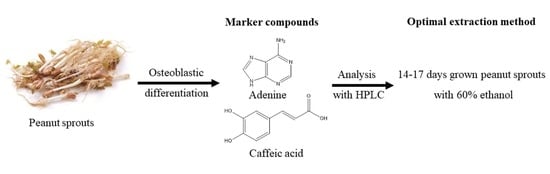
Isolation of Osteoblastic Differentiation-Inducing Constituents from Peanut Sprouts and Development of Optimal Extraction Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Peanut Sprout Extract
2.2. Isolation of Active Components
2.3. Cell Culture and Viability
2.4. Alkaline Phosphatase (ALP) Assay
2.5. Analysis of Nucleobase Content in Peanut Sprouts
2.6. Analysis of Phenolic Acid Content in Peanut Sprouts
2.7. Statistical Analysis
3. Results
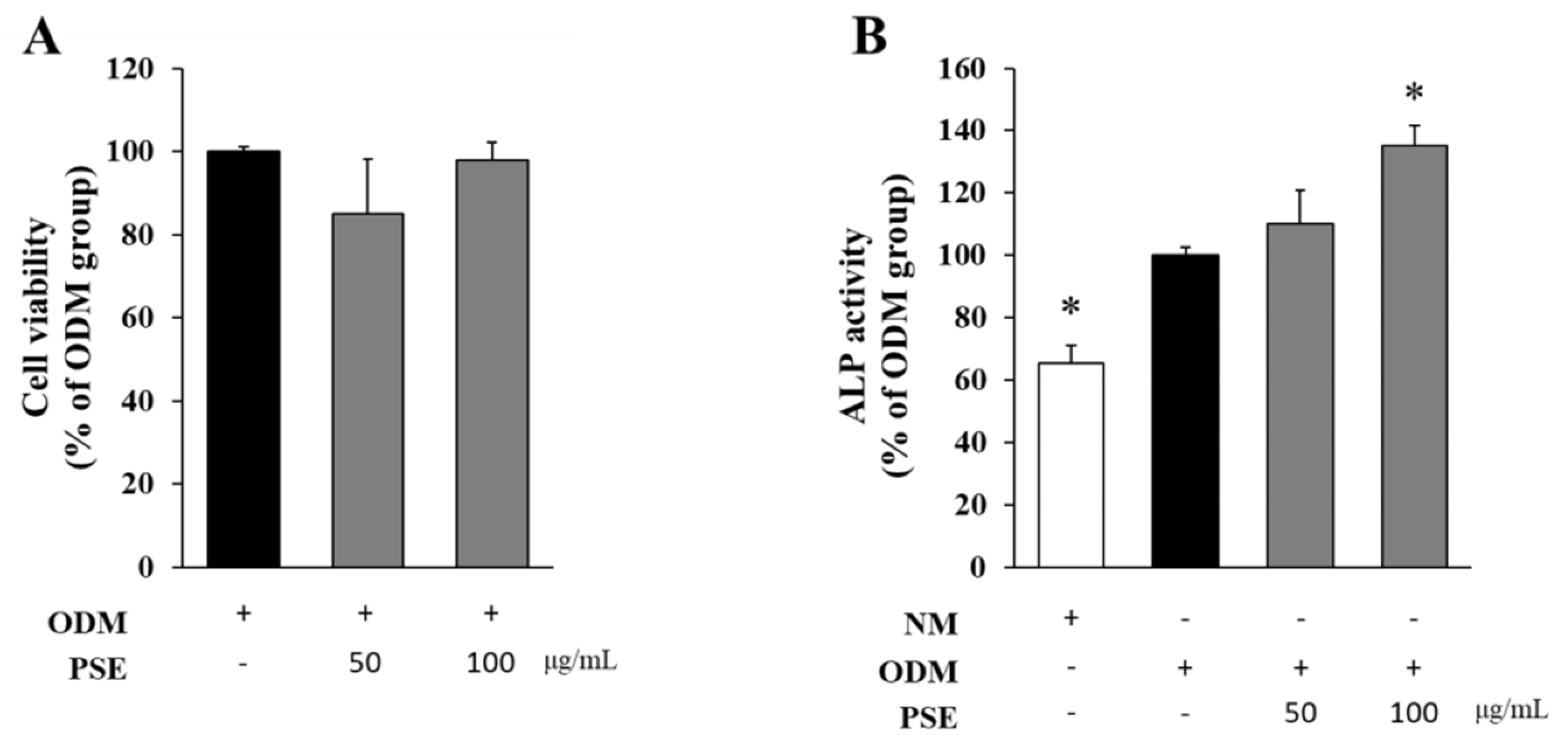
3.1. PSE and Its EA Layer Upregulated ALP Activity
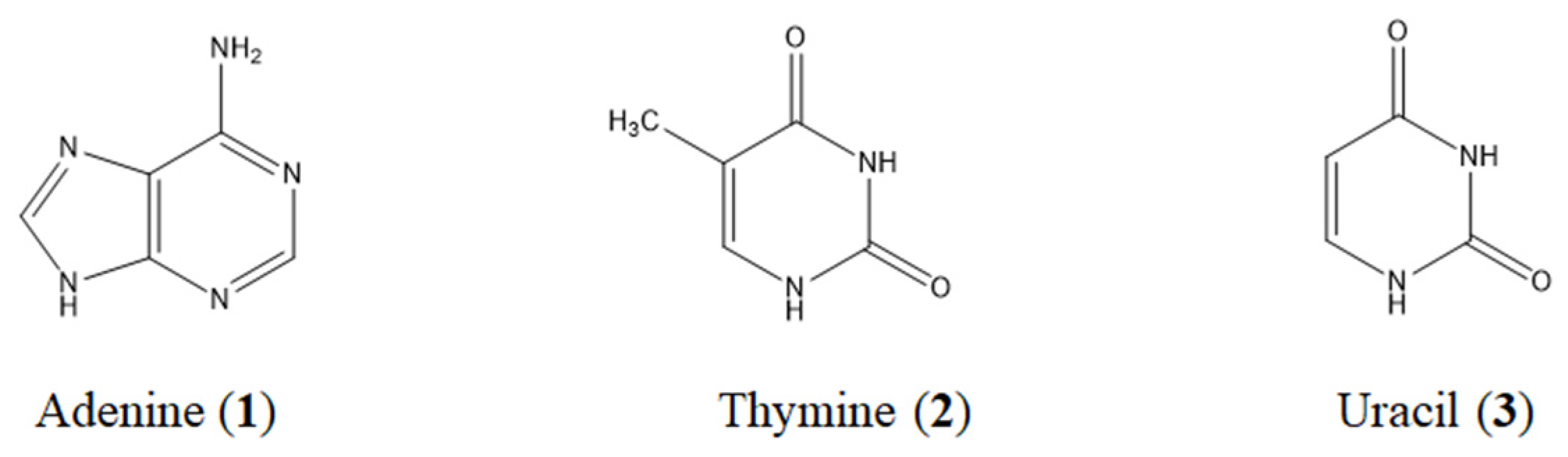
3.2. Nucleobases and Phenoli Acids as Active Components Isolated from PSE
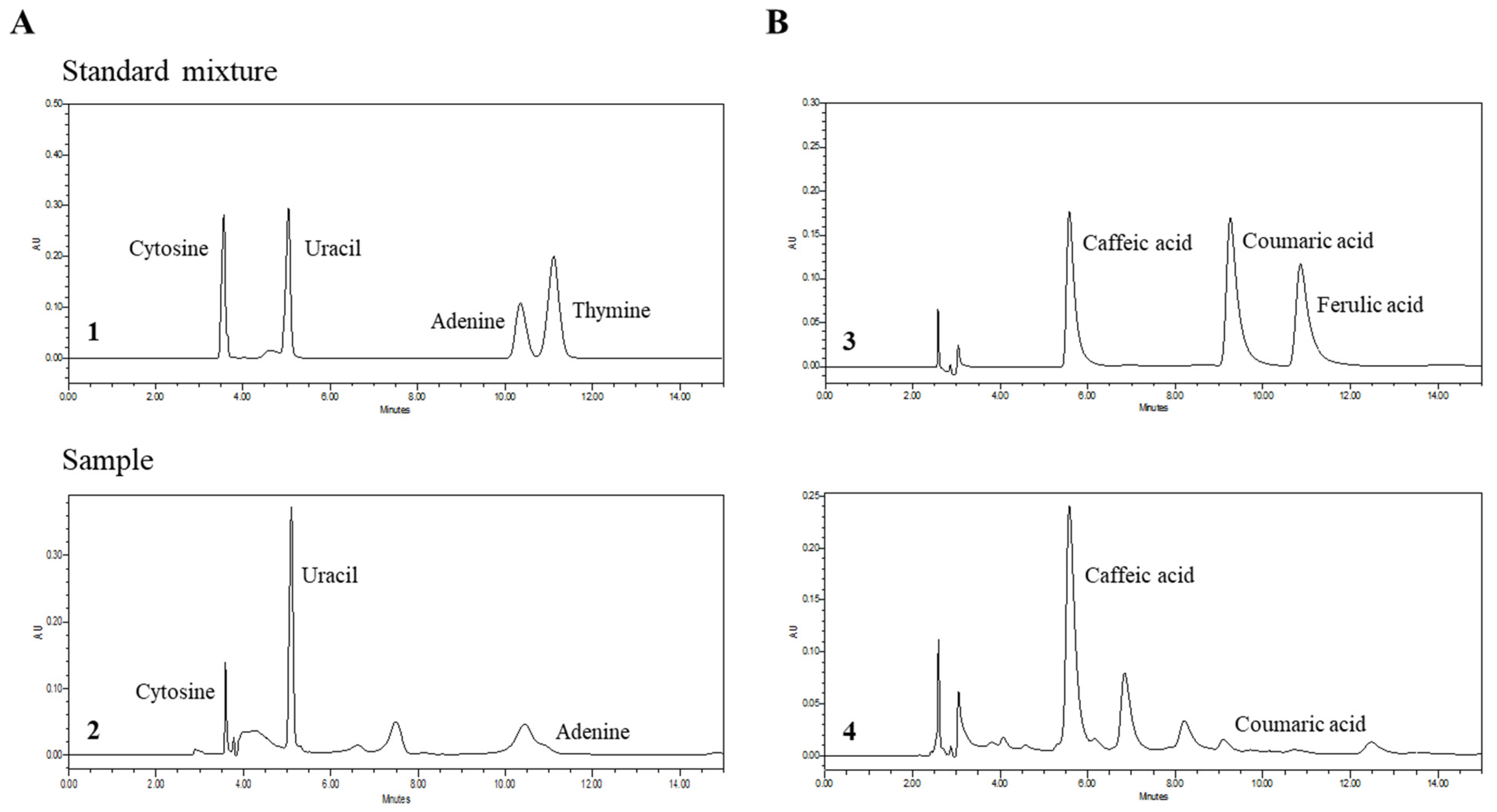
3.3. Qualitative Analyses of Nucleobases and Phenolic Acids in PSE
3.4. Determination of Optimal Extraction Conditions for PSE
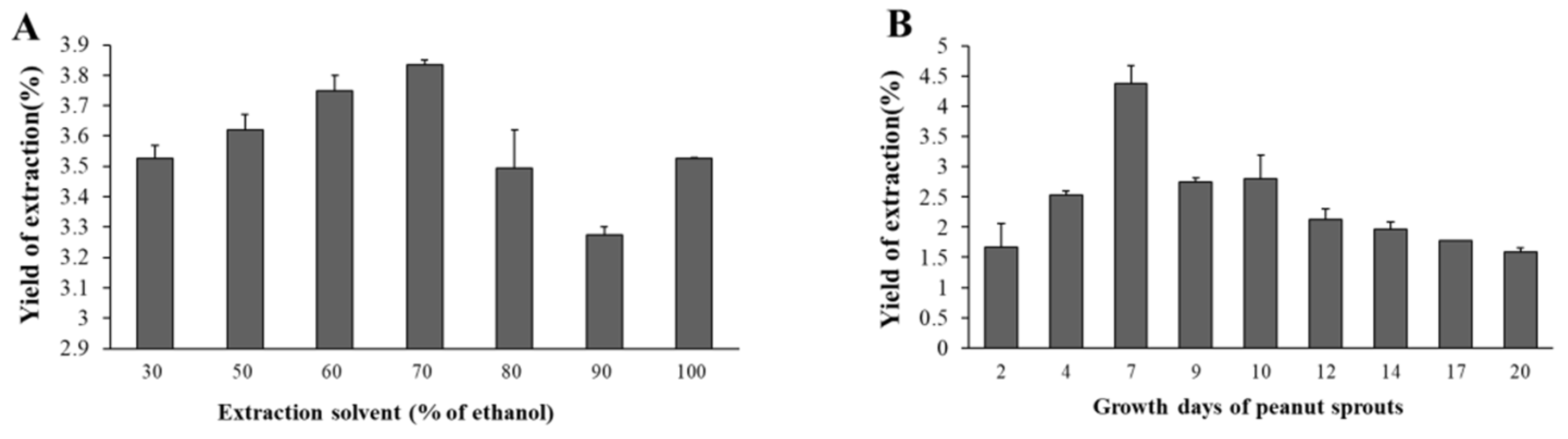
3.4.1. The Yield of PSE
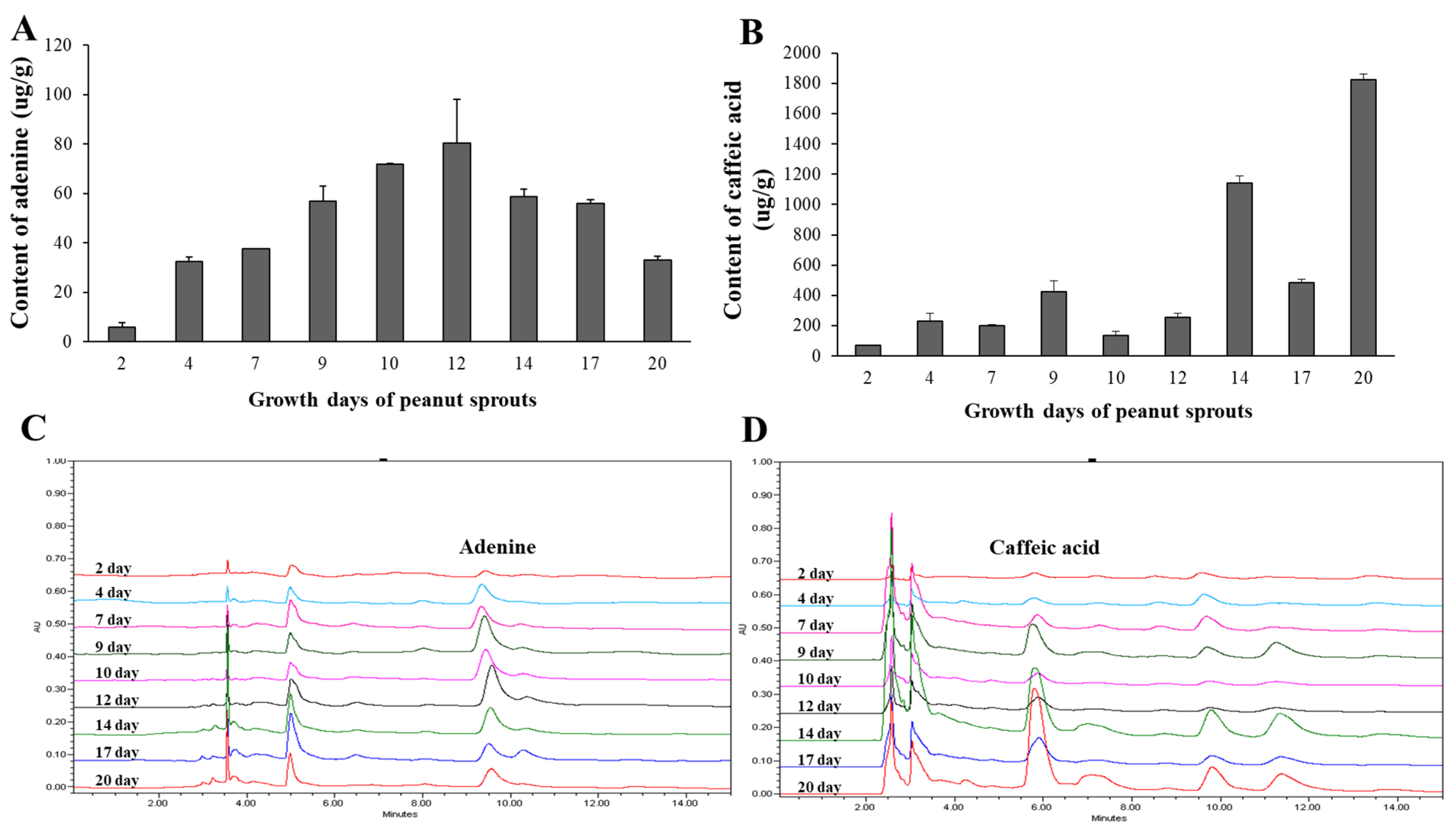
3.4.2. Contents of Marker Compounds with Different Extraction Solvents
3.4.3. Contents of Marker Compounds in Peanut Sprouts Grown Different Days
3.4.4. Optimal Extraction Condition for PSE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and Economic Burden of Osteoporosis-Related Fractures in the United States. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef]
- KIM, T.H.; Jung, J.W.; Ha, B.G.; Hong, J.M.; Park, E.K.; Kim, H.J.; Kim, S.Y. The Effects of Luteolin on Osteoclast Differentiation, Function in Vitro and Ovariectomy-Induced Bone Loss. J. Nutr. Biochem. 2011, 22, 8–15. [Google Scholar] [CrossRef]
- Shiraishi, A.; Muyabe, S.; Nakano, T.; Umakoshi, Y.; Ito, M.; Mihara, M. The Combination Therapy with Alfacalcidol and Risedronate Improves the Mechanical Property in Lumbar Spine by Affecting the Material Properties in an Ovariectomized Rat model of Osteoporosis. BMC Musculoskelet. Disord. 2009, 10, 66. [Google Scholar] [CrossRef]
