Enhanced Oil Spill Remediation Using Environmentally Asymmetric Dicationic Ionic Liquids: Synthesis, Characterization, and Evaluation
Abstract
1. Introduction
2. Methodology
2.1. Materials and Characterizations
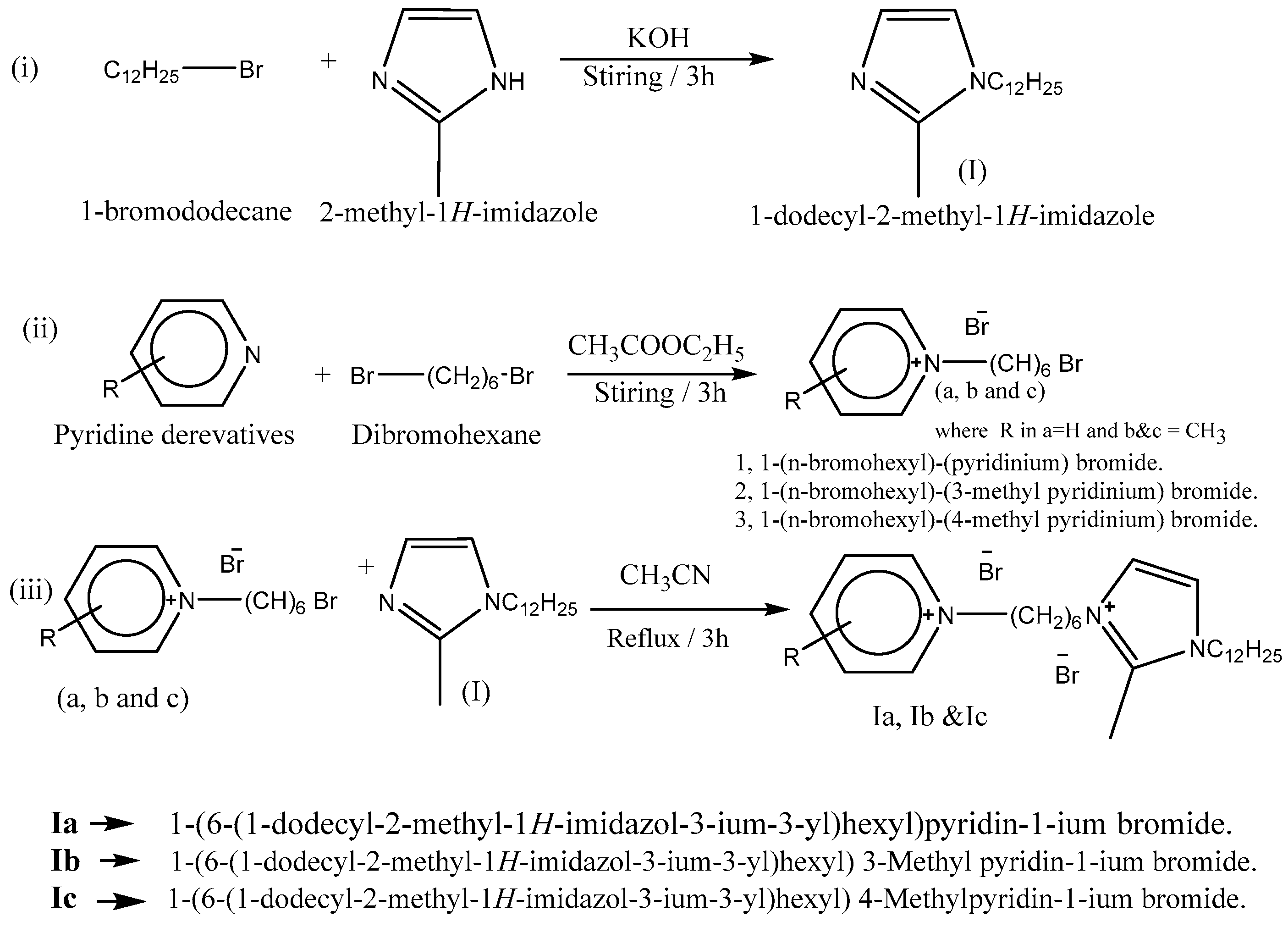
2.2. Synthesis of Amphiphilic Assemetric Dicationic Ionic Liquids
- Compound I was previously prepared in our previous work [20,21] by stirring 0.1 mL of 2-methyl imidazole with potassium hydroxide dissolved in acetonitrile. Following the addition of 0.1 of bromododecane, which was completely miscible, drop by drop, the stirring stopped when the white precipitate was noticed (after about 3 h). By filtration, the white precipitate of KBr was eliminated, and the filtrate was vaporized in an oven under vacuum.
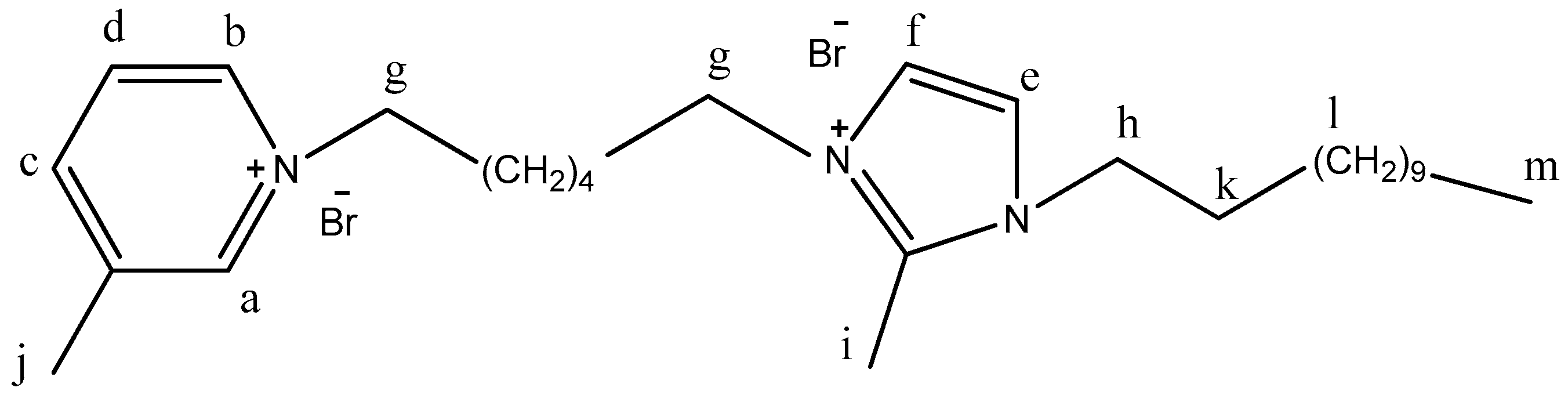
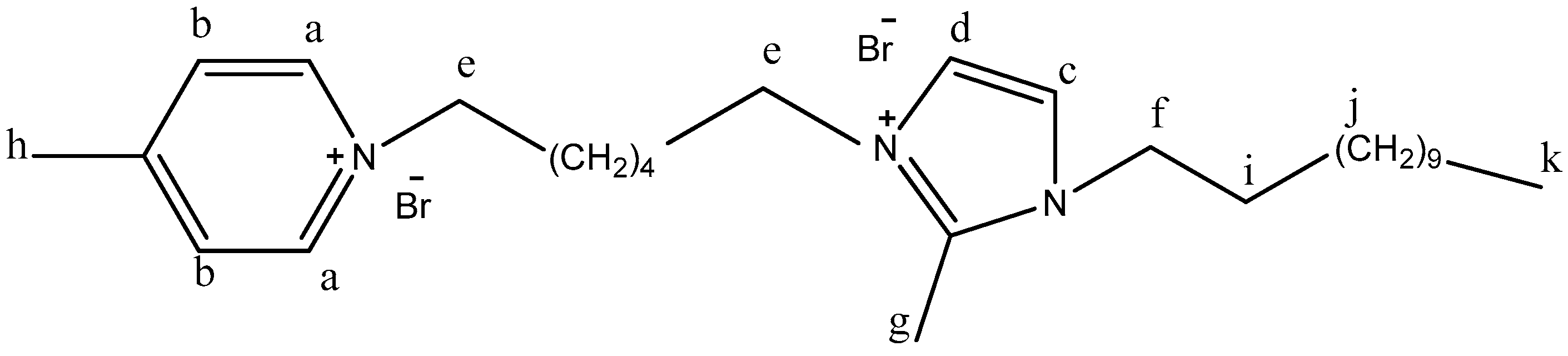
- a, b, and c were prepared by mixing pyridine, 3-methylpyridine, or 4-methyl pyridine (0.1 mol) with 0.1 mol of 1,6-dibromohexane for 3 h at ambient temperature. [22]. Ia, Ib, and Ic (dicationic ILs) were synthesized by the compounding of compound I (1:1) with a, b, and c under refluxing for 3 h. The products were collected and dried via evaporation under vacuum (89.2, 87.5, and 89.4% yield). The completion of the reaction was monitored and confirmed by the TLC technique in Scheme 1.
