Anticorrosive Effects of Essential Oils Obtained from White Wormwood and Arâr Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction of Essential Oils
2.3. EO Chemical Analysis (GC-MS)
2.4. Anti-Corrosion Activity
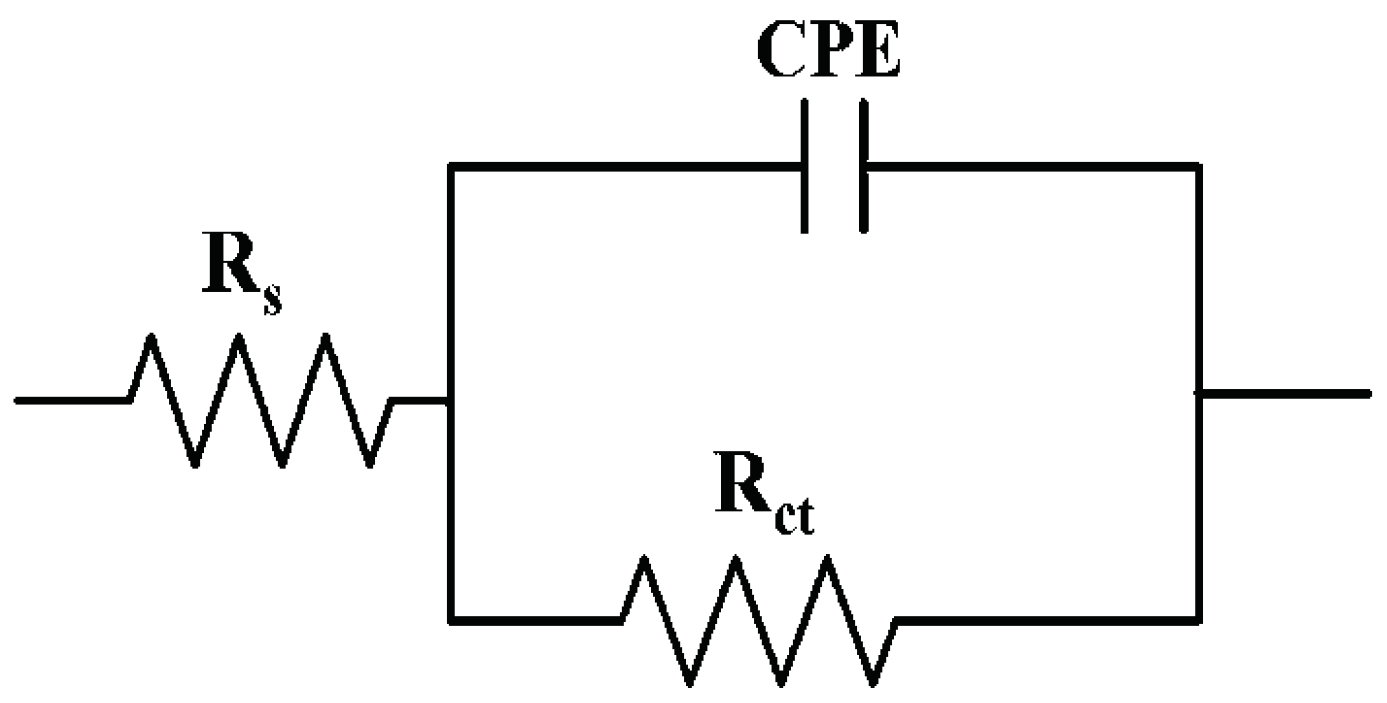
2.4.1. Electrochemical Analysis
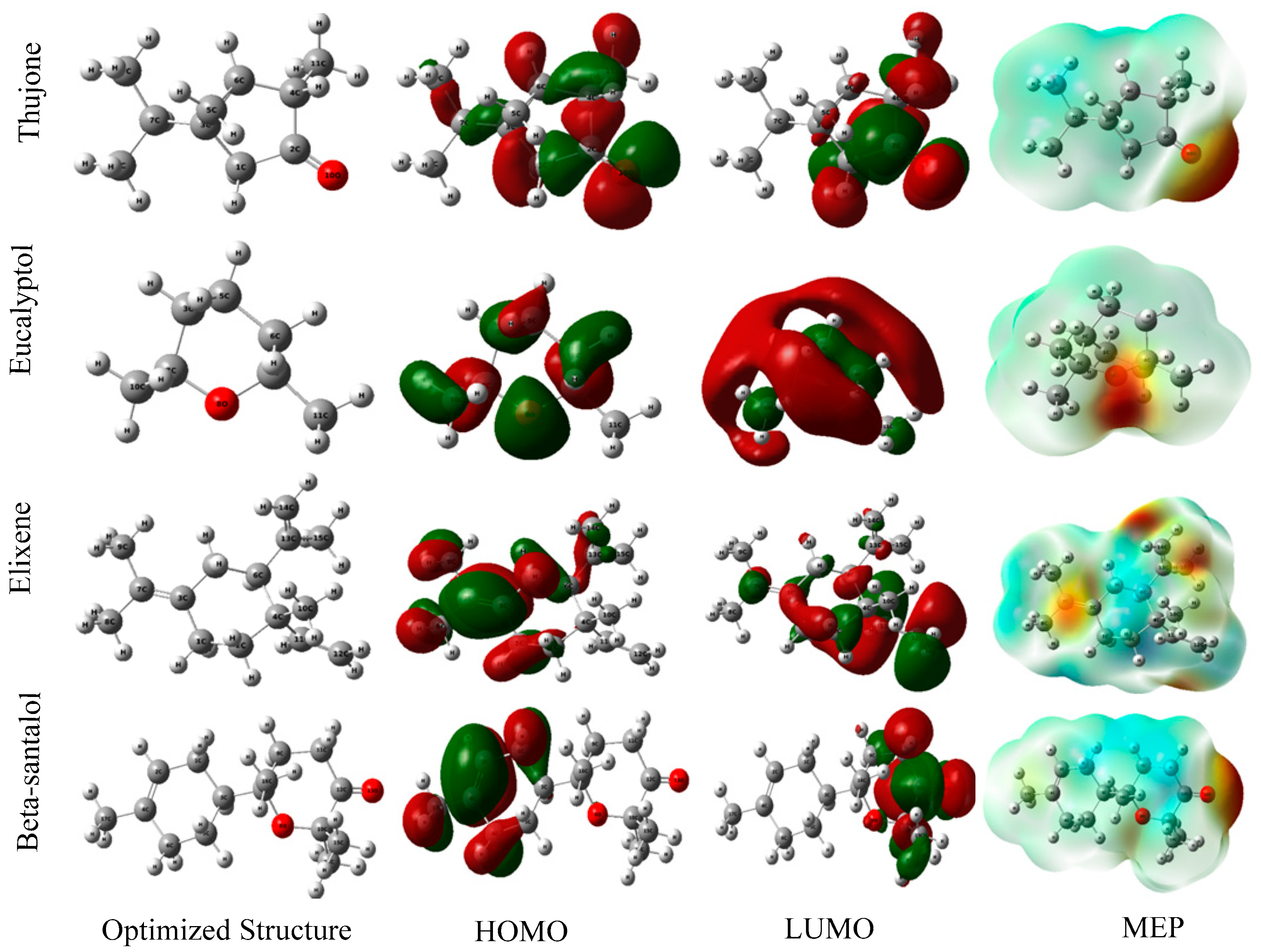
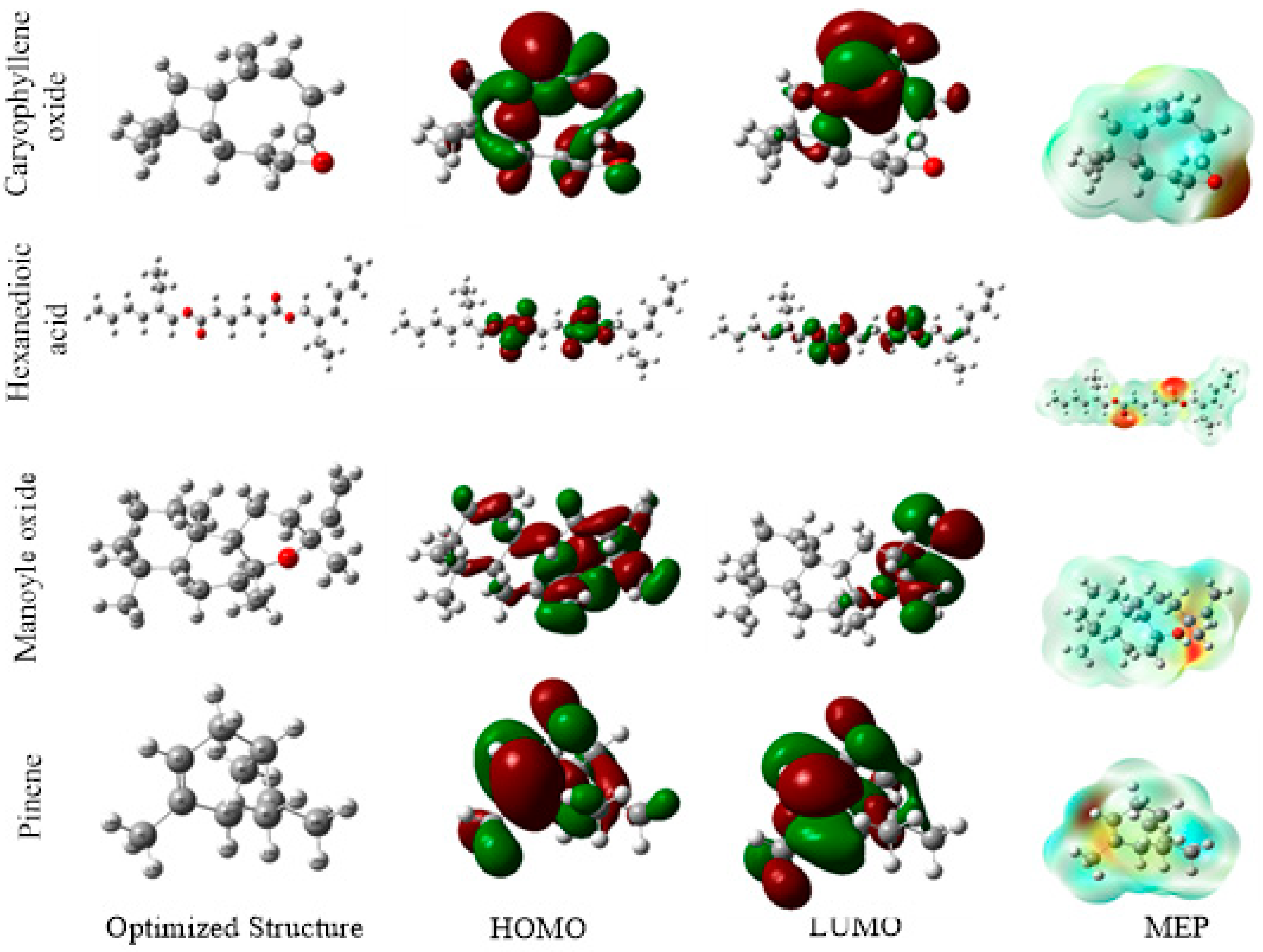
2.4.2. Theoretical Calculation
Density-Functional Theory (DFT) to Define B3LYP
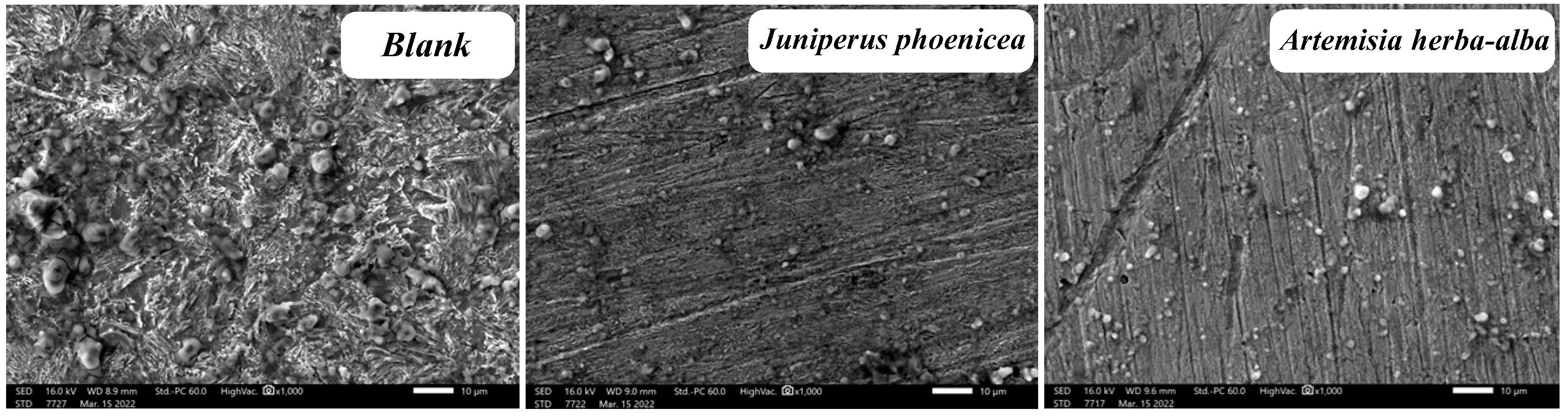
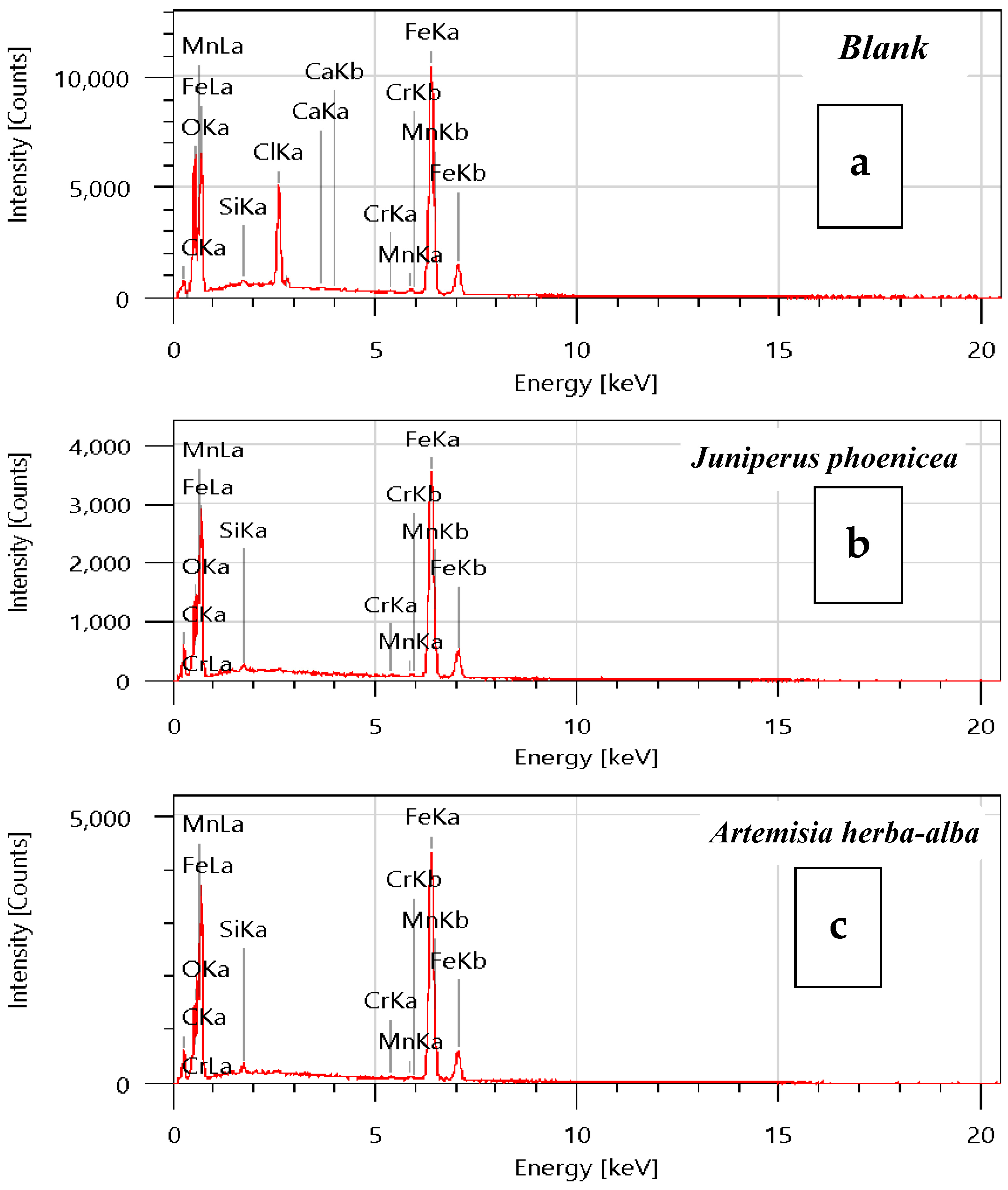
Surface Analysis
3. Results and Discussion
3.1. Chemical Composition of Artemisia herba-alba and Juniperus phoenicea EOs
3.1.1. Essential Oils from Artemisia herba-alba
3.1.2. Essential Oils from Juniperus phoenicea
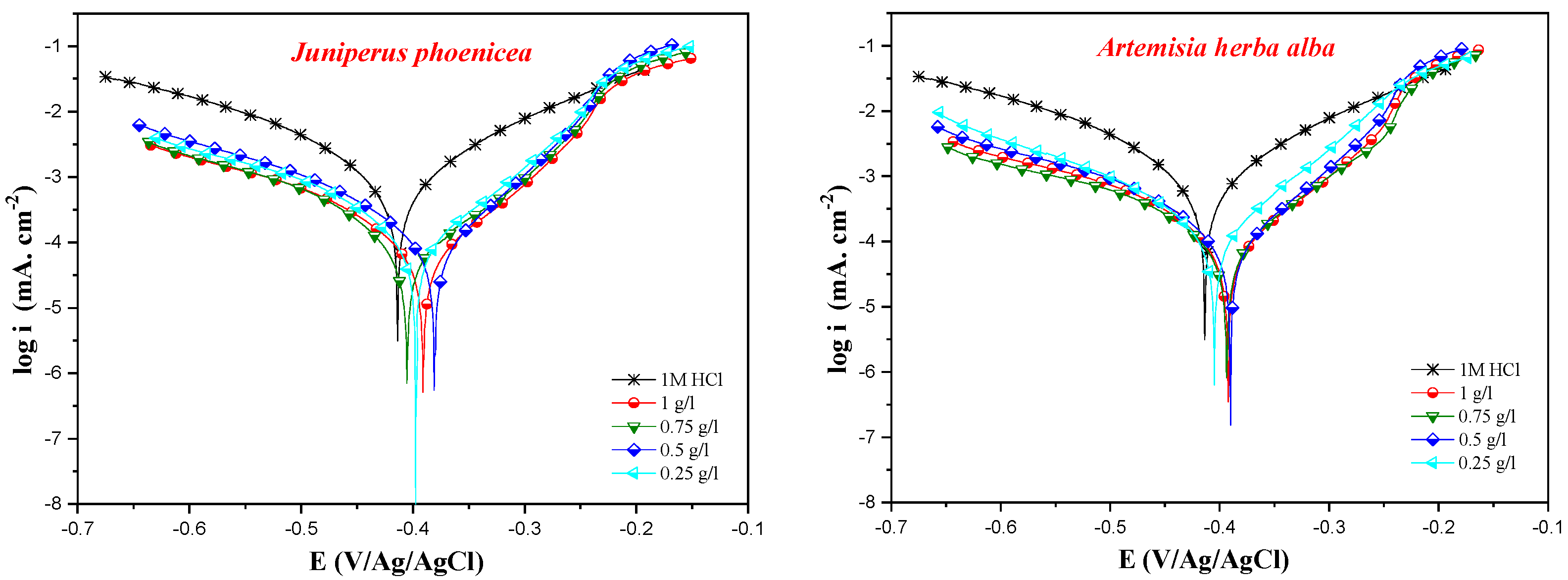
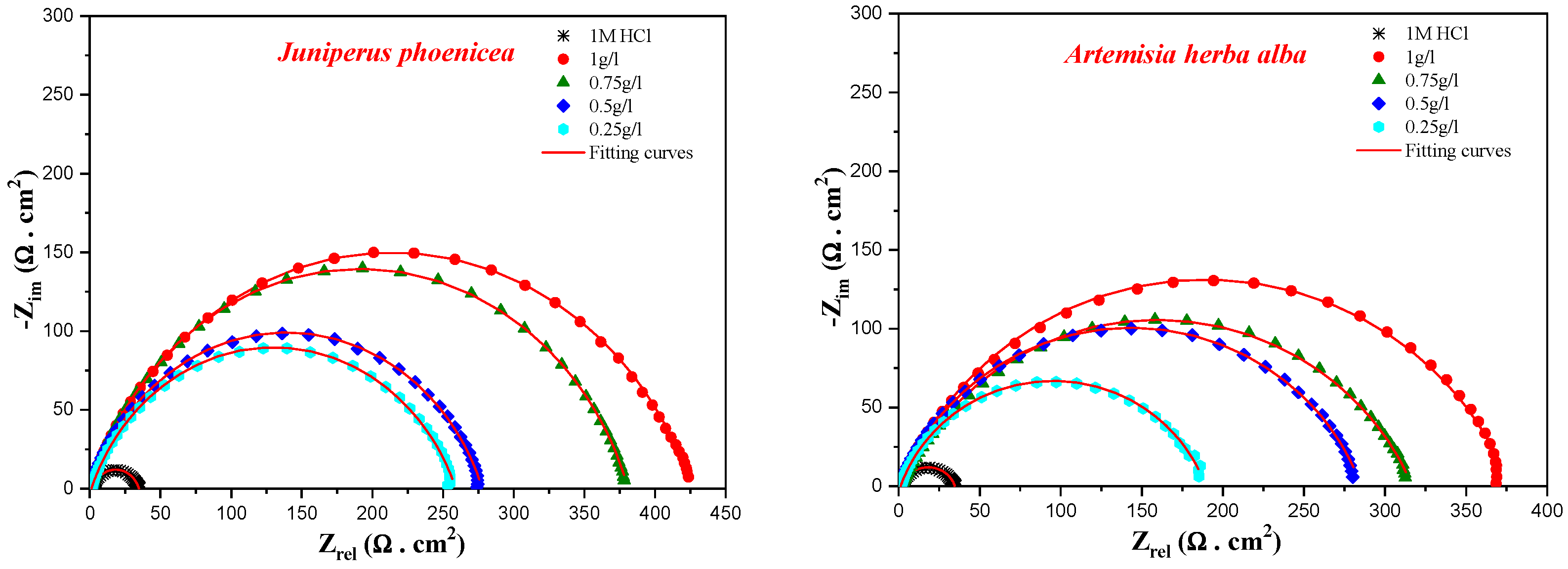
3.2. Anticorrosion Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lahsissene, H.; Kahouadji, A. Analyse ethnobotanique des plantes médicinales et aromatiques de la flore marocaine: Cas de la région de Zaër. Phythothérapie 2010, 8, 202–209. [Google Scholar] [CrossRef]
- Aimad, A.; Sanae, R.; Anas, F.; Abdelfattah, E.M.; Bourhia, M.; Mohammad Salamatullah, A.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Abdelkrim, A.; et al. Chemical Characterization and Antioxidant, Antimicrobial, and Insecticidal Properties of Essential Oil from Mentha pulegium L. Evid.-Based Complement. Altern. Med. 2021, 2021, e1108133. [Google Scholar] [CrossRef]
- Boudjouref, M.; Zerrouk, M.M.; Sétif, U.F.A. Etude de L’activité Antioxydante et Antimicrobienne D’extraits D’artemisiacampestres L. Master’s Thesis, Université Ferhat Abbes, Sétif, Algeria, 2011. [Google Scholar]
- Revie, R.W. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Shreir, L.L.; Burstein, G.T.; Jarman, R.A. Corrosion, 3rd ed.; Butterworth-Heinemann: Oxford, UK; Boston, MA, USA, 1994. [Google Scholar]
- Sastri, V.S. Corrosion Inhibitors: Principles and Applications; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Bouyanzer, A.; Hammouti, B. Naturally occurring ginger as corrosion inhibitor for steel in molar hydrochloric acid at 353 K. Bull. Electrochem. 2004, 20, 63–65. [Google Scholar]
- Hammouti, B.; Kertit, S.; Melhaoui, A. Electrochemical behavior of bgugaine as a corrosion inhibitor of iron in 1 M HCl. Bull. Electrochem. 1997, 13, 97–98. [Google Scholar]
