Abstract
Bioactive compounds from plants play an important role in slowing many neurodegenerative diseases, such as Alzheimer’s disease, by inhibiting cholinesterase enzymes. Studies have shown that oxidative stress is associated with the development of Alzheimer’s disease. In traditional medicine of Bosnia and Herzegovina, Teucrium montanum is used to treat numerous diseases. The chemical composition and biological activity of the essential oil (EO) and aqueous (AE) and methanol extract (ME) of this plant were studied. The chemical composition of EO was studied using GC-MS, while the composition of the extracts was studied using HPLC-DAD. Antioxidant activity was tested using the DPPH and FRAP methods. The protection of lipids and proteins from oxidation was tested using the ammonium thiocyanate and BSA oxidation methods. The ability to inhibit cholinesterases was tested by the Ellman method. The main identified EO compounds were α-cadinol, ß-selinene, δ-cadinene, epi-α-cadinol, germacrene D-4-ol, and α-pinene. The main phenolic compounds of the extracts were p-coumaric acid, ellagic acid and caffeic acid. The tested extracts showed good antioxidant radical scavenging and reducing potential and a very good ability to protect lipids and proteins from oxidation. The EO showed moderate AChE and BChE inhibition potential, while the extracts showed weak or no ability.
1. Introduction
Lamiaceae (Labiatae) is a family of flowering plants with cosmopolitan distribution. They are rich in essential oils, which is why many of them are used as spices and medicinal plants, as well as other secondary metabolites that have known pharmacological properties [1,2]. The family includes 236 genera, of which the genus Teucrium has 250 species worldwide, especially in the Mediterranean region [3]. Teucrium species have been used for centuries in folk medicine for their cholagogue, diuretic, antispasmodic, antidiabetic, antirheumatic, anti-inflammatory, antiseptic and vermicidal properties [4]. They are used against flatulence, as aromatics, as antipyretics, and as stimulants. Some of the Teucrium species are used in the traditional medicine of Bosnia and Herzegovina and neighboring countries [5]. T. montanum L. (Mountain germander, Mediterranean Germander, Iva grass) is a shrub with a height of 5 to 25 cm. It is found in Europe and Asia Minor and blooms from June to August [6]. In traditional medicine of Bosnia and Herzegovina, it is used for treatment of the liver, stomach and other diseases [7]. Research has shown that some T. montanum extracts have antibacterial, antifungal, anti-inflammatory and antioxidant activity [5]. T. montanum and its harvest are on the UNESCO list of Intangible Cultural Heritage. It is harvested on the Ozren Mountain in Bosnia and Herzegovina. It is believed that the harvest on the day of the beheading of St. John the Baptist in the summer has a special medicinal effect. It is consumed in different ways (as a tea, soaked in brandy, mixed with honey) both for its curative and preventive effects. Moreover, a wide-spread belief is that T. montanum can cure any disease, hence the local saying Iva grass brings the dead back to life [8].
Plant secondary metabolites play an important role in plant development, especially in their adaptation and survival under adverse conditions. They have beneficial effects on human health. For example, phenols have been shown to play an important role in combating chronic human diseases, and numerous terpenes also exhibit biological activity and are used to prevent and treat various pathological conditions [9].
Foods rich in phytochemicals contain alkaloids as well as terpenes and polyphenols, which alone or in synergy with each other can be good cholinesterase inhibitors [10]. The key enzyme in the hydrolysis of acetylcholine is acetylcholinesterase (AChE), so its inhibition is one of the strategies in the treatment of Alzheimer’s disease and other similar disorders. Plants are considered as potential sources of inhibitors, so many of them, as well as various secondary plant compounds, are the subject of extensive research and represent a model for the development of new drugs, ChE inhibitors [11]. Oxidative stress is associated with some of the major pathological processes and antioxidants play an essential role in the pathogenesis of Alzheimer’s disease [12,13].
In this work, the chemical composition and biological activity of the essential oil and aqueous and methanol extract of T. montanum L. from Bosnia and Herzegovina were studied. The chemical composition of the essential oils was studied by the coupled technique of gas chromatography–mass spectrometry (GC-MS), while the chemical composition of the aqueous and methanol extracts was studied by the coupled technique of high-performance liquid chromatography–diode array detector (HPLC-DAD). The total phenolic content of the extracts was determined by the Folin–Ciocalteu method, while the total flavonoid content was determined by the aluminum chloride method. As for biological activity, antioxidant activity was tested by DPPH and FRAP methods, protection of lipids and proteins from oxidation by the ammonium thiocyanate method and bovine serum albumin oxidation monitoring method. The ability to inhibit the enzymes acetylcholinesterase AChE) and butyrylcholinesterase (BChE) was tested by the Ellman method.
To our knowledge, there are no data on the chemical composition of the essential oil and the chemical composition of the phenolic compounds of T. montanum from Bosnia and Herzegovina. The ability of the essential oil and extracts of T. montanum from Bosnia and Herzegovina to scavenge free radicals and protect lipids and proteins from oxidation, as well as inhibit AChE and BChE, has also been tested for the first time.
2. Materials and Methods
2.1. Chemicals
The analytical-grade reagents and solvents ascorbic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), linoleic acid and Tween-20 (polyethylene glycol sorbitan monolaurate) were purchased from Sigma-Aldrich (St. Louis, MO, USA); acetylcholinesterase (AChE, from Electrophorus electricus, electric eel, type V-S), acetylthiocholine iodide (ATChI), butyrylcholinesterase (BChE, from equine serum), butyrylthiocholine iodide (BTChI), and 5,5-dithiobis (2-nitrobenzoicacid) (DTNB, Ellman’s reagent) were purchased from Sigma-Aldrich GmbH (Steinheim, Germany); butylated hydroxyanisol (BHA), butylated hydroxytoluene (BHT), quercetin and rutin were purchased from Acros Organics, Morris Plains, NJ, USA; DMSO (dimethyl sulfoxide) and methanol were purchased from J. T. Baker (Center Valley, Philadelphia, PA, USA); gallic acid was purchased from Carl Roth, GmbH; ethanol and formic acid were purchased from Kemika, Zagreb, Croatia; bovine serum albumin (BSA) and Folin–Ciocalteu reagent were purchased from Merck (Darmstadt, Germany); apigenin, caffeic acid, chlorogenic acid, chrysoeriol, p-coumaric acid, diosmetin, diosmin, ellagic acid, eriodyctiol, ferulic acid, kaempferol, luteolin, myricetin, naringenin, protocatechuic acid, rosmarinic acid and syringic acid were HPLC grade and purchased from Extrasynthese, Genay, France. Methanol was HPLC grade and purchased from J.T. Baker, Phillipsburg, NJ, USA.
2.2. Plant Material
T. montanum, including its aerial parts, was collected during full flowering in summer 2015 from the locality Lipa (44°41′15.5″ N 16°06′34.9″ E), near Bihac, Bosnia and Herzegovina. Identification of the plant material was performed by PhD Mirko Ruscic (Faculty of Natural Sciences, Split, Croatia). Voucher specimens of the plant material were deposited in the herbarium of the Department of Biochemistry, Faculty of Chemistry and Technology, University of Split (TI _2015). Fresh plant material was used for the isolation of essential oils.
2.3. Isolation of the Essential Oil
The essential oil of T. montanum was isolated from the fresh plant material by hydrodistillation in a Clevenger apparatus using 50 g of plant material, 500 mL of water, and a solvent trap (pentane/diethyl ether = 2/1, v/v) to prevent migration of lipophilic components into the water layer. The isolated essential oil was filled into vials, dried over anhydrous sodium sulphate and stored at 4 °C until analysis.
2.4. Preparation of Aqueous and Methanol Extracts
For the preparation of the aqueous T. montanum extract, fresh plant material (10 g) was covered with boiling water (100 mL) and allowed to stand for 24 h, while for the preparation of the methanol T. montanum extract, the fresh plant material (10 g) was macerated in methanol (100 mL, 70%, w/w) for 48 h at room temperature with occasional stirring. Subsequently, the extracts were filtered and liophilized. The extracts were stored in glass vials at −20 °C. For analysis, the liophilized extracts were dissolved in water or 86% ethanol.
2.5. Identification and Quantification of the Chemical Constituents of the Essential Oil by GC-MS
Identification and quantification of the chemical constituents of the essential oil were performed by GC-MS using a gas chromatograph (gas chromatograph model Varian 3900 (Lake Forest, SAD), and a tandem mass spectrometer (MS) model 2100 T. Chromatographic separation was performed on the nonpolar VF-5MS column (30 m × 0.25 mm × 0.25 µm). Helium was used as the carrier gas at a flow rate of 1.0 mL min, and the sample injection volume was 1 µL. Analyses were performed using MS full scans (40–350 m/z). The ion source temperature was set at 200 °C, the interface temperature was set at 250 °C, and the ion voltage was set at 70 eV. The column temperature program was set at 60 °C for the first 3 min and then heated to 246 °C at 3 °C/min and kept isothermal for 25 min.
The compounds of the essential oil were identified by comparing their retention indices with the series of n-hydrocarbons (C9–C40) analyzed under the same conditions as the essential oil. Individual components were identified by comparing their mass spectra to library entries from two commercial databases, Wiley 7 MS library (Wiley, NY, USA) and NIST02 (Gaithersburg, MD, USA), and by comparing their mass spectra and retention indices to published data [14]. The relative proportions of oil components (%) were calculated based on the peak areas on the chromatography column. Retention indices (RI) were calculated using the equation of van den Dool and Kratz [15].
