GC-MS Analysis and Bioactivity Screening of Leaves and Fruits of Zanthoxylum armatum DC.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
2.3. Experiment Apparatus
2.4. Extraction of Essential Oil
2.5. Extraction of Aroma
2.6. GC-MS Detection
2.7. Preliminary Screening of Antitumor Activity In Vitro
2.8. Primary Screening of Tyrosinase Inhibition Activity In Vitro
2.9. Primary Screening of HMGR Inhibition Activity In Vitro
2.10. Primary Screening of Nitric Oxide (NO) Production Inhibition Activity In Vitro
3. Results
3.1. The Main Chemical Composition of Essential Oils and Aromas from Leaves and Fruits
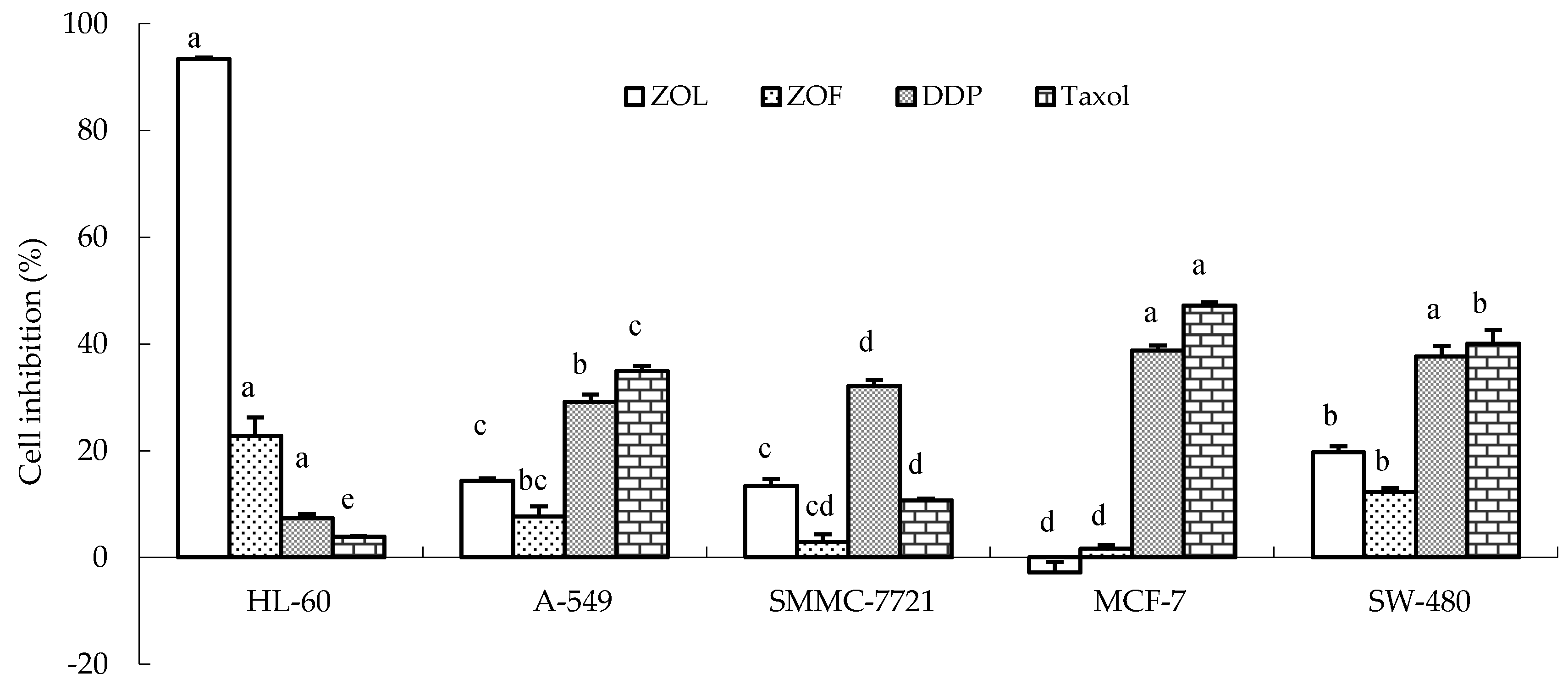
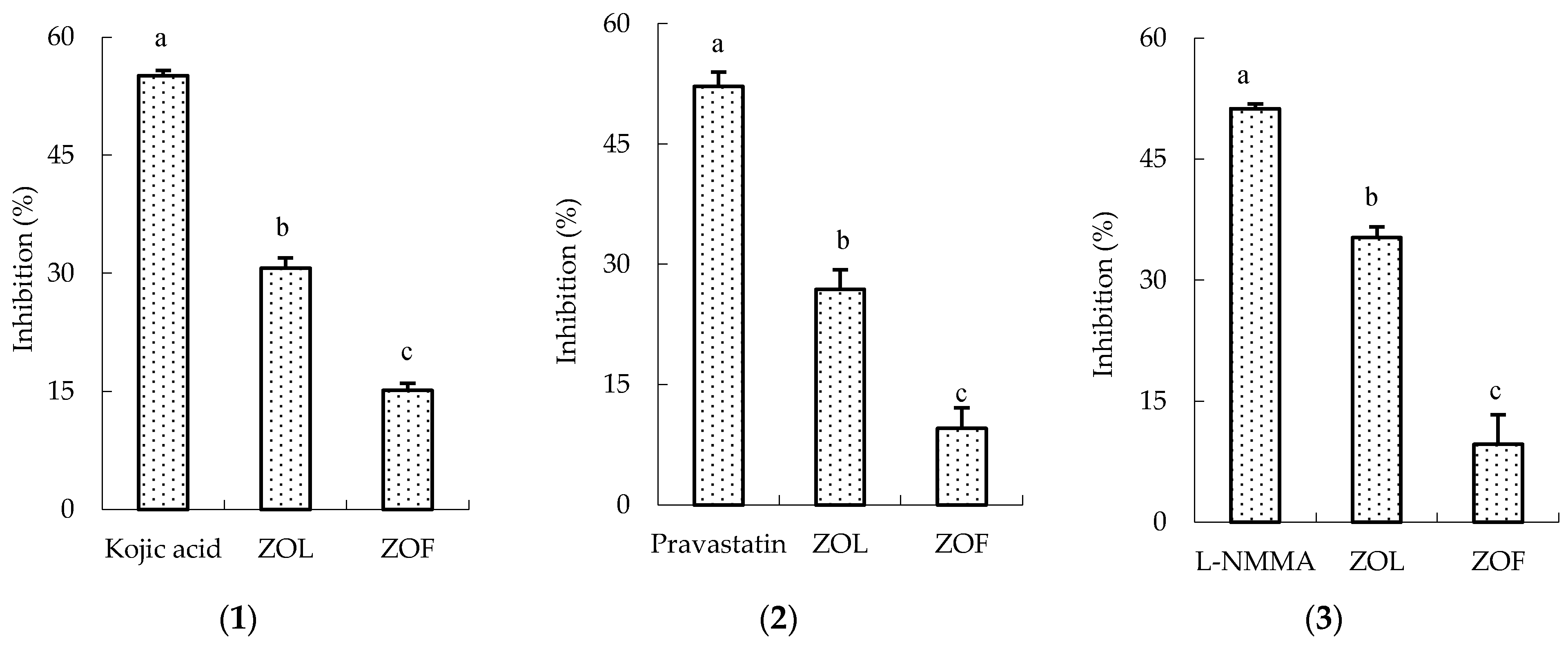
3.2. In Vitro Bioactivity of ZLO and ZFO
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cai, M.X. Hot and Spicy World: Sichuan Pepper; China Textile Press: Beijing, China, 2022; pp. 84–86. [Google Scholar]
- Barua, C.C.; Yasmin, N.; Elancheran, R. A review on effective utilization, phytochemical compounds, pharmacological intervention of a popularly used plant for developing a new drug: Zanthoxylum armatum with reference to its anticancer activity. MOJ Bioequiv. Availab. 2018, 5, 156–167. [Google Scholar]
- Oinam, J.; Raleng, I.; Meitankeishangbam, P.; Kumari, B.R.; Laishram, S. A study on hepatoprotective activity of ethanol extract of Zanthoxylum armatum DC (Mukthrubi) leaves in experimental animal. Int. J. Pharm. Sci. Res. 2017, 8, 3025–3029. [Google Scholar]
- Rynjah, C.V.; Devi, N.N.; Khongthaw, N.; Syiem, D.; Majaw, S. Evaluation of the antidiabetic property of aqueous leaves extract of Zanthoxylum armatum DC. using in vivo and in vitro approaches. J. Tradit. Complement. Med. 2017, 25, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Kaleeswaran, G.; Firake, D.; Behere, G.; Challa, G.; Sanjukta, R.; Baiswar, P. Insecticidal potential of traditionally important plant, Zanthoxylum armatum DC (Rutaceae) against cabbage butterfly, Pieris brassicae (Linnaeus). Indian J. Tradit. Know. 2019, 18, 304–311. [Google Scholar]
- Mirza, A.U.; Khan, M.S.; Nami, S.A.A.; Kareem, A.; Rehman, S.; Bhat, S.A.; Nishat, N. Copper oxide nanomaterials derived from Zanthoxylum armatum DC. and Berberis lycium Royle plant species: Characterization, assessment of free radical scavenging and antibacterial activity. Indian J. Tradit. Know. 2019, 50, 159–164. [Google Scholar] [CrossRef]
- Alam, F.; Shah, A.J. Butyrlycholine esterase inhibitory activity and effects of extracts (fruit, bark and leaf) from Zanthoxylum armatum DC in gut, airways and vascular smooth muscles. BMC Complement. Altern. Med. 2019, 19, 180. [Google Scholar] [CrossRef]
