Abstract
Unreasonable storage of phosphate ore is becoming an important pathway causing phosphate pollution in the surrounding aquatic environment. However, there is little research on the influence of dissolved organic matter (DOM) in water on the fate of phosphate ore. Here, we collected phosphate ores from two phosphate mines along the coast of Tanglang River and studied the effects of DOM concentrations and pH on the release of soluble active phosphorus (SRP) and fluoride ion (F−) from phosphate ores using humic acid (HA) as the representative of DOM. Based on the analysis of ZP, FTIR, XPS, and SEM, the influence mechanism of HA was revealed. The results showed that HA efficiently promoted the release of SRP and F− from phosphate ore. With decreasing pH, the P release increased in both water and HA solutions in general. The beneficial influence of HA on the release of SRP and F− from phosphate ore was ascribed to the introduction of oxygen-containing functional groups by HA, which altered the surface properties and enhanced the dispersion stability of phosphate ore. These findings provided new insights into the dispersion behavior of phosphate ore, which is helpful in promoting the pollution control and management strategy of phosphate ore.
1. Introduction
The eutrophication of surface water bodies such as lakes, reservoirs, slow-flow channels, and ponds has become one of the most concerning environmental problems in many contemporary countries [1,2,3]. Phosphorus (P) is recognized consistently as the dominant limiting nutrient in rivers and other freshwater, and plays an critical role in the growth of phytoplankton [4,5]. Elevated phosphorus (P) concentrations can lead to eutrophication, resulting in algae overgrowth, thus leading to a series of negative responses, including harmful algae blooms, hypoxia, and in severe cases fish kills [4].
The eutrophication of many lakes in China is mainly due to excessive P inputs [6,7,8]. Non-point source pollution caused by rainwater runoff erosion over the surface has become an important factor of water environment pollution and ecological degradation, and is the third major source of pollution of river and lake pollution [9]. Controlling the input of exogenous phosphorus is an effective measure to reduce the phosphorus content in surface water bodies [10]. Continuous efforts to better comprehend and manage P pollution have led to significant reductions in total P concentrations in Chinese lakes [11,12,13,14,15]. However, P pollution within Southwest China, particularly in the upper and middle reaches of the Yangtze River basin, remains critical for regional water quality protection [15,16].
Southwest China contains the largest geological phosphorus-rich mountain range in the world [15], but it is located in ecologically fragile areas, where human activities result in environmental degradation [17]. Waste phosphate ore in phosphorus-rich areas usually accumulates at the surface of the phosphate ore areas. After weathering, precipitation erosion, and surface runoff, the phosphorus originally existing in the solid phase is gradually transferred to the liquid phase or migrated into the nearby water with the solid state, thus forming potential phosphorus pollution. However, the potential phosphorus pollution caused by the large amount of phosphate ore stored in phosphate chemical plants and phosphate mining areas has not been paid enough attention for a long time. The Tanglang River is an important tributary of the Chin-sha River (the upper reaches of the Yangtze River), and it is also the only outlet of Dianchi Lake. In recent decades, the land use in the Tanglang River basin has changed significantly. The urban and rural residential land has significantly increased, and the cultivated land area has decreased. The basin is rich in phosphate mineral resources, and it is also the most intensive open-pit mining area in the Chin-sha River basin. Large-scale mining of phosphate mineral resources leads to serious soil erosion and non-point source pollution, and water eutrophication is increasingly serious. During the rainy season, the phosphate ore accumulated in the phosphorization plants and other places will mix with the rain and enter the Tanglang River environment, causing eutrophication. The potential pollution risk of accumulated phosphate ore has been evaluated. For example, Jiang et al. [18] evaluated the potential pollution risk of phosphorus leaching from phosphate waste ores in the Xiangxi River watershed. The results indicated that the phosphate waste ores deposited in the study area could threaten the adjacent surface-water environment, and should be considered as phosphorus point pollution sources. However, coexisting components in water bodies may affect phosphorus release and exacerbate ecological risk. There are insufficient studies on this aspect.
Dissolved organic matter (DOM) is present in almost all aquatic ecosystems at concentrations typically ranging from 0.1 to 10 mg L−1 and depending on biogeochemical conditions and climate [19]. There are three most important components of DOM in surface waters: humic substances, proteins, and polysaccharides [19,20,21]. Not only surface water contains DOM, but also abundant dissolved organic carbon (DOC) in global rainfall, with an annual settlement flux up to 430 × 1012 g·a−1 [22]. DOM plays a crucial role in the transport of various colloids such as hematite, clays, Fe(0), Cu(0), TiO2, ZnO, and hydroxyapatite in the soil by altering the surface properties and stability of colloids [19,23]. Humic acid (HA) is often used as a representative model for DOM. A previous study found that HA was able to inhibit phosphate mineral precipitation and improve the bioavailability of phosphorus because the adsorption of HA onto the mineral surfaces blocked the growth site of the active crystals [24]. Antonietti et al. [25] found that the modification of primary iron phosphate crystals by humic acid was associated with organic acid etching, but also with a redox process. It is worth noting that these effects on phosphate mines are usually not mutually exclusive, but jointly lead to an increased P bioavailability. The research on the influence mechanism of HA on phosphate ores mainly focuses on the soil level, but there is little similar research on aqueous systems. HA has been shown to increase the stability of colloidal suspensions of engineered carbon nanoparticles [26]. The carboxyl or phenolic functional groups of HA can interact with nano-inorganic oxides such as TiO2, SiO2, Al2O3, and ZnO by electrostatic attraction, ion exchange, hydrophobic effect and complexation [27,28,29,30,31,32]. The interaction between DOM and colloids affects the behavior of colloids in aquatic system. A better understanding of the interaction mechanism between DOM and phosphate ore is crucial to fully elucidate the fate and transport mechanisms of colloids and pollutants (e.g., P, F−) in the environment.
