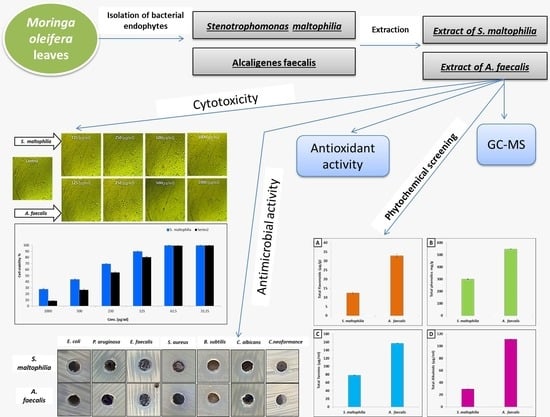
Bacterial Endophytes from Moringa oleifera Leaves as a Promising Source for Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Molecular Identification of Endophytic Bacteria
2.2. Extraction of Bioactive Compounds from Bacterial Endophytes
2.3. Screening of Bacterial Phytochemicals
2.4. Gas Chromatography-Mass Spectroscopy (GC–MS) Analysis
2.5. Antimicrobial Activity
2.6. Antioxidant Activity
2.7. In Vitro Cytotoxicity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Identification of Bacterial Strains
3.2. Screening of Bacterial Phytochemicals
3.3. Gas Chromatography–Mass Spectroscopy (GC–MS) Analysis
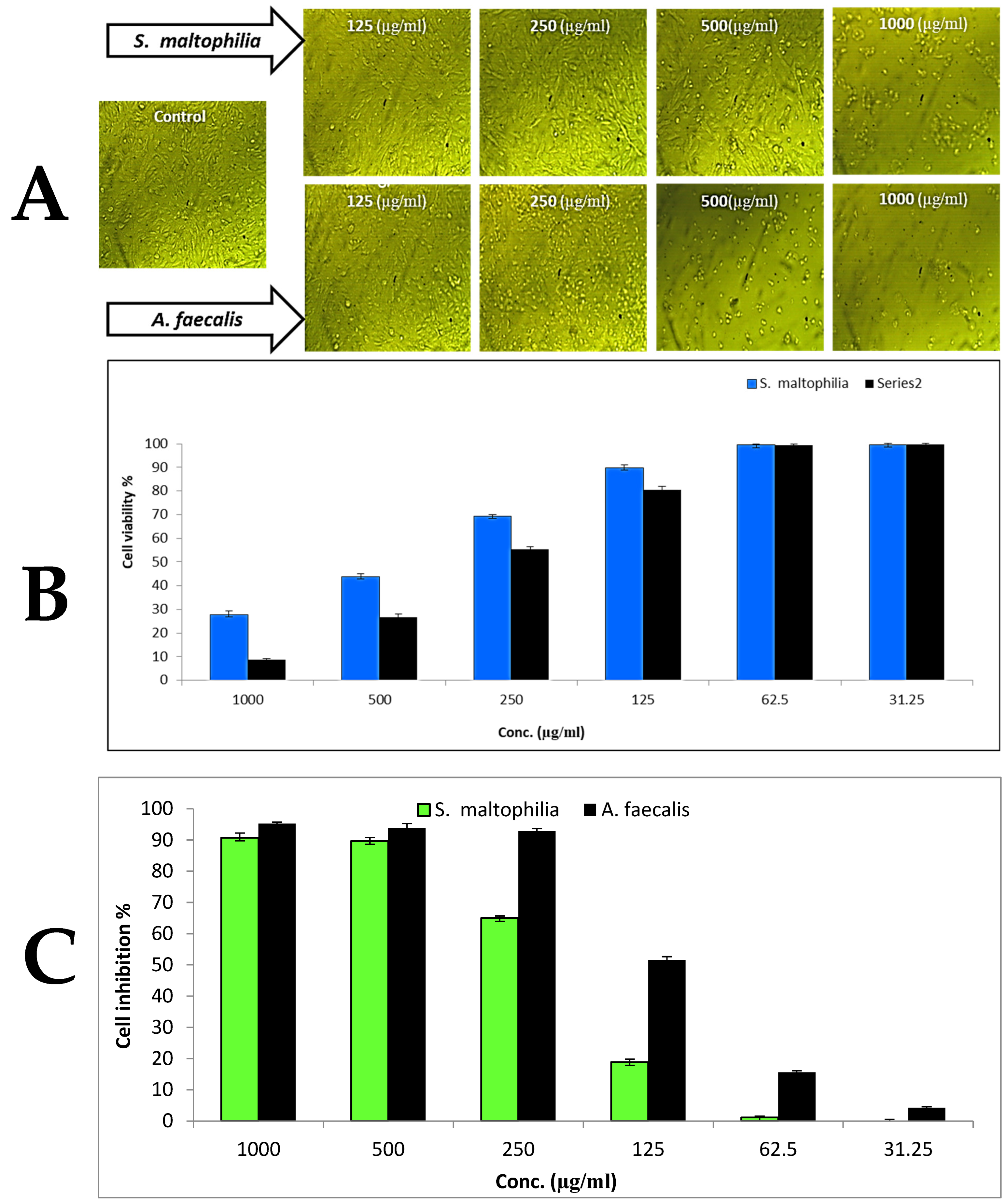
3.4. Cytotoxicity
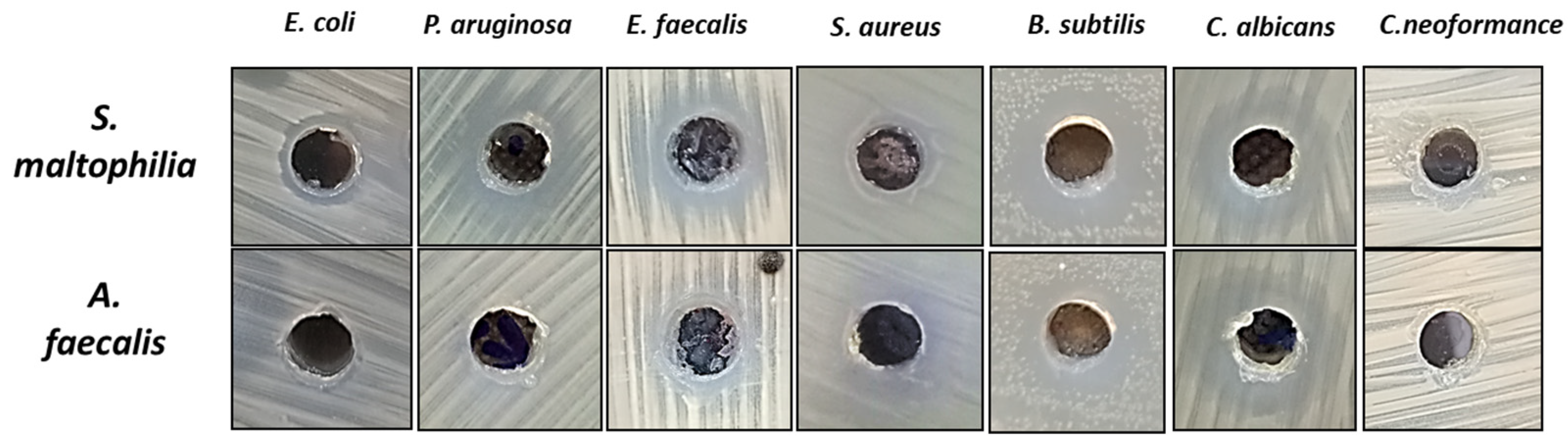
3.5. Antimicrobial Activity
3.6. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [PubMed]
- Abd Alhakim, A.; Hashem, A.; Abdelaziz, A.M.; Attia, M.S. Impact of plant growth promoting fungi on biochemical defense performance of tomato under fusarial infection. Egypt. J. Chem. 2022, 65, 291–301. [Google Scholar] [CrossRef]
- Sciarretta, K.; Røttingen, J.-A.; Opalska, A.; Van Hengel, A.J.; Larsen, J. Economic incentives for antibacterial drug development: Literature review and considerations from the Transatlantic Task Force on Antimicrobial Resistance. Clin. Infect. Dis. 2016, 63, 1470–1474. [Google Scholar] [CrossRef]
- Elbahnasawy, M.A.; Shehabeldine, A.M.; Khattab, A.M.; Amin, B.H.; Hashem, A.H. Green biosynthesis of silver nanoparticles using novel endophytic Rothia endophytica: Characterization and anticandidal activity. J. Drug Deliv. Sci. Technol. 2021, 62, 102401. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; El-Wakil, D.A.; Hashem, A.H.; Al-Askar, A.A.; AbdElgawad, H.; Attia, M.S. Efficient Role of Endophytic Aspergillus terreus in Biocontrol of Rhizoctonia solani Causing Damping-off Disease of Phaseolus vulgaris and Vicia faba. Microorganisms 2023, 11, 1487. [Google Scholar] [CrossRef]
- Attia, M.S.; Salem, M.S.; Abdelaziz, A.M. Endophytic fungi Aspergillus spp. reduce fusarial wilt disease severity, enhance growth, metabolism and stimulate the plant defense system in pepper plants. Biomass Convers. Biorefinery 2022, 1–11. [Google Scholar] [CrossRef]
- Sharaf, M.H.; Abdelaziz, A.M.; Kalaba, M.H.; Radwan, A.A.; Hashem, A.H. Antimicrobial, antioxidant, cytotoxic activities and phytochemical analysis of fungal endophytes isolated from ocimum basilicum. Appl. Biochem. Biotechnol. 2022, 194, 1271–1289. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; El-Wakil, D.A.; Attia, M.S.; Ali, O.M.; AbdElgawad, H.; Hashem, A.H. Inhibition of Aspergillus flavus Growth and Aflatoxin Production in Zea mays L. Using Endophytic Aspergillus fumigatus. J. Fungi 2022, 8, 482. [Google Scholar] [CrossRef]
- Toghueo, R.M.K.; Boyom, F.F. Endophyte enzymes and their applications in industries. Bioprospecting Plant Biodivers. Ind. Mol. 2021, 99–129. [Google Scholar] [CrossRef]
- Shoayb, M.; Soliman, H.G.; Abdelghany, T.M.; Abdelaziz, A.M. Occurrence of heavy metals in Qarun Lake and its influence on microbial biodiversity. Al-Azhar J. Agric. Res. 2023. [Google Scholar] [CrossRef]
- Anand, U.; Pal, T.; Yadav, N.; Singh, V.K.; Tripathi, V.; Choudhary, K.K.; Shukla, A.K.; Sunita, K.; Kumar, A.; Bontempi, E.; et al. Current Scenario and Future Prospects of Endophytic Microbes: Promising Candidates for Abiotic and Biotic Stress Management for Agricultural and Environmental Sustainability. Microb. Ecol. 2023, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.H.; Attia, M.S.; Kandil, E.K.; Fawzi, M.M.; Abdelrahman, A.S.; Khader, M.S.; Khodaira, M.A.; Emam, A.E.; Goma, M.A.; Abdelaziz, A.M. Bioactive compounds and biomedical applications of endophytic fungi: A recent review. Microb. Cell Factories 2023, 22, 107. [Google Scholar] [CrossRef]
- Matic, I.; Guidi, A.; Kenzo, M.; Mattei, M.; Galgani, A. Investigation of medicinal plants traditionally used as dietary supplements: A review on Moringa oleifera. J. Public Health Afr. 2018, 9, 841. [Google Scholar] [CrossRef]
- Oladeji, O.S.; Odelade, K.A.; Oloke, J.K. Phytochemical screening and antimicrobial investigation of Moringa oleifera leaf extracts. Afr. J. Sci. Technol. Innov. Dev. 2020, 12, 79–84. [Google Scholar] [CrossRef]