2.3. Quantum Chemical Parameters
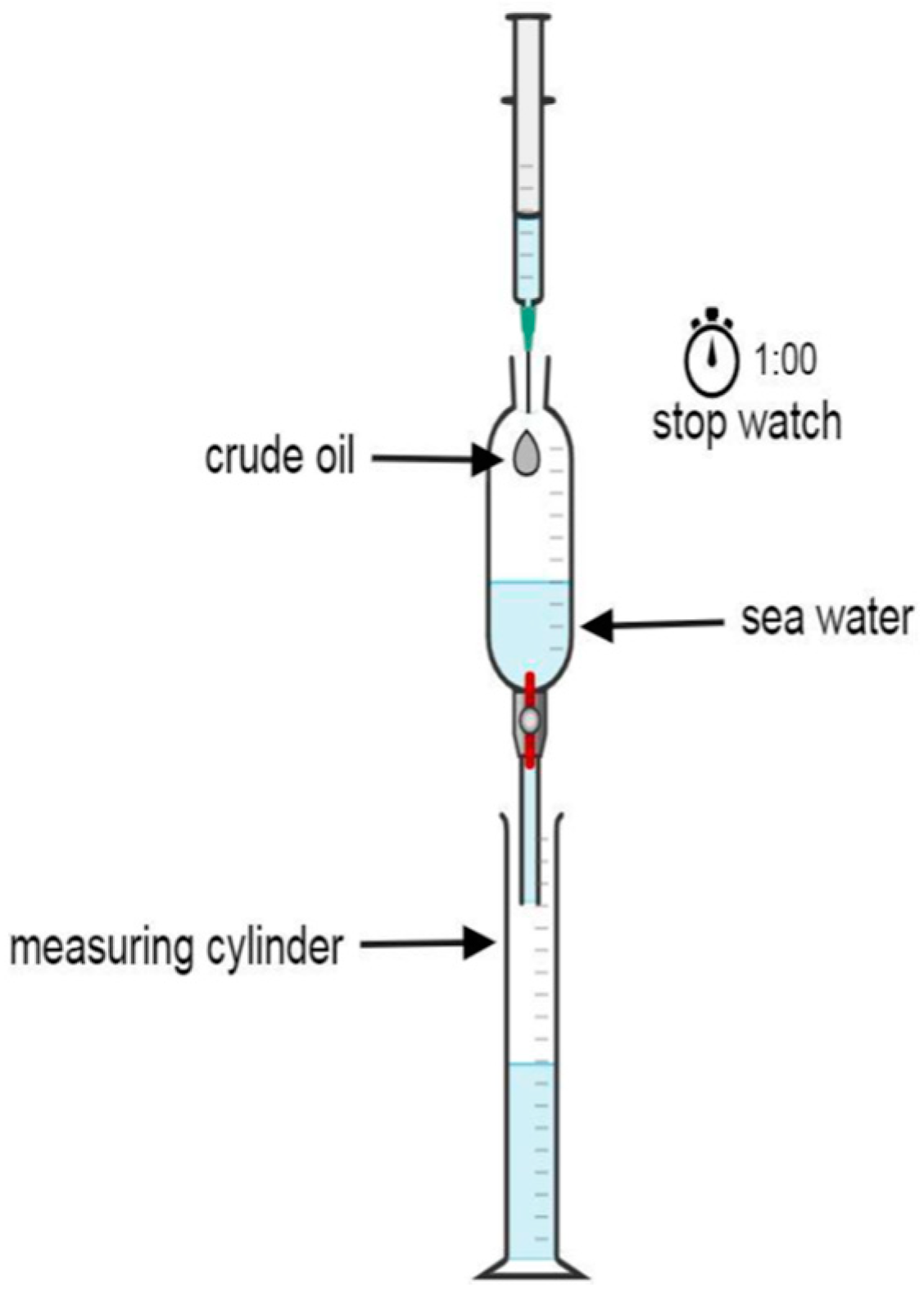
2.4. Evaluation ot the Prepared ILs for Oil Spill Remidiation
Efficiency Test
2.5. Acute Toxicity Test
3. Results and Discussions
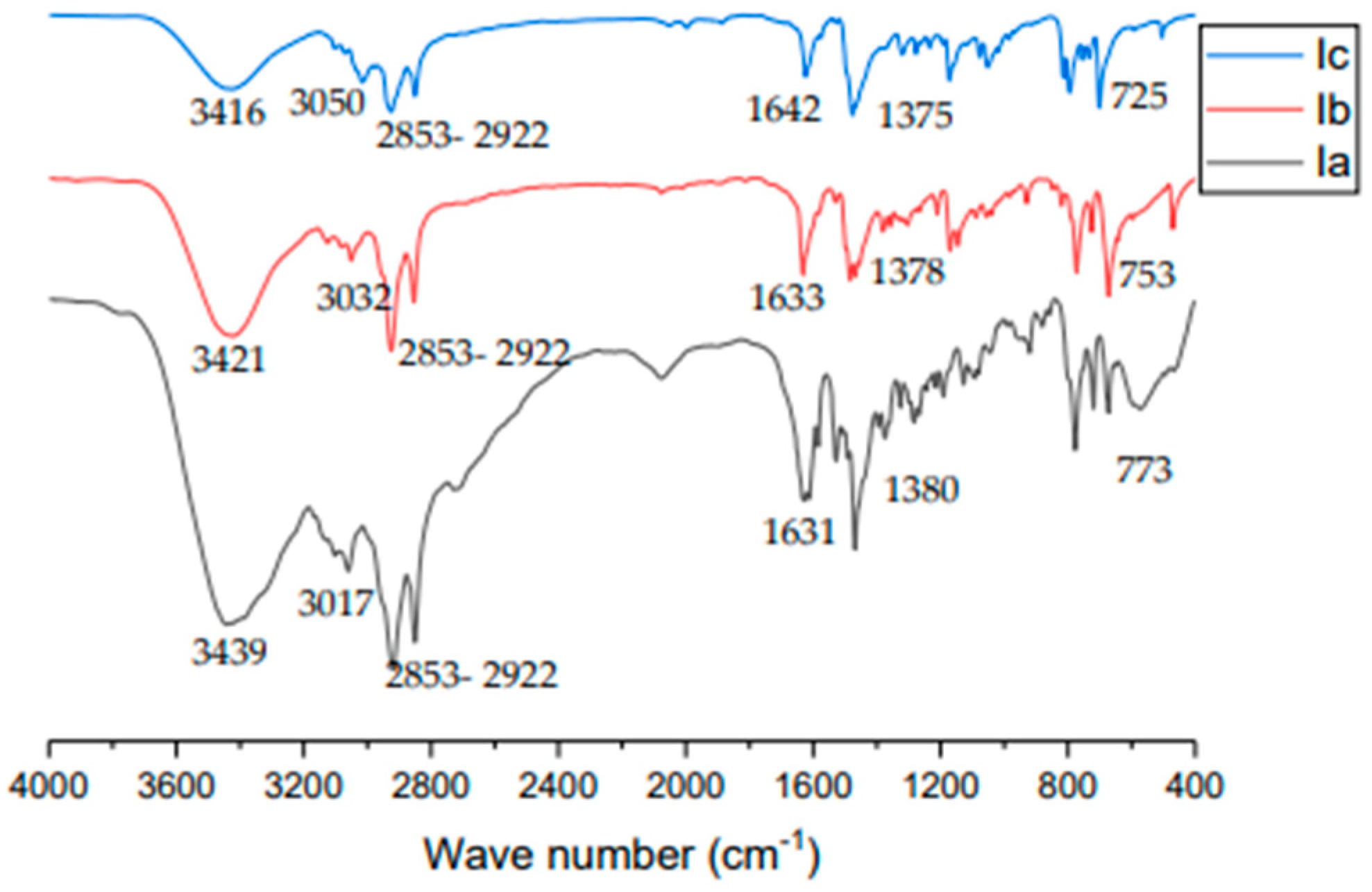
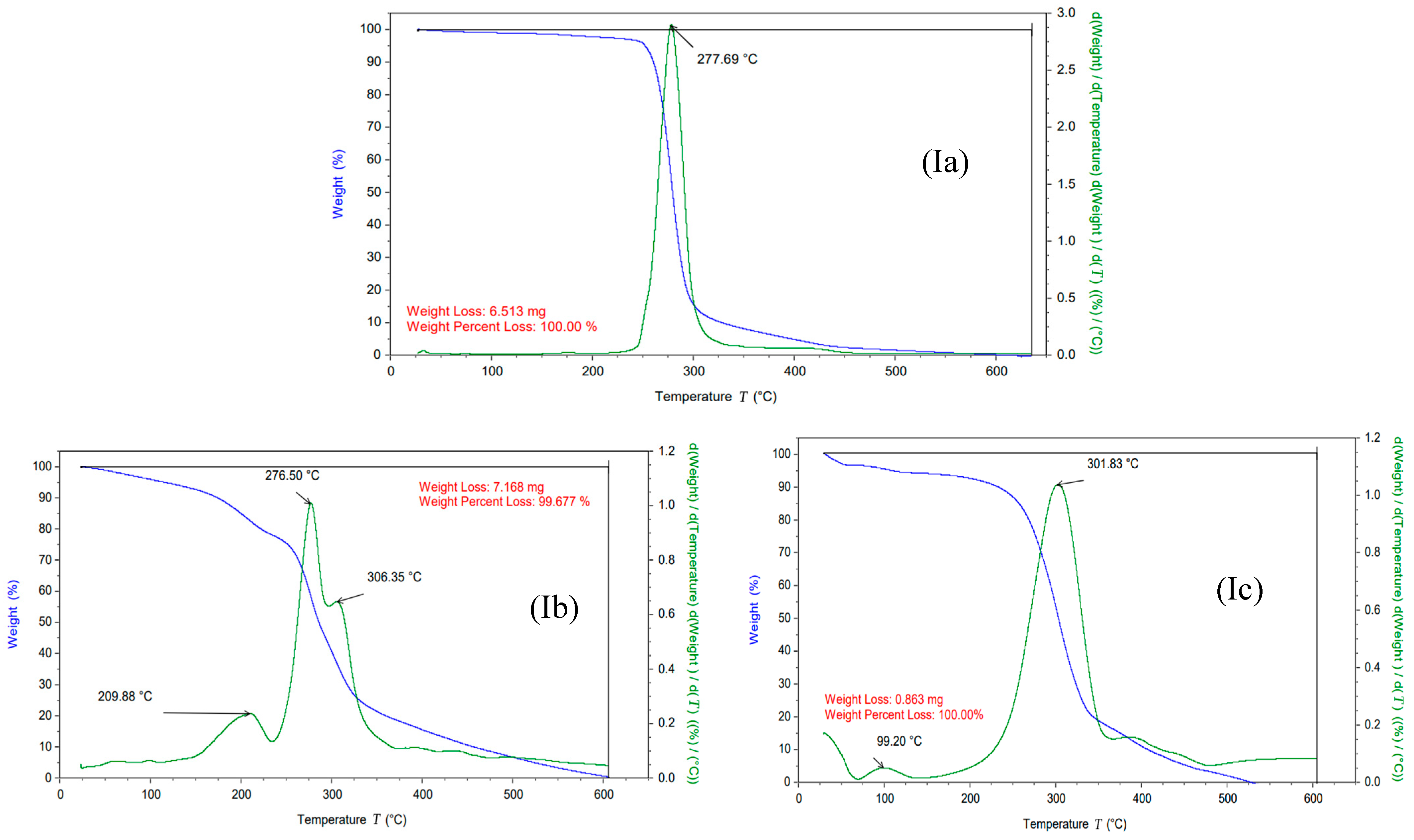
3.1. Characterization of the Synthesized ILs
3.2. Surface and Physical Characteristics of ILs
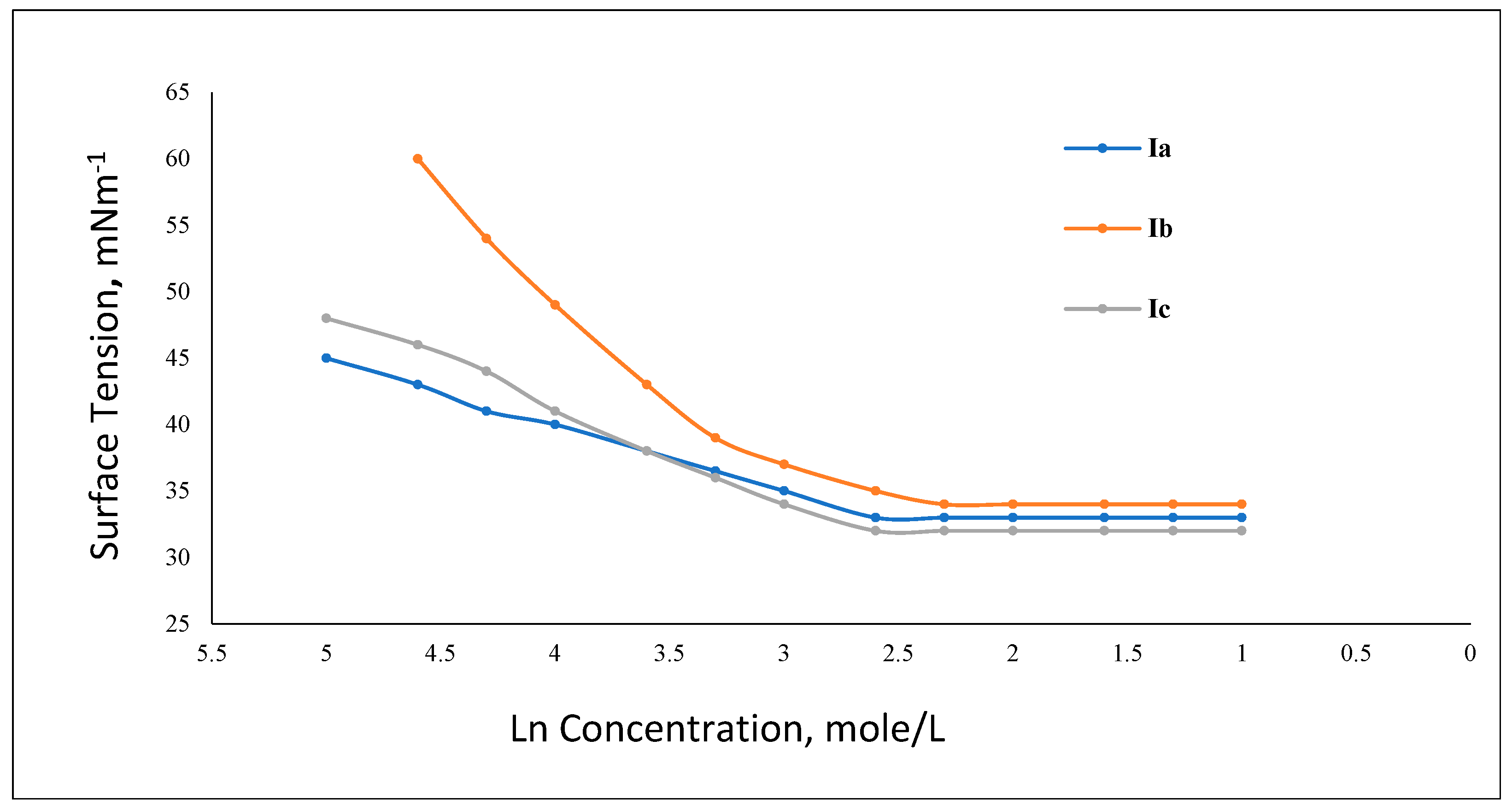
3.2.1. Surface Tension and Critical Micelle Concentration (CMC)
3.2.2. Thermodynamics of Micellization and Adsorption
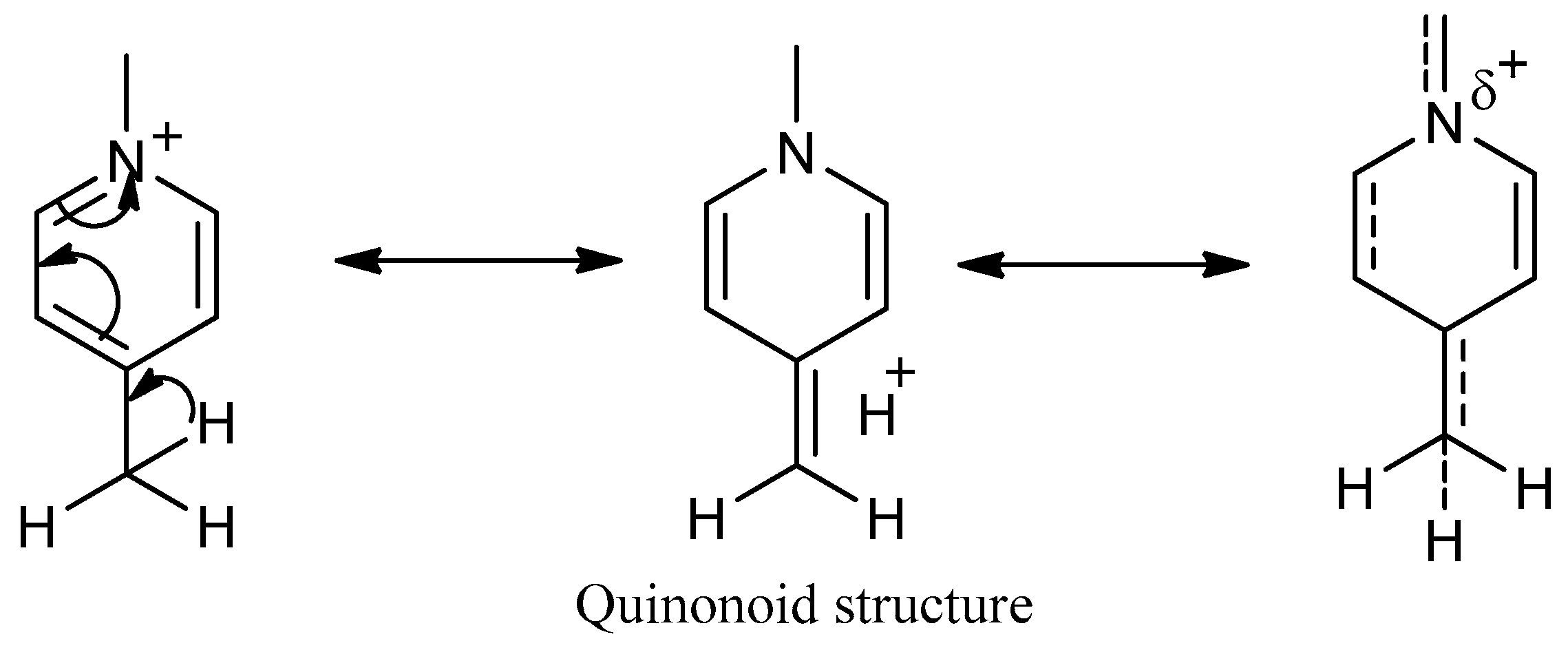
3.3. Quantum Chemical Calculations
3.4. Evaluation of the Synthesized ILs as Oil Dispersants:
3.4.1. Effect of Temperatures
3.4.2. Effect of DOR
3.4.3. Effect of Cation Structure
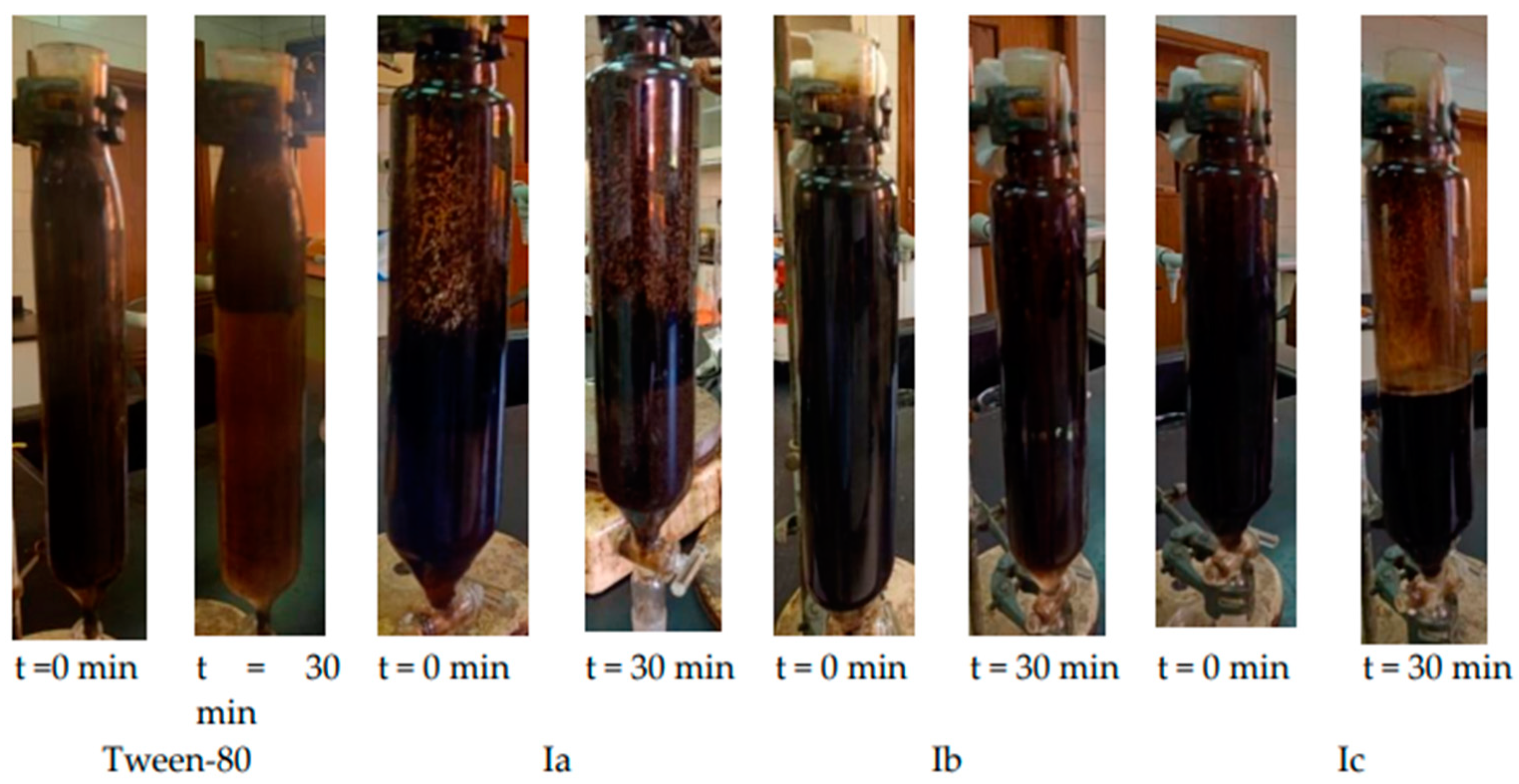
3.5. Stability of Crude Oil & Water Emulsions
3.6. Group Composition of Undispersed Crude Oil
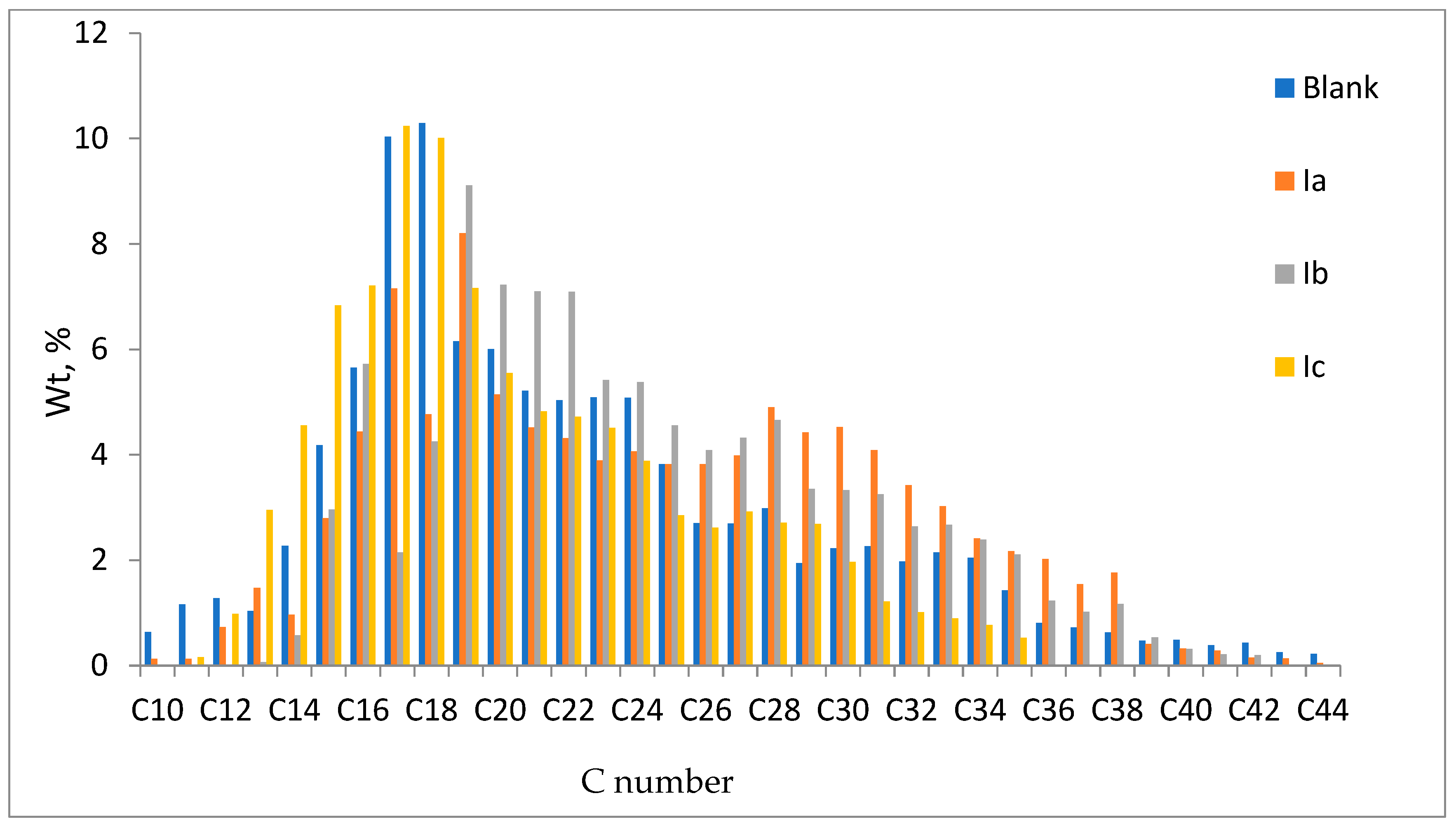
3.6.1. Gas Chromatography Analysis of Saturates Fraction
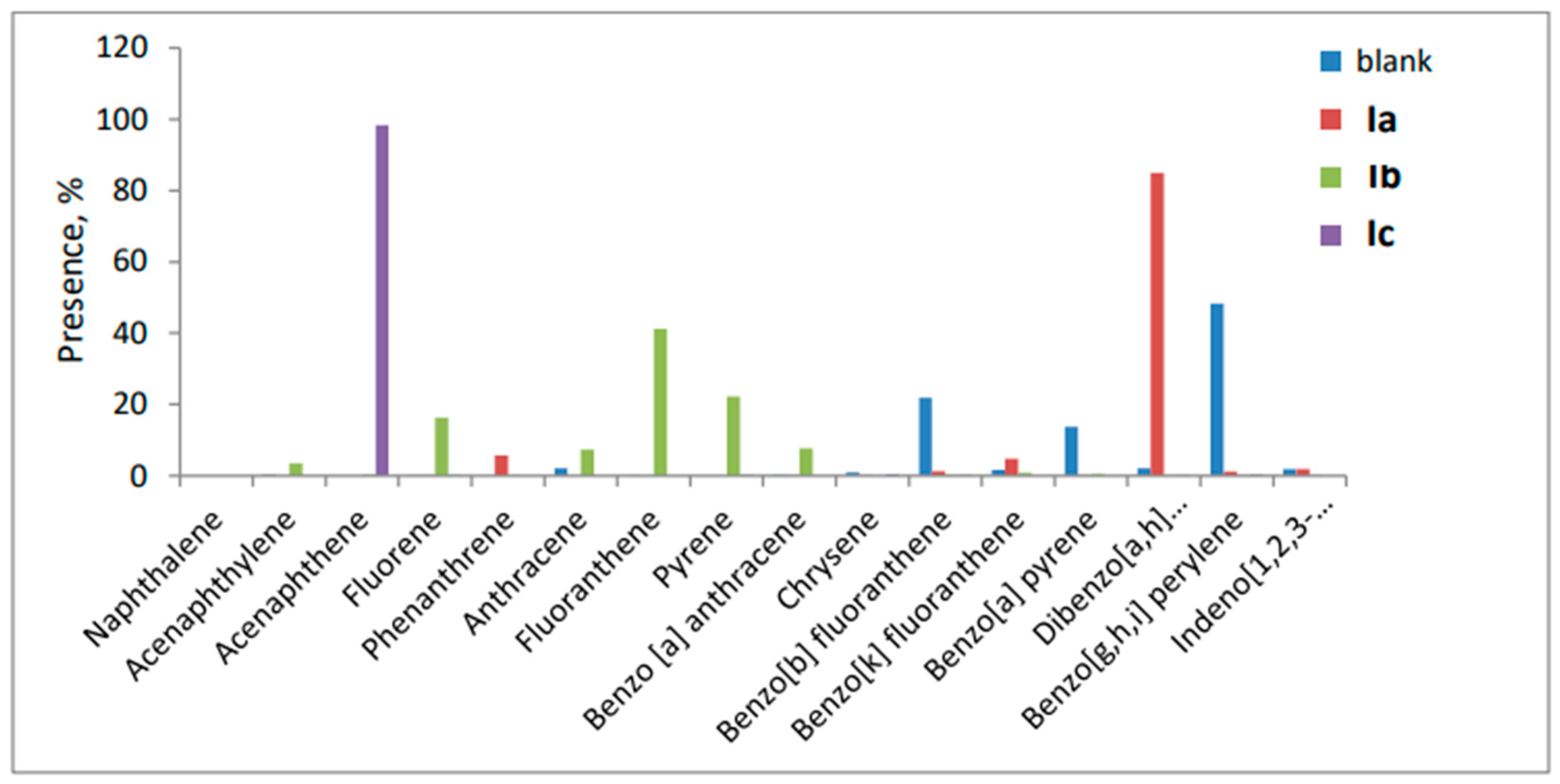
3.6.2. HPLC for Polyaromatic Hydrocarbons (PAHs)
3.7. Acute Toxicity of the Prepared Dispersants
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, P.; Cai, Q.; Lin, W.; Chen, B.; Zhang, B. Offshore oil spill response practices and emerging challenges. Mar. Pollut. Bull. 2016, 110, 6–27. [Google Scholar] [CrossRef]
- Burrows, P.; Rowley, C.K.; Owen, D. Torrey 711 canyon: A case study in accidental pollution. Scott. J. Political Econ. 1974, 21, 237–258. [Google Scholar] [CrossRef]
- Brakstad, O.G.; Lewis, A.; Beegle-Krause, C.J. A critical review of marine snow in the context of oil spills and oil spill dispersant treatment with focus on the Deepwater Horizon oil spill. Mar. Pollut. Bull. 2018, 135, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Nissanka, I.D.; Yapa, P.D. Calculation of oil droplet size distribution in ocean oil spills: A review. Mar. Pollut. Bull. 2018, 135, 723–734. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Li, S.; Zhang, F.; Zhu, Y.; Huang, X. Identifying critical factors of oil spill in the tanker shipping industry worldwide. J. Clean. Prod. 2017, 180, 1–10. [Google Scholar] [CrossRef]