- Pavone, V.; Testa, G.; Giardina, S.M.C.; Vescio, A.; Restivo, D.A.; Sessa, G. Pharmacological Therapy of Osteoporosis: A systematic Current Review of Literature. Front. Pharmacol. 2017, 8, 803. [Google Scholar]
- Riggs, B.L.; Khosla, S.; Melton, L.J., 3rd. Sex Steroids and the Construction and Conservation of the Adult Skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, S.Y.; Cheon, J.H.; Lee, J.Y.; Kim, B.K.; Lee, S.H.; Kong, C.S.; Kim, Y.Y.; Kim, M.H. Effects of Scytosiphon lomentaria on Osteoblastic Proliferation and Differentiation of MC3T3-E1 cells. Nutr. Res. Pract. 2016, 10, 148–153. [Google Scholar] [PubMed]
- Lane, N.E.; Kelman, A. A Review of Anabolic Therapies for Osteoporosis. Arthritis Res. Ther. 2003, 5, 214–222. [Google Scholar] [PubMed]
- Teitelbaum, S.L. Bone Resorption by Osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Seo, C.R.; Kim, M.A.; Kim, Y.J.; Song, N.J.; Jang, W.S.; Kim, B.J.; Lee, J.H.; Hong, J.W.; Nho, C.W.; et al. Dichloromethane Extracts of Sophora japonica L. Stimulate Osteoblast Differentiation In mesenchymal Stem Cells. Nutr. Res. 2013, 33, 1053–1062. [Google Scholar]
- Golub, E.E.; Boesze-Battaglia, K. The Role of Alkaline Phosphatase in Mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Gupta, T.; Das, N.; Imran, S. The Prevention and Therapy of Osteoporosis. A Review on Emerging Trends from Hormonal Therapy to Synthetic Drugs to Plant-Based Bioactives. J. Diet. Suppl. 2019, 16, 699–713. [Google Scholar] [CrossRef]
- Putnam, S.E.; Scutt, A.M.; Bicknell, K.; Priestley, C.M.; Williamson, E.M. Natural Products as Alternative Treatments for Metabolic Bone Disorders and for Maintenance of Bone Health. Phytother. Res. 2007, 21, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.H.; Kiem, P.V.; Nhiem, N.X.; Tung, N.H.; Quang, T.H.; Minh, C.V.; Kim, J.W.; Choi, E.M.; Kim, Y.H. A New Monoterpene Glycoside from the Roots of Paeonia lactiflora Increases the Differentiation of Osteoblastic MC3T3-E1 cells. Arch. Pharm. Res. 2007, 30, 1179–1185. [Google Scholar] [CrossRef]