- Mehta, K.; Balaraman, R.; Amin, A.; Bafna, P.; Gulati, O. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J. Ethnopharmacol. 2003, 86, 191–195. [Google Scholar] [CrossRef]
- Arora, D.S.; Kaur, N. Antimicrobial potential of fungal endophytes from Moringa oleifera. Appl. Biochem. Biotechnol. 2019, 187, 628–648. [Google Scholar] [CrossRef]
- Ilmi, N. Molecular Identification of Endophytic Bacteria from the Stem’s Bark of Moringa Plant and Their Antibacterial Activities. Ph.D. Thesis, Universitas Mataram, Mataram, Indonesia, 2018. [Google Scholar]
- Attia, M.S.; Abdelaziz, A.M.; Al-Askar, A.A.; Arishi, A.A.; Abdelhakim, A.M.; Hashem, A.H. Plant growth-promoting fungi as biocontrol tool against fusarium wilt disease of tomato plant. J. Fungi 2022, 8, 775. [Google Scholar] [CrossRef]
- Attia, M.S.; Hashem, A.H.; Badawy, A.A.; Abdelaziz, A.M. Biocontrol of early blight disease of eggplant using endophytic Aspergillus terreus: Improving plant immunological, physiological and antifungal activities. Bot. Stud. 2022, 63, 26. [Google Scholar] [CrossRef]
- Badawy, A.A.; Alotaibi, M.O.; Abdelaziz, A.M.; Osman, M.S.; Khalil, A.M.; Saleh, A.M.; Mohammed, A.E.; Hashem, A.H. Enhancement of seawater stress tolerance in barley by the endophytic fungus Aspergillus ochraceus. Metabolites 2021, 11, 428. [Google Scholar] [CrossRef] [PubMed]
- Elghaffar, R.Y.A.; Amin, B.H.; Hashem, A.H.; Sehim, A.E. Promising Endophytic Alternaria alternata from Leaves of Ziziphus spina-christi: Phytochemical Analyses, Antimicrobial and Antioxidant Activities. Appl. Biochem. Biotechnol. 2022, 194, 3984–4001. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Seo, J.-S.; Wang, J.; Keum, Y.-S.; Li, J.; Li, Q.X. Multiple degradation pathways of phenanthrene by Stenotrophomonas maltophilia C6. Int. Biodeterior. Biodegrad. 2013, 79, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Nandakumar, R.; Madayiputhiya, N.; Li, X. Proteomic analysis of 17β-estradiol degradation by Stenotrophomonas maltophilia. Environ. Sci. Technol. 2012, 46, 5947–5955. [Google Scholar] [CrossRef] [PubMed]
- Dunne, C.; Crowley, J.J.; Moënne-Loccoz, Y.; Dowling, D.N.; Bruijn, S.; O'Gara, F. Biological control of Pythium ultimum by Stenotrophomonas maltophilia W81 is mediated by an extracellular proteolytic activity. Microbiology 1997, 143, 3921–3931. [Google Scholar] [CrossRef]
- Hasan-Beikdashti, M.; Forootanfar, H.; Safiarian, M.; Ameri, A.; Ghahremani, M.; Khoshayand, M.; Faramarzi, M. Optimization of culture conditions for production of lipase by a newly isolated bacterium Stenotrophomonas maltophilia. J. Taiwan Inst. Chem. Eng. 2012, 43, 670–677. [Google Scholar] [CrossRef]
- Nowak, A.; Greń, I.; Mrozik, A. Changes in fatty acid composition of Stenotrophomonas maltophilia KB2 during co-metabolic degradation of monochlorophenols. World J. Microbiol. Biotechnol. 2016, 32, 198. [Google Scholar] [CrossRef]
- Berg, G.; Marten, P.; Ballin, G. Stenotrophomonas maltophilia in the rhizosphere of oilseed rape—Occurrence, characterization and interaction with phytopathogenic fungi. Microbiol. Res. 1996, 151, 19–27. [Google Scholar] [CrossRef]
- Nauton, L.; Kahn, R.; Garau, G.; Hernandez, J.-F.; Dideberg, O. Structural insights into the design of inhibitors for the L1 metallo-β-lactamase from Stenotrophomonas maltophilia. J. Mol. Biol. 2008, 375, 257–269. [Google Scholar] [CrossRef]
- Larik, I.; Qazi, M.; Phulpoto, A.; Haleem, A.; Ahmed, S.; Kanhar, N. Stenotrophomonas maltophilia strain 5DMD: An efficient biosurfactant-producing bacterium for biodegradation of diesel oil and used engine oil. Int. J. Environ. Sci. Technol. 2019, 16, 259–268. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, P. Genomic potential of Stenotrophomonas maltophilia in bioremediation with an assessment of its multifaceted role in our environment. Front. Microbiol. 2016, 7, 967. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Liu, B.; Zhao, L.; Zhang, Y.; Cheng, D.; Yan, X.; Jiang, J.; Hong, Q.; He, J. A novel degradation mechanism for pyridine derivatives in Alcaligenes faecalis JQ135. Appl. Environ. Microbiol. 2018, 84, e00910–e00918. [Google Scholar] [CrossRef]
