Biomass-Based Silica/Calcium Carbonate Nanocomposites for the Adsorptive Removal of Escherichia coli from Aqueous Suspensions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Adsorbents
2.3. Bacterial Adsorption Studies
2.4. Instrumental Analysis
3. Results and Discussion
3.1. Characterization Studies
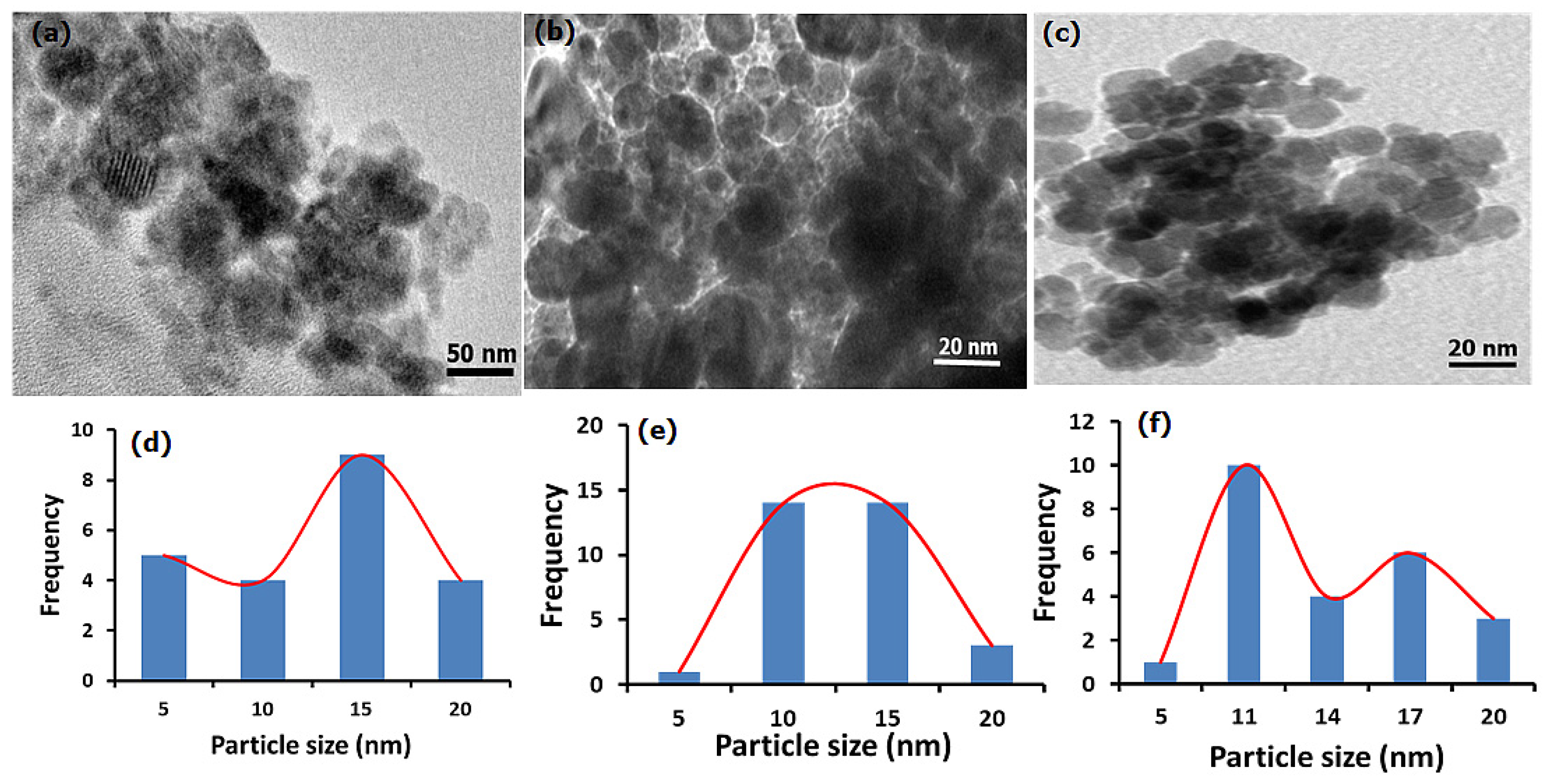
3.1.1. HRTEM and Particle Size Analysis
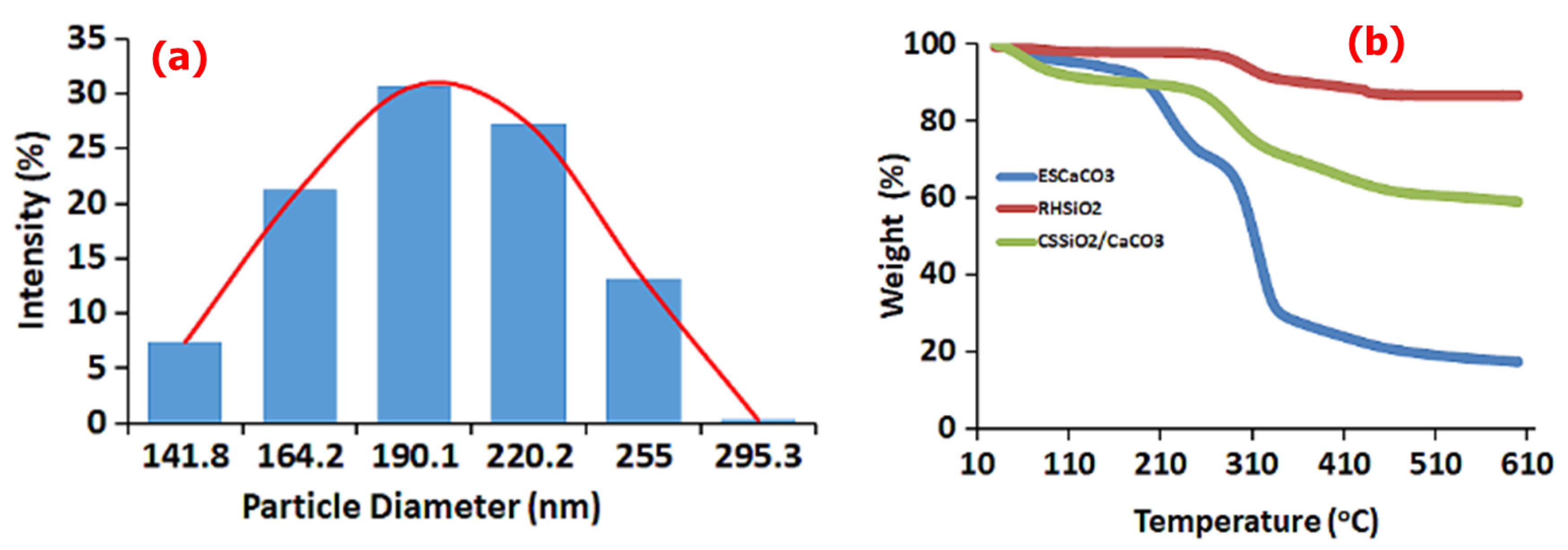
3.1.2. DLS and TGA Studies
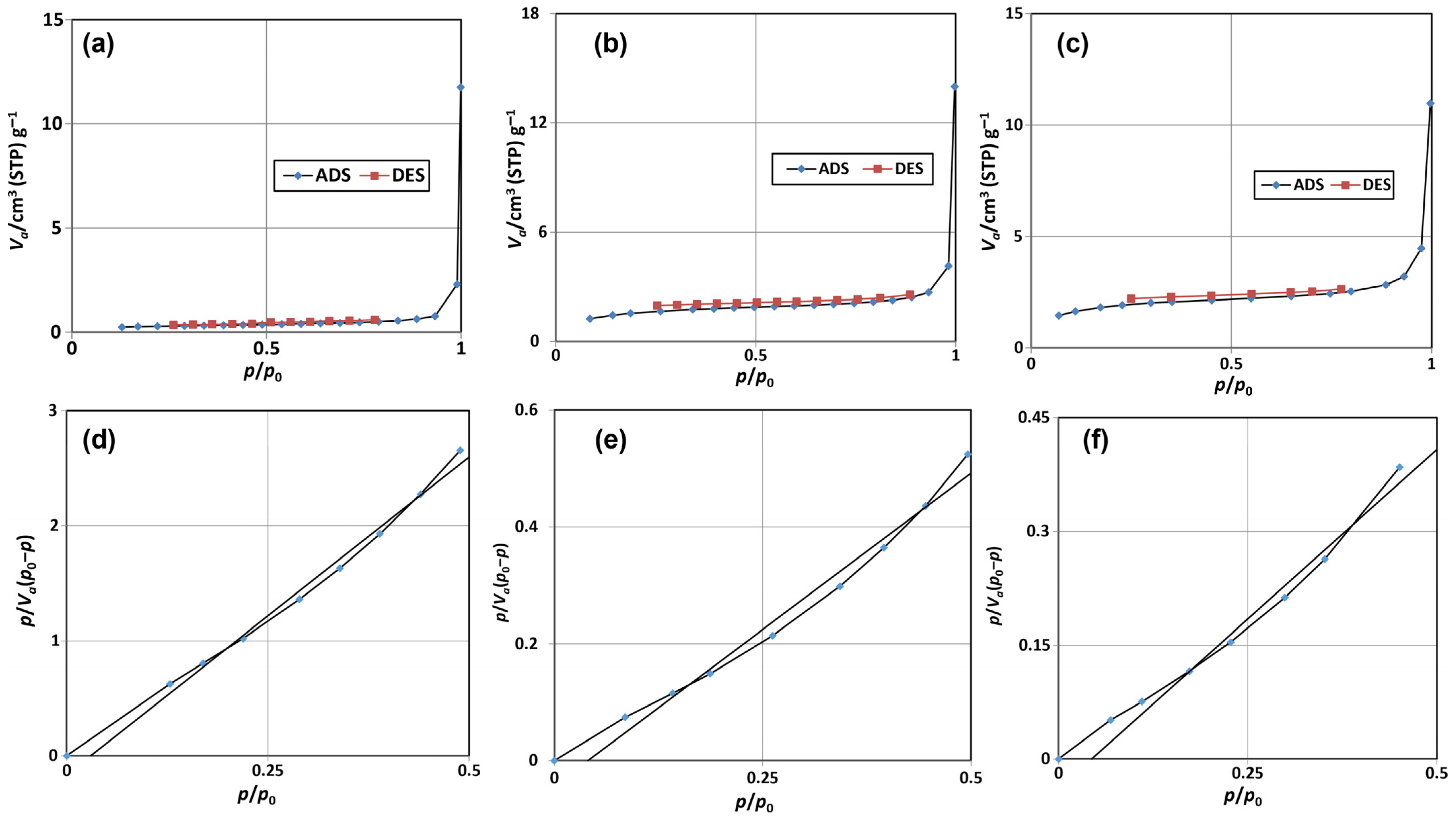
3.1.3. BET Adsorption/Desorption Isotherms
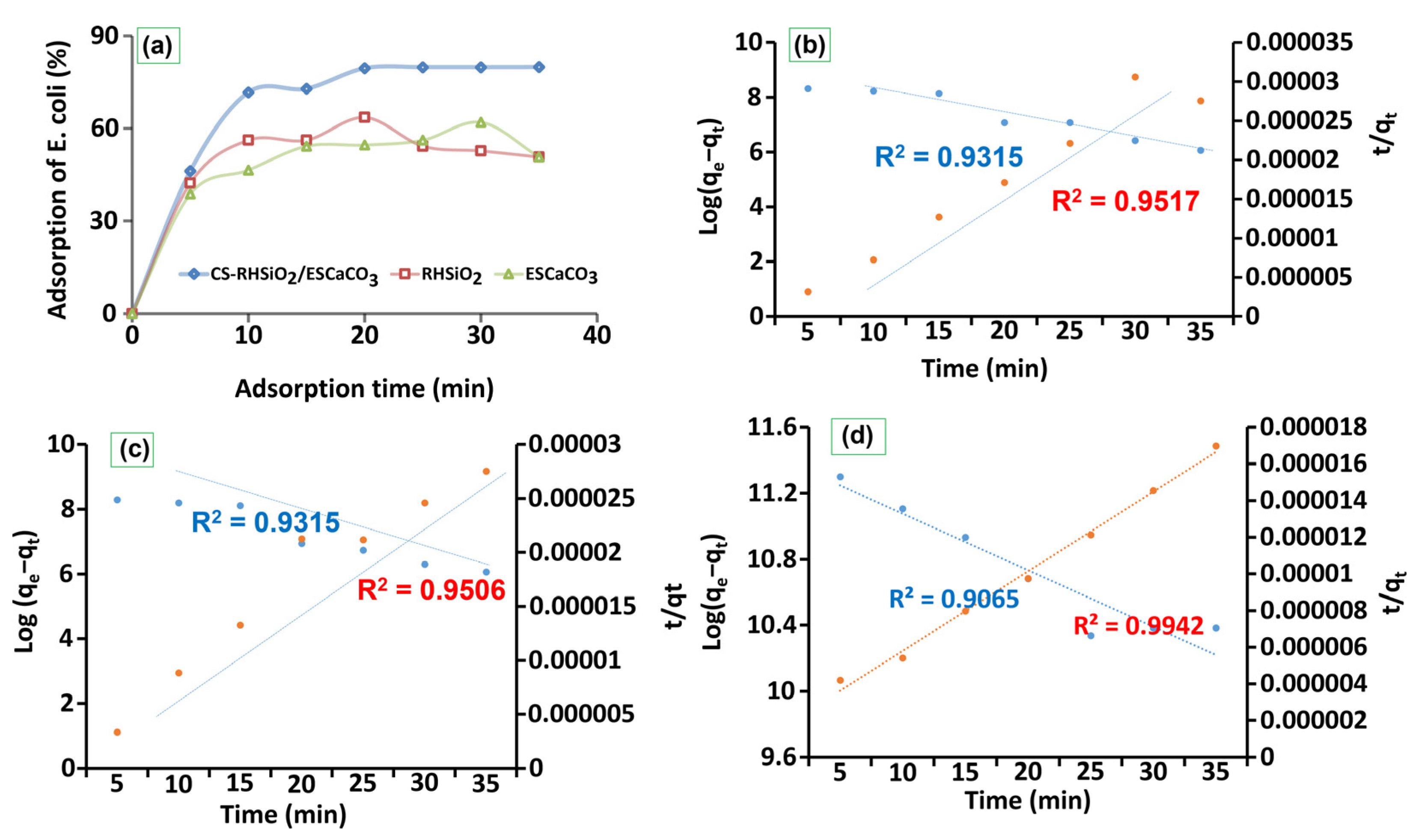
3.1.4. Kinetic and Adsorption Study
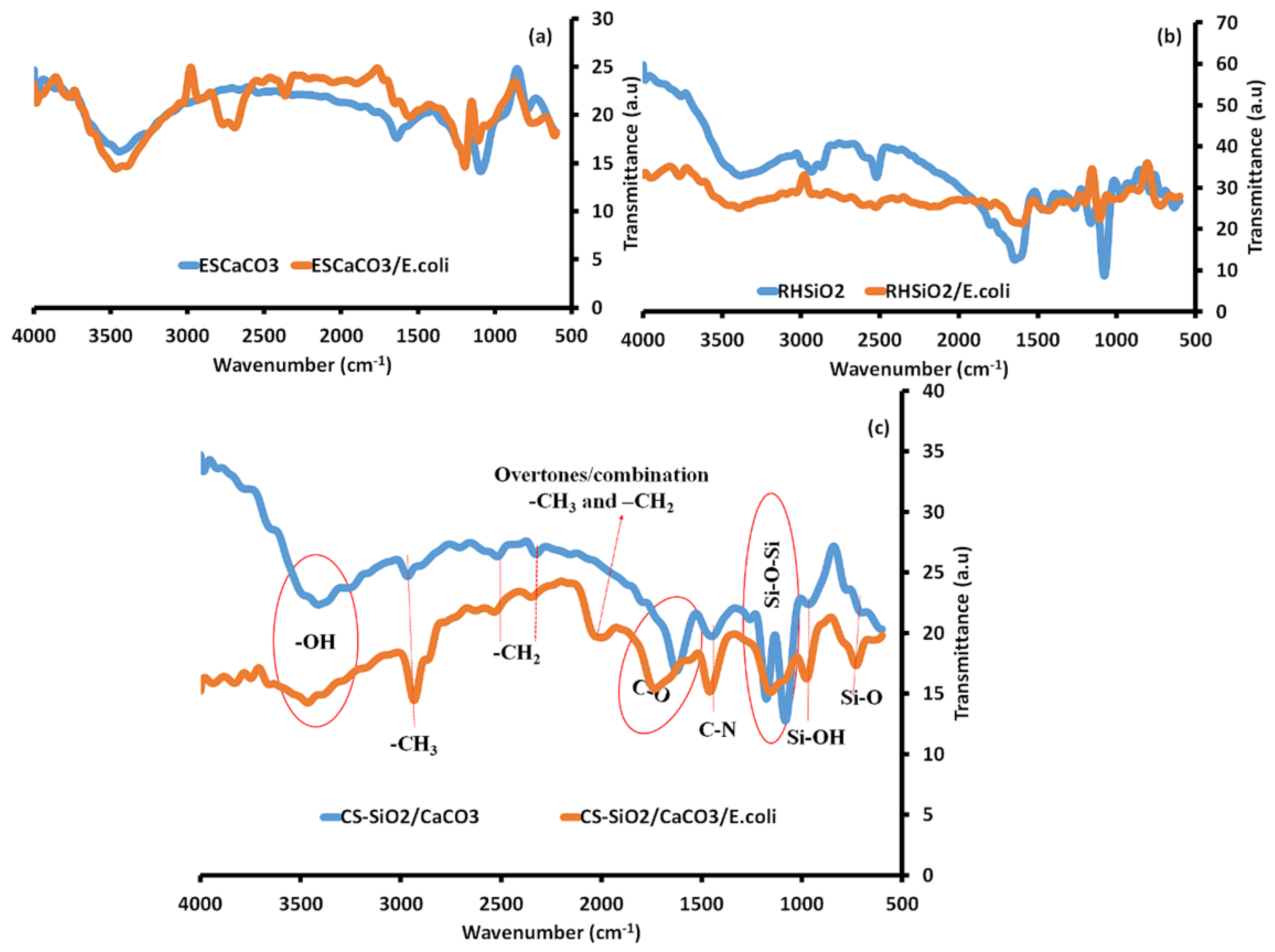
Adsorption Mechanism of E. coli—NPs Using Fourier Transform Infrared Spectroscopy
Comparison of the Maximum Sorption Capacity (qm) of E. coli onto CS-SiO2/CaCO3, RHSiO2, ESCaCO3 with Those of Other Adsorbents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darabdhara, G.; Boruah, P.K.; Hussain, N.; Borthakur, P.; Sharma, B.; Sengupta, P.; Das, M.R. Magnetic nanoparticles towards efficient adsorption of gram positive and gram negative bacteria: An investigation of adsorption parameters and interaction mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2017, 516, 161–170. [Google Scholar] [CrossRef]
- Oh, J.K.; Yegin, Y.; Yang, F.; Zhang, M.; Li, J.; Huang, S.; Verkhoturov, S.V.; Schweikert, E.A.; Perez-Lewis, K.; Scholar, E.A.; et al. The influence of surface chemistry on the kinetics and thermodynamics of bacterial adhesion. Sci. Rep. 2018, 8, 17247. [Google Scholar] [CrossRef] [PubMed]
- Da’ana, D.A.; Zouari, N.; Ashfaq, M.Y.; Abu-Dieyeh, M.; Khraisheh, M.; Hijji, Y.M.; Al-Ghouti, M.A. Removal of toxic elements and microbial contaminants from groundwater using low-cost treatment options. Curr. Pollut. Rep. 2021, 7, 300–324. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, K.; Vachet, R.W.; Xing, B. Interaction between oxide nanoparticles and biomolecules of the bacterial cell envelope as examined by infrared spectroscopy. Langmuir 2010, 26, 18071–18077. [Google Scholar] [CrossRef] [PubMed]
- Obijole, O.; Mugera, G.W.; Mudzielwana, R.; Ndungu, P.; Samie, A.; Babatunde, A. Hydrothermally treated aluminosilicate clay (HTAC) for remediation of fluoride and pathogens from water: Adsorbent characterization and adsorption modelling. Water Resour. Ind. 2021, 25, 100144. [Google Scholar] [CrossRef]
- Bwatanglang, I.B.; Yonnana, E.; Ibrahim, L.D.; Kubo, A.I.; Elijah, B.K.; Ayagwa, C.A.; Abdulkarim, J.; Yerima, Y. Appraisal of public pipe-borne water quality in Jimeta/Yola Adamawa State (Nigeria): From the treatment-plants to end-user points. Int. Res. J. Pure Appl. Chem. 2022, 21, 53–65. [Google Scholar] [CrossRef]
- Ojha, A. Nanomaterials for removal of waterborne pathogens: Opportunities and challenges. Waterborne Pathog. 2020, 1, 385–432. [Google Scholar]