2.6. Identification and Quantification of Phenolic Compounds
Identification and quantification of phenolic compounds were performed by HPLC-DAD, Agilent 1200 series. Chromatographic separation was performed using the InertSustain C18 column, with the dimensions 250 × 4.6 mm and a stationary phase particle size of 5 µm (GL sciences Inc., Mainz, Germany; S/N 2BR98021, C/N 5020-07346). The standards used were methanol with HPLC purity and formic acid (98–100%) with p.a. purity. A reversed-phase method was used for the separation and identification of phenolic compounds [16]. In the preparation of each sample, 25 mg of the extract was dissolved in 10 mL of 50% methanol solution. Samples were sonicated for 30 s and then filtered through a PTFE filter (CHROMAFIL Xtra PTFE-45/25, Macherey-Nagel, GmbH & Co, Dueren, Germany). Next, 0.06 mL of methanol was added to 0.4 mL of the prepared sample solution so that the final concentration of extract in the solution was 2.1739 mg/mL. The standards kaempferol, myricetin, quercetin, rutin, chrysoeriol, naringenin, eriodictyol, gallic acid, syringic acid, ellagic acid, ferulic acid, caffeic acid, chlorogenic acid, p-coumaric acid, rosmarinic acid and protocatechuic acid were prepared by dissolving in methanol, while four standards, namely luteolin, diosmetin, apigenin and diosmin, were dissolved in dimethyl sulfoxide (DMSO). A starting solution was prepared in which the concentration of each standard was 0.5 mg/mL. A standard addition was prepared by mixing 0.4 mL of the sample solution and 0.06 mL of the initial standard solution, called spikes. The final concentration of each standard in the standard addition was 0.0652 mg/mL. The following method was used: 0.1% formic acid (mobile phase A), methanol (mobile phase B); 0–60 min. Gradient leaching: 90% of phase A to 90% of phase B; 60–65 min. Isocratic wash: 10% A and 90% B; 65.1–70 min. Isocratic wash 90% A and 10% B. The total analysis time was 70 min. The mobile phase flow was 0.5 mL/min, the column temperature 30 °C and the injected sample volume was 20 µL. Chromatograms were recorded at 280 nm.
Identification was performed by comparing the retention times and UV spectra of the components in the sample with the corresponding standards. Quantification of the components in the samples was performed by comparing the integrated areas under the peaks of each component in the sample with the corresponding integrated areas under the peaks in the spike solution using the following formula: Δ = Aspike − Asample, γcompound = (γstandard in spike × Acompound in sample)/Δ, where A is the surface. The results were expressed in mg per g of the dry extract.
2.7. Determination of Total Phenolic Content in Extracts
The content of the total phenolic components in the extracts was determined by the Folin–Ciocalteu method [17]. In addition, 0.25 mL of the extract was pipetted into 25 mL volumetric flasks, and 15 mL of deionized water and 1.25 mL of Folin–Ciocalteu reagent, previously diluted 1:2 with water, were added. The prepared solutions were mixed well, and then 3.75 mL of a 20% Na2CO3 solution was added at a time interval of three to eight minutes. The volumetric flasks were filled to the mark with deionized water. The solutions were allowed to stand at room temperature for 2 h, after which the absorbance was measured at a wavelength of 765 nm. Gallic acid solutions were prepared as standards at the following concentrations: 0, 50, 100, 150, 250, and 500 mg/L. From the measured absorbances of the gallic acid solutions, a calibration curve was prepared, based on which the values of total phenols in the samples were read, expressed as mg of gallic acid equivalents (GAE) per 1 g of extract.
2.8. Determination of Total Flavonoid Content
The content of the total flavonoid content in the extracts was determined by the aluminum chloride method [18]. Then, 0.5 mL of the extract solution was placed in a 5 mL volumetric flask. IN addition, 1.5 mL of ethanol, 0.1 mL of 10% AlCl3 and 0.1 mL of 1 M CH3COOK were added. The total volume was made up to 5 mL by adding water. The controls were prepared along with the samples, adding 0.1 mL of water instead of 0.1 mL of AlCl3. After 30 min, the absorbance of the solution was measured at a wavelength of 415 nm using a spectrophotometer. Quercetin was used as a standard in the following concentration ranges: 0, 25, 50, 75, 100, and 200 mg/L; the values of total flavonoids in the extracts were expressed as mg quercetin equivalent (QE) per 1 g extract.
2.9. Methods for Testing the Biological Potential of Essential Oils and Extracts Isolated from T. montanum
2.9.1. Methods for Testing the Antioxidant Potential of Essential Oils and Extracts from T. montanum
Four in vitro methods were selected for testing the antioxidant activity of the essential oil and extracts of T. montanum: the DPPH radical scavenging method (DPPH method), the reduction potential testing method (FRAP method), the method for testing the role of the extracts in protecting lipids from peroxidation using linoleic acid as a model, and the method for testing the ability of the antioxidant to protect proteins from carbonylation. In addition to testing the antioxidant potential of plant extracts, the antioxidant potential of commercially available antioxidants known to be good was also tested, including vitamin C, BHA and/or BHT.
The DPPH Radical Scavenging Method, DPPH Method
The antioxidant activity of the essential oils and extracts in scavenging DPPH free radicals was determined by the DPPH method [19]. The reaction mixture consisted of 1 mL of DPPH radical solution with a concentration of 0.04 g/L and 50 µL of a sample solution (essential oil or extract) with a known concentration. In addition, 96% ethanol was used to prepare the DPPH radical solutions and the samples, which also served to set the zero point of the apparatus. The absorbance of the reaction mixture was measured after 60 min at a wavelength of 517 nm using a spectrophotometer. The ability to inhibit DPPH radicals, expressed as percentage inhibition of DPPH radicals, was calculated according to the following formula: % inhibition = [(A0 − Asample)/A0] × 100, where A0 is the absorbance of the DPPH solution without the sample, measured at the beginning of the measurement (t = 0), and Asample is the absorbance of the sample (essential oil or extract), measured after 60 min.
The Reduction Potential Testing Method, FRAP Method
The reducing potential of the essential oils and extracts was tested using the FRAP method (ferric ion reducing antioxidant power) [20], which is based on a comparison of the reducing potential of the tested samples with that of solutions of known concentrations of Fe2+ ions. In the measurement of antioxidant potential by the method FRAP, 30 μL of water and then 10 μL of the sample (essential oil or extract with a mass concentration of 1 mg/mL) were pipetted into the wells of the microtiter plate. Then, 300 μL of the previously prepared FRAP reagent was added, which was prepared by mixing 25 mL of 0.3 M acetate buffer (pH = 3.6), 2.5 mL of TPTZ solution (10 mM in 40 mM HCl), and 2.5 mL of fresh 0.02 M solution of ferric chloride hexahydrate (FeCl3 × 6H2O). After 4 min, the absorbance was measured at a wavelength of 593 nm. In parallel with the samples, the absorbance values of the controls (10 μL of the sample and 330 μL of water) were measured in the same way and their values were subtracted from the absorbance values obtained for the samples. The obtained values were compared with the calibration curve prepared with solutions of iron(II)sulphate heptahydrate (FeSO4 × 7H2O) in the concentration range 0–750 μM.
The Method for Testing the Role of the Extracts in Protecting Lipids from Peroxidation
The ammonium thiocyanate method was used to test the ability of plant extracts to protect lipids from peroxidation [21]. The extract solution with a volume of 0.5 mL and a concentration of 1 mg/mL was homogenized with 2.5 mL of linoleic acid emulsion and 2 mL of Na phosphate buffer (0.2 M; pH 7). The linoleic acid emulsion was prepared by mixing 0.2804 g linoleic acid, 0.2804 g Tween-20 emulsifier, and 50 mL Na-phosphate buffer. The reaction mixture was incubated at 37 °C for 24 h. The degree of oxidation of linoleic acid was measured by adding it to an aliquot of 0.1 mL of the sample ethanol (4.7 mL; 75%), ammonium thiocyanate (0.1 mL; 30%), and iron(II)chloride (0.1 mL; 0.02 M in 3.5% HCl). After allowing the reaction mixture to stand for 3 min, the peroxide content in the samples was determined by reading the absorbance at a wavelength of 500 nm. Controls were prepared by adding water, i.e., 86% ethanol, instead of the extract solution. The ability to inhibit lipid oxidation was calculated according to the following formula: % of inhibition of lipid peroxidation = [1 − (Asample − A0)] × 100, where A0 is the absorbance of the solution without the sample and Asample is the absorbance of the sample.
The Method for Testing the Ability of the Antioxidant to Protect Proteins from Carbonylation
This method is based on monitoring the oxidation of bovine serum albumin (BSA) in a metal-catalyzed reaction with or without the presence of the tested extract. The reaction of the resulting aldehyde or keto functional groups with the reagent dinitrophenylhydrazine (DNPH) produces DNP-hydrazone with an absorption maximum at a wavelength of 370 nm [22,23]. The reaction mixture contained BSA (4 mg/mL), FeCl3 (0.05 mM), ascorbic acid (0.1 mM), H2O2 (1 mM), and a plant extract with a concentration of 167 µg/mL. The total volume of the reaction mixture was 600 µL. Water was used for the aqueous extract and 86% ethanol was used as a blank for the methanol extract. The mixture was incubated at 37 °C for 1 h or 24 h. The amount of carbonyl groups formed was determined spectrophotometrically [23]. After incubation, 1 mL of DNPH solution (10 mM) was added to the mixture and the solution was incubated at room temperature for 60 min. Then, 1 mL of 10% trichloroacetic acid (TCA) was added to the sample and the solution was placed on ice for 15 min. The samples were then centrifuged at 5000 rpm for 40 min. The supernatant was discarded, and the precipitate was washed three times with 2 mL of an ethanol/ethyl acetate mixture (1:1 v/v). After the last centrifugation, the precipitates were dissolved by vortexing in 6M guanidine-HCl in 2 mL of 2 M HCl (pH 2) and incubated at 37 °C. After the sediments were resuspended, they were transferred to cuvettes suitable for measurement in the UV region of the spectrum, and absorbance was measured spectrophotometrically at a wavelength of 370 nm. The ability of antioxidants to protect proteins from carbonylation was calculated according to the following formula: % of inhibition of protein oxidation = [(A0 − Asample)/A0] × 100, where A0 is the absorbance of the blank and Asample is the absorbance of the sample.