- Gong, A.-D.; Wu, N.-N.; Kong, X.-W.; Zhang, Y.-M.; Hu, M.-J.; Gong, S.-J.; Dong, F.-Y.; Wang, J.-H.; Zhao, Z.-Y.; Liao, Y.-C. Inhibitory effect of volatiles emitted from Alcaligenes faecalis N1-4 on Aspergillus flavus and aflatoxins in storage. Front. Microbiol. 2019, 10, 1419. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X.; Zhang, K.; Li, G. Bioactive constituents from the bacteirium Alcaligenes faecalis YMF 3.175. Appl. Biochem. Microbiol. 2015, 51, 52–57. [Google Scholar] [CrossRef]
- Misaki, A.; Saito, H.; Ito, T.; Harada, T. Structure of succinoglucan and exocellular acidic polysaccharide of Alcaligenes faecalis var myxogenes. Biochemistry 1969, 8, 4645–4650. [Google Scholar] [CrossRef]
- Nageshwar, Y.; Sheelu, G.; Shambhu, R.R.; Muluka, H.; Mehdi, N.; Malik, M.S.; Kamal, A. Optimization of nitrilase production from Alcaligenes faecalis MTCC 10757 (IICT-A3): Effect of inducers on substrate specificity. Bioprocess Biosyst. Eng. 2011, 34, 515–523. [Google Scholar] [CrossRef]
- Khalil, A.; Abdelaziz, A.; Khaleil, M.; Hashem, A. Fungal endophytes from leaves of Avicennia marina growing in semi-arid environment as a promising source for bioactive compounds. Lett. Appl. Microbiol. 2021, 72, 263–274. [Google Scholar] [CrossRef]
- Nxumalo, C.I.; Ngidi, L.S.; Shandu, J.S.E.; Maliehe, T.S. Isolation of endophytic bacteria from the leaves of Anredera cordifolia CIX1 for metabolites and their biological activities. BMC Complement. Med. Ther. 2020, 20, 300. [Google Scholar] [CrossRef]
- Ashitha, A.; Midhun, S.; Sunil, M.; Nithin, T.; Radhakrishnan, E.; Mathew, J. Bacterial endophytes from Artemisia nilagirica (Clarke) Pamp., with antibacterial efficacy against human pathogens. Microb. Pathog. 2019, 135, 103624. [Google Scholar] [CrossRef]
- Kim, H.Y.; Choi, G.; Lee, H.; Lee, S.W.; Lim, H.; Jang, K.; Son, S.; Lee, S.; Cho, K.; Sung, N. Some fungal endophytes from vegetable crops and their anti-oomycete activities against tomato late blight. Lett. Appl. Microbiol. 2007, 44, 332–337. [Google Scholar] [CrossRef]
- Attia, M.S.; Elsayed, S.M.; Abdelaziz, A.M.; Ali, M.M. Potential impacts of Ascophyllum nodosum, Arthrospira platensis extracts and calcium phosphite as therapeutic nutrients for enhancing immune response in pepper plant against Fusarium wilt disease. Biomass Convers. Biorefinery 2023, 1–10. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Attia, M.S.; Salem, M.S.; Refaay, D.A.; Alhoqail, W.A.; Senousy, H.H. Cyanobacteria-mediated immune responses in pepper plants against fusarium wilt. Plants 2022, 11, 2049. [Google Scholar] [CrossRef]
- Kumar, R.S.; Balasubramanian, P.; Govindaraj, P.; Krishnaveni, T. Preliminary studies on phytochemicals and antimicrobial activity of solvent extracts of Coriandrum sativum L. roots (Coriander). J. Pharmacogn. Phytochem. 2014, 2, 74–78. [Google Scholar]
- Passari, A.K.; Chandra, P.; Leo, V.V.; Mishra, V.K.; Kumar, B.; Singh, B.P. Production of potent antimicrobial compounds from Streptomyces cyaneofuscatus associated with fresh water sediment. Front. Microbiol. 2017, 8, 68. [Google Scholar]
- Hsueh, P.-R.; Ko, W.-C.; Wu, J.-J.; Lu, J.-J.; Wang, F.-D.; Wu, H.-Y.; Wu, T.-L.; Teng, L.-J. Consensus statement on the adherence to Clinical and Laboratory Standards Institute (CLSI) Antimicrobial Susceptibility Testing Guidelines (CLSI-2010 and CLSI-2010-update) for Enterobacteriaceae in clinical microbiology laboratories in Taiwan. J. Microbiol. Immunol. Infect. 2010, 43, 452–455. [Google Scholar] [CrossRef]
- Hashem, A.H.; Abdelaziz, A.M.; Askar, A.A.; Fouda, H.M.; Khalil, A.M.A.; Abd-Elsalam, K.A.; Khaleil, M.M. Bacillus megaterium-Mediated Synthesis of Selenium Nanoparticles and Their Antifungal Activity against Rhizoctonia solani in Faba Bean Plants. J. Fungi 2021, 7, 195. [Google Scholar] [CrossRef]
- Dacrory, S.; Hashem, A.H.; Hasanin, M. Synthesis of cellulose based amino acid functionalized nano-biocomplex: Characterization, antifungal activity, molecular docking and hemocompatibility. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100453. [Google Scholar] [CrossRef]
