrossRef]
- Carbone, A.; Zignani, S.C.; Gatto, I.; Pedicini, R.; Oldani, C.; Cattaneo, A.; Aricò, A.S. Aquivion-based anion exchange membranes: Synthesis optimization via dispersant agents and reaction time. Chem. Eng. J. 2023, 455, 140765. [Google Scholar] [CrossRef]
- Afsar, N.U.; Li, X.; Zhu, Y.; Ge, Z.; Zhou, Y.; Zhao, Z.; Hussain, A.; Ge, L.; Fu, R.; Liu, Z.; et al. In-situ interfacial polymerization endows surface enrichment of –COOH groups on anion exchange membranes for efficient Cl−/SO42− separation. J. Polym. Sci. 2022, 60, 3022–3034. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Lang, Q.; Tan, M.; Zhang, Y. Composite anion exchange membrane made by layer-by-layer method for selective ion separation and water migration control. Sep. Purif. Technol. 2018, 192, 278–286. [Google Scholar] [CrossRef]
- Wang, W.; Yang, C.; Wang, W.; Fu, R.; Wang, H. Novel compact ion exchange membranes through suppressing reverse permeation for high-efficiency recovery of inorganic acids. Sep. Purif. Technol. 2022, 289, 120725. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.; Ji, G.; Zhong, J.; Zhang, F.; Chen, M.; Tong, S.; Wang, P.; Wu, Z.; Han, B.; et al. Partially Nitrided Ni Nanoclusters Achieve Energy-Efficient Electrocatalytic CO2 Reduction to CO at Ultralow Overpotential. Adv. Mater. 2023, 35, 2205262. [Google Scholar] [CrossRef]
- Hao, Q.; Liu, D.-X.; Deng, R.; Zhong, H.-X. Boosting Electrochemical Carbon Dioxide Reduction on Atomically Dispersed Nickel Catalyst. Front. Chem. 2022, 9, 837580. [Google Scholar] [CrossRef]
- Hao, J.; Zhuang, Z.; Hao, J.; Cao, K.; Hu, Y.; Wu, W.; Lu, S.; Wang, C.; Zhang, N.; Wang, D.; et al. Strain Relaxation in Metal Alloy Catalysts Steers the Product Selectivity of Electrocatalytic CO2 Reduction. ACS Nano 2022, 16, 3251–3263. [Google Scholar] [CrossRef]
- Cui, Y.-f.; Zhu, Y.-h.; Du, J.-y.; Zhang, Y.-l.; Li, K.; Liu, W.-q.; Huang, G.; Zhang, X.-b. A high-voltage and stable zinc-air battery enabled by dual-hydrophobic-induced proton shuttle shielding. Joule 2022, 6, 1617–1631. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Luo, L.; Li, H.; He, J.; Zu, H.; Liu, L.; Liu, H.; Wang, F.; Song, J. Blocking polysulfides with a Janus Fe3C/N-CNF@RGO electrode via physiochemical confinement and catalytic conversion for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2021, 9, 2205–2213. [Google Scholar] [CrossRef]
- Yuan, J.; Yin, H.; Ge, X.; Pan, R.; Huang, C.; Chen, D.; Hu, L.; Xie, H. Superior efficiency hydrogen peroxide production in acidic media through epoxy group adjacent to Co-O/C active centers on carbon black. Chem. Eng. J. 2023, 465, 142691. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, B.; Seo, J.Y.; Kim, Y.S.; Yoo, P.J.; Chung, C.-H. Water electrolysis and desalination using an AEM/CEM hybrid electrochemical system. Appl. Surf. Sci. 2023, 610, 155524. [Google Scholar] [CrossRef]
- Richard, I.; Masel, Q.C.; Liu, Z.; Kutz, R. Electrochemical Device for Converting Carbon Dioxide to a Reaction Product. U.S. Patent US9481939B2, 1 November 2016. [Google Scholar]