- Yun, H.M.; Park, K.R.; Quang, T.H.; Oh, H.; Hong, J.T.; Kim, Y.C.; Kim, E.C. 2,4,5-Trimethoxyldalbergiquinol Promotes Osteoblastic Differentiation and Mineralization Via the BMP and Wnt/beta-catenin Pathway. Cell Death. Dis. 2015, 6, e1819. [Google Scholar] [CrossRef] [PubMed]
- Son, H.E.; Kim, T.H.; Jang, W.G. Curculactones A and B Induced the Differentiation of C3H10T1/2 and MC3T3-E1 Cells to Osteoblasts. Bioorg. Med. Chem. Lett. 2017, 27, 1301–1303. [Google Scholar] [CrossRef]
- Thu, H.E.; Hussain, Z.; Mohamed, I.N.; Shuid, A.N. Eurycoma Longifolia, a Promising Suppressor of RANKL-Induced Differentiation and Activation of Osteoclasts: An in Vitro Mechanistic Evaluation. J. Ayurveda Integr. Med. 2019, 10, 102–110. [Google Scholar] [CrossRef]
- Li, W.D.; Yan, C.P.; Wu, Y.; Weng, Z.B.; Yin, F.Z.; Yang, G.M.; Cai, B.C.; Chen, Z.P. Osteoblasts Proliferation and Differentiation Stimulating Activities of the Main Components of Fructus Psoraleae corylifoliae. Phytomedicine 2014, 21, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Zhou, K.; Wang, S.; Zhang, H.; Fan, N.; Li, J.; Tan, X.; Hu, L.; Fan, X. Psoralen and Bakuchiol Ameliorate M-CSF Plus RANKL-Induced Osteoclast Differentiation and Bone Resorption Via Inhibition of AKT and AP-1 Pathways in Vitro. Cell. Physiol. Biochem. 2018, 48, 2123–2133. [Google Scholar] [CrossRef]
- Ha, A.W.; Kim, W.K.; Kim, J.H.; Kang, N.E. The Supplementation Effects of Peanut Sprout on Reduction of Abdominal Fat and Health Indices in Overweight and Obese Women. Nutr. Res. Pract. 2015, 9, 249–255. [Google Scholar] [CrossRef]
- Ha, A.W.; Kang, N.E.; Kim, W.K. Ethanol Extract of Peanut Sprout Lowers Blood Triglyceride Levels, Possibly Through a Pathway Involving SREBP-1c in Rats Fed a High-Fat Diet. J. Med. Food 2015, 18, 850–855. [Google Scholar] [CrossRef]