- Johannsdottir, L.; Cook, D. Systemic risk of maritime-related oil spills viewed from an Arctic and insurance perspective. Ocean Coast. Manag. 2019, 179, 104853. [Google Scholar] [CrossRef]
- Cirer-costa, J.C. Tourism and its hypersensitivity to oil spills. Mar. Pollut. Bull. 2015, 91, 65–72. [Google Scholar] [CrossRef]
- Nelson, J.R.; Grubesic, T.H.; Sim, L.; Rose, K. A geospatial evaluation of oil spill impact potential on coastal tourism in the Gulf of Mexico. Comput. Environ. Urban Syst. 2018, 68, 26–36. [Google Scholar] [CrossRef]
- Hoof, L.V.; Den Burg, S.W.; Banach, J.L.; Rockmann, C.; Goossen, M. Can multi-use of the sea be safe? A framework for risk assessment of multi-use at sea. Ocean Coast. Manag. 2020, 184, 105030. [Google Scholar] [CrossRef]
- Shah, M.H.; Reddy, A.V.B.; Yusup, S.; Goto, M.; Moniruzzaman, M. Ionic liquid-biosurfactant blends as effective dispersants for oil spills: Effect of carbon chain length and degree of saturation. Environ. Pollut. 2021, 284, 117–119. [Google Scholar]
- Shah, M.H.; Reddy, A.V.B.; Moniruzzaman, M. Ionic liquid–based surfactants for oil spill remediation. In Ionic Liquid-Based Technologies for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780128245453. [Google Scholar]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques–classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, X.; O’Reilly, S.E.; Qian, T.; Zhao, D. Effects of oil dispersant and oil on sorption and desorption of phenanthrene with Gulf Coast marine sediments. Environ. Pollut. 2014, 185, 240–249. [Google Scholar] [CrossRef]
- Nabipour, M.; Ayatollahi, S.; Keshavarz, P. Application of different novel and newly designed commercial ionic liquids and surfactants for more oil recovery from an Iranian oil field. J. Mol. Liq. 2017, 230, 579–588. [Google Scholar] [CrossRef]
- Santos, J.M., Jr.; Wisniewski, A.; Eberlin, M.N.; Schrader, W. Comparing crude oils with different API Gravities on a molecular level using mass spectrometric analysis—Part 1: Whole Crude Oil. Energies 2018, 11, 2766. [Google Scholar] [CrossRef]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. Med. Chem. Commun. 