- Díaz-Sainz, G.; Alvarez-Guerra, M.; Irabien, A. Continuous electroreduction of CO2 towards formate in gas-phase operation at high current densities with an anion exchange membrane. J. CO2 Util. 2022, 56, 101822. [Google Scholar] [CrossRef]
- Yang, W.; Xue, Z.; Yang, J.; Xian, J.; Liu, Q.; Fan, Y.; Zheng, K.; Liao, P.; Su, H.; Liu, Q.; et al. Fe nanoparticles embedded in N-doped porous carbon for enhanced electrocatalytic CO2 reduction and Zn-CO2 battery. Chin. J. Catal. 2023, 48, 185–194. [Google Scholar] [CrossRef]
- Moreno-González, M.; Mardle, P.; Zhu, S.; Gholamkhass, B.; Jones, S.; Chen, N.; Britton, B.; Holdcroft, S. One year operation of an anion exchange membrane water electrolyzer utilizing Aemion+® membrane: Minimal degradation, low H2 crossover and high efficiency. J. Power Sources Adv. 2023, 19, 100109. [Google Scholar] [CrossRef]
- Fortin, P.; Khoza, T.; Cao, X.; Martinsen, S.Y.; Oyarce Barnett, A.; Holdcroft, S. High-performance alkaline water electrolysis using Aemion™ anion exchange membranes. J. Power Sources 2020, 451, 227814. [Google Scholar] [CrossRef]
- Gangrade, A.S.; Cassegrain, S.; Chandra Ghosh, P.; Holdcroft, S. Permselectivity of ionene-based, Aemion® anion exchange membranes. J. Membr. Sci. 2022, 641, 119917. [Google Scholar] [CrossRef]
- Khalid, H.; Najibah, M.; Park, H.S.; Bae, C.; Henkensmeier, D. Properties of Anion Exchange Membranes with a Focus on Water Electrolysis. Membranes 2022, 12, 989. [Google Scholar] [CrossRef]
- Faqeeh, A.H.; Symes, M.D. A standard electrolyzer test cell design for evaluating catalysts and cell components for anion exchange membrane water electrolysis. Electrochim. Acta 2023, 444, 142030. [Google Scholar] [CrossRef]
- Caprì, A.; Gatto, I.; Lo Vecchio, C.; Trocino, S.; Carbone, A.; Baglio, V. Anion Exchange Membrane Water Electrolysis Based on Nickel Ferrite Catalysts. ChemElectroChem 2023, 10, e202201056. [Google Scholar] [CrossRef]
- Gatto, I.; Caprì, A.; Lo Vecchio, C.; Zignani, S.; Patti, A.; Baglio, V. Optimal operating conditions evaluation of an anion-exchange-membrane electrolyzer based on FUMASEP® FAA3-50 membrane. Int. J. Hydrogen Energy 2023, 48, 11914–11921. [Google Scholar] [CrossRef]
- Carbone, A.; Zignani, S.C.; Gatto, I.; Trocino, S.; Aricò, A.S. Assessment of the FAA3-50 polymer electrolyte in combination with a NiMn2O4 anode catalyst for anion exchange membrane water electrolysis. Int. J. Hydrogen Energy 2020, 45, 9285–9292. [Google Scholar] [CrossRef]
- Tham, D.D.; Kim, D. C2 and N3 substituted imidazolium functionalized poly(arylene ether ketone) anion exchange membrane for water electrolysis with improved chemical stability. J. Membr. Sci. 2019, 581, 139–149. [Google Scholar] [CrossRef]
- Guo, W.; Kim, J.; Kim, H.; Hong, S.; Kim, H.; Kim, S.Y.; Ahn, S.H. Electrochemically activated Ni@Ni(OH)2 heterostructure as efficient hydrogen evolution reaction electrocatalyst for anion exchange membrane water electrolysis. Mater. Today Chem. 2022, 24, 100994. [Google Scholar] [CrossRef]