- Murcia-Salvador, A.; Pellicer, J.A.; Rodríguez-López, M.I.; Gómez-López, V.M.; Núñez-Delicado, E.; Gabaldón, J.A. Egg by-products as a tool to remove direct Blue 78 dye from wastewater: Kinetic, equilibrium modeling, thermodynamics and desorption properties. Materials 2020, 13, 1262. [Google Scholar] [CrossRef] [PubMed]
- Şenol, Z.M.; Messaoudi, N.E.; Fernine, Y.; Keskin, Z.S. Bioremoval of rhodamine B dye from aqueous solution by using agricultural solid waste (almond shell): Experimental and DFT modeling studies. Biomass Convers. Biorefin. 2023, 17, 1–4. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Pajerski, W.; Ochonska, D.; Brzychczy-Wloch, M.; Indyka, P.; Jarosz, M.; Golda-Cepa, M.; Sojka, Z.; Kotarba, A. Attachment efficiency of gold nanoparticles by Gram-positive and Gram-negative bacterial strains governed by surface charges. J. Nanopart. Res. 2019, 21, 1–2. [Google Scholar] [CrossRef]
- Ye, M.; Sun, M.; Chen, X.; Feng, Y.; Wan, J.; Liu, K.; Tian, D.; Liu, M.; Wu, J.; Schwab, A.P.; et al. Feasibility of sulfate-calcined eggshells for removing pathogenic bacteria and antibiotic resistance genes from landfill leachates. Waste Manag. 2017, 63, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N.; Kashefi, M.; Mirjalili, M.; Afsharnezhad, S. On the adsorption kinetics and mechanism of enhanced photocatalytic activity of Fe3O4-SiO2-TiO2 core-multishell nanoparticles against E. coli. J. Biomed. Mater. Res. Part A 2021, 109, 181–192. [Google Scholar] [CrossRef]
- Borkowski, A.; Szala, M.; Cłapa, T. Adsorption studies of the Gram-negative bacteria onto nanostructured silicon carbide. Appl. Biochem. Biotechnol. 2015, 175, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Santamaría, L.; Korili, S.A.; Vicente, M.A.; Barbosa, L.V.; De Souza, S.D.; Marçal, L.; De Faria, E.H.; Ciuffi, K.J. A review of organic-inorganic hybrid clay based adsorbents for contaminants removal: Synthesis, perspectives and applications. J. Environ. Chem. Eng. 2021, 9, 105808. [Google Scholar] [CrossRef]
- Kubota, M.; Nakabayashi, T.; Matsumoto, Y.; Shiomi, T.; Yamada, Y.; Ino, K.; Yamanokuchi, H.; Matsui, M.; Tsunoda, T.; Mizukami, F.; et al. Selective adsorption of bacterial cells onto zeolites. Colloids Surf. B Biointerfaces 2008, 64, 88–97. [Google Scholar] [CrossRef]
- Rong, X.; Chen, W.; Huang, Q.; Cai, P.; Liang, W. Pseudomonas putida adhesion to goethite: Studied by equilibrium adsorption, SEM, FTIR and ITC. Colloids Surf. B Biointerfaces 2010, 80, 79–85. [Google Scholar] [CrossRef]