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Lotfi, M.; Moniruzzamana, M.; Sivapragasam, M.; Kandasamy, S.; Mutalib, M.I.A.; Alitheen, N.B.; Gotode, M. Solubility of acyclovir in nontoxic and biodegradable ionic liquids: COSMO-RS prediction and experimental verification. J. Mol. Liq. 2017, 243, 124–131. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Ono, T. Separation and characterization of cellulose fibers from cypress wood treated with ionic liquid prior to laccase treatment. Bioresour. Technol. 2013, 127, 132–137. [Google Scholar] [CrossRef]
- Mustahil, N.A.; Baharuddin, S.H.; Abdullah, A.A.; Reddy, A.V.B.; Mutalib, M.I.A.; Moniruzzaman, M. Synthesis, characterization, ecotoxicity and biodegradability evaluations of novel biocompatible surface active lauroyl sarcosinate ionic liquids. Chemosphere 2019, 229, 349–357. [Google Scholar] [CrossRef] [PubMed]
- El-Nagar, R.A.; Ghanem, A.A.; Nessim, M.I. Capture of CO2 from Natural Gas Using Ionic Liquids. In Shale Gas-New Aspects and Technologies; IntechOpen: London, UK, 2018; ISBN 978-1-78923-618-7. [Google Scholar]
- El shafiee, C.E.; El Nagar, R.A.; Nessim, M.I.; Khalil, M.M.H.; Shaban, M.E.; Alharthy, R.D.; Aismail, D.; Abdallah, R.I.; Moustafa, Y.M. Application of asymmetric dicationic ionic liquids for oil spill remediation in sea water. Arab. J. Chem. 2021, 14, 103123. [Google Scholar] [CrossRef]
- El-Nagar, R.A.; Nessim, M.; El-Wahab, A.A.; Ibrahim, R.; Faramawy, S. Investigating the efficiency of newly prepared imidazolium ionic liquids for carbon dioxide removal from natural gas. J. Mol. Liquids 2017, 237, 484–489. [Google Scholar] [CrossRef]
- Dost, K.; Ideli, C. Determination of polycyclic aromatic hydrocarbons in edible oils and barbecued food by HPLC/UV–Vis detection. Food Chem. 2012, 133, 193–199. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, C.; Lu, C.; Li, W.; Li, B.; Wang, J.; Wang, J.; Du, Z.; Zhu, L. Comparison of the toxic effects of non-task-specific and task-specific ionic liquids on zebrafish. Chemosphere 2022, 294, 133643. [Google Scholar] [CrossRef]
- Brycki, B.; Metecka, I.; Kozirog, A.; Otlewska, A. Synthesis, structure and antimicrobial properties of novel benzalkonium chloride analogues with pyridine rings. Molecules 2017, 22, 130. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wheelhouse, R.T.; Honey, M.A.; Karodia, N. Synthesis and characterisation of novel nopyl-derived phosphonium ionic liquids. J. Mol. Liq. 2020, 316, 113857. [Google Scholar] [CrossRef]