2.9.2. Method for Determining the Ability to Inhibit the Enzyme Cholinesterases
To determine the ability to inhibit the cholinesterase enzymes, AChE and BChE, the Ellman method was used, based on the reaction of Ellman reagent (DTNB) and thiocholine, yielding a yellow-colored product [24]. The reaction mixture consisted of 180 μL phosphate buffer (0.1 M; pH = 8), 10 μL 5,5′-dithiobis-(2-nitrobenzoic acid, DTNB), 10 μL acetylthiocholine iodide (ATChI), 10 μL acetylcholinesterase (AChE) from electric eel electrophores, and 10 μL samples at a specific concentration. The samples of essential oil and methanol extracts were dissolved in 86% ethanol, while the aqueous extracts were dissolved in water. Water and 86% ethanol were used for the control measurement. The total volume of the reaction mixture was 220 μL. A DTNB stock solution (6.6 mM) was prepared with phosphate buffer (0.1 M; pH = 7). Stock solutions of AChE (0.66 U/mL) and ATChI (11 mM) were prepared with phosphate buffer (0.1 M; pH = 8). Butyrylthiocholine iodide (BTChI) and the enzyme butyrylcholinesterase (BChE) from horse serum were used to test the ability to inhibit the enzyme butyrylcholinesterase (BChE). Stock solutions of BTChI (11 mM) and BChE (0.66 U/mL) were prepared with phosphate buffer (0.1 M; pH = 8). The other components of the reaction mixture and the reaction mechanism were the same as for the AChE enzyme inhibition test. Measurements were performed at 25 °C and a wavelength of 412 nm. Enzyme activity measurements were performed under the conditions of a constant amount of substrate and lasted 6 min with three replicates each. The percentage of AChE/BChE enzyme inhibition by essential oils or extracts was calculated according to the following formula: % inhibition of AChE/BChE = {[(Ae − Abe) − (Au − Abu)]/(Ae − Abe)} × 100; Ae—absorbance of enzyme without an inhibitor, Abe—absorbance of a blank for enzyme without a substrate, Au—absorbance of enzyme with an inhibitor, Abu—absorbance of blank for enzyme without an inhibitor.
3. Results and Discussion
In this work, the phytochemical composition as well as antioxidant and anticholinesterase potential of the essential oil (EO) and aqueous (AE) and methanol extract (ME) of T. montanum L. from Bosnia and Herzegovina were studied.
3.1. Chemical Composition and Content of Volatile Components of Essential Oil from T. montanum
The composition of volatile components of the EO from T. montanum from Bosnia and Herzegovina was determined using a coupled gas chromatography–mass spectrometry (GC-MS) system. The results of the chemical composition of the EO of T. montanum from Bosnia and Herzegovina are summarized in Table 1, while the corresponding chromatogram is shown in Figure 1.

Table 1.
GC-MS analysis of the essential oil isolated from T. montanum.
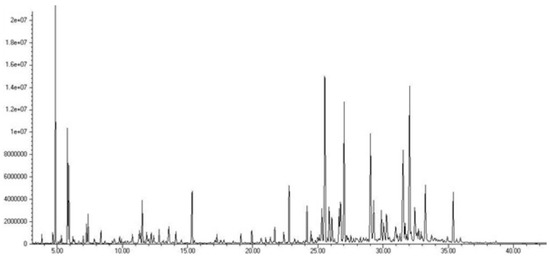
Figure 1.
GC-MS total ion chromatogram of essential oil of T. montanum.
A total of forty seven compounds were identified by GC-MS analysis of EOs isolated from fresh aerial T. montanum plant material. Among the identified compounds were oxygenated sesquiterpenes (36%), non-oxygenated sesquiterpenes (29.7%), non-oxygenated monoterpenes (15.3%) and oxygenated monoterpene compounds (10.0%). The major compounds identified were α-cadinol (9%), ß-selinene (9%), δ-cadinene (6.9%), epi-α-cadinol (6.9%), germacrene D-4-ol (6.8%), α-pinene (6.4%), (E)-caryophyllene (2.8%) and ß-pinene (2.7%). The high occurrence of sesquiterpene compounds is generally a feature of most Teucrium species [25,26].
To our knowledge, this is the first analysis of the chemical composition of EO of T. montanum from Bosnia and Herzegovina and the second analysis of EO isolated from fresh plant material. The only chemical analysis of the EO of this plant from fresh plant material was performed for the plant from Sicily [27]. In comparison with the previously analyzed EOs of T. montanum from other areas, the studied EO from Bosnia and Herzegovina had a similar composition with a difference in the content of the main components.
The EO of T. montanum isolated from fresh aerial plant material collected in Sicily in June is also very rich in sesquiterpenes (94.3%). The oxygenated sesquiterpenes constitute the main class of the oil (63.5%), with longifolenaldehyde (14.5%), epiglobulol (13.5%), and ledene oxide (12.1%) being the most important compounds. β-Cedrene (8.9%) was the main component among sesquiterpene hydrocarbons (30.8%) [27].
The EO from air-dried aerial parts of T. montanum collected in spring in Dalmatia, Croatia, contained germacrene D (17.2%), β-pinene (12.3%) and β-caryophyllene (7.1%) as the major constituents among 37 identified compounds [26].
The comparative study of the EOs of dried T. montanum from the Serbian region during flowering and from two different types of substrates showed that sesquiterpenes are the main components of the essential oils studied. The compound with the highest relative content in the calcareous populations was tetracosane (9.02%), while in the serpentinite populations it was limonene-10-ol (4.81%) [28]. Sesquiterpene compounds were also the main fraction (72.7%) of the EO, isolated from the material of aerial plants from southwestern Serbia (Jabuka, Prijepolje) in the flowering stage and previously dried, with δ-cadinene (8.1%), ß-caryophyllene (5.1%), τ-muurolol (4.2%) and α-pinene (4.0%) being the most abundant [29]. The EO of aerial and air-dried T. montanum collected in August in the mountains of Jadovnik, Serbia, showed the presence of δ-cadinene (17.19%) and ß-selinene (8.16%) as the main constituents [5,30].
The chemical profile of the EO of T. montanum subsp. jailae collected in August and previously dried from eastern Slovakia shows that the sesquiterpene fraction (76.3%) dominated over the monoterpene fraction (10.1%). The most important compounds were germacrene D (12.8%), two unknown oxygenated sesquiterpenes with MW of 220 (10.9 and 8.4%, respectively) and (E)-caryophyllene (8.0%) [31].
Kovacevic et al. [32] analyzed the chemical composition of EO of air-dried T. montanum collected from flowering plants on Mount Orjen in Montenegro. The results showed germacrene D (15.0%), α-pinene (12.4%), ß-eudesmol (10.1%) and ß-caryophyllene (6.9%) as the quantitatively most important compounds.
The EO from air-dried material of T. montanum from Turkey contained sabinene (11.3%), δ-cadinene (6.3%), germacrene D (5.8%), α-copaene (5.7%), (E)-ß-farnesene (5.5%) and α-pinene (5.2%) as the major compounds [25].
3.2. The Content of Phenol Compounds in Extracts of T. montanum
The content of selected phenol compounds (twenty different phenolic compounds) from AE and ME from fresh plant material of T. montanum was investigated using the HPLC-DAD system and the total phenol content of the plant extract was tested using the Folin–Ciocalteu method [17], while the total flavonoid content was tested using the method with AlCl3 [18]. The results are shown in Table 2 and the corresponding chromatograms in Figure 2.

Table 2.
Content of selected phenol compounds, total phenol content and total flavonoid content of aqueous and methanol extracts of T. montanum.
