A Novel UHPLC-MS/MS-Based Bioanalytical Method Developed for S-Allyl Cysteine in the Establishment of a Comparative Pharmacokinetic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticles Preparations for S-Allyl-L-Cysteine
2.2. Morphology of S-Allyl-L-Cysteine
2.3. TEM Study for SC PLGA NPs
2.4. Other Characterization of SC PLGA NPs
2.5. The Release Amount of In Vitro for S-Allyl Cysteine
2.6. Animal Examination
2.7. Standard and Quality Control Samples Preparation
2.8. A Sample Preparation in Plasma
2.9. LC-MS Bioanalytical Development and Their Validation for S-Allyl-L-Cysteine
2.10. Pharmacokinetic Evaluation
2.11. A Comparative Study of SC PLGA NPs for Antimicrobial Activity by Agar Well Diffusion Method
2.11.1. Strains of Bacteria
2.11.2. Agar Well Diffusion Method
2.11.3. Statistical Analysis
3. Results
3.1. NPs Preparation and Their Characterization
3.2. Nanoparticles Analysis by TEM
3.3. Release Kinetics (In Vitro)
3.4. Bioanalytical Method Development and Validation
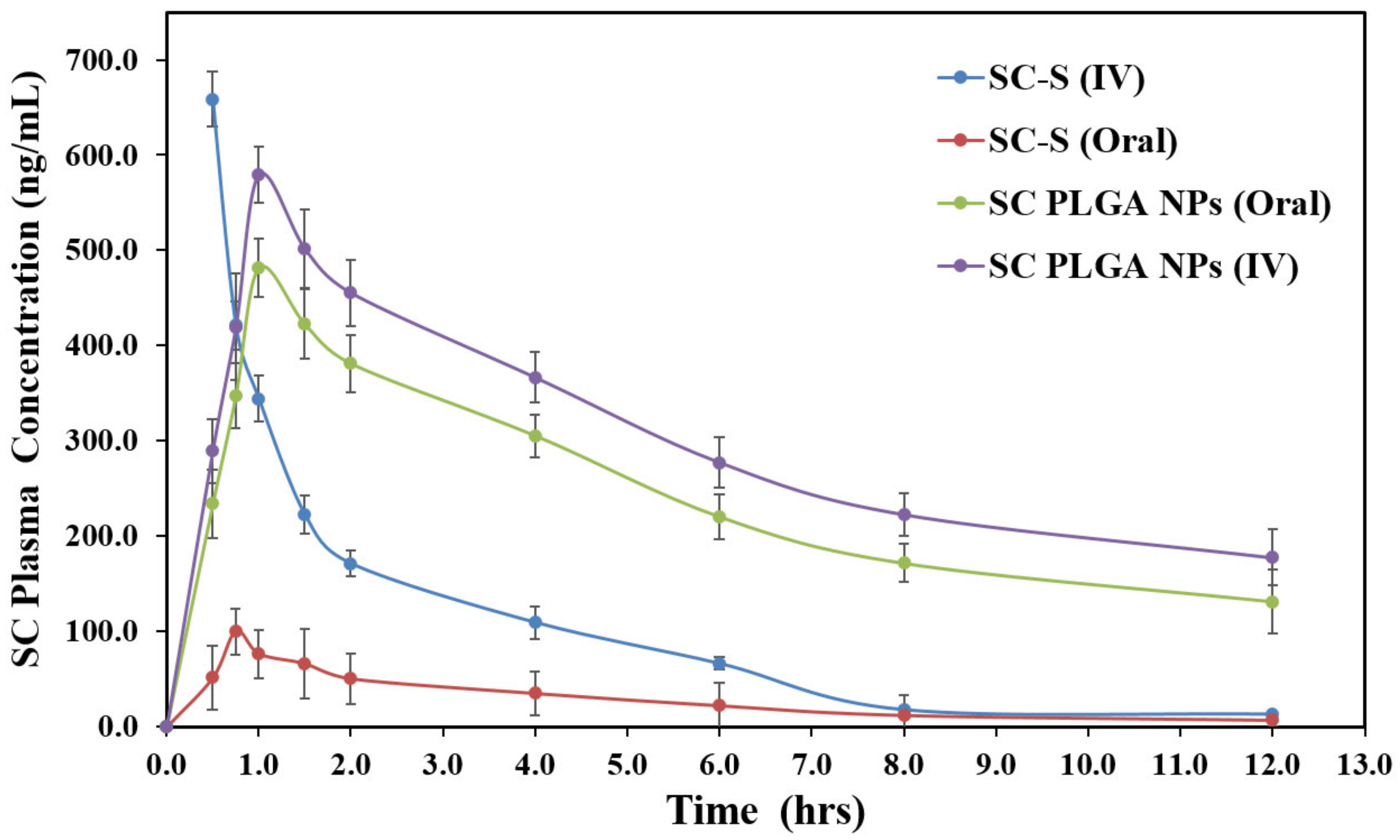
3.5. In Vivo Bioavailability and Pharmacokinetics
3.6. Antibacterial Activity
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.; Chang, N.I.; Yoo, M.; Choi, J.H.; Shin, D. Development and Validation of S-Allyl-L-Cysteine in Rat Plasma Using a Mixed-Mode Reversed-Phase and Cation-Exchange LC–ESI–MS/MS Method: Application to Pharmacokinetic Studies. J. Chromatogr. Sci. 2015, 53, 54–59. [Google Scholar] [CrossRef][Green Version]
- Numagami, Y.; Ohnishi, S.T. S-Allylcysteine inhibits free radical production, lipid peroxidation and neuronal damage in rat brain ischemia. J. Nutr. 2001, 131, 1100S–1105S. [Google Scholar] [CrossRef] [PubMed]
- Ide, N.; Lau, B.H.S. S-Allylcysteine attenuates oxidative stress in endothelial cells. Drug Dev. Ind. Pharm. 1999, 25, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, M.; Chen, K.; Yang, J.; Chen, R.; Wang, T.; Liu, J.; Yang, W.; Ye, Z. S-Allylcysteine induces cell cycle arrest and apoptosis in androgenindependent human prostate cancer cells. Mol. Med. Rep. 2012, 5, 439–443. [Google Scholar] [PubMed]
- Pai, M.H.; Kuo, Y.H.; Chiang, E.P.; Tang, F.Y. S-Allylcysteine inhibits tumour progression and the epithelial-mesenchymal transition in a mouse xenograft model of oral cancer. Br. J. Nutr. 2012, 108, 28–38. [Google Scholar] [CrossRef]
- Nakagawa, S.; Kasuga, S.; Matsuura, H. Prevention of liver damage by aged garlic extract and its components in mice. Phytother. Res. 1989, 3, 50–53. [Google Scholar] [CrossRef]
- Nishiyama, N.; Moriguchi, T.; Morihara, N.; Saito, H. Ameliorative effect of S-allylcysteine, a major thioallyl constituent in aged garlic extract, on learning deficits in senescence accelerated mice. J. Nutr. 2001, 131, 1093S–1095S. [Google Scholar] [CrossRef]