- Mills, A.L.; Herman, J.S.; Hornberger, G.M.; DeJesús, T.H. Effect of solution ionic strength and iron coatings on mineral grains on the sorption of bacterial cells to quartz sand. Appl. Environ. Microbiol. 1994, 60, 3300–3306. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Zhao, Y.; Bai, H.; Wen, T.; Kang, S.; Song, G.; Song, S.; Komarneni, S. Removal of heavy metals and dyes by clay-based adsorbents: From natural clays to 1D and 2D nano-composites. Chem. Eng. J. 2021, 420, 127574. [Google Scholar] [CrossRef]
- Zemnukhova, L.; Kharchenko, U.; Beleneva, I. Biomass derived silica containing products for removal of microorganisms from water. Int. J. Environ. Sci. Technol. 2015, 12, 1495–1502. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Wu, L.; Younes, M.; Hincke, M. Biotechnological applications of eggshell: Recent advances. Front. Bioeng. Biotechnol. 2021, 9, 675364. [Google Scholar] [CrossRef]
- Bullo, T.A.; Bayisa, Y.M.; Bultum, M.S. Optimization and biosynthesis of calcined chicken eggshell doped titanium dioxide photocatalyst based nanoparticles for wastewater treatment. SN Appl. Sci. 2022, 4, 17. [Google Scholar] [CrossRef]
- Alhasan, H.S.; Alahmadi, N.; Yasin, S.A.; Khalaf, M.Y.; Ali, G.A.M. Low-Cost and Eco-Friendly Hydroxyapatite Nanoparticles Derived from Eggshell Waste for Cephalexin Removal. Separations 2022, 9, 10. [Google Scholar] [CrossRef]
- Mori, N.; Kawasaki, H.; Nishida, E.; Kanemoto, Y.; Miyaji, H.; Umeda, J.; Kondoh, K. Rose bengal-decorated rice husk-derived silica nanoparticles enhanced singlet oxygen generation for antimicrobial photodynamic inactivation. J. Mater. Sci. 2023, 25, 1–3. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Chen, Z.; Zhao, X.; Luo, X.; Wang, L.; Li, Y.; Teng, F. Preparation of magnetic mesoporous silica from rice husk for aflatoxin B1 removal: Optimum process and adsorption mechanism. PLoS ONE 2020, 15, e0238837. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Huang, K. Biobased Chicken Eggshell Powder for Efficient Delivery of Low-Dose Silver Nanoparticles (AgNPs) to Enhance Their Antimicrobial Activities against Foodborne Pathogens and Biofilms. ACS Appl. Bio Mater. 2022, 5, 4390–4399. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Rao, N.V.; Omidifar, N.; Gholami, A.; Ghahramani, Y.; Chiang, W.H.; Kalashgrani, M.Y. Antibacterial and cytotoxic efficacy of Nano-Hydroxyapatite Synthesized from Eggshell and Sheep bones bio Waste. Res. Sq. 2022, 1–23. [Google Scholar] [CrossRef]
- Bashir, F.; Irfan, M.; Ahmad, T.; Iqbal, J.; Butt, M.T.; Sadef, Y.; Umbreen, M.; Shaikh, I.A.; Moniruzzaman, M. Efficient utilization of low cost agro materials for incorporation of copper nanoparticles to scrutinize their antibacterial properties in drinking water. Environ. Technol. Innov. 2021, 21, 101228. [Google Scholar] [CrossRef]
- Alhadhrami, A.; Mohamed, G.G.; Sadek, A.H.; Ismail, S.H.; Ebnalwaled, A.A.; Almalki, A.S. Behavior of silica nanoparticles synthesized from rice husk ash by the sol–gel method as a photocatalytic and antibacterial agent. Materials 2022, 15, 8211. [Google Scholar] [CrossRef] [PubMed]