In addition, the occurrence state of phosphorus in phosphate ore is mainly the minerals containing phosphorus and fluorine, such as gum phosphate ore and fluorophospapatite, so phosphate ore is an important source of fluoride ions. Although fluorine is one of the essential trace elements for the normal development of the human body, the intake of fluorine has a very narrow range between beneficial and harmful changes. The loss of phosphate ore may lead to excessive fluoride concentration in water. Long-term intake of high doses of fluoride can lead to fluorosis, endangering the ecological environment and human health. Therefore, the effect of DOM on the release of phosphate ore fluoride ion (F−) was also tested.
Accordingly, the purpose of this study is to reveal the influence mechanism of DOM on the release of soluble phosphorus and F− from phosphate ore from a new perspective, such as whether the surface properties of phosphate ore are changed by adsorption, thus affecting its dispersity, and to further provide some basis for the occurrence and control of water bloom in the phosphorus-rich area. Here, phosphate ores were collected from two phosphate mines along the Tanglang River basin to (I) study the effect of DOM concentration and pH on the release of SRP and F− from phosphate ore with HA as the representative of DOM; (II) analyze the chemical composition and mineral phase composition of phosphate ore using XRF and XRD; (III) explore the surface properties changes of phosphate ore based on ZP, FTIR, XPS and SEM methods; and (IV) discuss the new mechanism of the influence of the DOM. The result of his study can provide a basis for algae bloom prevention or control in phosphorus-rich basins, which could be significant for better phosphating plant management, comprehensive treatment of phosphorus pollution, and the technical system of water bloom control in the future.
2. Materials and Methods
2.1. Materials
Two kinds of phosphate ore samples in this study were collected from Sanming Xinjiang Mining Co., Ltd. (Yunnan, China), material field I of Jinwan Agricultural Science and Technology Co., Ltd. (Yunnan, China), which are distributed in the Tanglang River basic. The samples were crushed, ground, and then sieved to obtain a fraction of <0.75 µm after passing through a 200-mesh standard sieve. Two kinds of phosphate ore powder were taken into two sealed plastic bags signed S1 and S2, respectively, for reserve.
HA purchased from J&K Scientific Ltd. (Beijing, China) was used as a model aquatic DOM. To obtain 200 mg L−1 of the stock HA, 50 mg HA was weighed into 1 M NaOH solution and shaken overnight at a constant temperature of 25 °C. Subsequently, the HA solution was filtered through a 0.45 μm cellulose acetate membrane filter and then added to 250 mL with ultrapure (UP) water in a reaction vessel.
2.2. Phosphate Release Experiments
Two sets of indoor static simulation experiments were conducted to investigate the influence of HA on the release of soluble phosphorus from phosphate ore in water. A first set of experiments examined the effect of pH on the release of soluble phosphorus from phosphate ore at fixed HA concentration (0 and 2 mg L−1, respectively) over a pH range of 5.0–9.0. The pH was adjusted using 10% HCl and/or 1.0 mM NaOH to achieve pH values of 5.0 ± 0.02, 6.0 ± 0.02, 7.0 ± 0.02, 8.0 ± 0.02, and 9.0 ± 0.02. The second set investigated the effect of HA concentration (0, 2, 5, and 10 mg L−1) on the release of soluble phosphorus from phosphate ore at pH 6 (near the pH of the water environment at CO2 saturation).
The batch experiments were carried out using a constant dose by adding 1.00 g phosphate ore powder into 150 mL solution with a solid-to-liquid ratio of 1:150. The tested solutions with the desired solution pH and HA concentrations were prepared by mixing appropriate volumes of the HCl and/or NaOH solution and HA stock solutions. The phosphate powder was added to the beakers with the test solutions and subsequently the mixture was stirred for 30s and mixed well. The beakers were sealed with sealed films with a hole in the middle to prevent evaporation of the solution and placed statically at room temperature. At regular intervals, use a syringe to extract 5 mL of suspension within 2 cm below the liquid level. The suspension was passed through a 0.45 μm membrane filter, and the phosphate concentration in the filtrate was determined using the molybdenum blue method with a UV-visible spectrophotometer (Evolution 201, Thermo Scientific, Zhejiang, China) at 700 nm. Ultrapure water was used in all experiments.
2.3. Fluoron-Ion Leaching Test
To avoid too low F− concentration making the detection inconvenient, a solid-to-liquid ratio of 1:2 was used for multiple leaching. Add 5.0 g of phosphate ore powder and 10 mL test solutions of pH 6 (HA concentration is 0, 2, 5 and 10 mg L−1, respectively) were added into the centrifuge tubes. After thorough mixing, the tubes were shaked at constant temperature (25 °C, 170 r min−1) for 2 hours. Two hours later, they were removed and centrifuged for 15 min at 3000 rpm. The supernatant was carefully extracted and filtered with a 0.45 μm filter membrane. The fluoride ion concentration in the filtrate was determined by using an ion chromatograph (IC1800, Shun Yu Hengping, Shanghai, China). Then add HA solutions again and repeat the above steps time after time.