- Valgas, C.; Souza, S.M.D.; Smânia, E.; Smânia, A. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- Hashem, A.H.; Khalil, A.M.A.; Reyad, A.M.; Salem, S.S. Biomedical Applications of Mycosynthesized Selenium Nanoparticles Using Penicillium expansum ATTC 36200. Biol. Trace Elem. Res. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Van de Loosdrecht, A.; Beelen, R.; Ossenkoppele, g.; Broekhoven, M.; Langenhuijsen, M. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immunol Methods 1994, 174, 311–320. [Google Scholar] [CrossRef]
- Ray, S.; Swapnil, P.; Singh, P.; Singh, S.; Sarma, B.K.; Singh, H.B. Endophytic Alcaligenes faecalis mediated redesigning of host defense itinerary against Sclerotium rolfsii through induction of phenolics and antioxidant enzymes. Biol. Control 2020, 150, 104355. [Google Scholar] [CrossRef]
- Mastan, A.; Rane, D.; Dastager, S.G.; Vivek Babu, C.S. Plant Probiotic Bacterial Endophyte, Alcaligenes faecalis, Modulates Plant Growth and Forskolin Biosynthesis in Coleus forskohlii. Probiotics Antimicrob. Proteins 2020, 12, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Alikhani, H.A. Suppression of the fungal pathogen Magnaporthe grisea by Stenotrophomonas maltophilia, a seed-borne rice (Oryza sativa L.) endophytic bacterium. Arch. Agron. Soil Sci. 2016, 62, 1271–1284. [Google Scholar] [CrossRef]
- Ray, S.; Singh, V.; Singh, S.; Sarma, B.K.; Singh, H.B. Biochemical and histochemical analyses revealing endophytic Alcaligenes faecalis mediated suppression of oxidative stress in Abelmoschus esculentus challenged with Sclerotium rolfsii. Plant Physiol. Biochem. 2016, 109, 430–441. [Google Scholar] [CrossRef]
- Forchetti, G.; Masciarelli, O.; Alemano, S.; Alvarez, D.; Abdala, G. Endophytic bacteria in sunflower (Helianthus annuus L.): Isolation, characterization, and production of jasmonates and abscisic acid in culture medium. Appl. Microbiol. Biotechnol. 2007, 76, 1145–1152. [Google Scholar] [CrossRef]
- Araújo, W.L.; Maccheroni Jr, W.; Aguilar-Vildoso, C.I.; Barroso, P.A.; Saridakis, H.O.; Azevedo, J.L. Variability and interactions between endophytic bacteria and fungi isolated from leaf tissues of citrus rootstocks. Can. J. Microbiol. 2001, 47, 229–236. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Ayangbenro, A.; Babalola, O.O. Effect of endophytic bacterium, Stenotrophomonas maltophilia JVB5 on sunflowers. Plant Prot. Sci. 2022, 58, 185–198. [Google Scholar] [CrossRef]
- Ryan, R.P.; Monchy, S.; Cardinale, M.; Taghavi, S.; Crossman, L.; Avison, M.B.; Berg, G.; Van Der Lelie, D.; Dow, J.M. The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat. Rev. Microbiol. 2009, 7, 514–525. [Google Scholar] [CrossRef]
- Ramos, P.L.; Van Trappen, S.; Thompson, F.L.; Rocha, R.C.; Barbosa, H.R.; De Vos, P.; Moreira-Filho, C.A. Screening for endophytic nitrogen-fixing bacteria in Brazilian sugar cane varieties used in organic farming and description of Stenotrophomonas pavanii sp. nov. Int. J. Syst. Evol. Microbiol. 2011, 61, 926–931. [Google Scholar] [CrossRef]
- Hashem, A.H.; Shehabeldine, A.M.; Abdelaziz, A.M.; Amin, B.H.; Sharaf, M.H. Antifungal activity of endophytic Aspergillus terreus extract against some fungi causing mucormycosis: Ultrastructural study. Appl. Biochem. Biotechnol. 2022, 194, 3468–3482. [Google Scholar] [CrossRef]
- Photolo, M.M.; Mavumengwana, V.; Sitole, L.; Tlou, M.G. Antimicrobial and antioxidant properties of a bacterial endophyte, methylobacterium radiotolerans MAMP 4754, isolated from combretum erythrophyllum seeds. Int. J. Microbiol. 2020, 2020, 9483670. [Google Scholar] [CrossRef] [PubMed]
- Makowski, W.; Królicka, A.; Nowicka, A.; Zwyrtková, J.; Tokarz, B.; Pecinka, A.; Banasiuk, R.; Tokarz, K.M. Transformed tissue of Dionaea muscipula J. Ellis as a source of biologically active phenolic compounds with bactericidal properties. Appl. Microbiol. Biotechnol. 2021, 105, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res 2019, 10, 1567–1574. [Google Scholar]
- Qian, S.-Y.; Yang, C.-L.; Khan, A.; Chen, R.-X.; Wu, M.-S.; Tuo, L.; Wang, Q.; Liu, J.-G.; Cheng, G.-G. New pyrazinoquinazoline alkaloids Isolated from a culture of Stenotrophomonas maltophilia QB-77. Nat. Prod. Res. 2019, 33, 1387–1391. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef]