- Unglaube, F.; Lammers, A.; Kreyenschulte, C.R.; Lalk, M.; Mejía, E. Preparation, Characterization and Antimicrobial Properties of Nanosized Silver-Containing Carbon/Silica Composites from Rice Husk Waste. Chem. Open 2021, 10, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.; Truong, T.T.; Nguyen, H.L.; Hoang, L.B.; Bui, V.P.; Tran, T.T.; Dinh, T.D.; Le, T.D. Synthesis and Characterization of Novel Core–Shell ZnO@ SiO2 Nanoparticles and Application in Antibiotic and Bacteria Removal. ACS Omega 2022, 7, 42073–42082. [Google Scholar] [CrossRef] [PubMed]
- Riana, U.; Ramli, M.; Iqrammullah, M.; Raharjo, Y.; Wibisono, Y. Development of chitosan/rice husk-based silica composite membranes for biodiesel purification. Membranes 2022, 12, 435. [Google Scholar]
- Morsy, F.A.; El-Sheikh, S.M.; Barhoum, A. Nano-silica and SiO2/CaCO3 nanocomposite prepared from semi-burned rice straw ash as modified papermaking fillers. Arab. J. Chem. 2019, 12, 1186–1196. [Google Scholar] [CrossRef]
- Bwatanglang, I.B.; Magili, S.T.; Kaigamma, I. Adsorption of phenol over bio-based silica/calcium carbonate (CS-SiO2/CaCO3) nanocomposite synthesized from waste eggshells and rice husks. PeerJ Phys. Chem. 2021, 2, e17. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1996, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H. Uber die adsorption in losungen, zeitschrift fur phtsikalische chemie. Z. Phys. Chem. 1906, 62, 121–125. [Google Scholar]
- Abin-Bazaine, A.; Trujillo, A.C.; Olmos-Marquez, M. Adsorption Isotherms: Enlightenment of the Phenomenon of Adsorption. In Wastewater Treatment; Intech Open: London, UK, 2022. [Google Scholar] [CrossRef]
- Minakshi, M.; Higley, S.; Baur, C.; Mitchell, D.R.; Jones, R.T.; Fichtner, M. Calcined chicken eggshell electrode for battery and supercapacitor applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef]
- Ahmad, W.; Sethupathi, S.; Munusamy, Y.; Kanthasamy, R. Valorization of raw and calcined chicken eggshell for sulfur dioxide and hydrogen sulfide removal at low temperature. Catalysts 2021, 11, 295. [Google Scholar] [CrossRef]
- Le, V.H.; Thuc, C.N.; Thuc, H.H. Synthesis of silica nanoparticles from Vietnamese rice husk by sol–gel method. Nanoscale Res. Lett. 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Phoohinkong, W.; Kitthawee, U. Low-cost and fast production of nano-silica from rice husk ash. In Advanced Materials Research; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2014; Volume 979, pp. 216–219. [Google Scholar]
- Mohammad, F.; Arfin, T.; Al-Lohedan, H.A. Enhanced biological activity and biosorption performance of trimethyl chitosan-loaded cerium oxide particles. J. Ind. Eng. Chem. 2017, 45, 33–43. [Google Scholar] [CrossRef]