2.4. Sample Analysis and Data Evaluation
The mineral phase composition and chemical composition of the raw mineral powder were analyzed using X-ray diffraction (XRD, X’Pert PRO MPD, Panalytical, Almelo, Netherlands) and X-ray fluorescence (XRF, Zetium, Panalytical, Almelo, The Netherlands). Phase identification was performed by comparison with the reference spectra of the International Diffraction Data Center (ICDD). The morphology of the samples was observed and analyzed with a field emission scanning electron microscope (SEM, MIRA LMS, Jieke TESCAN, Hong Kong), and all samples were sprayed with gold for 30 s. The distribution of the elements was studied using the element mapping method.
The zeta potential of the particles in the suspension was measured using a particle size and zeta potential analyzer (Zetasizer Nano ZS, Malvern Instruments Technical Ltd., Malvern, UK). The surface functional groups of the samples were analyzed using the Fourier-transformed infrared spectrum (FTIR, iN10, Thermo Scientific, Waltham, MA, USA). The measurement resolution was set at 4 cm−1 and the spectra were collected in the range of 600–4000 cm−1. The surface elements of the samples were analyzed using an X-ray photoelectron spectrometer (XPS, K-Alpha, Thermo Scientific, Waltham, MA, USA). A laboratory pH meter (PHSJ-3C, glass composite electrode, Leici, Shanghai, China) was used for pH measurement. The surface contact angles of the samples were measured with the Contact Angle Measuring System (JC2000D1, Zhongchen POWEREACH, Shanghai, China). To prepare dry powders of phosphate-ore–HA complexes, the precipitate was freeze-dried and collected.
3. Results and Discussion
3.1. Humic Acid Efficiently Promotes the Release of SRP and F− from Phosphate Ore
The results of the phosphate release experiment showed that HA had a significant promoting effect on phosphate release at five pH levels, and the amount of phosphorus release decreased with the increasing pH of the HA solution (Figure 1). For S1, at pH 9, there was still a significant increase in the phosphate release in the presence of HA (Figure 1a). We speculate that the adsorption of HA changes the phosphate ore surface properties and makes the amount of phosphate ore released change. Perhaps influenced by alkaline solution, ionization of HA will reduce adsorption on its surface, but still have small adsorption onto phosphate ore. This inference is consistent with previous studies, that is, the adsorption of minerals to humus is affected by medium pH, and the adsorption amount is weakened with the increase of solution pH [33,34,35].
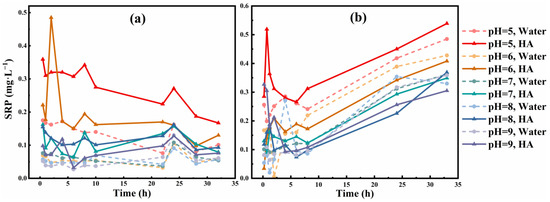
Figure 1.
SRP release concentration of phosphate ore (a) S1 and (b) S2 at 2 mg·L−1 of HA concentration at five pH levels. Water and HA represent pure water and HA solution, respectively.
We can see a significant pH dependence of the effect of HA. For S2, with increasing pH, the promoting effect of HA on phosphate ore P release began to become less apparent (Figure 1b). Previous studies have shown that with increasing pH, DOM becomes more deprotonated and hydrophilic [36]. However, at the same time, the surface of phosphate ore in pure water is more negative and more hydrophilic, which may make the dispersion effect of phosphate ore in pure water higher than HA.
As a whole, the soluble phosphorus release increases with increasing HA concentration at pH 6 (Figure 2). The concentration ranges of SRP released from phosphate ore S1 (Figure 2a) are 0.027~0.111 mg·L−1, 0.180~0.350 mg·L−1, 0.207~0.370 mg·L−1, and 0.291~0.412 mg·L−1 at HA concentrations of 0, 2, 5, and 10 mg·L−1, respectively. The concentration ranges of SRP released from phosphate ore S2 (Figure 2b) are 0.084~0.219 mg·L−1, 0.148~0.315 mg·L−1, 0.108~0.268 mg·L−1, and 0.320~1.021 mg·L−1 at HA concentrations of 0, 2, 5, and 10 mg·L−1, respectively.
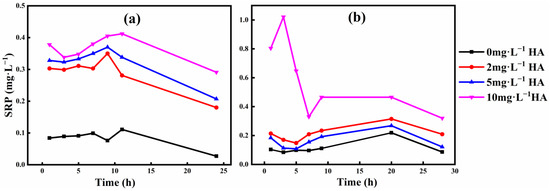
Figure 2.
SRP release concentration of phosphate ore (a) S1 and (b) S2 at different HA concentrations at pH 6.
The net release of fluoride ions after each leaching for 2 h is shown in Figure 3. From the first leaching to the third leaching, the amount of F− released gradually decreased. After the third leaching, the release of F− showed a steady fluctuation trend, and the concentration was about 0.6~0.8 mg·L−1. The release of fluoride ions from phosphate ore S1 also effectively increased in the presence of HA (Figure 3). The results lead us to pay more attention to water safety. As for the difference in the regularity of F− and SRP release at different HA concentrations, it is possible that the low solid-to-liquid ratio (1:2) and the different experimental conditions (constant temperature shaking) affected the function of HA on the dispersion of phosphate ore.