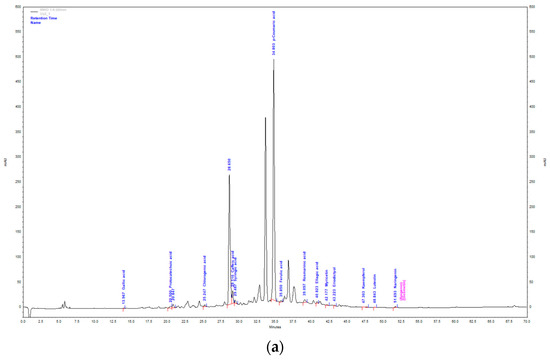
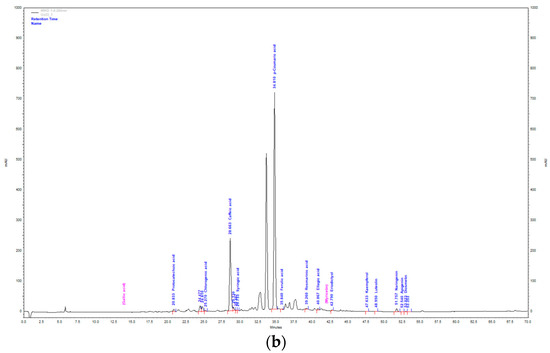
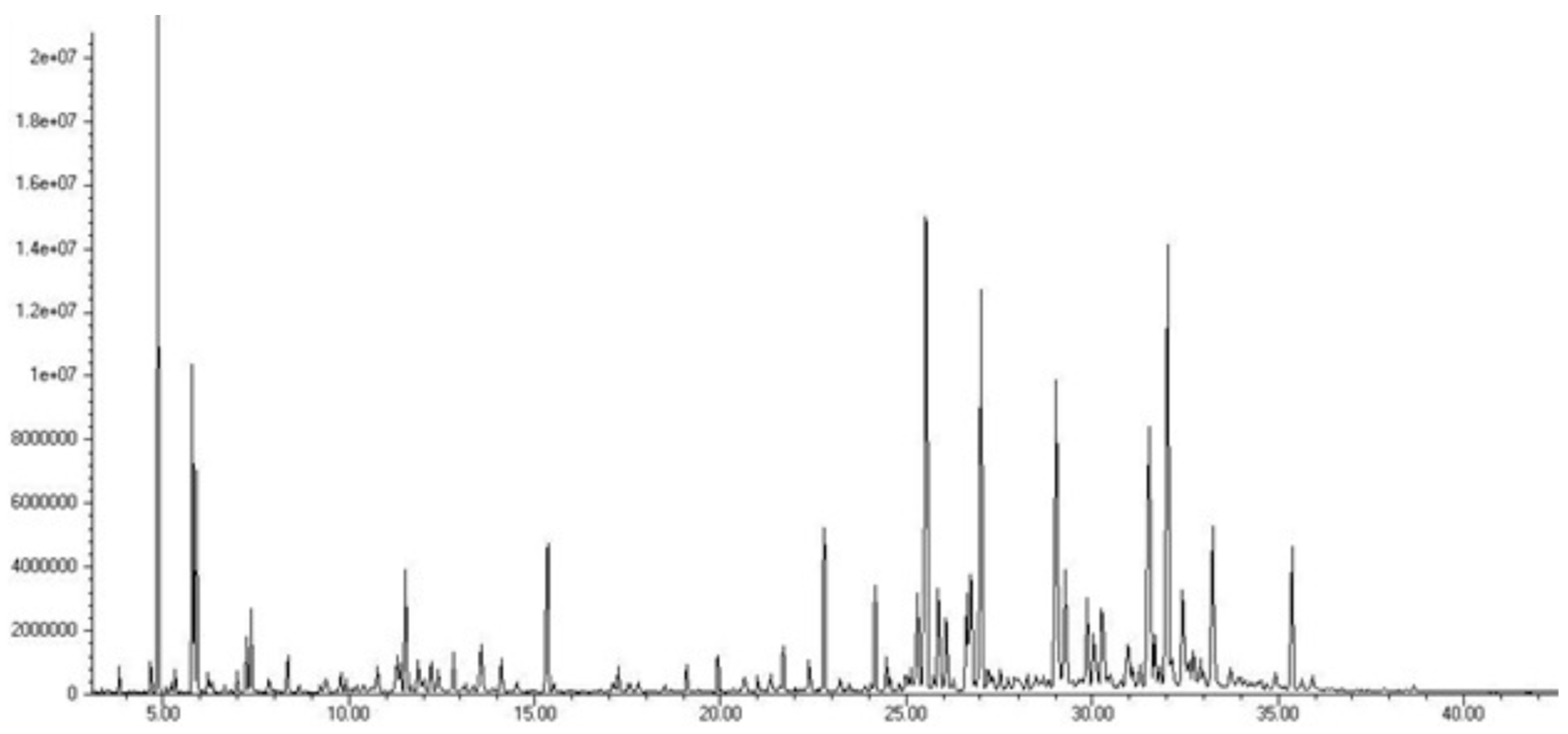
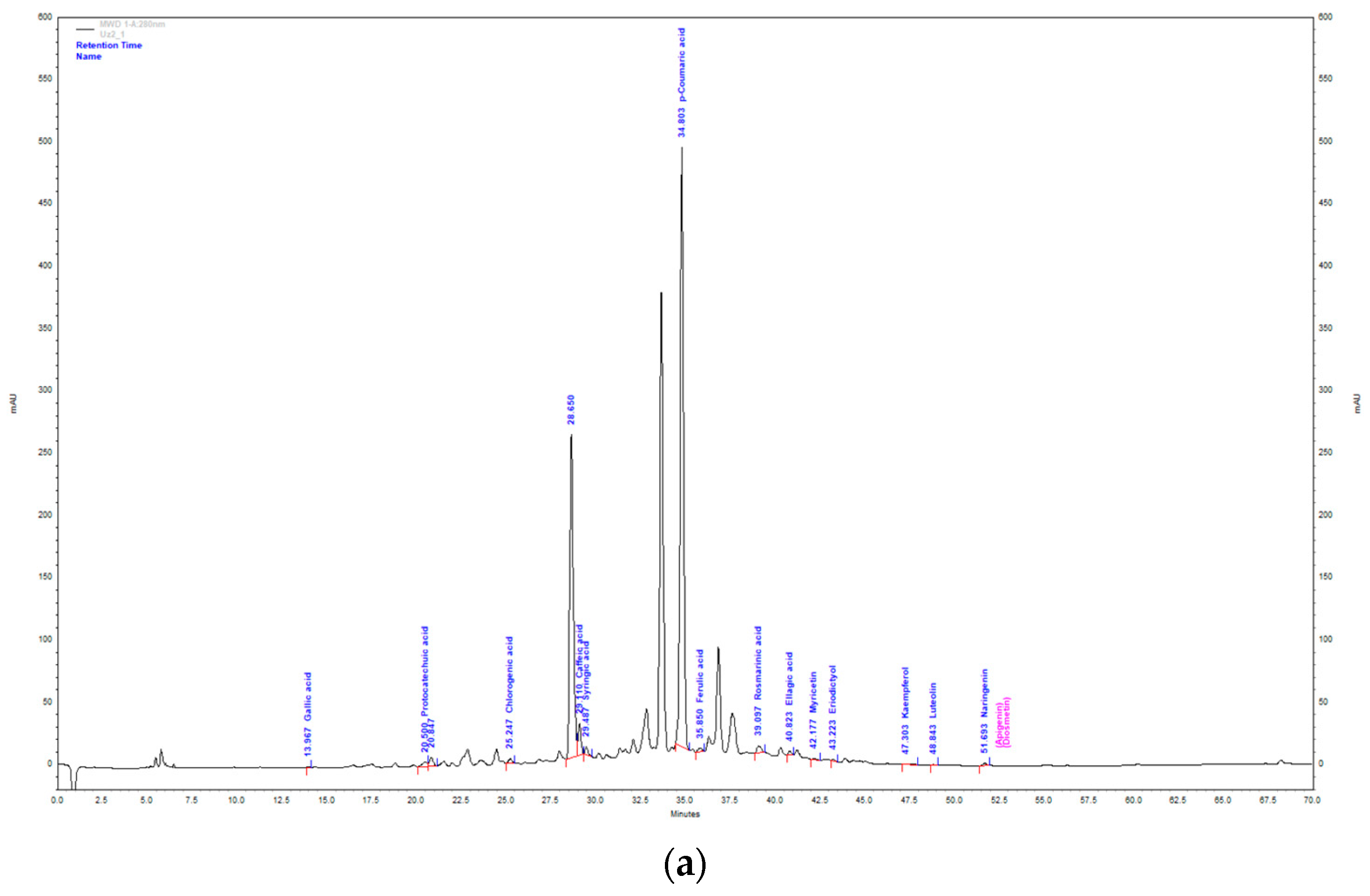
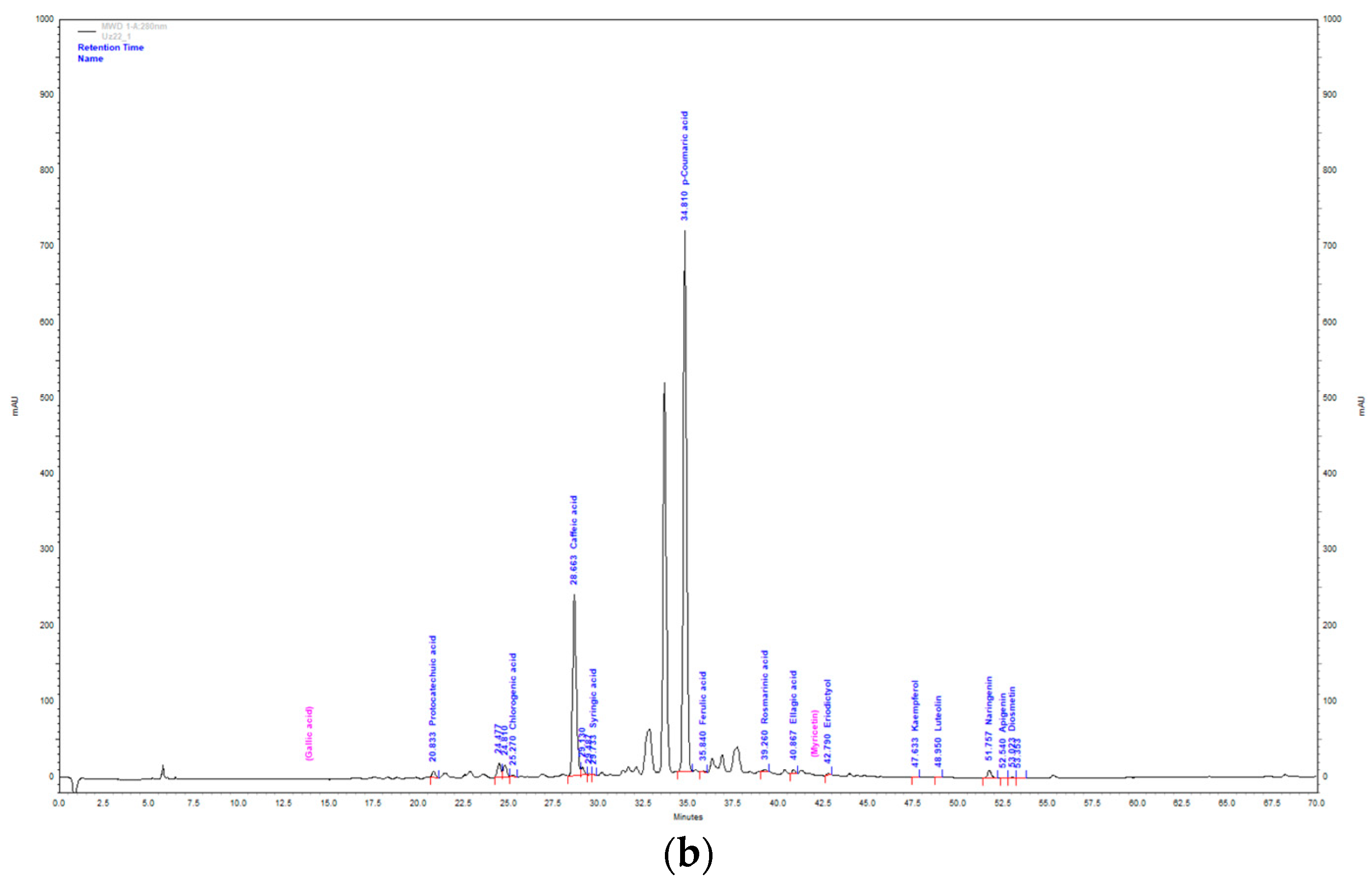
Figure 2.