- Akrout, A. Etude des huiles essentielles de quelques plantes pastorales de la région de Matmata (Tunisie). Cah. Options Méditerranéennes 2004, 62, 289–292. [Google Scholar]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef]
- Ahuja, A.; Yi, Y.-S.; Kim, M.-Y.; Cho, J.Y. Ethnopharmacological properties of Artemisia asiatica: A comprehensive review. J. Ethnopharmacol. 2018, 220, 117–128. [Google Scholar] [CrossRef]
- Benabid, A. Flore et Écosystèmes du Maroc: Evaluation et Préservation de la Biodiversité; Librairie et éditions Kalila Wa Dimna: Casablanca, Morocco, 2000. [Google Scholar]
- Fennane, M.; Ibn-Tattou, M. Flore Pratique du Maroc. Vol. 2. Angiospermae (Leguminosae-Lentibulariaceae); Inst. Scientifique, Université Mohammed V-Agdal: Rabat, Morocco, 2007. [Google Scholar]
- Avato, P.; Laquale, S.; Argentieri, M.P.; Lamiri, A.; Radicci, V.; D’Addabbo, T. Nematicidal activity of essential oils from aromatic plants of Morocco. J. Pest Sci. 2017, 90, 711–722. [Google Scholar] [CrossRef]
- Meloni, M.; Perini, D.; Filigheddu, R.; Binelli, G. Genetic variation in five Mediterranean populations of Juniperus phoenicea as revealed by inter-simple sequence repeat (ISSR) markers. Ann. Bot. 2006, 97, 299–304. [Google Scholar] [CrossRef]
- Nedjimi, B.; Beladel, B.; Guit, B. Multi-element determination in medicinal Juniper tree (Juniperus phoenicea) by instrumental neutron activation analysis. J. Radiat. Res. Appl. Sci. 2015, 8, 243–246. [Google Scholar] [CrossRef]
- Le Floc’h, E. Contribution à Une étude Ethnobotanique de la Flore Tunisienne; Ministère de l’Enseignement Supériur et de la Recherche Scientifique: Tunis, Tunisia, 1983.
- Adams, R.P. Identifcation of Essential Oil Components by Gas Chromatograpy/Mass Spectrometry, 4th ed.; Carol Stream, Allured Pub. Corp.: Illinois, IL, USA, 2007. [Google Scholar]
- Arrousse, N.; Mabrouk, E.H.; IsmailyAlaoui, K.; El Hajjaji, F.; Rais, Z.; Taleb, M. Economical synthesis strategy, characterization and theoretical study of the organic dye 3-oxo-3H-spiro [isobenzofuran-1, 9′-xanthene]-3′, 6′-diyl dibenzoate. SN Appl. Sci. 2020, 2, 1019. [Google Scholar] [CrossRef]
- Abdellaoui, O.; Skalli, M.K.; Haoudi, A.; Rodi, Y.K.; Arrousse, N.; Taleb, M.; Ghibate, R.; Senhaji, O. Study of the inhibition of corrosion of mild steel in a 1 M HCl solution by a new quaternary ammonium surfactant. Moroc. J. Chem. 2021, 9, 44–56. [Google Scholar]
- Alaoui Mrani, S.; Ech-chihbi, E.; Arrousse, N.; Rais, Z.; El Hajjaji, F.; El Abiad, C.; Radi, S.; Mabrouki, J.; Taleb, M.; Jodeh, S. DFT and Electrochemical Investigations on the Corrosion Inhibition of Mild Steel by Novel Schiff’s Base Derivatives in 1 M HCl Solution. Arab. J. Sci. Eng. 2021, 46, 5691–5707. [Google Scholar] [CrossRef]