- Mandoli, C.; Pagliari, F.; Pagliari, S.; Forte, G.; Di Nardo, P.; Licoccia, S.; Traversa, E. Stem cell aligned growth induced by CeO2 nanoparticles in PLGA scaffolds with improved bioactivity for regenerative medicine. Adv. Funct. Mater. 2010, 20, 1617–1624. [Google Scholar] [CrossRef]
- Islam, K.; Haque, M.; Kumar, A.; Hoq, A.; Hyder, F.; Hoque, S.M. Manganese ferrite nanoparticles (MnFe2O4): Size dependence for hyperthermia and negative/positive contrast enhancement in MRI. Nanomaterials 2020, 10, 2297. [Google Scholar] [CrossRef]
- Du, H.; Amstad, E. Water: How does it influence the CaCO3 formation? Angew. Chem. Int. Ed. 2020, 59, 1798–1816. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.H.; Reis, T.V.; Rovani, S.; Fungaro, D.A. Green synthesis and characterization of biosilica produced from sugarcane waste ash. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Bwatanglang, I.B.; Mohammad, F.; Yusof, N.A.; Abdullah, J.; Hussein, M.Z.; Alitheen, N.B.; Abu, N. Folic acid targeted Mn: ZnS quantum dots for theranostic applications of cancer cell imaging and therapy. Int. J. Nanomed. 2016, 11, 413. [Google Scholar]
- Gautam, M.; Santhiya, D.; Dey, N. Zein coated calcium carbonate nanoparticles for the targeted controlled release of model antibiotic and nutrient across the intestine. Mater. Today Commun. 2020, 25, 101394. [Google Scholar] [CrossRef]
- Ge, M.; Wang, X.; Du, M.; Liang, G.; Hu, G.; SM, J.A. Adsorption analyses of phenol from aqueous solutions using magadiite modified with organo-functional groups: Kinetic and equilibrium studies. Materials 2018, 12, 96. [Google Scholar] [CrossRef]
- Halder, M.; Meikap, A.K. Dielectric relaxation and magnetodielectric response of mesoporous terbium manganate nanopaticles. SN Appl. Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Ng, K.C.; Burhan, M.; Shahzad, M.W.; Ismail, A.B. A universal isotherm model to capture adsorption uptake and energy distribution of porous heterogeneous surface. Sci. Rep. 2017, 7, 10634. [Google Scholar] [CrossRef]
- Rahmani, S.; Bouchmella, K.; Budimir, J.; Raehm, L.; Cardoso, M.B.; Trens, P.; Durand, J.O.; Charnay, C. Degradable hollow organosilica nanoparticles for antibacterial activity. ACS Omega 2019, 4, 1479–1486. [Google Scholar] [CrossRef]
- Aldahash, S.A.; Higgins, P.; Siddiqui, S.; Uddin, M.K. Fabrication of polyamide-12/cement nanocomposite and its testing for different dyes removal from aqueous solution: Characterization, adsorption, and regeneration studies. Sci. Rep. 2022, 12, 13144. [Google Scholar] [CrossRef] [PubMed]
- Gedam, A.H.; Dongre, R.S. Adsorption characterization of Pb(II) ions onto iodate doped chitosan composite: Equilibrium and kinetic studies. RSC Adv. 2015, 5, 54188–54201. [Google Scholar] [CrossRef]