HPLC chromatogram of aqueous extract (a) and methanol extract (b) of T. montanum.
AE and ME obtained by isolation from fresh plant material of T. montanum were analyzed by the HPLC-DAD system. The extracts were analyzed for the content of twenty different phenolic compounds. Sixteen phenolic compounds were determined qualitatively and quantitatively, while quercetin, rutin, diosmin and chrysoeriol were not detected.
In the total mass of identified compounds in the AE of T. montanum, hydroxycinnamic acids are the most abundant with 81.01% (17.75 mg/g extract). They are followed by hydroxybenzoic acids with 17.28% (3.79 mg/g extract), flavonols with 0.9% (0.2 mg/g extract), flavanones 0.73% (0.16 mg/g extract) and flavones with 0.14% (0.03 mg/g extract). The most abundant compound is p-coumaric acid (15.95 ± 0.11 mg/g extract). It is followed by ellagic acid (3.51 ± 0.99 mg/g extract), caffeic acid (0.71 ± 0.01 mg/g extract) and rosmarinic acid (0.51 ± 0.07 mg/g extract).
With the largest percentage of the total mass of identified compounds in the ME of T. montanum, hydroxycinnamic acids are the most abundant with 80.26% (33.18 mg/g extract). They are followed by hydroxybenzoic acids with 17.59% (7.27 mg/g extract), flavanones with 1.84% (0.76 mg/g extract), flavones with 0.24% (0.1 mg/g extract) and flavonols with 0.05% (0.02 mg/g extract). The most abundant compound is p-coumaric acid (21.98 ± 0.23 mg/g extract). It is followed by caffeic acid (10.73 ± 0.09 mg/g extract) and ellagic acid (7.25 ± 0.42 mg/g extract).
To date, three analyses of the phenolic composition of WE and ME of plants from Serbia and one analysis of ME from Romania have been performed, but none from fresh plant material. No analysis has yet been performed for plants from Bosnia and Herzegovina.
The contents of luteolin and apigenin in the ME of air-dried T. montanum from Romania were 1.1775 and 0.6531 mg/g dry extract, respectively, while the content of caffeic acid was 1.6185 mg/g dry extract. No content of rosmarinic acid was detected in the studied extracts [33].
The contents of rosmarinic acid, caffeic acid and chlorogenic acid were 1.51 ± 0.45, 0.34 ± 0.02 and 1.31 mg/g dry extract in the AE and 0.51 ± 0.07, 5.67 ± 0.23 and 1.41 ± 0.05 mg/g dry extract in the ME of T. montanum from the Stara Planina Mountains, Serbia [34]. The contents of identified phenolic compounds detected by HPLC-PDA in subcritical AE of commercial and dried T. montanum (Adonis D.O.O., Sokobanja, Serbia) were as follows: gallic acid 345 ± 34, protocatechuic acid 117 ± 12, chlorogenic acid 79.9 ± 8.0, caffeic acid 56.0 ± 6.2 and ferulic acid 48.9 ± 4.9 mg/100 g DE (dry extract) [35]. Gallic acid was absent in the AE and ME of dried T. montanum from Serbia (Zlatibor), while protocatechic and ferulic acids were present in the ME in amounts of 0.09 mg/g and 0.05 mg/g, respectively. Chlorogenic acid was present in the AE at a concentration of 0.15 mg/g extract. Caffeic acid was present in the ME and AE at a concentration of 0.13 and 0.06 mg/g extract, respectively [36].
In the ethanol extract of T. montanum from Croatia, the content of hydroxycinnamic acids was as follows: chlorogenic acid 2.25, caffeic acid 2.36, and ferulic acid 1.70 mg/g of extract [37].
The most abundant flavonoids in the aerial parts of various extracts (extracted with 70% ethanol and after evaporation of ethanol, the water phase was subsequently extracted with diethyl ether, ethyl acetate or n-butanol) of T. montanum from Bulgaria were luteolin and diosmetin [38].
In the AE and ME of T. montanum investigated by this work, the most abundant were p-coumaric acid (15.95 ± 0.11 and 21.98 ± 0.23 mg/g extract), ellagic acid (3.51 ± 0.99 and 7.25 ± 0.42 mg/g extract) and caffeic acid (0.71 ± 0.01 and 10.73 ± 0.09 mg/g extract). The content of caffeic acid in ME was higher than in ethanol extracts from Croatia and AE and ME from Serbia (Zlatibor). The content of ferulic acid was higher than the content of the same compounds in the previously tested extracts. The content of chlorogenic acid was generally lower in the extracts from Bosnia and Herzegovina than in the other tested extracts. Only the AE extract from Serbia (Zlatibor) had a lower content of chlorogenic acid. The most abundant flavonoids in ME were naringenin, diosmetin and luteolin.
The total phenol content of ME of T. montanum investigated in this study was higher (214.75 ± 1.98 mg GAE/g extract) than that of AE (136.97 ± 1.65 mg GAE/g extract). Total flavonoid content as well as total phenolic content were higher in the ME of T. montanum (24.72 mg QE/g extract, QE—quercetin equivalent) than in the AE (15.66 mg QE/g extract).
The total phenol content of ME of the plant from the northeastern region of Romania was 164.42 ± 0.03 mg GAE/g dry extract, while the total flavonoid content was 51.42 ± 0.15 mg GAE/g dry extract [33].
The total phenol content of AE and ME of the dried plant from Stara Planina Mountains, Serbia, was 10.19 ± 0.85 and 25.32 ± 3.04 mg GAE/g of the extract, while the total flavonoid content was 2.39 ± 0.43 and 4.69 ± 0.46 mg QE/g [34]. The total content of phenol compounds in the ME of previously dried T. montanum collected during full flowering at serpentine sites (Goč and Kamenica, Stolovi Mountains, Serbia) ranged from 160.21 to 190.20 mg GA/g of the extract, while the amount of phenols from calcareous (Kopaonik and Durmitor Mountains, Serbia and Montenegro) was 143.42 and 148.21 mg GA/g of the extract. The values of flavonoid content in the plant extracts of the species T. montanum from serpentine areas ranged from 53.82 to 54.19 mg Ru/g of the extract, while the flavonoid content of the extracts from the samples from calcareous sites reached the values 46.50 and 49.53 mg Ru/g of the extract (Ru, rutin equivalent) [39]. The content of total phenols in the AE and ME of different parts of T. montanum (whole plants, leaves, flowers and stems) from central Serbia, expressed in GAE/g dry extract, was tested. The order of concentrations of phenolic compounds was leaves > flowers > stems. Among the tested extracts from whole plants as well as extracts from plant parts, the methanol extract of the whole plant (169.06 mg GAE/g) and the AE of leaves (154.81 mg GAE/g) contained the highest phenolic content. The content of phenol compounds in the AE from the whole plant was 110 mg GAE/g [40,41]. Similar results were obtained for the total flavonoid content in this plant. The order of concentrations of flavonoid compounds was whole plant > leaves > flowers > stems and in the range of 20 to 50 mg Ru/g extract [40]. The total phenol content in the extracts of T. montanum L. from Zlatibor, Serbia, was 59.8 ± 2.54 mg/g CAE (chlorogenic acid equivalents per gram dry weight) for the AE and 154 ± 6.7 mg/g CAE for the ME [42]. The contents of total phenol compounds in the AE and ME of T. montanum from Serbia (Zlatibor) were 59.8 and 154 mg CAE/g extract (CAE, chlorogenic acid equivalents) [36].