- Arrousse, N.; Mabrouk, E.; Hammouti, B.; El Hajjaji, F.; Rais, Z.; Taleb, M. Synthesis, characterization, anti-corrosion behavior and theoretical study of the new organic dye: 3-oxo-3H-spiro [isobenzofuran-1,9-xanthene]-3,6-diyl bis (3-methyl-benzenesulfonate). Int. J. Corros. Scale Inhib. 2020, 9, 661–687. [Google Scholar]
- Hudaib, M.M.; Aburjai, T.A. Composition of the essential oil from Artemisia herba-alba grown in Jordan. J. Essent. Oil Res. 2006, 18, 301–304. [Google Scholar] [CrossRef]
- Zaim, A.; El Ghadraoui, L.; Farah, A. Effets des huiles essentielles d’Artemisia herba-alba sur la survie des criquets adultes d’Euchorthippusalbolineatus (Lucas, 1849). Bull. L’institut Sci. Rabat Sect. Sci. Vie 2012, 34, 127–133. [Google Scholar]
- Ghanmi, M.; Satrani, B.; Aafi, A.; Isamili, M.R.; Houti, H.; El Monfalouti, H.; Benchakroun, K.H.; Aberchane, M.; Harki, L.; Boukir, A.; et al. Effet de la date de récolte sur le rendement, la composition chimique et la bioactivité des huiles essentielles de l’armoise blanche (Artemisia herba-alba) de la région de Guerçif (Maroc oriental). Phytothérapie 2010, 8, 295–301. [Google Scholar] [CrossRef]
- Bezza, L.; Mannarino, A.; Fattarsi, K.; Mikail, C.; Abou, L.; Hadji-Minaglou, F.; Kaloustian, J. Chemical composition of the essential oil of Artemisia herba-alba issued from the district of Biskra (Algeria). Phytothérapie 2010, 8, 277. [Google Scholar] [CrossRef]
- Dob, T.; Benabdelkader, T. Chemical composition of the essential oil of Artemisia herba-alba Asso grown in Algeria. J. Essent. Oil Res. 2006, 18, 685–690. [Google Scholar] [CrossRef]
- Dhifallah, A.; Rouissi, H.; Selmi, H. Evaluation of the antioxidant and antibacterial activities of Tunisian Artemisia herba-alba essential oil. Moroc. J. Agric. Sci. 2021, 2, 114–117. [Google Scholar]
- Elmhalli, F.; Garboui, S.S.; Karlson, A.K.B.; Mozūraitis, R.; Baldauf, S.L.; Grandi, G. Acaricidal activity against Ixodesricinus nymphs of essential oils from the Libyan plants Artemisia herba alba, Origanummajorana and Juniperus phoenicea. Vet. Parasitol. Reg. Stud. Rep. 2021, 24, 100575. [Google Scholar]
- Boumhara, K.; Harhar, H.; Tabyaoui, M.; Bellaouchou, A.; Guenbour, A.; Zarrouk, A. Corrosion inhibition of mild steel in 0.5 M H2SO4 solution by Artemisia herba-alba oil. J. Bio-Tribo-Corros. 2019, 5, 8. [Google Scholar] [CrossRef]