- Javed, H.; Khan, M.M.; Khan, A.; Vaibhav, K.; Ahmad, A.; Khuwaja, G.; Ahmed, M.E.; Raza, S.S.; Ashafaq, M.; Tabassum, R.; et al. S-Allyl cysteine attenuates oxidative stress associated cognitive impairment and neurodegeneration in mouse model of streptozotocininduced experimental dementia of Alzheimer’s type. Brain Res. 2011, 1389, 133–142. [Google Scholar] [CrossRef]
- Nagae, S.; Ushijima, M.; Hatono, S.; Imai, J.; Kasuga, S.; Matsuura, H.; Itakura, Y.; Higashi, Y. Pharmacokinetics of the garlic compound S-allylcysteine. Planta Med. 1994, 60, 214–217. [Google Scholar] [CrossRef]
- Rosen, R.T.; Hiserodt, R.D.; Fukuda, E.K.; Ruiz, R.J.; Zhou, Z.; Lech, J.; Rosen, S.L.; Hartman, T.G. The determination of metabolites of garlic preparations in breath and human plasma. Biofactors 2000, 13, 241–249. [Google Scholar] [CrossRef]
- Kyung, K.H. Antimicrobial properties of allium species. Curr. Opin. Biotechnol. 2012, 23, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Katsuzaki, H.; Ohta, R.; Ishikawa, K.; Fukuda, H.; Fujino, T.; Suzuki, A. Antimicrobial Activity of the Thiosulfinates Isolated from Oil-Macerated Garlic Extract. Biosci. Biotechnol. Biochem. 1999, 63, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Mi-Kyung, C.; Kyung-Yeon, C.; Joo-Young, L.; Kyu-Hang, K. Antimicrobial Activity of Chemical Substances Derived from S-Alk(en)yl-L-Cysteine Sulfoxide (Alliin) in Garlic, Allium sativum L. Food Sci. Biotechnol. 2007, 16, 1–7. [Google Scholar]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive components. J. Nutr. 2001, 131, 955S–962S. [Google Scholar] [CrossRef]
- Bae, S.E.; Cho, S.Y.; Won, Y.D.; Lee, S.H.; Park, H.J. A comparative study of the different analytical methods for analysis of S-allyl cysteine in black garlic by HPLC. Food Sci. Technol. 2012, 46, 532–535. [Google Scholar] [CrossRef]
- Pathak, K.; Raghuvanshi, S. Oral bioavailability: Issues and solutions via nanoformulations. Clin. Pharmacokinet 2015, 54, 325–357. [Google Scholar] [CrossRef]
- Cherniakov, I.; Domb, A.J.; Hoffman, A. Self-nano-emulsifying drug delivery systems: An update of the biopharmaceutical aspects. Expert Opin. Drug Deliv. 2015, 12, 1121–1133. [Google Scholar] [CrossRef]
- Ozeki, T.; Tagami, T. Functionally engineered nanosized particles in pharmaceutics: Improved oral delivery of poorly water-soluble drugs. Curr. Pharm. Des. 2013, 19, 6259–6269. [Google Scholar] [CrossRef]
- Zhao, W.; Li, J.; Jin, K.; Liu, W.; Qiu, X.; Li, C. Fabrication of functional PLGA-based electrospun scaffolds and their applications in biomedical engineering. Mater Sci. Eng. C Mater Biol. Appl. 2016, 59, 1181–1194. [Google Scholar] [CrossRef]