- Kolodziej, H.; Kayser, O.; Latté, K.P.; Kiderlen, A.F. Enhancement of antimicrobial activity of tannins and related compounds by immune modulatory effects. Plant Polyphen. 2 Chem. Biol. Pharmacol. Ecol. 1999, 575–594. [Google Scholar] [CrossRef]
- Brader, G.; Compant, S.; Mitter, B.; Trognitz, F.; Sessitsch, A. Metabolic potential of endophytic bacteria. Curr. Opin. Biotechnol. 2014, 27, 30–37. [Google Scholar] [CrossRef]
- Zote, J.; Passari, A.K.; Siddaiah, C.N.; Kumar, N.S.; Abd_Allah, E.F.; Hashem, A.; Alqarawi, A.A.; Malik, J.A.; Singh, B.P. Phylogenetic affiliation and determination of bioactive compounds of bacterial population associated with organs of mud crab, Scylla olivacea. Saudi J. Biol. Sci. 2018, 25, 1743–1754. [Google Scholar] [CrossRef]
- Cakir, A. Essential oil and fatty acid composition of the fruits of Hippophae rhamnoides L. (Sea Buckthorn) and Myrtus communis L. from Turkey. Biochem. Syst. Ecol. 2004, 32, 809–816. [Google Scholar] [CrossRef]
- Singh, M.; Pandey, K.D. Endophytic bacterial strains modulated synthesis of lycopene and bioactive compounds in Solanum lycopersicum L. fruit. Biocatal. Agric. Biotechnol. 2021, 35, 102088. [Google Scholar] [CrossRef]
- Abd Sharad, A.; Usupb, G.; Sahrani, F.K.; Ahmad, A. Bioactivity of Natural Compounds Produced by Marine Alcaligenes faecalis as Antimicrobial, Antibiofilm Formation and Anti-biocorrosion Effect against Desulfovibrio sp. Isol. Crude Oil Fluid 2018, 6, 134–148. [Google Scholar]
- Momodu, I.; Okungbowa, E.; Agoreyo, B.; Maliki, M. Gas Chromatography–Mass Spectrometry Identification of Bioactive Compounds in Methanol and Aqueous Seed Extracts of Azanza garckeana Fruits. Niger. J. Biotechnol. 2022, 38, 25–38. [Google Scholar] [CrossRef]
- Brooke, J.S.; Di Bonaventura, G.; Berg, G.; Martinez, J.-L. A multidisciplinary look at Stenotrophomonas maltophilia: An emerging multi-drug-resistant global opportunistic pathogen. Front. Media SA 2017, 8, 1511. [Google Scholar]
- Dilika, F.; Bremner, P.; Meyer, J. Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia 2000, 71, 450–452. [Google Scholar] [CrossRef]
- Olejníková, P.; Birošová, L.; Švorc, Ľ.; Vihonská, Z.; Fiedlerová, M.; Marchalín, Š.; Šafář, P. Newly synthesized indolizine derivatives–antimicrobial and antimutagenic properties. Chem. Pap. 2015, 69, 983–992. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Lammers, A.; Zweers, H.; Sandfeld, T.; Bilde, T.; Garbeva, P.; Schramm, A.; Lalk, M. Antimicrobial compounds in the volatilome of social spider communities. Front. Microbiol. 2021, 12, 700693. [Google Scholar] [CrossRef] [PubMed]
- Qanash, H.; Yahya, R.; Bakri, M.M.; Bazaid, A.S.; Qanash, S.; Shater, A.F.; TM, A. Anticancer, antioxidant, antiviral and antimicrobial activities of Kei Apple (Dovyalis caffra) fruit. Sci. Rep. 2022, 12, 5914. [Google Scholar] [CrossRef]
- Giovagnoni, G.; Tugnoli, B.; Piva, A.; Grilli, E. Dual Antimicrobial Effect of Medium-Chain Fatty Acids against an Italian Multidrug Resistant Brachyspira hyodysenteriae Strain. Microorganisms 2022, 10, 301. [Google Scholar] [CrossRef]
- Asilbekova, D.; KhM, B.; Sasmakov, S.; Abdurakhmanov, J.; ShS, A.; Abdullaev, N.; Sh, S. Composition and antimicrobial activity of essential oils from Daucus carota L. subsp. carota, growing in Uzbekistan. Am. J. Essen. Oils Nat. Prod. 2017, 5, 9–13. [Google Scholar]
- Di Stefano, V.; Pitonzo, R.; Schillaci, D. Antimicrobial and antiproliferative activity of Athamanta sicula L. (Apiaceae). Pharmacogn. Mag. 2011, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Langa-Lomba, N.; Casanova-Gascón, J.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Characterization and antimicrobial activity of a halophyte from the Asturian coast (Spain): Limonium binervosum (GE Sm.) CE Salmon. Plants 2021, 10, 1852. [Google Scholar] [CrossRef]
- Mohadjerani, M.; Hosseinzadeh, R.; Hosseini, M. Chemical composition and antibacterial properties of essential oil and fatty acids of different parts of Ligularia persica Boiss. Avicenna J. Phytomedicine 2016, 6, 357. [Google Scholar]
- Kalaiarasan, A.; Kumar, P.; John, S. GC/MS determination of bioactive components of Bulbophyllum kaitense Reichib leaves Estern ghats in India. N. Y. Sci. J 2011, 4, 46–49. [Google Scholar]