- Tilaoui, M.; Ait Mouse, H.; Jaafari, A.; Zyad, A. Comparative phytochemical analysis of essential oils from different biological parts of Artemisia herba alba and their cytotoxic effect on cancer cells. PLoS ONE 2015, 10, e0131799. [Google Scholar] [CrossRef]
- Ennajar, M.; Bouajila, J.; Lebrihi, A.; Abderraba, M.F.; Raies, A.; Romdhane, A. Chemical Composition and Antimicrobial and Antioxidant Activities of EssentialOils and Various Extracts of Juniperus phoenicea L. (Cupressacees). J. Food Sci. 2009, 74, M364–M371. [Google Scholar] [CrossRef]
- Keskes, H.; Mnafgui, K.; Hamden, K.; Damak, M.; El Feki, A.; Allouche, N. In vitro anti-diabetic, anti-obesity and antioxidant proprieties of Juniperus phoenicea L. leaves from Tunisia. Asian Pac. J. Trop. Biomed. 2014, 4, S649–S655. [Google Scholar] [CrossRef]
- Aafi, A.; Taleb, M.S.; Fechtal, M. Espèces Remarquables de la Flore du Maroc; CNRF: Mechanicsville, VA, USA, 2002; Volume 146. [Google Scholar]
- Ait-Ouazzou, A.; Lorán, S.; Arakrak, A.; Laglaoui, A.; Rota, C.; Herrera, A.; Pagán, R.; Conchello, P. Evaluation of the chemical composition and antimicrobial activity of Mentha pulegium, Juniperus phoenicea, and Cyperus longus essential oils from Morocco. Food Res. Int. 2012, 45, 313–319. [Google Scholar] [CrossRef]
- Bahri, F.; Romane, A.; Arjouni, Y.; Harrak, R.; Ahmed El Alaoui El Fels, M. Chemical composition and antibacterial activity of the essential oil of Moroccan Juniperus phoenicea. Nat. Prod. Commun. 2011, 6, 1515–1518. [Google Scholar]
- Bouzouita, N.; Kachouri, F.; Ben Halima, M.; Chaabouni, M.M. Composition chimique et activités antioxydante, antimicrobienne et insecticide de l’huile essentielle de Juniperusphoenicea. J. Soc. Chim. Tunis 2008, 10, 119–125. [Google Scholar]
- El-Sawi, S.; Motawae, H.; Ali, M. Chemical composition, cytotoxic activity and antimicrobialactivity of essential oils of leaves and berries of Juniperus phoenicea growin in Egypt. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 417. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P.; Barrero, A.F.; Lara, A. Comparisons of the leaf essential oils of Juniperus phoenicea, J. phoenicea subsp. eu-mediterranea Lebr. &Thiv. and J. phoenicea var. turbinata (Guss.) Parl. J. Essent. Oil Res. 1996, 8, 367–371. [Google Scholar]
- Ghouti, D.; Rached, W.; Abdallah, M.; Pires, T.C.; Calhelha, R.C.; Alves, M.J.; Abderrahmane, L.H.; Barros, L.; Ferreira, I.C. Phenolic profile and in vitro bioactive potential of Saharan Juniperus phoenicea L. and Cotula cinerea (Del) growing in Algeria. Food Funct. 2018, 9, 4664–4672. [Google Scholar] [CrossRef] [PubMed]