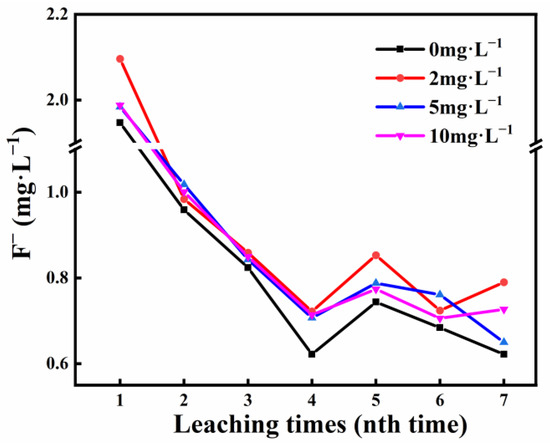
Figure 3.
F− release concentration of phosphate ore S1 at different HA concentrations at pH 6.
3.2. Characterization of Phosphate Ore S1
We used phosphate ore S1 to explore the mechanism. The results of XRF indicated that the phosphate ore mainly contained CaO (45.875%), P2O5 (29.954%), SiO2 (15.910%), F (1.936%), and a small amount of oxide (Table S1). The X-ray diffraction patterns of phosphate ore demonstrated that the major mineral phases include fluorapatite (Ca5(PO4)3F) and quartz (SiO2) (Figure 4). The main peaks of fluorapatite were at 2θ = 26.6°, 28.5°, 32.9°, 34.6°, and 36.8°, while the main peaks of quartz were at 2θ = 20.9°, 26.6°, 50.4°, and 62.4°. This result is consistent with the major component of the XRF. The surface morphology of the phosphate ore is shown in Figure 5 and is described below.
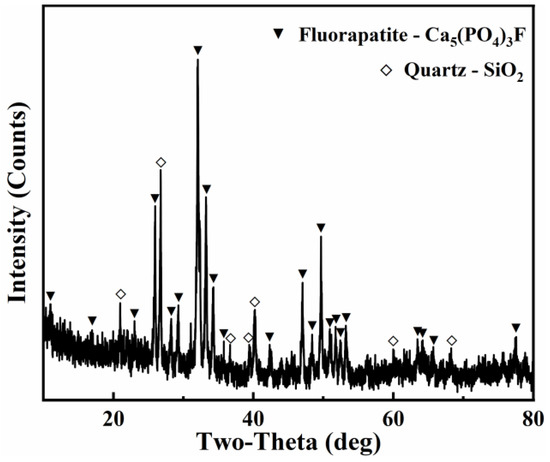
Figure 4.
X-ray diffraction patterns of phosphate ore S1.
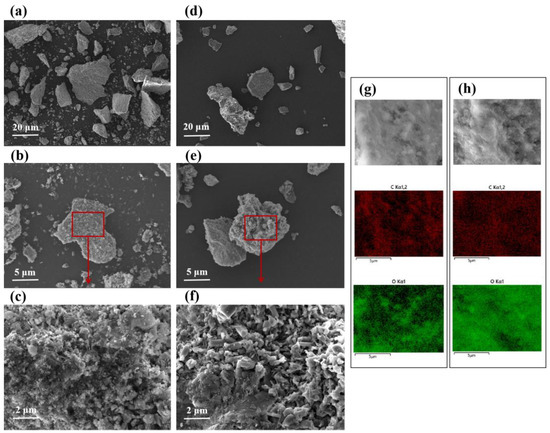
Figure 5.
Representative SEM images of (a–c) raw phosphorous ore and (d–f) phosphorous ore after reacting with 10 mg·L−1 HA; element mapping of (g) raw phosphorous ore and (h) phosphorous ore after reacting with 10 mg·L−1 HA.
3.3. Humic Acid Alters the Surface Properties and Enhances Dispersion Stability of Phosphate Ore by Adsorption
The morphology of the phosphate ore was observed before and after reacting with HA (Figure 5). As can be seen from Table S1 and Figure 5, the ore composition is complex, and the useful mineral fluoroapatite is closely symbiotic with other gangue minerals (quartz). The surface is relatively flat and compact with small mineral particles showing flocculent agglomeration, and the size of pronophosphate ore particles are different. It can be observed that the mineral particles with large particle sizes show an unbroken structure and no fine pores (Figure 5a,b). The element mapping of phosphate ore before and after interaction with HA was investigated (Figure 5g,h). The results showed that elements C and O are widely distributed in the original phosphate ore. After the interaction with HA, the distribution of element C is more uniform, suggesting that HA may adsorb onto the surface of phosphate ore. Because apatite is mixed with trace carbon or organic matter, the aggregate is often gray-black or brown. It may be that the step sequestration of minerals gradually embedded soil organic matter into the interior [37].
After the interaction with HA, many corrosion pits appeared on the originally smooth surface, with significantly fewer microparticles attached to the phosphate ore surface (Figure 5a–f), and the hexagonal prism-like crystals appeared with an obvious irregular arrangement (Figure 5c,f). This may be due to the adsorption of HA making the depolymerization of tiny mineral aggregates on the mineral surface, thus increasing the contact area with the solution and accelerating the dissolution of phosphate ore. When the erosion pit appears after mineral dissolution, the contact area between HA and minerals will be further increased, and the effect of continuous dispersion will appear.
Zeta potentials were measured to explore the surface charge of phosphate ores with different concentration levels of HA in aqueous solutions. phosphate ores were negatively charged at pH 6 in water (Figure 6a). When the absolute value of zeta potential is small, the electrostatic repulsion between the fine particles is weak, and the fine particles are more likely to agglomerate by van der Waals gravity and form larger aggregates, which are more likely to descend under gravity with poor dispersion and stability.