The total polyphenol content of T. montanum ME (30%) from Croatia varied from 12.8% in cultivated to 13.7% in dried wild plants [7].
The content of flavonoids in the 70% ethanol extract of the air-dried aerial parts of T. montanum from Bulgaria was 0.15% [38].
The total content of flavonoids in the AE and ME of T. montanum from Bosnia and Herzegovina was higher than reported in the literature for extracts of the same plant species from Serbia. In other samples, the content is given in other units and cannot be compared. Differences in phenol and flavonoid content may be caused by the duration of the extraction process, the characteristics of the plant itself, the origin of the plant and the time of collection [43]. The amount of sample used for extraction may also have an influence, as well as the storage conditions, the determination method, the choice of standards, and the presence of interfering substances (waxes, lipids, terpenes, and chlorophyll).
ME showed a higher content of total phenols and flavonoids. Some authors also pointed out that alcohols are suitable solvents for the extraction of phenol and flavonoid compounds. Methanol or ethanol are most commonly used for the separation of this class of natural products because they dissolve best in alcohols, especially methanol [44,45,46].
3.3. Antioxidant Potential of Essential Oil and/or Extracts Isolated from T. montanum from Bosnia and Herzegovina
The antioxidant potential of EO and/or AE and ME isolated from T. montanum was tested by four different methods. These are the DPPH radical scavenging method (DPPH method) [19], the reduction potential testing method (FRAP method) [20], the method for testing the role of the extracts in protecting lipids from peroxidation using linoleic acid as a model [21], and the method for testing the ability of the antioxidant to protect proteins from carbonylation [22]. The results obtained were compared with those of known good antioxidants and are shown in Table 3.

Table 3.
Antioxidant potential of the essential oil and/or aqueous and methanol extract of T. montanum from Bosnia and Herzegovina.
The ME of T. montanum showed the highest potential in scavenging DPPH radicals (IC50 = 21.43 µg/mL). Followed by the AE (IC50 = 38.09 µg/mL) and the EO of the plant (IC50 = 201.91 µg/mL). The same results were obtained when the reduction potential of the plant extracts was tested using the FRAP method. The ME showed the best reduction potential (1548.00 ± 16.16 Fe2+ µmol/g), followed by the AE (761.33 ± 11.89 Fe2+ µmol/g) and EO (13.02 ± 4.31 Fe2+ µmol/g).
The obtained results were compared with those of the known good antioxidants BHA and ascorbic acid. The ability of BHA to scavenge DPPH free radicals is IC50 = 17.62 µg/mL, while the reduction potential tested by the method FRAP is 5586.29 ± 174.76 Fe2+ µmol/g sample. The ability of ascorbic acid to scavenge DPPH free radicals is IC50 = 16.67 µg/mL, while the reduction potential tested by the method FRAP is 5568.43 ± 125.26 Fe2+ µmol/g. Comparison of these results with those of the extracts isolated from T. montanum showed that the tested extracts have a good antioxidant potential, but it is weaker compared to BHA and ascorbic acid.
Antioxidant activity has already been evaluated for the EOs of several members of the genus Teucrium [47,48], but not for EO of T. montanum.
The antioxidant potential tested by the DPPH and FRAP method was previously tested for the AE and ME of the plant from Serbia, while the antioxidant activity tested by FRAP method was presented for AE from Tuzla and Brcko (BiH) and for the subcritical water extracts of the commercially available plant from Serbia.
The antioxidant activity of ME of T. montanum from northeastern Romania tested by the DPPH method was IC50 = 50.91 ± 0.21 μg/mL [33].
In the DPPH assay, IC50 values for the AE of T. montanum from Serbia were 0.240 mg/mL [49]. The IC50 values for extracts of the plant from Serbia (Goč, serpentine substrate and Kopaonik, calcareous substrate) were 92.49 ± 0.44 and 102.21 ± 0.8 µg/mL for AE and 38.76 ± 0.18 and 44.99 ± 0.11 µg/mL for ME, respectively [39]. The IC50 values for testing the antioxidant potential of the extracts by the DPPH method for the plant from Goc Mt. in central Serbia were 29.41 ± 0.76 µg/mL for AE and 45.41 ± 0.85 µg/mL for ME. Both extracts showed good DPPH radical scavenging activity compared to BHA (IC50 = 5.39 ± 0.31 µg/mL) [40]. The subcritical AE, obtained from the commercially available (Adonis D.O.O., Sokobanja, Serbia) aerial parts of dry T. montanum material from Serbia, showed DPPH radical scavenging activity between 92.83 ± 4.56 and 155.83 ± 14.19 (mg TE/g DE; TE = Trolox equivalent) at different extraction pressures (10–100 bar) and between 80 and 175 mg TE/g DE at different temperatures (60–200 °C). The influence of different extraction pressures (10–100 bar) on the reduction potential tested by the FRAP method for this sample ranged from 119.96 ± 1.81 to 131.40 ± 5.83 TE/g DE, while the influence of extraction temperature was 115–140 mg AAE/g DE (AAE = ascorbic acid equivalent) [35]
The IC50 value for the ethanolic extract of T. montanum from Croatia was 7.78 ± 0.13 µg/mL [37].
The reducing power of AE of T. montanum from Tuzla Canton (TC) and Brcko District (BD) tested by the method FRAP was 3960.90 and 10,974.50 μmol Fe2+/L extract, respectively [50]. This is the second sample of T. montanum AE tested by this method, and the results are lower than those obtained in this study.
Comparing the results obtained in this work with those previously reported and mentioned above, we found that the ability to scavenge DPPH radicals is higher or similar in AE, while it is slightly higher in ME.
The antioxidant potential of AE and ME of this plant correlates with the content of total phenols and the content of flavonoids in this plant. These are known to be compounds that have strong antioxidant activity [42,51,52]. A particularly good correlation exists between the content of total phenols and flavonoids and the ability to scavenge free radicals, as well as the reduction potential of the plant, which has already been shown in other examples [53].
This work also tested the ability of AE and ME to protect lipids and proteins from oxidation. To our knowledge, this is the first work to investigate this type of antioxidant potential.
The ability of AE and ME to protect lipids from oxidation was tested at two concentrations of stock solution, 0.1 mg/mL and 1 mg/mL (the concentration in the reaction mixture was 0.01 and 0.1 mg/mL). The results obtained were as follows: 52.47 ± 2.73 and 58.79 ± 6.60% for the AE and 34.82 ± 0.17 and 39.58 ± 3.11% for the ME. The results showed that the AE of the plant has a slightly better ability to protect lipids from peroxidation than the ME at both concentrations tested. Comparison of the obtained results with those of the standards tested at the same concentrations, BHA (59.90 and 64.78%) and BHT (60.36 and 64.58%), suggests that the AE of T. montanum shows very good results in protecting lipids from oxidation, while the ME shows only slightly weaker results. In all these samples, increasing the concentration did not lead to a significant increase in inhibition. Ascorbic acid (vitamin C) showed a prooxidant effect at an initial concentration of 0.1 mg/mL, while at a concentration of 1 mg/mL, it inhibited the peroxidation of linoleic acid by 95.5%. The results obtained for ascorbic acid are in accordance with the already known fact that ascorbic acid can participate in the Haber–Weiss reaction as a prooxidant. At higher concentrations, its antioxidant capacity outweighs its ability to accelerate oxidation, resulting in an overall antioxidant effect [54].
The AE and ME of T. montanum were also tested for protection of proteins against oxidation by spectrophotometric measurement of the amount of carbonyls formed. Oxidation of bovine serum albumin (BSA) is induced by heating the protein in the presence of Fe3+, ascorbic acid, and hydrogen peroxide. The results of inhibition of BSA carbonylation (% inhibition) in the presence of the tested extracts and the commercial antioxidants (BHT and BHA) are shown. The incubation time at 37 °C with metal-induced oxidation was 1 and 24 h.
The ability of AE and ME to protect proteins from carbonylation was tested at a stock solution concentration of 1 mg/mL (the concentration in the reaction system was 167 µg/mL), and the results for testing after 1 h and after 24 h were as follows: 8.49 ± 0.94% and prooxidant activity for the AE as well as 11.11 ± 5.05% and prooxidant activity for the ME.
The results obtained after 1 h incubation showed a very good protective effect of both the AE and ME of T. montanum compared with the standard antioxidant BHT, which showed an inhibition of carbonylation of 11.12%. BHA showed prooxidant activity.
After incubation for 24 h, the extracts showed prooxidant activity, while BHT and BHA inhibited the carbonylation of BSA by 13.54% and 8.04%, respectively.
The prooxidant activity of the extracts has already been described in the literature. Polyphenols from foods act as prooxidants that catalyze oxidative damage to DNA, proteins, and carbohydrates, although they protect lipids from oxidation. The study by Dorman and Hiltunen (2011) [55] showed the complexity, i.e., the paradox of certain phytochemicals with antioxidant activity that can play a protective role by protecting some biologically important molecules, e.g., lipids, from oxidative damage, but also exhibit prooxidant activity toward other biologically important molecules, e.g., proteins. In the above study, gallic acid showed prooxidant activity, as did extracts of juniper (Juniperus communis L.), basil (Ocimum basilicum L.), caraway (Carum carvi L.), and laurel (Laurus nobilis L.).