- Saikia, B.; Barua, C.C.; Sarma, J.; Haloi, P.; Tamuli, S.M.; Kalita, D.J.; Purkayastha, A.; Barua, A.G. Zanthoxylum alatum ameliorates scopolamine-induced amnesia in rats: Behavioral, biochemical and molecular evidences. Indian J. Pharmacol. 2018, 50, 30–38. [Google Scholar] [CrossRef]
- Yadav, C.K.; Poudel, K.; Mehta, R.; Shrivastava, A.K. Anti-depressant activity of the leaves of Zanthoxylum armatum on Swiss albino mice. J. Univ. Coll. Med. Sci. 2020, 8, 46–50. [Google Scholar] [CrossRef]
- Kennedy, D.; Wightman, E.; Khan, J.; Grothe, T.; Jackson, P. The acute and chronic cognitive and cerebral blood-flow effects of Nepalese pepper (Zanthoxylum armatum DC.) extract-a randomized, double-blind, placebo-controlled study in healthy humans. Nutrients 2019, 11, 3022. [Google Scholar] [CrossRef] [PubMed]
- Gajurel, P.R.; Kashung, S.; Nopi, S.; Singh, B. Present status of distribution, utilization and commercialization of Zanthoxylum armatum DC. -a socio economically potential species in Arunachal Pradesh. India. Plant Sci. Today 2021, 8, 210–217. [Google Scholar] [CrossRef]
- Phuyal, N.; Jha, P.K.; Raturi, P.P.; Gurung, S.; Rajbhandary, S. Essential oil composition of Zanthoxylum armatum leaves as a function of growing conditions. Int. J. Food Prop. 2019, 22, 1873–1885. [Google Scholar] [CrossRef]
- Schmiderer, C.; Grassi, P.; Novak, J.; Franz, C. Diversity of essential oil glands of Spanish sage (Salvia Lavandulifolia Vahl, Lamiaceae). Nat. Prod. Commun. 2008, 3, 1155–1160. [Google Scholar] [CrossRef]
- Li, Q. Forest Medicine; Science Press: Beijing, China, 2013; pp. 142–149. [Google Scholar]
- Ferhat, M.; Muhammad, A.; Farkhanda, M. A comparative study of in vitro total antioxidant capacity, in vivo antidiabetic and antimicrobial activity of essential oils from leaves and seeds of Zanthoxylum armatum DC. Asian J. Chem. 2013, 25, 10221–10224. [Google Scholar]
- Akbar, S.; Majid, A.; Hassan, S.; Rehman, A.U. Comparative in vitro activity of ethanol and hot water extracts of Zanthoxylum armatum to some selective human pathogenic bacterial strains. Int. J. Biosci. 2014, 4, 285–291. [Google Scholar]
- Muhammad, I.; Naveed, M.; Barkat, U.; Haroon, K.; Faryal, J.; Nadeem, A. Antinociceptive and anticonvulsant activities of essential oils of Zanthoxylum alatum. Phytopharmacology 2012, 3, 191–198. [Google Scholar]
- Muhammad, N.; Khan, A.Z.; Barkatullah, A.; Ibrar, M. Behavioral properties of essential oils of Zanthoxylum armatum DC leaves: Augmented by chemical profile using GC/GC-MS. J. Chem. Soc. Pakistan 2013, 35, 1593–1598. [Google Scholar]
- Bisht, M.; Mishra, D.; Sah, M.L.; Joshi, S.; Mishra, S. Biological activities of the essential oil of Zanthoxylum armatum DC. leave. Nat. Prod. J. 2014, 4, 229–232. [Google Scholar] [CrossRef]