- López-Fernández, E.; Gómez-Sacedón, C.; Gil-Rostra, J.; Espinós, J.P.; Brey, J.J.; González-Elipe, A.R.; de Lucas-Consuegra, A.; Yubero, F. Optimization of anion exchange membrane water electrolyzers using ionomer-free electrodes. Renew. Energy 2022, 197, 1183–1191. [Google Scholar] [CrossRef]
- Chen, N.; Paek, S.Y.; Lee, J.Y.; Park, J.H.; Lee, S.Y.; Lee, Y.M. High-performance anion exchange membrane water electrolyzers with a current density of 7.68 A cm−2 and a durability of 1000 h. Energy Environ. Sci. 2021, 14, 6338–6348. [Google Scholar] [CrossRef]
- Motealleh, B.; Liu, Z.; Masel, R.I.; Sculley, J.P.; Richard Ni, Z.; Meroueh, L. Next-generation anion exchange membrane water electrolyzers operating for commercially relevant lifetimes. Int. J. Hydrogen Energy 2021, 46, 3379–3386. [Google Scholar] [CrossRef]
- Guo, W.; Kim, J.; Kim, H.; Ahn, S.H. Cu–Co–P electrodeposited on carbon paper as an efficient electrocatalyst for hydrogen evolution reaction in anion exchange membrane water electrolyzers. Int. J. Hydrogen Energy 2021, 46, 19789–19801. [Google Scholar] [CrossRef]
- Park, Y.S.; Jeong, J.; Noh, Y.; Jang, M.J.; Lee, J.; Lee, K.H.; Lim, D.C.; Seo, M.H.; Kim, W.B.; Yang, J.; et al. Commercial anion exchange membrane water electrolyzer stack through non-precious metal electrocatalysts. Appl. Catal. B Environ. 2021, 292, 120170. [Google Scholar] [CrossRef]
- Cho, M.K.; Park, H.-Y.; Lee, H.J.; Kim, H.-J.; Lim, A.; Henkensmeier, D.; Yoo, S.J.; Kim, J.Y.; Lee, S.Y.; Park, H.S.; et al. Alkaline anion exchange membrane water electrolysis: Effects of electrolyte feed method and electrode binder content. J. Power Sources 2018, 382, 22–29. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.; Zhao, Z.; Shi, L.; Wu, B.; Zeng, L. Iron-doped metal-organic framework with enhanced oxygen evolution reaction activity for overall water splitting. Int. J. Hydrogen Energy 2021, 46, 34565–34573. [Google Scholar] [CrossRef]
- Khataee, A.; Shirole, A.; Jannasch, P.; Krüger, A.; Cornell, A. Anion exchange membrane water electrolysis using Aemion™ membranes and nickel electrodes. J. Mater. Chem. A 2022, 10, 16061–16070. [Google Scholar] [CrossRef]
- Ruck, S.; Körner, A.; Hutzler, A.; Bierling, M.; Gonzalez, J.; Qu, W.; Bock, C.; Thiele, S.; Peach, R.; Pham, C.V. Carbon supported NiRu nanoparticles as effective hydrogen evolution catalysts for anion exchange membrane water electrolyzers. J. Phys. Energy 2022, 4, 044007. [Google Scholar] [CrossRef]
- Xia, L.; Jiang, W.; Hartmann, H.; Mayer, J.; Lehnert, W.; Shviro, M. Multistep Sulfur Leaching for the Development of a Highly Efficient and Stable NiSx/Ni(OH)2/NiOOH Electrocatalyst for Anion Exchange Membrane Water Electrolysis. ACS Appl. Mater. Interfaces 2022, 14, 19397–19408. [Google Scholar] [CrossRef]
- Caielli, T.; Ferrari, A.R.; Bonizzoni, S.; Sediva, E.; Caprì, A.; Santoro, M.; Gatto, I.; Baglio, V.; Mustarelli, P. Synthesis, characterization and water electrolyzer cell tests of poly(biphenyl piperidinium) Anion exchange membranes. J. Power Sources 2023, 557, 232532. [Google Scholar] [CrossRef]