- Muhammad, N.; Gao, Y.; Iqbal, F.; Ahmad, P.; Ge, R.; Nishan, U.; Rahim, A.; Gonfa, G.; Ullah, Z. Extraction of biocompatible hydroxyapatite from fish scales using novel approach of ionic liquid pretreatment. Sep. Purif. Technol. 2016, 161, 129–135. [Google Scholar] [CrossRef]
- Nakahara, H.; Nishino, A.; Tanaka, A.; Fujita, Y.; Shibata, O. Interfacial behavior of gemini surfactants with different spacer lengths in aqueous medium. Colloid Polym. Sci. 2019, 297, 183–189. [Google Scholar] [CrossRef]
- Baoli, S. The strengths of van der Waals and electrostatic forces in 1-alkyl-3-methylimidazolium ionic liquids obtained through Lifshitz theory and Coulomb formula. J. Mol. Liq. 2020, 320, 114412. [Google Scholar]
- Ahmed, S.M.; Khidr, T.T.; Ismail, D.A. Effect of gemini surfactant additives on pour point depressant of crude oil. J. Dispers. Sci. Technol. 2018, 39, 1160–1164. [Google Scholar] [CrossRef]
- Ghanem, A.; Alharthy, R.D.; Desouky, S.M.; El-Nagar, R.A. Synthesis and Characterization ofImidazolium-Based Ionic Liquidsand Evaluating Their Performance asAsphaltene Dispersants. Materials 2022, 15, 1600. [Google Scholar] [CrossRef]
- Baharuddin, S.H.; Mustahil, N.A.; Reddy, A.V.B.; Abdullah, A.A.; Mutalib, M.I.A.; Moniruzzaman, M. Development, formulation and optimization of a novel biocompatible ionic liquids dispersant for the effective oil spill remediation. Chemosphere 2020, 249, 126125. [Google Scholar] [CrossRef]
- Jin, J.; Wang, H.; Jing, Y.; Liu, M.; Wang, D.; Li, Y.; Bao, M. An Efficient and environmental-friendly dispersant based on the synergy of amphiphilic surfactants for oil spill remediation. Chemosphere 2018, 215, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, M.; Shah, U.H.; Yahya, W.Z.N.; Goto, M.; Moniruzzaman, M. Surface active ionic liquid and Tween-80 blend as an effective dispersant for crude oil spill remediation. Environ. Technol. Innov. 2021, 24, 101868. [Google Scholar]
- Ashoori, S.; Sharifi, M.; Masoumi, M.; Salehi, M.M. The relationship between SARA fractions and crude oil stability. Egypt. J. Pet. 2017, 26, 209–213. [Google Scholar] [CrossRef]