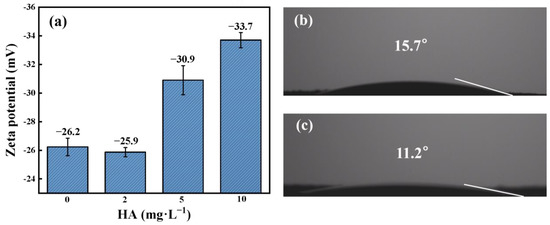
Figure 6.
(a) The zeta potential on the phosphate ore S1 surface at different HA concentrations at pH 6; contact angles of (b) raw phosphate ore and (c) phosphate ore after reacting with 10 mg·L−1 HA at pH 6.
Higher concentrations of humic acid can cause enhanced negative charge on the phosphate ore surface at pH 6. That is, humic acid can enhance the dispersion of phosphate ore. At pH 6, in the presence of HA, the zeta potentials of phosphate ore were more negative than bare phosphate ore, which indicates that the electrostatic repulsion of HA-coated phosphate ore is responsible for the observed HA stabilization. Moreover, HA sorption onto phosphate ore surfaces may increase the steric hindrance between phosphate ores and more strongly inhibit their aggregation [26]. In addition, after interaction with HA, the surface contact angle of the phosphate ore decreased from 15.7° to 11.2° (Figure 6b,c), which further indicated increased hydrophilicity and enhanced suspension stability. As we all know, the enhanced dispersion stability of phosphate ore in the liquid phase will contribute to more release of SRP and F−. Wei Ren et al. reported that there are some well-fitted and negative linear relationships between the zeta potential and total oxygen content or levels of each oxygen group at the surfaces of carbon nanotubes, indicating that the carbonyl and carboxyl groups are the main groups to make the zeta potential more negative [38]. Therefore, it may be that the introduction of oxygen-containing hydrophilic groups on the phosphate ore surface has important implications for the increased electronegativity and hydrophilicity on the phosphate ore surface. Next, we will discuss the application of FTIR and XPS to further interpret the oxygen-containing functional groups.
The changes in functional groups in phosphate ore samples were first examined using FTIR analysis (Figure 7). It can be observed that the CO3 vibration bands of phosphate ore were located at approximately 872 cm−1 (out-of-plane bending vibration band), 714 cm−1 (in-plane bending vibration band), and 1391 cm−1 (stretching vibration band), respectively. The absorption peaks occurring at 1033 cm−1 and 1092 cm−1 are the characteristic peaks of PO43−. The characteristic peaks of PO43− and CO32− were generally weakened after the interaction with HA.
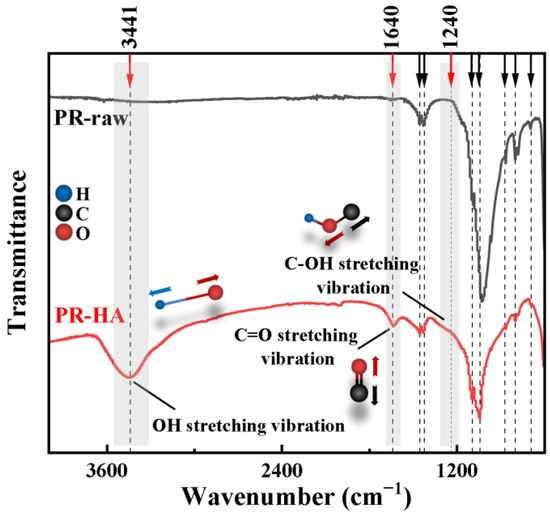
Figure 7.
Fourier-transform infrared spectra for phosphate ore samples at pH 6. PR-raw represents the primary phosphate ore, and PR-HA represents the phosphate ore after reacting with 10 mg·L−1 HA.
There are three new peaks that appear after reacting with HA. The structure of the functional groups is shown in Figure 7. The O-H wide absorption peak is at 3441 cm−1, and the strong characteristic peak of C=O stretch vibration is at 1650~1850 cm−1. The phosphate ore after reaction with HA showed C=O stretch characteristic peak near 1640 cm−1, indicating that the C=O is conjugated to other structures (enene bond, the aromatic rings, etc.), which decreases the absorption frequency. The vibration band at 1240 cm−1 was attributed to the C-C stretching vibrations of humic acid [39]. The stretching vibration of C-C (linked with C=O bond) enhanced with the increase in the electron cloud density of the C-C bond, which was attributed to the conjugation effect between C-O and the aromatic ring. This finding indicates that HA adsorption occurs, introducing O-H and C=O functional groups [40].
To further confirm the reaction mechanisms, the types and concentrations of oxygen groups were analyzed with XPS. The detailed C 1s analyses of phosphate ore after reaction with HA are shown in Figure 8. The broad C 1s peak can be fitted into three components with binding energies at 284.8, 286.6~286.7, and 288.8~289.2 eV, which correspond to C-C, C-O, and C=O, respectively. The raw ore contains C element containing 19.74 at.%, which is consistent with the element mapping results. The C content after interaction with HA was 24.1 at.%. Kleber et al. [41] proposed the concept of “organic coatings on mineral surfaces”. The author believes that the mineral surface does not adsorb special sites for organic matter at first, but the organic molecular layer formed on the mineral surface creates potential adsorption points for organic matter, which has been agreed upon by other researchers [42]. However, FTIR cannot detect organo-functional groups on raw phosphate ore surfaces that XPS can detect. This phenomenon could be because FTIR was not as sensitive as XPS to show some change in surface organic matter.