- Tan, Y.H.; Davis, J.A.; Fujikawa, K.; Ganesh, N.V.; Demchenko, A.V.; Stine, K.J. Surface area and pore size characteristics of nanoporous gold subjected to thermal, mechanical, or surface modification studied using gas adsorption isotherms, cyclic voltammetry, thermogravimetric analysis, and scanning electron microscopy. J. Mater. Chem. 2012, 22, 6733–6745. [Google Scholar] [CrossRef]
- Bai, K.; Hao, J.; Yang, Y.; Qian, A. The effect of hydrothermal temperature on the properties of SBA-15 materials. Heliyon 2020, 6, e04436. [Google Scholar] [CrossRef]
- Zhang, W.; Li, C.; Ma, Z.; Yang, L.; He, H. Effects of calcination temperature on properties of 0.5% Al-3% In-TiO2 photocatalyst prepared using sol-gel method. J. Adv. Oxid. Technol. 2016, 19, 119–124. [Google Scholar] [CrossRef]
- Qiu, J.; Li, J.; Du, X.; Zhou, T.; Xie, B.; He, L. Synthesis and characterization of colistin-functionalized silica materials for rapid capture of bacteria in water. Molecules 2022, 27, 8292. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Tang, C.Y.; Ma, J.; Liu, M.; Ping, M.; Chen, M.; Wu, Z. Modification of microfiltration membranes by alkoxysilane polycondensation induced quaternary ammonium compounds grafting for biofouling mitigation. J. Membr. Sci. 2018, 549, 165–172. [Google Scholar] [CrossRef]
- Omar, K.; Aziz, N.; Amr, S.; Palaniandy, P. Removal of lindane and Escherichia coli (E. coli) from rainwater using photocatalytic and adsorption treatment processes. Glob. Nest J. 2017, 19, 191–198. [Google Scholar]
- Said, R.B.; Rahali, S.; Ben Aissa, M.A.; Albadri, A.; Modwi, A. Uptake of BF Dye from the aqueous phase by CaO-g-C3N4 Nananosorbent: Construction, descriptions, and recyclability. Inorganics 2023, 11, 44. [Google Scholar] [CrossRef]
- Thamilselvi, V.; Radha, K.V. Silver nanoparticle loaded silica adsorbent for wastewater treatment. Korean J. Chem. Eng. 2017, 34, 1801–1812. [Google Scholar] [CrossRef]
- Kim, S.; Song, M.H.; Wei, W.; Yun, Y.S. Selective biosorption behavior of Escherichia coli biomass toward Pd(II) in Pt(IV)–Pd(II) binary solution. J. Hazard. Mater. 2015, 283, 657–662. [Google Scholar] [CrossRef]
- Markovski, J.S.; Marković, D.D.; Đokić, V.R.; Mitrić, M.; Ristić, M.Đ.; Onjia, A.E.; Marinković, A.D. Arsenate adsorption on waste eggshell modified by goethite, α-MnO2 and goethite/α-MnO2. Chem. Eng. J. 2014, 237, 430–442. [Google Scholar] [CrossRef]
- Faghihzadeh, F.; Anaya, N.M.; Schifman, L.A.; Oyanedel-Craver, V. Fourier transform infrared spectroscopy to assess molecular-level changes in microorganisms exposed to nanoparticles. Nanotechnol. Environ. Eng. 2016, 1, 1–6. [Google Scholar] [CrossRef]
- Sukprasert, J.; Thumanu, K.; Phung-On, I.; Jirarungsatean, C.; Erickson, L.E.; Tuitemwong, P.; Tuitemwong, K. Synchrotron FTIR light reveals signal changes of biofunctionalized magnetic nanoparticle attachment on Salmonella sp. J. Nanomater. 2020, 2020, 1–2. [Google Scholar] [CrossRef]