- Wan, L.; Xu, Z.; Wang, B. Green preparation of highly alkali-resistant PTFE composite membranes for advanced alkaline water electrolysis. Chem. Eng. J. 2021, 426, 131340. [Google Scholar] [CrossRef]
- Patra, S.; Soman, B.; Vineesh, T.; Shyaga, N.; Narayanan, T.N. Eggshell membrane-based water electrolysis cells. Mater. Chem. Front. 2020, 4, 567–573. [Google Scholar] [CrossRef]
- Vincent, I.; Kruger, A.; Bessarabov, D. Development of efficient membrane electrode assembly for low cost hydrogen production by anion exchange membrane electrolysis. Int. J. Hydrogen Energy 2017, 42, 10752–10761. [Google Scholar] [CrossRef]
- Aguedo, J.; Lorencova, L.; Barath, M.; Farkas, P.; Tkac, J. Electrochemical Impedance Spectroscopy on 2D Nanomaterial MXene Modified Interfaces: Application as a Characterization and Transducing Tool. Chemosensors 2020, 8, 127. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, B.; Gu, X.; Liu, H.; Feng, L. Selective structure transformation for NiFe/NiFe2O4 embedded porous nitrogen-doped carbon nanosphere with improved oxygen evolution reaction activity. Chem. Eng. J. 2020, 395, 125170. [Google Scholar] [CrossRef]
- Wang, J.; Teng, X.; Niu, Y.; Guo, L.; Kong, J.; He, X.; Chen, Z. In situ autologous growth of self-supporting NiFe-based nanosheets on nickel foam as an efficient electrocatalyst for the oxygen evolution reaction. RSC Adv. 2019, 9, 21679–21684. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, Y.; Chen, X.; Li, Y.; Su, Z.; Zhao, C. Vertical Growth of Porous Perovskite Nanoarrays on Nickel Foam for Efficient Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2020, 8, 4863–4870. [Google Scholar] [CrossRef]
- Gao, J.; Xu, C.-Q.; Hung, S.-F.; Liu, W.; Cai, W.; Zeng, Z.; Jia, C.; Chen, H.M.; Xiao, H.; Li, J.; et al. Breaking Long-Range Order in Iridium Oxide by Alkali Ion for Efficient Water Oxidation. J. Am. Chem. Soc. 2019, 141, 3014–3023. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Kirk, D.W. Platinum Stability at the Cathode of an Anion Exchange Membrane Fuel Cell. Electrocatalysis 2020, 11, 292–300. [Google Scholar] [CrossRef]
- López-Fernández, E.; Gómez-Sacedón, C.; Gil-Rostra, J.; Espinós, J.P.; González-Elipe, A.R.; Yubero, F.; de Lucas-Consuegra, A. Ionomer-Free Nickel-Iron bimetallic electrodes for efficient anion exchange membrane water electrolysis. Chem. Eng. J. 2022, 433, 133774. [Google Scholar] [CrossRef]
- Roschger, M.; Wolf, S.; Billiani, A.; Mayer, K.; Hren, M.; Gorgieva, S.; Genorio, B.; Hacker, V. Study on Commercially Available Membranes for Alkaline Direct Ethanol Fuel Cells. ACS Omega 2023, 8, 20845–20857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sajjad, S.D.; Gao, Y.; Yang, H.; Kaczur, J.J.; Masel, R.I. The effect of membrane on an alkaline water electrolyzer. Int. J. Hydrogen Energy 2017, 42, 29661–29665. [Google Scholar] [CrossRef]