- Achak, N.; Romane, A.; Abbad, A.; Ennajar, M.; Romdhane, M.; Abderrabba, A. Essential oil composition of Juniperus phoenicea from Morocco and Tunisia. J. Essent. Oil Bear. Plants 2008, 11, 137–142. [Google Scholar] [CrossRef]
- Fernine, Y.; Ech-chihbi, E.; Arrousse, N.; El Hajjaji, F.; Bousraf, F.; Touhami, M.E.; Rais, Z.; Taleb, M. Ocimumbasilicium seeds extract as an environmentally friendly antioxidant and corrosion inhibitor for aluminium alloy 2024-T3 corrosion in 3 wt% NaCl medium. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127232. [Google Scholar] [CrossRef]
- Abdelahi, M.M.; Elmsellem, H.; Benchidmi, M.; Sebbar, N.K.; Belghiti, M.A.; El Ouasif, L.; Jilalat, A.E.; Kadmi, Y.; Essassi, E.M. A DFT and molecular dynamics study on inhibitory action of indazole derivative on corrosion of mild steel. J. Mater. Environ. Sci. 2017, 8, 1860–1876. [Google Scholar]
- Zaidi, S.Z.J.; Hassan, S.; Raza, M.; Walsh, F.C. Transition metal oxide as possible electrode materials for Li-ion batteries: A DFT Analysis. Int. J. Electrochem. Sci. 2021, 16, 210322. [Google Scholar] [CrossRef]
- Mrani, S.A.; Arrousse, N.; Haldhar, R.; Lahcen, A.A.; Amine, A.; Saffaj, T.; Kim, S.-C.; Taleb, M. In Silico Approaches for Some Sulfa Drugs as Eco-Friendly Corrosion Inhibitors of Iron in Aqueous Medium. Lubricants 2022, 10, 43. [Google Scholar] [CrossRef]
- Obot, I.B.; Haruna, K.; Saleh, T.A. Atomistic Simulation: A Unique and Powerful Computational Tool for Corrosion Inhibition Research. Arab. J. Sci. Eng. 2019, 44, 1–32. [Google Scholar] [CrossRef]
- Ricky, E.X.; Mpelwa, M.; Xu, X. The study of m-pentadecylphenol on the inhibition of mild steel corrosion in 1 M HCl solution. J. Ind. Eng. Chem. 2021, 101, 359–371. [Google Scholar] [CrossRef]
- Goyal, M.; Kumar, S.; Bahadur, I.; Verma, C.; Ebenso, E.E. Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review. J. Mol. Liq. 2018, 256, 565–573. [Google Scholar] [CrossRef]
- Galai, M.; Rbaa, M.; Ouakki, M.; Dahmani, K.; Kaya, S.; Arrousse, N.; Dkhireche, N.; Briche, S.; Lakhrissi, B.; Touhami, M.E. Functionalization effect on the corrosion inhibition of novel eco-friendly compounds based on 8-hydroxyquinoline derivatives: Experimental, theoretical, and surface treatment. Chem. Phys. Lett. 2021, 776, 138700. [Google Scholar] [CrossRef]