- Barkatullah, I.M.; Muhammad, N.; Rehman, I.U.; Rehman, M.U.; Khan, A.Z. Chemical composition and biological screening of essential oils of Zanthoxylum armatum DC leaves. J. Clinic. Toxicol. 2013, 3, 172. [Google Scholar]
- Wang, Y.L.; Luo, J.J.; Hou, X.Y.; Xu, H.J.; Li, Q.Y.; Li, S.S.; Luo, Q.Y.; Li, M.L.; Liu, X.Y.; Shen, G.H.; et al. Physicochemical, antibacterial, and biodegradability properties of green Sichuan pepper (Zanthoxylum armatum DC.) essential oil incorporated starch films. LWT 2022, 161, 113392. [Google Scholar] [CrossRef]
- Slathia, S.; Sharma, Y.P.; Hakla, H.; Urfan, M.; Yadav, N.S.; Pal, S. Post-harvest management of Alternaria induced rot in tomato fruits with essential oil of Zanthoxylum armatum DC. Front. Sustain. Food Syst. 2021, 5, 679830. [Google Scholar] [CrossRef]
- Pathak, I.; Rokaha, S.; Bajracharya, K.B. Phytoconstituents and biological activities of Zanthoxylum armatum fruit extract. J. Nepal Chem. Soc. 2021, 42, 125–131. [Google Scholar] [CrossRef]
- Prajapati, N.; Ojha, P.; Karki, T.B. Antifungal property of essential oil extracted from Zanthoxylum armatum (Timur). J. Nutr. Health Food Eng. 2015, 3, 266–270. [Google Scholar]
- Lu, D. Revision and supplement of the account of the pepper in the tombs of the Han dynasty in Flora of China. Negative 2014, 5, 30–33. [Google Scholar]
- Li, Y.X.; Pei, X.H.; Jiang, D.Q.; Yuan, K. Phytochemical compositions of the essential oil from Blumea balsamifera. Asian J. Chem. 2013, 25, 6361–6365. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Meeran, M.F.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective potential of limonene and limonene containing natural products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef]
- Song, Y.; Seo, S.; Lamichhane, S.; Seo, J.; Hong, J.T.; Cha, H.J.; Yun, J. Limonene has anti-anxiety activity via adenosine A2A receptor-mediated regulation of dopaminergic and GABAergic neuronal function in the striatum. Phytomedicine 2021, 83, 153474. [Google Scholar] [CrossRef]
- Araújo-Filho, H.G.; Dos Santos, J.F.; Carvalho, M.T.B.; Picot, L.; Fruitier-Arnaudin, I.; Groult, H.; Quintans-Júnior, L.J.; Quintans, J.S.S. Anticancer activity of limonene: A systematic review of target signaling pathways. Phytother. Res. 2021, 35, 4957–4970. [Google Scholar] [CrossRef]
- Jong-Ho, A.; Hyun-Hee, L.; Seong-Gu, H.; In-Sik, N. Studies on efficient extraction of limonene from citron and immune-modulation activity for development of environmentally friendly material. Korean Assoc. Org. Agri. 2020, 28, 591–604. [Google Scholar]