- Driskell, W.B.; Payne, J.R. Macondo oil in northern Gulf of Mexico waters—Part 2: Dispersant-accelerated PAH dissolution in theDeepwater Horizon plume. Mar. Pollut. Bulletin 2018, 129, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Pie, H.V.; Mitchelmore, C.L. Acute toxicity of current and alternative oil spill chemical dispersants to early life stage blue crabs (Callinectes sapidus). Chemosphere 2015, 128, 14–20. [Google Scholar] [CrossRef]
| Experiment | Method | Result |
|---|---|---|
| Density@ 15.56 °C | ASTM D-4052 | 0.9558 |
| Specific gravity | 0.9568 | |
| API | 16.3 | |
| Kinematic Viscosity @40 °C, cSt | ASTM D-445 | 1820.35 |
| Wax content, wt.% | UOP-64 | 1.37 |
| Water content, vol.% | ASTM D-4006 | 7.5 |
| Pour point, °C | ASTM D-97 | 15 |
| Flash point, °C | ASTM D-93 | <−22 |
| Group composition (SARA Analysis) | ||
| Saturates | 18.18 | |
| Aromatics | 37.99 | |
| Resin | 30.24 | |
| Asphaltene | IP-143 | 13.59 |
| Total Dissolved Solids (T.D.S.) | 46,191.9 mg/L | Density @ 60 F | 1.03207 g/mL |
| Salinity (as NaCl) | 45,568.1 mg/L | Specific gravity | 1.03310 |
| Alkalinity (as CaCO3) | 150.1 mg/L | pH @ 25 °C | 7.8 |
| Total Hardness (as CaCO3) | 8760.1 mg/L | Conductivity | 6.46 × 10−2 mhos/cm @17.8 °C |
| Resistivity | 0.1548Ohm-m @17.8 °C |
| Cation | mg/L | meq/L | Anion | mg/L | meq/L |
|---|---|---|---|---|---|
| Lithium | 4.21 | 0.607 | Fluoride | 0.50 | 0.026 |
| Sodium | 14,132.00 | 614.459 | Chloride | 27,617.00 | 777.971 |
| Ammonium | <0.0005 | <0.00001 | Bromide | 40.00 | 0.501 |
| Potassium | 507.12 | 12.972 | Nitrate | <5 | <0.2 |
| Magnesium | 1654.30 | 136.133 | Nitrite | 111.00 | 2.408 |
| Calcium | 779.99 | 38.921 | Phosphate | <0.07 | <0.0022 |
| Strontium | 58.37 | 1.332 | Sulfate | 1032.00 | 21.497 |
| Barium | 72.37 | 1.054 | Hydroxide | >1.81 × 10−7 | >2 × 10−8 |
| Iron | >0.5 | >0.018 | Carbonate | >100 | >3.27 |
| Copper | >0.001 | >3.17 × 10−5 | Bicarbonate | 183.00 | 2.999 |
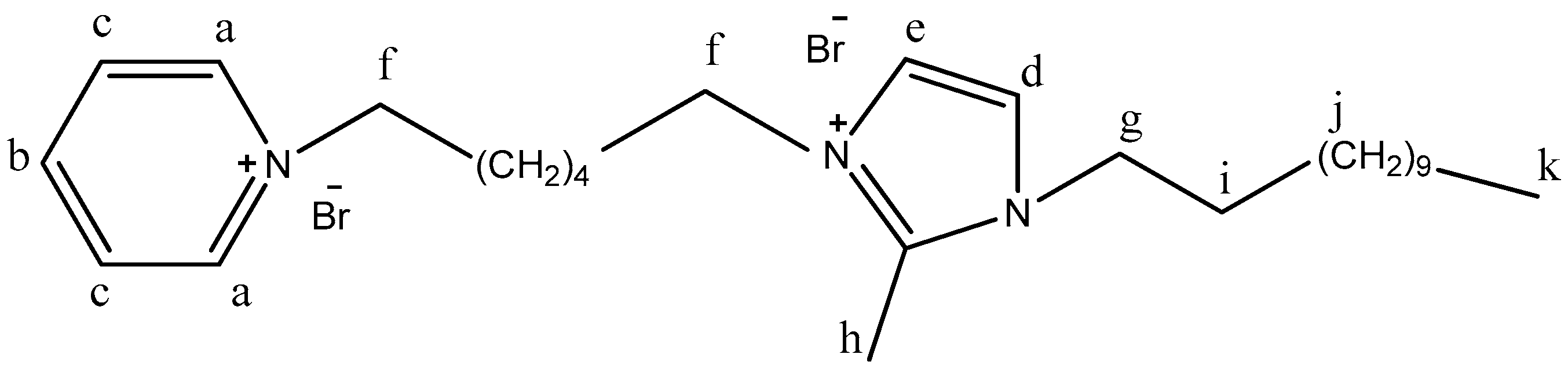
| Comp | A | B | C | d | e | F | g | h | i | J | K | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (d) | (t) | (t) | (d) | (d) | (t) | (t) | (s) | (m) | (s) | (t) | ||