- Moureaux, F.; Stevens, P.; Toussaint, G.; Chatenet, M. Timely-activated 316L stainless steel: A low cost, durable and active electrode for oxygen evolution reaction in concentrated alkaline environments. Appl. Catal. B Environ. 2019, 258, 117963. [Google Scholar] [CrossRef]
- Campagna Zignani, S.; Faro, M.L.; Carbone, A.; Italiano, C.; Trocino, S.; Monforte, G.; Aricò, A.S. Performance and stability of a critical raw materials-free anion exchange membrane electrolysis cell. Electrochim. Acta 2022, 413, 140078. [Google Scholar] [CrossRef]
- Zhang, P.; Shen, B.; Pu, H. Robust, dimensional stable, and self-healable anion exchange membranes via quadruple hydrogen bonds. Polymer 2022, 245, 124698. [Google Scholar] [CrossRef]
- Tang, Q.; Li, F.; Chen, H.; Yin, X.; Tang, Y.; Zeng, Q. Synthesis of (Enantioenriched) Dibenzyl Thioethers and Disulfides via SN2 Nucleophilic Substitution of Quaternary Ammonium Salts. Asian J. Org. Chem. 2021, 10, 1687–1690. [Google Scholar] [CrossRef]
- Sang, J.; Yang, L.; Wang, F.; Wang, Z.; Zhu, H. SEBS-Based Anion Exchange Membrane Grafted with N-Spirocyclic Quaternary Ammonium Cations for Fuel Cells. ACS Appl. Energy Mater. 2022, 5, 12347–12358. [Google Scholar] [CrossRef]
- Peltier, C.R.; You, W.; Fackovic Volcanjk, D.; Li, Q.; Macbeth, A.J.; Abruña, H.D.; Coates, G.W. Quaternary Ammonium-Functionalized Polyethylene-Based Anion Exchange Membranes: Balancing Performance and Stability. ACS Energy Lett. 2023, 8, 2365–2372. [Google Scholar] [CrossRef]
- Kleijwegt, R.J.T.; Winkenwerder, W.; Baan, W.; van der Schaaf, J. Degradation kinetics and solvent effects of various long-chain quaternary ammonium salts. Int. J. Chem. Kinet. 2022, 54, 16–27. [Google Scholar] [CrossRef]
- Hall, D.S.; Bock, C.; MacDougall, B.R. The Electrochemistry of Metallic Nickel: Oxides, Hydroxides, Hydrides and Alkaline Hydrogen Evolution. J. Electrochem. Soc. 2013, 160, F235. [Google Scholar] [CrossRef]
- Safaei, Z.; Shiroudi, A.; Zahedi, E.; Sillanpää, M. Atmospheric oxidation reactions of imidazole initiated by hydroxyl radicals: Kinetics and mechanism of reactions and atmospheric implications. Phys. Chem. Chem. Phys. 2019, 21, 8445–8456. [Google Scholar] [CrossRef]
- Lee, B.; Yun, D.; Lee, J.-S.; Park, C.H.; Kim, T.-H. Development of Highly Alkaline Stable OH−-Conductors Based on Imidazolium Cations with Various Substituents for Anion Exchange Membrane-Based Alkaline Fuel Cells. J. Phys. Chem. C 2019, 123, 13508–13518. [Google Scholar] [CrossRef]
- Olsson, J.S.; Pham, T.H.; Jannasch, P. Functionalizing Polystyrene with N-Alicyclic Piperidine-Based Cations via Friedel–Crafts Alkylation for Highly Alkali-Stable Anion-Exchange Membranes. Macromolecules 2020, 53, 4722–4732. [Google Scholar] [CrossRef] [PubMed]
- Holdcroft, S.; Fan, J. Sterically-encumbered ionenes as hydroxide ion-conducting polymer membranes. Curr. Opin. Electrochem. 2019, 18, 99–105. [Google Scholar] [CrossRef]