- Fang, T.T.; Li, X.; Wang, Q.S.; Zhang, Z.J.; Liu, P.; Zhang, C.C. Toxicity evaluation of CdTe quantum dots with different size on Escherichia coli. Toxicol. Vitr. 2012, 26, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Guo, J.; Qu, J.; Hu, X. Photocatalytic degradation of pathogenic bacteria with AgI/TiO2 under visible light irradiation. Langmuir 2007, 23, 4982–4987. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Liu, P.; Wang, P.; Zhang, L. Photocatalytic degradation of E. coli membrane cell in the presence of ZnO nanowires. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2011, 26, 222–225. [Google Scholar] [CrossRef]
- Iwamoto, R.; Nara, A.; Matsuda, T. Near-infrared combination and overtone bands of the CH2 sequence in CH2X2, CH2XCHX2, and CH3(CH2)5CH3 and their characteristic frequency zones. Appl. Spectrosc. 2006, 60, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Zeroual, W.; Choisy, C.; Doglia, S.M.; Bobichon, H.; Angiboust, J.F.; Manfait, M. Monitoring of bacterial growth and structural analysis as probed by FT-IR spectroscopy. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1994, 1222, 171–178. [Google Scholar] [CrossRef]
- Davis, R.; Mauer, L.J. Fourier transform infrared (FT-IR) spectroscopy: A rapid tool for detection and analysis of foodborne pathogenic bacteria. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 2, 1582–1594. [Google Scholar]
- Riding, M.J.; Martin, F.L.; Trevisan, J.; Llabjani, V.; Patel, I.I.; Jones, K.C.; Semple, K.T. Concentration-dependent effects of carbon nanoparticles in gram-negative bacteria determined by infrared spectroscopy with multivariate analysis. Environ. Pollut. 2012, 163, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Nadtochenko, V.A.; Rincon, A.G.; Stanca, S.E.; Kiwi, J. Dynamics of E. coli membrane cell peroxidation during TiO2 photocatalysis studied by ATR-FTIR spectroscopy and AFM microscopy. J. Photochem. Photobiol. A Chem. 2005, 169, 131–137. [Google Scholar] [CrossRef]
- Kim, M.; Yoon, S.H.; Choi, E.; Gil, B. Comparison of the adsorbent performance between rice hull ash and rice hull silica gel according to their structural differences. LWT-Food Sci. Technol. 2008, 41, 701–706. [Google Scholar] [CrossRef]
- Chen, G.; Rockhold, M.; Strevett, K.A. Equilibrium and kinetic adsorption of bacteria on alluvial sand and surface thermodynamic interpretation. Res. Microbiol. 2003, 154, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Upadhyayula, V.K.; Smith, G.B.; Mitchell, M.C. Adsorption equilibrium and kinetics of microorganisms on single-wall carbon nanotubes. IEEE Sens. J. 2008, 8, 954–962. [Google Scholar] [CrossRef]
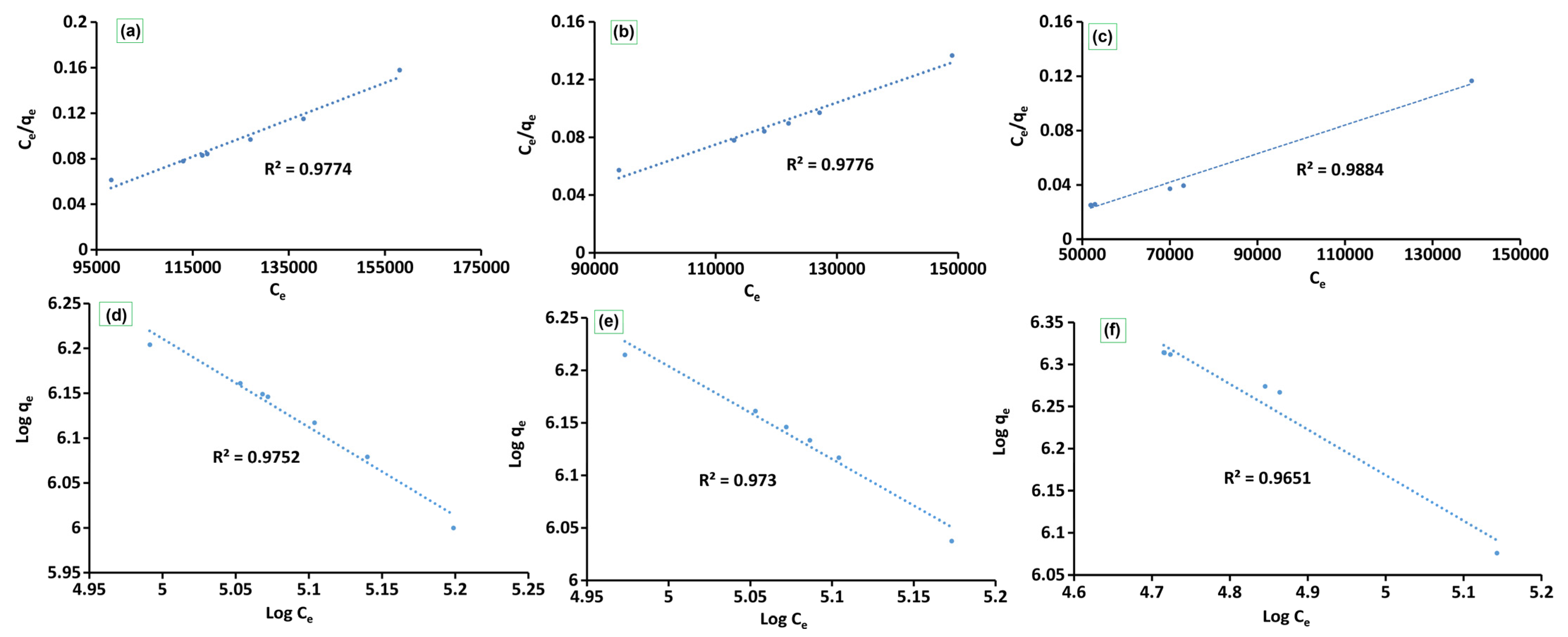

| Models | Parameters | Adsorbates | ||