| Ia | 9.22 | 8.63 | 8.10 | 7.78 | 7.24 | f1 | f2 | 3.98 | 3.63 | 2.54 | 1.19 | 0.84 |
| 4.32 | 3.98 | |||||||||||
| Comp | a | b | C | d | e | f | g | h | i | j | K | L | m | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (s) | (d) | (d) | (t) | (d) | (d) | (t) | (t) | (s) | (s) | (m) | (s) | (t) | ||
| Ib | 9.15 | 9.03 | 8.06 | 7.70 | 7.23 | 6.94 | g1 | g2 | 3.91 | 3.47 | 2.51 | 1.53 | 1.23 | 0.85 |
| 4.54 | 3.89 | |||||||||||||
| Comp | a | B | C | d | e | f | g | h | i | j | k | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (d) | (d) | (d) | (d) | (t) | (t) | (s) | (s) | (m) | (s) | (t) | ||
| Ic | 9.34 | 8.00 | 7.44 | 7.37 | e1 | e2 | 4.23 | 3.40 | 2.82 | 2.63 | 1.71 | 0.85 |
| 4.55 | 4.13 | |||||||||||
| Elements | ||||||||
|---|---|---|---|---|---|---|---|---|
| C% | H% | N% | Br % | |||||
| Comp. | Calc. | Obs. | Calc. | Obs. | Calc. | Obs. | Calc. | Obs. |
| Ia | 56.55 | 56.60 | 8.26 | 8.31 | 7.33 | 7.37 | 27.87 | 27.91 |
| Ib | 57.24 | 57.27 | 8.41 | 8.45 | 7.15 | 7.18 | 27.20 | 27.25 |
| Ic | 57.24 | 57.03 | 8.41 | 8.54 | 7.15 | 7.26 | 27.20 | 27.17 |
| ILs | CMC mol/L | γCMC mN/m | πCMC mN/m | Pc20 | Γmax × 1011 mol/cm2 | Amin nm2 | ΔGomic KJ/mol | ΔGoads KJ/mol |
|---|---|---|---|---|---|---|---|---|
| Ia | 2.5 × 10−3 | 33 | 39 | 5.5 × 10−6 | 2.921 | 56.840 | −14.847 | −28.199 |
| Ib | 1.6 × 10−3 | 32 | 40 | 2.5 × 10−6 | 4.286 | 38.738 | −15.953 | −23.886 |
| Ic | 1.0 × 10−3 | 34 | 38 | 6 × 10−5 | 8.050 | 20.633 | −17.118 | −21.840 |
| ILs | E HOMO (eV) | E LUMO (eV) | ΔE (eV) | Ionizatio-n Potential I (eV) | Electron Affinity A (eV) | Dipole Moment µ (Debye) | Electronegativi-ty (eV mol−1) | Hardness (η) (eV mol−1) | Softness eV−1 |
|---|---|---|---|---|---|---|---|---|---|
| Ia | −1.542 | −1.230 | 0.312 | 1.542 | 1.230 | 52.4894 | 1.386 | 0.156 | 6.4102564 |
| Ib | −1.395 | −0.930 | 0.465 | 1.395 | 0.930 | 49.0859 | 1.1625 | 0.2325 | 4.3010753 |
| Ic | −1.219 | −1.119 | 0.100 | 1.219 | 1.119 | 49.2601 | 1.169 | 0.05 | 20 |
| Comp. | Dispersion Efficiency % at 10 °C | |||
|---|---|---|---|---|
| (Dispersant: Crude Oil) wt.% | ||||
| (0.4:10) | (0.8:10) | (1.2:10) | (1.6:10) | |
| Ia | 2.49 | 5.32 | 4.02 | 3.88 |