- Santana, H.S.R.; de Carvalho, F.O.; Silva, E.R.; Santos, N.G.L.; Shanmugam, S.; Santos, D.N.; Wisniewski, J.O.; Junior, J.S.C.; Nunes, P.S.; Araujo, A.A.S.; et al. Anti-inflammatory activity of limonene in the prevention and control of injuries in the respiratory system: A systematic review. Curr. Pharm. Design. 2020, 26, 2182–2189. [Google Scholar] [CrossRef]
- Bai, X.D.; Tang, J. Myrcene exhibits antitumor activity against lung cancer cells by inducing oxidative stress and apoptosis mechanisms. Nat. Prod. Commun. 2020, 15, 1934578X2096118. [Google Scholar] [CrossRef]
- Du, Y.H.; Luan, J.; Jiang, R.P.; Liu, J.; Ma, Y. Myrcene exerts anti-asthmatic activity in neonatal rats via modulating the matrix remodeling. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420954948. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, R.; Ukeda, H.; Sawamura, M. Tyrosinase inhibitory activity of citrus essential oils. J. Agric. Food Chem. 2006, 54, 2309–2313. [Google Scholar] [CrossRef]
- Ryu, Y.; Lee, D.; Jung, S.H.; Lee, K.; Jin, H.; Kim, S.J.; Lee, H.M.; Kim, B.; Won, K.J. Sabinene prevents skeletal muscle atrophy by inhibiting the MAPK-MuRF-1 pathway in rats. Int. J. Mol. Sci. 2019, 20, 4955. [Google Scholar] [CrossRef]
- Elbe, H.; Ozturk, F.; Yigitturk, G.; Baygar, T.; Cavusoglu, T. Anticancer activity of linalool: Comparative investigation of ultrastructural changes and apoptosis in breast cancer cells. Ultrastruct. Pathol. 2022, 46, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Altınok-Yipel, F.; Ozan Tekeli, İ.; Özsoy, Ş.Y.; Güvenç, M.; Kaya, A.; Yipel, M. Hepatoprotective activity of linalool in rats against liver injury induced by carbon tetrachloride. Int. J. Vitam. Nutr. Res. 2020, 90, 302–308. [Google Scholar] [CrossRef]
- Peana, A.T.; Marzocco, S.; Popolo, A.; Pinto, A. (-)-Linalool inhibits in vitro NO formation: Probable involvement in the antinociceptive activity of this monoterpene compound. Life Sci. 2006, 78, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Asada, K.; Satoh, R.; Takada, K.; Kitajima, J. Capillin, a major constituent of Artemisia capillaris Thunb. flower essential oil, induces apoptosis through the mitochondrial pathway in human leukemia HL-60 cells. Phytomedicine 2015, 22, 545–552. [Google Scholar] [CrossRef]
- Saleh, A.M.; Aljada, A.; Rizvi, S.A.; Nasr, A.; Alaskar, A.S.; Williams, J.D. In vitro cytotoxicity of Artemisia vulgaris L. essential oil is mediated by a mitochondria-dependent apoptosis in HL-60 leukemic cell line. BMC Complement. Altern Med. 2014, 14, 226. [Google Scholar] [CrossRef]
- Kumar, A.; Malik, F.; Bhushan, S.; Sethi, V.K.; Shahi, A.K.; Kaur, J.; Taneja, S.C.; Qazi, G.N.; Singh, J. An essential oil and its major constituent isointermedeol induce apoptosis by increased expression of mitochondrial cytochrome c and apical death receptors in human leukaemia HL-60 cells. Chem. Biol. Interact. 2008, 171, 332–347. [Google Scholar] [CrossRef]