- Ayuba, A.M.; Abubakar, M. A Theoretical Study on Isolated Compounds from the Leaves Extract of Guiera Senegalensis as Aluminium Corrosion Inhibitor. J. Sci. Technol. 2021, 13, 47–56. [Google Scholar]
- Chile, N.E.; Haldhar, R.; Godffrey, U.K.; Chijioke, O.C.; Umezuruike, E.A.; Ifeoma, O.P.; Oke, M.O.; Ichou, H.; Arrousse, N.; Kim, S.-C.; et al. Theoretical Study and Adsorption Behavior of Urea on Mild Steel in Automotive Gas oil (AGO) Medium. Lubricants 2022, 10, 157. [Google Scholar] [CrossRef]

| Peaks | Retention Time | Kovats Index | Compounds | Area (%) | ||
|---|---|---|---|---|---|---|
| Literature | Calculated | A. herba-alba | J. phoenicea | |||
| 1 | 3.435 | 737 | 733 | Norbornene | 0.70% | - |
| 2 | 7.989 | 939 | 939 | alpha-Pinene | - | 43.61% |
| 3 | 8. 999 | 930 | 925 | Thujene | 1.41% | - |
| 4 | 9. 138 | 975 | 976 | Sabinene | 0.75% | - |
| 5 | 10.433 | 1024 | 1024 | p-Cymene | 0.70% | - |
| 6 | 10.661 | 979 | 979 | p-Menthane, 1,8-epoxy | 2.53% | - |
| 8 | 10.761 | 1014 | 1014 | p-Cineole | 2.10% | - |
| 9 | 10.906 | 1031 | 1032 | 1,8-Cineole | 4.67% | - |
| 10 | 12.593 | 1096 | 1095 | Linalool | - | 0.83% |
| 11 | 12.904 | 1096 | 1092 | Linalool | - | 2.88% |
| 12 | 12.949 | 1138 | 1140 | Thujanone | 0.84% | - |
| 13 | 13.152 | 1102 | 1102 | Isothujone | 13.12% | - |
| 14 | 13.310 | 1102 | 1004 | Alpha-thujone | 13.32% | 0.82% |
| 15 | 13.550 | 1114 | 1114 | Beta-thujone | 30.07% | - |
| 16 | 13.961 | 1141 | 1141 | cis-Verbenol | - | 0.68% |
| 17 | 14.105 | 1142 | 1142 | cis-Sabinol | 0.75% | - |
| 18 | 14.177 | 1141 | 1141 | 2-Pinen-4-ol, trans- | - | 1.04% |
| 19 | 14.312 | 1144 | 1144 | trans-Verbenol | - | 3.16% |
| 20 | 15.068 | 1169 | 1169 | endo-Borneol | 0.64% | - |
| 21 | 15.315 | 1177 | 1174 | Terpinen-4-ol | 2.09% | - |
| 22 | 15.750 | 1195 | 1196 | Myrtenal | - | 1.37% |
| 23 | 16.093 | 1205 | 1205 | Verbenone | - | 1.52% |
| 24 | 17.441 | 1363 | 1363 | cis-4-Decenol | - | 0.68% |
| 25 | 18.580 | 1290 | 1290 | Thymol | 0.71% | - |
| 26 | 20.833 | 1260 | 1262 | Benzyl propanoate | - | 1.22% |
| 27 | 21.128 | 1390 | 1390 | beta-Elemene | - | 1.18% |
| 28 | 21.955 | 1408 | 1410 | Caryophyllene | - | 2.01% |
| 29 | 22.152 | 1561 | 1561 | Germacrene B | - | 1.18% |
| 30 | 22.906 | 1288 | 1290 | Bornyl acetate | 0.76% | - |
| 31 | 23.040 | 1137 | 1135 | cis-p-Mentha-2,8-dienol | 0.69% | - |
| 32 | 23.205 | 1485 | 1488 | Germacrene D | 0.80% | - |
| 33 | 23.824 | 1290 | 1290 | Sabinyl acetate | 1.01% | - |
| 34 | 24.183 | 1689 | 1688 | Shyobunol | - | 0.82% |