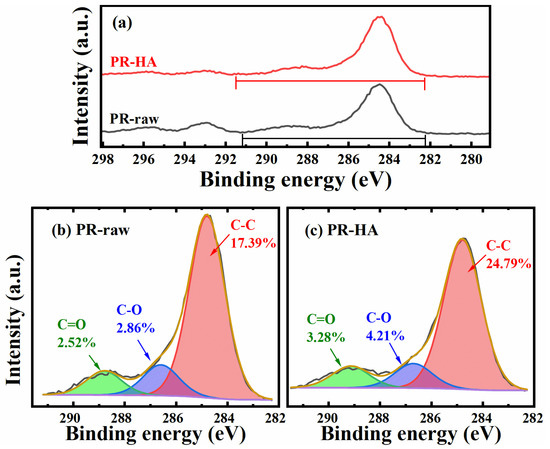
Figure 8.
The XPS spectra of C 1s. The survey and high-resolution scans of XPS spectra of phosphate ore before and after reacting with HA. (a) Total survey scans of C 1s; (b) C 1s peaks of phosphate ore; (c) C 1s peaks of phosphate-ore–HA. PR-raw represents the raw phosphate ore, and PR-HA represents the phosphate ore after reacting with HA.
Not surprisingly, the oxygen-containing functional groups of the surface of phosphate ore increased after acting with HA. The C-C content on the surface of phosphate ore increased from 17.39 at.% to 24.79 at.%, the C=O content increased from 2.52 at.% to 3.28 at.%, and the C-O content increased from 2.86 at.% to 4.21 at.%. Smith et al. [43] found that colloidal suspension and stability of acid-treated MWCNTs had a positive dependence linear relationship on the oxygen content on their surface, which was attributed to the energetically favorable interactions between the hydrophilic functional groups and water molecules. This suggests that the adsorption of HA introduced more sorbed O-containing groups into the surface of phosphate ores, enhancing their surface interaction with water molecules through hydrogen bonding, thereby enhancing the suspension of phosphate ores.
3.4. Possible Path of the Effects of Humic Acid on Phosphate Ore
Here, the possible underlying mechanism of phosphate ore SRP release by HA is briefly explained (Figure 9). As the phosphate ore enters the liquid phase containing HA, the HA layer surrounds the phosphate ore surface, changing its surface charge and the abundance of the hydrophilic functional groups. On the one hand, the suspension stability is enhanced by steric resistance and electrostatic repulsion, and the aggregation and deposition of phosphate ore are inhibited. On the other hand, the enhanced surface hydrophilicity breaks down the aggregation structure with small forces, increases their contact area with the liquid phase, and the hydrophilic functional groups originally wrapped in the bundle interact with water molecules, which increases the contact area with the liquid phase, and the hydrophilic functional groups originally wrapped in the bundle interact with water molecules.
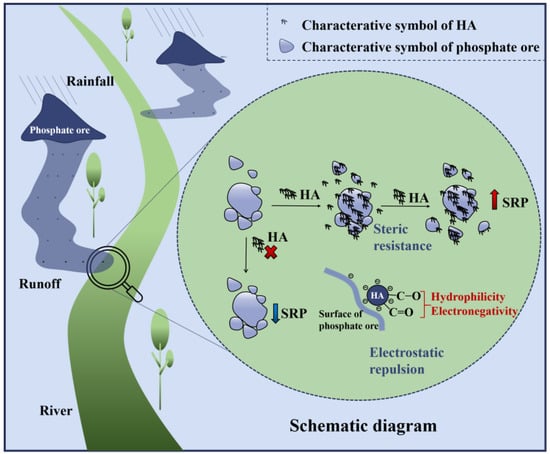
Figure 9.
Schematic diagram of the main formation pathway of blooms in phosphorus-rich areas and the possible mechanism of humic acid on phosphate ore P release.
4. Conclusions
Phosphate ore in the phosphate-rich area may increase the release of SRP due to the presence of DOM in the liquid phase during rainwater dissolution and after entering the river, resulting in more than the predicted ecological risk in the case of complex DOM. The reason why DOM increases the release of soluble phosphorus from phosphate ore may be that the adsorption of DOM on its surface introduces O-containing ionizable functional groups, which enhances surface electronegativity and hydrophilicity. The surface-bound functional groups or organic molecules enhance the electrostatic repulsion and steric hindrance between individual phosphate ores, increasing the dispersion stability and the SRP concentration in the upper liquid accordingly. These findings were important for better predicting the fate and transport of phosphate ore in the presence of DOM, and potentially provide insight into other related mechanisms controlling ecological and health risks of loss of phosphate ore in the environment. Based on the mechanism, we can gradually explore the appropriate methods to alleviate phosphorus release. However, the aquatic environment is complex and changeable. In order to create a more harmonious ecological environment, our best choice is prevention. Therefore, it is necessary to strengthen the risk assessment, consider the influence of factors such as the DOM in rain and river water, and standardize the management of phosphate chemical plants and phosphate ore sites from the source, so as to reduce phosphorus loss as much as possible.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations10080425/s1, Table S1: Results of chemical composition analysis for phosphate ore S1/%.