- Lin, X.; Shi, X.; Zheng, X.; Shen, L.; Feng, Y. Injectable long-acting systems for Radix Ophiopogonis polysaccharide based on mono-PEGylation and in situ formation of a PLGA depot. Int. J. Nanomed. 2014, 9, 5555–5563. [Google Scholar] [CrossRef][Green Version]
- Chen, F.; Zhang, J.; He, Y.; Fang, X.; Wang, Y.; Chen, M. Glycyrrhetinic acid-decorated reduction-sensitive micelles to enhance the bioavailability antihepatocellular carcinoma efficacy of tanshinone, IIA. Biomater. Sci. 2016, 4, 167–182. [Google Scholar] [CrossRef]
- Matsutomo, T.; Stark, T.D.; Hofmann, T. Targeted screening and quantitative analyses of antioxidant compounds in aged-garlic extract. Eur. Food Res. Technol. 2018, 244, 1803–1814. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.-L.; Lee, S.; Lee, S.-Y.; Ko, S.; Yoo, M. UPLC/ESI-MS/MS analysis of compositional changes for organosulfur compounds in garlic (Allium sativum L.) during fermentation. Food Chem. 2016, 211, 555–559. [Google Scholar] [CrossRef]
- Zhu, Q.; Kakino, K.; Nogami, C.; Ohnuki, K.; Shimizu, K. An LC-MS/MS-SRM Method for Simultaneous Quantification of Four Representative Organosulfur Compounds in Garlic Products. Food Anal. Methods 2016, 9, 3378–3384. [Google Scholar] [CrossRef]
- Park, T.; Oh, J.-H.; Lee, J.H.; Park, S.C.; Jang, Y.P.; Lee, Y.-J. Oral Administration of (S)-Allyl-L-Cysteine and Aged Garlic Extract to Rats: Determination of Metabolites and Their Pharmacokinetics. Planta Med. 2017, 83, 1351–1360. [Google Scholar] [CrossRef]
- Ahmad, N.; Al-Ghamdi, M.J.A.; Alnajjad, H.S.M.; Al Omar, B.B.A.; Khan, M.F.; Almalki, Z.S.; Albassam, A.A.; Ullah, Z.; Khalid, M.S.; Ashraf, K. A comparative Brain Toxico-Pharmacokinetics study of a developed Tannic Acid nanoparticles in the treatment of epilepsy. J. Drug Deliv. Sci. Technol. 2022, 76, 103772. [Google Scholar] [CrossRef]
- Ahmad, N.; Khalid, M.S.; Al Ramadhan, A.M.; Alaradi, M.Z.; Al Hammad, M.R.; Ansari, K.; Alqurashi, Y.D.; Khan, M.F.; Albassam, A.A.; Ansari, M.J.; et al. Preparation of melatonin novel-mucoadhesive nanoemulsion used in the treatment of depression. Polym. Bull. 2022, 80, 8093–8132. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Mi, Y.; Zhang, B.; Gu, S.; Liu, G.; Li, X. PLGA nanoparticles for the oral delivery of nuciferine: Preparation, physicochemical characterization and in vitro/in vivo studies. Drug Deliv. 2017, 24, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L.; Gergova, G.; Nikolov, R.; Derejian, S.; Lazarova, E.; Katsarov, N.; Mitov, I.; Krastev, Z. Activity of Bulgarian propolis against 94 Helicobacter pylori strains in vitro by agar-well diffusion, agar dilution and disc diffusion methods. J. Med. Microbiol. 2005, 54, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Feroze, N.; Arshad, B.; Younas, M.; Afridi, M.I.; Saqib, S.; Ayaz, A. Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microsc. Res. Tech. 2020, 83, 72–80. [Google Scholar] [CrossRef]