- Noor Azam, A.M.; Ragunathan, T.; Zulkefli, N.N.; Masdar, M.S.; Majlan, E.H.; Mohamad Yunus, R.; Shamsul, N.S.; Husaini, T.; Shaffee, S.N. Investigation of Performance of Anion Exchange Membrane (AEM) Electrolysis with Different Operating Conditions. Polymers 2023, 15, 1301. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.J.; Yang, S.H.; Park, M.G.; Jeong, J.; Cha, M.S.; Shin, S.-H.; Lee, K.H.; Bai, Z.; Chen, Z.; Lee, J.Y.; et al. Efficient and Durable Anion Exchange Membrane Water Electrolysis for a Commercially Available Electrolyzer Stack using Alkaline Electrolyte. ACS Energy Lett. 2022, 7, 2576–2583. [Google Scholar] [CrossRef]
- Hwang, M.; Sun, R.; Willis, C.; Elabd, Y.A. Solid-State Alkaline Fuel Cell Performance of Pentablock Terpolymer with Methylpyrrolidinium Cations as Anion Exchange Membrane and Ionomer. Fuel Cells 2020, 20, 624–633. [Google Scholar] [CrossRef]
- Son, T.Y.; Kim, T.-H.; Nam, S.Y. Crosslinked Pore-Filling Anion Exchange Membrane Using the Cylindrical Centrifugal Force for Anion Exchange Membrane Fuel Cell System. Polymers 2020, 12, 2758. [Google Scholar] [CrossRef]
- Son, T.Y.; Ko, T.H.; Vijayakumar, V.; Kim, K.; Nam, S.Y. Anion exchange composite membranes composed of poly(phenylene oxide) containing quaternary ammonium and polyethylene support for alkaline anion exchange membrane fuel cell applications. Solid State Ion. 2020, 344, 115153. [Google Scholar] [CrossRef]
- Vecchio, C.L.; Lyu, X.; Gatto, I.; Zulevi, B.; Serov, A.; Baglio, V.; Vecchio, C.L.; Gatto, I.; Baglio, V. Performance investigation of alkaline direct methanol fuel cell with commercial PGM-free cathodic materials. J. Power Sources 2023, 561, 232732. [Google Scholar] [CrossRef]
- Sebastián, D.; Lemes, G.; Luque-Centeno, J.M.; Martínez-Huerta, M.V.; Pardo, J.I.; Lázaro, M.J. Optimization of the Catalytic Layer for Alkaline Fuel Cells Based on Fumatech Membranes and Ionomer. Catalysts 2020, 10, 1353. [Google Scholar] [CrossRef]
- Linge, J.M.; Erikson, H.; Mooste, M.; Piirsoo, H.-M.; Kaljuvee, T.; Kikas, A.; Aruväli, J.; Kisand, V.; Tamm, A.; Kannan, A.M.; et al. Ag nanoparticles on mesoporous carbon support as cathode catalyst for anion exchange membrane fuel cell. Int. J. Hydrogen Energy 2023, 48, 11058–11070. [Google Scholar] [CrossRef]
- Novalin, T.; Pan, D.; Lindbergh, G.; Lagergren, C.; Jannasch, P.; Lindström, R.W. Electrochemical performance of poly(arylene piperidinium) membranes and ionomers in anion exchange membrane fuel cells. J. Power Sources 2021, 507, 230287. [Google Scholar] [CrossRef]
- Reshetenko, T.; Odgaard, M.; Schlueter, D.; Serov, A. Analysis of alkaline exchange membrane fuel cells performance at different operating conditions using DC and AC methods. J. Power Sources 2018, 375, 185–190. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, Y.S. Quaternized aryl ether-free polyaromatics for alkaline membrane fuel cells: Synthesis, properties, and performance—A topical review. J. Mater. Chem. A 2018, 6, 15456–15477. [Google Scholar] [CrossRef]
- Salma, U.; Nagao, Y. Alkaline stability of ether bond free fluorene-based anion exchange polymer containing cycloaliphatic quaternary ammonium groups. Polym. Degrad. Stab. 2020, 179, 109299. [Google Scholar] [CrossRef]