In the study by Mayo et al. (2003) [56] on the protection of proteins from oxidative damage, trolox and ascorbic acid showed prooxidant activity. Ascorbic acid is known to be a strong reducing agent and is used to reduce transition metals such as Fe3+ or Cu2+ and generate •OH radicals. The combination of vitamin C and Cu2+ causes significant protein damage. In the same study, resveratrol showed no protective effect [56].
The results obtained can be explained by the reaction mechanism. Metal-catalyzed oxidation of proteins is a process that occurs mainly at specific metal-binding sites of the protein, where one or more amino acids are oxidized. The amino acid residues that are most sensitive to oxidation are histidine, proline, arginine, and lysine. The reaction proceeds as a “closed” process in which iron undergoes a redox cycle from Fe2+ to Fe3+ in reaction with H2O2 and again by protein-dependent reduction to Fe2+. During this process, the oxygen radical formed as an intermediate does not diffuse into the surrounding medium as it reacts with the aforementioned amino acid residues of the protein [56].
Since potential antioxidants cannot come into contact with free radicals, they cannot compete with amino acids for them, so their protective effect is absent.
3.4. Cholinesterase Inhibition Potential of Essential Oil and Extracts Isolated from T. montanum
The ability of the EO and extracts of the selected plant to inhibit the enzymes acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) was tested by the Ellman method [24]. The tested stock solution concentration of the EO was 1 and 2 mg/mL, while the tested concentration of the extracts was 1 mg/mL. The concentrations in the reaction system were 22 times lower. The results were compared with those obtained with the known inhibitor eserine, which was tested at the stock solution concentration of 0.1 mg/mL. The results are shown in Table 4.

Table 4.
The ability of the essential oil and extracts of T. montanum from Bosnia and Herzegovina to inhibit the enzymes AChE and BChE.
The EO of T. montanum at concentrations of 1 and 2 mg/mL of the stock solution (45 and 90 µg/mL in the reaction system) showed inhibition of AChE of 51.92 and 59.32% and inhibition of BChE of 35.65 and 49.54%. The AE of this plant showed a weaker ability to inhibit both AChE (27.77%) and BChE (4.30%) at the tested stock solution concentration of 1 mg/mL compared to the EO, while the ME of the plant showed a weaker ability to inhibit AChE (10.05%) and no inhibitory effect on BChE.
The known ChE inhibitor eserine showed 95.92% inhibition of AChE and 79.12% inhibition of BChE at a concentration of 0.1 mg/mL.
From the obtained results, it can be concluded that T. montanum EO has a moderate ability to inhibit both AChE and BChE, while the plant extracts have weak or no ability to inhibit these two enzymes. Among the compounds of the essential oil of this plant, α-pinene is a compound that has been shown to have the ability to inhibit these enzymes [57].
As expected, the results of testing the ability of EO and AE and ME to inhibit AChE were slightly better than the ability to inhibit BChE.
To date, this is the only study that has examined the ability of these enzymes to be inhibited by the essential oil or AE of T. montanum.
The inhibition of AChE and BChE was tested by the ME of T. montanum from Romania. The extract concentration for 50% inhibition (IC50) of these enzymes was 296.35 ± 2.37 and 190.78 ± 1.03 μg/mL, while the IC50 for galantamine was 28.70 ± 0.20 and 23.20 ± 0.09 μg/mL, respectively [33].
The ethanol extract of T. montanum from Croatia at the tested concentrations of 0.25, 0.5, and 1 g/L inhibited AChE by about 30, 40, and 80%, respectively [37].
4. Conclusions
In this work, a detailed and comprehensive analysis of the volatile and non-volatile extracts of the plant T. montanum was carried out, as well as a detailed evaluation of the anticholinesterase and antioxidant potential of the extracts of this plant using various analytical techniques and methods. For the first time, the chemical composition of EO and the phenolic composition of the AE and ME of T. montanum from Bosnia and Herzegovina were analyzed.
The main identified EO components of T. montanum from Bosnia and Herzegovina were α-cadinol, ß-selinene, δ-cadinene, epi-α-cadinol, germacrene D-4-ol, α-pinene, (E)-caryophyllene and ß-pinene.
In the AE and ME, among the twenty phenol compounds studied, the most abundant were p-coumaric acid, ellagic acid and caffeic acid.
The total phenolic content of ME was higher than that of AE. The total flavonoid content was also higher in the ME than in the AE.
The antioxidant activity of the essential oil of T. montanum was studied for the first time, and the ability of AE and ME to protect lipids and proteins from oxidation was also tested for the first time.
The ME showed the highest potential in scavenging DPPH radicals, followed by the AE and the EO of the plant. The same results were obtained when the reduction potential of the plant extracts was tested using the FRAP method. The ME showed the best reduction potential, followed by AE and EO. The comparison of these results with those of the known good antioxidants BHA and ascorbic acid shows that the tested extracts have good antioxidant potential, but it is weaker compared to BHA and ascorbic acid.
The AE has a slightly better ability to protect lipids from peroxidation than ME. Comparison of the obtained results with those of the standards BHA and BHT shows that AE offers very good results in protecting lipids from oxidation, while ME has only slightly weaker results.
The results of inhibition of protein carbonylation in the presence of the tested extracts showed very good protective effects in comparison with the standard antioxidants BHT and BHA.
The EO was moderately able to inhibit both AChE and BChE, whereas the plant extracts were weakly or not able to inhibit these two enzymes at the tested concentration in comparison with the known ChE inhibitor eserine. This is the only study that has examined the ability of these enzymes to be inhibited by the EO or AE of T. montanum.
Author Contributions
Conceptualization, O.P., M.B. and M.R.; methodology, O.P., M.B. and M.J.; validation, O.P., M.B. and M.J; formal analysis, O.P., M.B. and M.J.; investigation, O.P., M.B. and M.J.; resources, O.P., M.B. and M.R.; writing—original draft preparation, O.P. and M.B.; writing—review and editing, O.P., M.B., M.R. and M.J.; visualization, O.P. and M.B.; supervision, O.P., M.B. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was part of Croatian Science Foundation, grant number HRZZ-IP-2016-06-1316.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are within the paper.
Acknowledgments
The authors would like to thank the botanist Mirko Ruscic, Faculty of Science, University of Split, Croatia, for identification of the plant material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Da Silva, L.R.R.; Ferreira, O.O.; Cruz, J.N.; Franco, C.J.P.; Dos Anjos, T.O.; Cascaes, M.M.; Da Costa, W.A.; De Aguiar Andrade, E.H.; De Oliveira, M.S. Lamiaceae Essential Oils, Phytochemical Profile, Antioxidant, and Biological Activities. Evid. Based Complement. Alternat. Med. 2021, 2021, 7203934. [Google Scholar] [CrossRef] [PubMed]
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Pardossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrial, prospects and role of “positive-stress”. Ind. Crop. Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
- Raja, R.R. Medicinally potential plants of Labiatae (Lamiaceae) family: An overview. Res. J. Med. Plant 2012, 6, 203–213. [Google Scholar] [CrossRef]
- Zlatic, N.M.; Stankovic, M. Anticholinesterase, Antidiabetic and Anti-inflammatory Activity of Secondary Metabolites of Teucrium Species. In Teucrium Species: Biology and Applications; Stankovic, M., Ed.; Springer Nature: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Vukovic, N.; Milosevic, T.; Sukdolak, S.; Solujic, S. Antimicrobial Activities of Essential Oil and Methanol Extract of Teucrium montanum. Evid.-Based Compl. Alt. 2007, 4, 17–20. [Google Scholar] [CrossRef]
- Soljan, D.; Muratovic, E.; Abadzic, S. Biljke planina BiH; Šahinpašić: Sarajevo, Bosnia and Herzegovina, 2009. [Google Scholar]
- Jurisic Grubesic, R.; Kremer, D.; Vladimir-Knezevic, S.; Vukovic Rodríguez, J. Analysis of polyphenols, phytosterols, and bitter principles in Teucrium L. species. Cent. Eur. J. Biol. 2012, 7, 542–550. [Google Scholar] [CrossRef]
- Redzic, S. Wild medicinal plants and their usage in traditional human therapy (Southern Bosnia and Herzegovina, W. Balkan). J. Med. Plants Res. 2010, 4, 1003–1027. [Google Scholar] [CrossRef]
- Demain, A.L.; Zhang, L. Natural Products and Drug Discovery. In Natural Products Drug Discovery and Therapeutic Medicine; Zhang, L., Demain, A.L., Eds.; Humana Press: Totow, NJ, USA, 2005; pp. 3–33. [Google Scholar] [CrossRef]