|---|---|---|---|---|
| CS-SiO2/CaCO3 | RHSiO2 | ESCaCO3 | ||
| Pseudo–first order | qm (CFU g−1) | 2.63 × 1011 | 8.71 × 108 | 9.33 × 108 |
| K1 (S-1) | 1.37 × 10−3 | −3.40 × 10−3 | −3.27 × 10−3 | |
| R2 | 0.9065 | 0.9315 | 0.9355 | |
| Pseudo–second order | qm (CFU g−1) | 2.50 × 106 | 1.25 × 106 | 1.11 × 106 |
| K2 (S−1) | 6.25 × 1018 | 1.56 × 1018 | 1.23 × 1018 | |
| R2 | 0.9942 | 0.9506 | 0.9517 | |
| Langmuir | qm (CFU g−1) | 3.18 × 10 | 1.18 × 10 | 9.54 × 100 |
| KL (CFU g−1) | 3.18 × 107 | 1.18 × 107 | 4.77 × 106 | |
| R2 | 0.9884 | 0.9776 | 0.9774 | |
| RL | 8.22 × 1012 | 3.04 × 1012 | 1.23 × 1012 | |
| Freundlich | Kf (CFU g−1) | 5.87 × 108 | 4.21 × 1010 | 1.39 × 1011 |
| n | −5.42 × 10−1 | −8.84 × 10−1 | −9.87 × 10−1 | |
| R2 | 0.9651 | 0.973 | 0.9752 | |
| qm (CFU g−1) | 1.59 × 106 | 1.41 × 106 | 1.48 × 106 | |
| Dubinin–Radushkevich | ED-R (KJ/mol) | 3.14 × 10−2 | 3.65 × 10−2 | 3.04 × 10−2 |
| R2 | 0.9772 | 0.9124 | 0.9042 | |
| Adsorbents | Adsorption Maximum (qm) | Reference |
|---|---|---|
| Silicon carbide | 8.26 × 1010 cells/g | [14] |
| Sulphate calcined ES | 1.56 × 109 cells/g | [12] |
| Pt(IV) Binary solution | 3.32 × 10 mg/g | [64] |
| Pd (II) binary solution | 7.32 × 100 mg/g | |
| Fe3O4B | 6.16 × 1010 mg/g | [1] |
| Limestone | 2.50 × 103 cells/g | [61] |
| Laterite soil | 3.33 × 103 cells/g | |
| SiO2@NH2@COOHCST | 5.20 × 109 CFU/g | [59] |
| Single-Wall Carbon Nanotubes | 3.33 × 1010 CFU/g | [78] |
| ESCaCO3 | 9.54 × 100 CFU/g | This study |
| RHSiO2 | 1.18 × 10 CFU/g | |
| CS-SiO2/CaCO3 | 3.18 × 10 CFU/g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bwatanglang, I.B.; Magili, S.T.; Mohammad, F.; Al-Lohedan, H.A.; Soleiman, A.A. Biomass-Based Silica/Calcium Carbonate Nanocomposites for the Adsorptive Removal of Escherichia coli from Aqueous Suspensions. Separations 2023, 10, 212. https://doi.org/10.3390/separations10030212
Bwatanglang IB, Magili ST, Mohammad F, Al-Lohedan HA, Soleiman AA. Biomass-Based Silica/Calcium Carbonate Nanocomposites for the Adsorptive Removal of Escherichia coli from Aqueous Suspensions. Separations. 2023; 10(3):212. https://doi.org/10.3390/separations10030212
Chicago/Turabian StyleBwatanglang, Ibrahim Birma, Samuel T. Magili, Faruq Mohammad, Hamad A. Al-Lohedan, and Ahmed A. Soleiman. 2023. "Biomass-Based Silica/Calcium Carbonate Nanocomposites for the Adsorptive Removal of Escherichia coli from Aqueous Suspensions" Separations 10, no. 3: 212. https://doi.org/10.3390/separations10030212
APA StyleBwatanglang, I. B., Magili, S. T., Mohammad, F., Al-Lohedan, H. A., & Soleiman, A. A. (2023). Biomass-Based Silica/Calcium Carbonate Nanocomposites for the Adsorptive Removal of Escherichia coli from Aqueous Suspensions. Separations, 10(3), 212. https://doi.org/10.3390/separations10030212