- Hata, T.; Sakaguchi, I.; Mori, M.; Ikeda, N.; Kato, Y.; Minamino, M.; Watabe, K. Induction of apoptosis by Citrus paradisi essential oil in human leukemic (HL-60) cells. Vivo 2003, 17, 553–559. [Google Scholar]
- Maeda, H.; Yamazaki, M.; Katagata, Y. Kuromoji (Lindera umbellata) essential oil-induced apoptosis and differentiation in human leukemia HL-60 cells. Exp. Ther. Med. 2012, 3, 49–52. [Google Scholar] [CrossRef]
- Gunia-Krzyżak, A.; Popiol, J.; Marona, H. Melanogenesis inhibitors: Strategies for searching for and evaluation of active compounds. Curr. Med. Chem. 2016, 23, 3548–3574. [Google Scholar] [CrossRef]
- Murray, A.F.; Satooka, H.; Shimizu, K.; Chavasiri, W.; Kubo, I. Polygonum odoratum essential oil inhibits the activity of mushroom derived tyrosinase. Heliyon 2019, 5, e02817. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.Y.; Jung, J.Y.; Yang, J.K. Camellia japonica essential oil inhibits α-MSH-induced melanin production and tyrosinase activity in B16F10 melanoma cells. Evid. Based Complement. Alternat Med. 2021, 2021, 6328767. [Google Scholar] [CrossRef]
- Capetti, F.; Tacchini, M.; Marengo, A.; Cagliero, C.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Citral-containing essential oils as potential tyrosinase inhibitors: A bio-guided fractionation approach. Plants 2021, 10, 969. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qi, X.; Faulkner, R.A.; Schumacher, M.M.; Donnelly, L.M.; De Bose-Boyd, R.A.; Li, X. Regulated degradation of HMG CoA reductase requires conformational changes in sterol-sensing domain. Nat. Commun. 2022, 13, 4273. [Google Scholar] [CrossRef] [PubMed]
- Bahr, T.; Butler, G.; Rock, C.; Welburn, K.; Allred, K.; Rodriguez, D. Cholesterol-lowering activity of natural mono- and sesquiterpenoid compounds in essential oils: A review and investigation of mechanisms using in silico protein-ligand docking. Phytother. Res. 2021, 35, 4215–4245. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Ko, Y.J.; Ahn, G.; Ham, Y.M.; Song, S.M.; Ko, E.Y.; Cho, S.H.; Yoon, W.J.; Kim, K.N. Anti-inflammatory effect and mechanism of action of Lindera erythrocarpa essential oil in lipopolysaccharide-stimulated RAW264.7 cells. EXCLI J. 2017, 16, 1103–1113. [Google Scholar]