- Zhang, F.; Li, T.; Chen, W.; Yan, X.; Wu, X.; Jiang, X.; Zhang, Y.; Wang, X.; He, G. High-Performance Anion Exchange Membranes with Para-Type Cations on Electron-Withdrawing C=O Links Free Backbone. Macromolecules 2020, 53, 10988–10997. [Google Scholar] [CrossRef]
- Wei, C.; Yu, W.; Wu, L.; Ge, X.; Xu, T. Physically and Chemically Stable Anion Exchange Membranes with Hydrogen-Bond Induced Ion Conducting Channels. Polymers 2022, 14, 4920. [Google Scholar] [CrossRef]
- Arges, C.G.; Wang, L.; Jung, M.-s.; Ramani, V. Mechanically Stable Poly(arylene ether) Anion Exchange Membranes Prepared from Commercially Available Polymers for Alkaline Electrochemical Devices. J. Electrochem. Soc. 2015, 162, F686. [Google Scholar] [CrossRef]
- Basso Peressut, A.; Montagna, J.; Moretti, P.; Arrigoni, A.; Latorrata, S.; Bertarelli, C.; Dotelli, G. Development and characterization of crosslinked PPO-based anion exchange membranes for AEM fuel cells. Solid State Ion. 2023, 394, 116212. [Google Scholar] [CrossRef]
- Sung, S.; Mayadevi, T.S.; Min, K.; Lee, J.; Chae, J.E.; Kim, T.-H. Crosslinked PPO-based anion exchange membranes: The effect of crystallinity versus hydrophilicity by oxygen-containing crosslinker chain length. J. Membr. Sci. 2021, 619, 118774. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, J.; Ding, A.; Nie, Y.; Li, Z.; Shen, C.; Gao, S. Synthesis and properties of novel crosslinking anion exchange membranes based on quaternary poly(fluorene-piperidine). Colloid Interface Sci. Commun. 2022, 46, 100584. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, B.; Wu, H.; Cao, L.; He, X.; Jiang, Z. Imidazolium-functionalized carbon nanotubes crosslinked with imidazole poly(ether ether ketone) for fabricating anion exchange membranes with high hydroxide conductivity and dimension stability. Electrochim. Acta 2019, 318, 572–580. [Google Scholar] [CrossRef]
- Jang, S.-C.; Tsen, W.-C.; Chuang, F.-S.; Gong, C. Simultaneously enhanced hydroxide conductivity and mechanical properties of quaternized chitosan/functionalized carbon nanotubes composite anion exchange membranes. Int. J. Hydrogen Energy 2019, 44, 18134–18144. [Google Scholar] [CrossRef]
- Lo, C.-F.; Wu, J.-F.; Li, H.-Y.; Hung, W.-S.; Shih, C.-M.; Hu, C.-C.; Liu, Y.-L.; Lue, S.J. Novel polyvinyl alcohol nanocomposites containing carbon nano-tubes with Fe3O4 pendants for alkaline fuel cell applications. J. Membr. Sci. 2013, 444, 41–49. [Google Scholar] [CrossRef]
- Lim, T.; Kim, S.-K. Non-precious hydrogen evolution reaction catalysts: Stepping forward to practical polymer electrolyte membrane-based zero-gap water electrolyzers. Chem. Eng. J. 2022, 433, 133681. [Google Scholar] [CrossRef]
- Hossen, M.M.; Hasan, M.S.; Sardar, M.R.I.; Haider, J.B.; Mottakin; Tammeveski, K.; Atanassov, P. State-of-the-art and developmental trends in platinum group metal-free cathode catalyst for anion exchange membrane fuel cell (AEMFC). Appl. Catal. B Environ. 2023, 325, 121733. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).