- Roseiro, L.B.; Rauter, A.P.; Mourato Serralheiro, M.L. Polyphenols as acetylcholinesterase inhibitors: Structural specificity and impact on human disease. Nutr. Aging 2012, 1, 99–111. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef]
- Berkoz, M. The role of oxidative stress in Alzheimer’s disease. In Oxidative Stress and Antioxidant Defense System; Guven, A., Ed.; Livre de Lyon: Lyon, France, 2021; pp. 23–46. [Google Scholar]
- Huang, W.-J.; Zhang, X.; Chen, W.-W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2017. [Google Scholar]
- Dool, H.V.D.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Davies, K.M.; Schwinn, K.E. Chemistry, Biochemistry and Applications. In Flavonoids; Andersen, Q.M., Markham, K.R., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 143–219. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. Use of a free radical method to evaluate antioxidant activity. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Yen, G.C.; Hsieh, C.L. Antioxidant activity of extracts from du-zhong (Eucommia ulmoides) toward various lipid peroxidation models in vitro. J. Agric. Food Chem. 1998, 46, 3952–3957. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.; Ahn, B.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Natarajan, M.; Lopez-Burillo, S.; Reiter, R.J. Protection against oxidative protein damage induced by metal-catalyzed reaction or alkylperoxyl radicals: Comparative effects of melatonin and other antioxidants. Biochim. Biophys. Acta 2003, 1620, 139–150. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.; Demircakmak, B.; Duman, H. Composition of the Essential Oils of Three Teucrium Species from Turkey. J. Essent. Oil Res. 1997, 9, 545–549. [Google Scholar] [CrossRef]
- Bezic, N.; Vuko, E.; Dunkic, V.; Ruscic, M.; Blazevic, I.; Burcul, F. Antiphytoviral Activity of Sesquiterpene-Rich Essential Oils from Four Croatian Teucrium Species. Molecules 2011, 16, 8119–8129. [Google Scholar] [CrossRef]
- Catinella, G.; Badalamenti, N.; Ilardi, V.; Rosselli, S.; De Martino, L.; Bruno, M. The Essential Oil Compositions of Three Teucrium Taxa Growing Wild in Sicily: HCA and PCA Analyses. Molecules 2021, 26, 643. [Google Scholar] [CrossRef] [PubMed]
- Zlatic, N.; Mihailovic, V.; Ljesevic, M.; Beskoski, V.; Stankovic, M. Geological substrate-related variability of Teucrium montanum L. (Lamiaceae) essential oil. Biochem. Syst. Ecol. 2022, 100, 104372. [Google Scholar] [CrossRef]
- Radulovic, N.; Dekic, M.; Joksovic, M.; Vukicevic, R. Chemotaxonomy of Serbian Teucrium Species Inferred from Essential Oil Chemical Composition: The Case of Teucrium scordium L. ssp. scordioides. Chem. Biodivers. 2012, 9, 106–122. [Google Scholar] [CrossRef]
- Vukovic, N.; Milosevic, T.; Sukdolak, S.; Solujic, S. The chemical composition of the essential oil and the antibacterial activities of the essential oil and methanol extract of Teucrium montanum. J. Serb. Chem. Soc. 2008, 73, 299–305. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G.; Canale, A.; Maggi, F.; Mártonfi, P. Exploring essential oils of Slovak medicinal plants for insecticidal activity: The case of Thymus alternans and Teucrium montanum subsp. jailae. Food Chem. Toxicol. 2020, 138, 111203. [Google Scholar] [CrossRef]
- Kovacevic, N.N.; Lakusic, B.S.; Ristic, M.S. Composition of the Essential Oils of Seven Teucrium Species from Serbia and Montenegro. J. Essent. Oil Res. 2001, 13, 163–165. [Google Scholar] [CrossRef]
- Humulescu, I.; Flutur, M.-M.; Cioanca, O.; Mircea, C.; Robu, S.; Marin-Batir, D.; Spac, A.; Corciova, A.; Hancianu, M. Comparative Chemical and Biological Activity of Selective Herbal Extracts. Farmacia 2021, 69, 861–866. [Google Scholar] [CrossRef]
- Oaldje, M.M.; Kolarevic, S.M.; Zivkovic, J.C.; Vukovic-Gacic, B.S.; Jovanovic Maric, J.M.; Kracun Kolarevic, M.J.; Djordjevic, J.Z.; Alimpic Aradski, A.Z.; Marin, P.D.; Savikin, K.P.; et al. The impact of different extracts of six Lamiaceae species on deleterious effects of oxidative stress assessed in acellular, prokaryotic and eukaryotic models in vitro. Saudi Pham. J. 2020, 28, 1592–1604. [Google Scholar] [CrossRef]
- Nastic, N.; Svarc-Gajic, J.; Delerue-Matos, C.; Morais, S.; Barroso, M.F.; Moreira, M.M. Subcritical water extraction of antioxidants from mountain germander (Teucrium montanum L.). J. Supercrit. Fluid 2018, 138, 200–206. [Google Scholar] [CrossRef]
- Tumbas, V.T.; Mandic, A.I.; Cetkovic, G.S.; Djilas, S.M.; Canadanovic-Brunet, J.M. HPLC analysis of phenolic acids in mountain germander (Teucrium montanum L.) extracts. Acta Period. Technol. 2004, 35, 265–273. [Google Scholar] [CrossRef]
- Vladimir-Knezevic, S.; Blazekovic, B.; Kindl, M.; Vladic, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase Inhibitory, Antioxidant and Phytochemical Properties of Selected Medicinal Plants of the Lamiaceae Family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Kadifkova Panovska, T.; Kulevanova, S.; Stefova, M. In vitro antioxidant activity of some Teucrium species (Lamiaceae). Acta Pharm. 2005, 55, 207–214. [Google Scholar] [PubMed]
- Zlatic, N.M.; Stankovic, S.; Simic, Z.S. Secondary metabolites and metal content dynamics in Teucrium montanum L. and Teucrium chamaedrys L. from habitats with serpentine and calcareous substrate. Environ. Monit. Assess. 2017, 189, 110. [Google Scholar] [CrossRef]
- Stankovic, M.S.; Niciforovic, N.; Topuzovic, M.; Solujic, S. Total phenolic content, flavonoid concentrations and antioxidant activity, of the whole plant and plant parts extracts from Teucrium montanum L. var. montanum, f. supinum (L.) Reichenb. Biotechnol. Biotechnol. Equip. 2011, 25, 2222–2227. [Google Scholar] [CrossRef]
- Stankovic, M.S.; Curcic, M.G.; Zizic, J.B.; Topuzovic, M.D.; Solujic, S.R.; Markovic, S.D. Teucrium Plant Species as Natural Sources of Novel Anticancer Compounds: Antiproliferative, Proapoptotic and Antioxidant Properties. Int. J. Mol. Sci. 2011, 12, 4190–4205. [Google Scholar] [CrossRef] [PubMed]
- Canadanovic-Brunet, J.M.; Djilas, S.M.; Cetkovic, G.S.; Tumbas, V.T.; Mandic, A.I.; Canadanovic, V.M. Antioxidant activities of different Teucrium montanum L. extracts. Int. J. Food Sci. Tech. 2006, 41, 667–673. [Google Scholar] [CrossRef]
- Teixeira, B.; Marquesa, A.; Ramos, C.; Batista, I.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. European pennyroyal (Mentha pulegium) from Portugal: Chemical composition of essential oil and antioxidant and antimicrobial properties of extracts and essential oil. Ind. Crops Prod. 2012, 36, 81–87. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Khettaf, A.; Belloula, N.; Dridi, S. Antioxidant activity, phenolic and flavonoid contents of some wild medicinal plants in southeastern Algeria. Afr. J. Biotechnol. 2016, 15, 524–530. [Google Scholar] [CrossRef]
- Nino, J.; Anjum, N.; Tripathi, Y.C. Phytochemical screening and evaluation of polyphenols, flavonoids and antioxidant activity of Prunus cerasoids. D. Don leaves. J. Pharm. Res. 2016, 10, 502–508. [Google Scholar]
- Candela, R.G.; Rosselli, S.; Bruno, M.; Fontana, G.A. Review of the Phytochemistry, Traditional Uses and Biological Activities of the Essential Oils of Genus Teucrium. Planta Med. 2020, 87, 432–479. [Google Scholar] [CrossRef]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Lyoussi, B.; Oumokhtar, B.; Abdellaoui, A. Phytochemistry, antioxidant and antibacterial activities of two Moroccan Teucrium polium L. subspecies: Preventive approach against nosocomial infections. Arab. J. Chem. 2020, 13, 3866–3874. [Google Scholar] [CrossRef]
- Djilas, S.M.; Markov, S.L.; Cvetkovic, D.D.; Canadanovic-Brunet, J.M.; Cetkovic, G.S.; Tumbas, V.T. Antimicrobial and free radical scavenging activities of Teucrium montanum. Fitoterapia 2006, 77, 401–403. [Google Scholar] [CrossRef]
- Srabovic, M.; Poljakovic, M.; Hodzic, Z.; Banjanin, B.; Saletovic, M.; Salimovic, C.; Pehlic, E. Antioxidant capacity in some medicinal plants and fruits extracts. Healthmed 2011, 5, 2252–2257. [Google Scholar]
- Kefayati, Z.; Motamed, S.M.; Shojaii, A.; Noori, M.; Ghods, R. Antioxidant Activity and Phenolic and Flavonoid Contents of the Extract and Subfractions of Euphorbia splendida Mobayen. Pharmacogn. Res. 2017, 9, 362–365. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Lyoussi, B.; Abdellaoui, A. Antioxidant activity of two wild Teucrium species from Morocco. Int. J. Pharm. Sci. Res. 2019, 10, 723–2729. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A. Lipids. In Fennema’s Food Chemistry, 4th ed.; Damodaran, S., Parkin, K.L., Fennema, O.R., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2008; pp. 155–217. [Google Scholar]
- Dorman, H.J.; Hiltunen, R. Antioxidant and pro-oxidant in vitro evaluation of water-soluble food-related botanical extracts. Food Chem. 2011, 129, 1612–1618. [Google Scholar] [CrossRef]
- Stadtman, E.R. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu. Rev. Biochem. 1993, 62, 797–821. [Google Scholar] [CrossRef]
- Burcul, F.; Blazevic, I.; Radan, M.; Politeo, O. Terpenes, Phenylpropanoids, Sulfur and Other Essential Oil Constituents as Inhibitors of Cholinesterases. Curr. Med. Chem. 2020, 27, 4297–4343. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).