- Limmongkon, A.; Nopprang, P.; Chaikeandee, P.; Somboon, T.; Wongshaya, P.; Pilaisangsuree, V. LC-MS/MS Profiles and Interrelationships Between the Anti-Inflammatory Activity, Total Phenolic Content and Antioxidant Potential of Kalasin 2 cultivar Peanut Sprout Crude Extract. Food Chem. 2018, 239, 569–578. [Google Scholar] [CrossRef]
- Lee, Y.M.; Son, E.J.; Kim, D.S. Treatment with Peanut Sprout Root Extract Alleviates Inflammation in a Lipopolysaccharide-Stimulated Mouse Macrophage Cell Line by Inhibiting the MAPK Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 5907. [Google Scholar] [CrossRef] [PubMed]
- Mediero, A.; Cronstein, B.N. Adenosine and Bone Metabolism. Trends Endocrinol. Metab. 2013, 24, 290–300. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Ijerman, A.P.; Jacobson, K.A.; Linden, J.; Muller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and Classification of Adenosine Receptors-an Update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef]
- Strazzulla, L.C.; Cronstein, B.N. Regulation of Bone and Cartilage by Adenosine Signaling. Purinergic Signal. 2016, 12, 583–593. [Google Scholar] [CrossRef]
- Jacobsen, N.E. NMR Data Interpretation Explained: Understanding 1D and 2D NMR Spectra of Organic Compounds and Natural Products; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 348–349. [Google Scholar]
- Mohamed, T.A.; Shabaan, I.A.; Zoghaib, W.M.; Husband, J.; Farag, R.S.; Alajhaz, A.M.A. Tautomerism, Normal Coordinate Analysis, Vibrational Assignments, Calculated IR, Raman and NMR Spectra of Adenine. J. Mol. Struct. 2009, 938, 263–276. [Google Scholar] [CrossRef]
- Benoit, R.L.; Frechette, M. 1H and 13C nuclear Magnetic Resonance and Ultraviolet Studies of the Protonation of Cytosine, Uracil, Thymine, and Related Compounds. Can. J. Chem. 1986, 64, 2348–2352. [Google Scholar] [CrossRef]