- Watanabe, T.; Yano, S.; Kawai, T.; Jinbo, Y.; Nonomura, Y. Selective antibacterial activity of palmitoleic acid in emulsions and other formulations. J. Surfactants Deterg. 2021, 24, 973–979. [Google Scholar] [CrossRef]
- Alabi, K.; Lajide, L.; Owolabi, B. Biological activity of oleic acid and its primary amide: Experimental and Computational studies. J. Chem. Soc. Niger. 2018, 43, 1–10. [Google Scholar]
- Comlekcioglu, N. Bioactive compounds and antioxidant activity in leaves of endemic and native Isatis spp in Turkey. Braz. Arch. Biol. Technol. 2019, 62. [Google Scholar] [CrossRef]
- Mohamad, O.A.; Li, L.; Ma, J.-B.; Hatab, S.; Xu, L.; Guo, J.-W.; Rasulov, B.A.; Liu, Y.-H.; Hedlund, B.P.; Li, W.-J. Evaluation of the antimicrobial activity of endophytic bacterial populations from Chinese traditional medicinal plant licorice and characterization of the bioactive secondary metabolites produced by Bacillus atrophaeus against Verticillium dahliae. Front. Microbiol. 2018, 9, 924. [Google Scholar] [CrossRef]
- Abdel-Wareth, M.T.A.; Ghareeb, M.A.; Abdel-Aziz, M.S.; El-Hagrassi, A.M. Snailicidal, antimicrobial, antioxidant and anticancer activities of Beauveria bassiana, Metarhizium anisopliae and Paecilomyces lilacinus fungal extracts. Egypt. J. Aquat. Biol. Fish. 2019, 23, 195–212. [Google Scholar] [CrossRef]
- Pinto, M.E.; Araujo, S.G.; Morais, M.I.; Sa, N.P.; Lima, C.M.; Rosa, C.A.; Siqueira, E.P.; Johann, S.; Lima, L.A. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. An. Da Acad. Bras. De Ciências 2017, 89, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Helesbeux, J.-J.; Peyronnet, D.; Labaïed, M.; Grellier, P.; Frappier, F.; Seraphin, D.; Richomme, P.; Duval, O. Synthesis and antimalarial activity of some new 1, 2-dioxolane derivatives. J. Enzym. Inhib. Med. Chem. 2002, 17, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Mazziotta, J.C.; Newman, N.J.; Pomeroy, S.L. Diagnosis of neurological disease. In Bradley and Daroff's Neurology in Clinical Practice, 8th ed.; Elsevier: Philadelphia, PA, USA, 2022. [Google Scholar]
- Sinha, N.; Singh, B.K.; Dutta, P. Research on Antibacterial Screening and Drug Delivery using Chitosan-Stearic Acid Derivative. J. Polym. Mater. 2017, 34, 11–20. [Google Scholar]
- Malathi, K.; Ramaiah, S. Ethyl iso-allocholate from a medicinal rice Karungkavuni inhibits dihydropteroate synthase in Escherichia coli: A molecular docking and dynamics study. Indian J. Pharm. Sci. 2017, 78, 780–788. [Google Scholar] [CrossRef]
- Naz, I.; Khan, M. Nematicidal activity of nonacosane-10-ol and 23a-homostigmast-5-en-3β-ol isolated from the roots of Fumaria parviflora (Fumariaceae). J. Agric. Food Chem. 2013, 61, 5689–5695. [Google Scholar] [CrossRef]
- Değirmenci, H.; Erkurt, H. Relationship between volatile components, antimicrobial and antioxidant properties of the essential oil, hydrosol and extracts of Citrus aurantium L. flowers. J. Infect. Public Health 2020, 13, 58–67. [Google Scholar] [CrossRef]
- Benli, M.; Yiğit, N.; Geven, F.; Güney, K.; Bingöl, Ü. Antimicrobial activity of endemic Digitalis lamarckii Ivan from Turkey. IJEB 2009, 47, 218–221. [Google Scholar]
- Freitas, C.S.; Lage, D.P.; Oliveira-da-Silva, J.A.; Costa, R.R.; Mendonça, D.V.; Martins, V.T.; Reis, T.A.; Antinarelli, L.M.; Machado, A.S.; Tavares, G.S. In vitro and in vivo antileishmanial activity of β-acetyl-digitoxin, a cardenolide of Digitalis lanata potentially useful to treat visceral leishmaniasis. Parasite 2021, 28. [Google Scholar] [CrossRef]
- Cai, H.; Wang, H.-Y.L.; Venkatadri, R.; Fu, D.-X.; Forman, M.; Bajaj, S.O.; Li, H.; O’Doherty, G.A.; Arav-Boger, R. Digitoxin analogues with improved anticytomegalovirus activity. ACS Med. Chem. Lett. 2014, 5, 395–399. [Google Scholar] [CrossRef]
- Gautam, V.; Kohli, S.K.; Arora, S.; Bhardwaj, R.; Kazi, M.; Ahmad, A.; Raish, M.; Ganaie, M.A.; Ahmad, P. Antioxidant and antimutagenic activities of different fractions from the leaves of Rhododendron arboreum Sm. and their GC-MS profiling. Molecules 2018, 23, 2239. [Google Scholar] [CrossRef]
- Johnson, T.O.; Odoh, K.D.; Nwonuma, C.O.; Akinsanmi, A.O.; Adegboyega, A.E. Biochemical evaluation and molecular docking assessment of the anti-inflammatory potential of Phyllanthus nivosus leaf against ulcerative colitis. Heliyon 2020, 6, e03893. [Google Scholar] [CrossRef]