- Lee, J.H.; Chang, K.M.; Kim, G.H. Composition and anti-inflammatory activities of Zanthoxylum schinifolium essential oil: Suppression of inducible nitric oxide synthase, cyclooxygenase-2, cytokines and cellular adhesion. J. Sci. Food Agric. 2019, 89, 1762–1769. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, Y.J.; Li, Y.S.; Zhang, W.K.; Tang, H.B. α-Pinene, linalool, and 1-octanol contribute to the topical anti-inflammatory and analgesic activities of frankincense by inhibiting COX-2. J. Ethnopharmacol. 2016, 179, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Adeel, S.; Mumtaz, A.; Mia, R.; Aftab, M.; Hussaan, M.; Amin, N.; Khan, S.R.; Khattak, S.P. Microwave-assisted sustainable coloration of wool fabric using Rheum emodi- based natural dye. Surf. Innov. 2023, 1–12. [Google Scholar] [CrossRef]
- Adeel, S.; Azeem, M.; Habib, N.; Hussaan, M.; Kiran, A.; Haji, A.; Haddar, W. Sustainable application of microwave assisted extracted tea based tannin natural dye for chemical and bio-mordanted wool fabric. J. Nat. Fibers 2023, 20, 2136322. [Google Scholar] [CrossRef]
- Adeel, S.; Rehman, F.; Pervaiz, M.; Hussaan, M.; Amin, N.; Majeed, A.; Rehman, H. Microwave assisted green isolation of laccaic acid from Lac Insect (Kerria lacca) for wool dyeing. Prog. Color Color. Coat. 2021, 14, 293–299. [Google Scholar]


| No. | Compound Name | Chemical Formula | Essential Oil | Aroma | ||||
|---|---|---|---|---|---|---|---|---|
| Retention Time (min) | Fruits Area (%) | Leaves Area (%) | Retention Time (min) | Fruits Area (%) | Leaves Area (%) | |||
| 1 | 1,3-Hexadiene | C6H10 | ND | ND | ND | 2.523 | ND | 0.14 |
| 2 | 2-Ethylfuran | C6H8O | ND | ND | ND | 3.205 | ND | 0.37 |
| 3 | 3-Hexenal | C6H10O | ND | ND | ND | 5.096 | 0.64 | 0.97 |
| 4 | 2-Hexenal | C6H10O | ND | ND | ND | 6.363 | 0.33 | ND |
| 5 | 3-Hexen-1-ol | C6H12O | ND | ND | ND | 6.63 | 0.43 | ND |
| 6 | cis-Hex-3-en-1-ol | C6H12O | ND | ND | ND | 6.667 | ND | 2.98 |
| 7 | 1,2-Xylene | C8H10 | ND | ND | ND | 7.047 | ND | 0.33 |
| 8 | Phenylethylene | C8H8 | ND | ND | ND | 7.047 | ND | 0.8 |
| 9 | α-Thujene | C10H16 | 5.282 | 0.08 | ND | 8.817 | 2.17 | 2.94 |
| 10 | α-Pinene | C10H16 | 6.292 | 0.2 | ND | 9.019 | 1.01 | 0.17 |
| 11 | Sabinene | C10H16 | 7.253 | 6.77 | 0.55 | 10.313 | 13.56 | 8.17 |
| 12 | β-Pinene | C10H16 | 7.347 | 0.28 | ND | ND | ND | ND |
| 13 | β-Myrcene | C10H16 | 7.651 | 1.25 | 0.4 | 10.944 | 8.64 | 15.8 |
| 14 | α-Phellandrene | C10H16 | ND | ND | ND | 11.284 | 0.43 | 1.11 |
| 15 | Leaf acetate | C8H14O2 | ND | ND | ND | 11.389 | 0.19 | 2.11 |
| 16 | Octanal | C8H16O | 7.948 | 0.13 | ND | ND | ND | ND |
| 17 | α-Terpinene | C10H16 | 8.352 | 0.53 | ND | 11.683 | 0.99 | 1.94 |
| 18 | Limonene | C10H16 | 8.685 | 8.05 | 3.75 | 12.225 | 28.43 | 39.15 |
| 19 | trans-Ocimene | C10H16 | ND | ND | ND | 12.362 | 0.27 | 0.3 |
| 20 | trans-β-Ocimene | C10H16 | 9.17 | 0.18 | ND | 12.691 | 3.29 | 2.63 |