| 35 | 24.866 | 1017 | 1017 | geranyl-.alpha.-terpinene | - | 1.55% |
| 36 | 25.022 | 1578 | 1574 | Spathulenol | - | 1.08% |
| 37 | 25.171 | 1549 | 1544 | Elemol | - | 2.31% |
| 38 | 25.855 | 1602 | 1602 | Ledol | 0.63% | 2.55% |
| 39 | 26.024 | 1578 | 1576 | Spathulenol | 1.08% | 1.22% |
| 40 | 26.200 | 1583 | 1583 | Caryophyllene oxide | 0.93% | 4.34% |
| 41 | 26.881 | 1590 | 1592 | Globulol | 1.93% | - |
| 42 | 27.025 | 1648 | 1644 | AgarospiroI | 2.95% | - |
| 43 | 27.339 | 1640 | 1640 | Alpha Cadinol | 0.96% | - |
| 44 | 27.526 | 1630 | 1630 | Iso-spathulenol | 4.45% | - |
| 45 | 27.596 | 1338 | 1332 | Gamma-Elemene | 4.45% | - |
| 46 | 27.738 | 1900 | 1900 | Columellarin | 1.89% | - |
| 47 | 28.242 | 1658 | 1655 | Bisabolol oxide B | 1.13% | - |
| 48 | 28.465 | 1675 | 1675 | alpha-Santalol | 0.78% | - |
| 49 | 29.816 | 2206 | 2202 | Isospathulenol | 0.83% | |
| 50 | 29.973 | 1702 | 1702 | beta-Santalol | 3.38% | - |
| 51 | 30.704 | 1685 | 1680 | alpha-Bisabololoxide A | 0.92% | - |
| 52 | 31.253 | 1460 | 1458 | Allo-aromadendrene oxide | - | 1.83% |
| 53 | 31.598 | 1490 | 1490 | 6-Eudesmen-4-α-ol | 1.15% | - |
| 54 | 32.286 | 1466 | 1466 | Caryophyllene epoxide | - | 1.41% |
| 55 | 32.410 | 1600 | 1662 | Rosifoliol | - | 0.61% |
| 56 | 32.605 | 1490 | 1485 | beta-Selinene | - | 0.63% |
| 57 | 32.685 | 1607 | 1603 | beta-Oplopenone | - | 0.96% |
| 58 | 36.392 | 2216 | 2214 | Manool oxide | - | 11.50% |
| 59 | 40.151 | 2468 | 2468 | Abictol | - | 0.94% |
| 60 | 43.429 | 1032 | 1030 | Hexanedioic acid | - | 4.41% |
| Conc (g/L) | -Ecorr (mV/Ag/AgCl) | icorr (µA cm−2) | βc (mV dec−1) | ηPDP (%) | |
|---|---|---|---|---|---|
| 1 M HCl | ** | 413 | 944 | 139 | 00 |
| Juniperus phoenicea | 1 | 391 | 75 | 108 | 92 |
| 0.75 | 405 | 87 | 104 | 91 | |
| 0.5 | 381 | 97 | 107 | 90 | |
| 0.25 | 396 | 105 | 102 | 89 | |
| Artemisia herba-alba | 1.00 | 392 | 81 | 138 | 91 |
| 0.75 | 394 | 96 | 135 | 90 | |
| 0.50 | 389 | 105 | 130 | 89 | |
| 0.25 | 404 | 162 | 128 | 83 |
| Conc. | Rs | Rct | Cdl | ndl | Q | ƞimp | |
|---|---|---|---|---|---|---|---|
| (g/L) | (Ω cm2) | (Ωcm2) | (µF·cm−2) | (µF·Sn−1) | % | ||
| 1 M HCl | ** | 1.76 | 33.2 | 89.10 | 0.784 | 312.7 | 00 |
| Juniperusphoenicea | 1 | 0.94 | 425.4 | 32.78 | 0.781 | 83.23 | 92 |
| 0.75 | 1.06 | 379.9 | 41.03 | 0.806 | 91.76 | 91 | |
| 0.5 | 0.53 | 277.2 | 46.80 | 0.791 | 116.1 | 88 | |
| 0.25 | 1.04 | 258.0 | 53.79 | 0.773 | 141.6 | 87 | |
| Artemisia herba-alba | 1 | 1.7 | 375.3 | 31.6 | 0.777 | 85 | 91 |