Author Contributions
Conceptualization, H.L.; data curation, F.Z.; formal analysis, F.Z.; funding acquisition, Y.L.; investigation, F.Z., C.T. and H.L.; methodology, H.L., Y.L. and F.Z.; project administration, Y.L. and Y.M.; resources, Q.Z. and Y.M.; supervision, Y.L. and C.T.; validation, F.Z.; visualization, F.Z. and Q.Z.; writing—original draft preparation, F.Z.; writing—review and editing, H.L. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Yunnan Fundamental Research Projects (202201AT070097).
Data Availability Statement
The main data of this study have been included in this article and the Supporting Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morgane, L.M.; Chantal, G.O.; Alain, M.; Yves, S.; Claire, É.; Alix, L.; Florentina, M.; Alexandrine, P.; Philippe, S.; Alain, L.; et al. Eutrophication: A new wine in an old bottle? Sci. Total Environ. 2019, 651, 1–11. [Google Scholar]
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Reyes, E.; Anex, R.P. A life cycle impact assessment method for freshwater eutrophication due to the transport of phosphorus from agricultural production. J. Clean. Prod. 2018, 177, 474–482. [Google Scholar] [CrossRef]
- Li, B.; Brett, M.T. The influence of dissolved phosphorus molecular form on recalcitrance and bioavailability. Environ. Pollut. 2013, 182, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.M.; Wurtsbaugh, W.A. Control of Lacustrine Phytoplankton by Nutrients: Erosion of the Phosphorus Paradigm. Int. Rev. Hydrobiol. 2008, 93, 446–465. [Google Scholar] [CrossRef]
- Huang, J.; Xu, C.-c.; Ridoutt, B.G.; Wang, X.-c.; Ren, P.-a. Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J. Clean. Prod. 2017, 159, 171–179. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H. Mitigation of lake eutrophication: Loosen nitrogen control and focus on phosphorus abatement. Prog. Nat. Sci. 2009, 19, 1445–1451. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, S.; Wang, Y. Characteristics of bioavailable organic phosphorus in sediment and its contribution to lake eutrophication in China. Environ. Pollut. 2016, 219, 537–544. [Google Scholar] [CrossRef]
- Amir, T.; Ronald, L.D. Pollution loads in urban runoff and sanitary wastewater. Sci. Total Environ. 2004, 327, 175–184. [Google Scholar]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Stone, R. China aims to turn tide against toxic lake pollution. Science 2011, 333, 1210. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Y.; Lin, D.; Shan, K.; Luo, Y.; Zhao, L.; Tan, Z.; Song, L. The relationships of meteorological factors and nutrient levels with phytoplankton biomass in a shallow eutrophic lake dominated by cyanobacteria, Lake Dianchi from 1991 to 2013. Environ. Sci. Pollut. Res. 2016, 23, 15616. [Google Scholar] [CrossRef]
- Liu, X. The identification of nutrient limitations on eutrophication in Dianchi Lake, China. Water Environ. J. 2017, 31, 45–54. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, W.; Wang, X.; Couture, R.M.; Larssen, T.; Zhao, Y.; Li, J.; Liang, H.; Liu, X.; Bu, X. Decline in Chinese lake phosphorus concentration accompanied by shift in sources since 2006. Nat. Geosci. 2017, 10, 12–17. [Google Scholar] [CrossRef]
- Yan, K.; Yuan, Z.; Goldberg, S. Phosphorus mitigation remains critical in water protection: A review and meta-analysis from one of China’s most eutrophicated lakes. Sci. Total Environ. 2019, 689, 1336–1347. [Google Scholar] [CrossRef]
- Powers, S.M.; Bruulsema, T.W.; Burt, T.P.; Chan, N.I.; Elser, J.J.; Haygarth, P.M.; Howden, N.J.K.; Jarvie, H.P.; Yang, L.; Peterson, H.M. Long-term accumulation and transport of anthropogenic phosphorus in three river basins. Nat. Geosci. 2016, 9, 353–356. [Google Scholar] [CrossRef]
- Peng, J.; Xu, Y.Q.; Cai, Y.L.; Xiao, H.L. The role of policies in land use/cover change since the 1970s in ecologically fragile karst areas of Southwest China: A case study on the Maotiaohe watershed. Environ. Sci. 2011, 14, 408–418. [Google Scholar] [CrossRef]
- Jiang, L.; Liang, B.; Xue, Q.; Yin, C. Characterization of phosphorus leaching from phosphate waste rock in the Xiangxi River watershed, Three Gorges Reservoir, China. Chemosphere 2016, 150, 130–138. [Google Scholar] [CrossRef]
- Allan, P.; Gabriele, E.S. Interactions of dissolved organic matter with natural and engineered inorganic colloids: A review. Environ. Sci. Technol. 2014, 48, 8946–8962. [Google Scholar]
- Nebbioso, A.; Piccolo, A. Molecular characterization of dissolved organic matter (DOM): A critical review. Anal. Bioanal. Chem. 2013, 405, 109–124. [Google Scholar] [CrossRef]
- Schulze, W.X. Protein analysis in dissolved organic matter: What proteins from organic debris, soil leachate and surface water can tell us—A perspective. Biogeosciences 2005, 2, 75–86. [Google Scholar] [CrossRef]