- Akhbari, M.; Yaghoobei, M.; Hamedi, S. Composition of the oily compounds, phytochemical screening and biological activity of different aerial parts of Smirnovia turkestana Bunge. Nat. Prod. Res. 2018, 32, 2697–2700. [Google Scholar] [CrossRef]
- Labbozzetta, M.; Poma, P.; Tutone, M.; McCubrey, J.A.; Sajeva, M.; Notarbartolo, M. Phytol and Heptacosane are Possible Tools to Overcome Multidrug Resistance in an In Vitro Model of Acute Myeloid Leukemia. Pharmaceuticals 2022, 15, 356. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Dagur, H.S.; Khan, M.; Malik, N.; Alam, M.; Mushtaque, M. Therapeutic role of flavonoids and flavones in cancer prevention: Current trends and future perspectives. Eur. J. Med. Chem. Rep. 2021, 3, 100010. [Google Scholar] [CrossRef]
- Pandi, M.; SenthilKumaran, R.; Rajapriya, P.; Yogeswari, S.; Muthumary, J. Taxol, A potential drug for the treatment of cancer. Biores Bull 2013, 2, 1–9. [Google Scholar]
- Uzma, F.; Mohan, C.D.; Hashem, A.; Konappa, N.M.; Rangappa, S.; Kamath, P.V.; Singh, B.P.; Mudili, V.; Gupta, V.K.; Siddaiah, C.N. Endophytic fungi—Alternative sources of cytotoxic compounds: A review. Front. Pharmacol. 2018, 9, 309. [Google Scholar] [CrossRef]
- Monowar, T.; Rahman, M.S.; Bhore, S.J.; Raju, G.; Sathasivam, K.V. Silver nanoparticles synthesized by using the endophytic bacterium Pantoea ananatis are promising antimicrobial agents against multidrug resistant bacteria. Molecules 2018, 23, 3220. [Google Scholar] [CrossRef] [PubMed]
- Mai, P.-Y.; Levasseur, M.; Buisson, D.; Touboul, D.; Eparvier, V. Identification of antimicrobial compounds from Sandwithia guyanensis-associated endophyte using molecular network approach. Plants 2019, 9, 47. [Google Scholar] [CrossRef]
- Rojas-Solís, D.; Zetter-Salmón, E.; Contreras-Pérez, M.; Rocha-Granados, M.d.C.; Macías-Rodríguez, L.; Santoyo, G. Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR71 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal. Agric. Biotechnol. 2018, 13, 46–52. [Google Scholar] [CrossRef]
- Legrifi, I.; Al Figuigui, J.; El Hamss, H.; Lazraq, A.; Belabess, Z.; Tahiri, A.; Amiri, S.; Barka, E.A.; Lahlali, R. Potential for Biological Control of Pythium schmitthenneri Root Rot Disease of Olive Trees (Olea europaea L.) by Antagonistic Bacteria. Microorganisms 2022, 10, 1635. [Google Scholar] [CrossRef]
- Zheng, C.J.; Yoo, J.-S.; Lee, T.-G.; Cho, H.-Y.; Kim, Y.-H.; Kim, W.-G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.T.; Ghaly, M.F.; Fahmi, S.M. Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. J. Basic Microbiol. 2021, 61, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Cai, Y.-Z.; Xing, J.; Corke, H.; Sun, M. A potential antioxidant resource: Endophytic fungi from medicinal plants. Econ. Bot. 2007, 61, 14–30. [Google Scholar] [CrossRef]
- Seifried, H.E.; Anderson, D.E.; Fisher, E.I.; Milner, J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007, 18, 567–579. [Google Scholar] [CrossRef]
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef]


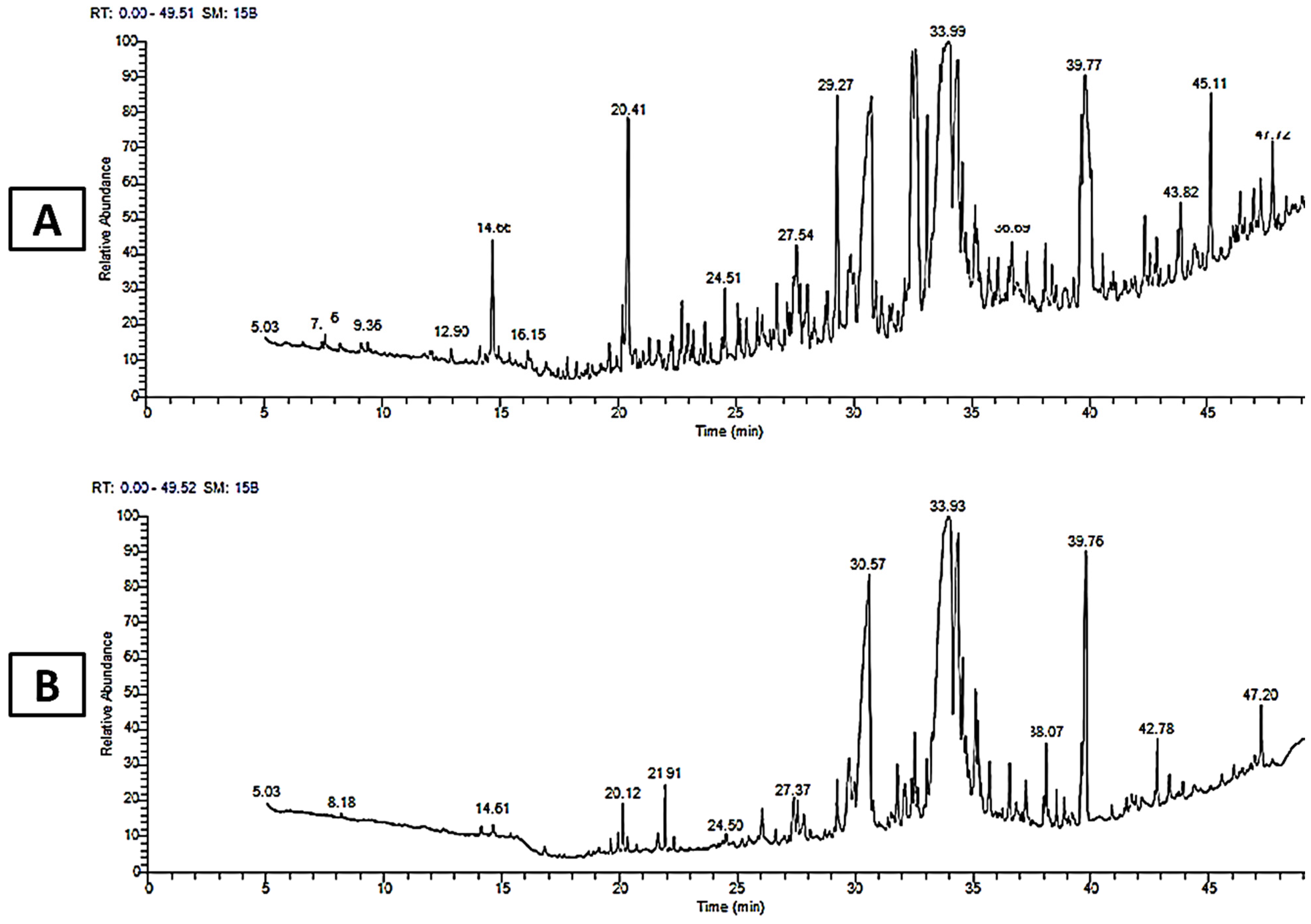
| No. | Compound Name | RT (min) | Peak Area % | Activity | Ref | |
|---|---|---|---|---|---|---|
| Bacterial Strain | ||||||
| A. faecalis | S. maltophilia | |||||
| 1 | Indolizine | 14.67 | 1.64 | - | Antimicrobial and antimutagenic | [77] |
| 2 | Caryophyllene | 17.82 | 0.24 | - | Anticancer, antioxidant, antimicrobial properties | [78] |
| 3 | Docosane | 19.61 | 0.38 | - | Antimicrobial activity | [79] |
| 4 | Dotriacontane | 20.72 | 0.17 | - | Cytotoxic effects against hepatocarcinoma, antioxidant activity, antibacterial and antiviral | [80] |
| 5 | Dodecanoic acid | 21.70 | 0.32 | - | Antimicrobial | [81] |
| 6 | Carotol | 22.27 | 0.40 | - | Antifungal | [82] |
| 7 | Apiol | 23.89 | 0.24 | - | Cancer, chemotherapy antimicrobial | [83] |
| 8 | Tetradecanoic acid | 26.03 | - | 0.73 | Antibacterial activity | [84] |
| 9 | Pentadecanoic acid | 27.37 | - | 1.10 | Antibacterial | [85] |
| 10 | Heptatriacotanol | 27.93 | 0.44 | - | Antimicrobial | [86] |
| 11 | Hexadecanoic acid | 29.28 | 8.06 | 5.35 | Antioxidant, antibacterial, anti-inflammatory, antimicrobial. | [9] |