- Colín-González, A.L.; Santamaría, A. Chapter 20—Garlic, Gastrointestinal Protection and Oxidative Stress. In Gastrointestinal Tissue. Oxidative Stress and Dietary Antioxidants; Elsevier: Amsterdam, The Netherlands, 2017; pp. 275–288. [Google Scholar] [CrossRef]
- Sun, S.-B.; Liu, P.; Shao, F.-M.; Miao, Q.-L. Formulation and evaluation of PLGA nanoparticles loaded capecitabine for prostate cancer. Int. J. Clin. Exp. Med. 2015, 8, 19670–19681. [Google Scholar]
- USFDA. Guidance for Industry Bioanalytical Method Validation. 2001. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf (accessed on 24 May 2018).
- Ahmad, N. Rasagiline-encapsulated chitosan-coated PLGA nanoparticles targeted to the brain in the treatment of parkinson’s disease. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 677–690. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Vincent, S.; Saravanan, M.; Lee, Y.; Oh, Y.K.; Kim, K.H. Green synthesis of silver nanoparticles using Rheum palmatum root extract and their antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa. Artif. Cells Nanomed. Biotechnol. 2017, 45, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Qureshi, M.; Jabeen, S.; Ahmad, R.; Alabdalall, A.H.; Aljafary, M.A.; Al-Suhaimi, E. Synthesis, characterization and antibacterial activity of silver nanoparticles using Rhazya stricta. PeerJ 2018, 6, e6086. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, S.; Prasad, S.; Gambhir, I.S. Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Dig. J. Nanomater. Biostructures 2018, 3, 115–122. [Google Scholar]
- Arasoglu, T.; Derman, S.; Mansuroglu, B. Comparative evaluation of antibacterial activity of caffeic acid phenethyl ester and PLGA nanoparticle formulation by different methods. Nanotechnology 2016, 27, 025103. [Google Scholar] [CrossRef]
- Sana, S.S.; Dogiparthi, L.K. Green synthesis of silver nanoparticles using Givotia moluccana leaf extract and evaluation of their antimicrobial activity. Mater. Lett. 2018, 226, 47–51. [Google Scholar] [CrossRef]
- Kaviya, S.; Santhanalakshmi, J.; Viswanathan, B. Green synthesis of silver nanoparticles using Polyalthia longifolia leaf extract along with D-sorbitol: Study of antibacterial activity. J. Nanotechnol. 2011, 2011, 152970. [Google Scholar] [CrossRef]
- Rauf, A.; Khan, A.; Dawood, Z.; Uddin, G.; Farooq, U.; Maalikb, A. Synthesis, characterization and bioactivities of silver nanoparticles using ethanolic and aqueous extracts of Rhazya strictica. In Proceedings of the Nanosmat, Arlington, TX, USA, May 2016; pp. 1–704. [Google Scholar]