- Wiley, J.D.; Kieber, R.J.; Eyman, M.S.; Avery, G.B. Rainwater dissolved organic carbon: Concentrations and global flux. Glob. Biogeochem. Cycles 2000, 14, 139–148. [Google Scholar] [CrossRef]
- Aiken, G.R.; Hsu-Kim, H.; Ryan, J.N. Influence of dissolved organic matter on the environmental fate of metals, nanoparticles, and colloids. Environ. Sci. Technol. 2011, 45, 3196–3201. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.G.; William, P.I. Kinetics of octacalcium phosphate crystal growth in the presence of organic acids. Geochim. Cosmochim. Ac. 1992, 56, 1955–1961. [Google Scholar]
- Antonietti, M.; Yang, F.; Zhang, S.; Song, J.; Tarakina, N.V. Tackling the world’s phosphate problem: Synthetic humic acids solubilize otherwise insoluble phosphates for fertilization. Angew. Chem. Int. Ed. 2019, 58, 18813–18816. [Google Scholar]
- Zhou, X.Z.; Shu, L.; Zhao, H.B.; Guo, X.Y.; Wang, X.L.; Tao, S.; Xing, B.S. Suspending multi-walled carbon nanotubes by humic acids from a peat soil. Environ. Sci. Technol. 2012, 46, 1793–1802. [Google Scholar] [CrossRef]
- Yang, K.; Lin, D.H.; Xing, B.S. Interactions of humic acid with nanosized inorganic oxides. Langmuir 2009, 25, 3571–3576. [Google Scholar] [CrossRef]
- Wang, X.L.; Lu, J.L.; Xu, M.G.; Xing, B.S. Sorption of pyrene by regular and nanoscaled metal oxide particles: Influence of adsorbed organic matter. Environ. Sci. Technol. 2008, 42, 7267–7272. [Google Scholar] [CrossRef]
- Kang, S.; Xing, B.S. Phenanthrene sorption to sequentially extracted soil humic acids and humins. Environ. Sci. Technol. 2005, 39, 134–140. [Google Scholar] [CrossRef]
- Chen, K.L.; Elimelech, M. Influence of humic acid on the aggregation kinetics of fullerene (C60) nanoparticles in monovalent and divalent electrolyte solutions. J. Colloid Interface Sci. 2007, 309, 126–134. [Google Scholar] [CrossRef]
- Chen, K.L.; Elimelech, M. Interaction of fullerene (C60) nanoparticles with humic acid and alginate coated silica surfaces: Measurements, mechanisms, and environmental implications. Environ. Sci. Technol. 2008, 42, 7607–7614. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, X.L.; Zhao, T.H.; Wang, H.; Wang, P.F. Magnetic nanoparticles interaction with humic acid: In the presence of surfactants. Environ. Sci. Technol. 2016, 50, 8640–8648. [Google Scholar] [CrossRef]
- Zhou, J.L.; Rowland, S.; Fauzi, R.; Mantoura, C.; Braven, J. The formation of humic coatings on mineral particles under simulated estuarine conditionsâ—A mechanistic study. Water Res. 1994, 28, 571–579. [Google Scholar] [CrossRef]
- Vermeer, A.W.P.; Koopal, L.K. Adsorption of humic acids to mineral particles. 2. Polydispersity effects with polyelectrolyte Adsorption. Langmuir 1998, 14, 4210–4216. [Google Scholar]
- Vermeer, A.W.P.; Riemsdijk, W.H.; Koopal, L.K. Adsorption of humic acids to mineral particles. 1. Specific and electrostatic interactions. Langmuir 1998, 14, 2810–2819. [Google Scholar]
- Sutton, R.; Sposito, G. Molecular structure in soil humic substances: The new view. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef]
- Chi, J.L.; Fan, Y.K.; Wang, L.J.; Putnis, C.V.; Zhang, W.J. Retention of soil organic matter by occlusion within soil minerals. Rev Environ. Sci. Bio. 2022, 21, 727–746. [Google Scholar] [CrossRef]
- Ren, W.; Xiong, L.; Nie, G.; Zhang, H.; Duan, X.; Wang, S. Insights into the Electron-Transfer Regime of Peroxydisulfate Activation on Carbon Nanotubes: The Role of Oxygen Functional Groups. Environ. Sci. Technol. 2019, 54, 1267–1275. [Google Scholar] [CrossRef]
- Huang, J.H.; Elzinga, E.J.; Brechbuehl, Y.; Voegelin, A.; Kretzschmar, R. Impacts of shewanella putrefaciens strain CN-32 cells and extracellular polymeric substances on the sorption of As(V) and As(III) on Fe(III)-(Hydr)oxides. Environ. Sci. Technol. 2011, 45, 2804–2810. [Google Scholar] [CrossRef]
- Xing, B.; Graham, N.; Yu, W. Transformation of siderite to goethite by humic acid in the natural environment. Commun. Chem. 2020, 3, 7461–7469. [Google Scholar] [CrossRef]
- Kleber, M.; Sollins, P.; Sutton, R. A conceptual model of organo-mineral interactions in soils: Self-assembly of organic molecular fragments into zonal structures on mineral surfaces. Biogeochemistry 2007, 85, 9–24. [Google Scholar] [CrossRef]
- Sollins, P.; Swanston, C.; Kramer, M. Stabilization and destabilization of soil organic matter—A new focus. Biogeochemistry 2007, 85, 1–7. [Google Scholar] [CrossRef]
- Smith, B.; Wepasnick, K.; Schrote, K.E.; Cho, H.H.; Ball, W.P.; Fairbrother, D.H. Influence of surface oxides on the colloidal stability of multi-walled carbon nanotubes: A structure-property relationship. Langmuir 2009, 25, 9767–9776. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).