- Exarchou, V.; Troganis, A.; Gerothanassis, I.P.; Tsimidou, M.; Boskou, D. Identification and Quantification of Caffeic and Rosmarinic Acid in Complex Plant Extracts by the Use of Variable-Temperature Two-Dimensional Nuclear Magnetic Resonance Spectroscopy. J. Agric. Food Chem. 2001, 49, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, S.E.; Shokoohinia, Y.; Moayedi, N.S. Isolation and Identification of Ferulic Acid from Aerial Parts of Kelussia odoratissima Mozaff. Jundishapur J. Nat. Pharm. 2012, 7, 159–162. [Google Scholar] [CrossRef]
- Shimomura, H.; Sashida, Y.; Mimaki, Y.; Kudo, Y.; Maeda, K. New Phenylpropanoid Glycerol Glucosides from the Bulbs of Lilium Species. Chem. Pharm. Bull. 1988, 36, 4841–4848. [Google Scholar] [CrossRef]
- Wang, K.H.; Lai, Y.H.; Chang, J.C.; Ko, T.F.; Shyu, S.L.; Chiou, R.Y. Germination of Peanut Kernels to Enhance Resveratrol Biosynthesis and Prepare Sprouts as a Functional Vegetable. J. Agric. Food Chem. 2005, 53, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.I.; Kim, J.Y.; Kwon, S.J.; Park, K.W.; Kang, J.S.; Seo, K.I. Antioxidative Effects of Peanut Sprout Extracts. J. Korean Soc. Food Sci. Nutr. 2010, 39, 941–946. [Google Scholar] [CrossRef]
- Wang, G.; Lei, Z.; Zhong, Q.; Wu, W.; Zhang, H.; Min, T.; Wu, H.; Lai, F. Enrichment of Caffeic acid in Peanut Sprouts and Evaluation of its In vitro Effectiveness Against Oxidative Stress-Induced Erythrocyte Hemolysis. Food Chem. 2017, 217, 332–341. [Google Scholar] [CrossRef]
- Choi, D.I.; Choi, J.Y.; Kim, Y.J.; Lee, J.B.; Kim, S.O.; Shin, H.T.; Lee, S.C. Ethanol Extract of Peanut Sprout Exhibits a Potent Anti-Inflammatory Activity in Both an Oxazolone-Induced Contact Dermatitis Mouse Model and Compound 48/80-Treated HaCaT Cells. Ann. Dermatol. 2015, 27, 142–151. [Google Scholar] [CrossRef]
- Song, J.H.; Hwang, B.D.; Chung, H.J.; Moon, B.K.; Kim, J.W.; Ko, K.S.; Kim, B.W.; Kim, W.R.; Kim, W.J.; Myung, S.C.; et al. Peanut Sprout Extracts Cultivated with Fermented Sawdust Medium Inhibits Benign Prostatic Hyperplasia In vitro and In vivo. World J. Men’s Health 2020, 38, 385–396. [Google Scholar] [CrossRef]