| 12 | Palmitoleic acid | 29.84 | 1.22 | - | Antibacterial properties | [87] |
| 13 | Oleic Acid | 29.98 | 0.89 | 29.44 | Antibacterial activity and antifungal activity. | [76,88] |
| 14 | cis-11-Eicosenoic acid | 31.13 | 0.49 | 0.98 | Antioxidant, antimicrobial and anticancer | [89] |
| 15 | Hepatadecanoic acid | 31.48 | 0.31 | - | Antimicrobial and antifungal | [90] |
| 16 | Octadecenoic acid, methyl ester | 32.66 | 3.24 | - | Antimicrobial, antioxidant and anticancer | [91] |
| 17 | Methyl stearate | 33.08 | 2.33 | - | Antibacterial, antioxidant and antifungal | [92] |
| 18 | Linoleic acid ethyl ester | 33.62 | 3.38 | 0.95 | Antifungal | [92] |
| 19 | Octadecenoic acid | 34.01 | 17.49 | 9.57 | Antimicrobial, antioxidant and anticancer | [91] |
| 20 | Cis-2-phenyl-1, 3-dioxolane-4-methyl | 34.52 | - | 1.87 | Antimalarial | [93] |
| 21 | Ergotamine | 36.70 | 0.78 | 1.34 | Pharmacological activity as vasoconstriction, adrenergic blockade. | [94] |
| 22 | Stearic anhydride | 38.51 | - | 0.73 | Antibacterial | [95] |
| 23 | Ethyl Iso-allocholate | 41.70 | - | 0.35 | Antimicrobial | [96] |
| 24 | Nonacosane | 45.12 | 2.24 | - | Nematicides | [97] |
| 25 | Isochiapin-B | 46.05 | 0.35 | - | Antimicrobial and antioxidant | [98] |
| 26 | Digitoxin | 46.35 | 0.65 | - | Cardiac drugs, antileishmanial, anticytomegalovirus | [99,100,101] |
| 27 | Methyl commate | 46.91 | 0.58 | - | Antioxidant and antimutagenic | [102] |
| 28 | Cholest-22-ene-21-ol, 3,5-dehydro-6-methoxy-, pivalate | 47.23 | 0.77 | - | Anti-inflammatory | [103] |
| 29 | Heptacosane | 47.73 | 1.13 | - | Antimicrobial, anti-multidrug resistance | [104,105,106] |
| Test Microorganism | EA | EA Extract of S. maltophilia | EA Extract of A. faecalis | AMC/FLU | |||
|---|---|---|---|---|---|---|---|
| IZ */mm | MIC90 | IZ/mm | MIC90 | IZ/mm | MIC90 | ||
| E. coli | 0.0 | 11.8 ± 0.35 cd | 500 | 0.0 ± 0.00 e | N D | 9.65 ± 0.65 c | 1000 |
| P. aeruginosa | 0.0 | 14.9 ± 0.90 b | 250 | 0.0 ± 0.00 e | N D | 10.5 ± 0.3 bc | 1000 |
| E. faecalis | 0.0 | 15.0 ± 1.00 b | 250 | 12.9 ± 1.10 bc | 500 | 9.2 ± 0.5 c | 1000 |
| S. aureus | 0.0 | 10.3 ± 0.58 d | 1000 | 11.1 ± 1.10 cd | 500 | 10.45 ± 0.55 bc | 1000 |
| B. subtilis | 0.0 | 18.2 ± 0.76 a | 125 | 15.0 ± 1.00 ab | 250 | 12.7 ± 0.7 a | 500 |
| C. albicans | 0.0 | 14.1 ± 1.21 bc | 250 | 17.3 ± 1.32 a | 125 | 11.75 ± 0.75 b | 500 |
| C. neoformans | 0.0 | 11.9 ± 0.90 cd | 500 | 10.3 ± 0.58 d | 1000 | 9.7 ± 0.3 c | 1000 |
| Conc (µg/mL) | Antioxidant Activity % | IC50 (µg/mL) | ||||
|---|---|---|---|---|---|---|
| AA | S. maltophilia | A. faecalis | AA | S. maltophilia | A. faecalis | |
| 1000 | 99.27 ± 0.46 a | 88.67 ± 1.53 a | 81.17 ± 1.26 a | 6.32 | 146.2 | 247.6 |
| 500 | 98.67 ± 0.58 a | 80.67 ± 1.15 b | 73.33 ± 1.15 b | |||
| 250 | 95.00 ± 1.00 b | 61.03 ± 1.05 c | 50.90 ± 0.85 c | |||
| 125 | 89.93 ± 0.90 c | 47.83 ± 1.26 d | 40.10 ± 1.15 d | |||
| 62.5 | 80.33 ± 0.76 d | 30.67 ± 1.15 e | 20.07 ± 0.90 e | |||
| 31.25 | 73.47 ± 1.29 e | 20.47 ± 1.75 f | 11.67 ± 0.58 f | |||
| 15.62 | 64.27 ± 0.64 f | 6.33 ± 0.58 g | 4.33 ± 0.58 g | |||
| 7.81 | 52.33 ± 1.53 g | 0.00 h | 0.00 h | |||
| 3.9 | 41.27 ± 1.10 h | 0.00 h | 0.00 h | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashem, A.H.; Al-Askar, A.A.; Abd Elgawad, H.; Abdelaziz, A.M. Bacterial Endophytes from Moringa oleifera Leaves as a Promising Source for Bioactive Compounds. Separations 2023, 10, 395. https://doi.org/10.3390/separations10070395
Hashem AH, Al-Askar AA, Abd Elgawad H, Abdelaziz AM. Bacterial Endophytes from Moringa oleifera Leaves as a Promising Source for Bioactive Compounds. Separations. 2023; 10(7):395. https://doi.org/10.3390/separations10070395
Chicago/Turabian StyleHashem, Amr H., Abdulaziz A. Al-Askar, Hamada Abd Elgawad, and Amer M. Abdelaziz. 2023. "Bacterial Endophytes from Moringa oleifera Leaves as a Promising Source for Bioactive Compounds" Separations 10, no. 7: 395. https://doi.org/10.3390/separations10070395
APA StyleHashem, A. H., Al-Askar, A. A., Abd Elgawad, H., & Abdelaziz, A. M. (2023). Bacterial Endophytes from Moringa oleifera Leaves as a Promising Source for Bioactive Compounds. Separations, 10(7), 395. https://doi.org/10.3390/separations10070395