| 21 | γ-Terpinene | C10H16 | 9.487 | 0.83 | 0.21 | 13.016 | 1.42 | 2.45 |
| 22 | trans-4-Thujanol | C10H18O | 9.724 | 0.82 | ND | 13.262 | 0.14 | ND |
| 23 | Terpinolene | C10H16 | 10.309 | 0.25 | ND | 13.921 | 0.98 | 1.51 |
| 24 | 2,4-Dimethylstyrene | C10H12 | ND | ND | ND | 14.027 | ND | 0.1 |
| 25 | Linalool | C10H18O | 10.8 | 72.17 | 62.01 | 14.027 | 11.47 | 5.25 |
| 26 | β-Thujone | C10H16O | 10.898 | 0.96 | ND | 14.842 | 0.07 | ND |
| 27 | neo-Alloocimene | C10H16 | ND | ND | ND | 15.165 | 0.18 | 0.38 |
| 28 | β-Ocimene | C10H16 | ND | ND | ND | 15.527 | 0.19 | ND |
| 29 | α-Thujone | C10H16O | 11.168 | 0.6 | ND | ND | ND | ND |
| 30 | trans-p-Menth-2-en-1-ol | C10H18O | 11.279 | 0.22 | 0.18 | ND | ND | ND |
| 31 | cis-p-Menth-2-en-1-ol | C10H18O | 11.749 | 0.15 | 0.18 | ND | ND | ND |
| 32 | Citronellal | C10H18O | 12.09 | 0.12 | 0.26 | ND | ND | ND |
| 33 | Terpinen-4-ol | C10H18O | 12.82 | 3.41 | 3.61 | 16.608 | 0.06 | 0.12 |
| 34 | cis-3-Hexenyl butyrate | C10H18O2 | ND | ND | ND | 16.849 | 0.04 | 0.14 |
| 35 | α-Terpineol | C10H18O | 13.17 | 0.97 | 2.27 | 16.996 | 0.14 | 0.07 |
| 36 | (-)-Myrtenal | C10H14O | 13.354 | 0.41 | ND | ND | ND | ND |
| 37 | Piperitone | C10H16O | ND | ND | ND | 18.827 | 0.12 | ND |
| 38 | Nerol | C10H18O | 14.159 | 0.05 | 0.4 | ND | ND | ND |
| 39 | Citronellol | C10H20O | ND | ND | ND | 18.057 | ND | 0.21 |
| 40 | cis-3-Hexenyl 2-methylbutanoate | C11H20O2 | ND | ND | ND | 18.171 | ND | 0.13 |
| 41 | Linalyl acetate | C12H20O2 | 14.93 | ND | 0.19 | 18.833 | ND | 0.29 |
| 42 | Geraniol | C10H18O | 14.86 | 0.07 | 0.46 | ND | ND | ND |
| 43 | Phenethyl acetate | C10H12O2 | 14.952 | 0.34 | ND | ND | ND | ND |
| 44 | 2-Undecanone | C11H22O | 15.951 | ND | 9.83 | 19.876 | 0.07 | 0.51 |
| 45 | γ-Pyrone | C5H4O2 | ND | ND | ND | 21.13 | 0.36 | 0.1 |
| 46 | 2,6-Dimethyl-2,6-octadiene | C10H18 | ND | ND | ND | 21.464 | ND | 0.11 |
| 47 | (-)-α-Cubebene | C15H24 | ND | ND | ND | 21.455 | 0.13 | ND |
| 48 | (-)-α-Copaene | C15H24 | ND | ND | ND | 22.172 | 0.15 | 0.06 |
| 49 | (-)-β-Elemene | C15H24 | 22.99 | ND | 0.72 | 22.584 | 3.46 | 0.92 |
| 50 | Tetradecane | C14H30 | ND | ND | ND | 22.675 | 0.19 | 0.21 |
| 51 | β-Caryophyllene | C15H24 | 23.81 | ND | 2.02 | 23.335 | 4.91 | 1.79 |
| 52 | β-Cubebene | C15H24 | ND | ND | ND | 22.695 | 0.19 | ND |
| 53 | (-)-γ-Elemene | C15H24 | ND | ND | ND | 23.635 | 0.1 | 0.05 |
| 54 | (+)-Aromandendrene | C15H24 | ND | ND | ND | 23.819 | 0.29 | 0.11 |
| 55 | α-Caryophyllene | C15H24 | 24.72 | ND | 0.57 | 24.187 | 1.47 | 0.49 |
| 56 | Decanohydrazide | C10H22N2O | 25.1 | ND | 0.34 | ND | ND | ND |
| 57 | β-Ionone | C13H20O | 25.25 | ND | 0.57 | ND | ND | ND |
| 58 | (-)-Isoledene | C15H24 | ND | ND | ND | 24.359 | 0.16 | 0.08 |