| 0.75 | 2.2 | 314.7 | 31.9 | 0.753 | 99 | 89 | |
| 0.5 | 0.9 | 284.4 | 43.7 | 0.784 | 113 | 88 | |
| 0.25 | 1.2 | 188.2 | 51.2 | 0.787 | 137 | 82 |
| EOs | Descriptors | EHOMO (eV) | ELUMO (eV) | ∆Egap (eV) | η (eV) | σ (eV−1) | χ (eV) | ∆N (eV) |
|---|---|---|---|---|---|---|---|---|
| Artemisia herba-alba | Thujone | −6.860 | -0.829 | 6.031 | 3.015 | 0.331 | 3.845 | 0.161 |
| Eucalyptol | −6.601 | 0.873 | 7.474 | 3.737 | 0.267 | 2.863 | 0.261 | |
| Beta-santalol | −6.428 | -0.909 | 5.519 | 2.759 | 0.362 | 3.668 | 0.208 | |
| Juniperusphoenicea | Pinene | −6.1964 | 0.4729 | 6.6693 | 3.3346 | 0.2998 | 2.8617 | 0.2936 |
| Manoyl oxide | −6.7698 | 0.1404 | 6.9102 | 3.4551 | 0.2894 | 3.3146 | 0.2178 | |
| Hexanedioicacid | −7.7058 | 0.0897 | 7.7956 | 3.8978 | 0.2565 | 3.8080 | 0.1298 | |
| Caryophylleneoxide | −6.6356 | 0.0059 | 6.6416 | 3.3208 | 0.3011 | 3.3148 | 0.2266 |
| Element | Juniperusphoenicea | Artemisia herba-alba | Blank |
|---|---|---|---|
| C | 7.94 | 7.36 | 0.30 |
| O | 6.61 | 6.02 | 7.53 |
| Si | 0.24 | 0.44 | 0.00 |
| Cr | 0.35 | 0.29 | 0.25 |
| Mn | 0.83 | 0.79 | 0.60 |
| Fe | 84.02 | 85.09 | 77.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beniaich, G.; Beniken, M.; Salim, R.; Arrousse, N.; Ech-chihbi, E.; Rais, Z.; Sadiq, A.; Nafidi, H.-A.; Bin Jardan, Y.A.; Bourhia, M.; et al. Anticorrosive Effects of Essential Oils Obtained from White Wormwood and Arâr Plants. Separations 2023, 10, 396. https://doi.org/10.3390/separations10070396
Beniaich G, Beniken M, Salim R, Arrousse N, Ech-chihbi E, Rais Z, Sadiq A, Nafidi H-A, Bin Jardan YA, Bourhia M, et al. Anticorrosive Effects of Essential Oils Obtained from White Wormwood and Arâr Plants. Separations. 2023; 10(7):396. https://doi.org/10.3390/separations10070396
Chicago/Turabian StyleBeniaich, Ghada, Mustapha Beniken, Rajae Salim, Nadia Arrousse, Elhachmia Ech-chihbi, Zakia Rais, Asmae Sadiq, Hiba-Allah Nafidi, Yousef A. Bin Jardan, Mohammed Bourhia, and et al. 2023. "Anticorrosive Effects of Essential Oils Obtained from White Wormwood and Arâr Plants" Separations 10, no. 7: 396. https://doi.org/10.3390/separations10070396
APA StyleBeniaich, G., Beniken, M., Salim, R., Arrousse, N., Ech-chihbi, E., Rais, Z., Sadiq, A., Nafidi, H.-A., Bin Jardan, Y. A., Bourhia, M., & Taleb, M. (2023). Anticorrosive Effects of Essential Oils Obtained from White Wormwood and Arâr Plants. Separations, 10(7), 396. https://doi.org/10.3390/separations10070396