- Pyo, K.H.; Lee, Y.W.; Lee, S.H.; Xin, C.F.; Shin, J.H.; Shin, E.H. Preventive Effects of Resveratrol Enriched Extract of Peanut Sprout on Bacteria and Estradiol Induced Prostatitis in Mice. Nat. Prod. Commun. 2017, 12, 73–78. [Google Scholar] [CrossRef]
- Boonsong, T.; Pakwan, S.; Chawnawa, W. Anti-Adipogenesis and Antioxidative Defense Status of a Crude Extract of ‘Kalasin 2’ Peanut Sprouts in 3T3-L1 Mouse Adipocytes. Biochem. Cell Biol. 2019, 97, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Bonku, R.; Yu, J. Health Aspects of Meanuts as an Outcome of its Chemical Composition. Food Sci. Hum. Wellness 2020, 9, 21–30. [Google Scholar] [CrossRef]
- Kim, S.H.; Yuk, H.J.; Ryu, H.W.; Oh, S.R.; Song, D.Y.; Lee, K.S.; Park, K.I.; Choi, S.W.; Seo, W.D. Biofunctional Soyasaponin Bb in Peanut (Arachis hypogaea L.) Sprouts Enhances Bone Morphogenetic Protein-2-Dependent Osteogenic Differentiation via Activation of Runt-Related Transcription Factor 2 in C2C12 Cells. Phytother. Res. 2019, 33, 1490–1500. [Google Scholar] [CrossRef]
- Wong, X.K.; Yeong, K.Y. From Nucleic Acids to Drug Discovery. Nucleobases as Emerging Templates for Drug Candidates. Curr. Med. Chem. 2021, 28, 7076–7121. [Google Scholar] [CrossRef]
- Hosoi, T.; Ino, S.; Ohnishi, F.; Todoroki, K.; Yoshii, M.; Kakimoto, M.; Muller, C.E.; Ozawa, K. Mechanisms of the Action of Adenine on Anti-Allergic Effect in Mast Cells. Immun. Inflamm. Dis. 2018, 6, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Silwal, P.; Lim, K.; Heo, J.Y.; Park, J.I.; Namgung, U.; Park, S.K. Adenine Attenuates Lipopolysaccharide Induced Inflammatory Reactions. Korean J. Physiol. Pharmacol. 2018, 22, 379–389. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chu, Y.L.; Tsuang, Y.H.; Wu, Y.; Kuo, C.Y.; Kuo, Y.J. Anti-Inflammatory Effects of Adenine Enhance Osteogenesis on the Osteoblast Like MG-63 Cells. Life 2020, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic Acids and Other Cinnamates—Nature, Occurrence and Dietary Burden. J. Sci. Food Agric. 1999, 79, 3. [Google Scholar] [CrossRef]
- Tsao, R.; Deng, Z. Separation Procedures for Naturally Occurring Antioxidant Phytochemicals. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 812, 85–99. [Google Scholar] [CrossRef]
- Oh, T.W.; Park, K.-I.; Do, H.J.; Kim, K.; Yang, H.J.; Cho, W.K.; Ma, J.Y. Acer tegmentosum Maxim Prevents Bone Loss by Inhibiting Osteoclastogenesis and Promoting Osteoblast Mineralization in Ovariectomized Mice. Nat. Prod. Sci. 2020, 26, 83–89. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Y.; Jiang, X.; Zhang, X.; Xia, L.; Lin, K.; Xu, Y. The Effect of Quercetin on the Osteogenesic Differentiation and Angiogenic Factor Expression of Bone Marrow-Derived Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0129605. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Ho, C.T. Antioxidant Activities of Caffeic Acid and Its Related Hydroxycinnamic Acid Compounds. J. Agric. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Mallik, S.B.; Mudgal, J.; Nampoothiri, M.; Hall, S.; Anoopkumar-Dukie, S.; Grant, G.; Rao, C.M.; Arora, D. Caffeic Acid Attenuates Lipopolysaccharide-Induced Sickness Behaviour and Neuroinflammation in Mice. Neurosci. Lett. 2016, 6, 218–223. [Google Scholar] [CrossRef]
- Wang, W.; Sun, W.; Jin, L. Caffeic Acid Alleviates Inflammatory Response in Rheumatoid Arthritis Fibroblast-Like Synoviocytes by Inhibiting Phosphorylation of IκB Kinase α/β and IκBα. Int. Immunopharmacol. 2017, 48, 61–66. [Google Scholar] [CrossRef]