- Chen, J.; Mu, Z.; Chen, D.; Huang, C.; Jin, T.; Li, L.; Zeng, Y.; Zhou, Q.; Zhang, Y.; Mao, H.; et al. H2S-releasing versatile hydrogel dressing with potent antimicrobial, anti-inflammatory, epithelialization and angiogenic capabilities for diabetic wound healing. Chem. Eng. J. 2023, 469, 143985. [Google Scholar] [CrossRef]







| Intra–Batch | Inter–Batch | % Recovery | ||||||
|---|---|---|---|---|---|---|---|---|
| QC ID | Theoretical Content (ng mL−1) | Mean Concentration Observed (ng mL−1) | Accuracy a (%) | CV b (%) | Mean Concentration Observed (ng mL−1) | Accuracy a (%) | CV b (%) | |
| LOQQC | 5.01 | 4.98 ± 0.19 | 99.40 | 3.82 | 4.96 ± 0.21 | 99.00 | 4.23 | 77.09 ± 5.07 |
| LQC | 14.90 | 13.86 ± 0.26 | 93.02 | 1.88 | 13.79 ± 0.36 | 92.55 | 2.61 | 78.63 ± 3.37 |
| MQC | 445.00 | 433.81 ± 15.26 | 97.49 | 3.52 | 428.64 ± 16.38 | 96.32 | 3.82 | 80.51 ± 4.86 |
| HQC | 840.00 | 817.65 ± 23.09 | 97.34 | 2.82 | 808.91 ± 21.53 | 96.30 | 2.66 | 81.28 ± 3.96 |
| Conditions | LQC (14.90 ng mL−1) | HQC (840.0 ng mL−1) |
|---|---|---|
| Long-term stability; recovery (ng) after storage (−80 °C) | ||
| Previous day | 14.88 ± 0.13 | 835.13 ± 21.29 |
| 30th Day | 14.79 ± 0.15 (99.40%) | 817.37 ± 20.67 (97.87%) |
| Freeze–thaw stress; recovery (ng) after freeze–thaw cycles (−40 °C to 25 °C) | ||
| Pre-Cycle | 14.87 ± 0.15 | 836.16 ± 21.17 |
| First Cycle | 14.83 ± 0.14 (99.73%) | 821.38 ± 19.98 (98.23%) |
| Second Cycle | 14.78 ± 0.19 (99.39%) | 808.64 ± 21.11 (96.71%) |
| Third Cycle | 14.74 ± 0.21 (99.13%) | 795.09 ± 19.38 (95.09) |
| Heating–cooling stress; recovery (ng) after heating–cooling cycles (50 °C to 4 °C) | ||
| Pre-Cycle | 14.89 ± 0.14 | 836.88 ± 21.38 |
| First Cycle | 14.74 ± 0.25 (98.99%) | 823.04 ± 20.06 (98.35%) |
| Second Cycle | 14.65 ± 0.26 (98.39%) | 807.37 ± 21.64 (96.47%) |
| Third Cycle | 13.61 ± 0.36 (91.40%) | 798.33 ± 21.11 (95.39%) |
| Bench top stability; recovery (ng) at room temperature (25 °C) | ||
| 0 h | 14.85 ± 0.16 | 837.29 ± 20.18 |
| 24 h | 13.99 ± 0.22 (94.21%) | 824.69 ± 19.67 (98.50%) |
| Post processing stability; recovery (ng) after storage in the autosampler (4 °C) | ||
| 0 h | 14.86 ± 0.17 | 835.66 ± 19.69 |
| 24 h | 14.26 ± 0.29 (95.96%) | 833.09 ± 21.09 (99.69%) |
| Parameters | Cmax (ng/mL) | Tmax (h) | t1/2 | Keli (h−1) | AUC0−t (ng h/mL) | AUC0−∞ (ng h/mL) |
|---|---|---|---|---|---|---|
| SC-S (i.v.) | 658.61 ± 43.67 | 0.50 | 2.08 ± 0.078 | 0.33273 ± 0.00011 | 1073.75 ± 53.98 | 1113.30 ± 59.87 |
| SC-S (Oral) | 99.68 ± 16.37 | 0.75 | 3.19 ± 0.056 | 0.21757 ± 0.00009 | 319.85 ± 12.94 | 352.07 ± 13.64 |
| SC PLGA NPs (Oral) | 481.64 ± 30.28 ** | 1.00 | 7.38 ± 0.316 ** | 0.09393 ± 0.00006 | 2813.50 ± 121.64 *** | 4211.50 ± 171.68 *** |
| SC PLGA NPs (i.v.) | 579.21 ± 36.13 *** | 1.00 | 8.26 ± 0.485 ** | 0.08395 ± 0.00008 | 3487.91 ± 138.09 *** | 5600.38 ± 190.35 *** |