| Ib | 16.88 | 20.45 | 18.54 | 17.82 |
| Ic | 13.47 | 33.61 | 23.61 | 11.57 |
| Comp. | Dispersion Efficiency % at 30 °C | |||
|---|---|---|---|---|
| (Dispersant: Crude Oil) wt.% | ||||
| (0.4:10) | (0.8:10) | (1.2:10) | (1.6:10) | |
| Ia | 6.40 | 12.28 | 9.48 | 7.91 |
| Ib | 20.65 | 52.55 | 28.88 | 27.75 |
| Ic | 21.90 | 66.80 | 61.08 | 54.32 |
| IL Dispersants | % of Dispersed Crude Oil | % of Undispersed Crude Oil |
|---|---|---|
| Ia | 12.08 | 87.92 |
| Ib | 53.17 | 46.83 |
| Ic | 67.01 | 32.99 |
| Chemical Composition, % | ||||
|---|---|---|---|---|
| Asphaltene | Maltene, wt.% | |||
| Oil, wt.% | ||||
| Resin | Saturate | Aromatic | ||
| Ia | 25.08 | 31.99 | 13.24 | 29.69 |
| Ib | 29.74 | 41.45 | 9.01 | 19.80 |
| Ic | 32.45 | 43.42 | 6.45 | 17.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharthy, R.D.; El Shafiee, C.E.; Nessim, M.I.; Abdallah, R.I.; Moustafa, Y.M.; Wafeek, M.; Ismail, D.A.; Khalil, M.M.H.; El-Nagar, R.A. Enhanced Oil Spill Remediation Using Environmentally Asymmetric Dicationic Ionic Liquids: Synthesis, Characterization, and Evaluation. Separations 2023, 10, 397. https://doi.org/10.3390/separations10070397
Alharthy RD, El Shafiee CE, Nessim MI, Abdallah RI, Moustafa YM, Wafeek M, Ismail DA, Khalil MMH, El-Nagar RA. Enhanced Oil Spill Remediation Using Environmentally Asymmetric Dicationic Ionic Liquids: Synthesis, Characterization, and Evaluation. Separations. 2023; 10(7):397. https://doi.org/10.3390/separations10070397
Chicago/Turabian StyleAlharthy, Rima D., C. E. El Shafiee, M. I. Nessim, R. I. Abdallah, Y. M. Moustafa, M. Wafeek, D. A. Ismail, M. M. H. Khalil, and R. A. El-Nagar. 2023. "Enhanced Oil Spill Remediation Using Environmentally Asymmetric Dicationic Ionic Liquids: Synthesis, Characterization, and Evaluation" Separations 10, no. 7: 397. https://doi.org/10.3390/separations10070397
APA StyleAlharthy, R. D., El Shafiee, C. E., Nessim, M. I., Abdallah, R. I., Moustafa, Y. M., Wafeek, M., Ismail, D. A., Khalil, M. M. H., & El-Nagar, R. A. (2023). Enhanced Oil Spill Remediation Using Environmentally Asymmetric Dicationic Ionic Liquids: Synthesis, Characterization, and Evaluation. Separations, 10(7), 397. https://doi.org/10.3390/separations10070397