- Ekeuku, S.O.; Pang, K.L.; Chin, K.Y. Effects of Caffeic Acid and Its Derivatives on Bone: A Systematic Review. Drug Des. Dev. Ther. 2021, 15, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Hapidin, H.; Romli, N.A.A.R.; Abdullah, H. Proliferation Study and Microscopy Evaluation on the Effects of Tannic Acid in Human Fetal Osteoblast Cell Line (hFOB 1.19). Microsc. Res. Tech. 2019, 82, 1928–1940. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Wankhade, U.D.; Alund, A.W.; Blackburn, M.L.; Shankar, K.; Lazarenko, O.P. 3-(3-Hydroxyphenyl)-Propionic Acid (PPA) Suppresses Osteoblastic Cell Senescence to Promote Bone Accretion in Mice. JBMR Plus 2019, 3, e10201. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lazarenko, O.P.; Chen, J.R. Hippuric Acid and 3-(3-Hydroxyphenyl) Propionic Acid Inhibit Murine Osteoclastogenesis through RANKL-RANK Independent Pathway. J. Cell. Physiol. 2020, 235, 599–610. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.-S.; Lee, C.H.; Ko, M.S.; Choi, J.Y.; Hwang, K.W.; Park, S.-Y. Isolation of Osteoblastic Differentiation-Inducing Constituents from Peanut Sprouts and Development of Optimal Extraction Method. Separations 2023, 10, 435. https://doi.org/10.3390/separations10080435
Cho E-S, Lee CH, Ko MS, Choi JY, Hwang KW, Park S-Y. Isolation of Osteoblastic Differentiation-Inducing Constituents from Peanut Sprouts and Development of Optimal Extraction Method. Separations. 2023; 10(8):435. https://doi.org/10.3390/separations10080435
Chicago/Turabian StyleCho, Eun-Sang, Chung Hyeon Lee, Min Sung Ko, Jee Yeon Choi, Kwang Woo Hwang, and So-Young Park. 2023. "Isolation of Osteoblastic Differentiation-Inducing Constituents from Peanut Sprouts and Development of Optimal Extraction Method" Separations 10, no. 8: 435. https://doi.org/10.3390/separations10080435
APA StyleCho, E.-S., Lee, C. H., Ko, M. S., Choi, J. Y., Hwang, K. W., & Park, S.-Y. (2023). Isolation of Osteoblastic Differentiation-Inducing Constituents from Peanut Sprouts and Development of Optimal Extraction Method. Separations, 10(8), 435. https://doi.org/10.3390/separations10080435