| Bacterial Species | Diameter of Inhibition Zone DIZ (mm) for SC PLGA NPs | Diameter of Inhibition Zone DIZ (mm) for SC-S |
|---|---|---|
| Staphylococcus aureus | 36.66 ± 0.57 *** | 26.33 ± 0.61 |
| Bacillus cereus | 29.66 ± 1.52 * | 23.0 ± 1.0 |
| Escherichia coli | 29.66 ± 1.53 ** | 20.33 ± 0.57 |
| Salmonella typhi | 23.33 ± 0.57 * | 20.66 ± 1.15 |
| Pseudomonas aeruginosa | 24.0 ± 1.0 * | 18.66 ± 0.57 |
| Proteus mirabilis | 20.66 ± 1.0 * | 16.0 ± 1.0 |
| Bacterial Species | Diameter of Inhibition Zone DIZ (mm) for SC PLGA NPs | Diameter of Inhibition Zone DIZ (mm) for SC-S | ||||
|---|---|---|---|---|---|---|
| 30 µL | 50 µL | 100 µL | 30 µL | 50 µL | 100 µL | |
| Staphylococcus aureus | 29.33 ± 0.57 | 31.33 ± 0.57 | 34.33 ± 0.57 | 20.33 ± 0.57 | 22.33 ± 0.57 | 25.33 ± 0.57 |
| Bacillus cereus | 24.33 ± 0.57 | 26.0 ± 1.0 | 28.0 ± 1.0 | 19.33 ± 0.57 | 21.0 ± 1.0 | 21.66 ± 0.57 |
| Escherichia coli | 19.66 ± 1.15 | 20.33 ± 0.57 | 22.0 ± 1.0 | 12.0 ± 1.0 | 16.0 ± 1.0 | 20.33 ± 0.57 |
| Salmonella typhi | 19.66 ± 0.57 | 20.66 ± 1.15 | 22.66 ± 1.15 | 15.0 ± 1.0 | 16.66 ± 0.57 | 19.66 ± 1.15 |
| Pseudomonas aeruginosa | 17.66 ± 1.15 | 19.33 ± 0.57 | 23.33 ± 0.57 | 13.00 ± 0.57 | 15 ± 1.0 | 18.33 ± 0.57 |
| Proteus mirabilis | 14.33 ± 0.57 | 16.0 ± 1.0 | 19.0 ± 1.0 | 10 ± 1.0 | 12.00 ± 0.57 | 15.0 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.F.; Ahmad, N.; Alkholifi, F.K.; Ullah, Z.; Farooqui, S.; Khan, N.; Khalid, M.S.; Ali, M.N.; Tabassum, H. A Novel UHPLC-MS/MS-Based Bioanalytical Method Developed for S-Allyl Cysteine in the Establishment of a Comparative Pharmacokinetic Study. Separations 2023, 10, 423. https://doi.org/10.3390/separations10080423
Khan MF, Ahmad N, Alkholifi FK, Ullah Z, Farooqui S, Khan N, Khalid MS, Ali MN, Tabassum H. A Novel UHPLC-MS/MS-Based Bioanalytical Method Developed for S-Allyl Cysteine in the Establishment of a Comparative Pharmacokinetic Study. Separations. 2023; 10(8):423. https://doi.org/10.3390/separations10080423
Chicago/Turabian StyleKhan, Mohd Faiyaz, Niyaz Ahmad, Faisal K. Alkholifi, Zabih Ullah, Sadaf Farooqui, Nazia Khan, Mohammed Saifuddin Khalid, Mir Naiman Ali, and Hajera Tabassum. 2023. "A Novel UHPLC-MS/MS-Based Bioanalytical Method Developed for S-Allyl Cysteine in the Establishment of a Comparative Pharmacokinetic Study" Separations 10, no. 8: 423. https://doi.org/10.3390/separations10080423
APA StyleKhan, M. F., Ahmad, N., Alkholifi, F. K., Ullah, Z., Farooqui, S., Khan, N., Khalid, M. S., Ali, M. N., & Tabassum, H. (2023). A Novel UHPLC-MS/MS-Based Bioanalytical Method Developed for S-Allyl Cysteine in the Establishment of a Comparative Pharmacokinetic Study. Separations, 10(8), 423. https://doi.org/10.3390/separations10080423