| 59 | Bicyclosesquiphellandrene | C15H24 | ND | ND | ND | 24.413 | 0.19 | 0.06 |
| 60 | γ-Muurolene | C15H24 | ND | ND | ND | 24.726 | 0.68 | 0.3 |
| 61 | Germacrene D | C15H24 | 25.36 | ND | 0.51 | 24.867 | 3.11 | 0.37 |
| 62 | β-Eudesmene | C15H24 | ND | ND | ND | 25.006 | 1.59 | 0.55 |
| 63 | 2-Tridecanone | C13H26O | 25.63 | ND | 5.47 | 25.09 | ND | 0.29 |
| 64 | γ-Selinene | C15H24 | ND | ND | ND | 25.219 | 3.72 | 1.34 |
| 65 | α-Farnesene | C15H24 | 25.84 | ND | 0.25 | ND | ND | ND |
| 66 | Elemicin | C12H16O3 | 26.88 | ND | 0.12 | ND | ND | ND |
| 67 | cis-Nerolidol | C15H26O | 27.23 | ND | 0.93 | ND | ND | ND |
| 68 | Caryophyllene Oxide | C15H24O | 27.84 | ND | 0.36 | ND | ND | ND |
| 69 | (-)-α-Amorphene | C15H24 | ND | ND | ND | 25.655 | 0.52 | 0.23 |
| 70 | δ-Cadinene | C15H24 | ND | ND | ND | 25.871 | 0.92 | ND |
| 71 | cis -Calamenene | C15H22 | ND | ND | ND | 25.862 | ND | 0.54 |
| 72 | α-Cadinene | C15H24 | ND | ND | ND | 26.208 | 0.23 | 0.1 |
| 73 | Hexadecanal | C16H32O | ND | ND | ND | 27.525 | 0.07 | 0.14 |
| 74 | cis-9-Octadecenal | C18H34O | 37.87 | ND | 0.16 | ND | ND | ND |
| 75 | 9,17-Octadecadienal, (Z)- | C18H32O | ND | ND | 0.14 | ND | ND | ND |
| 76 | Oleic alcohol | C18H36O | 39.24 | ND | 0.18 | ND | ND | ND |
| 77 | Phytol | C20H40O | 40.13 | ND | 0.68 | ND | ND | ND |
| Grouped compounds | ||||||||
| Monoterpene hydrocarbons | 18.42 | 4.91 | 61.56 | 76.55 | ||||
| Oxygenated monoterpenes | 79.95 | 69.37 | 12.04 | 5.79 | ||||
| Sesquiterpene hydrocarbons | 4.07 | 21.82 | 6.99 | |||||
| Oxygenated sesquiterpenes | 1.29 | |||||||
| Others | 0.47 | 17.68 | 2.28 | 9.58 | ||||
| Total percentage of identified components | 98.84 | 97.32 | 97.7 | 98.91 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Ning, L.; Wang, J.; Gong, W.; Gao, Y.; Li, M. GC-MS Analysis and Bioactivity Screening of Leaves and Fruits of Zanthoxylum armatum DC. Separations 2023, 10, 420. https://doi.org/10.3390/separations10080420
Ma J, Ning L, Wang J, Gong W, Gao Y, Li M. GC-MS Analysis and Bioactivity Screening of Leaves and Fruits of Zanthoxylum armatum DC. Separations. 2023; 10(8):420. https://doi.org/10.3390/separations10080420
Chicago/Turabian StyleMa, Jie, Liping Ning, Jingyan Wang, Wei Gong, Yue Gao, and Mei Li. 2023. "GC-MS Analysis and Bioactivity Screening of Leaves and Fruits of Zanthoxylum armatum DC." Separations 10, no. 8: 420. https://doi.org/10.3390/separations10080420
APA StyleMa, J., Ning, L., Wang, J., Gong, W., Gao, Y., & Li, M. (2023). GC-MS Analysis and Bioactivity Screening of Leaves and Fruits of Zanthoxylum armatum DC. Separations, 10(8), 420. https://doi.org/10.3390/separations10080420





